Abstract
We previously reported that the combinatorial use of T20 and T1144, the first and next generations of HIV fusion inhibitors, containing different functional domains resulted in synergistic anti-HIV-1 effect, but this effect diminished when T20 and T1144 were covalently linked together. To elucidate the mechanism underlying this synergistic anti-HIV-1 effect, we studied the interactions between T20 and T1144 either in a mixture state or in a covalently linked state. T20 alone in solution was largely featureless, while T1144 alone was in α-helical trimeric conformation. When mixed in solution, T20 and T1144 showed a loose and transient interaction, with a moderate 10% α-helical content increase, but this interaction was greatly enhanced in the linked state, and T20 and T1144 showed ∼100% α-helical content. These results suggested that the loose and transient interaction between T20 and T1144 may destabilize the T1144 trimer, which makes its otherwise shielded binding sites more accessible to N-terminal heptad repeat (NHR) and increases its associating rate, thus increasing its anti-HIV-1 potency against the temporarily exposed target in NHR and causing the synergistic anti-HIV-1 effect. However, the strong interaction between T20 and T1144 in the covalently linked state may shield their NHR-binding sites, resulting in reduction of the synergistic effect.—Cai, L., Pan, C., Xu, L., Shui, Y., Liu, K., Jiang, S. Interactions between different generation HIV-1 fusion inhibitors and the putative mechanism underlying the synergistic anti-HIV-1 effect resulting from their combination.
Keywords: peptide, gp41, TLT, T20, T1144
Human immunodeficiency virus type 1 (HIV-1) enters and infects host cells through an envelope protein (Env)-mediated viral and host-cell membrane fusion (1, 2). HIV-1 Env is a complex of noncovalent associated gp120 and gp41 surface and transmembrane subunits, respectively (2). The fusion process is initiated by the binding of gp120 to the cellular surface receptor CD4 and a coreceptor, CCR5 or CXC4, triggering a cascade of large conformational changes of the gp120/gp41 complex, from a native state to a prehairpin intermediate (PHI) state and then to a hairpin fusion state. The fusion core formed at the fusion state contains a 6-helical bundle (6-HB), in which 3 gp41 N-terminal heptad repeats (NHRs) form a trimeric inner core, and 3 C-terminal heptad repeats (CHRs) pack in an antiparallel fashion against the inner NHR trimer (3). The energy released by 6-HB drives the apposition and subsequent fusion of viral and target cell membranes. Peptides derived from the NHR and CHR sequences can bind to their counterparts in gp41 to form an unproductively hetero 6-HB and prevent fusogenic 6-HB core formation, thus inhibiting HIV-1 host-cell membrane fusion and blocking viral infection (4–6).
T20 (generic name, enfuvirtide; brand name, Fuzeon) is the first-generation HIV-1 fusion inhibitor approved for salvage therapy for HIV-1-infected patients refractory to current antiretroviral drugs (7, 8). However, its application has been limited by high cost of peptide synthesis, rapid proteolysis, and poor efficacy against emerging T20-resistant strains. These drawbacks call for a new generation of fusion inhibitors with improved antiviral and pharmacokinetic profiles. In response, researchers at Trimeris (Durham, NC, USA) designed and developed the next-generation HIV-1 fusion inhibitors T1249 (6) and T1144 (9), respectively.
Although derived from HIV-1 gp41 CHRs, these different generations of peptide HIV-1 fusion inhibitors contain different functional domains and interact differently with gp41 NHR or lipid membrane (10). We have reported that the combinatorial use of different generations of peptide fusion inhibitors elicited strong synergistic anti-HIV-1 effects (11, 12). For instance, the combination of T20 and T1144 results in an increase in potency against HIV-1 infection by 5- to 20-fold (11). Preliminary studies showed that the different and complementary functional domains between T20 and T1144, as well as their different interaction models, may contribute to this synergistic anti-HIV-1 effect (11). Recently, we designed a chimera peptide, T20-T1144 linked (TLT), by covalently linking T20 and T1144 through a flexible amino acid linker (13). We found that TLT folds into a stable protein that is resistant to proteolysis and has high α-helix content. Although TLT showed high potency against HIV-1 gp41-mediated cell-cell fusion and HIV-1 infection, its activity was significantly lower than that of the T20/T1144 combination, indicating that the covalent linkage between the two peptide sequences in TLT somehow abolished the synergistic anti-HIV-1 effect otherwise observed from the combinational use of the isolated peptides T20 and T1144.
To get insight into the mechanism underlying the synergistic anti-HIV-1 effect resulting from the combinational use of T20 and T1144, here we used different biochemical and biophysical methods to study the interaction between T20 and T1144, in the T20-T1144 mixture (T20/T1144 mix) state or in the TLT state, as well as their interaction with gp41 NHRs. We found a loose and transient interaction between T20 and T1144 in the T20/T1144 mix state and a strong intramolecular interaction between T20 and T1144 in the TLT state, while T1144 is in a self-associated trimeric conformation. The self-association of T1144 trimer may shield its binding sites and result in slow associating rate with the NHRs, and restrain its antiviral activity against a temporarily exposed target. A loose and transient interaction between T20 and T1144 may result in a short-term associated T1144-T20 pair, which destabilizes the T1144 trimer, makes the binding site more accessible to the target and greatly increases its association rate with gp41 NHRs, and causes the synergistic anti-HIV-1 effect. But under the TLT state, the intramolecular interaction between T1144 and T20 may be too strong, resulting in the shielding of their NHR-binding sites and the reduction of their inhibitory potencies. Our observations provide clues for the design and development of new HIV fusion inhibitors as more effective anti-HIV therapeutics.
MATERIALS AND METHODS
Peptides
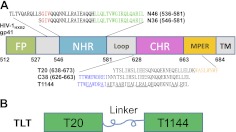
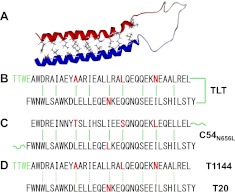
The sequences of the peptides used in this study and the different functional domains in HIV-1 gp41 are shown in Fig. 1. T20, T1144, N36, and N46 were synthesized by a standard solid-phase fluorenylmethyloxycarbonyl (FMOC) method using an Applied Biosystems model 433A peptide synthesizer(Applied Biosystems, Inc., Foster City, CA, USA). All peptides were acetylated at the N termini and amidated at the C termini. All peptides were >95% pure by high-performance liquid chromatography (HPLC) and identified by laser desorption mass spectrometry (PerSeptive Biosystems, Framingham, MA, USA). The peptide concentration was determined using Edelhoch's method, by measuring UV absorbance of the peptide using a ND 2000 microspectrometer (Thermo Scientific, Waltham, MA, USA) in 6 M GuHCl solution and calculating the molar-extinction coefficient ε (280 nm) of 5500 and 1490 M−1 cm−1 based on the number of tryptophan (Trp) residues and tyrosine (Tyr) residues, respectively (11).
Figure 1.
HIV-1 gp41 ectodomain and TLT design. A) HIV-1 gp41 ectodomain contains several functional domains, including fusion peptide (FP), N-terminal heptad repeat (NHR), C-terminal heptad repeat (CHR), and membrane proximal external region (MPER); the amino acid sequence number is based on HIV-1HXB2 gp160 sequence. C38 and T1144 occupy the CHR sequence, including pocket binding domain (PBD, in blue) and helix binding domain (HBD, in black); T20 includes HBD and lipid binding domain (LBD, in orange), which extends halfway into MPER. N36 occupies the NHR sequence, containing a deep pocket (sequence in green), which is considered a hotspot in NHR/CHR interaction, a helix region (in black), and a T20-resistant determinant GIV motif (in red). N46 includes the NHR sequence and an additional 10 amino acid residues upstream which are putative targets for LBD in the HIV-1-cell fusion processes. B) TLT was designed by covalently linking T20 and T1144 with a flexible peptide linker.
Recombinant protein expression and purification
The expression plasmid pTLTx (where x is the number of amino acid residues in the linker) was constructed as described previously (13). To express the GST-TLTx fusion proteins, the Escherichia coli Rosetta (DE3) plysS strain (Novagen, San Diego, CA, USA) was infected with plasmid pTLTx and scanned for overexpression stable colonies. The proteins were overexpressed in the bacteria, which were lysed with PBS (pH 7.2) using sonication. After centrifugation, the supernatants containing the GST-fused protein were collected. The GST-TLTx fusion proteins were then purified by Glutathione-Sepharose 4B affinity columns (GE Healthcare, Piscataway, NJ, USA) and treated by PreScission Protease (GE Healthcare) to release the chimeras from GST. TLTxs were further purified by fast protein liquid chromatography (FPLC; GE Healthcare Life Sciences) and analyzed by SDS-PAGE with Novex 10–20% tricine gel (Invitrogen, Carlsbad, CA, USA).
HIV-1 infection assay
The inhibitory activity of the HIV-1 fusion inhibitors, alone or in combination, on infection by HIV-1 Bal (subtype B, R5) in TZM-bl cells was determined as described previously (11, 13, 14). Briefly, 50 μl of an inhibitor alone or 2 inhibitors in combination was incubated with an equal volume of the HIV-1 Bal at an MOI of 0.01 at 37°C for 30 min, followed by addition of 100 μl of TZM-bl cells (5×105/ml). After incubation at 37°C overnight, the culture medium was replaced with fresh medium without inhibitors. On d 3 postinfection, the cells were harvested and lysed for analysis of luciferase activity, using a luciferase assay kit (Promega, Madison, WI, USA) and a luminometer (Ultra 386; Tecan, Durham, NC, USA) according to the manufacturer's instructions. The percentage inhibition of luciferase activity, the 50% inhibitory concentration (IC50) values, and the synergistic effect were calculated by using the CalcuSyn program (15), kindly provided by Dr. T. C. Chou (Memorial Sloan-Kettering Cancer Center, New York, NY, USA).
Circular dichroism (CD) measurement
CD measurements were performed as described previously (11). Briefly, individual peptides at 10 μM, or mixtures of 10 μM of each peptide in PBS (50 mM sodium phosphate and 150 mM NaCl, pH 7.2), were incubated at 37°C for 30 min. The CD spectrum was acquired on a Jasco spectropolarimeter (J-715; Jasco Inc., Easton, MD, USA) at 20°C using a 5-nm bandwidth, 0.5-nm resolution, 0.1-cm path length, and an average time of 5.0 s. Spectra were corrected by subtraction of a blank corresponding to the solvent composition of each sample. The α-helical content was calculated from the CD signal by dividing the mean residue ellipticity at 222 nm by the value expected for 100% helix formation (33,000° cm2 dmol−1), according to previous studies. Thermal denaturation was monitored at 222 nm by applying a thermal gradient of 2°C/min in the range of 20–98°C. The melting curve was smoothed, and the midpoint of the thermal unfolding transition (Tm) values was calculated using Jasco software utilities.
Native polyacrylamide gel electrophoresis (N-PAGE) analysis
N-PAGE was carried out to determine the 6-HB formation between the N and C peptides, as described previously (16). Briefly, TLTx (20 μM), C peptide (40 μM) alone, or mixtures with 40 μM (or indicated molecular ratio) of N36 were incubated at 37°C for 30 min. The peptide samples were mixed with the same volume of 2× Tris-glycine native sample buffer (Invitrogen), and the mixture was loaded onto 10- × 1.0-cm precast 18% Tris-glycine gels (Invitrogen) at 25 μl/well. Gel electrophoresis was carried out with 125 V of constant voltage at room temperature for 2 h. The gel was then stained with Coomassie blue and imaged with a FluorChem 8800 imaging system (Alpha Innotech Corp., San Leandro, CA, USA).
Computational modeling
The conformation of the coiled-coil chimera protein TLT was studied by a molecular dynamics simulation using Amber 8.0 (17) on a SGI Altix 350 workstation (Silicon Graphics, Inc., Sunnyvale, CA, USA). Molecular models were constructed using Swiss-Model web servers based on the structure of C54N656L (PDB: 3H01; ref. 18). The peptide was immersed in a rectangular box with TIP3P water before energy minimization. The first minimization was conducted for 500 steps of steepest descent followed by 500 steps of conjugate gradient minimization with restraints of 30 kcal mol−1 Å−2 on α-helix. Thereafter, the second energy minimization was conducted without any constraints to obtain an initial state for molecular dynamics. The system was warmed from 0 to 300 K and equilibrated with a Langevin temperature equilibration scheme for 20 ps. The bonds linked to hydrogen were constrained using the Shake algorithm, and a constant volume periodic boundary was used. A restraint of 10 kcal/mol was applied on α-helix, and the molecular dynamics calculation was carried out to rationalize the system to equilibrium for simulation. A further 5 nm simulation was conducted at constant temperature of 300 K and at a constant pressure of 1 atm, without restraints on the system. The coordinates of the entire system at each time point (2 ps) of the output trajectories were saved.
RESULTS
T20 and T1144 showed strong synergistic anti-HIV-1 effect when used in combination; synergy was lost when covalently linked in TLT
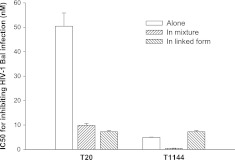
We previously demonstrated that combinational use of T20 and T1144 resulted in potent synergistic antiviral effect against HIV-1-mediated cell-cell fusion and HIV-1 infection (11). Here we assessed the inhibitory activity of T20 and T1144 when tested alone or in combination, and TLT on HIV-1 Bal infection. T20 and T1144 showed a 5-fold (IC50 50 vs. 10 nM) and 12-fold (IC50 5 vs. 0.4 nM) potency increase, respectively, when they were tested in combination, while TLT exhibited an IC50 of 7.3 nM, at a similar level as T1144 when it was tested alone (Fig. 2), further confirming that T20 and T1144 in combination exhibit synergistic anti-HIV-1 effect, while the covalent link of T20 and T1144 fragments in TLT causes the loss of their synergistic anti-HIV-1 effect.
Figure 2.
Inhibitory activities of T20 and T1144, tested alone or in combination, and TLT on HIV-1 Bal infection. Inhibitors were tested in triplicate; data are presented as means ± sd.
T20 and T1144 showed loose, transient interaction in solution, resulting in a complicated interaction network in T20-T1144-N46 mixtures
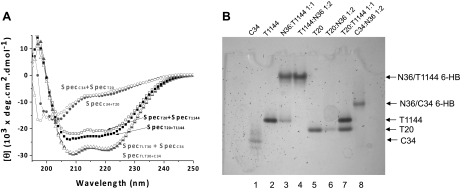
Peptide-peptide or protein-protein interactions can be studied using CD spectra by monitoring their secondary structure changes (19, 20). CD spectra of single peptides or their mixture at identical concentration and experimental condition were recorded. The sum of the CD signals of the single peptides (SpecA+SpecB) was used as a reference mixure of noninteracting peptides, which was compared with the spectrum of their mixture (SpecA+B); a difference between SpecA+SpecB and SpecA+B indicates the interaction between these peptides, which causes secondary structure change on interaction. As indicated by CD spectra, the interaction between T20 and T1144 resulted in the induction of 10% more α-helix structure when the two peptides were mixed in solution. The secondary structure change, though moderate, was very reproducible; controls using noninteracting peptide pairs, such as T20-C34 or TLT-C34, showed no such interaction, since the SpecA+SpecB was identical with SpecA+B for these peptide pairs (Fig. 3A).
Figure 3.
T20, T1144, and NHR interactions. A) T20 and T1144 show weak interaction in solution. CD spectrum of peptide mixtures (SpecA+B, solid symbols) and the sum of the spectra of the related isolated peptides (SpecA+SpecB, open symbols) are shown for comparison. T20-T1144 interaction induces more α-helix structure (SpecT20+T1144) than the sum of the single peptides (SpecT20+SpecT1144), which is distinguishable from noninteracting peptide pairs, such as T20-C34 or TLT-C34, in which the SpecA+B is identical to SpecA+SpecB. B) Interactions among T20, T1144, and NHR in N-PAGE. T1144 forms typical 6-HB with N36, similar to C34; T20 shows stable interaction with N36, causing the T20 band to disappear in the gel, while no 6-HB formed; no stable interaction between T20 and T1144, as shown by two intact bands of both peptides in the gel.
The interaction between T20 and T1144 was uncommon, since they were both derived from the HIV-gp41 CHR region and were assumed to bind to NHR to prevent fusogenic 6-HB formation rather than interact with each other (3). Therefore, to further characterize the nature of the induced α-helix, we tested two possible causations of CD signal change. First, if the α-helix structure was induced by a concentration-dependent self-association of samples with the same or similar properties, increasing the concentration of either T20 or T1144 separately would result in a similar α-helical enhancement. Second, if the interaction between the two peptides was stable, while the binding between them was weak, then only a small fraction of complex with high α-helical content would be formed at the concentration tested. In this case, we would expect higher α-helical content of T20/T1144 mix at higher concentrations. However, both possibilities were ruled out, because when we measured CD spectra using T20 and T1144 concentrations from 10 to 30 μM, separately or in mixture, no concentration-dependent α-helical content differences were observed (data not shown). Thus, we concluded that T20 and T1144 may have a loose, transient interaction in solution.
The interactions among T20, T1144, and N36 were further characterized by N-PAGE. In N-PAGE, 6-HB was characterized by a new band formed upward from C peptide; the N-peptide band did not show up because of its net positive charge properties, which may migrate into solution (11), as evidenced by the interaction between N36 and C34 (Fig. 3B). T1144 forms stable 6-HB with N36, as does C34, concomitant with the disappearance of the T1144 band, which is consistent with the literature (11). T20-NHR interaction was not straightforward and somewhat controversial (20, 21). It could not form stable 6-HB in the N-PAGE, as did T1144 or C34; however, the T20 band disappeared in the presence of N36 (Fig. 3B), indicating a stable interaction. T20/T1144 mix displayed 2 identical bands in N-PAGE, with the same intensity and at the same position as the separated peptides, indicating no stable interactions between T20 and T1144 in solution. Combining the CD and N-PAGE results suggested that T20 and T1144 had a loose and transient interaction when mixed; this interaction may change the nature of their interaction with NHRs and results in a complicated interacting network in the mixture of T20, T1144, and an NHR peptide.
T20 and T1144 have strong interaction in TLT when they are covalently linked and TLT folds into a stable protein with high α-helical content
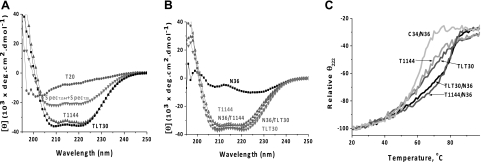
A peptide chimera TLT was designed and produced by linking T20 and T1144 with a flexible 30mer (GGGGS)6 amino acid linker. The linker greatly enhances the otherwise loose and transient interaction between T20 and T1144. CD spectra showed a high α-helical content of TLT (Fig. 4A) in that the calculated α-helix content in TLT based on the CD signal at 222 nm was 111% if we only accounted for amino acid residues in T20 and T1144 sequences in the calculation, assuming that the (GGGGS)6 linker was largely unstructured in TLT. This assumption was justified based on linker design and was also supported by the evidence. That is, when using different lengths of the (GGGGS)n linker (n=2–8), no linker length dependence of the α-helical content was observed for these TLTxs, based on the same calculation (data not shown). This indicated that the T20 and T1144 fragments were fully α-helical in TLT, in striking contrast to the unstructured character of T20 in solution. The calculated α-helical content of >100% suggested that one or two amino acid residues of the linker that directly connected with T20 and T1144 may adapt α-helical conformation.
Figure 4.
T20 and T1144 formed 100% α-helix structure in TLT and can form 6-HB with N36. For comparison, residues in the linker sequence were not included in [θ] calculation, considering its unstructured nature. A) T20 is unstructured in solution, contains 21% helical structure based on [θ]222; T1144 is 99.9% helical in solution; TLT is fully helical (111% helix), indicating that the unstructured T20 sequence adapted a fully helical structure in the TLT environment. B) TLT forms a fully α-helical complex with N36 (108% helix), as does T1144 (100.2% helix). C) Thermal stability of T1144, TLT, and their complex with N36. N36/C34 complex was used as a control.
Since TLT was highly active against HIV-1 gp41-mediated cell-cell fusion and virus infection, we tested its interaction with N36. CD showed that TLT interacting with N36 resulted in 100% α-helix in the complex (Fig. 4B, same calculation as above), suggesting the formation of a 6-HB-like structure. We also tested the thermal stabilities of the peptides and their 6-HB with N36 by monitoring the CD signal at 222 nm. As shown in Fig. 4C, TLT showed thermal denature behavior identical to that of T1144 in all the tests. Both showed Tm of 62°C by themselves and Tm of ∼81°C when bound with N36. As a control, C34/N36 6-HB had a Tm of 63°C. These results indicate that the 6-HBs formed between N36 and TLT or T1144 are more stable than that formed by N36 and C34.
TLT contains 2 functional domains and showed 1:2 binding stoichiometry with N36
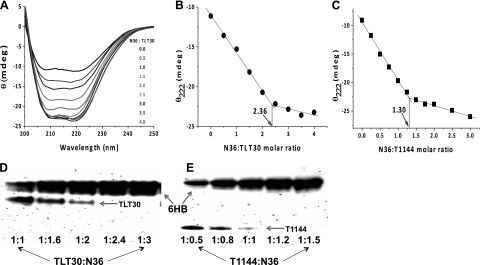
TLT contains T1144 and T20 fragments, both having the potential to interact with NHR peptides. Measuring the binding stoichiometry may provide new insight into structural information, leading to a better understanding of the inhibition mechanism. The binding stoichiometry between N36 and TLT was studied by CD using a constant concentration of TLT mixed with different molar ratio of N36 (Fig. 5). N36 is partially structured by itself and will form ∼100% α-helix structure on binding with TLT to form 6-HB. As expected, the CD signal increased linearly with increasing concentration of N36 at the beginning, and the signal changes corresponding to ∼100% α-helix structure formed for the added N36, indicating interaction between N36 and TLT. As N36 molecular ratio increased, a saturation of the CD signal was observed, indicating that no more 6-HB formed because all available TLT had bound with N36 and no more α-helix structure was induced for the added N36 (Fig. 5A). A binding isothermal curve was drawn between the CD signal at 222 nm and the N36:TLT molecular ratio (Fig. 5B). An intersection was observed in the binding isothermal curve at N36:T1144 ratio at 2.36, indicating that 1 TLT molecule can bind 2 N36 molecules to form 6-HB. The method was verified by using N36-T1144 binding as a control, and a 1.3 binding stoichiometry was observed (Fig. 5C), consistent with a 1:1 binding stoichiometry between them.
Figure 5.
TLT shows 1:2 binding stoichiometry with N36. A) CD spectra of solution containing constant concentration of TLT with increasing molecular ratio of N36. Saturation is observed as the molecular ratio of N36 increases. B) θ222 changes in solution containing 4 μM TLT with different N36 molecular ratio; an inflection point at TLT:N36 at 1:2.36 indicates that 1 TLT can bind 2 N36. C) Control using the same method shows a 1:1 binding stoichiometry between T1144 and N36. D) N-PAGE shows that 1 TLT can bind 2 N36 to form 6-HB. E) Control N-PAGE shows 1:1 binding stoichiometry between T1144 and N36.
Native-PAGE assay was also used to confirm the binding stoichiometry. N36 could form 6-HB with TLT, causing the TLT band in N-PAGE to disappear. As shown in Fig. 5D, the TLT band began to completely disappear at an N36 M ratio of 2.4, concomitant with a saturated 6-HB band thereafter, indicating that 1 TLT molecule can interact with 2 N36 peptides to form the 6-HB complex. N36 and T1144 control showed a 1:1 binding stoichiometry at the same condition (Fig. 5E). Based on the CD and N-PAGE results, we concluded that both T20 and T1144 fragments could interact with gp41 NHR to form 6-HB in the TLT environment. This indicated that the T20 fragment showed a interaction similar to that of T1144 to form stable 6-HB with N36, in striking contrast to that of isolated T20, which seems to play a disruptive role against the α-helix structure of NHR (20).
DISCUSSION
Synergy in drug combination is very useful for developing efficient therapy; it results in dosage reduction and increases the therapeutic window of drugs. Different drugs that interfere with the same biological process, but having different interaction models or binding sites, or having favorable interaction with each other, may cause potent synergy when used in combination. We previously discovered a synergistic effect when T20 and T1144, the first and new generation of HIV fusion inhibitors, respectively, were used in combination (11, 12). This synergy resulted in potent increase by hundreds of folds. Biochemical and biophysical approaches were used to elucidate the mechanism of the synergistic anti-HIV-1 effect.
T20 and T1144 have different functional domains and interact with NHRs in different ways to inhibit HIV-1 gp41-mediated virus-cell fusion. Specifically, T1144 forms typical 6-HB with N36 (11, 22); T20, on the other hand, cannot form 6-HB with N peptides and instead seems to disrupt the secondary structure of NHR peptides (20). CD and N-PAGE showed only weak and transient interaction between T20 and T1144 in solution, but the interaction was greatly enhanced when T20 and T1144 were covalently linked by a flexible amino acid linker. The interaction between T20 and T1144 was unexpected, since these fusion inhibitors are derived from gp41 CHR and have been assumed to interact with gp41 NHRs rather than with each other. The enhanced interaction between T20 and T1144 when covalently linked in TLT did not promote the inhibitory activity of the two peptide fragments; instead, the synergy between T20 and T1144 was largely lost in TLT, suggesting that the weak and transient interaction between T20 and T1144 is a key for understanding this strong synergy.
Therefore, based on a recently published X-ray crystal structure of a C54N656L dimer, including T20 sequence and C38 sequence from which T1144 was derived, the interaction between T20 and T1144 was studied by computational modeling (18). The computational modeling structure of TLT is shown in Fig. 6A; T20 and T1144 interact with each other in an antiparallel manner in TLT to form a stable coiled coil in which T20 and T1144 adapt fully α-helical structures. The residues involved in T20-T1144 interaction are shown in Fig. 6B, based on the modeling structure. Residues at a, e positions in T20 and T1144 contact each other and contribute to the interaction. Compared with crystal structure of the C54N656L dimer (Fig. 6C), most of the residues at a, e positions involved in T20-T1144 interaction were conserved between TLT and C54N656L, and 14 of 18 residues were identical. For the 4 discrepancies, as highlighted in Fig. 6B, C, 2 residues were more hydrophobic in TLT, and 2 were more hydrophobic in C54N656L dimer, suggesting a similar interaction pattern between T20 and T1144 in TLT and that in C54N656L dimer. Although T20 and T1144 may interact in mixture, such interaction may only be short term, resulting in a T20/T1144 transient interaction pair, as shown in Fig. 6D. The loose interaction between T20 and T1144 was consistent with that in C54N656L dimer, where obvious α-helical structure was observed only at low temperature (18).
Figure 6.
T20 and T1144 interaction. A) Computational modeling structure of TLT based on C54N656L dimer (PDB: 3H01). B) T20 and T1144 interaction in TLT; strong interactions are dominant, resulting in a very stable complex with high helical content. C) Interactions between C54N656L coiled-coil dimer. Different residues between TLT and C54N656L dimer involving the interactions are highlighted in red. D) T20 and T1144 interaction in combination; short-term interactions are dominant, resulting in transient T20-T1144 pairs.
T20 and T1144 contain partially complementary binding sites to interact with gp41 NHRs. However, these complementary binding sites in sequence do not appear to sufficiently explain the peptides' synergistic effect, because an expected synergistic enhancement was not observed when the two fragments were covalently linked in TLT with the enhanced interaction. Therefore, other factors must contribute to the synergy.
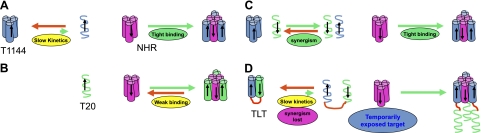
In HIV-1 fusion inhibitor design, there is a well-accepted notion that increasing the α-helical content and 6-HB thermal stability of C peptides will result in higher anti-HIV-1 potencies. Conforming to this idea, peptide engineering was subsequently applied to design and modify C-peptide sequences to increase their helicity and 6-HB stability, resulting in highly potent fusion inhibitors (22–27). Specifically, systematic approaches were applied by Trimeris that replaced the hydrophilic amino acid residues at the a, d position with hydrophobic residues (22). These replacements resulted in peptides with increased α-helical content and the formation of more stable 6-HB with NHR peptides. As a consequence, inhibitory activities also increased for some peptides, especially against drug-resistant HIV-1 strains (22). The increased stability of 6-HB may have resulted from more hydrophobic residues being buried, providing a stronger driving force for 6-HB formation, thus increasing the inhibitory potency of the C peptides. Further hydrophobic replacement, although resulting in fully α-helical peptides with very strong 6-HB stability, had an adverse effect on anti-HIV activities. C-peptide T267229, with completely hydrophobic replacement at the a, d position, was largely inactive, despite its fully α-helical structure and superthermal 6-HB stability with Tm > 100°C, even in 8 M urea (22). Ultracentrifuge analysis showed that these highly stable C-peptide fusion inhibitors formed trimers in solution. C-peptide fusion inhibitors bind NHRs through a, d residues, but the same hydrophobic residues are also used to form a hydrophobic core in the self-associating C-peptide trimers. Therefore, while hydrophobic replacements in a, d residues of C peptides increase the 6-HB stability of C-peptide-NHR complexes and favor the inhibitory potency of the C peptides, they also promote trimerized self-association of the C peptide, thus shielding the same a, d residues from contact with NHRs and reducing the binding kinetics of C-peptide fusion inhibitors, since a dissociation step is requested before efficient binding. In HIV-1-cell fusion processes, the gp41 NHR target is temporarily exposed, and fusion inhibitors have only a couple of minutes to access the target. Therefore, besides binding stability, associated kinetics is also a determinant of fusion inhibitor activity. For peptide inhibitors with relative weak binding affinities, the potency is largely determined by their target binding affinities; when binding affinities reach a certain degree, the association rate begins to play a role and, in fact, becomes the main determinant of the potency of a fusion inhibitor in certain circumstances (28, 29).
Moderate and short-term interactions without forming stable complexes between two peptide fusion inhibitors with different models of interaction may cause strong synergistic anti-HIV-1 effect against a temporarily exposed target, such as gp41 PHI. T1144, bearing two more hydrophobic residues at the a, d position than the wild sequence, can form very stable complexes with NHRs. T267221, an analog of T1144 with an I/L mutation, has biophysical and biochemical properties identical to T1144 (22). This class of fusion inhibitor forms extraordinarily stable 6-HB with NHR peptides, such that the secondary structure can be maintained even in 8 M urea (22). These inhibitors form stable self-associated α-helical trimers in solution, using the hydrophobic residues at a, d positions to form the hydrophobic core. The self-association blocks the binding sites, and this requires a dissociation step to expose the binding sites for efficient NHR binding. Therefore, T1144's inhibitory activity is determined by its rate of association with the NHR target (Fig. 7A), and making its binding site more accessible to the target and increasing its binding kinetics are keys for improving its potency. T20 adapts a largely random coil structure in solution, which favors the binding kinetics when interacting with NHRs. However, since the T20/NHR complex is less stable, a thermodynamic equilibrium between the associated and free T20 determines its inhibitory activity (Fig. 7B). Based on this evidence, when T20 and T1144 are used in combination, their moderate interaction may cause the formation of transient T20-T1144 pairs in a manner that reduces self-association of T1144 and makes its binding site more accessible to gp41 NHRs, thus greatly increasing its association rate. The T20-T1144 pair binds to NHRs and forms dead-end stable complexes using T1144 to terminate HIV-1 gp41-mediated virus-cell fusion processes (Fig. 7C). The resultant unleashing of the kinetic restraint of T1144-NHR binding causes a synergistic anti-HIV-1 effect. T20 may also interact with NHRs to form stable hetero 6-HB when 1 or 2 grooves of the NHR trimer are preoccupied by T1144 in T20 and T1144 combination, as those in TLT-N36 complex, although this notion needs further verification. The lipid binding property of T20 may also promote the T1144-T20 pair to associate with the lipid membrane where the fusion process occurs, thereby providing additional benefit to increase the inhibitory potency of the dual-drug combination. On the other hand, in cases where inter- or intramolecular interaction from C-peptide inhibitors is too strong, for example, either TLT or T1144, binding sites may be blocked, preventing these inhibitors from efficiently binding with their target and, in turn, reducing the association rate, while, at the same time, abolishing the otherwise potent synergy between T20 and T1144 (Fig. 7D).
Figure 7.
Putative mechanism of the synergy of the T20-T1144 combination in inhibiting HIV-1 gp41-mediated virus-cell fusion. Stable α-helical structures are shown as cylinders, and unstructured peptides as helix lines; arrows indicate peptide chain direction from N to C terminus. C-peptide fusion inhibitors interact with NHR to form hetero-6-HB to prevent fusogenic 6-HB formation and stop virus-cell fusion processes. Two features in C-peptide-NHR interaction include the temporary exposure of NHR for a couple of minutes and the use of a, d residues of C-peptide fusion inhibitors to interact with NHR and form an external layer of 6-HB where 3 C peptides are separated from each other. A) T1144 forms very stable 6-HB with NHR, which can be considered irreversible binding. However, since its trimer dominates in solution and thus requires dissociation into monomer to expose its a, d position binding sites for effective interaction with NHR, its inhibitory activity is restrained by its slow association rate with the NHR target. B) On the other hand, T20's loose association in solution enables its direct interaction with NHR; however, since the T20/NHR complex is not sufficiently stable and the interaction is reversible, its inhibitory activity is restrained by its weak binding affinity with NHR target. C) In the T20/T1144 combination, loose and transient T20-T1144 pairs are formed as a result of their weak interaction, and the loose T20-T1144 pair rapidly interacts with NHR to form dead-end T1144/NHR 6-HB, unleashing the kinetic restraint of T1144 and causing synergy. D) T20-T1144 interaction in TLT forms a stable structure and shields binding sites of both T20 and T1144; meanwhile, the kinetic benefit gained from the loose and transient interaction between T20 and T1144 is lost, thus abolishing the synergistic anti-HIV-1 effect observed in the T20/T1144 combination.
In summary, we report the characterization of a moderate and transient interaction between T20 and T1144, the first and new generation of peptide fusion inhibitors, respectively. The interaction may weaken the self-association of T1144, making its otherwise shielded binding sites more easily accessible to the temporarily exposed NHR target and greatly increasing its association rate. As a result, the unleashing of the kinetic restraint of T1144 by this transient interaction causes the synergy of the T20-T1144 combination. In contrast, either inter- or intramolecular interaction between peptide fusion inhibitors that is too strong may cause the reduction of inhibitory kinetics and, hence, weak potency. Our work may shed new light on drug design and therapeutic development against a temporarily exposed target, such as virus-cell fusion processes.
Acknowledgments
This research was supported by U.S. National Institutes of Health grant AI046221 to S.J., National Natural Science Foundation of China grants 81072581 to L.C. and 81173098 to S.J., and National Science and Technology Major Project of Original New Drug Research Key Technology grant 2009ZX09301-002 to K.L.
Footnotes
- 6-HB
- 6-helical bundle
- CD
- circular dichroism
- CHR
- C-terminal heptad repeat
- Env
- envelope
- FMOC
- fluorenylmethyloxycarbonyl
- HIV-1
- human immunodeficiency virus type 1
- NHR
- N-terminal heptad repeat
- PHI
- prehairpin intermediate
- TLT
- T20-T1144 linked
- T20/T1144 mix
- T20-T1144 mixture
REFERENCES
- 1. Cai L., Jiang S. (2010) Development of peptide and small-molecule HIV-1 fusion inhibitors that target gp41. ChemMedChem 5, 1813–1824 [DOI] [PubMed] [Google Scholar]
- 2. Eckert D. M., Kim P. S. (2001) Mechanisms of viral membrane fusion and its inhibition. Ann. Rev. Biochem. 70, 777–810 [DOI] [PubMed] [Google Scholar]
- 3. Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 [DOI] [PubMed] [Google Scholar]
- 4. Jiang S. B., Lin K., Strick N., Neurath A. R. (1993) HIV-1 inhibition by a peptide. Nature 365, 113–113 [DOI] [PubMed] [Google Scholar]
- 5. Wild C., Oas T., McDanal C., Bolognesi D., Matthews T. (1992) A synthetic peptide inhibitor of human-immunodeficiency-virus replication - correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. U. S. A. 89, 10537–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wild C., Dubay J. W., Greenwell T., Baird T., Oas T. G., McDanal C., Hunter E., Matthews T. (1994) Propensity for a leucine zipper-like domain of human-immunodeficiency-virus type-1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc. Natl. Acad. Sci. U. S. A. 91, 12676–12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kilby J. M., Hopkins S., Venetta T. M., DiMassimo B., Cloud G. A., Lee J. Y., Alldredge L., Hunter E., Lambert D., Bolognesi D., Mathews T., Johnson M. R., Nowak M. A., Shaw G. M., Saag M. S. (1998) Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4, 1302–1307 [DOI] [PubMed] [Google Scholar]
- 8. Lalezari J. P., Henry K., O'Hearn M., Montaner J. S. G., Piliero P. J., Trottier B., Walmsley S., Cohen C., Kuritzkes D. R., Eron J. J., Chung J., DeMasi R., Donatacci L., Drobnes C., Delehanty J., Salgo M., for the TORO 1 Study Group (2003) Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. New Engl. J. Med. 348, 2175–2185 [DOI] [PubMed] [Google Scholar]
- 9. Delmedico M., Bray B., Cammack N., Davison D., Dwyer J., Frick L., Tvermoes N., Wring S., Zhang H., Greenberg M. (2006) Next generation HIV peptide fusion inhibitor candidates achieve potent, durable suppression of virus replication in vitro and improved pharmacokinetic properties. 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, USA [Google Scholar]
- 10. Liu S. W., Jing W. G., Cheung B., Lu H., Sun J., Yan X. X., Niu J. K., Farmar J., Wu S. G., Jiang S. B. (2007) HIV gp41 C-terminal heptad repeat contains multifunctional domains - relation to mechanisms of action of anti-HIV peptides. J. Biol. Chem. 282, 9612–9620 [DOI] [PubMed] [Google Scholar]
- 11. Pan C. G., Cai L. F., Lu H., Qi Z., Jiang S. B. (2009) Combinations of the first and next generations of human immunodeficiency virus (HIV) fusion inhibitors exhibit a highly potent synergistic effect against enfuvirtide-sensitive and -resistant HIV type 1 strains. J. Virol. 83, 7862–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan C., Lu H., Qi Z., Jiang S. B. (2009) Synergistic efficacy of combination of enfuvirtide and sifuvirtide, the first- and next-generation HIV-fusion inhibitors. Aids 23, 639–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan C., Cai L., Lu H., Lu L., Jiang S. (2011) A novel chimeric protein-based HIV-1 fusion inhibitor targeting gp41 with high potency and stability. J. Biol. Chem. 286, 28425–28434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong M. X., Zhang J. A., Peng X. Q., Lu H., Yun L. H., Jiang S. B., Dai Q. Y. (2010) Tricyclononene carboxamide derivatives as novel anti-HIV-1 agents. Eur. J. Med. Chem. 45, 4096–4103 [DOI] [PubMed] [Google Scholar]
- 15. Chou T. (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681 [DOI] [PubMed] [Google Scholar]
- 16. Liu S. W., Zhao Q., Jiang S. B. (2003) Determination of the HIV-1 gp41 fusogenic core conformation modeled by synthetic peptides: applicable for identification of HIV-1 fusion inhibitors. Peptides 24, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 17. Case D. A., Darden T. A., Cheatham T. E., III, .Simmerling C. L., Wang J., Duke R. E., Luo R., Merz K. M., Wang B., Pearlman D. A., Crowley M., Brozell S., Tsui V., Gohlke H., Mongan J., Hornak V., Cui G., Beroza P., Schafmeister C., Caldwell J. W., Ross W. S., Kollman P. A. (2004) AMBER 8, University of California, San Francisco [Google Scholar]
- 18. Liu J., Deng Y. Q., Li Q. N., Dey A. K., Moore J. P., Lu M. (2010) Role of a putative gp41 dimerization domain in human immunodeficiency virus type 1 membrane fusion. J. Virol. 84, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai L. F., Balogh E., Gochin M. (2009) Stable extended human immunodeficiency virus type 1 gp41 coiled coil as an effective target in an assay for high-affinity fusion inhibitors. Antimicrob. Agents Chemother. 53, 2444–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawless M. K., Barney S., Guthrie K. I., Bucy T. B., Petteway S. R., Merutka G. (1996) HIV-1 membrane fusion mechanism: Structural studies of the interactions between biologically-active peptides from gp41. Biochemistry 35, 13697–13708 [DOI] [PubMed] [Google Scholar]
- 21. Wild C., Greenwell T., Shugars D., Rimskyclarke L., Matthews T. (1995) The inhibitory activity of an hiv type-1 peptide correlates with its ability to interact with a leucine-zipper structure. Aids Res. Hum. Retrov. 11, 323–325 [DOI] [PubMed] [Google Scholar]
- 22. Dwyer J. J., Wilson K. L., Davison D. K., Freel S. A., Seedorff J. E., Wring S. A., Tvermoes N. A., Matthews T. J., Greenberg M. L., Delmedico M. K. (2007) Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc. Natl. Acad. Sci. U. S. A. 104, 12772–12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naito T., Izumi K., Kodama E., Sakagami Y., Kajiwara K., Nishikawa H., Watanabe K., Sarafianos S. G., Oishi S., Fujii N., Matsuoka M. (2009) SC29EK, a peptide fusion inhibitor with enhanced alpha-helicity, inhibits replication of human immunodeficiency virus type 1 mutants resistant to enfuvirtide. Antimicrob. Agents Chemother. 53, 1013–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishikawa H., Nakamura S., Kodama E., Ito S., Kajiwara K., Izumi K., Sakagami Y., Oishi S., Ohkubo T., Kobayashi Y., Otaka A., Fujii N., Matsuoka M. (2009) Electrostatically constrained alpha-helical peptide inhibits replication of HIV-1 resistant to enfuvirtide. Int. J. Biochem. Cell Biol. 41, 891–899 [DOI] [PubMed] [Google Scholar]
- 25. Nishikawa H., Oishi S., Fujita M., Watanabe K., Tokiwa R., Ohno H., Kodama E., Izumi K., Kajiwara K., Naitoh T., Matsuoka M., Otaka A., Fujii N. (2008) Identification of minimal sequence for HIV-1 fusion inhibitors. Bioorg. Med. Chem. 16, 9184–9187 [DOI] [PubMed] [Google Scholar]
- 26. Oishi S., Ito S., Nishikawa H., Watanabe K., Tanaka M., Ohno H., Izumi K., Sakagami Y., Kodama E., Matsuoka M., Fujii N. (2008) Design of a novel HIV-1 fusion inhibitor that displays a minimal interface for binding affinity. J. Med. Chem. 51, 388–391 [DOI] [PubMed] [Google Scholar]
- 27. Otaka A., Nakamura M., Nameki D., Kodama E., Uchiyama S., Nakamura S., Nakano H., Tamamura H., Kobayashi Y., Matsuoka M., Fujii N. (2002) Remodeling of gp41–C34 peptide leads to highly effective inhibitors of the fusion of HIV-1 with target cells. Angew. Chem. Int. Ed. 41, 2938–2940 [DOI] [PubMed] [Google Scholar]
- 28. Steger H. K., Root M. J. (2006) Kinetic dependence to HIV-1 entry inhibition. J. Biol. Chem. 281, 25813–25821 [DOI] [PubMed] [Google Scholar]
- 29. Kahle K. M., Steger H. K., Root M. J. (2009) Asymmetric deactivation of HIV-1 gp41 following fusion inhibitor binding. PLoS Pathogens 5 [DOI] [PMC free article] [PubMed] [Google Scholar]