Review on mechanisms of monocyte differentiation/maturation, HIV infectivity, and transmigration into the CNS parenchyma that contribute to cognitive impairment in HIV infected individuals.
Keywords: brain, CD, dementia, HAND
Abstract
HIV continues to be a global health crisis with more than 34 million people infected worldwide (UNAIDS: Report on the Global AIDS Epidemic 2010, Geneva, World Health Organization). HIV enters the CNS within 2 weeks of infection and establishes a spectrum of HAND in a large percentage of infected individuals. These neurologic deficits greatly impact the quality of life of those infected with HIV. The establishment of HAND is largely attributed to monocyte transmigration, particularly that of a mature CD14+CD16+ monocyte population, which is more susceptible to HIV infection, across the BBB into the CNS parenchyma in response to chemotactic signals. To enter the CNS, junctional proteins on the monocytes must participate in homo- and heterotypic interactions with those present on BMVECs of the BBB as they transmigrate across the barrier. This transmigration is responsible for bringing virus into the brain and establishing chronic neuroinflammation. While there is baseline trafficking of monocytes into the CNS, the increased chemotactic signals present during HIV infection of the brain promote exuberant monocyte transmigration into the CNS. This review will discuss the mechanisms of monocyte differentiation/maturation, HIV infectivity, and transmigration into the CNS parenchyma that contribute to the establishment of cognitive impairment in HIV-infected individuals. It will focus on markers of monocyte subpopulations, how differentiation/maturation alters HIV infectivity, and the mechanisms that promote their increased transmigration across the BBB into the CNS.
Introduction
The advent of cART rendered systemic HIV infection more manageable, successfully increasing survival of infected individuals, lowering viral load, and maintaining CD4 cell counts [1–4]. Despite the success of cART in quelling peripheral infection, HIV continues to enter the CNS, resulting in the development of cognitive, behavioral, and motor deficits associated with HAND in 40–60% of HIV-infected individuals [5, 6]. This may be due, at least in part to the fact that cART is not usually administered immediately upon exposure to HIV. As HIV enters the CNS very early after peripheral infection [7], it is likely that the virus is present in the CNS of many infected individuals before they even receive antiretroviral therapy. Postmortem analyses demonstrate that 80–90% of HIV-infected individuals undergo neuropathological changes, suggesting that HAND may be more frequent than current epidemiology indicates [8]. HAND, the collective term for a spectrum of cognitive impairments that affect HIV-infected individuals, presents as a range of clinical manifestations. It is classified according to the severity of cognitive dysfunction, as determined by neuropsychological testing, and the extent to which it impacts the daily living of those affected. The three classifications of HAND, in order of increasing severity, are asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HAD [9]. In the past, HAD was commonly seen in HIV-infected individuals, but the use of antiretroviral therapies has decreased the incidence of this severe form of HAND. Now milder types of impairment are more frequent. As HIV-infected individuals live longer, the prevalence of HAND is increasing [10–13].
HAND is often difficult to diagnose, as neurocognitive testing provides a snapshot of an individual's cognitive function and may not accurately reflect the dynamic nature of the disorder [14]. Complicating things further, HAND does not follow a linear course of events. Rather than progressively declining over time, neurocognitive ability fluctuates and can plateau, improve, and/or worsen throughout the course of HIV infection [15].
PHENOTYPE OF THE MATURING MONOCYTE
HAND continues to develop in a large percentage of HIV-infected individuals. A mature monocyte subpopulation is believed to play a critical role in promoting the series of events that leads to neurocognitive impairment, namely by transmigrating across the BBB and establishing infection and chronic inflammation within the CNS. While the human monocyte classification system is mainly based on surface expression of CD14—the LPS receptor—it has been known for 20 years that peripheral blood monocytes are heterogeneous cells with differing sizes, granularities, levels of maturation and activation, and functions [16, 17]. An additional marker among monocyte populations is CD16—the FcγIIIR—although most peripheral blood monocytes of healthy individuals are CD14+CD16− [18]. CD14+ monocytes that also have surface CD16 are believed to be a more mature population [19]. There is additional heterogeneity within the CD14+CD16+ population based on differing surface amounts of each protein: some double-positive monocytes have higher levels of CD14 (CD14++CD16+), whereas others are dimmer (CD14+CD16++), as determined by flow cytometry. There is some variability in the percentages of these two populations in the blood among individuals. In this review, the discussion of CD14 and CD16 antigens, as well as of other markers on monocytes, refers only to cell-surface expression, unless stated otherwise.
Until recently, there was no consensus in the literature to distinguish among monocyte subpopulations, and there was no uniformity in monocyte description in the majority of studies. Updated monocyte nomenclature by the NC-IUIS assigned new terminology to each subset. The major population of monocytes in the peripheral blood is termed classical monocytes and expresses only CD14 (CD14++CD16−). Those that maintain high levels of CD14, while gaining CD16 (CD14++ CD16+), are termed intermediate monocytes, and the monocytes with high CD16 and lower levels of CD14 (CD14+CD16++) are termed nonclassical monocytes [20]. The NC-IUIS standardized the parameters used to distinguish among these three subsets, based on the levels of CD14 and CD16, compared with IgG-negative controls in flow cytometry. For simplicity, this review will term all monocytes with CD14 and CD16 as being CD14+CD16+, regardless of the relative amounts of each antigen.
Heterogeneity also exists among mouse peripheral blood monocytes, and the NC-IUIS issued nomenclature regarding their respective subpopulations as well. Mouse monocytes differ from human monocytes in identifying markers and perhaps in their roles in immune defense. Mouse monocytes are characterized by their expression of M-CSFR (CD115), F4/80, and integrin alpha M (CD11b) [21]. Within these CD115+F4/80+CD11b+ cells are two subsets with differing levels of Ly-6C and CD43 [20]. Mouse cells will not be discussed further here. For an excellent review of these cells and the differences between human and mouse monocytes, refer to Robbins and Swirski [22].
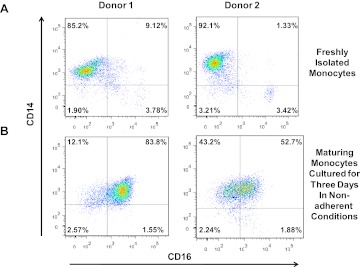
The CD14+CD16+ monocyte subpopulation makes up only 5–10% of all peripheral blood monocytes as shown in Fig. 1A; however, this percentage can expand to ∼40% in HIV-infected individuals [23–25]. The expansion of the CD14+CD16+ monocyte subpopulation in the blood may be predictive of HIV-associated neurocognitive decline [26–29]. Whereas the mature CD14+CD16+ subset is implicated in CNS HIV pathogenesis and the establishment of HAND, the phenotype of the monocyte that enters the CNS and is susceptible to HIV infection is not fully characterized. As the CD14+CD16+ monocyte population is present in such low numbers in the peripheral blood of healthy individuals, analysis of the phenotypic markers specific to this subset is difficult. We therefore developed a culture method that enables the enrichment of this monocyte subset, as monocytes do not proliferate. Using this system, we characterized markers associated with monocyte maturation, susceptibility to HIV infection, and transmigration [30]. This system is established as follows. PBMCs are isolated by Ficoll-Paque density gradient, and monocytes are obtained by positive CD14 selection. They are then cultured nonadherently in Teflon flasks to prevent adherence-mediated monocyte differentiation into macrophages, with M-CSF for 3 days, as monocytes circulate in the periphery before entering tissues for this time frame [31–33]. In agreement with data from others [18, 23, 34], we found the majority of freshly isolated peripheral blood monocytes to express CD14, and 5–10% of these cells also express CD16 (Fig. 1A). However, upon maturation with 3 days in nonadherent culture, the number of monocytes with CD16 increased (Fig. 1B). The extent of CD16 expansion was donor-dependent.
Figure 1. CD14+CD16+ monocytes increase in numbers in nonadherent culture conditions over 3 days.
Human monocytes were isolated by CD14-positive selection from PBMCs (two representative individuals are shown, Donor 1 and 2). (A) Freshly isolated and (B) monocytes maturing for 3 days in nonadherent conditions with M-CSF in our culture system were stained with CD14 APC and CD16 PECy7-coupled antibodies. CD14+CD16+ monocytes are present in low numbers in freshly isolated, circulating monocytes. Their numbers significantly increase after 3 days in nonadherent culture conditions. This increase varies among donors.
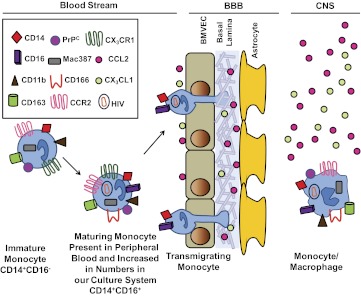
In addition to CD14 and CD16, other markers used to identify monocyte subsets include CD11b, CD163, CD166, Mac387, PrPC, CCR2, and CX3CR1. Figure 2 is a schematic representation of these markers on freshly isolated monocytes and maturing monocytes in our culture system, as compared with in vivo, and of the transmigration process into the CNS. These markers have been found to be important in monocyte function and some may also have implications in HIV neuropathogenesis. As we could generate sufficient numbers of CD14+CD16+ monocytes for analysis using our culture system, we found that this subpopulation also expresses CD11b, CD163, CD166, and Mac387 [30]. Additionally, we found that CX3CR1 and CCR2 were expressed on the CD14+CD16+ population (see detailed description below), as well as was PrPC [30] (Table 1). Another study examined the CD14+CD16− and CD14+CD16+ monocyte populations by microarray analyses and identified markers that further distinguish phenotypic differences between these subsets, including the CSF-1R [40].
Figure 2. Schematic representation of the correlation of monocyte maturation in vivo with our nonadherent culture system of CD14+ monocytes.
Circulating peripheral blood monocytes are mainly CD14+, and a small population also expresses CD16. In the blood, these CD14+CD16+ cells represent 5–10% of the monocyte population. In our nonadherent culture system, these cells greatly increase in numbers (see text and Fig. 1). The CD14+CD16+ cells in the blood and in our model culture system also express CD11b, CD163, PrPC, Mac387, CD166, CCR2, and CX3CR1. This CD14+CD16+ maturing monocyte subset is susceptible to HIV infection and preferentially transmigrates across the BBB in response to specific chemokine gradients. Upon transmigration across the BBB, these cells differentiate into monocyte/macrophages.
Table 1. Markers Found on Immature CD14+CD16− Monocytes and on Maturing CD14+CD16+ Cells in the Blood and on Monocytes Cultured Nonadherently for 3 Days in Our Model System.
| Peripheral blood |
CD14+CD16+ Day 3 and transmigrating monocyte in our system | |||
|---|---|---|---|---|
| CD14+CD16− immature monocytes in blood: 90–95% of monocytes | CD14+CD16+ maturing monocytes in blood: 5–10% of monocytes | References | ||
| CD11b | ++ | ++ | [35–39] | ++ [30] |
| CD163 | +/− | ++ | [35, 36, 40] | ++ [30] |
| CD166 | Not yet distinguished among subpopulations | +/++a [30] | ||
| CD31 | ++ | ++/+++ | [35, 37, 39, 40] | ++/+++a [30] |
| CD99 | +/++ | + | [40, 41] | +/++a |
| CCR2 | ++ | +/++ | [35, 36, 39, 40, 42] | +/++a [30] |
| CX3CR1 | +/− | ++ | [35, 39, 40] | ++a |
Unpublished results. +/−, variable expression; +, low expression; ++, intermediate expression; +++, high expression.
CD11b is a monocyte-specific adhesion molecule that facilitates monocyte adherence to ECs and its subsequent migration into tissues [43]. As the alpha M component of the Mac1 integrin complex, CD11b mediates binding to ECs by its interactions with ICAM-1 [44]. While it has not been implicated directly in monocyte transmigration into the CNS during the pathogenesis of NeuroAIDS, it has been shown that upon monocyte activation, which may occur with inflammation and/or HIV infection, this ligand increases avidity for its receptor [45, 46]. This may facilitate tighter binding to the BBB, enabling the series of events leading to diapedesis.
CD163 is a hemoglobin scavenger receptor and a member of the scavenger receptor cysteine-rich group B family; it is expressed exclusively on cells of the monocytic lineage, including microglia and perivascular macrophages of the CNS [47–49]. CD163-positive macrophages are implicated in inflammation and are found within inflamed tissues, including the CNS. During the development of HAND, an accumulation of CD163-positive monocytes/macrophages in the CNS may occur, at least in part, as a result of the increased numbers of CD14+CD16+CD163+ monocytes in the blood of those with detectable HIV infection as compared with uninfected or HIV-infected individuals with undetectable viral loads [50]. CD163-positive monocytes/macrophages also accumulate in nonhuman primate SIVE perivascularly within nodular lesions [49, 51, 52]. CD14+CD16+ monocytes/macrophages, which are also CD163+, may therefore be an important biomarker for HAND and AIDS progression.
Mac387 is an intracellular marker of recently infiltrated monocytes/macrophages into tissues [53–55]. In one study, Mac387+ macrophages were arranged in cell clusters with T cells that may have initiated their activation, enabling them to become more susceptible to SIV infection [56]. Mac387+ cells have also been found in high levels in the brains of mildly encephalitic macaques [57], suggesting that these cells had recently infiltrated the CNS. Mac387 is present in freshly isolated monocytes, as well as in the maturing monocyte population in our culture system [30]. This marker may therefore be an important tool in assessing the monocyte population that enters the CNS.
PrPC is the cellular isoform of the protease-resistant protein of the nonpathogenic human prion protein localized to membrane raft microdomains, where it participates in signal transduction [58, 59]. PrPC also serves as an adhesion molecule and is highly expressed in the CNS, where it is constitutively found on BMVECs, glial cells, neurons, and macrophages/microglia [60, 61]. PrPC is present on monocytes, and PrPC-PrPC homotypic interactions facilitate transmigration across BMVECs [62]. We found PrPC to be a potential biomarker of HIV-associated neurocognitive impairment [63]. PrPC is increased in the CNS of those with HAND but not HIV-positive individuals without cognitive impairment. This increase was mirrored in the CSF, where shed PrPC levels correlated with the level of cognitive function [63]. PrPC is also increased in the brain of macaques with SIVE [63].
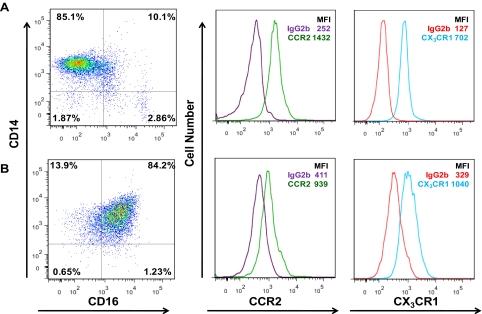
The expression of CCR2, the receptor for the chemokine CCL2 (MCP-1), and CX3CR1, the receptor for the chemokine CX3CL1 (fractalkine), on monocyte subpopulations is controversial. CCR2 is generally associated with the classical CD14++CD16− monocyte, whereas CX3CR1 is believed to be expressed primarily by the CD14+CD16+ monocyte subpopulation [64]. The presence of CX3CR1 on the classical CD14++CD16− monocyte and CCR2 on the CD14+CD16+ monocyte subset is believed to be very low, at almost undetectable levels. However, we showed that CCR2 [30] and CX3CR1 are expressed on freshly isolated (Fig. 3A), as well as on Day 3 (Fig. 3B), maturing monocytes cultured in our system (Fig. 3). The receptors on the monocytes obtained from our in vitro model system are functional. Our data indicate that the same mature monocyte population transmigrates across our BBB model (see description below) to a similar extent in response to CCL2 or CX3CL1 (unpublished results). Our data are in agreement [65] and differ with those found by other groups [64, 66, 67]. It is possible that we detect these receptors, as we use sensitive methods of flow cytometry, and our culture system enables us to examine large numbers of cells. However, our data do not distinguish among the additional subpopulations within the CD14+CD16+ monocyte subset. As the sensitivity of antibodies and multicolor flow cytometric analyses improves, a more detailed study of these chemokine receptors on monocyte subpopulations will be possible.
Figure 3. Both CCR2 and CX3CR1 are present on freshly isolated and mature monocytes.
(A) Freshly isolated monocytes or (B) mature monocytes cultured nonadherently with M-CSF for 3 days in our system were stained with CD14 PE and CD16 PECy7 and CCR2 AlexaFluor 647 or CX3CR1 FITC-coupled antibodies and analyzed by flow cytometry. Histograms of CCR2 and CX3CR1 include the mean fluorescence intensity (MFI) as compared with its IgG isotype-matched control antibody.
STEPS IN MONOCYTE TRANSMIGRATION
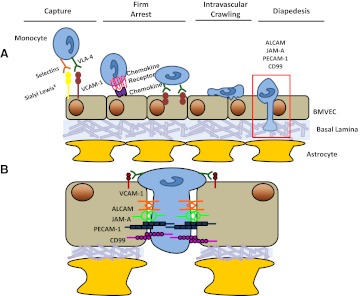
The transmigration of monocytes into the CNS establishes the neuroinflammation associated with HAND and is a multistep process involving the interaction of adhesion molecules and tight junction proteins, which we will term junctional proteins, on ECs with the transmigrating monocyte. Much of the understanding of monocyte transmigration and diapedesis is from studies of the peripheral vasculature. There is less information regarding transmigration across the highly specialized BBB. Many of the components involved in these processes are likely conserved between the CNS and the periphery, although there certainly may be differences between the two. The steps in transmigration include monocyte capture from the circulating blood flow onto the EC, rolling, firm arrest, intravascular crawling, and ultimately, diapedesis through the EC layer into the underlying tissue [68] (Fig. 4).
Figure 4. Monocyte transmigration across the BBB.
Monocyte transmigration is a multistep process, (A) consisting of capture and rolling, chemokine-mediated activation of the monocyte and subsequent firm arrest, intravascular crawling with the formation of focal adhesions to the vasculature, and ultimately, diapedesis. (B) An enlarged image illustrating some of the homotypic monocyte–EC interactions, which mediate diapedesis.
Capture—the loose tethering and initial binding step of monocytes to the EC—is mediated by selectins. This tethering slows the monocyte, and selectins, as well as the integrin VLA-4, promote rolling along the vasculature [69]. Monocyte firm arrest is triggered by chemokines and mediated by ICAM-1 and VCAM-1 on the EC and LFA-1, CD11b, and VLA-4 on the monocyte. The final step in transmigration is diapedesis and involves the opening of junctions of the endothelium to interact with the junctional proteins on monocytes in a zipper-like manner, quickly sealing as the monocytes diapedese, and can occur as rapidly as 90 s [68].
Transmigration can occur between ECs (paracellular transmigration) or through the EC (transcellular transmigration), although the latter mechanism is less frequent, accounting for only 9–30% of total transmigrated events [70]. The conditions under which cells transmigrate para- or transcellularly are unclear. They may be related to the strength of the junctions present in the EC layer and/or to the activation state of the transmigrating monocyte [71]. The predominant mechanism of transmigration across the BBB is not known. Furthermore, it is unknown whether HIV alters the prevalence of one pathway over the other. Regardless, many of the same junctional proteins are used to facilitate both forms of diapedesis [71].
The CD14+CD16+ monocyte preferentially transmigrated across our in vitro model of the BBB in response to CCL2 [30]. Our model consists of human BMVECs and human astrocytes cocultured on opposite sides of a gelatin-coated tissue culture insert with 3 μm pores [72, 73]. The cocultures grow to confluence over 3 days, at which time, the astrocytic processes penetrate the insert and contact the BMVEC to seal the barrier. To characterize further this monocyte subpopulation that transmigrates across the BBB, we performed microarray analyses from the RNA of maturing monocytes added to the coculture system and of transmigrated monocytes and found 474 differentially regulated genes involved in chemotaxis and metastasis, including connective tissue growth factor; CCL18/pulmonary and activation-regulated chemokine; EGF-like, module-containing, mucin-like hormone receptor-like sequence 1; and the mannose receptor. We also found that upon transmigration, the mature monocyte population cultured in our system had increased expression of CCR2 relative to that before transmigration [30].
MONOCYTE DIAPEDESIS AND THE BBB
To enter into the CNS, monocytes must first transmigrate across the BBB, a selectively permeable barrier consisting primarily of BMVECs and astrocytes, which separate the periphery from the CNS parenchyma [74, 75]. The BBB forms a physical and chemical barrier as a result of tight junction proteins, which inhibit paracellular entry, and a diverse group of transporters and low rates of pinocytosis, which inhibit transcellular entry, of small molecules into the CNS [76]. As part of immune surveillance, there is low-level leukocyte, including monocyte, trafficking across the BBB [77, 78]. Entry into the CNS is highly regulated, and immune surveillance in the brain occurs 100-fold less than in other organs, such as the spleen [79].
Diapedesis across the BBB is mediated by interactions between the junctional molecules on monocytes and the endothelium, some of which include ALCAM (CD166), JAM-A (F11R/CD321), PECAM-1 (CD31), and CD99. We found that in addition to CD16, the junctional molecules ALCAM, JAM-A, CD99, and PECAM-1 increase on the cell surface during monocyte maturation (unpublished results). We showed that the higher levels of at least some of these junctional molecules promote transmigration in greater numbers across our model of the human BBB in response to CCL2. Blocking antibodies to ALCAM and JAM-A significantly decrease the transmigration of this maturing monocyte subset, when both HIV-infected or uninfected, below baseline levels (unpublished results). This suggests the importance of junctional molecules on the monocyte in facilitating transmigration and warrants additional study, as it may represent novel therapeutic opportunities toward decreasing monocyte transmigration and the subsequent development of HAND. It may also be an important mechanism for monocyte transmigration into the CNS during other inflammatory processes.
ALCAM, JAM-A, CD99, and PECAM-1 are also expressed on BMVECs and mediate monocyte transmigration. Additionally, they are integral components of the BBB, helping to maintain its integrity. ALCAM is a member of the Ig superfamily and is expressed by T cells and monocytes, as well as ECs, particularly the BMVECs of the BBB, where it is localized to lipid rafts [80]. Unlike other junctional molecules involved in monocyte transmigration, ALCAM has higher expression in the endothelium of the BBB compared with those from other anatomical locations [81]. The role of ALCAM in baseline conditions is not well understood, but in inflammatory environments, its main role is to facilitate transendothelial migration [81]. ALCAM is essential for the transmigration of monocytes in atherosclerosis and is also implicated in the migration of regulatory T cells in pancreatic tumors [82]. ALCAM was found initially as the ligand of CD6, a glycoprotein found on activated T cells, and ALCAM expressed on the BMVECs can interact heterotypically with CD6 on those cells [83, 84]. Monocytes do not express detectable surface CD6, however, and are believed to participate solely in ALCAM–ALCAM homotypic interactions [81]. During transmigration, ALCAM is localized at endothelial cell-cell junctions and also at the transmigratory cup surrounding the monocyte. Its importance in mediating transmigration was demonstrated in studies using anti-ALCAM-blocking antibodies. In experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, mice that received ALCAM-blocking antibody had better clinical scores, less inflammatory lesions, and reduced monocyte and T cell infiltration than control animals [81]. Furthermore, the same antibody completely inhibited migration of CD4+ T cells and monocytes in vitro but not that of CD8+ T cells. Cocaine was shown to increase ALCAM expression in BMVECs, which led to increased numbers of monocyte transmigration across the BBB [85]. Anti-ALCAM antibody also blocked this process [85]. This may have implications for HIV-infected individuals, as substance abuse is a risk factor for acquiring HIV. In addition, many drugs of abuse alter BBB integrity, and a history of drug abuse may increase the rate of CNS inflammation and neurologic decline in HIV-infected individuals [86–90].
Similar to ALCAM, JAM-A is an Ig family member, as well as a member of the JAM family, including JAM-B, JAM-C, JAM-4, and JAM-L. Of these, JAM-A was the first to be identified and is the most widely expressed. JAM-A is found at the lateral borders between adjacent endothelial and epithelial cells of many tissues, including kidney, liver, and brain. JAM-A is also expressed on platelets, neutrophils, lymphocytes, and monocytes [91]. Homophilic interactions between JAM-A molecules of adjacent peripheral ECs are critical for maintaining EC integrity by stabilizing the cell junction and decreasing paracellular permeability [92]. A similar role is found in the BMVEC of the BBB, where JAM-A acts as a tight junction protein that interacts with cell polarity and cytoskeleton-associated proteins [91]. JAM-A is often upregulated under inflammatory conditions and is redistributed to the apical surface, making it available to interact with transmigrating monocytes [91]. The role of JAM-A in transendothelial cell migration was implicated with a blocking antibody, which inhibited baseline and chemokine-induced monocyte migration [92]. There have been many subsequent studies, during which JAM-A is blocked with antibody or reduced with genetic knockdown, some of which have confirmed its role in monocyte transmigration [93–98], whereas others have had opposite results [99–102]. JAM-A, on the EC, can also mediate transmigration by interacting heterotypically with LFA-1 on monocytes [95]. The role of JAM-A in mediating monocyte transmigration across the BBB during baseline conditions or in the context of HIV infection still needs to be characterized fully.
PECAM-1, another Ig superfamily member, is expressed on monocytes and at EC borders, albeit at a lower density in monocytes [103]. The PECAM-1 localized to EC lateral borders interacts with PECAM-1 present on monocytes. These homophilic interactions are required for transendothelial migration, as during this process, PECAM-1 is recruited to the site at which migration occurs [104]. One of the first studies to demonstrate the role of PECAM-1 in facilitating monocyte transmigration was with the use of an anti-PECAM-1 mAb, which decreased transendothelial migration by 80% [105]. Additional blocking studies, in vitro and in vivo, validated the essential role of PECAM-1 in transmigration. PECAM-1-blocking antibody was also shown to inhibit neutrophil recruitment to the lung, peritoneal cavity, and in human skin grafts [106]. When monocytes do not engage with PECAM-1 on the lateral border of ECs, they remain adherent to the apical surface, unable to pass through the intracellular junction [105]. One strain of mouse appears to be refractory to the anti-PECAM-1 transmigration blockade, however, and it is not known whether additional molecules play compensatory roles [107]. Transfection of PECAM-1 into ECV304 cells that do not endogenously express it confers their ability to support transendothelial migration [108]. This has not yet been demonstrated with other junctional molecules. The role of PECAM-1 at the BBB is less well-characterized [109]. In one study that knocked out PECAM-1, BMVECs showed a decrease in barrier integrity, followed by an increase in activated monocyte migration [110]. PECAM-1 has also been implicated in monocyte extravasation into the basal lamina once diapedesis is completed [111]. PECAM-1 was shown to act sequentially to JAM-A during monocyte migration in vivo [96]. Our laboratory showed that in vitro, HIV-infected PBMCs shed PECAM-1 when treated with CCL2, and in vivo, there is an accumulation of cleaved, sPECAM-1 in the brain tissue of individuals with HIVE. This increase is reflected in the sera of HIV-infected individuals [112]. These elevated levels of sPECAM-1 in the CNS may compete with surface PECAM–PECAM interactions between EC–EC and/or between EC and the monocyte, resulting in BBB permeability and increased monocyte transmigration, facilitating the development of HAND.
Unlike the previously discussed junctional proteins, CD99 is not an Ig family member or a member of any other protein family. CD99 has no homology with any gene in the human genome, with the exception of CD99L2 [113]. CD99 is expressed on most monocytes and at the lateral borders between adjacent ECs. Similar to other junctional molecules, CD99 participates in homophilic interactions to mediate monocyte transmigration. This interaction is necessary for transmigration in vitro and in vivo. Blocking CD99 on the monocyte or EC with mAb essentially inhibited all transendothelial migration [114]. In vitro studies showed a block of diapedesis by 90% [115]. In vivo studies with anti-CD99 inhibited T cell migration into the skin, as well as neutrophil and monocyte entry into the peritoneal cavity [116, 117]. It has been suggested that CD99 acts at the step in diapedesis directly after PECAM-1 [115]. Furthermore, it has been shown that CD99 cannot mediate migration when PECAM-1 is not present [108]. The sequential role of PECAM-1 and CD99 has been challenged recently, however, suggesting that CD99 may work independently of PECAM-1 [118]. Although closely related, CD99 and CD99L2 are not redundant proteins, as the latter may mediate the transmigration of fewer PBMC subsets than CD99. CD99L2 has been shown to mediate neutrophil but not T cell transmigration, unlike CD99, which has been implicated in the diapedesis of both of these cell types, as well as of monocytes [119].
BMVECs cannot be infected with HIV. EC damage from the virus may occur instead upon the release of viral proteins. The effects of these viral components on ALCAM, JAM-A, PECAM-1, and CD99 in the BBB have not been examined. However, studies implicate the effects of viral proteins, such as tat and gp120, on tight junctions, including occludin, claudin-5, and zonula occludens-1, in disrupting barrier integrity [120–122]. Interestingly, occludin and claudin-5 have been found on monocytes and may play a role in mediating their transmigration [123]. We showed the mRNA expression of tight junctions on the maturing monocyte [30]. A different study demonstrated ezrin, another tight junction protein, mRNA to be increased in the CD14+CD16+ monocyte subset as compared to CD14+CD16- cells [40]. The role of tight-junction interactions between monocytes and BMVECs in transmigration needs to be examined further.
HIV proteins may also interact with astrocytes, another cell type that plays a critical role in establishing the BBB, thereby compromising its integrity. The viral protein tat induces astrocytic PDGF, which in turn, causes production of CCL2 and IL-1β [124]. This could result in additional monocyte chemotaxis and possible damage to the BBB. We showed that tat also directly induces production of CCL2 in astrocytes and promotes transmigration across the BBB [125, 126]. Additionally, HIV itself may lead to barrier breakdown by infecting astrocytes. We demonstrated that HIV infects ∼5% of cultured astrocytes and that the infection of these few cells leads to BBB damage [127].
During acute infection, it is possible that the barrier may be breached prior to monocyte transmigration, as a result of viremia and the viral proteins mentioned above, as well as by the production of cytokines and chemokines by PBMCs and/or CNS cells. The diminished integrity of the BBB may then allow access of HIV-infected monocytes into the CNS. The damage that occurs during acute HIV infection often resolves, and BBB properties are reestablished during the chronic phase of HIV infection. Thus, entry of HIV-infected cells into the CNS can also exacerbate existing BBB damage and promote chronic neuroinflammation.
It is important to understand the signals that promote the entry of the mature monocyte population into the CNS during HIV infection. CCL2, a potent monocyte chemoattractant highly elevated in the CNS and CSF of individuals with cognitive impairment [128–130], is well-characterized in the neuropathology of HIV. Another chemokine, CX3CL1, is also present in the CNS and CSF of those with HIV infection [131, 132]. These two chemokines may coordinately recruit monocytes to the CNS. The CD14+CD16+ monocyte subpopulation secretes CCL2 when in contact with CX3CL1-expressing ECs [133]. This interaction also promotes monocyte production of matrix metalloproteinase 9, an essential component for entry into the basement membrane underlying the EC layer, and IL-6, an inflammatory cytokine implicated in HIV-related CNS pathology [133].
HIV INFECTION OF MONOCYTES
Monocyte transmigration across the BBB results in HIV seeding within the CNS and also establishes neuroinflammation during the pathogenesis of HAND [134]. As monocytes, which were infected with HIV in the circulation, enter the CNS, the virus too enters as an integrated provirus within the monocyte genome or as a nonintegrated circular form [135]. Infectious viral particles are released as the mature monocyte enters the CNS and also as it differentiates into a macrophage. This released virus can then infect other cells of the CNS, namely microglia, perivascular macrophages, and a small percentage of astrocytes [136, 137]. These infected cells can shed and secrete viral proteins, such as tat and gp120, which activate surrounding macrophages, microglia, and astrocytes. The activated cells produce inflammatory cytokines TNF-α, IL-1β, and IL-6 and chemokines, which recruit additional cells into the CNS, and small molecules, such as arachidonic acid, quinolinic acid, and NO. All of these factors can alter BBB permeability, as well as cause astrocytic glutamate dysregulation, ultimately resulting in the neuronal damage and loss associated with HAND [138]. This elaboration of neurotoxic and chemotactic factors also perpetuates the cycle of chronic inflammation within the CNS.
While HIV infection of monocytes and their entry into the CNS are believed to initiate the series of events that lead to HAND, there are many discrepancies regarding the susceptibility of monocytes to HIV infection. HIV infection of monocytes occurs predominantly by CD4 and CCR5 host protein interactions with the envelope of the virus, but there is evidence that CXCR4 may also be used as a coreceptor [139]. The importance of CCR5 in facilitating HIV infection is demonstrated by individuals who have a homozygous, 32-bp deletion in CCR5 (CCR5Δ32) and are not infected by HIV [140, 141]. Although monocytes express the receptor and coreceptors necessary to mediate viral entry, in the past, they were considered to be refractory to HIV infection, at least in vitro [142–146]. However, many groups showed that infectious virus can be isolated from the monocytes of seropositive individuals, particularly from the mature CD14+CD16+ monocyte subset, often expanded in the peripheral blood of those infected with HIV [147–156]. This monocyte subpopulation has increased susceptibility to HIV infection and has been shown to harbor integrated viral DNA [30, 157–159]. This suggests that there may be a maturation-dependent component to the susceptibility of monocytes to HIV and that one of the reasons for the reported lack of permissiveness of monocytes to HIV infection is the low numbers of the mature CD14+CD16+ subpopulation in the peripheral blood of healthy individuals, which was used for in vitro infectivity studies.
As there are conflicting data regarding monocyte susceptibility to HIV, we characterized the infectivity of freshly isolated monocytes compared with the CD14+CD16+ maturing monocyte subset expanded in our system. We found that the mature monocyte population was easily infected and had a robust production of HIV p24, the viral capsid antigen routinely used as an indicator of HIV replication, whereas freshly isolated monocytes had minimal virus production [30]. To determine gene expression that may facilitate infection of the maturing monocyte population, we performed microarray analyses using RNA from freshly isolated monocytes and monocytes cultured nonadherently for 3 days in our system, which were highly vulnerable to HIV infection. There were several genes up-regulated in the maturing monocyte population, whose protein products have been shown to interact with HIV proteins, including fibronectin-1, syndecan-2, and integrin beta 5 [30]. It will be important to determine the role that these proteins and others identified by our microarray play in HIV infection.
Studies that focused on determining the cause of the blockade of HIV production in freshly isolated monocytes did not specifically examine this maturing population and have attributed the low level of infection to many factors, some of which include inhibition prior to reverse transcription, a defect in tat transactivation, low-viral cDNA synthesis, decreased levels of CCR5, “anti-HIV” microRNAs, the TRIM family member TRIM5α, and the apolipoprotein B mRNA-editing enzyme catalytic polypeptide family of cytidine deaminases [159–170]. While all of these may be important mechanisms leading to inhibition of monocyte infection by HIV, additional studies with the maturing monocyte population are needed to determine factors contributing to their susceptibility.
In HIV neuropathogenesis, HIV-infected monocytes transmigrate across the BBB, exposing the CNS to virus. We used our human in vitro BBB model to characterize the transmigration of HIV-infected cells. Our initial studies showed that PBMC transmigrate in very high numbers across the BBB in response to CCL2 and disrupt barrier integrity, in part by reducing BMVEC tight junctions [73]. This high level of transmigration did not occur when HIV-infected cells were added to the barrier in the absence of CCL2 or with uninfected PBMCs, suggesting that HIV and CCL2 have a combined effect on increased transmigration and disrupting barrier integrity. Our additional studies using monocytes from our nonadherent culture system demonstrated that when infected with HIV, the maturing monocyte population also transmigrates across the BBB in response to CCL2 in much higher numbers than when uninfected (Fig. 5). As with the PBMCs used in our initial studies, CCL2 was required for this exuberant transmigration.
Figure 5. HIV-infected monocytes transmigrate exuberantly as compared to uninfected monocytes across the BBB in response to CCL2.
Maturing monocytes cultured nonadherently with M-CSF in our system, either HIV-infected or uninfected, were added to the top of our in vitro model of the BBB and transmigrated in response to media alone or 200 ng/mL CCL2 for 24 h. CCL2 resulted in significantly higher numbers of HIV-infected transmigrated CD14+CD16+ monocytes relative to baseline and to the transmigration of uninfected cells to CCL2 (*P<0.03; n=5). There was also significant transmigration of uninfected monocytes in response to CCL2 (*P<0.03; n=5). Baseline transmigration was similar for the HIV-infected and uninfected mature monocytes.
MONOCYTES AND HAND IN THE ANTIRETROVIRAL ERA
In the present cART era, peripheral and central viral loads are low or undetectable [171]. The question is now whether neuroinflammation occurs in the current era and if the presence of monocytes/macrophages in the CNS correlates closely to the neuropathogenesis of HIV, as was seen prior to antiretroviral therapy [172–174]. There is evidence that neuroinflammation persists in the CNS of HIV-infected individuals despite cART, even if sometimes at low levels, and that monocytes/macrophages remain critical to the pathogenesis of HAND [175–178]. It was suggested in one study that there are “surprising” levels of neuroinflammation in those receiving cART and that the extent of this inflammation mirrors that seen in HIVE cases pre-cART [179]. In contrast, another group found minimal HIV brain pathology in the antiretroviral era, even though 88% of individuals in the cohort presented with HAND [180]. Others suggest that inflammation within the CNS has not decreased with therapeutics, but instead that the sites at which it occurs have shifted [181–183].
The peripheral infection of monocytes has been shown to correlate with HAND in cART-treated individuals [184], and the levels of HIV DNA in the CD14+CD16+ cells remain high, even after 1 year of therapy [185]. The sequences of recovered HIV RNA from plasma more closely relate to CD14+CD16+ monocytes than to CD4+T lymphocytes, suggesting that even during successful therapy, monocytes may contribute to the production of virus [186].
The entry of monocytes into the brain plays a key role in initiating the series of events that lead to HAND, and although antiretrovirals hinder viral replication, they may have minimal effects on the continued transmigration of monocytes into the brain. One study showed that a single exposure of the CNS to the neurotoxic viral protein HIV tat led to the infiltration of peripheral blood monocytes and resulted in prolonged disruption of CNS function [187]. The effects of continuous exposure to viral proteins in the brain over the course of HIV infection, even if cART is administered, can therefore not be discounted. This suggests that the entry of even only a few HIV-infected monocytes into the CNS early during the acute phase of HIV infection and prior to the administration of cART may be enough to irreparably alter the CNS [188, 189].
MACAQUE MODELS OF CNS NEUROPATHOGENESIS
Nonhuman primate models of SIV have greatly expanded our knowledge of HIV infection. These models address many questions concerning the neuropathogenesis of HIV, in part, because of the ability to access CSF and CNS samples during the dynamic course of the disease, for which in humans there is limited availability. SIV is a well-accepted alternative for the study of HIV, because of the genetic similarities, phylogenetic proximity, and similar progression of the neuropathologies [190–193]. HIV and SIV also infect many of the same cells, peripherally and in the CNS [194–197]. Macaque models of SIVE provided much knowledge about the pathogenesis of HAND, as SIV-infected animals progress toward disease in a reproducible, reliable time frame. Commonly used strains include SIVmac251 for rhesus macaques and the simultaneous use of an immunosuppressive strain SIV/deltaB670 and a neurovirulent SIV/17E-Fr strain in pigtailed macaques [198, 199]. This pigtailed macaque model results in an accelerated disease process, during which 90% of animals develop SIVE after infection. Another model uses an anti-CD8 antibody in conjunction with injection of SIVmac251 to promote rapid infection [200]. Studies performed with SIV corroborated many of the findings implicated in promoting HIV neuropathogenesis, such as the importance of CCL2 in mediating neuroinflammation in HAND, phenotypic changes in peripheral blood monocytes, and infection of perivascular macrophages within the CNS [35, 198, 201–203]. SIV-infected macaques have been a useful tool in studying viral latency within the CNS and also in establishing the role of monocytes as an indicator of AIDS progression [204, 205]. Studies of SIV infection within the CNS clearly demonstrate the relationships among infection of monocytes, neuronal injury, and viremia [206]. SIV facilitates the study of therapeutics, such as the use of minocycline, an antibiotic now in clinical trials as a treatment for HAND [207, 208]. Animals treated with minocycline had decreased viral loads, which were attributed to decreased numbers of CD14+CD16+ monocytes in the blood, suggesting that fewer of these cells would be available to enter the CNS [36]. As a result of early viral entry into the brain and subsequent chronic inflammation, it has been suggested that CNS-penetrating drugs should also be administered as soon as an individual begins cART, even before any evidence of neurologic impairment [209].
CONCLUDING REMARKS
HAND occurs in 40–60% of HIV-infected individuals and is largely attributed to the influx of monocytes into the brain and the subsequent development of chronic inflammation that occurs, despite antiretroviral therapy. The CD14+CD16+ subset represents a mature monocyte population, which increases in the blood of HIV-infected individuals, and due to CCL2 (and perhaps CX3CL1) chemotactic signals, transmigrates across the BBB, accumulates in the CNS parenchyma, and is believed to play a critical role in promoting the series of events that leads to neurocognitive impairment. This transmigration may be facilitated by maturation-associated markers, as well as increased junctional proteins on the monocyte, which interact with the BMVECs of the BBB upon diapedesis. Monocytes were considered refractory to HIV infection, but recent studies demonstrate that the mature CD14+CD16+ monocyte population is susceptible to HIV infection and can be associated with high levels of virus production. As this monocyte subset is preferentially infected by HIV, upon entry into the CNS, it may produce infectious virus and viral proteins and establish neuroinflammation. Identification of markers specific to this mature monocyte subpopulation, the factors that contribute to their HIV susceptibility, and the mechanisms that mediate transmigration into the CNS are critical to the development of novel therapeutics targeted toward preventing the neuropathogenesis of HIV.
ACKNOWLEDGMENTS
This work was supported by National Institute of Mental Health grants (MH075679, MH090958, and DA025567 to J.W.B. and T.M.C.), a KO1 grant from the National Institute of Mental Health (MH076679 to E.A.E.), and Geographic Medicine and Emerging Infections training grant (T32AI070117 to D.W.W.). We thank the NIH Centers for AIDS Research (CFAR) grant AI-051519 at the Albert Einstein College of Medicine. We thank Drs. Peter Gaskill, Clarisa Buckner, and Loreto Carvallo, as well as Jacqueline Coley and Lillie Lopez, for their help with experiments and for their thoughtful discussions. We also thank Dr. Brad Poulos of the Fetal Tissue Repository, the New York Blood Center for providing leukopaks, and Dr. Lydia Tesfa of the FACS Core Facility.
Footnotes
- ALCAM
- activated leukocyte cellular adhesion molecule
- BBB
- blood brain barrier
- BMVEC
- brain microvascular endothelial cell
- cART
- combined antiretroviral therapy
- CD99L2
- CD99-like 2
- CSF1-R
- colony stimulating factor receptor 1
- EC
- endothelial cell
- HAD
- HIV-associated dementia
- HAND
- HIV-associated neurocognitive disorder(s)
- HIVE
- HIV encephalitis
- ICAM-1
- intercellular adhesion molecule 1
- JAM-A
- junctional adhesion molecule A
- LFA-1
- lymphocyte function-associated antigen 1
- LPS
- lipopolysaccharide
- NC-IUIS
- Nomenclature Committee of the International Union of Immunological Societies
- PDGF
- platelet-derived growth factor
- PrPC
- cellular prion protein
- SIVE
- SIV encephalitis
- sPECAM-1
- soluble PECAM-1
- TRIM
- tripartite motif
- VCAM-1
- vascular cell adhesion molecule 1
- VLA-4
- very late antigen-4
REFERENCES
- 1. Graham N. M. H., Zeger S. L., Park L. P., Vermund S. H., Detels R., Rinaldo C. R., Phair J. P. (1992) The effects on survival of early treatment of human immunodeficiency virus infection. N. Engl. J. Med. 326, 1037–1042 [DOI] [PubMed] [Google Scholar]
- 2. Hammer S. M., Squires K. E., Hughes M. D., Grimes J. M., Demeter L. M., Currier J. S., Eron J. J., Jr., Feinberg J. E., Balfour H. H., Jr., Deyton L. R., Chodakewitz J. A., Fischl M. A. (1997) A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337, 725–733 [DOI] [PubMed] [Google Scholar]
- 3. Li T. S., Tubiana R., Katlama C., Calvez V., Mohand H. A., Autran B. (1998) Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351, 1682–1686 [DOI] [PubMed] [Google Scholar]
- 4. Hunt P. W., Deeks S. G., Rodriguez B., Valdez H., Shade S. B., Abrams D. I., Kitahata M. M., Krone M., Neilands T. B., Brand R. J., Lederman M. M., Martin J. N. (2003) Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 17, 1907–1915 [DOI] [PubMed] [Google Scholar]
- 5. Ances B., Ellis R. (2007) Dementia and neurocognitive disorders due to HIV-1 infection. Semin. Neurol. 27, 086–092 [DOI] [PubMed] [Google Scholar]
- 6. Kaul M., Zheng J., Okamoto S., Gendelman H. E., Lipton S. A. (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 12, 878–892 [DOI] [PubMed] [Google Scholar]
- 7. Davis L. E., Hjelle B. L., Miller V. E., Palmer D. L., Llewellyn A. L., Merlin T. L., Young S. A., Mills R. G., Wachsman W., Wiley C. A. (1992) Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42, 1736–1739 [DOI] [PubMed] [Google Scholar]
- 8. Williams K. C., Hickey W. F. (2002) Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Ann. Rev. Neurosci. 25, 537–562 [DOI] [PubMed] [Google Scholar]
- 9. Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., Clifford D. B., Cinque P., Epstein L. G., Goodkin K., Gisslen M., Grant I., Heaton R. K., Joseph J., Marder K., Marra C. M., McArthur J. C., Nunn M., Price R. W., Pulliam L., Robertson K. R., Sacktor N., Valcour V., Wojna V. E. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cysique L. A., Vaida F., Letendre S., Gibson S., Cherner M., Woods S. P., McCutchan J. A., Heaton R. K., Ellis R. J. (2009) Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology 73, 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sevigny J. J., Albert S. M., McDermott M. P., McArthur J. C., Sacktor N., Conant K., Schifitto G., Selnes O. A., Stern Y., McClernon D. R., Palumbo D., Kieburtz K., Riggs G., Cohen B., Epstein L. G., Marder K. (2004) Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63, 2084–2090 [DOI] [PubMed] [Google Scholar]
- 12. Nath A., Schiess N., Venkatesan A., Rumbaugh J., Sacktor N., McArthur J. (2008) Evolution of HIV dementia with HIV infection. Int. Rev. Psychiatry 20, 25–31 [DOI] [PubMed] [Google Scholar]
- 13. Sacktor N., McDermott M. P., Marder K., Schifitto G., Selnes O. A., Mcarthur J. C., Stern Y., Albert S., Palumbo D., Kieburtz K., De Marcaida J. A., Cohen B., Epstein L. (2002) HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 8, 136–142 [DOI] [PubMed] [Google Scholar]
- 14. Ances B. M., Clifford D. B. (2008) HIV-associated neurocognitive disorders and the impact of combination antiretroviral therapies. Curr. Neurol. Neurosci. Rep. 8, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis R., Langford D., Masliah E. (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 8, 33–44 [DOI] [PubMed] [Google Scholar]
- 16. Figdor C., Bont W., Touw I., de Roos J., Roosnek E., de Vries J. (1982) Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood 60, 46–53 [PubMed] [Google Scholar]
- 17. Yasaka T., Mantich N., Boxer L., Baehner R. (1981) Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J. Immunol. 127, 1515–1518 [PubMed] [Google Scholar]
- 18. Passlick B., Flieger D., Ziegler-Heitbrock H. (1989) Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 [PubMed] [Google Scholar]
- 19. Clarkson S. B., Ory P. A. (1988) CD16. Developmentally regulated IgG Fc receptors on cultured human monocytes. J. Exp. Med. 167, 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J. M., Liu Y-J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80 [DOI] [PubMed] [Google Scholar]
- 21. Tacke F., Randolph G. (2006) Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211, 609–618 [DOI] [PubMed] [Google Scholar]
- 22. Robbins C. S., Swirski F. K. (2010) The multiple roles of monocyte subsets in steady state and inflammation. Cell. Mol. Life Sci. 67, 2685–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziegler-Heitbrock H. W. L., Fingerle G., Ströbel M., Schraut W., Stelter F., Schütt C., Passlick B., Pforte A. (1993) The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur. J. Immunol. 23, 2053–2058 [DOI] [PubMed] [Google Scholar]
- 24. Thieblemont N., Weiss L., Sadeghi H. M., Estcourt C., Haeffner-Cavaillon N. (1995) CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur. J. Immunol. 25, 3418–3424 [DOI] [PubMed] [Google Scholar]
- 25. Allen J. B., Wong H. L., Guyre P. M., Simon G. L., Wahl S. M. (1991) Association of circulating receptor Fc γ RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-β. J. Clin. Invest. 87, 1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M. S. (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349, 692–695 [DOI] [PubMed] [Google Scholar]
- 27. Fischer-Smith T., Croul S., Sverstiuk A. E., Capini C., L'Heureux D., Regulier E. G., Richardson M. W., Amini S., Morgello S., Khalili K., Rappaport J. (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 7, 528–541 [DOI] [PubMed] [Google Scholar]
- 28. Fischer-Smith T., Croul S., Adeniyi A., Rybicka K., Morgello S., Khalili K., Rappaport J. (2004) Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am. J. Pathol. 164, 2089–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clay C. C., Rodrigues D. S., Ho Y. S., Fallert B. A., Janatpour K., Reinhart T. A., Esser U. (2007) Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J. Virol. 81, 12040–12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buckner C. M., Calderon T. M., Willams D. W., Belbin T. J., Berman J. W. (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 267, 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Furth R., Cohn Z. A. (1968) The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haskill S., Johnson C., Eierman D., Becker S., Warren K. (1988) Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J. Immunol. 140, 1690–1694 [PubMed] [Google Scholar]
- 33. Shaw R. J., Doherty D. E., Ritter A. G., Benedict S. H., Clark R. A. (1990) Adherence-dependent increase in human monocyte PDGF(B) mRNA is associated with increases in c-fos, c-jun, and EGR2 mRNA. J. Cell. Biol. 111, 2139–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziegler-Heitbrock H. W. L. (1996) Heterogeneity of human blood monocytes: the CD14+CD16+ subpopulation. Immunol. Today 17, 424–428 [DOI] [PubMed] [Google Scholar]
- 35. Kim W-K., Sun Y., Do H., Autissier P., Halpern E. F., Piatak M., Lifson J. D., Burdo T. H., McGrath M. S., Williams K. (2010) Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J. Leukoc. Biol. 87, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell J. H., Burdo T. H., Autissier P., Bombardier J. P., Westmoreland S. V., Soulas C., González R. G., Ratai E-M., Williams K. C. (2011) Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV NeuroAIDS. PLoS One 6, e18688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strauss-Ayali D., Conrad S. M., Mosser D. M. (2007) Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 82, 244–252 [DOI] [PubMed] [Google Scholar]
- 38. Sánchez-Torres C., García-Romo G. S., Cornejo-Cortés M. A., Rivas-Carvalho A., Sánchez-Schmitz G. (2001) CD16+ and CD16− human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int. Immunol. 13, 1571–1581 [DOI] [PubMed] [Google Scholar]
- 39. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 40. Ancuta P., Liu K-Y., Misra V., Wacleche V., Gosselin A., Zhou X., Gabuzda D. (2009) Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genomics 10, 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Imbert A-M., Belaaloui G., Bardin F., Tonnelle C., Lopez M., Chabannon C. (2006) CD99 expressed on human mobilized peripheral blood CD34+ cells is involved in transendothelial migration. Blood 108, 2578–2586 [DOI] [PubMed] [Google Scholar]
- 42. Grage-Griebenow E., Flad H-D., Ernst M. (2001) Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 [PubMed] [Google Scholar]
- 43. Stewart M., Thiel M., Hogg N. (1995) Leukocyte integrins. Curr. Opin. Cell. Biol. 7, 690–696 [DOI] [PubMed] [Google Scholar]
- 44. Todd R. F., Petty H. R. (1997) β2(CD11/CD18) Integrins can serve as signaling partners for other leukocyte receptors. J. Lab. Clin. Med. 129, 492–498 [DOI] [PubMed] [Google Scholar]
- 45. Most J., Schwaeble W., Drach J., Sommerauer A., Dierich M. (1992) Regulation of the expression of ICAM-1 on human monocytes and monocytic tumor cell lines. J. Immunol. 148, 1635–1642 [PubMed] [Google Scholar]
- 46. Zhang W-Y., Schwartz E., Wang Y., Attrep J., Li Z., Reaven P. (2006) Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 514–519 [DOI] [PubMed] [Google Scholar]
- 47. Lau S. K., Chu P. G., Weiss L. M. (2004) CD163. Am. J. Clin. Pathol. 122, 794–801 [DOI] [PubMed] [Google Scholar]
- 48. Nguyen T. T., Schwartz E. J., West R. B., Warnke R. A., Arber D. A., Natkunam Y. (2005) Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am. J. Surg. Pathol. 29, 617–624 [DOI] [PubMed] [Google Scholar]
- 49. Roberts E. S., Eleizeir M., Howard F. (2004) CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE). J. Neuropathol. Exp. Neurol. 63, 1255–1264 [DOI] [PubMed] [Google Scholar]
- 50. Fischer-Smith T., Tedaldi E. M., Rappaport J. (2008) CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res. Hum. Retrovir. 24, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim W-K., Alvarez X., Fisher J., Bronfin B., Westmoreland S., McLaurin J., Williams K. (2006) CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am. J. Pathol. 168, 822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burdo T. H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M. J., Williams K. C. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 6, e1000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Odink K., Cerletti N., Bruggen J., Clerc R. G., Tarcsay L., Zwadlo G., Gerhards G., Schlegal R., Sorg C. (1987) Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 330, 80–82 [DOI] [PubMed] [Google Scholar]
- 54. Rugtveit J., Brandtzaeg P., Halstensen T. S., Fausa O., Scott H. (1994) Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut 35, 669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brandtzaeg P., Flavell D. J., Fagerhol M. K. (1988) Mac 387 antibody and detection of formalin resistant myelomonocytic L1 antigen. J. Clin. Pathol. 41, 963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Otani I., Mori K., Sata T., Terao K., Doi K., Akari H., Yoshikawa Y. (1999) Accumulation of MAC387+ macrophages in paracortical areas of lymph nodes in rhesus monkeys acutely infected with simian immunodeficiency virus. Microb. Infect. 1, 977–985 [DOI] [PubMed] [Google Scholar]
- 57. Soulas C., Conerly C., Kim W-K., Burdo T. H., Alvarez X., Lackner A. A., Williams K. C. (2011) Recently infiltrating MAC387+ monocytes/macrophages. Am. J. Pathol. 178, 2121–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mouillet-Richard S., Ermonval M., Chebassier C., Laplanch J. L., Lehmann S., Launay J. M., Kellermann O. (2000) Signal transduction through prion protein. Science 289, 1925–1928 [DOI] [PubMed] [Google Scholar]
- 59. Schneider B., Mutel V., Pietri M., Ermonval M., Mouillet-Richard S., Kellermann O. (2003) NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc. Natl. Acad. Sci. USA 100, 13326–13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moser M., Colello R. J., Pott U., Oesch B. (1995) Developmental expression of the prion protein gene in glial cells. Neuron 14, 509–517 [DOI] [PubMed] [Google Scholar]
- 61. Jeffrey M., Goodsir C. M., Race R. E., Chesebro B. (2004) Scrapie-specific neuronal lesions are independent of neuronal PrP expression. Ann. Neurol. 55, 781–792 [DOI] [PubMed] [Google Scholar]
- 62. Viegas P., Chaverot N., Enslen H., Perrière N., Couraud P-O., Cazaubon S. (2006) Junctional expression of the prion protein PrPC by brain endothelial cells: a role in trans-endothelial migration of human monocytes. J. Cell Sci. 119, 4634–4643 [DOI] [PubMed] [Google Scholar]
- 63. Roberts T. K., Eugenin E. A., Morgello S., Clements J. E., Zink M. C., Berman J. W. (2010) PrPC, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. Am. J. Pathol. 177, 1848–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 65. Merino A., Buendia P., Martin-Malo A., Aljama P., Ramirez R., Carracedo J. (2011) Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J. Immunol. 186, 1809–1815 [DOI] [PubMed] [Google Scholar]
- 66. Ancuta P., Rao R., Moses A., Mehle A., Shaw S. K., Luscinskas F. W., Gabuzda D. (2003) Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weber C., Belge K., von Hundelshausen P., Draude G., Steppich B., Mack M., Frankenberger M., Weber K., Ziegler-Heitbrock H. (2000) Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 67, 699–704 [DOI] [PubMed] [Google Scholar]
- 68. Muller W. A. (2003) Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24, 327–334 [DOI] [PubMed] [Google Scholar]
- 69. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 70. Millan J., Hewlett L., Glyn M., Toomre D., Clark P., Ridley A. J. (2006) Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8, 113–123 [DOI] [PubMed] [Google Scholar]
- 71. Muller W. A. (2011) Mechanisms of leukocyte transendothelial migration. Ann. Rev. Pathol. Mech. Dis. 6, 323–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eugenin E. A., Berman J. W. (2003) Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 29, 351–361 [DOI] [PubMed] [Google Scholar]
- 73. Eugenin E. A., Osiecki K., Lopez L., Goldstein H., Calderon T. M., Berman J. W. (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 26, 1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Janzer R. C., Raff M. C. (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325, 253–257 [DOI] [PubMed] [Google Scholar]
- 75. Abbott N. J., Ronnback L., Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 [DOI] [PubMed] [Google Scholar]
- 76. Gloor S. M., Wachtel M., Bolliger M. F., Ishihara H., Landmann R., Frei K. (2001) Molecular and cellular permeability control at the blood-brain barrier. Brain Res. Brain Res. Rev. 36, 258–264 [DOI] [PubMed] [Google Scholar]
- 77. Hickey W. F., Hsu B. L., Kimura H. (1991) T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28, 254–260 [DOI] [PubMed] [Google Scholar]
- 78. Hickey W. F. (1999) Leukocyte traffic in the central nervous system: the participants and their roles. Semin. Immunol. 11, 125–137 [DOI] [PubMed] [Google Scholar]
- 79. Greenwood J., Heasman S. J., Alvarez J. I., Prat A., Lyck R., Engelhardt B. (2011) Review: leucocyte–endothelial cell crosstalk at the blood–brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 37, 24–39 [DOI] [PubMed] [Google Scholar]
- 80. Masedunskas A., King J. A., Tan F., Cochran R., Stevens T., Sviridov D., Ofori-Acquah S. F. (2006) Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 580, 2637–2645 [DOI] [PubMed] [Google Scholar]
- 81. Cayrol R., Wosik K., Berard J. L., Dodelet-Devillers A., Ifergan I., Kebir H., Haqqani A. S., Kreymborg K., Krug S., Moumdjian R., Bouthillier A., Becher B., Arbour N., David S., Stanimirovic D., Prat A. (2008) Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 9, 137–145 [DOI] [PubMed] [Google Scholar]
- 82. Rauch S. J., Rosenkranz A. C., Böhm A., Meyer-Kirchrath J., Hohlfeld T., Schrör K., Rauch B. H. Cholesterol induces apoptosis-associated loss of the activated leukocyte cell adhesion molecule (ALCAM) in human monocytes. Vasc. Pharmacol. 54, 93–99 [DOI] [PubMed] [Google Scholar]
- 83. Gimferrer I., Calvo M., Mittelbrunn M., Farnós M., Sarrias M. R., Enrich C., Vives J., Sánchez-Madrid F., Lozano F. (2004) Relevance of CD6-mediated interactions in T cell activation and proliferation. J. Immunol. 173, 2262–2270 [DOI] [PubMed] [Google Scholar]
- 84. Zimmerman A. W., Joosten B., Torensma R., Parnes J. R., van Leeuwen F. N., Figdor C. G. (2006) Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 107, 3212–3220 [DOI] [PubMed] [Google Scholar]
- 85. Yao H., Kim K., Duan M., Hayashi T., Guo M., Morgello S., Prat A., Wang J., Su T-P., Buch S. (2011) Cocaine hijacks ω1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J. Neurosci. 31, 5942–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bouwman F. H., Skolasky R. L., Hes D., Selnes O. A., Glass J. D., Nance-Sproson T. E., Royal W., Dal Pan G. J., McArthur J. C. (1998) Variable progression of HIV[b]-associated dementia. Neurology 50, 1814–1820 [DOI] [PubMed] [Google Scholar]
- 87. Dhillon N. K., Peng F., Bokhari S., Callen S., Shin S.-H., Zhu H., Kim K., Buch S. J. (2008) Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J. Neuroimmune Pharmacol. 3, 52–56 [DOI] [PubMed] [Google Scholar]
- 88. Kapadia F., Vlahov D., Donahue R. M., Friedland G. (2005) The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin. Infect. Dis. 41, 1027–1034 [DOI] [PubMed] [Google Scholar]
- 89. Mahajan S. D., Aalinkeel R., Sykes D. E., Reynolds J. L., Bindukumar B., Adal A., Qi M., Toh J., Xu G., Prasad P. N., Schwartz S. A. (2008) Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 1203, 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shapshak P., Kangueane P., Fujimura R. K., Commins D., Chiappelli F., Singer E., Levine A. J., Minagar A., Novembre F. J., Somboonwit C., Nath A., Sinnott J. T. (2011) Editorial NeuroAIDS review. AIDS 25, 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weber C., Fraemohs L., Dejana E. (2007) The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 7, 467–477 [DOI] [PubMed] [Google Scholar]
- 92. Martìn-Padura I., Lostaglio S., Schneemann M., Williams L., Romano M., Fruscella P., Panzeri C., Stoppacciaro A., Ruco L., Villa A., Simmons D., Dejana E. (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Del Maschio A., De Luigi A., Martin-Padura I., Brockhaus M., Bartfai T., Fruscella P., Adorini L., Martino G., Furlan R., De Simoni M. G., Dejana E. (1999) Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis Is inhibited by an antibody to junctional adhesion molecule (Jam). J. Exp. Med. 190, 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zernecke A., Liehn E. A., Fraemohs L., von Hundelshausen P., Koenen R. R., Corada M., Dejana E., Weber C. (2006) Importance of junctional adhesion molecule-A for neointimal lesion formation and infiltration in atherosclerosis-prone mice. Arterioscl. Thromb. Vasc. Biol. 26, e10–e13 [DOI] [PubMed] [Google Scholar]
- 95. Ostermann G., Weber K. S. C., Zernecke A., Schroder A., Weber C. (2002) JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3, 151–158 [DOI] [PubMed] [Google Scholar]
- 96. Woodfin A., Reichel C. A., Khandoga A., Corada M., Voisin M. B., Scheiermann C., Haskard D. O., Dejana E., Krombach F., Nourshargh S. (2007) JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood 110, 1848–1856 [DOI] [PubMed] [Google Scholar]
- 97. Khandoga A., Kessler J. S., Meissner H., Hanschen M., Corada M., Motoike T., Enders G., Dejana E., Krombach F. (2005) Junctional adhesion molecule-A deficiency increases hepatic ischemia-reperfusion injury despite reduction of neutrophil transendothelial migration. Blood 106, 725–733 [DOI] [PubMed] [Google Scholar]
- 98. Corada M., Chimenti S., Cera M. R., Vinci M., Salio M., Fiordaliso F., De Angelis N., Villa A., Bossi M., Staszewsky L. I., Vecchi A., Parazzoli D., Motoike T., Latini R., Dejana E. (2005) Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 102, 10634–10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lechner F., Sahrbacher U., Suter T., Frei K., Brockhaus M., Koedel U., Fontana A. (2000) Antibodies to the junctional adhesion molecule cause disruption of endothelial cells and do not prevent leukocyte influx into the meninges after viral or bacterial infection. J. Infect. Dis. 182, 978–982 [DOI] [PubMed] [Google Scholar]
- 100. Liu Y., Nusrat A., Schnell F., Reaves T., Walsh S., Pochet M., Parkos C. (2000) Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113, 2363–2374 [DOI] [PubMed] [Google Scholar]
- 101. Shaw S. K., Ma S., Kim M. B., Rao R. M., Hartman C. U., Froio R. M., Yang L., Jones T., Liu Y., Nusrat A., Parkos C. A., Luscinskas F. W. (2004) Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 200, 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schenkel A. R., Mamdouh Z., Muller W. A. (2004) Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5, 393–400 [DOI] [PubMed] [Google Scholar]
- 103. Nourshargh S., Krombach F., Dejana E. (2006) The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J. Leukoc. Biol. 80, 714–718 [DOI] [PubMed] [Google Scholar]
- 104. Imhof B. A., Aurrand-Lions M. (2004) Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 4, 432–444 [DOI] [PubMed] [Google Scholar]
- 105. Muller W. A., Weigl S. A., Deng X., Phillips D. M. (1993) PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178, 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vaporciyan A., DeLisser H. M., Yan H., Mendiguren I. I., Thorn S. R., Jones M. L., Ward P. A., Albelda S. M. (1993) Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 262, 1580–1582 [DOI] [PubMed] [Google Scholar]
- 107. Schenkel A. R., Chew T. W., Muller W. A. (2004) Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J. Immunol. 173, 6403–6408 [DOI] [PubMed] [Google Scholar]
- 108. Dasgupta B., Dufour E., Mamdouh Z., Muller W. A. (2009) A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J. Immunol. 182, 5041–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Roberts T. K., Buckner C. M., Berman J. W. (2010) Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Front. Biosci. 15, 478–536 [DOI] [PubMed] [Google Scholar]
- 110. Graesser D., Solowiej A., Bruckner M., Osterweill E., Juedes A., Davis S., Ruddle N. H., Engelhardt B., Madri J. A. (2002) Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 109, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liao F., Huynh H. K., Eiroa A., Greene T., Polizzi E., Muller W. A. (1995) Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 182, 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Eugenin E. A., Gamss R., Buckner C., Buono D., Klein R. S., Schoenbaum E. E., Calderon T. M., Berman J. W. (2006) Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J. Leukoc. Biol. 79, 444–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Suh Y. H., Shin Y. K., Kook M., Oh K. I., Park W. S., Kim S. H., Lee I.-S., Park H. J., Huh T., Park S. H. (2003) Cloning, genomic organization, alternative transcripts and expression analysis of CD99L2, a novel paralog of human CD99, and identification of evolutionary conserved motifs. Gene 307, 63–76 [DOI] [PubMed] [Google Scholar]
- 114. Lou O., Alcaide P., Luscinskas F. W., Muller W. A. (2007) CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 178, 1136–1143 [DOI] [PubMed] [Google Scholar]
- 115. Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. (2002) CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3, 143–150 [DOI] [PubMed] [Google Scholar]
- 116. Bixel G., Kloep S., Butz S., Petri B., Engelhardt B., Vestweber D. (2004) Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood 104, 3205–3213 [DOI] [PubMed] [Google Scholar]
- 117. Dufour E. M., Deroche A., Bae Y. M., Muller W. A. (2008) CD99 Is essential for leukocyte diapedesis in vivo. Cell Commun. Adhes. 15, 351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bixel M. G., Li H., Petri B., Khandoga A. G., Khandoga A., Zarbock A., Wolburg-Buchholz K., Wolburg H., Sorokin L., Zeuschner D., Maerz S., Butz S., Krombach F., Vestweber D. (2010) CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116, 1172–1184 [DOI] [PubMed] [Google Scholar]
- 119. Bixel M. G., Petri B., Khandoga A. G., Khandoga A., Wolburg-Buchholz K., Wolburg H., März S., Krombach F., Vestweber D. (2007) A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood 109, 5327–5336 [DOI] [PubMed] [Google Scholar]
- 120. Nakamuta S., Endo H., Higashi Y., Kousaka A., Yamada H., Yano M., Kido H. (2008) Human immunodeficiency virus type 1 gp120–mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J. Neurovirol. 14, 186–195 [DOI] [PubMed] [Google Scholar]
- 121. King J. E., Eugenin E. A., Buckner C. M., Berman J. W. (2006) HIV tat and neurotoxicity. Microbes Infect. 8, 1347–1357 [DOI] [PubMed] [Google Scholar]
- 122. Kanmogne G. D., Schall K., Leibhart J., Knipe B., Gendelman H. E., Persidsky Y. (2006) HIV-1 gp120 compromises blood-brain barrier integrity and enhance monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab. 27, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Alexander J. S., Dayton T., Davis C., Hill S., Jackson T. H., Blaschuk O., Symonds M., Okayama N., Kevil C. G., Laroux F. S., Berney S. M., Kimpel D. (1998) Activated T-lymphocytes express occludin, a component of tight junctions. Inflammation 22, 573–582 [DOI] [PubMed] [Google Scholar]
- 124. Bethel-Brown C., Yao H., Callen S., Lee Y. H., Dash P. K., Kumar A., Buch S. (2011) HIV-1 tat-mediated induction of platelet-derived growth factor in astrocytes: role of early growth response gene 1. J. Immunol. 186, 4119–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Weiss J. M., Downie S. A., Lyman W. D., Berman J. W. (1998) Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J. Immunol. 161, 6896–6903 [PubMed] [Google Scholar]
- 126. Weiss J. M., Nath A., Major E. O., Berman J. W. (1999) HIV-1 tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human bood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163, 2953–2959 [PubMed] [Google Scholar]
- 127. Eugenin E. A., Clements J. E., Zink M. C., Berman J. W. (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood–brain barrier integrity by a gap junction-dependent mechanism. J. Neurosci. 31, 9456–9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cinque P., Vago L., Mengozzi M., Torri V., Ceresa D., Vicenzi E., Transidico P., Vagani A., Sozzani S., Mantovani A., Lazzarin A., Poli G. (1998) Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS 12, 1327–1332 [DOI] [PubMed] [Google Scholar]
- 129. Conant K., Garzino-Demo A., Nath A., McArthur J. C., Halliday W., Power C., Gallo R. C., Major E. O. (1998) Induction of monocyte chemoattractant protein-1 in HIV-1 tat-stimulated astrocytes and elevation in AIDS dementia. Proc. Natl. Acad. Sci. USA 95, 3117–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kelder W., McArthur J. C., Nance-Sproson T. E., McClernon D. R., Griffin D. E. (1998) B-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann. Neurol. 44, 831–835 [DOI] [PubMed] [Google Scholar]
- 131. Tong N., Perry S. W., Zhang Q., James H. J., Guo H., Brooks A., Bal H., Kinnear S. A., Fine S., Epstein L. G., Dairaghi D., Schall T. J., Gendelman H. E., Dewhurst S., Sharer L. R., Gelbard H. A. (2000) Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J. Immunol. 164, 1333–1339 [DOI] [PubMed] [Google Scholar]
- 132. Cotter R., Williams C., Ryan L., Erichsen D., Lopez A., Peng H., Zheng J. (2002) Fractalkine (CX3CL1) and brain inflammation: implications for HIV-1-associated dementia. J. Neurovirol. 8, 585–598 [DOI] [PubMed] [Google Scholar]
- 133. Ancuta P., Wang J., Gabuzda D. (2006) CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J. Leukoc. Biol. 80, 1156–1164 [DOI] [PubMed] [Google Scholar]
- 134. Ho D. D., Rota T. R., Hirsch M. S. (1986) Infection of monocyte/macrophages by human T lymphotropic virus type III. J. Clin. Invest. 77, 1712–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wu Y. (2004) HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology 1, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Eugenin E. A., Berman J. W. (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J. Neurosci. 27, 12844–12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brack-Werner R. (1999) Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13, 1–22 [DOI] [PubMed] [Google Scholar]
- 138. Kaul M., Garden G. A., Lipton S. A. (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 [DOI] [PubMed] [Google Scholar]
- 139. Verani A., Pesenti E., Polo S., Tresoldi E., Scarlatti G., Lusso P., Siccardi A. G., Vercelli D. (1998) Cutting edge: CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 Isolates. J. Immunol. 161, 2084–2088 [PubMed] [Google Scholar]
- 140. Samson M., Libert F., Doranz B. J., Rucker J., Liesnardt C., Farber C., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C., Muyldermans G., Verhofstede C., Burtonboy G., Georges M., Imai T., Rana S., Yi Y., Smyth R. J., Collman R. G., Doms R. W., Vassart G., Parmentier M. (1996) Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 [DOI] [PubMed] [Google Scholar]
- 141. Liu R., Paxton W. A., Choe S., Ceradini D., Martin S. R., Horuk R., MacDonald M. E., Stuhlmann H., Koup R. A., Landau N. R. (1996) Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 [DOI] [PubMed] [Google Scholar]
- 142. McElrath M. J., Pruett J. E., Cohn Z. A. (1989) Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc. Natl. Acad. Sci. USA 86, 675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Coleman C. M., Wu L. (2009) HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology 6, 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Arfi V., Riviere L., Jarrosson-Wuilleme L., Goujon C., Rigal D., Darlix J. L., Cimarelli A. (2008) Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1. J. Virol. 82, 6557–6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Weinberg J. B., Matthews T. J., Cullen B. R., Malim M. H. (1991) Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 174, 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bergamaschi A., Pancino G. (2010) Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology 7, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sonza S., Mutimer H. P., Oelrichs R., Jardine D., Harvey K., Dunne A., Purcell D. F., Birch C., Crowe S. M. (2001) Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 15, 17–22 [DOI] [PubMed] [Google Scholar]
- 148. Zhu T., Muthui D., Holte S., Nickle D., Feng F., Brodie S., Hwangbo Y., Mullins J. I., Corey L. (2002) Evidence for human immunodeficiency virus type 1 replication in vivo in CD14 monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Furtado M. R., Callaway D. S., Phair J. P., Kunstman K. J., Stanton J. L., Macken C. A., Perelson A. S., Wolinsky S. M. (1999) Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340, 1614–1622 [DOI] [PubMed] [Google Scholar]
- 150. Crowe S. M., Sonza S. (2000) HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. J. Leukoc. Biol. 68, 345–350 [PubMed] [Google Scholar]
- 151. Valcour V. G., Shiramizu B. T., Sithinamsuwan P., Nidhinandana S., Ratto-Kim S., Ananworanich J., Siangphoe U., Kim J. H., de Souza M., Degruttola V., Paul R. H., Shikuma C. M. (2009) HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology 72, 992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lambotte O., Taoufik Y., de Goer M. G., Wallon C., Goujard C., Delfraissy J. F. (2000) Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 23, 114–119 [DOI] [PubMed] [Google Scholar]
- 153. Llewellyn N., Zioni R., Zhu H., Andrus T., Xu Y., Corey L., Zhu T. (2006) Continued evolution of HIV-1 circulating in blood monocytes with antiretroviral therapy: genetic analysis of HIV-1 in monocytes and CD4+ T cells of patients with discontinued therapy. J. Leukoc. Biol. 80, 1118–1126 [DOI] [PubMed] [Google Scholar]
- 154. Patterson B. K., Mosiman V. L., Cantarero L., Furtado M., Bhattacharya M., Goolsby C. (1998) Detection of HIV-RNA-positive monocytes in peripheral blood of HIV-positive patients by simultaneous flow cytometric analysis of intracellular HIV RNA and cellular immunophenotype. Cytometry 31, 265–274 [DOI] [PubMed] [Google Scholar]
- 155. Saksela K., Stevens C., Rubinstein P., Baltimore D. (1994) Human immunodeficiency virus type 1 mRNA expression in peripheral blood cells predicts disease progression independently of the numbers of CD4+ lymphocytes. Proc. Natl. Acad. Sci. USA 91, 1104–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ancuta P., Kunstman K., Autissier P., Zaman T., Stone D., Wolinsky S., Gabuzda D. (2006) CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344, 267–276 [DOI] [PubMed] [Google Scholar]
- 157. Ellery P. J., Tippett E., Chiu Y. L., Paukovics G., Cameron P. U., Solomon A., Lewin S. R., Gorry P. R., Jaworowski A., Greene W. C., Sonza S., Crowe S. M. (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 178, 6581–6589 [DOI] [PubMed] [Google Scholar]
- 158. Han J., Wang B., Han N., Zhao Y., Song C., Feng X., Mao Y., Zhang F., Zhao H., Zeng H. (2009) CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J. Acquir. Immune Defic. Syndr. 52, 553–559 [DOI] [PubMed] [Google Scholar]
- 159. Pulliam L., Sun B., Rempel H. (2004) Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 157, 93–98 [DOI] [PubMed] [Google Scholar]
- 160. Sonza S., Maerz A., Deacon N., Meanger J., Mills J., Crowe S. (1996) Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 70, 3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Eisert V., Kreutz M., Becker K., Königs C., Alex U., Rübsamen-Waigmann H., Andreesen R., von Briesen H. (2001) Analysis of cellular factors influencing the replication of human immunodeficiency virus type I in human macrophages derived from blood of different healthy donors. Virology 286, 31–44 [DOI] [PubMed] [Google Scholar]
- 162. O'Brien W. A., Namazi A., Kalhor H., Mao S.-H., Zack J. A., Chen I. S. Y. (1994) Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 68, 1258–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Srichatrapimuk S., Auewarakul P. (2007) Resistance of monocyte to HIV-1 infection is not due to uncoating defect. Virus Res. 126, 277–281 [DOI] [PubMed] [Google Scholar]
- 164. Triques K., Stevenson M. (2004) Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J. Virol. 78, 5523–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Naif H. M., Li S., Alali M., Sloane A., Wu L., Kelly M., Lynch G., Lloyd A., Cunningham A. L. (1998) CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72, 830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tuttle D. L., Harrison J. K., Anders C., Sleasman J. W., Goodenow M. M. (1998) Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J. Virol. 72, 4962–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wang X., Ye L., Hou W., Zhou Y., Wang Y. J., Metzger D. S., Ho W. Z. (2009) Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood 113, 671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sung T. L., Rice A. P. (2009) miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 5, e1000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Peng G., Greenwell-Wild T., Nares S., Jin W., Lei K. J., Rangel Z. G., Munson P. J., Wahl S. M. (2007) Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood 110, 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Stremlau M., Owens C. M., Perron M. J., Kiessling M., Autissier P., Sodroski J. (2004) The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 [DOI] [PubMed] [Google Scholar]
- 171. Kraft-Terry S. D., Stothert A. R., Buch S., Gendelman H. E. (2010) HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol. Dis. 37, 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Langford T. D., Letendre S. L., Larrea G. J., Masliah E. (2003) Changing patterns in the neuropathogenesis of HIV during the HAART Era. Brain Pathol. 13, 195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yadav A., Collman R. G. (2009) CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J. Neuroimmune Pharmacol. 4, 430–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zheng J., Gendelman H. E. (1997) The HIV-1 associated dementia complex: a metabolic encephalopathy fueled by viral replication in mononuclear phagocytes. Curr. Opin. Neurol. 10, 319–326 [PubMed] [Google Scholar]
- 175. Liner K., Ro M., Robertson K. (2010) HIV, antiretroviral therapies, and the brain. Curr. HIV/AIDS Rep. 7, 85–91 [DOI] [PubMed] [Google Scholar]
- 176. Harezlak J., Buchthal S., Taylor M., Schifitto G., Zhong J., Daar E., Alger J., Singer E., Campbell T., Yiannoutsos C., Cohen R., Navia B., HIV Neuroimaging Consortium (2011) Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Edén A., Price, Richard W., Spudich S., Fuchs D., Hagberg L., Gisslén M. (2007) Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J. Infect. Dis. 196, 1779–1783 [DOI] [PubMed] [Google Scholar]
- 178. Gannon P., Khan M. Z., Kolson D. L. (2011) Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr. Opin. Neurol. 24, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Anthony I. C., Ramage S. N., Carnie F. W., Simmonds P., Bell J. E. (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation. J. Neuropathol. Exp. Neurol. 64, 529–536 [DOI] [PubMed] [Google Scholar]
- 180. Everall I., Vaida F., Khanlou N., Lazzaretto D., Achim C., Letendre S., Moore D., Ellis R., Cherner M., Gelman B., Morgello S., Singer E., Grant I., Masliah E. (2009) Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J. Neurovirol. 15, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Anthony I. C., Bell P. J. E. (2008) The neuropathology of HIV/AIDS. Int. Rev. Psychiatry 20, 15–24 [DOI] [PubMed] [Google Scholar]
- 182. Simioni S., Cavassini M., Annoni J-M., Hirschel B., Du Pasquier R. A. (2010) HIV-associated neurocognitive disorders: a changing pattern. Future Neurol. 6, 81–95 [Google Scholar]
- 183. Heaton R., Franklin D., Ellis R., McCutchan J., Letendre S., LeBlanc S., Corkran S., Duarte N., Clifford D., Woods S., Collier A., Marra C., Morgello S., Mindt M., Taylor M., Marcotte T., Atkinson J., Wolfson T., Gelman B., McArthur J., Simpson D., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T., Wong J., Grant I., Charter f. t., Groups H. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Shiramizu B., Paul R., Williams A., Shikuma C., Watters M., Grove J., Valcour V. (2007) HIV proviral DNA associated with decreased neuropsychological function. J. Neuropsychiatry Clin. Neurosci. 19, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Valcour V. G., Shiramizu B. T., Shikuma C. M. (2010) HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J. Leukoc. Biol. 87, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lopez C., Vazquez M., Hill M., Del C., Colon M., Porrata-Doria T., Johnston I., Lorenzo E. (2010) Characterization of HIV-1 RNA forms in the plasma of patients undergoing successful HAART. Arch. Virol. 155, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lu S-M., Tremblay M-É., King I. L., Qi J., Reynolds H. M., Marker D. F., Varrone J. J. P., Majewska A. K., Dewhurst S., Gelbard H. A. (2011) HIV-1 tat-induced microgliosis and synaptic damage via interactions between peripheral and central myeloid cells. PLoS One 6, e23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. McPhail M., Robertson K. (2011) Neurocognitive impact of antiretroviral treatment: thinking long-term. Curr. HIV/AIDS Rep. 8, 249–256 [DOI] [PubMed] [Google Scholar]
- 189. Schouten J., Cinque P., Gisslen M., Reiss P., Portegies P. (2011) HIV-1 infection and cognitive impairment in the cART era: a review. AIDS 25, 561–575 [DOI] [PubMed] [Google Scholar]
- 190. Desrosiers R. C., Daniel M. D., Li Y. (1989) HIV-related lentiviruses of nonhuman primates. AIDS Res. Hum. Retrovir. 5, 465–473 [DOI] [PubMed] [Google Scholar]
- 191. Murray E., Rausch D. M., Lendvay J., Sharer L. R., Eiden L. E. (1992) Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science 255, 1246–1249 [DOI] [PubMed] [Google Scholar]
- 192. Zink M. C., Spelman J. P., Robinson R. B., Clements J. E. (1998) SIV infection of macaques—modeling the progression to AIDS dementia. J. Neurovirol. 4, 249–259 [DOI] [PubMed] [Google Scholar]
- 193. Apetrei C., Robertson D. L., Marx P. A. (2004) The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 9, 225–254 [DOI] [PubMed] [Google Scholar]
- 194. Clements J. E., Anderson M. G., Zink M. C., Joag S. V., Narayan O. (1994) The SIV model of AIDS encephalopathy. Role of neurotropic viruses in diseases. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 72, 147–157 [PubMed] [Google Scholar]
- 195. Lackner A. A., Vogel P., Ramos R. A., Kluge J. D., Marthas M. (1994) Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145, 428–439 [PMC free article] [PubMed] [Google Scholar]
- 196. Mankowski J. L., Spelman J. P., Ressetar H. G., Strandberg J. D., Laterra J., Carter D. L., Clements J. E., Zink M. C. (1994) Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro. J. Virol. 68, 8202–8208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fox H. S., Gold L. H., Henriksen S. J., Bloom F. E. (1997) Simian immunodeficiency virus: a model for NeuroAIDS. Neurobiol. Dis. 4, 265–274 [DOI] [PubMed] [Google Scholar]
- 198. Williams K. C., Corey S., Westmoreland S., Pauley D., Knight H., deBakker C., Alvarez X., Lackner A. A. (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zink M. C., Clements J. E. (2002) A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J. Neurovirol. 8 (Suppl. 2), 42–48 [DOI] [PubMed] [Google Scholar]
- 200. Schmitz J. E., Kuroda M. J., Santra S., Sasseville V. G., Simon M. A., Lifton M. A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B. J., Ghrayeb J., Forman M. A., Montefiori D. C., Rieber E. P., Letvin N. L., Reimann K. A. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 [DOI] [PubMed] [Google Scholar]
- 201. Zink M. C., Coleman G. D., Mankowski J. L., Adams R. J., Tarwater P. M., Fox K., Clements J. E. (2001) Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 184, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 202. Otani I., Akari H., Nam K.-H., Mori K., Suzuki E., Shibata H., Doi K., Terao K., Yosikawa Y. (1998) Phenotypic changes in peripheral blood monocytes of cynomolgus monkeys acutely infected with simian immunodeficiency virus. AIDS Res. Human Retrovir. 14, 1181–1186 [DOI] [PubMed] [Google Scholar]
- 203. Kuwata T., Kodama M., Sato A., Suzuki H., Miyazaki Y., Miura T., Hayami M. (2007) Contribution of monocytes to viral replication in macaques during acute infection with simian immunodeficiency virus. AIDS Res. Human Retrovir. 23, 372–380 [DOI] [PubMed] [Google Scholar]
- 204. Clements J. E., Gama L., Bullock B., Carruth L. M., Mankowski J. L., Zink M. C. (2005) The central nervous system is a viral reservoir in simian immunodeficiency virus-infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral theraby. J. Neurovirol. 11, 180–189 [DOI] [PubMed] [Google Scholar]
- 205. Hasegawa A., Liu H., Ling B., Borda J. T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W.-K., Williams K. C., Ribeiro R. M., Lackner A. A., Veazey R. S., Kuroda M. J. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Williams K., Westmoreland S., Greco J., Ratai E., Lentz M., Kim W. K., Fuller R. A., Kim J. P., Autissier P., Sehgal P. K., Schinazi R. F., Bischofberger N., Piatak M., Lifson J. D., Masliah E., Gonzalez R. G. (2005) Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J. Clin. Invest. 115, 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Follstaedt S., Barber S., Zink M. (2008) Mechanisms of minocycline-induced suppression of simian immunodeficiency virus encephalitis: inhibition of apoptosis signal-regulating kinase 1. J. Neurovirol. 14, 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Zink M. C., Uhrlaub J., DeWitt J., Voelker T., Bullock B., Mankowski J., Tarwater P., Clements J., Barber S. (2005) Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293, 2003–2011 [DOI] [PubMed] [Google Scholar]
- 209. Witwer K. W., Gama L., Li M., Bartizal C. M., Queen S. E., Varrone J. J., Brice A. K., Graham D. R., Tarwater P. M., Mankowski J. L., Zink M. C., Clements J. E. (2009) Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One 4, e8129 [DOI] [PMC free article] [PubMed] [Google Scholar]