Abstract
Islet amyloid polypeptide (IAPP) is a peptide hormone cosecreted with insulin by pancreatic β-cells. In type II diabetes, IAPP aggregates in a process that is associated with β-cell dysfunction and loss of β-cell mass. The relationship between IAPP's conformational landscape and its capacity to mediate cell death remains poorly understood. We have addressed these unknowns by comparing the cytotoxic effects of sequence variants with differing α-helical and amyloid propensities. IAPP was previously shown to oligomerize cooperatively on binding to lipid bilayers. Here, comparable transitions are evident in cell culture and are associated with a change in subcellular localization to the mitochondria under toxic conditions. Notably, we find that this toxic gain of function maps to IAPP's capacity to adopt aggregated membrane-bound α-helical, and not β-sheet, states. Our findings suggest that upon α-helical mediated oligomerization, IAPP acquires cell-penetrating peptide (CPP) properties, facilitating access to the mitochondrial compartment, resulting in its dysfunction.—Magzoub, M. Miranker, A. D. Concentration-dependent transitions govern the subcellular localization of islet amyloid polypeptide.
Keywords: Alzheimer's disease, amylin, Parkinson's disease
Type II diabetes is characterized by loss of blood glucose homeostasis and systemic insulin resistance. A critical feature of diabetic pathogenesis is the presence of proteinaceous deposits, termed amyloid, that are associated with the death of insulin secreting β-cells. These amyloid deposits are composed primarily of protein fibers formed from islet amyloid polypeptide (IAPP; also known as amylin; refs. 1–3). IAPP is a 37-residue, natively unstructured peptide hormone that is copackaged with insulin in the secretory granules of the β cells (4, 5).
In mammals that spontaneously develop type II diabetes, such as primates and cats, the sequence variant of IAPP is competent to self-assemble and form fibers in vitro (6). In contrast, IAPP from rodents (rIAPP) does not readily aggregate, and wild-type rodents do not spontaneously develop type II diabetes (7). Significantly, rodents transgenic for human IAPP (hIAPP) develop symptoms closely similar to type II diabetes (8). In addition, studies on type I diabetes models have linked hIAPP misfolding to the failure of transplanted human islets (9). These findings clearly implicate IAPP misfolding in β-cell death and pathogenic elements of both type I and type II diabetes.
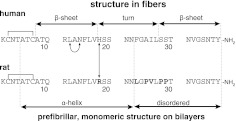
The ability of IAPP to form amyloid fibrils is cooperatively dependent on two regions of its primary sequence (see Fig. 1). Residues 20–29 (IAPP20–29) have long been associated with amyloid formation by IAPP, as it represents the subsegment of the protein that most readily polymerizes in isolation. However, the concentrations and timescales for independent aggregation by IAPP20–29 are orders of magnitude greater than those required for full-length IAPP. This suggests that regions outside residues 20–29 are responsible for increasing the nucleation potential of the 20–29 segment (10). Mutagenesis and related efforts have led to suggestions that the residues N-terminal to 20–29 mediate this catalysis (11). Specifically, oligomerization in both parallel (12) and antiparallel orientations (13) can be mediated by interactions of an ∼22-residue structured N-terminal subdomain (14). Surprisingly, the monomer within these oligomers appears to maintain an α-helical region spanning residues 5–19, which we first identified in rat IAPP (15). Several groups have now shown this structure to be sampled on a variety of alternative membrane mimics (14, 16–18). We previously suggested that this catalysis results from a combination of raising the effective local concentration and relative orientation of the nucleating peptide sequence, IAPP20–29 (14, 19).
Figure 1.
Primary sequence of IAPP. Shown are human and rat sequences of IAPP, with amino acid differences indicated in bold. Large horizontal arrows indicate areas of unambiguous secondary structure reported for both hIAPP and rIAPP on membranes (14) and for fibrillar states of hIAPP (32). Small arrows indicate additional constructs assayed in this work: hIAPPL12N/N14L, hIAPPH18R, and rIAPPR18H.
It has long been known that a poor correlation exists between amyloid burden and disease pathogenesis. For example, in Alzheimer's disease, familial mutations in the Aβ peptide have been identified for which amyloid burden is high and yet dementia is low (and vice versa; ref, 20). A similar absence of correlation of amyloid burden and β-cell death has been noted for IAPP (1). Indeed, the rate of β-cell death has been reported as anticorrelated with the rate of amyloid deposition and inhibition of fiber formation does not necessarily prevent β-cell death (21–23). In addition, fibrillar preparations of IAPP appear to be less toxic than soluble IAPP (24). Thus, it has become generally accepted that fibers themselves are not the toxic state. Rather, it is the process of amyloid formation and, in particular, the formation of soluble intermediate states that represent the origin of toxicity (11).
The current paradigm is that IAPP toxicity is mediated by the formation of membrane-bound protein assemblies. Previously, we discovered that IAPP binds to lipid bilayers to form monodisperse and two classes of heterogeneous oligomer (25). The latter are rich in α-helix content and have the capacity to render model membranes permeable to small molecules. Notably, we showed that targeting of these α-helical states with structure-specific, small-molecule, α-helical mimetics is protective against cytotoxicity (19). In this work, we seek to illuminate the molecular basis for cellular dysfunction, identify the subcellular target for cytotoxicity, and determine the molecular mechanism by which IAPP gains access to its subcellular target.
MATERIALS AND METHODS
Reagents
rIAPP and hIAPP were synthesized by standard t-Boc method and purified by the W. M. Keck facility (Yale University, New Haven, CT, USA). hIAPP mutants, hIAPPL12N/N14L and hIAPPH18R, and rIAPP mutant, rIAPPR18H, were synthesized by the W. M. Keck facility using standard Fmoc methods. All synthetic IAPPs were purified in-house and represent fractions pooled from a single peak of reverse-phase separation on C18 medium. Purity and identification were confirmed by electrospray mass spectrometry. Melittin, from honeybee (Apis mellifera) venom, was purchased from Sigma (St. Louis, MO, USA) and used as is. MTT Cell Proliferation Assay kit was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). Cell Titer-Blue (CTB) Cell Viability Assay and CytoTox-One Homogeneous Membrane Integrity Assay [lactate dehydrogenase (LDH)] kits were both from Promega (Madison, WI, USA). Organelle markers ER-Tracker Red (BODIPY TR glibenclamide), MitoTracker Red (CM-H2TMRos), and LysoTracker Red (DND-99) were from Invitrogen (Carlsbad, CA, USA).
Peptide preparation
hIAPP stocks were prepared by dissolving lyophilized peptide in 7 M guanidine hydrochloride (GuHCl), filtering through a Whatman 0.2-μm PTFE syringe filter (Whatman, Piscataway, NJ,USA), then loading onto Vydac C-18 microspin columns (Amika Bioscience, Holliston, MA, USA). The column was washed with 10% acetonitrile/0.1% TFA, followed by MilliQ water, then eluted with DMSO. rIAPP was dissolved in MilliQ water and filtered through a 0.22-μm Tuffryn syringe filter (Pall Corp., Port Washington, NY, USA). hIAPP (1 mM) and rIAPP (1–2 mM) stocks were stored at 4°C until needed. Peptide concentrations were determined by absorbance measurements at 280 nm (ε=1280 M−1·cm−1).
Peptide labeling
Amine coupling was used to attach Alexa Fluor 488-succinimidylester (Invitrogen) to the N terminus of IAPP. Labeling was done by loading 0.5 mg IAPP, dissolved in GuHCL, on a Vydac C-18 microspin column, followed by the addition of the dye solution (in 10 mM KPi buffer) to the column, vortexing, and then incubating on a rotator for 2–4 h on a rotator. Separation of IAPP488 from unlabeled peptide was done by reverse-phase HPLC. Extent of labeling was assessed using electrospray ionization-mass spectrometry (Platform LCT; Waters Corp., Milford, MA, USA), and concentration of labeled protein was determined using absorbance at 494 nm (ε=73,000 M−1·cm−1).
Cell culture
Rat insulinoma INS-1 cells (from Dr. Gary W. Cline, Department of Internal Medicine, Yale University) were cultured in 5% CO2 in RPMI 1640 (Invitrogen) supplemented with 10% FBS (Gemini Bio-Products, West Sacramento, CA, USA), 1% penicillin/streptomycin (Invitrogen), and 20% INS-1 stock solution (0.5 mM HEPES, 100 mM l-glutamine, 100 mM sodium pyruvate, and 2.5 mM β-mercaptoethanol, all from Sigma). COS-1 cells (from immortalized African green monkey kidney; CRL-1650; ATCC) were cultured in 5% CO2 in DMEM (Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. Once the cells reached ∼95% confluence, they were split (using 0.25% trypsin-EDTA; Invitrogen) into fractions and propagated or used in experiments.
Cell viability and toxicity assays
Cell viability and toxicity were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (26), which involves reduction of the tetrazolium salt MTT to purple formazan crystals in living cells; CTB assay, which measures the metabolic reduction of a nonfluorescent compound, resazurin, in living cells, resulting in a fluorescent product, resofurin; and CytoTox-One assay, in which the amount of LDH released from cells in response to membrane damage is measured with a coupled enzymatic assay that results in a fluorescent product.
Cells were plated at a density of 2 × 104 cells/well in 500 μl medium in 24-well plates (Falcon; BD Biosciences, San Diego, CA, USA). After culturing for 48 h, the medium was replaced with medium containing IAPP at the desired concentration, and incubation was continued for the indicated duration. Thereafter, the medium was replaced with fresh medium, and 50 μl MTT or 100 μl CTB reagent was added to each well and incubated for 4 h, at 37°C. The formazan crystals produced by MTT reduction were dissolved overnight at room temperature using a detergent reagent (ATCC; 500 μl added/well), and the absorbance (λ=595 nm) was measured on an Emax microplate reader (Molecular Devices, Sunnyvale, CA, USA), with a reference wavelength of 650 nm to subtract background. Fluorescence of the resofurin product (λex/λem 560/620) of CTB reduction was measured on a FluoDia T70 fluorescence plate reader (Photon Technology International, Birmingham, NJ, USA). For the LDH assay, plates were first allowed to equilibrate to 22°C, at which point 10 μl of lysis solution was added to the control wells. CytoTox-One reagent (500 μl) was then added to each well, and the plates were shaken for 30 s and incubated at 22°C for 10 min. Finally, 250 μl of stop solution was added to each well, and the fluorescence was recorded (λem/λex 560/590 nm). Wells treated with peptide-free carrier were used as a control, and wells with medium alone served as a blank. MTT or CTB reduction, or LDH release, was determined from the ratio of the absorbance or fluorescence of the treated wells to the control wells.
Scrape-loading
Scrape-loading is an established method for efficient introduction of peptides and compounds into live cells (27) and has been used in many cell types, from fibroblasts to neurons (28). The method involves tearing cells from their culture substratum in the presence of the compound of interest, resulting in transient, survivable plasma membrane disruptions, which allow rapid introduction of the compound into the cytoplasm (29, 30). Cells (2×104) were cultured in 1 ml of medium in a 12-well plate for 48 h. Following culturing, medium was replaced with medium containing IAPP at specified concentrations or DMSO. The cells were then scraped off of their substratum using a rubber spatula and allowed to incubate in the loading medium for 10–20 min. Cells were then washed by repeated mixing with an excess of PBS and spinning down, resuspended in fresh culture medium, and replated. Following incubation for the desired duration, the toxicity was determined using the cell viability assays.
Fluorescence correlation spectroscopy (FCS)
FCS was used to monitor binding and uptake of IAPPA488. FCS measurements were made on a laboratory-built instrument based around an inverted microscope using an Olympus IX71 microscope (Olmpus, Tokyo, Japan), as described previously (31). Briefly, a continuous-emission 488-nm diode-pumped solid-state 50 mW laser was set to 5–20 mW output power and further adjusted with neutral density filters to 5 μW power just prior to entering the microscope. Fluorescence was collected through an objective and separated from the excitation laser using a Z488rdc long-pass dichroic and an HQ600/200m bandpass filter (Chroma, Bellows Falls, VT, USA). Fluorescence was focused onto the aperture of a 50-μm optical fiber coupled to an avalanche photodiode (Perkin Elmer, Waltham, MA, USA). A digital correlator (Flex03LQ-12; Correlator.com, Bridgewater, NJ, USA) was used to generate the autocorrelation curve.
Measurements were made in 8-well chambered coverglasses (Nunc, Rochester, NY, USA) that were precoated with polylysine-conjugated polyethylene glycol (PEG-PLL), prepared from a modified Pierce PEGylation protocol (Pierce, Rockford, IL, USA; ref. 31), to prevent IAPP from adsorbing to chamber surfaces. Cells were plated in phenol red-free medium in the wells and cultured for 48 h prior to measurements. Control wells contained the same volume of medium without cells. IAPPA488 (100 nM), with or without an excess of unlabeled peptide (10 or 200 μM for hIAPP or rIAPP, respectively), was added to the wells at the start of the experiment, and the autocorrelation curves were taken in the medium well above the cells, at a height of 100 μm from the bottom surface of the wells. The curves were collected at regular intervals (10–30 min), over a period ranging from 2 to 16 h, and each autocorrelation curve was 10 s, repeated 10 times. In between measurements, the chambers were returned to the incubators to maintain the temperature in the wells at 37°C. For certain experiments, the cells were maintained at 4°C.
For the endocytosis inhibition experiments, INS-1 cells grown in complete medium in the 8-well chambered PEG-coated coverglasses were pretreated for 30 min in serum-free medium, with 30 μM chlorpromazine, 5 mM methyl-β-cyclodextrin, or 10 μM cytochalasin D (all from Sigma). Following pretreatment, the cells were washed with PBS, and finally incubated in complete medium at 37°C. IAPPA488 (100 nM) with an excess of unlabeled peptide (10 μM), was then added to the extracellular medium, and loss of peptide from the medium was measured by FCS.
Measurement of peptide uptake
We developed a novel method to measure the concentration of intracellular peptide, and applied it to determine the amount of hIAPP that is incorporated into cells by scrape-loading. Cells were scrape-loaded in the presence of 10 μM hIAPP (doped with 100 nM hIAPPA488) and cultured, as described earlier. Following culturing, the cells were harvested by trypsinization, spun down, and resuspended in PBS/0.02% EDTA. IAPP-positive cells were isolated using fluorescence-activated cell sorting (FACS), done on a BD FACS Aria III cell sorter, followed by lysis of a measured number of the IAPP-positive cells (lysis buffer: 50 mM Tris, pH 7.4; 1% Triton X-100; 0.1% SDS; 150 mM NaCl; and 1 mM EDTA) and measurement of total probe concentration in the sample using FCS.
Intracellular imaging and colocalization
Cells were plated in 35-mm glass-bottom Willco Wells cell culture dishes (Warner Instruments, Hamden, CT, USA). After culturing for 48 h, the medium was replaced with phenol red-free medium containing 100 nM IAPPA488 with or without an excess of unlabeled peptide (10 or 200 μM for hIAPP or rIAPP, respectively) and incubated for 72 h. At 30 min to 2 h prior to imaging, the medium was replaced with fresh medium containing organelle marker (50 nM MitoTracker or LysoTracker, or 1 μM ER-Tracker) or vehicle. Finally, immediately prior to imaging, the medium was once again replaced with fresh medium to remove any extracellular markers. Imaging was carried out at the Department of Molecular, Cellular, and Developmental Biology imaging facility (Yale University), on a Zeiss LSM 510 confocal microscope, using a ×63 Plan-Apo/1.4-NA oil-immersion objective with DIC capability (Carl Zeiss, Oberkochen, Germany). Image acquisition and processing were achieved using Zeiss Efficient Navigation (ZEN) software.
Statistical analysis
Unless otherwise indicated, all confidence intervals in this work represent the means ± sd of ≥3 independent experiments.
The FCS autocorrelation curves were averaged to obtain statistical variations used for weighting in the fitting outlined below. All fitting was done using Matlab (The MathWorks, Natick, MA, USA). The average curve was fit to Eq. 1 for 1-component diffusion to obtain N and τ:
| (1) |
where N is the number of IAPP molecules, and τD is the characteristic translational diffusion time. The structure factor s, the ratio of the radial to axial dimensions of the focal volume, was determined as a free parameter for solutions of free Alexa Fluor 488 hydrazide dye and then fixed to the experimentally determined value of 0.2 for all subsequent fittings.
RESULTS
Our overall approach is to make perturbations to IAPP's structural ensemble and relate these changes to alterations in cellular metabolism and subcellular localization. Specifically, we use established measures of cellular reductase activity to assess the metabolic effect of human IAPP. We then compare these results with sequence variants that either lack the capacity to form β-sheet fibers or have a perturbed capacity to form α-helix-rich, membrane-bound complexes (Fig. 1; refs. 14, 32). Notably, we have developed a novel approach to measuring cellular uptake. The approach is quantitative and complements imaging efforts performed on two cell lines: INS-1, which is an insulinoma-derived β-cell model notable for its robust glucose response (33); and COS-1, a kidney cell derived line that has established use in studies of IAPP trafficking, fibrillation, and toxicity (34, 35).
Inhibition of cellular reductase activity
Cellular mitochondrial reductase activity diminishes in a dose-dependent manner on exposure to hIAPP. Following treatment of INS-1 cells with 0.5 to 10 μM hIAPP for 24 h, mitochondrial reductase activity was assessed using MTT (Fig. 2A). At 0.5 μM, MTT response was 86 ± 3% that of controls using protein-free carrier. At 10 μM hIAPP, the response diminished to 52 ± 5%. The same assay applied to COS-1 cells gave 94 ± 3 and 68 ± 4% for 0.5 and 10 μM, respectively (Fig. 2B). Experiments performed using subconfluent parent stocks (36) of INS-1 cells (Supplemental Fig. S1A) showed a significantly greater effect, with mitochondrial reductase activities of 71 ± 5 and 35 ± 4% at 0.5 and 10 μM, respectively. Our results are within the range of toxicities published for IAPP, with reported viabilities ranging from 15 to 80% reported for exposure to 5–25 μM hIAPP for 24–48 h (37–40).
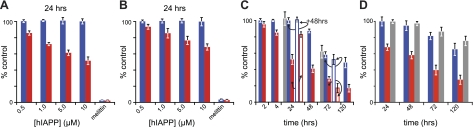
Figure 2.
Complementary measures of the effects of hIAPP on cultured cells. A, B) Dose-dependent loss of mitochondrial (MTT, red) or cytosolic reductase (CTB, blue) activity. INS-1 (A) and COS-1 (B) cells were treated with the indicated concentrations of hIAPP. Change in reductase activity is expressed as a percentage of control using protein-free carrier. For comparison, inhibition of mitochondrial and cytosolic reductase activity is also presented for 5 μM melittin. C, D) Time-dependent loss of reductase activity on incubation of INS-1 (C, solid bars) and COS-1 (D, solid bars) cells with 10 μM hIAPP (C, open bars). Reductase activity was measured 48 h after culture medium was exchanged with hIAPP-free medium. Gray bars show direct measure of viability/toxicity performed by manual counting of live, adherent cells (C) or release of cytosolic LDH to the culture medium (D).
Cytosolic reductase activity diminishes on exposure to hIAPP at later time points than mitochondrial reductases. The former was assessed using the CTB assay. In marked contrast to MTT, CTB response was not significantly affected in either INS-1 or COS-1 cells following treatment with 10 μM hIAPP for 24 h (Fig. 2A, B). Instead, significant loss of CTB response occurred at 72 h, with 58 ± 4 and 81 ± 3% for INS-1 and COS-1 cells, respectively (Fig. 2C, D). As a control, treatment of the cells with 5 μM of the peptide toxin, melittin, resulted in complete inhibition of both MTT and CTB response (Fig. 2A, B). Thus, while the effect of hIAPP on CTB occurs at comparable concentrations as MTT, it occurs at a later time. This suggests that loss of cytosolic reductase activity is a downstream consequence of the same processes that resulted in loss of mitochondrial reductase activity.
Loss of mitochondrial reductase activity precedes cell death. hIAPP cytotoxicity was independently measured using LDH release from COS-1 cells. Following treatment with 10 μM hIAPP, LDH release to the medium was not apparent until 48 h (Fig. 2D). By 72 h, viability was reduced 86 ± 4% relative to protein-free carrier controls. For INS-1 cells, cytotoxicity was instead measured by manual counting of adherent cells (pyruvate in INS-1 medium interferes with the LDH assay). At 10 μM hIAPP, this method gave viabilities of 100 and 62 ± 8% at 24 and 72 h, respectively. Note that both cell counting and LDH release agreed with the CTB data. In contrast, MTT response underestimated viability. Moreover, the loss of mitochondrial reductase activitywas, at first, rescuable. Replacing the medium after 24 h (where MTT response was 52±6%, but no loss of CTB reduction was observed) with IAPP-free medium resulted in recovery (48 h later) of MTT response to 83 ± 3% (Fig. 2C). In contrast, replacement of the medium after 72 h, where INS-1 cells showed 29 ± 3 and 58 ± 4% MTT and CTB reduction, respectively, resulted in recovery of neither CTB nor MTT (Fig. 2C). This suggests that loss of mitochondrial reductase activity reports on a cellular dysfunction that precedes cell death.
β-Sheet-independent toxicity
A similar mechanism of toxicity is apparent for the non-β-sheet-forming sequence variant of IAPP from rodents (Fig. 1). Following 24 h incubation with rIAPP, loss of mitochondrial reductase activity was apparent in INS-1 cells, but required 100–200 μM protein (Fig. 3A and Supplemental Fig. S1C). At 200 μM rIAPP, the time-dependent loss of MTT and CTB response in INS-1 cells showed similar trends to hIAPP (Fig. 2C), with loss of mitochondrial reductase activity preceding loss of cytosolic reductase activity (Fig. 3A). At 72 h, MTT and CTB response was 75 ± 4 and 93 ± 3%, respectively. This suggests that rIAPP exhibits mechanistically similar cytotoxic effects to hIAPP, albeit at a much higher concentration. This is comparable to previous findings, which showed that rIAPP, like hIAPP, can induce leakage in model liposomes (25, 41, 42). Notably, rIAPP requires significantly higher concentrations to induce leakage. Differences in the concentration dependence between hIAPP and rIAPP were attributed to differences in the capacity of these two peptides to populate oligomeric α-helical states (25, 41). Indeed, as rIAPP never forms amyloid fibers; our results shown here suggest that β-sheet formation is not a prerequisite for amyloidogenic cytotoxicity.
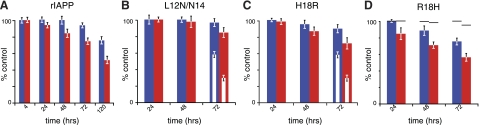
Figure 3.
Role of conformation in IAPP cytotoxicity. A) Time-dependent loss of MTT (red) or CTB (blue) response in INS-1 cells following incubation with 200 μM of the nonamyloidogenic rIAPP. B–D) Role of the helical subdomain was probed by measurement of reductase activity in INS-1 cells following incubation with 10 μM of the hIAPP mutants, L12N/N14L (B) or H18R (C), or 50 μM of the rIAPP mutant, R18H (D). Inset open bars (C) indicate loss of reductase activity at 10 μM hIAPP. Dashes (D) indicate loss of reductase activity at 50 μM rIAPP.
The N-terminal α-helical domain contributes to IAPP-mediated cytotoxicity. We examined the effects of mutations on this domain, as it is highly similar in the human and rodent proteins (Fig. 1) and mediates membrane interactions in vitro. The hIAPPL12N/N14L double mutation is notable as it is a physicochemically conservative change that does not prevent β-sheet fiber formation (43). This construct is conservative with respect to the parallel, in-register β-sheet stacking characteristic of amyloid fibers. It is not, however, conservative with respect to α-helical propensity, and indeed, hIAPPL12N/N14L shows reduced α-helical content (43). Here, treatment of INS-1 cells with 10 μM hIAPPL12N/N14L showed that the protein had lost its capacity to affect either MTT or CTB response (85±6 and 97±5%, respectively, at 72 h; Fig. 3B). Within the α-helical subdomain of IAPP, only residue 18 differed between rIAPP and hIAPP (arginine and histidine, respectively). Treatment of INS-1 cells with 10 μM hIAPPH18R yielded significantly less loss of reductase activity (73±7 and 91±5% for MTT and CTB at 72 h, respectively), compared to wild-type hIAPP (Fig. 3C). In contrast, the complementary mutation, rIAPPR18H, resulted in increased loss of reductase activity compared to wild-type rIAPP (57±5 and 78±4% for MTT and CTB at 50 μM and 72 h, respectively; Fig. 3D and Supplemental Fig. S1D). These results are in agreement with recent studies on IAPP1–19, which underscores the role of H18 in modulating both the membrane interaction and toxicity of the peptide (42, 44). These three mutations demonstrate the key importance of the α-helical domain in mediating cytoxicity of IAPP.
Mechanism of cellular uptake
At subtoxic concentrations, IAPP is internalized by an active, energy-dependent process. IAPP (100 nM), N-terminally labeled with Alexa Fluor 488 (IAPPA488), was added to the culture medium of INS-1 cells. No toxicity was evident at these concentrations (Supplemental Fig. S1B). FCS was then used to measure directly the diffusion time of IAPPA488 in the medium 100 μm above the cell layer at 37°C. All data fit to a single diffusing species with no evidence for high-molecular-weight components, which might result from IAPP self-assembly, or IAPP interactions with high-molecular-weight proteins in the culture medium. Confocal volume transit times of 233 ± 4 and 226 ± 3 μs were measured for hIAPPA488 and rIAPPA488, respectively. As transit times are directly proportional to hydrodynamic radius, these results suggest that rIAPP and hIAPP have comparable diffusion constants when measured in cell culture medium. We estimate the constant to be ∼3.1 × 10−10 m2/s, consistent with a freely diffusing monomeric peptide. This is closely similar to values reported for hIAPP determined by NMR (45) but is inconsistent with reports of diffusion constants for rIAPP (14, 45). Here, under matched conditions in culture medium, we observe closely similar diffusion behavior for rat and human variants of IAPP. The same analysis also yields the rate of loss of IAPPA488, which we observe to increase linearly with time (Fig. 4). This suggests an active uptake mechanism at a rate of 1.1 ± 0.1 × 10−3 and 1.0 ± 0.1 × 10−3 fmol/cell/min for hIAPP and rIAPP, respectively. Similar rates were apparent when measured at 4-fold higher cell density (Supplemental Fig. S2). No IAPPA488 was lost in control wells containing only medium and no cells (Supplemental Fig. S2). In marked contrast, at 4°C, where energy-dependent uptake processes are inhibited, no loss of peptide from the medium was observed (Fig. 4). Clearly, IAPP uptake under nontoxic conditions is through an active, energy-dependent mechanism.
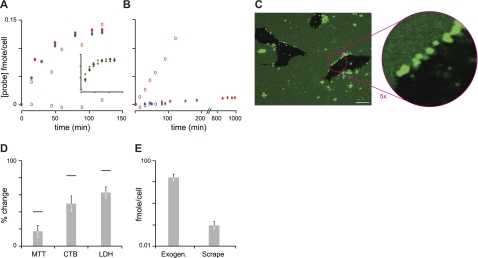
Figure 4.
Cellular uptake of IAPP. A, B) Loss of hIAPP (A) and rIAPP (B) from the extracellular medium of INS-1 cells. Open symbols indicate nontoxic levels (100 nM hIAPP488 or rIAPP488); solid symbols indicate toxic levels (100 nM labeled hIAPP488 or rIAPP488, with 10 or 200 μM unlabeled hIAPP or rIAPP, respectively). Comparisons are made between physiological temperature (37°C; red) and a temperature at which all endocytotic, and other energy-dependent processes, are inhibited (4°C; blue). Inset (A): effect of inhibition of endocytosis on hIAPP uptake, under toxic conditions (100 nM hIAPP488 with 10 μM unlabeled hIAPP). Cells were pretreated with an inhibitor of clathrin-mediated endocytosis (30 μM chlorpromazine, solid black diamonds), an inhibitor of caveolae-mediated endocytosis (5 mM methyl-β-cyclodextrin, solid orange diamonds), or an inhibitor of macropinocytosis (10 μM cytochalasin D, solid green diamonds). Data are plotted on the same scale as the main figure (A). Data are calculated from loss of probe from the extracellular medium, measured by FCS. This is expressed as amount of probe per unit cell per unit time (fmol/cell/min). C) Confocal imaging of hIAPP at toxic concentrations following a 20-min incubation at 4°C. D) Effect of introducing hIAPP directly to the cytoplasm. COS-1 cells were scrape-loaded in the presence of 10 μM hIAPP. Cells were then washed and replated with IAPP-free medium. Loss of reductase activity (MTT or CTB) or release of LDH was measured after 72 h and compared to controls scraped with IAPP-free carrier. Dashes indicate effect of 72 h continuous incubation of COS-1 cells with 10 μM hIAPP. E) Measurement of internalized IAPP. Scrape indicates peptide taken up by scrape loading and assessed by FACS/FCS method, as described in Materials and Methods. Exogen indicates peptide taken up over 72 h exposure to 10 μM hIAPP and 100 nM hIAPPA488 in culture medium, calculated by extrapolation from A (solid red ovals). Scale bar = 10 μm.
At toxic concentrations, hIAPP initially forms plasma membrane-bound aggregates. IAPPA488 (100 nM) and a toxic excess of nonlabeled peptide (10 μM hIAPP or 200 μM rIAPP) was added to INS-1 cells. For both variants, a high rate of loss was observed (Fig. 4). In the case of rIAPP (Fig. 4B), uptake was linear with time at a rate of 2.2 ± 0.4 × 10−2 fmol/cell/min, again suggesting an active uptake mechanism that is ∼20× that observed at 100 nM total rIAPP (Fig. 4A). Consistent with this, no loss of rIAPPA488 was apparent at 4°C (Fig. 4B). In marked contrast, loss of hIAPP was nonlinear with time, and the kinetic profile was unaffected by lowering the temperature to 4°C (Fig. 4A), indicating an energy-independent, uptake mechanism. Closely similar results were observed for cells kept at 37°C with endocytosis inhibited by chemical means (Fig. 4A, inset). Inhibition of clathrin-mediated endocystosis (30 μM chlorpromazine; ref. 46), caveolae-mediated endocytosis (5 mM methyl-β-cyclodextrin; ref. 47), or macropinocytosis (10 μM cytochalasin D; ref. 48) had no effect on the rate of peptide loss from the medium. These results demonstrate an endocytosis-independent uptake mechanism for hIAPP under toxic conditions. Confocal imaging of INS-1 cells at 20 min after addition of hIAPPA488 at 4°C, revealed discrete puncta at the cell surface (Fig. 4C), suggestive of membrane-bound peptide aggregates. Little probe was evident inside the cells at these short times. This suggests that membrane-bound hIAPP aggregates observed under toxic conditions represent an early first step in an energy- and endocytosis-independent uptake mechanism.
IAPP toxicity is dependent on the peptide accessing the cytoplasm. IAPP was directly introduced into the cytosol by transiently rupturing the plasma membrane in the presence of IAPP using scrape-loading techniques (27, 28). COS-1 cells were scraped in the presence of 10 μM hIAPP and compared to scraping with IAPP-free carrier (INS-1 cells were insufficiently adherent to be assayed in this way). Total exposure time to IAPP was 20 min, followed by 72 h incubation in IAPP-free medium. For exogenous exposure to hIAPP via the culture medium, a 20-min exposure time to 10 μM hIAPP is expected to result in no loss of reductase activity (Fig. 2C, D). In marked contrast, direct introduction of IAPP to the cytosol resulted in dramatic loss of both MTT and CTB response (17±6 and 49±9%, respectively) and a gain in LDH release (36±7%). Notably, these changes greatly exceed the changes observed when cells are continuously exposed to 10 μM hIAPP in the medium for 72 h (Fig. 4D).
A novel assay allows direct, quantitative measure of cellular uptake of IAPP by scrape-loading. To evaluate concentration-dependent changes in cytotoxicity, measurement of intracellular protein concentration is essential. This is typically performed in a semiquantitative manner using indirect immunochemical approaches. Recently, an approach has been put forward that capitalizes on the seeding potential of amyloid to extrapolate protein concentration (49). Here, we report the development of a method that enables us to directly measure intracellular peptide concentrations. Following the scrape-loading of COS-1 cells in the presence of 10 μM hIAPP doped with 100 nM hIAPPA488, fluorescent cells were isolated using FACS. A measured number of IAPP-positive cells was then lysed, and FCS was used to measure the total amount of hIAPPA488 in the sample. Using this method, we found that the total amount of hIAPP introduced into cells by scrape-loading was 0.09 ± 0.01 fmol/cell (Fig. 4E). For exogenously added IAPP, we can determine total protein uptake from the rate of protein loss from the medium (Fig. 4A, E). After 72 h, a total of ∼15 fmol of protein was processed by each cell. Thus, 2 orders of magnitude separate the difference in potency between direct and exogenous exposure to IAPP. This suggests that cytotoxicity is predicated on IAPP peptide accessing the cell cytoplasm. In the case of exogenous exposure to IAPP, this process is attenuated, most likely by degradative pathways.
Subcellular localization of IAPP
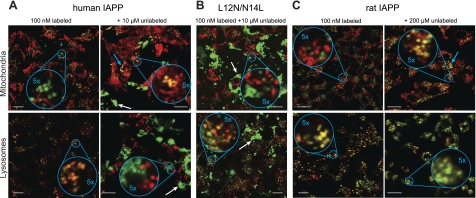
Under nontoxic conditions, IAPP is internalized through endocytosis. Confocal imaging of INS-1 cells following 72 h incubation with 100 nM IAPPA488 revealed colocalization of hIAPP and rIAPP with lysosomes (Fig. 5A, C). This suggests that IAPP is taken up by endocytosis and trafficked into late endosomes or lysosomes, consistent with the FCS uptake experiments, which indicates an energy-dependent uptake process under these conditions (Fig. 4). Colocalization is not observed with other compartments (Fig. 5C and Supplemental Fig. S3), indicating that once taken up, the peptide is simply degraded.
Figure 5.
Intracellular localization of IAPP. Colocalization of hIAPPA488 (A), hIAPPL12N/N14L (B), or rIAPP (C) with MitoTracker (top panels) or LysoTracker (bottom panels) in INS-1 cells. Conditions were 100 nM labeled IAPP with or without indicated concentration of unlabeled protein. Incubations were conducted for 72 h prior to imaging. For hIAPP and rIAPP, the addition of 10 and 200 μM unlabeled material, respectively, reflects conditions when toxicity is observed (Figs. 2 and 3A). White and cyan arrows indicate examples of extracellular aggregates and mitochondrial colocalization, respectively. Scale bars = 10 μm.
Cytotoxicity is associated with localization of IAPP to the mitochondria. Following 72 h incubation of INS-1 cells with toxic levels of IAPP (100 nM hIAPPA488 or rIAPPA488, with 10 or 200 μM of hIAPP or rIAPP, respectively), both hIAPP and rIAPP localized to the mitochondria (Fig. 5A, C). For rIAPP, colocalization to lysosomes was observed, even under toxic conditions (Fig. 5C), consistent with an energy-dependent uptake mechanism (Fig. 4B). In contrast, hIAPP did not localize to lysosomes under these conditions (Fig. 5A), consistent with the uptake data (Fig. 4A). This suggests that at these toxic concentrations, uptake into the cytosol proceeds via a direct and likely energy-independent mechanism. In comparison, the nontoxic variant (100 nM hIAPPA488,L12N/N14L and 10 μM hIAPPL12N/N14L) showed abundant extracellular fibers, localization to lysosomes, and no colocalization with mitochondria (Fig. 5B). Thus, lysosomal localization and fiber formation can be apparent under toxic and nontoxic conditions; mitochondrial localization is only observed under toxic conditions.
DISCUSSION
Identifying the cellular target of IAPP-mediated toxicity, determining the nature of this mechanism, and characterizing the structure of the toxic species of IAPP, is central to deciphering the role of IAPP in the diabetic loss of insulin-secreting β-cells. Here, we have carried out a number of cell viability and toxicity assays, as well as fluorescence spectroscopy and microscopy studies, in tandem with a novel method we developed to directly and quantitatively measure intracellular protein concentration, to address these pertinent questions. Our results show that IAPP cytotoxicity is correlated with mitochondrial dysfunction and that this toxicity is a consequence of concentration-dependent formation of nonamyloid conformers.
Toxicity is a downstream consequence of mitochondrial dysfunction induced directly by contact with IAPP. We have shown that metabolic assays dominated by mitochondrial reductases are sensitive to change long before cell death (Figs. 2 and 3). This sensitivity is increased in rapidly dividing INS-1 cells (Supplemental Fig. S1), in agreement with the reported increased vulnerability of replicating β-cells to hIAPP toxicity (50), which may account for the failure to adaptively increase β-cell mass, leading to β-cell deficiency, observed in type II diabetes. Recent efforts have similarly suggested that IAPP-induced apoptosis is associated with disruption of mitochondrial membranes (51), as well as mitochondrial fragmentation and loss of mitochondrial membrane potential (52). Here we have shown for both hIAPP and nonamyloidogenic rIAPP that localization to mitochondria occurs only under conditions where toxicity is observed (Fig. 5). As mitochondrial reductase activity is correlated with these effects, this suggests that IAPP acts directly on mitochondria, most likely by disruption of mitochondrial membrane integrity.
IAPP gains toxic function from nonamyloid oligomeric assembly. At toxic concentrations, hIAPP can be seen to first associate with the plasma membrane as oligomeric puncta within 20 min of incubation (Fig. 4C). These are comparable to previously reported cell membrane bound states (24, 53). While we cannot definitively determine the extent of fibers in these puncta, we note that the kinetic profile for lipid catalyzed fiber formation under these conditions has a lag phase longer than 1 h (41). Moreover, at later time points where toxicity is evident, fiber-like aggregates are apparent extracellularly, while a fraction of the peptide colocalizes with mitochondria (Fig. 5A). Here, we assess a mutant, hIAPPL12NN14L, which retains the capacity to forms fibers (Fig. 5B) but does not cause toxicity (Fig. 3B). This strongly suggests that while toxicity and fiber formation can be related processes, fibers themselves are nontoxic. Remarkably, nonamyloidogenic rIAPP is also toxic. It is typically reported that rIAPP is nontoxic, leading to a general assumption that β-sheet formation is the origin of toxicity (24, 42, 54). However, experimental concentrations of rIAPP are typically matched to hIAPP, ranging from 250 nM to 10 μM. Our data are consistent, and further show that rIAPP becomes toxic at elevated concentrations. Previously, we reported that while rIAPP cannot form fibers, it can undergo membrane-associated α-helical aggregation (25, 41). This occurs for both rIAPP and hIAPP, and is associated with their capacity to induce membrane leakage. Notably, rIAPP requires >20 μM protein for oligomerization to become apparent on synthetic liposomes. Others have gone further to show that protein fragments from the α-helical domains of both rIAPP and hIAPP have little capacity to independently form β-sheet fibers but retain the ability to induce membrane leakage (42). These observations are qualitatively mirrored in this work with the rodent protein showing toxicity at concentrations >10-fold higher than that required for hIAPP. Others have reported β-cell death, even in rodent models of diabetes (55, 56), where amyloid is not apparent. We conjecture that the increases in β-cell apoptosis in rodents (57, 58), while modest compared to rodents transgenic for human IAPP, are due to α-helical subdomain-mediated aggregation.
Gain of toxic function by IAPP includes the acquisition of physicochemical properties typically associated with cell-penetrating peptides (CPPs; also known as protein transduction domains or PTDs; ref. 59). We suggest that oligomeric IAPP gains the capacity to behave as a CPP and, as such, can independently traverse cellular membranes. In the case of hIAPP, the plasma membrane is crossed directly (Fig. 4A), through a mechanism initiated by formation of membrane-bound aggregates (visible as puncta, Fig. 4C), enabling the peptide to bypass the endosomal-lysosomal pathway (Fig. 5C). For rIAPP, continuous uptake by endocytosis leads to a buildup of the peptide within late endosomes and lysosomes, resulting in formation of oligomers (ref. 60 and Fig. 5C). These intralysosomal oligomers may behave in a similar fashion to plasma membrane-bound hIAPP oligomers, enabling them to escape the lysosomes. CPPs, such as the so-called TAT peptide, derived from the HIV-1 transcription-activating (TAT) protein, acquire their functionality, in part, from short (typically 8 or more), positively charged regions that act on anionic cellular membranes (59). IAPP is wholly cationic, but with only 3–4 charges in the N-terminal α-helical domain of IAPP (refs. 14, 15 and Fig. 1). Oligomerization of IAPP necessarily concentrates multiple sets of cations, giving it a charge density comparable to a CPP. It has been shown that CPP internalization follows a process that includes Ca2+-influx-triggered resealing of the plasma membrane (61). This is intriguing, as Ca2+ influx reported (42) following cellular exposure to IAPP follows a kinetic profile that is very similar. Therefore, a concentration-dependent acquisition of CPP-like properties would account for our observation of mitochondrial access at elevated protein concentrations.
Our newly developed method for quantifying peptide uptake enabled us to show that cytoplasmic exposure is >100-fold more sensitive than extracellular exposure to protein. This not only supports our assertion of a CPP-like gain of function, it also provides a framework for reconciling disparate reports regarding the origins of IAPP toxicity. Some studies introduce IAPP to culture medium and implicate the plasma membrane as the site of toxicity (62). Others, using transfection, have attributed toxicity to intracellular IAPP (63, 64). Still others have used oligomer-specific antibodies to suggest toxicity along the secretory pathway and/or the mitochondrial membranes (51). These diverse origins are easily reconciled if the concentration-dependent acquisition of CPP-like properties permit IAPP to traverse any biological membrane. This permits access to mitochondria where membrane disruption first causes dysfunction and later causes toxicity. Indeed, this may be a common mechanism for all amyloids derived from disordered precursors. Loss of MTT reduction preceding cell death has also been reported for Aβ1–42 (65). More recently, it has been proposed that Aβ exerts its neurotoxic effects through disruption of mitochondrial function via direct interaction (66, 67). Mitochondria have also been implicated in Parkinson's disease as a target for the toxic actions of the amyloidogenic α-synuclein (68). α-Synuclein mutants, which exhibit accelerated oligomer formation (69, 70), have recently been shown to directly interact with, and fragment, mitochondria (71). Toxicity in all three systems, which exhibit similar disordered-to-ordered transitions in the presence of membranes, may well be predicated on gaining access to the cytoplasm through CPP-like effects, followed by interaction and disruption of the mitochondrial membrane.
Supplementary Material
Acknowledgments
The authors thank Dr. Gary W. Cline and Rebecca Pongratz (Department of Internal Medicine, Yale University) for the gift of INS-1 cells, valuable discussions, and technical assistance with cell culture. The authors thank A. Trexler for assistance with the FCS, Dr. V. Horsley and Dr. K. Nelson for assistance with FACS, Dr. J. Wolenski for assistance with confocal imaging, and N. Last and Dr. G. W. Cline for critical reading of the manuscript.
This work was supported by grants from the U.S. National Institutes of Health (DK079829 and GM094693).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CTB
- Cell Titer-Blue
- CPP
- cell-penetrating peptide
- hIAPP
- human islet amyloid polypeptide
- IAPP
- islet amyloid polypeptide
- FACS
- fluorescence-activated cell sorting
- FCS
- fluorescence correlation spectroscopy
- LDH
- lactate dehydrogenase
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- rIAPP
- rodent islet amyloid polypeptide.
REFERENCES
- 1. Haataja L., Gurlo T., Huang C. J., Butler P. C. (2008) Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr. Rev. 29, 303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoppener J. W., Ahren B., Lips C. J. (2000) Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 343, 411–419 [DOI] [PubMed] [Google Scholar]
- 3. Kahn S. E., Andrikopoulos S., Verchere C. B. (1999) Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48, 241–253 [DOI] [PubMed] [Google Scholar]
- 4. Clark A., Nilsson M. R. (2004) Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 47, 157–169 [DOI] [PubMed] [Google Scholar]
- 5. Hull R. L., Westermark G. T., Westermark P., Kahn S. E. (2004) Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643 [DOI] [PubMed] [Google Scholar]
- 6. Kapurniotu A. (2001) Amyloidogenicity and cytotoxicity of islet amyloid polypeptide. Biopolymers 60, 438–459 [DOI] [PubMed] [Google Scholar]
- 7. Westermark P., Engstrom U., Johnson K. H., Westermark G. T., Betsholtz C. (1990) Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc. Natl. Acad. Sci. U. S. A. 87, 5036–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matveyenko A. V., Butler P. C. (2006) Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55, 2106–2114 [DOI] [PubMed] [Google Scholar]
- 9. Potter K. J., Abedini A., Marek P., Klimek A. M., Butterworth S., Driscoll M., Baker R., Nilsson M. R., Warnock G. L., Oberholzer J., Bertera S., Trucco M., Korbutt G. S., Fraser P. E., Raleigh D. P., Verchere C. B. (2010) Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc. Natl. Acad. Sci. U. S. A. 107, 4305–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruschak A. M., Miranker A. D. (2007) Fiber-dependent amyloid formation as catalysis of an existing reaction pathway. Proc. Natl. Acad. Sci. U. S. A. 104, 12341–12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hebda J. A., Miranker A. D. (2009) The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: insights from type II diabetes. Annu. Rev. Biophys. 38, 125–152 [DOI] [PubMed] [Google Scholar]
- 12. Wiltzius J. J., Sievers S. A., Sawaya M. R., Eisenberg D. (2009) Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 18, 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nath A., Miranker A. D., Rhoades E. (2011) A membrane-bound antiparallel dimer of rat islet amyloid polypeptide. Angew. Chem. Int. Ed. Engl. 50, 10859–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williamson J. A., Loria J. P., Miranker A. D. (2009) Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J. Mol. Biol. 393, 383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson J. A., Miranker A. D. (2007) Direct detection of transient alpha-helical states in islet amyloid polypeptide. Protein Sci. 16, 110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patil S. M., Xu S., Sheftic S. R., Alexandrescu A. T. (2009) Dynamic alpha-helix structure of micelle-bound human amylin. J. Biol. Chem. 284, 11982–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apostolidou M., Jayasinghe S. A., Langen R. (2008) Structure of alpha-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J. Biol. Chem. 283, 17205–17210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanga R. P., Brender J. R., Xu J., Hartman K., Subramanian V., Ramamoorthy A. (2009) Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J. Am. Chem. Soc. 131, 8252–8261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hebda J. A., Saraogi I., Magzoub M., Hamilton A. D., Miranker A. D. (2009) A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem. Biol. 16, 943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neve R. L., Robakis N. K. (1998) Alzheimer's disease: a re-examination of the amyloid hypothesis. Trends Neurosci. 21, 15–19 [DOI] [PubMed] [Google Scholar]
- 21. Butler A. E., Janson J., Soeller W. C., Butler P. C. (2003) Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52, 2304–2314 [DOI] [PubMed] [Google Scholar]
- 22. Butler A. E., Jang J., Gurlo T., Carty M. D., Soeller W. C., Butler P. C. (2004) Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): a new model for type 2 diabetes. Diabetes 53, 1509–1516 [DOI] [PubMed] [Google Scholar]
- 23. Meier J. J., Kayed R., Lin C. Y., Gurlo T., Haataja L., Jayasinghe S., Langen R., Glabe C. G., Butler P. C. (2006) Inhibition of human IAPP fibril formation does not prevent beta-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am. J. Physiol. Endocrinol. Metab. 291, E1317–E1324 [DOI] [PubMed] [Google Scholar]
- 24. Janson J., Ashley R. H., Harrison D., McIntyre S., Butler P. C. (1999) The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes 48, 491–498 [DOI] [PubMed] [Google Scholar]
- 25. Last N. B., Rhoades E., Miranker A. D. (2011) Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc. Natl. Acad. Sci. U. S. A. 108, 9460–9465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 27. McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. (1984) A method for incorporating macromolecules into adherent cells. J. Cell Biol. 98, 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mcneil P. L. (1989) Incorporation of macromolecules into living cells. Methods Cell Biol. 29, 153–173 [DOI] [PubMed] [Google Scholar]
- 29. Terasaki M., Miyake K., McNeil P. L. (1997) Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J. Cell Biol. 139, 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNeil P. L. (2002) Repairing a torn cell surface: make way, lysosomes to the rescue. J. Cell Sci. 115, 873–879 [DOI] [PubMed] [Google Scholar]
- 31. Middleton E. R., Rhoades E. (2010) Effects of curvature and composition on alpha-synuclein binding to lipid vesicles. Biophys. J. 99, 2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luca S., Yau W. M., Leapman R., Tycko R. (2007) Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry 46, 13505–13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 34. O'Brien T. D., Butler P. C., Kreutter D. K., Kane L. A., Eberhardt N. L. (1995) Human islet amyloid polypeptide expression in COS-1 cells. A model of intracellular amyloidogenesis. Am. J. Pathol. 147, 609–616 [PMC free article] [PubMed] [Google Scholar]
- 35. Hiddinga H. J., Eberhardt N. L. (1999) Intracellular amyloidogenesis by human islet amyloid polypeptide induces apoptosis in COS-1 cells. Am. J. Pathol. 154, 1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riss T. L., Moravec R. A. (2004) Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2, 51–62 [DOI] [PubMed] [Google Scholar]
- 37. Bai J. Z., Saafi E. L., Zhang S., Cooper G. J. (1999) Role of Ca2+ in apoptosis evoked by human amylin in pancreatic islet beta-cells. Biochem. J. 343, 53–61 [PMC free article] [PubMed] [Google Scholar]
- 38. Rumora L., Hadzija M., Barisic K., Maysinger D., Grubiic T. Z. (2002) Amylin-induced cytotoxicity is associated with activation of caspase-3 and MAP kinases. Biol. Chem. 383, 1751–1758 [DOI] [PubMed] [Google Scholar]
- 39. Konarkowska B., Aitken J. F., Kistler J., Zhang S., Cooper G. J. (2006) The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 273, 3614–3624 [DOI] [PubMed] [Google Scholar]
- 40. Lim Y. A., Ittner L. M., Lim Y. L., Gotz J. (2008) Human but not rat amylin shares neurotoxic properties with Abeta42 in long-term hippocampal and cortical cultures. FEBS Lett. 582, 2188–2194 [DOI] [PubMed] [Google Scholar]
- 41. Knight J. D., Hebda J. A., Miranker A. D. (2006) Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry 45, 9496–9508 [DOI] [PubMed] [Google Scholar]
- 42. Brender J. R., Hartman K., Reid K. R., Kennedy R. T., Ramamoorthy A. (2008) A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry 47, 12680–12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koo B. W., Hebda J. A., Miranker A. D. (2008) Amide inequivalence in the fibrillar assembly of islet amyloid polypeptide. Protein Eng. Des. Sel. 21, 147–154 [DOI] [PubMed] [Google Scholar]
- 44. Nanga R. P., Brender J. R., Xu J., Veglia G., Ramamoorthy A. (2008) Structures of rat and human islet amyloid polypeptide IAPP(1–19) in micelles by NMR spectroscopy. Biochemistry 47, 12689–12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soong R., Brender J. R., Macdonald P. M., Ramamoorthy A. (2009) Association of highly compact type II diabetes related islet amyloid polypeptide intermediate species at physiological temperature revealed by diffusion NMR spectroscopy. J. Am. Chem. Soc. 131, 7079–7085 [DOI] [PubMed] [Google Scholar]
- 46. Wang L. H., Rothberg K. G., Anderson R. G. (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wadia J. S., Stan R. V., Dowdy S. F. (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10, 310–315 [DOI] [PubMed] [Google Scholar]
- 48. Sampath P., Pollard T. D. (1991) Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry 30, 1973–1980 [DOI] [PubMed] [Google Scholar]
- 49. Du D., Murray A. N., Cohen E., Kim H. E., Simkovsky R., Dillin A., Kelly J. W. (2011) A kinetic aggregation assay allowing selective and sensitive amyloid-beta quantification in cells and tissues. Biochemistry 50, 1607–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ritzel R. A., Butler P. C. (2003) Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes 52, 1701–1708 [DOI] [PubMed] [Google Scholar]
- 51. Gurlo T., Ryazantsev S., Huang C. J., Yeh M. W., Reber H. A., Hines O. J., O'Brien T. D., Glabe C. G., Butler P. C. (2010) Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am. J. Pathol. 176, 861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li X. L., Chen T., Wong Y. S., Xu G., Fan R. R., Zhao H. L., Chan J. C. (2011) Involvement of mitochondrial dysfunction in human islet amyloid polypeptide-induced apoptosis in INS-1E pancreatic beta cells: An effect attenuated by phycocyanin. Int. J. Biochem. Cell Biol. 43, 525–534 [DOI] [PubMed] [Google Scholar]
- 53. Casas S., Gomis R., Gribble F. M., Altirriba J., Knuutila S., Novials A. (2007) Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes 56, 2284–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mirzabekov T. A., Lin M. C., Kagan B. L. (1996) Pore formation by the cytotoxic islet amyloid peptide amylin. J. Biol. Chem. 271, 1988–1992 [DOI] [PubMed] [Google Scholar]
- 55. Tschop M., Heiman M. L. (2001) Rodent obesity models: an overview. Exp. Clin. Endocrinol. Diabetes. 109, 307–319 [DOI] [PubMed] [Google Scholar]
- 56. Hoppener J. W., Oosterwijk C., Nieuwenhuis M. G., Posthuma G., Thijssen J. H., Vroom T. M., Ahren B., Lips C. J. (1999) Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia 42, 427–434 [DOI] [PubMed] [Google Scholar]
- 57. Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. (1998) Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. U. S. A. 95, 2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Efanova I. B., Zaitsev S. V., Zhivotovsky B., Kohler M., Efendic S., Orrenius S., Berggren P. O. (1998) Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J. Biol. Chem. 273, 33501–33507 [DOI] [PubMed] [Google Scholar]
- 59. Magzoub M., Graslund A. (2004) Cell-penetrating peptides: [corrected] from inception to application. Q. Rev. Biophys. 37, 147–195 [DOI] [PubMed] [Google Scholar]
- 60. Soto C., Castano E. M. (1996) The conformation of Alzheimer's beta peptide determines the rate of amyloid formation and its resistance to proteolysis. Biochem. J. 314, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palm-Apergi C., Lorents A., Padari K., Pooga M., Hallbrink M. (2009) The membrane repair response masks membrane disturbances caused by cell-penetrating peptide uptake. FASEB J. 23, 214–223 [DOI] [PubMed] [Google Scholar]
- 62. Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 280, 17294–17300 [DOI] [PubMed] [Google Scholar]
- 63. Lin C. Y., Gurlo T., Kayed R., Butler A. E., Haataja L., Glabe C. G., Butler P. C. (2007) Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes 56, 1324–1332 [DOI] [PubMed] [Google Scholar]
- 64. Matveyenko A. V., Gurlo T., Daval M., Butler A. E., Butler P. C. (2009) Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes 58, 906–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu M. L., Hong S. T. (2005) Early phase of amyloid beta42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp. Mol. Med. 37, 559–566 [DOI] [PubMed] [Google Scholar]
- 66. Lustbader J. W., Cirilli M., Lin C., Xu H. W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., Trinchese F., Liu S., Gunn-Moore F., Lue L. F., Walker D. G., Kuppusamy P., Zewier Z. L., Arancio O., Stern D., Yan S. S., Wu H. (2004) ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science 304, 448–452 [DOI] [PubMed] [Google Scholar]
- 67. Lim Y. A., Rhein V., Baysang G., Meier F., Poljak A., Raftery M. J., Guilhaus M., Ittner L. M., Eckert A., Gotz J. (2010) Aβ and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics 10, 1621–1633 [DOI] [PubMed] [Google Scholar]
- 68. Nakamura K., Nemani V. M., Wallender E. K., Kaehlcke K., Ott M., Edwards R. H. (2008) Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J. Neurosci. 28, 12305–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Conway K. A., Harper J. D., Lansbury P. T. J. (2000) Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry 39, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 70. Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T. J. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U. S. A. 97, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., Sesaki H., Cheng Y., Finkbeiner S., Nussbaum R. L., Masliah E., Edwards R. H. (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286, 20710–20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.