Abstract
Abnormalities in T-lymphocyte populations and function are observed in autism. Soluble amyloid precursor protein α (sAPP-α) is elevated in some patients with autism and is known to be produced by immune cells. In light of the well-established role of sAPP-α in proliferation, growth, and survival of neurons, we hypothesized an analogous role in the immune system. Thus, we explored whether sAPP-α could modulate immune development and function, especially aspects of the pinnacle cell of the adaptive arm of the immune system: the T cell. To do this, we generated mice overexpressing human sAPP-α and characterized elements of T-cell development, signal transduction, cytokine production, and innate/adaptive immune functions. Here, we report that transgenic sAPP-α-overexpressing (TgsAPP-α) mice displayed increased proportions of CD8+ T cells, while effector memory T cells were decreased in the thymus. Overall apoptotic signal transduction was decreased in the thymus, an effect that correlated with dramatic elevations in Notch1 activation; while active-caspase-3/total-caspase-3 and Bax/Bcl-2 ratios were decreased. Greater levels of IFN-γ, IL-2, and IL-4 were observed after ex vivo challenge of TgsAPP-α mouse splenocytes with T-cell mitogen. Finally, after immunization, splenocytes from TgsAPP-α mice displayed decreased levels IFN-γ, IL-2, and IL-4, as well as suppressed ZAP70 activation, after recall antigen stimulation. Given elevated levels of circulating sAPP-α in some patients with autism, sAPP-α could potentially drive aspects of immune dysfunction observed in these patients, including dysregulated T-cell apoptosis, aberrant PI3K/AKT signaling, cytokine alterations, and impaired T-cell recall stimulation.—Bailey, A. R., Hou, H., Obregon, D. F., Tian, J., Zhu, Y., Zou, Q., Nikolic, W. V., Bengtson, M., Mori, T., Murphy, T., Tan, J. Aberrant T-lymphocyte development and function in mice overexpressing human soluble amyloid precursor protein-α: implications for autism.
Keywords: T cells, sAPP-α, transgenic mice
Autism is a neurodevelopmental disorder characterized by impaired social interaction, deficits in verbal and nonverbal communication, and restricted, repetitive patterns of stereotyped behaviors and interests (1). The disorder is diagnosed using standardized behavioral observation tools and has several comorbidities, including mental retardation and aggression. In addition to these traits, various immune abnormalities have been identified and observed in patients with autism. Anomalies of the immune system in autism manifest in the form of altered autoantibody and cytokine profiles, neuroinflammation, and changes in cellular populations and function (2). Autoimmunity and ongoing systemic immune activation have been associated with autism pathogenesis as well (3). Speculation that immune system malfunction may be related directly to the biological etiology of autism has yet to be conclusively corroborated.
Recent studies show that patients with severe autism express elevated levels of secreted soluble amyloid precursor protein α (sAPP-α) in their plasma (4). The amyloid precursor protein (APP) is a type 1 transmembrane protein that is expressed ubiquitously in numerous cell types. It is proteolytically processed via two pathways that involve a combination of cleavage enzymes called secretases; each pathway produces several soluble fragments. The amyloidogenic pathway generates amyloid β (Aβ; the focus of research in Alzheimer's disease), sAPP-β, and the APP-intracellular domain (AICD). The other pathway is termed the nonamyloidogenic pathway and produces sAPP-α along with the p3 and AICD fragments (5). The sAPP-α metabolite is well-known for its neurotrophic and neuroprotective properties (6, 7). In addition, the fragment is secreted from immune cells after stimulation, implying a role for sAPP-α in immune cell activation (8–10). Although the fragment is being considered as a candidate peripheral biomarker for autism (4, 11), its potential role in the pathophysiology of the disorder remains to be elucidated.
Among the immune cell features seen in patients with autism are anomalies in T-lymphocyte population and function (12). One team identified reduced numbers of CD4+ T-helper cells in serum from subjects with autism compared to controls (13). The same group provided further detail on the CD4+ T-cell subpopulations, showing a decrease in CD4RA+ T cells, which are responsible for inducing suppressor T cells (14). In another study, ∼35% of patients with autism had decreased CD4+ T cells in the serum (15). Furthermore, a shift is apparent in the CD4+-cell population from T-helper 1 (Th1) cells toward Th2 cells in patients with autism (16).
Concurrently, compelling experimental evidence suggests a potential role for sAPP-α in T-lymphocyte activation. Transcription, translation, and secretion of APP were induced on stimulation of peripheral mononuclear blood leukocytes with T-cell mitogens (9). Moreover, secreted APP is expressed by CD4+ and CD8+ T lymphocytes after stimulation with T-cell-specific mitogen phytohemagglutinin (PHA), suggesting that sAPP is involved in the initiation of the T-cell-mediated immune response (8). Also, human peripheral T cells, as well as Jurkat cells, expressed increased levels of APP mRNA when activated with a calcium ionophore (17).
Given that T-cell immunity is altered in patients with autism, and that sAPP-α is expressed at high levels in some patients and is potentially influential in T-cell activation, we hypothesize that increased levels of sAPP-α in the periphery may alter immune cell development and function, thereby contributing to this particular aspect of the immune anomalies observed in subsets of patients with autism. To investigate this theory, we created a transgenic sAPP-α-overexpressing (TgsAPP-α) mouse that overexpresses human sAPP-α (hsAPP-α) in several organs, including the brain, thymus, and spleen. Here, we report that the T-cell population is altered in TgsAPP-α mice. We demonstrate that T-cell development in the thymus is affected due to curious levels of protein expression, which suggests antiapoptotic signaling. Finally, we show that T-cell memory function is reduced in our model. We conclude that sAPP-α is instrumental in modifying T-cell development and function, and therefore may be directly involved in the pathophysiology of autism.
MATERIALS AND METHODS
Mice and genotyping
Generation of TgsAPP-α mice was carried out at the H. Lee Moffitt Cancer Center Animal Core Facility (Tampa, FL, USA) by standard pronuclear injection. A 1.8-kb genomic fragment transcribing hsAPP-α695 was subcloned into a MoPrP.Xho vector (18, 19). The pcDNA3.1 plasmid containing the hsAPP-α695 DNA fragment was graciously provided by Dr. Steven Barger (University of Arkansas, Little Rock, AR, USA). Several lines of TgsAPP-α mice were generated, and the line with the highest level of hsAPP-α protein expression was selected and maintained by heterozygous backcrossing on the C57BL6 mouse strain. Animals were genotyped by quantitative real-time PCR using the following sequences of primers: hsAPPα forward, 5′-GCCTGGACGATCTCCAGC-3′; hsAPP-α reverse, 5′-TGGCCCGGTGTTAGCACTGGC-3′; β-actin forward, 5-AGCTTGCTGTATTCCCCTCCATCGTG-3′; β-actin reverse, 5′-AATTCGGATGGCTACGTACATGGCTG-3′. Mice were housed in a 12-h light-dark cycle, and all experiments were conducted in accordance with institutional guidelines and were approved by the University of South Florida institutional animal care and use committee.
Tissue isolation and preparation
Mice were anesthetized using gaseous isoflourane. Blood was collected from the right ventricle of the heart and immediately placed into tubes containing 0.5 M EDTA (BD Biosciences, San Jose, CA, USA). Plasma was removed after centrifugation at 3000 rpm for 15 min at 4°C. Animals were then perfused transcardially with 0.01 M PBS (pH 7.4), and the thymus, spleen, and brain were removed. Single-cell suspensions of thymocytes and splenocytes were generated using a 40-μm nylon mesh cell strainer (Thermo Fisher Scientific, Waltham, MA, USA). Red blood cells were eliminated using ACK RBC lysis buffer (Invitrogen, Carlsbad, CA, USA). Subsequently, thymocytes and splenocytes were washed in 1× PBS (Mediatech, Manassas, VA, USA) before suspension in RPMI 1640 (Lonza, Walkersville, MD, USA) for culture or 1× PBS containing 5% fetal bovine serum (FBS) for flow cytometry analysis. Whole thymus organs were fixed in 4% paraformaldehyde at 4°C overnight, cryoprotected in concentrations of 10, 20, and 30% sucrose; embedded in Neg50 frozen section medium (Richard-Allan Scientific, Kalamazoo, MI, USA), and cut sagittally on a Microm HM 550 cryostat (Thermo Fisher Scientific) at 25-μm thickness. Brains were removed and sagittally bisected. Left brain hemispheres and thymus organs were homogenized in 1× lysis buffer (Cell Signaling, Boston, MA, USA) with 1% PMSF (Sigma-Aldrich, St. Louis, MO, USA), centrifuged at 14,000 rpm for 15 min and stored at −80°C.
Enzyme-linked immunosorbent assay (ELISA)
hsAPP-α expression in brain homogenates and plasma was quantified using a highly specific assay kit (IBL-America, Minneapolis, MN, USA). According to the manufacturer, this kit is 100% specific for hsAPP-α and cross-reacts at <0.1% with hsAPP-β wild-type (WT) and Swedish type. Splenocytes from untreated TgsAPP-α mice were suspended in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY, USA) plated in 24-well culture plates and incubated with 5 μg/ml concanavalin A (ConA; Sigma-Aldrich), or PBS for 18 h at 37°C in 5% CO2. Splenocytes from TgsAPP-α mice immunized with either myelin oligodendrocyte protein (MOG; Mimotopes, Melbourne, Australia) in complete Freund's adjuvant (CFA, Sigma-Aldrich) and pertussis toxin (PTX, Sigma-Aldrich) or PBS/CFA/PTX were suspended in DMEM, plated in 24-well plates, and incubated with 5 μg/ml ConA, 10 μg/ml MOG, or PBS for 36 h at 37°C in 5% CO2. Levels of cytokines: interferon γ, (IFN-γ), interleukin-2 (IL-2), IL-4, tumor necrosis factor α (TNF-α), IL-6, and IL-1β were measured in supernatants using commercially available ELISA kits (IFN-γ, R&D Systems, Minneapolis, MN, USA; all others, eBioscience, San Diego, CA, USA) according to manufacturer's instructions.
Flow cytometry
Single-cell suspensions of thymocytes and splenocytes in PBS containing 5% FBS were incubated with mouse antibodies: anti-CD19-APC, anti-CD3-FITC, anti-CD4-PE, anti-CD8-APC Cy7, anti-CD4-FITC, anti-CD8-PE, anti-CD44-PE Cy7, anti-CD25-APC, and/or anti-CD8-PE (BD Bioscience) for 20 min at room temperature. Cells were then washed twice with the same buffer, and relative fluorescence was measured using a BD LSR II flow cytometer (BD Bioscience).
Immunohistochemistry
Thymus sections were washed in PBS, blocked in 5% horse serum/PBS for 1 h at room temperature, and incubated with rabbit anti-caspase-3 (1:1000, Cell Signaling) overnight at 4°C in blocking solution. Sections were then washed and incubated for 1 h with biotinylated secondary antibody that was viewed by an ABC kit (Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine (DAB). For TdT-mediated dUTP-biotin nick end labeling (TUNEL) immunohistochemistry, thymus sections were washed for 10 min, then labeled and stained according to manufacturer's instructions using the FD Apop Kit (FD Neurotechnologies, Ellicott City, MD, USA).
Western blotting
Thymus homogenates and splenocyte lysates were subjected to SDS-PAGE on 10% glycine gels with Tris-glycine-SDS buffer (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to 0.45-μm nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA) in Tris-glycine buffer. After blocking with 5% milk in 1× TBS/0.01% Tween 20, blots were incubated overnight at 4°C with the following primary antibodies: mouse 6E10 (1:1000; Covance Research Products, Emeryville, CA, USA); rabbit anti-Bcl-2 (1:1000; Cell Signaling); rabbit anti-Bax (1:1000; Cell Signaling); rabbit anti-caspase-3 (1:1000; Cell Signaling); rabbit anti-Notch1 (1:1000; Epitomics, Burlingame, CA, USA); rabbit anti-NICD (1:500; Abcam, Cambridge, MA, USA); rabbit anti-phospho-PI3K (1:1000; Cell Signaling); rabbit anti-total-PI3K (1:1000, Cell Signaling); rabbit anti-phospho-AKT (1:1000, Cell Signaling); rabbit anti-total-AKT (1:1000; Cell Signaling); rabbit anti-phospho-ZAP-70 (1:1000; Cell Signaling); rabbit anti-total-ZAP-70 (1:1000, Cell Signaling); mouse anti-β-actin (1:4000, Sigma-Aldrich). After washing with ddH2O, blots were incubated for 1 h at room temperature with one of the following horseradish peroxidase-conjugated secondary anti-bodies: goat anti-mouse IgG (1:2000; Cell Signaling); anti-rabbit IgG (1:5000; Thermo Fisher Scientific). Blots were developed using Supersignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific).
Statistical analyses
Statistical differences between genotype groups were determined using 1-way analysis of variance (ANOVA) for multiple comparisons. Other statistical differences were determined using Student's t test. Analyses were performed on Microsoft Excel software (Microsoft, Redmond, WA, USA).
RESULTS
Human sAPP-α was detected in blood from both TgsAPP-α+/− and TgsAPP-α+/+ mice
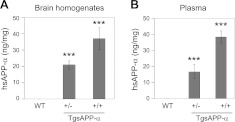
Studies have shown that a subset of patients with autism have elevated plasma levels of sAPP-α compared to typically developed children (4, 11, 20). Given that sAPP-α has neurotrophic properties, we sought to overexpress this target protein predominantly in the brain using a prion protein promoter. To initially characterize sAPP-α expression in TgsAPP-α mice, whole brain homogenates and plasma samples were collected from TgsAPP-α+/+ (n=3) and TgsAPP-α+/− (n=3) mice and WT littermates (n=4) at 6 wk of age and analyzed using a commercially available hsAPP-α ELISA kit (IBL-America). As expected, our data showed that hsAPP-α was detected, in a genotype-dependent manner, in both brain and plasma of TgsAPP-α mice and not WT littermates, with TgsAPP-α+/+ genotype expressing significantly higher levels of hsAPP-α compared to TgsAPP-α+/− genotype (Fig. 1). These findings confirm the successful generation of a mouse that may mimic the peripheral phenotype of patients with severe autism identified in the above-mentioned studies.
Figure 1.
Brain and plasma hsAPP-α levels in TgsAPP-α mice were measured by ELISA. Mouse brain homogenates and plasma were prepared from ten 6-wk-old TgsAPP-α mice and WT littermates [TgsAPP-α+/− (n=3), TgsAPP-α+/+ (n=3) and WT mice (n=4) are siblings from one family]. Human sAPP-α levels in homogenates (ng/mg protein; A) and plasma (ng/ml plasma; B) were measured by ELISA. Results are represented as means ± se. Similar results were also observed in other siblings from 4 families. ***P < 0.001.
Immune stimulation of splenocytes with either ConA or anti-CD3 antibody results in markedly increased levels of IFN-γ, IL-2, and IL-4 in TgsAPP-α mice
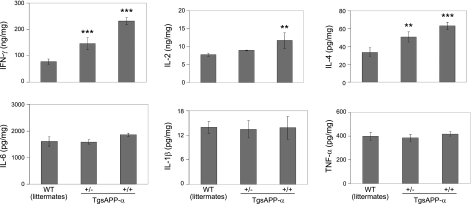
In addition to its neurotrophic properties, sAPP-α may play a role in immune cell activation. Stimulation of immune cells leads to the secretion of APP (8, 9). To see how elevated levels of sAPP-α could be affecting immune cell function, we measured cytokine levels produced from TgsAPP-α mouse and WT littermate splenocytes stimulated in vitro using ELISA. Splenocytes were challenged with ConA, a well-known T-cell mitogen (21). The secretion of 6 cytokines from challenged splenocytes was quantified using respective commercially available kits. We found that splenocytes from TgsAPP-α mice produced significantly higher levels of IFN-γ, IL-2, and IL-4 after challenge compared to WT littermates (Fig. 2, left panel), suggesting that hsAPP-α augments the T-cell immune response. Moreover, significant differences in IFN-γ and IL-4 levels between TgsAPP-α+/− and TgsAPP-α+/+ mice corroborate a genotype-dependent effect of hsAPP-α on the stimulation of T cells. The release of TNF-α, IL-1β, and IL-6 from mouse splenocytes after challenge was also measured; however, we did not see differences in the levels of each of these cytokines between TgsAPP-α mice and WT littermates (Fig. 2, right panel). In a parallel experiment, we challenged TgsAPP-α mouse splenocytes with anti-CD3 monoclonal antibody and found similar results to those above (data not shown).
Figure 2.
T-cell-derived cytokines are markedly increased in splenocytes cultured from TgsAPP-α mice after T-cell mitogen challenge. Splenocytes were isolated from TgsAPP-α and WT littermates at 6 wk of age, cultured at 5 × 106 in 24-well plates, and treated with ConA at 5 μg/ml for 18 h. Cell supernatants were collected and subjected to cytokine ELISA. Cell lysates were prepared and assayed for measurement of intracellular protein concentrations. Results are represented as means ± se (ng or pg/mg intracellular protein). Similar results were also observed in other littermates from 4 breeding pairs as well as with the anti-CD3 antibody stimulation (data not shown). *P < 0.05, **P < 0.01, ***P < 0.001.
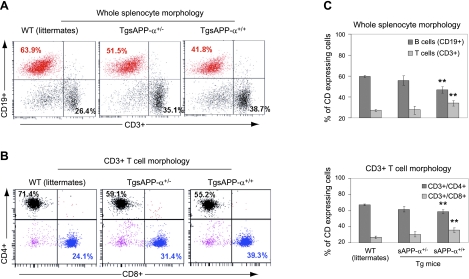
A significant increase of CD3+/CD8+ T cells in TgsAPP-α mouse splenocytes
In an attempt to understand the cause of the increased cytokine production in TgsAPP-α mice, we sought to establish the relative sizes of the immune cell populations represented in the spleen. To do this, we isolated splenocytes from TgsAPP-α mice and WT littermates and assessed B- and T-cell populations using flow cytometry. Antibodies against CD19 and CD3 were used to identify B and T cells, respectively. T cells were further characterized using antibodies against CD4 and CD8. Our results showed a marked decrease in the percentage of CD19+ cells from TgsAPP-α+/+ mouse splenocyte populations compared to those from WT littermates (Fig. 3A, C, top panel). In contrast, TgsAPP-α+/+ mouse splenocytes comprise a significantly increased percentage of CD3+ cells compared to splenocytes from WT littermates (Fig. 3A, C, top panel). Further classification of the CD3+ cell populations revealed opposing alterations in CD4+ and CD8+ cell populations from TgsAPP-α mouse splenocytes compared to WT littermate splenocytes. TgsAPP-α+/+ mice contain a significantly lower percentage of CD3+/CD4+ cells in the splenocyte population compared to WT littermates (Fig. 3B, C, bottom panel). Conversely, the percentage of CD3+/CD8+ cells is considerably greater in splenocytes from TgsAPP-α+/+ mice compared to those from WT littermates (Fig. 3B, C, bottom panel). These data demonstrate the presence of modifications to the immune cell populations in splenocytes from TgsAPP-α mice. They also imply that an increase in the population of CD3+/CD8+ cells may be inducing the increased cytokine production after splenocyte challenge (Fig. 2).
Figure 3.
Characterization of T and B cells in splenocytes isolated from TgsAPP-α mice and WT littermates. Splenocytes were prepared from TgsAPP-α mice and WT littermates and analyzed by flow cytometry. A) Percentage of CD3+ T and CD19+ B cells in whole splenocytes. B) Percentage of CD4+ and CD8+ T cells in total CD3+ T cells. C) Quantification of CD3+ T and CD19+ B cells in whole splenocytes (top panel) and CD4+ and CD8+ T cells in total CD3+ T cells (bottom panel). **P < 0.01.
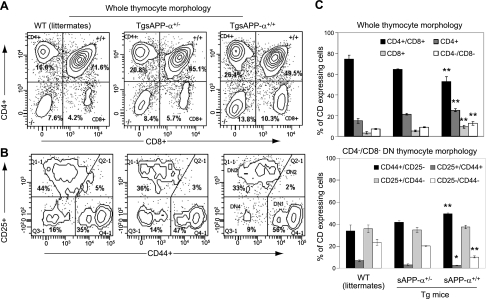
CD4+/CD8+ cells are significantly reduced in TgsAPP-α mouse thymocytes, whereas CD44+/CD25− cells are significantly increased in CD4−/CD8− TgsAPP-α mouse thymocytes
To explain our discovery that T-cell populations, in particular, are modified in TgsAPP-α mice, we examined T-cell populations in the thymus, the site of T-cell development (22, 23). For this investigation, thymocytes from TgsAPP-α mice and WT littermates were isolated and then analyzed with flow cytometry. Using a hierarchical detection strategy, the various populations were classified based on antibody colabeling according to the markers expressed by each type of cell at its respective stage of development in the thymus. First, mature and immature thymocyte populations were delineated using CD4 and CD8 antibodies. Subsequently, antibodies against CD44 and CD25 were used to characterize immature thymocytes at each of the 4 stages. Notably, our data revealed marked variations in mature and immature T-cell populations in the thymus of TgsAPP-α+/+ mice compared to WT littermates. Thymocytes from TgsAPP-α+/+ mice consisted of a significantly smaller population of CD4+/CD8+ T cells, which comprised a greater percentage of the individual CD4+ and CD8+ cell populations compared to WT littermate thymocytes (Fig. 4A, C, top panel). Justifiably, the CD4−/CD8− double-negative (DN) population in TgsAPP-α+/+ mouse thymocytes was considerably increased compared to the same population in WT littermate thymocytes (Fig. 4B, C, bottom panel).
Figure 4.
Characterization of thymocyte immune populations in TgsAPP-α mice. A, B) Thymocytes were prepared from TgsAPP-α mice and WT littermates and analyzed by flow cytometry analysis for CD4+/CD8+ double-positive thymocytes (A) and CD4−/CD8− double-negative (DN) thymocytes (B). C) Quantification of CD4+/CD8+ DP thymocytes in whole thymocytes (top panel) and CD44+/CD25−, CD44+/CD25+, CD44−/CD25+, and CD44−/CD25− thymocytes in CD4−/CD8− DN thymocytes (bottom panel). *P < 0.05, **P < 0.01.
Focus on this DN population of thymocytes in all 3 groups of mice demonstrated significant alteration in the percentage population of 3 of the 4 types of immature thymocytes. When compared to thymocytes from WT littermates, the percentages of the CD25+/CD44+ and the CD25−/CD44− populations of thymocytes are decreased in TgsAPP-α+/+ mice. In contrast, the percentage CD44+/CD25− population was greater in TgsAPP-α+/+ mouse thymocytes compared to WT littermate thymocytes. While differences were found in the percentages of the individual populations of whole thymocytes at the various stages of development, the overall pattern of representation was the same for all 3 groups (Fig. 4C, top panel). However, for the DN population specifically, TgsAPP-α mice exhibited a unique representation pattern in comparison to WT littermates, in which the CD44+/CD25− population appeared larger than the CD25+/CD44− population. In the WT littermate group, these two populations were similar in percentage population (Fig. 4C, bottom panel). Collectively, our observations suggest that T-cell development may be altered by the high levels of sAPP-α characteristically produced in TgsAPP-α mice.
Apoptotic signaling pathways are markedly reduced in TgsAPP-α mouse thymocytes
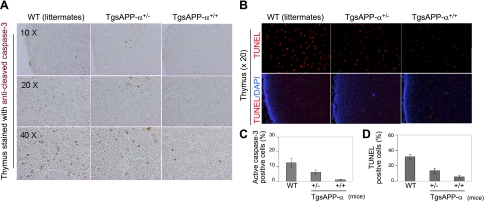
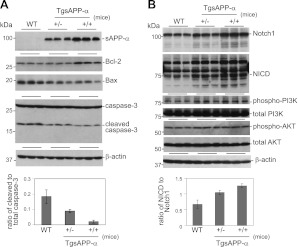
Apoptosis is a very important part of the T-cell development process in the thymus. Considering our finding that developing T-cell populations in the thymus are altered in TgsAPP-α mice, we investigated whether the expression of proteins involved in apoptotic signaling were affected in these mice. To do this, we stained thymus tissue from TgsAPP-α mice and WT littermates with an antibody against cleaved caspase-3 and identified cells undergoing apoptosis by TUNEL assay. Caspase-3, an effector protein in the apoptotic process, is widely used as a marker of apoptosis (24). We also used Western blot analysis to study the expression levels of several proteins that are inherent to the apoptotic signaling pathway. In immunohistochemical and biochemical analyses, TgsAPP-α+/+ mice displayed a marked reduction of cleaved caspase-3 expression in the thymus compared to both TgsAPP-α+/− mice and WT littermates (Figs. 5A, C and 6A), suggesting decreased apoptosis. This result was corroborated by the TUNEL assay, which detected fewer positively labeled thymus cells in TgsAPP-α+/+ mice compared to TgsAPP-α+/− mice and WT littermates (Fig. 5B, D). Western blot analyses of thymus tissue showed augmented expression of antiapoptotic protein Bcl-2 in TgsAPP-α+/+ mice, whereas expression of proapoptotic protein Bax in TgsAPP-α+/+ mice was reduced compared to TgsAPP-α +/− mice and WT littermates (Fig. 6A). Moreover, decreased levels of active caspase-3 were found in TgsAPP-α mice compared to WT littermates (Fig. 6A). Notch1, Notch intracellular domain (NICD), phosphorylated phosphoinositide 3-kinase (phospho-PI3K), and Akt were investigated by Western blot analyses as well. We discovered a genotype-dependent increase in Notch1 and NICD expression in TgsAPP-α mice and WT littermates (Fig. 6B). We also found increases in the expression of phospho-PI3K and phospho-Akt; however, these increases did not appear to be affected by genotype and the differences in expression of these proteins among the three groups of mice were not significant. Altogether, our findings indicate that TgsAPP-α+/+ mice exhibit a marked reduction of the occurrence of the apoptotic process.
Figure 5.
Reduced thymocytic apoptosis in TgsAPP-α mice. A, B) Thymus sections from TgsAPP-α +/+, TgsAPP-α +/−, and WT mice at 6 wk of age stained with anti-cleaved caspase-3 antibody (A; brown signal), TUNEL (B; top panel, red signal), and DAPI (B; bottom panel, blue signal). C) Percentages of anti-cleaved caspase-3 (active caspase-3) immunoreactive area from TgsAPP-α mouse and WT littermate thymocytic sections (n=3/group) were quantified by image analysis. D) Percentages of areas from TgsAPP-α mouse and WT littermate thymocytic sections (n=3/group) that stained positively with TUNEL were quantified using image analysis **P < 0.01, ***P < 0.001.
Figure 6.
Characterization of apoptotic molecular signals in thymocytes from TgsAPP-α mice and WT littermates. Thymus homogenates were prepared from 6-wk-old TgsAPP-α +/+ and TgsAPP-α +/− mice and WT littermates, and analyzed by Western blot. A) Apoptotic signaling pathways were examined by Western blot using antibodies against Bcl-2, Bax, caspase-3, and cleaved caspase-3. B) Notch1, activated Notch (NICD), and its related signaling pathways, including P13K and AKT, were also studied using Western blot. Densitometry analysis shows the band density ratios of cleaved caspase-3 to total caspase-3 and NICD to Notch1 (bottom panels). *P < 0.05, ***P < 0.001.
Cultured splenocytes from MOG-immunized TgsAPP-α mice exhibit impaired MOG recall immune responses
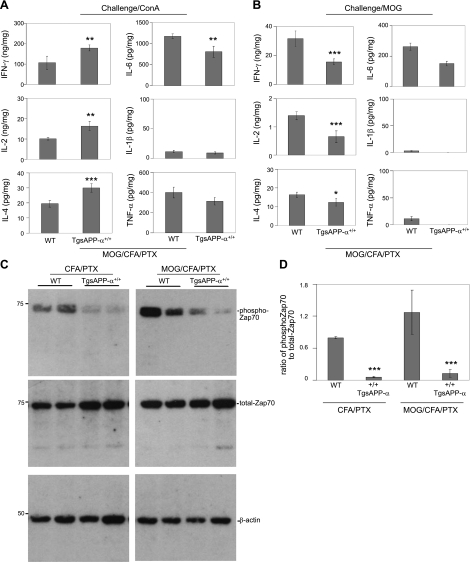
The mammalian adaptive immune response relies on the ability of T cells to remember antigens from initial challenges to the immune system. This unique “memory” capability in T cells allows them to mobilize the immune system for a faster and more potent response to previously encountered pathogenic attacks. As the means to test the effects of sAPP-α on immune cell function, we investigated the recall response in splenocytes isolated from MOG-immunized TgsAPP-α+/+ mice and WT littermates. To do this, isolated splenocytes were treated with ConA or MOG in vitro, and proinflammatory cytokine release was measured by ELISA. After treatment with ConA, splenocytes from MOG-immunized TgsAPP-α+/+ mice released significantly higher levels of IFN-γ, IL-2, and IL-4 compared to WT littermates (Fig. 7A). Curiously, ConA challenge produced decreased secretion of IL-6 in TgsAPP-α+/+ mice compared to WT littermates. However, as seen in the prior experiment (Fig. 2), no significant difference was found between transgenic and WT littermates in the release of IL-1β and TNF-α (Fig. 7A). Interestingly, splenocytes from MOG-immunized TgsAPP-α+/+ mice that were treated in vitro with MOG secreted significantly lower levels of IFN-γ, IL-2, and IL-4 compared to WT littermate splenocytes (Fig. 7B). Decreased secretion of IL-6 in TgsAPP-α+/+ mice after MOG treatment did not attain statistical significance, and levels of IL-1β and TNF-α were not significantly different from WT littermates (Fig. 7B). Therefore, we infer that the recall response in T cells from TgsAPP-α mice might be impaired.
Figure 7.
TgsAPP-α mouse-derived T cells have impaired recall immune responses. TgsAPP-α+/+ mice (n=4) and WT littermates (n=6) at 16 wk of age were immunized with MOG/CFA/PTX or PBS/CFA/PTX (control). At 14 d after the immunizations, splenocytes were isolated from these mice, cultured at 5 × 106 in 24-well-plates, and challenged with ConA (5 μg/ml) or MOG (1 μg/ml) for 36 h. Supernatants and cell lysates were collected and prepared from these splenocyte cultures. A, B) Cytokine expression levels in the supernatants were measured by ELISA, including T-cell-derived cytokines, IFN-γ, IL-2, and IL-4 in addition to monocyte-derived cytokines, IL-6, IL-1β, and TNF-α. Results are represented as means ± se (ng or pg/mg intracellular protein). C) Cell lysates were analyzed by Western blot for Syk family protein tyrosine kinase Zap-70 analysis. Densitometry shows ratio of phospho-Zap 70 to total Zap-70 as seen in panel D. *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, to comprehend why the T-cell memory response is abated, we examined expression of phosphorylated ζ-chain-associated protein kinase 70 (ZAP70) in splenocyte lysates of MOG-immunized TgsAPP-α mice and WT littermates by Western blot analysis. ZAP70 is expressed in T cells and is involved in the initiation of T-cell signaling (25). Our data show that lysates of splenocytes from TgsAPP-α+/+ mice expressed less phosphorylated ZAP70 compared to WT littermates, irrespective of immunization with adjuvant only (CFA/PTX) or with MOG and adjuvant (Fig. 7C). Densitometry showed that the ratio of phospho-ZAP70 to total ZAP70 is significantly reduced in TgsAPP-α+/+ mice compared to WT littermates (Fig. 7D), irrespective of initial immunization with adjuvant only or with MOG and adjuvant. These data imply that reduced ZAP70 expression and phosphorylation are characteristic of TgsAPP-α mice after immune challenge, and may be involved in the T-cell memory-response impairment seen in splenocytes from these mice.
DISCUSSION
Here, we report that in transgenic mice expressing elevated brain and plasma levels of hsAPP-α, there was increased production of IFN-γ, IL-2, and IL-4 after T-cell mitogen stimulation of splenocytes. We demonstrate that CD4+ T-cell populations were decreased, but CD8+ T-cell populations were increased in splenocytes of TgsAPP-α mice compared to WT littermates. Furthermore, we report variations in the double-positive (DP) and DN cell populations from the thymus of TgsAPP-α mice, consisting of fewer DP thymocytes and more DN1 thymocytes than WT littermates. In addition, cleaved caspase-3 and Bax were expressed at reduced levels in TgsAPP-α mouse thymocytes, while Bcl-2, Notch, and NICD were found at increased levels. Finally, the production of IFN-γ, IL-2, and IL-4 as part of the memory response of T-cells from splenocytes of TgsAPP-α mice was impaired. Taken together, these data suggest that sAPP-α reinforces initial T cell activation in the primary immune response but impairs the antigen-specific immunological memory, causing a weak secondary immune response. Also, our results demonstrate that sAPP-α modifies T cell development in the thymus by promoting antiapoptotic signaling. These findings have significant implications. First, they confirm the already-projected theory that sAPP-α is involved in the initiation of the T cell response. Secondly, these results propose a potential purpose for the elevated levels of sAPP-α seen in patients with autism. Additionally, our findings provide a possible explanation for the anomalies in cellular immunity and immune cell function found in patients with autism.
Sokol and colleagues first reported in 2006 that children with severe autism expressed elevated levels of sAPP-α in their plasma compared to typically developed, age-matched controls (4). A corroborative study of our own supported the above findings (11). The Sokol team later confirmed its own findings with a similar but stronger study with increased statistical power (20). These reports suggest a potential role for sAPP-α in autism pathophysiology. A mouse model mimicking the condition reported in these studies is necessary for identifying such a role. Therefore, we generated the TgsAPP-α mouse by standard pronuclear injection of a DNA-construct transcribing only the secreted portion of human APP which is produced after α-secretase cleavage. The current study confirms expression of hsAPP-α in both the brain and the plasma of TgsAPP-α mice (Fig. 1A, B). To the best of our knowledge, this is the first model of its kind to be created.
While the neurotrophic properties of sAPP-α have been well-documented in scientific literature, its immunological activity has been recognized and is still being explored. The APP fragment first showed signs of trophic activity in fibroblasts that require the presence of sAPP-α in order to proliferate and differentiate in vitro (26). Since then, the neurotrophic and neuroprotective activities of sAPP-α have been demonstrated by experiments proving its requirement for neurite extension (27–30) and neuron survival (31). Exogenous sAPP-α potentiates the neuritogenic activity of nerve growth factor in naive PC12 cells and cortical neurons (32, 33). The fragment also protected cultured rat hippocampal and septal neurons and human cortical neurons from hypoglycemic damage (7), and intracerebroventricular treatment of postischemic CA1 hippocampal neurons with sAPP-α increased their survival and rescued function (34). Most recently, PC-12 cells and slice cultures from Thy1-GFP mouse hippocampi were protected from epoxomycin-induced apoptosis and genotoxic stress, conditions of proteasomal stress, when treated with sAPP-α (35). It is believed that sAPP-α may contribute to brain overgrowth patterns seen in groups of patients with autism (36), presenting a possible role for the fragment in autism pathophysiology specific to the central nervous system.
Immunologically, the theory that sAPP-α plays a role in the stimulation of cellular immunity has been influenced by several studies. Among them are reports demonstrating the increased translation and expression of APP mRNA and protein, respectively, in activated immune cells (17, 37). Human peripheral blood leukocytes and splenocytes secrete sAPP-α on stimulation with T cell mitogens (9, 10) and CD4+ and CD8+ T-cells secrete comparable amounts of sAPP-α with stimulation (8). These reports provide evidence that sAPP-α is particularly involved in T cell activation; however, the nature of its contribution has yet to be elucidated. Given that the splenocytes from TgsAPP-α mice, which are already exposed to increased sAPP-α levels, display increased IFN-γ, IL-2, and IL-4 secretion (Fig. 2), the results of our study suggest that sAPP-α acts on T-cells by specifically potentiating the primary immunological response to challenge. Overall, sAPP-α may be a part of a positive-feedback mechanism during T cell activation in which, after stimulation, T-cells secrete sAPP-α leading to further activation of the T-cells.
It is important to note that the immunological features identified in patients with autism to date are heterogenous. Most studies concerning T-lymphocyte populations in these patients report decreased CD4+ cells in patients with autism (12, 13, 38) though studies report findings of the opposite in other patients (39, 40). In addition to decreased T cell population, reduced T cell activation recently has been identified in stimulated peripheral blood mononuclear cells isolated from patients with autism (41–43). Lymphocytes extracted from autistic children demonstrate reduced responsiveness when stimulated in vitro with phytohemagglutinin (44). A later study confirms the reduced lymphocyte responsiveness associated with autism and alludes to decreases in the number of lymphocytes present in these patients (45). Our results offer a prospective explanation for the cellular immunity features seen in the majority of the reports on patients with autism. The splenocytes from our transgenic mice consist of a decreased percentage of CD4+ T-cells and CD19+ B-cells than WT littermates, with a significant increase in CD8+ T cell percentage (Fig. 3C), suggesting that elevated levels of sAPP-α impact immune cell population. Experiments on patient plasma samples for elevated sAPP-α levels and T-lymphocyte population are necessary to confirm this association.
For T cell development, progenitor cells progressing along the four initial stages are DN for CD4 and CD8, (stages DN1–4). The thymocytes then become (CD4/CD8) DP, and finally mature into CD4 or CD8 single-positive (SP) T-cells. Along the maturation process, cells are eliminated by apoptosis during β, positive, and negative selection at the DN3, DP, and SP stages, respectively (25, 46–48). Our data show sAPP-α-associated variations in the percentage cell populations at each of the different stages and suggest a sAPP-α-dependent reduction in apoptotic signaling within thymocytes (Figs. 4–6). Based on our findings, the presence of the sAPP-α fragment at elevated levels does not appear to affect the percentage population of cells at the DN3 stage (Fig. 4C, bottom panel). The decreased populations of DN4 and DP thymocytes seen in TgsAPP-α mice, compared to WT littermates, appear to contradict the notion of reduced apoptosis at this stage of development in the thymus. However, increased percentage populations of CD4 and CD8 SP thymocytes substantiate a possible reduction in apoptosis at the DP and SP stages. The apparent inconsistency may be explained by the idea that apoptosis during the DN3 stage is less prominent than at the DP stage. Up to 97% of the cell populations at the DN3 stage do not survive positive selection and are eliminated (49, 50). Therefore, sAPP-α, most likely by reducing apoptotic signaling predominantly at the DP phase, modifies thymocyte development and affects the CD4+ and CD8+ T-cell populations in circulation. Another possible explanation is a global reduction in the population of cells undergoing apoptosis in the thymus of TgsAPP-α mice compared to WT littermates. This reduction may be supplied by the decrease in the percentage population of DP cells in TgsAPP-α mice, together with no significant change in the DN3 cell population of these mice compared to WT littermates. Fittingly, the thymus of the transgenic mice contain an increased percentage of the entire DN population as well as of the DN1 population in particular, which suggests that sAPP-α may be acting to hold the immature thymocytes in the first phase of development for an unusually lengthy period of time. A closer look at the mechanics of thymocyte development under the conditions of elevated sAPP-α expression would provide clarity. Nonetheless, it is obvious from our results that sAPP-α modifies thymocyte development, most likely by limiting apoptotic signaling directly or by way of the cells in which the signaling is occurring.
T cells are vital players in the secondary immune response of adaptive immunity because of their unique immunological memory feature. Considering that sAPP-α appears to strengthen the primary T-cell immune response (Fig. 2), note that, according to our results, TgsAPP-α mice demonstrate reduced T-cell memory function (Fig. 7A, B), suggesting that sAPP-α impairs the recall function in these lymphocytes. This is a novel manner in which sAPP-α may be affecting lymphocyte function. These data further emphasizes that sAPP-α is involved in T-cell function, but while its role appears to be robustly supportive for T cells activated by an initial immune challenge, the exact opposite seems true for T cells engaged in the secondary immune response. This contradictory phenomenon may be explained by a sAPP-α-associated decrease in phosphorylated ZAP70, a protein that is involved in the initiation of T-cell signaling (Fig. 7C, D). ZAP70 is a member of the Syk family of kinases that selectively interacts with the activated T-cell receptor CD3 complex on the surface of T cells (25, 51) to initiate further signaling downstream (52). In the thymus, ZAP70 deficiency causes the arrest of T-cell development at the DP phase (53, 54). The expression of ZAP70 appears unaffected by the DNA methylation patterns of the APP gene (55), and total ZAP70 expression is unchanged in TgsAPP-α mice compared to controls (Fig. 7C). It has been reported that sAPP-α acts by disrupting full-length APP dimers (56). Therefore, sAPP-α may be acting by hindering phosphorylation of ZAP70. Our findings of impairment in the T-cell memory function as a result of high levels of sAPP-α may explain why children with autism are more susceptible to infection (57, 58). Interestingly, reduced responses to specific antigens have been identified in patients with autism (44) and could be the result of impaired T-cell memory. Further research is required to corroborate the role of sAPP-α in the impairment of the T-cell memory response to comprehend how sAPP-α may be affecting the expression of T-cell-activating ZAP70 protein.
In summary, based on the data reported in this study, we propose that elevated peripheral levels of sAPP-α could be a cause of aberrations of T-cell population and function in patients with autism. Our data show that transgenic mice expressing elevated brain and plasma levels of hsAPP-α exhibit increased T-cell cytokine secretion along with decreased CD4+ and increased CD8+ T-cell populations in splenocytes compared to WT littermates. These transgenic mice also demonstrate unusual DP and DN thymocyte populations and protein expression profiles, suggesting reduced apoptotic signaling in the thymus. Third, the immunological memory response of T cells from splenocytes of transgenic mice is impaired. These findings are important because they substantiate the strengthening hypothesis that sAPP-α is involved in the initiation of the T-cell response, and they provide a theory for the possible association between two phenomena observed in patients with autism: elevated levels of sAPP-α and aberrant T-cell immunity. Future studies investigating this potential connection are imperative and will lead to the necessary development of individualized treatments for the unique subset of patients with autism for whom these studies are relevant.
Acknowledgments
This work was supported by the Silver Foundation and the U.S. National Institutes of Health/National Institute of Mental Health (R21MH087849, J.T.). J.T. holds the Sliver Chair in Developmental Neurobiology. T.M. holds the Rothman Chair for Neuropsychiatry. The authors particularly thank Steven Barger (University of Arkansas, Little Rock, AR, USA) for providing the sAPP-α DNA construct and Danielle T. Sutton for her helpful advice and discussion. The authors also thank Frank Fernandez for his support.
Footnotes
- Aβ
- amyloid β
- AICD
- APP-intracellular domain
- APP
- amyloid precursor protein
- CFA
- complete Freund's adjuvant
- ConA
- concanavalin A
- DN
- double negative
- DP
- double positive
- hsAPP-α
- human sAPP-α
- MOG
- myelin oligodendrocyte protein
- NICD
- Notch intracellular domain
- PHA
- phytohemagglutinin
- PI3K
- phosphoinositide 3-kinase
- PTX
- pertussis toxin
- sAPP-α
- soluble amyloid precursor protein α
- SP
- single positive
- TgsAPP-α
- transgenic sAPP-α-overexpressing
- Th1
- T-helper 1
- ZAP70
- ζ-chain-associated protein kinase 70
REFERENCES
- 1. APA (2000) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), American Psychiatric Publishing, Arlington, VA, USA [Google Scholar]
- 2. Goines P., Van de Water J. (2010) The immune system's role in the biology of autism. Curr. Opin. Neurol. 23, 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashwood P., Van de Water J. (2004) Is autism an autoimmune disease? Autoimmun. Rev. 3, 557–562 [DOI] [PubMed] [Google Scholar]
- 4. Sokol D. K., Chen D., Farlow M. R., Dunn D. W., Maloney B., Zimmer J. A., Lahiri D. K. (2006) High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J. Child Neurol. 21, 444–449 [DOI] [PubMed] [Google Scholar]
- 5. Turner P. R., O'Connor K., Tate W. P., Abraham W. C. (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 70, 1–32 [DOI] [PubMed] [Google Scholar]
- 6. Araki W., Kitaguchi N., Tokushima Y., Ishii K., Aratake H., Shimohama S., Nakamura S., Kimura J. (1991) Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochem. Biophys. Res. Commun. 181, 265–271 [DOI] [PubMed] [Google Scholar]
- 7. Mattson M. P., Cheng B., Culwell A. R., Esch F. S., Lieberburg I., Rydel R. E. (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10, 243–254 [DOI] [PubMed] [Google Scholar]
- 8. Monning U., Konig G., Banati R. B., Mechler H., Czech C., Gehrmann J., Schreiter-Gasser U., Masters C. L., Beyreuther K. (1992) Alzheimer beta A4-amyloid protein precursor in immunocompetent cells. J. Biol. Chem. 267, 23950–23956 [PubMed] [Google Scholar]
- 9. Monning U., Konig G., Prior R., Mechler H., Schreiter-Gasser U., Masters C. L., Beyreuther K. (1990) Synthesis and secretion of Alzheimer amyloid beta A4 precursor protein by stimulated human peripheral blood leucocytes. FEBS Lett. 277, 261–266 [DOI] [PubMed] [Google Scholar]
- 10. Suh Y. H., Choi W., Kim S. H., Kim J. S., Rhi B. Y., Chong Y. H., Woo J. I., Lee K. W. (1997) Expression of Alzheimer's amyloid precursor protein in human lymphocyte. Arch. Gerontol. Geriatr. 24, 1–7 [DOI] [PubMed] [Google Scholar]
- 11. Bailey A. R., Giunta B. N., Obregon D., Nikolic W. V., Tian J., Sanberg C. D., Sutton D. T., Tan J. (2008) Peripheral biomarkers in autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int. J. Clin. Exp. Med. 1, 338–344 [PMC free article] [PubMed] [Google Scholar]
- 12. Stern L., Francoeur M. J., Primeau M. N., Sommerville W., Fombonne E., Mazer B. D. (2005) Immune function in autistic children. Ann. Allergy Asthma Immunol. 95, 558–565 [DOI] [PubMed] [Google Scholar]
- 13. Yonk L. J., Warren R. P., Burger R. A., Cole P., Odell J. D., Warren W. L., White E., Singh V. K. (1990) CD4+ helper T cell depression in autism. Immunol. Lett. 25, 341–345 [DOI] [PubMed] [Google Scholar]
- 14. Warren R. P., Yonk L. J., Burger R. A., Cole P., Odell J. D., Warren W. L., White E., Singh V. K. (1990) Deficiency of suppressor-inducer (CD4+CD45RA+) T cells in autism. Immunol. Invest. 19, 245–251 [DOI] [PubMed] [Google Scholar]
- 15. Gupta S., Aggarwal S., Heads C. (1996) Dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. J. Autism Dev. Disord. 26, 439–452 [DOI] [PubMed] [Google Scholar]
- 16. Gupta S., Aggarwal S., Rashanravan B., Lee T. (1998) Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 85, 106–109 [DOI] [PubMed] [Google Scholar]
- 17. Fukuyama R., Murakawa Y., Rapoport S. I. (1994) Induction of gene expression of amyloid precursor protein (APP) in activated human lymphoblastoid cells and lymphocytes. Mol. Chem. Neuropathol. 23, 93–101 [DOI] [PubMed] [Google Scholar]
- 18. Borchelt D. R., Davis J., Fischer M., Lee M. K., Slunt H. H., Ratovitsky T., Regard J., Copeland N. G., Jenkins N. A., Sisodia S. S., Price D. L. (1996) A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet. Anal. 13, 159–163 [DOI] [PubMed] [Google Scholar]
- 19. Jankowsky J. L., Slunt H. H., Ratovitski T., Jenkins N. A., Copeland N. G., Borchelt D. R. (2001) Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol. Eng. 17, 157–165 [DOI] [PubMed] [Google Scholar]
- 20. Ray B., Long J. M., Sokol D. K., Lahiri D. K. (2011) Increased secreted amyloid precursor protein-alpha (sAPPalpha) in severe autism: proposal of a specific, anabolic pathway and putative biomarker. PLoS ONE 6, e20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dwyer J. M., Johnson C. (1981) The use of concanavalin A to study the immunoregulation of human T cells. Clin. Exp. Immunol. 46, 237–249 [PMC free article] [PubMed] [Google Scholar]
- 22. Hong R. (2001) The thymus. Finally getting some respect. Chest Surg. Clin. N. Am. 11, 295–310 [PubMed] [Google Scholar]
- 23. Nishino M., Ashiku S. K., Kocher O. N., Thurer R. L., Boiselle P. M., Hatabu H. (2006) The thymus: a comprehensive review. Radiographics 26, 335–348 [DOI] [PubMed] [Google Scholar]
- 24. Fernandes-Alnemri T., Litwack G., Alnemri E. S. (1994) CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 269, 30761–30764 [PubMed] [Google Scholar]
- 25. Wang H., Kadlecek T. A., Au-Yeung B. B., Goodfellow H. E., Hsu L. Y., Freedman T. S., Weiss A. (2010) ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb. Perspect. Biol. 2, a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saitoh T., Sundsmo M., Roch J. M., Kimura N., Cole G., Schubert D., Oltersdorf T., Schenk D. B. (1989) Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell 58, 615–622 [DOI] [PubMed] [Google Scholar]
- 27. Milward E. A., Papadopoulos R., Fuller S. J., Moir R. D., Small D., Beyreuther K., Masters C. L. (1992) The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron 9, 129–137 [DOI] [PubMed] [Google Scholar]
- 28. Masliah E., Mallory M., Ge N., Saitoh T. (1992) Amyloid precursor protein is localized in growing neurites of neonatal rat brain. Brain Res. 593, 323–328 [DOI] [PubMed] [Google Scholar]
- 29. Ninomiya H., Roch J. M., Sundsmo M. P., Otero D. A., Saitoh T. (1993) Amino acid sequence RERMS represents the active domain of amyloid beta/A4 protein precursor that promotes fibroblast growth. J. Cell Biol. 121, 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roch J. M., Jin L. W., Ninomiya H., Schubert D., Saitoh T. (1993) Biologically active domain of the secreted form of the amyloid beta/A4 protein precursor. Ann. N. Y. Acad. Sci. 695, 149–157 [DOI] [PubMed] [Google Scholar]
- 31. Roch J. M., Masliah E., Roch-Levecq A. C., Sundsmo M. P., Otero D. A., Veinbergs I., Saitoh T. (1994) Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proc. Natl. Acad. Sci. U. S. A. 91, 7450–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallace W. C., Akar C. A., Lyons W. E. (1997) Amyloid precursor protein potentiates the neurotrophic activity of NGF. Brain Res. Mol. Brain Res. 52, 201–212 [DOI] [PubMed] [Google Scholar]
- 33. Luo J. J., Wallace M. S., Hawver D. B., Kusiak J. W., Wallace W. C. (2001) Characterization of the neurotrophic interaction between nerve growth factor and secreted alpha-amyloid precursor protein. J. Neurosci. Res. 63, 410–420 [DOI] [PubMed] [Google Scholar]
- 34. Smith-Swintosky V. L., Pettigrew L. C., Craddock S. D., Culwell A. R., Rydel R. E., Mattson M. P. (1994) Secreted forms of beta-amyloid precursor protein protect against ischemic brain injury. J. Neurochem. 63, 781–784 [DOI] [PubMed] [Google Scholar]
- 35. Copanaki E., Chang S., Vlachos A., Tschape J. A., Muller U. C., Kogel D., Deller T. (2010) sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Mol. Cell. Neurosci. 44, 386–393 [DOI] [PubMed] [Google Scholar]
- 36. Sokol D. K., Maloney B., Long J. M., Ray B., Lahiri D. K. (2011) Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 76, 1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bullido M. J., Munoz-Fernandez M. A., Recuero M., Fresno M., Valdivieso F. (1996) Alzheimer's amyloid precursor protein is expressed on the surface of hematopoietic cells upon activation. Biochim. Biophys. Acta 1313, 54–62 [DOI] [PubMed] [Google Scholar]
- 38. Gupta S., Samra D., Agrawal S. (2010) Adaptive and innate immune responses in autism: rationale for therapeutic use of intravenous immunoglobulin. J. Clin. Immunol. 30, 90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plioplys A. V., Greaves A., Kazemi K., Silverman E. (1994) Lymphocyte function in autism and Rett syndrome. Neuropsychobiology 29, 12–16 [DOI] [PubMed] [Google Scholar]
- 40. Fiumara A., Sciotto A., Barone R., D'Asero G., Munda S., Parano E., Pavone L. (1999) Peripheral lymphocyte subsets and other immune aspects in Rett syndrome. Pediatr. Neurol. 21, 619–621 [DOI] [PubMed] [Google Scholar]
- 41. Denney D. R., Frei B. W., Gaffney G. R. (1996) Lymphocyte subsets and interleukin-2 receptors in autistic children. J. Autism Dev. Disord. 26, 87–97 [DOI] [PubMed] [Google Scholar]
- 42. Mostafa G. A., Al Shehab A., Fouad N. R. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J. Child Neurol. 25, 328–335 [DOI] [PubMed] [Google Scholar]
- 43. Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I. N., Van de Water J. (2011) Altered T cell responses in children with autism. Brain Behav. Immun. 25, 840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stubbs E. G., Crawford M. L. (1977) Depressed lymphocyte responsiveness in autistic children. J. Autism Child Schizophr. 7, 49–55 [DOI] [PubMed] [Google Scholar]
- 45. Warren R. P., Margaretten N. C., Pace N. C., Foster A. (1986) Immune abnormalities in patients with autism. J. Autism Dev. Disord. 16, 189–197 [DOI] [PubMed] [Google Scholar]
- 46. Zuniga-Pflucker J. C. (2004) T-cell development made simple. Nat. Rev. Immunol. 4, 67–72 [DOI] [PubMed] [Google Scholar]
- 47. Surh C. D., Sprent J. (1994) T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372, 100–103 [DOI] [PubMed] [Google Scholar]
- 48. Von Boehmer H. (2004) Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv. Immunol. 84, 201–238 [DOI] [PubMed] [Google Scholar]
- 49. Werlen G., Hausmann B., Naeher D., Palmer E. (2003) Signaling life and death in the thymus: timing is everything. Science 299, 1859–1863 [DOI] [PubMed] [Google Scholar]
- 50. Starr T. K., Jameson S. C., Hogquist K. A. (2003) Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 [DOI] [PubMed] [Google Scholar]
- 51. Hatada M. H., Lu X., Laird E. R., Green J., Morgenstern J. P., Lou M., Marr C. S., Phillips T. B., Ram M. K., Theriault K., Zoller M. J., Karas J. L. (1995) Molecular basis for interaction of the protein tyrosine kinase ZAP-70 with the T-cell receptor. Nature 377, 32–38 [DOI] [PubMed] [Google Scholar]
- 52. Williams B. L., Schreiber K. L., Zhang W., Wange R. L., Samelson L. E., Leibson P. J., Abraham R. T. (1998) Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18, 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Negishi I., Motoyama N., Nakayama K., Senju S., Hatakeyama S., Zhang Q., Chan A. C., Loh D. Y. (1995) Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature 376, 435–438 [DOI] [PubMed] [Google Scholar]
- 54. Kadlecek T. A., van Oers N. S., Lefrancois L., Olson S., Finlay D., Chu D. H., Connolly K., Killeen N., Weiss A. (1998) Differential requirements for ZAP-70 in TCR signaling and T cell development. J. Immunol. 161, 4688–4694 [PubMed] [Google Scholar]
- 55. Tong W. G., Wierda W. G., Lin E., Kuang S. Q., Bekele B. N., Estrov Z., Wei Y., Yang H., Keating M. J., Garcia-Manero G. (2010) Genome-wide DNA methylation profiling of chronic lymphocytic leukemia allows identification of epigenetically repressed molecular pathways with clinical impact. Epigenetics 5, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gralle M., Botelho M. G., Wouters F. S. (2009) Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J. Biol. Chem. 284, 15016–15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Niehus R., Lord C. (2006) Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. 27, S120–127 [DOI] [PubMed] [Google Scholar]
- 58. Atladottir H. O., Thorsen P., Schendel D. E., Ostergaard L., Lemcke S., Parner E. T. (2010) Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch. Pediatr. Adolesc. Med. 164, 470–477 [DOI] [PubMed] [Google Scholar]