Abstract
Background:
Postoperative nosocomial infections remain a major problem in health care facilities, resulting in extended length of stay, substantial morbidity and mortality, high excess of cost, and less frequent cause of death in the surgical patient.
Aims:
To determine the prevalence of aerobic nosocomial pathogens among patients with postoperative wound infections at Gadarif state which located in Eastern part of Sudan.
Materials and Methods:
109 wound swabs were collected from patients who had developed postoperative wound infection. Conventional technique for isolation of bacteria was applied with analytical profile index (API system) for identification to confirm primary and secondary isolates. Antibiotics susceptibility was applied for all isolated bacteria.
Results:
Aerobic bacterial isolates were S. aureus (n=55, 55.0%), P. mirabilis (n=35, 35.0%), E. coli (n=5, 5.0%), Ps. aeruginosa (n=3, 3.0%), and Pr. vulgaris (n=2, 2.0%). The prevalence rate of hospital acquired infection were 25.23%
Conclusion:
The highest prevalence rate of nosocomial postoperative wound infection, in Sudan was due to poor antibiotic selection, for prophylaxis during and after surgery and increased level of contamination in most part of the hospital.
Keywords: Antibiotics, Nosocomial, Prophylaxis, Surgery, Wound infection
Introduction
Hospital acquired infection (HAI) is a serious health hazard worldwide. World Health Organization (WHO)[1] described it as one of the major infectious diseases having huge economic impact despite advances in the control and prevention of Nosocomial infection, they continue to remain a major side effect of hospital treatment and contribute significantly to the rate of morbidity, mortality and cost of care. It is estimated that surgical site infections develop in 2%-5% of the 16 million patients undergoing surgical procedures each year.[2,3] They account for about 24% of all nosocomial infections. The problem is aggravated in developing countries where resources are scarce and staffs are always in short supply.[4]
Nosocomial infections invariably occur as a result of close environment where medical and paramedical staff is in close contact with the patient in various stages of treatment. It is very difficult to accurately pinpoint the source of infection. Nosocomial infections involve diverse anatomic sites, but the risk of these various types of infections and consequently their relative frequency appears to be similar in most hospitals.[5]
Infection of a wound is difficult to define and no clear rules can be given to distinguish it from contamination and colonization. Wounds and other lesions are prone to contamination with a multitude of organisms from the body surfaces and environment; the contaminating organisms are at first generally present in relatively small numbers, as originally introduced, and need not subsequently multiply.[6] Infection occurs when one or more of the contaminant evades the clearing effect of the host's defenses’, replicates in large numbers and attacks and harms the host's tissues. In the case of commensal or low-grade pathogen, the multiplication may cause little or no harm to the host and may be best described as colonization.[6] Whether harmful infection or harmless colonization occurs is dependent on the virulence of the organisms and the local and the general resistance of the host. Knowledge of the patient's general and local condition is therefore important in assessing the significance of bacteriological findings. Infection in a wound delays healing and may cause wound breakdown, herniation of the wound and complete wound dehiscence.[7] In spite of technological advances that have been made in surgery and wound management, wound infection has been regarded as the most common nosocomial infection especially in patients undergoing surgery.[8]
A wide variety of aerobic and anaerobic species of bacteria may be present, either singly or in combination, in infections of wounds, are generally associated with the production of pus and the bacteria involved are said to be “pyogenic” (pus producing).[6] Postoperative infection complications are a frequent cause of morbidity and mortality in surgical patients. These septic events usually involve the urinary and the respiratory tracts or occur in the operative wound. The overall incidence of the postoperative wound infections varies from surgeon to surgeon, from hospital to hospital and from one surgical procedure to anther.[9] The lowest infection rate (less than 2%) followed clean operations, such as elective orthopedic procedures, in which the possible sources of contamination were solely airborne or exogenous. Clean-contaminated operations that resulted in additional exposure of the operative site to the endogenous microflora had high rates of infections (10-20%).[9]
The average hospital stay doubled and the cost of hospitalization were thus increased when post-operative wound infection developed after any commonly performed operation.[10] The majority of post-operative wound infections are uncomplicated, involving only the skin and subcutaneous tissues. Infrequently, they progress to become necrotizing infection, which may involve the fascia and muscle 8. The usual clinical presentation of uncomplicated wound infection includes local incisional pain and tenderness, swelling, redness, and increased warmth and elevated body temperature, which most often begin between the fourth and the eighth postoperative day.[8]
The aims of this study are to determine the prevalence of aerobic Nosocomial pathogens among patients with post-operative wound infections and to evaluate the antibiotic susceptibility pattern of these pathogens to the most commonly antibiotics used in teaching Hospital at Gadarif State.
Materials and Methods
Patients and specimen collection
This study was prospective cross sectional study. 432 different operations took place at Gadarif Teaching Hospital during the period from April 2006 to April 2008, One hundred nine wound swabs were collected from patients who had developed postoperative wound infection with purulent discharge and clinically diagnosed as postoperative sepsis. Purulent materials were collected on sterile commercial cotton swabs aseptically and gently to avoid contamination of the specimens with normal microbial flora of the skin. Specimens were collected before redressing and administration of antibiotic therapy. Specimens were labeled, kept in a thermoflask containing ice and transferred immediately to the laboratory for bacteriological examination.
Cultural methods
Post-operative wound swabs were used to inoculate blood agar, nutrient agar, chocolate agar plates and mannitol salt agar (Plasmatic Ltd., UK). These inoculated plates were cultivated aerobically at optimum temperature 37°C for overnight (18-24 h).
Test control organisms
Organisms of American Type of Culture Collection (ATCC) were used as test control organisms. They were S. aureus (St. 25923), E. coli (25922), P. vulgaris (13315), P. mirabilis (35659), K. pneumoniae (35657), and Ps. aeruginosa (27853).
Examination of cultures and identification of isolates
All cultures were examined with the naked eye for growth and colonial morphology as well as any changes in the media. Plates, which showed visible growth, were subjected to subsequent bacteriological tests. Those which did not show visible growth were reincubated and examined daily for up to 7 days. Standard bacteriological identification of the isolates using Biochemical test previously describe.[11]
API System
All isolated bacteria were subjected to API system identification to confirm primary and secondary identification. API-Staph was used to identify S. aureus and API-20E to identify members of the family Enterobacteriaceae and associated organisms according to manufacturers’ procedures (BioMerieux, Inc. Hazelwood, MO., France.).
Pastorex staph plus test
This is a latex test kit supplied by Sanofi Diagnostics Pasteur (France). The test was used to detect and confirm isolated strains of MRSA.
Antibiotic susceptibility test
All isolated bacteria were subjected to a number of antibiotics by disc diffusion technique (Kirby-Bauer method). The growth was used to inoculate on a plate of Diagnostic Sensitivity Test (DST) agar medium. Commercially prepared antibiotic discs (Plasmatic Ltd., UK) were placed on the surface of the medium by sterile forceps. Discs were gently pressed to ensure full contact with the medium. Plates were incubated at 37°C for 24 h. The zone of growth inhibition around each disc was measured in millimeters and the result was reported either sensitive or intermediate or resistant. Antibiotics used in susceptibility test were Penicillin (1 IU), Gentamicin (10 μg), Ciprofloxacin (5 μg), Ceftazidime (30 μg), Cephalaxin (30 μg), Vancomycin (30 μg), Methicillin (10 μg), and Fusidic acid (10 μg).
β-lactamase test
β-lactamase test strips were used to examine β-lactamase production of the isolated bacteria. The test was performed according to the manufactures procedure (Abtek Biologicals Ltd., Liverpool).
Statistical analysis of the data
Statistical package for social sciences (SPSS) version 9.05 software was used for data analysis. The 2-tailed chi-squire test and Fisher exact test were used for categorical variables, Chi-square test and Student's t-test for continuous variables for the univariate comparison. A two-tailed P<0.05 was considered as statistically significant.
Results
Out of the 432 operations done at Gadarif Teaching Hospital; 109 Wound swabs were collected from patients who had developed post-operative wound infection with purulent discharge and clinically diagnosed as post-operative wound infection, these 100 hospitalized patients, were grown and identified as aerobic bacterial species with frequency rate of the infection 23.15%.
The mean age of hospitalized patients was 40.27 years (4-78 years); patients subjected to the study were 54% female patients with mean age 38.63 years and 46% male patients with mean age 42.20 years. Age confidence intervals 37.29-43.25 and the accepted P-value P<0.05.
Post-operative wound infections isolates
Aerobic bacteria isolated included S. aureus (n=55, 55.0%), Pr. mirabilis (n=35, 35.0%), E. coli (n=5, 5.0%), Ps. aeruginosa (n=3, 3.0%), and Pr. vulgaris (n=2, 2.0%).
Susceptibility of the isolates
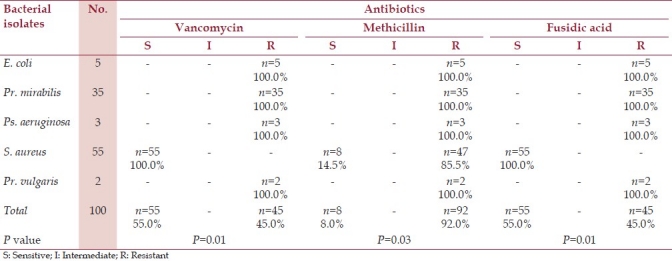
Antibiotic susceptibility testing was performed on the aerobic bacteria isolated from patients with post-operative wound infections. Tables 1 and 2 show sensitivity, patterns of the isolated bacteria to antibiotics, their number and percentage (the accepted P-value: P<0.05).
Table 1.
Antibiotic susceptibility tests for aerobic bacteria isolates from patients with postoperative wound infections
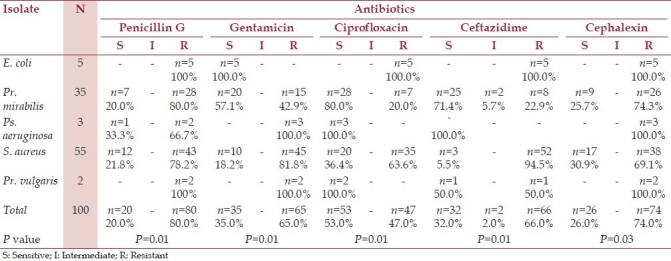
Table 2.
Antibiotic susceptibility tests for aerobic bacteria isolates from patients with postoperative wound infection
β-lactamase production
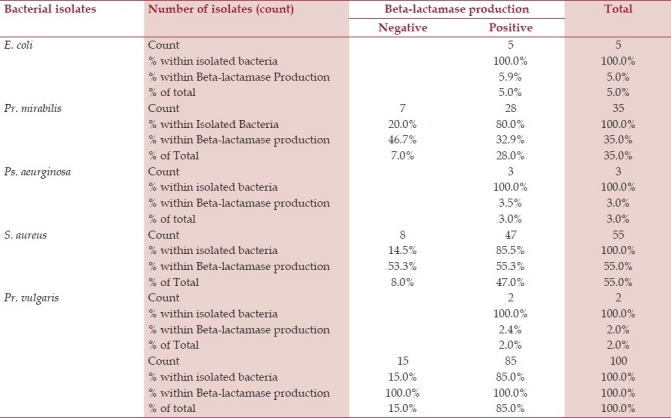
β-lactamase production test was performed to the isolated bacteria, all strains of E. coli (n=5, 100.0%) were positive, 28(80.0%) strains of Pr. mirabilis were positive and 7(20.0%) negative, all strains of Ps. aeruginosa (n=3, 100.0%) were positive, 47(85.5%) strains of S. aureus were positive and 8(14.5%) were negative, and all strains of Pr. vulgaris were positive (n=2, 100.0%) [Table 3].
Table 3.
βeta-lactamase production of aerobic bacteria isolated from patients with post-operative wound infections
Discussion
Hospital-acquired infections have increased worldwide, contributing considerably to morbidity of the hospitalized patients.[12] This can prolonging hospital stays, which can add significantly to the economic burden to manage the underlying disease. This study was the first research done in the Eastern Sudan as well as Gadarif state.
The mean age of patients in this study, who developed postoperative wound infections was 40.3 years (4-78 years). The majority of patients were >55 years of age, suggesting that advancing age is an important risk factor for this type of hospital-acquired infection. The infection may be acquired in the operating room, as 10/63 (15.87%) strains of S. aureus were identified as contaminants in these important site. Two of the strains (2/10) were identified as Methicillin-resistant S. aureus (MRSA). In Addition to aerobic bacterial strains that identified in wards which contribute to the infections about 28/63 (44.44%) of S. aureus n=13, 20.63%; Pr. mirabilis n=6, 9.53% and Ps. aeruginosa n=9, 14.29%. Similar observations had been recorded in studies done in Taiwan and Spain.[13] In these reports, the majority of patient's particularly elderly patients acquired infection in the operating room and in medical specialty wards.[13]
Our study showed that the prevalence rate of Hospital Acquired Infection (HAI) among post-operative wound infections in Gadarif Teaching Hospital (Eastern Sudan) was 25.2%, is higher than the A prevalence survey conducted by the World Health Organization (WHO) in 55 hospitals of 14 countries representing 4 WHO Regions (Europe, Eastern Mediterranean, South-East Asia and Western Pacific). This showed an average of 8.7% of hospital patients had HAIs. At any time, over 1.4 million people worldwide suffer from infectious complications acquired in hospital. The highest frequencies of HAIs were reported from hospitals in the Eastern Mediterranean and South-East Asia Regions (11.8% and 10.0%, respectively), with a prevalence of 7.7 and 9.0% respectively in the European and Western Pacific Regions.[14]
Another four surveys were conducted in Norway in 2002 and 2003. The total prevalence of the four recorded nosocomial infections varied between 5.1% and 5.4% in the four surveys. In all surveys, nosocomial infections were located most frequently in the surgical sites (28%) The prevalence surveys give a brief overview of the burden and distribution of nosocomial infections.[15] Moreover, the prevalence of HAI in Tanzania was particularly high in the medical intensive-care unit (40%), the surgical (orthopaedic and general surgery) wards (36.7%), and one of the general medical wards (22.2%). Factors associated with a patient having a HAI were hospitalization for >30 days.[16] Prevalence is higher than our study, but the risk factor is similar for prolonged hospitalization stay for more than 10 days.
Staphylococcus aureus is a major cause of infection in both healthcare and community settings. It is one of the most common causes of healthcare-associated infections reported to the National Nosocomial Infections Surveillance (NNIS) System, including surgical site infection and ventilator-associated pneumonia.[17] Our study revealed that the S. aureus strains were the major causes of HAI from post-operative wound infections. Forty-five strains were identified as MRSA. Other aerobic bacteria isolated were gram-negative bacteria in particular Pr. mirabilis, P. vulgaris, E. coli and P. aeruginosa. Faria et al., reported that MRSA has remained a major cause of nosocomial disease worldwide,[18] causing 50% or more of hospital-acquired S. aureus infections in several countries.[18] Gram-negative bacteria were also frequently responsible for post-operative wound infections in a study conducted in New Orleans, USA, among these organisms were P. mirabilis, P. vulgaris, E. coli and Ps. aeruginosa.
This study also looked at the susceptibility patterns of aerobic bacteria responsible for post-operative wound infections. Isolated strains of S. aureus were in considerable variation in term of antibiotic susceptibility, in which 47/55 of the isolated strains were identified as methicillin-resistant S. aureus (MRSA). Vancomycin and Fusidic acid appeared to be of greater choice because of their effectiveness against all isolated strains including MRSA (complete susceptibility). The susceptibility to Gentamicin and Ciprofloxacin to some extent accepted, as it was 81.8% and 63.6%, respectively. In study conducted in Denmark methicillin-resistant S. aureus (MRSA) isolated were typically resistant to fluoroquinolones (essentially ciprofloxacin) and macrolides. Furthermore, third-generation cephalosporins have poor activity against methicillin-resistant S. aureus (MRSA).[17] In the last 20 years, MRSA has spread throughout the world in healthcare settings, leading to an increased reliance on vancomycin for empiric treatment.[19] This study supported the strategy of that treatment, due to highly susceptibility of the all isolates of S. aureus, including MRSA to vancomycin, and fusidic acid. These data showed no standard methods can be used for surgical prophylaxis for the patients in Gadarif teaching hospital.
In this study aerobic gram-negative bacterium isolated from post-operative wound infections obviously differed in susceptibility to antibiotics; E. coli showed resistance to all antibiotics used except gentimicin (5/5 sensitive). P. mirabilis (35/100) was frequently susceptible to gentamicin (20/35), ciprofloxacin (28/35) and ceftazidime (25/35). P. vulgaris was susceptible to ciprofloxacin (2/2), ceftazidime (1/2) and resistant to the rest of other antibiotics used. Ps. aeruginosa also showed susceptibility only to ciprofloxacin (3/3) and ceftazidime (3/3), eliminating the pharse “and mounted resistance against others.”
Conclusion
The high prevalence rate of nosocomial post operative wound infection in the teaching hospital in Gadarif state was estimated due to poor antibiotic selection for prophylaxis during and after surgery. Moreover, the levels of contamination in the most part of the hospital were above the normal.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.WHO. Surveillance, control and prevention of hospital acquired (nosocomial) infections. Report of an advisory group. 1981 BAC/NIC/81.6. [Google Scholar]
- 2.Blomstedt GC. Infection in Neurosurgery: A restrospective study of 1143 patients and 1517 operations. Acta Neurochir (Wien) 1985;78:81–90. doi: 10.1007/BF01808684. [DOI] [PubMed] [Google Scholar]
- 3.Albright L, Reigel DH. Management Hydrocephalus secondary to posterior fossa tumours. J Neurosurg. 1977;46:52–5. doi: 10.3171/jns.1977.46.1.0052. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal M, Thomas P. Prevalence of post-op. nosocomial infection in neuro-surgical patients and associated risk factors - A prospective study of 2441 patients. (212).Nurs J India. 2003;94:197–8. [PubMed] [Google Scholar]
- 5.Hansis M, Arens S, Wingenfeld C. [Rate of infection in trauma surgery.An overview based on recent German language literature] Unfallchirurg. 1997;100:457–64. doi: 10.1007/s001130050142. [DOI] [PubMed] [Google Scholar]
- 6.Collee JG, Fraser AG, Marmion BP, Simmons A. Infections of wounds and other tissues. In: Mackie, McCartney, editors. Practical Medical Microbiology. 14th ed. Singapore: Longman Publishers (Pte) Ltd; 1996. pp. 66–7. [Google Scholar]
- 7.Alexander MF. Wound Infection. In: Alexander MF, Fawcett JN, Runciman PJ, editors. Nursing Practice Hospital and Home, the Adult. London, UK: Churchill Livingstone; 1994. p. 703. [Google Scholar]
- 8.Dionigi R, Rovera F, Dionigi G, Imperatori A, Ferrari A, Dionigi P, et al. Risk factors in Surgery. J Chemother. 2001;13:6–11. doi: 10.1179/joc.2001.13.Supplement-2.6. [DOI] [PubMed] [Google Scholar]
- 9.Mandell GL, Douglas RG, Bonnett JE. In: Principles and Practice of Infectious Diseases. 2nd Ed. Awiley Medical Publications; 1985. Post-operative wound infections and prophylaxis; pp. 1637–43. [Google Scholar]
- 10.Green JW, Wenzel RG. Post-operative wound infections: A controlled study on the duration of hospital stay and direct cost of hospitalization. Ann Surg. 1977;185:264. doi: 10.1097/00000658-197703000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochei J, Kolhatkar A. Medical Microbiology Science, theory and practice. New Delhi: Tata McGraw-Hill Publishing Company Limited; 2000. pp. 525–856. [Google Scholar]
- 12.Panhotra BR, Saxena AK, Al-Ghandi AM. Extended-spectrum Beta-lactamase-producing K. pneumoniae hospital-acquired bacteraemia, risk factors and clinical outcome. Saudi Med J. 2004;25:1871–6. [PubMed] [Google Scholar]
- 13.Tsay RW, Siu LK, Fung CP, Chang FY. Characteristic bacteraemia between community-acquired and nosocomial K. pneumoniae infections, risk factors for mortality and impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med. 2002;162:1021–7. doi: 10.1001/archinte.162.9.1021. [DOI] [PubMed] [Google Scholar]
- 14.Prevention of hospital-acquired infections: A practical guide. 2nd ed. 2002. WHO/CDS/CSR/EPH; p. 12. [Google Scholar]
- 15.Eriksen HM, Iversen BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40–5. doi: 10.1016/j.jhin.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Gosling R, Mbatia R, Savage A, Mulligan JA, Reyburn H. Prevalence of hospital-acquired infections in a tertiary referral hospital in northern Tanzania. Ann Trop Med Parasitol. 2003;97:69–73. doi: 10.1179/000349803125002724. [DOI] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992–June 2001, issued June 2001. Am J Infect Control. 2001;29:404–21. doi: 10.1067/mic.2001.119952. [DOI] [PubMed] [Google Scholar]
- 18.Faria NA, Oliveira DC, Westh H, Monnet DL, Larsen AR, Skov R, et al. Epidemiology of emerging methicillin-resistant Staphylococcus aureus (MRSA) in Denmark: A nationwide study in a country with low prevalence of MRSA infection. J Clin Microbiol. 2005;43:1836–42. doi: 10.1128/JCM.43.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–72. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]


