Abstract
A unilateral injection of botulinum toxin A (BTxA) in the calf induces paralysis and profound loss of ipsalateral trabecular bone within days. However, the cellular mechanism underlying acute muscle paralysis-induced bone loss (MPIBL) is poorly understood. We hypothesized that MPIBL arises via rapid and extensive osteoclastogenesis. We performed a series of in vivo experiments to explore this thesis. First, we observed elevated levels of the proosteoclastogenic cytokine receptor activator for nuclear factor-κB ligand (RANKL) within the proximal tibia metaphysis at 7 d after muscle paralysis (+113%, P<0.02). Accordingly, osteoclast numbers were increased 122% compared with the contralateral limb at 5 d after paralysis (P=0.04) and MPIBL was completely blocked by treatment with human recombinant osteoprotegerin (hrOPG). Further, conditional deletion of nuclear factor of activated T-cells c1 (NFATc1), the master regulator of osteoclastogenesis, completely inhibited trabecular bone loss (−2.2±11.9%, P<0.01). All experiments included negative control assessments of contralateral limbs and/or within-animal pre- and postintervention imaging. In summary, transient muscle paralysis induced acute RANKL-mediated osteoclastogenesis resulting in profound local bone resorption. Elucidation of the pathways that initiate osteoclastogenesis after paralysis may identify novel targets to inhibit bone loss and prevent fractures.—Aliprantis, A. O., Stolina, M., Kostenuik, P. J., Poliachik, S. L., Warner, S. E., Bain, S. D., Gross, T. S. Transient muscle paralysis degrades bone via rapid osteoclastogenesis.
Keywords: homeostasis, botulinum toxin A, NFATc1, RANKL
The ability of the skeletal system to remodel enables it to serve as a metabolic reservoir, a niche for hematopoietic cell development, and an adaptable structure for locomotion and visceral protection. Skeletal homeostasis is achieved via a balance of bone formation and resorption by osteoblasts and osteoclasts, respectively, the activities of which are modulated by both systemic and local stimuli. Following the completion of skeletal development and growth, it is clear that the skeleton demonstrates a heightened response to stimuli that signal for bone removal rather than those that signal for accretion (1–4). It is therefore not surprising that pharmacological interventions for bone loss pathologies, such as osteoporosis- and cancer-induced osteolysis, have focused on inhibiting bone resorption (5, 6).
Osteoclasts are the only cells in the body capable of bone resorption. These multinucleated giant cells are derived from myeloid precursors in the bone marrow microenvironment and differentiate in response to the receptor activator for nuclear factor-κB ligand (RANKL; refs. 7, 8). Osteoprotegerin (OPG) is a soluble decoy receptor for RANKL that acts to mitigate osteoclastogenesis. Thus, animals deficient in RANKL or OPG demonstrate a marked increase or decrease in bone mass, respectively (9–11). Moreover, treatment with excess recombinant OPG or antibodies against RANKL can block osteoclastogenesis and attenuate bone resorption in vivo (12). Activation of osteoclast precursors with RANKL through its receptor RANK initiates a series of intracellular signaling events culminating in the up-regulation and activation of the transcription factor nuclear factor of activated T-cells c1 (NFATc1), which promotes the expression of proosteoclastogenic genes and ultimately osteoclast formation (13, 14). In the genetic absence of NFATc1, osteoclasts cannot differentiate, leading to severe osteopetrosis (15–17). Interestingly, RANKL is needed for both the differentiation of osteoclasts from precursors and for activation of resorption by existing osteoclasts. A wide range of cells within the bone microenvironment are capable of producing RANKL, including, but not limited, to bone marrow stromal cells, osteoblasts, osteocytes, T lymphocytes, and endothelial cells (18–21).
Given that mechanical loading of the skeleton holds clear potential for anabolic augmentation of bone mass and morphology, there has been extensive consideration of how the skeleton interacts with muscle, the tissue responsible for inducing large magnitude bone deformation during locomotion in gravity environments (22). Epidemiological studies have clearly demonstrated a positive relation between muscle and bone mass (23, 24). In general, activities that increase muscle mass appear to also augment skeletal morphology, while conditions in which muscle mass declines (e.g., disease or aging) are associated with diminished bone mass (25, 26). The necessary role of muscle function in maintaining bone homeostasis is revealed when individuals suffer spinal cord injury (SCI), stroke, or partial paralysis. Here, loss of muscle and bone in affected portions of the musculoskeletal system are rapid and extensive (1, 27). For context, the rate of bone loss induced by SCI greatly exceeds that occurring during menopause. Such prolific bone loss elevates fracture risk and increases the potential for severe secondary complications, like kidney stones, providing additional challenges to the patient already coping with a life-altering clinical event (28).
Specific signaling pathways that link muscle function and bone homeostasis have been difficult to clarify, in part due to challenges associated with separating the influence of loss of muscle function from concomitant loss of bone loading due to diminished locomotion. We recently developed a model of transient muscle paralysis in the mouse, which we believe has potential to explore why normal muscle function is required to maintain bone homeostasis. In an initial study, we observed that bone loss following transient muscle paralysis was morphologically characterized by trabecular perforation and endocortical expansion, suggesting a process initially dominated by osteoclastic resorption (29). A subsequent time-course study revealed that the profound trabecular bone loss within the proximal tibia metaphysis following calf paralysis occurred much more rapidly (−51% by 5 d and −76% by 12 d) than initially anticipated (30). We therefore hypothesized that acute muscle paralysis-induced bone loss (MPIBL) arises via rapid and extensive osteoclastogenesis. To explore this hypothesis, we determined whether RANKL and osteoclast numbers were acutely upregulated following muscle paralysis and assessed whether pharmacologic or genetic inhibition of osteoclastogenesis mitigates MPIBL.
MATERIALS AND METHODS
The study was comprised of 4 in vivo experiments on separate groups of mice. With the exception of the NFATc1 study, as noted below, all mice were 16-wk-old female C57Bl6/J. Each experiment utilized a single injection of botulinum toxin A (BTxA; 2 U/100 g, 10 μl total volume; Allergan, Irvine, CA, USA) to induce transient muscle paralysis (with an equal volume saline injection for control mice). We assessed the acute alteration in RANKL and OPG expression following muscle paralysis, acute up-regulation of osteoclasts following muscle paralysis, mitigation of MPIBL via human recombinant (hr)OPG, and mitigation of MPIBL via postnatal deletion of NFATc1. The experiments were initiated with the injection of BTxA into the calf muscle group, with the exception of the hrOPG study, in which both the calf and quadriceps muscles were paralyzed. We have previously reported that bone loss in the proximal tibia metaphysis, the outcome morphology site for all of the experiments, is equivalent for either intervention (30). All experiments were approved by the University of Washington Institutional Animal Care and Use Committee.
Assessment of RANKL following muscle paralysis
RANKL and OPG protein levels were determined in the proximal tibial metaphysis at 1, 3, and 7 d following paralysis of the calf muscles (n=4/group) using previously established procedures (31). Briefly, proximal tibial metaphyses were isolated and flash frozen in liquid nitrogen. The samples were individually pulverized, and protein was extracted using a digestion buffer (50 mM Tris buffer, pH 7.4, containing 0.1 M NaCl and 0.1% Triton X-100). The concentration of RANKL and OPG in protein extracts was evaluated by using single-plex Luminex kits (Millipore, Billerica, MA, USA) and normalized to the total protein concentration, which was acquired by a standard BCA protein assay (Pierce, Rockford, IL, USA). While bone loss in the BTxA model appears focal to the limb undergoing paralysis (29), it is possible that some level of cellular signaling is also present in contralateral limbs. To minimize animal use, we confirmed that there were no differences between RANKL or OPG expression in treated vs. contralateral tibiae 1 d following muscle paralysis. Accordingly, these data were pooled as a baseline to assess differential expression at later time points.
Determination of osteoclast numbers following muscle paralysis
Female C57Bl6/J mice (16 wk, n=5) underwent transient paralysis of the right calf muscles and were euthanized 5 d later. The left (contralateral) and right (experimental) proximal tibial metaphyses were removed at death, fixed, decalcified, and paraffin embedded. Coronal sections through the metaphyseal region were obtained from each specimen and stained for tartrate-resistant acid phosphatase (TRAP); ref. 32). Images of stained sections were obtained and TRAP-positive osteoclast numbers/total area were determined via point counting (33).
Mitigation of MPIBL via hrOPG
Thirty-six female C57Bl6/J mice (16 wk) were randomized into 3 treatment groups (n=12 each): saline, BTxA, and BTxA + OPG. At d 0, all mice received either saline (saline group) or BTxA injections in both the quadriceps and calf of the right leg. Mice that received BTxA injections were then treated with equal volume saline (BTxA group) or recombinant human OPG (BTxA+OPG group) via s.c. injection (OPGFc; 5 mg/kg in 12.5 μl saline, 2/wk, beginning d 0 through d 18; Amgen). The BTxA+OPG mice also received injections of recombinant human OPG (OPGFc; 5 mg/kg in 12.5 μl saline s.c., 2/wk, beginning d 0 through d 18), while saline and BTxA mice received same-day saline injections. One mouse in the BTxA group inadvertently received an hrOPG injection at 14 d and was excluded from the analysis. Following euthanasia, bilateral quadriceps and calf wet weights were determined, and the left (contralateral) and right (experimental) proximal tibial metaphyses were imaged ex vivo via high-resolution microCT (0.8-mm-thick region spanning the proximal tibia metaphysis, 10.5-μm voxel nominal resolution; Viva CT40; Scanco, Wayne, PA, USA). Image analysis was performed as in previous studies by our group, using a fixed image threshold of 275 (30). The following trabecular parameters were characterized for each tibia (34): trabecular volume (mm3), bone volume (mm3), bone volume/tissue volume (BV/TV; %), trabecular number (n/mm), thickness (μm), and spacing (μm).
Mitigation of MPIBL via postnatal deletion of NFATc1 in osteoclasts
In this experiment, transient calf paralysis via a single injection of BTxA was superimposed on a mouse model of conditional temporal deletion of NFATc1 (15). Mice (n=9 Nfatc1fl/fl and n=10 Nfatc1fl/fl,Mx1-Cre) were allowed to age until 21 wk, at which time n = 4 Nfatc1fl/fl and n = 5 Nfatc1fl/fl,Mx1-Cre mice received polyinosinic:polycytidylic acid (poly I:C) injections (conditionally deleting NFATc1 in the Nfatc1fl/fl,Mx1-Cre mice to generate Nfatc1Δ/Δ mice). The remaining mice (n=5 Nfatc1fl/fl and n=5 Nfatc1fl/fl,Mx1-Cre) received equal volume saline injections. Four weeks later, at 25 wk of age, the right proximal tibias of all mice were imaged via high-resolution in vivo microCT, and the right calf muscles were injected with BTxA immediately following imaging (d 0). At 21 d after BTxA injection, all mice underwent repeat imaging of the proximal tibia metaphysis (i.e., each mouse was imaged at d 0 and 21). Left and right calf muscle wet weights were determined following euthanasia, and trabecular bone morphology analyses were performed as noted above.
Statistical analysis
Statistical analyses for experiments 1 and 3 utilized a 1-way ANOVA. When ANOVA indicated significance (P<0.05), a Bonferroni post hoc multiple comparison was used to identify individual treatment differences. For experiment 2, a paired t test was employed to determine the effect of muscle paralysis on osteoclast numbers (P<0.05). For experiment 4, a 1-way ANCOVA using d 0 microCT values as the covariate was performed (P<0.05). In addition, paired t tests were used to compare d 0 and 21 microCT changes within each group following BTxA-induced muscle paralysis.
RESULTS
Assessment of RANKL following muscle paralysis
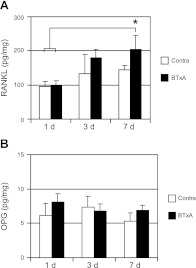
At 1 d following BTxA injection into the calf muscles, RANKL expression did not differ between contralateral and experimental tibiae, supporting the pooling of data (Fig. 1A). RANKL expression levels in the tibiae isolated from paralyzed limbs began to increase at 3 d and reached statistical significance by 7 d (113%, P<0.02). At both 3 and 7 d postparalysis, RANKL expression in contralateral tibiae trended toward increase vs. 1 d, but these differences were not statistically significant. In contrast, OPG expression was not altered within the experimental time course (Fig. 1B).
Figure 1.
Up-regulation of RANKL following muscle paralysis. Mean ± se expression of RANKL (A) and OPG (B) within the proximal tibia microenvironment adjacent to BTxA-induced calf paralysis and in the contralateral tibia. RANKL was significantly increased at 7 d postparalysis in the BTxA-injected side. OPG expression was unchanged. *P < 0.02 vs. 1 d combined data.
Determination of osteoclast numbers following muscle paralysis
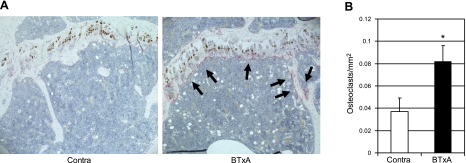
Qualitatively, sections from tibiae isolated from limbs injected with BTxA demonstrated increased staining for TRAP at the metaphysis (Fig. 2). At 5 d following muscle paralysis, experimental tibiae demonstrated a 122% increase in osteoclast number per square millimeter of bone (P=0.04) compared with tibiae isolated from the contralateral leg.
Figure 2.
Increased osteoclastogenesis following muscle paralysis. Representative photomicrographs of TRAP stains (A; red, noted by arrows) and mean ± se number of TRAP positive osteoclasts from the left (contralateral) and right (BTxA) proximal tibial metaphysis (B) of a mouse at 5 d after induction of calf muscle paralysis. *P = 0.04.
Mitigation of MPIBL via hrOPG
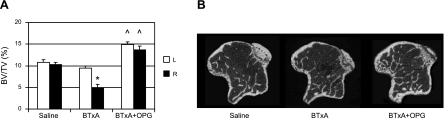
We then systemically blocked osteoclast differentiation and activation with OPG, an antagonist of RANKL, in the MPIBL model. Compared with the saline group, BTxA injection caused a significant decrease in quadriceps and calf muscle mass independent of OPG treatment (Table 1). Mice in the BTxA + OPG group lost 52.9 and 50.4% of muscle mass in their quadriceps and calf, respectively, which was comparable with 53.6 and 49.8% losses in these muscle groups in the BTxA-only group. As expected, trabecular BV/TV was equivalent between the left (contralateral to the injection) and right (adjacent to injection) tibiae in the saline group (Fig. 3A), as were all other trabecular morphological parameters (Table 1). In the contralateral tibiae, the BTxA group demonstrated slight, but not statistically significant, catabolic alterations in morphology compared with the saline group (Table 1 and Fig. 3A). The contralateral tibiae of the BTxA + OPG group demonstrated an increase in BV/TV (38.1%, P=0.01) and trabecular number (14.4%, P=0.01) and a decrease in trabecular spacing (−16.9%, P=0.01) compared with the contralateral tibiae of the saline group (Fig. 3A). In the experimental limbs, the BTxA group experienced a 51.4% decrease in metaphyseal BV/TV compared with the saline group (P<0.01; Table 1 and Fig. 3). In contrast, the BTxA + OPG group demonstrated a 33.3% increase in BV/TV (P=0.01). Trabecular thickness was significantly diminished (−21.2%, P<0.01) by BTxA, while trabecular number (−6.5%) and trabecular spacing were not significantly altered (8.5%). Finally, treatment with hrOPG preserved trabecular thickness (−0.7%), while increasing trabecular number (+15.5%) and decreasing trabecular spacing vs. saline mice (−16.3%; both P=0.01, Table 1).
Table 1.
Muscle wet mass and proximal metaphysis trabecular morphology in hrOPG
| Parameter | Saline | BTxA | BTxA + OPG |
|---|---|---|---|
| n | 12 | 11 | 12 |
| Calf (mg) | |||
| L | 149.3 ± 1.8 | 124.6 ± 4.4* | 122.2 ± 3.5* |
| R | 144.8 ± 2.0 | 73.1 ± 9.5* | 71.8 ± 8.8* |
| Quadriceps (mg) | |||
| L | 210.7 ± 2.1 | 171.5 ± 6.0* | 168.5 ± 4.7* |
| R | 206.5 ± 2.0 | 95.9 ± 10.8* | 97.2 ± 12.4* |
| Trabecular volume (mm3) | |||
| L | 1.807 ± 0.030 | 1.855 ± 0.021 | 1.778 ± 0.044 |
| R | 1.805 ± 0.038 | 1.947 ± 0.020 | 1.767 ± 0.055# |
| Bone volume (mm3) | |||
| L | 0.196 ± 0.013 | 0.175 ± 0.006 | 0.264 ± 0.013*,# |
| R | 0.188 ± 0.010 | 0.096 ± 0.014* | 0.242 ± 0.013*,# |
| BV/TV (%) | |||
| L | 10.8 ± 0.6 | 9.5 ± 0.4 | 14.9 ± 0.6*,# |
| R | 10.3 ± 0.5 | 5.0 ± 0.7* | 13.7 ± 0.8*,# |
| Trabecular number (n/mm) | |||
| L | 3.289 ± 0.133 | 3.296 ± 0.082 | 3.762 ± 0.072*,# |
| R | 3.157 ± 0.123 | 2.953 ± 0.086 | 3.646 ± 0.084*,# |
| Trabecular thickness (mm) | |||
| L | 0.063 ± 0.001 | 0.060 ± 0.001 | 0.065 ± 0.001# |
| R | 0.063 ± 0.001 | 0.050 ± 0.003* | 0.062 ± 0.002# |
| Trabecular spacing (mm) | |||
| L | 0.311 ± 0.012 | 0.301 ± 0.007 | 0.258 ± 0.007*,# |
| R | 0.320 ± 0.012 | 0.346 ± 0.011 | 0.268 ± 0.007*,# |
Values are means ± se. L, left; R, right.
P = 0.01 vs. saline;
P = 0.01 vs. BTxA.
Figure 3.
OPG treatment prevents MPIBL. A) Mean (±se) BV/TV in the left (contralateral) and right (BTxA) tibial metaphysis. BV/TV was reduced in BTxA-treated limbs compared with saline-treated mice, with no difference between the contralateral tibae. BV/TV was significantly increased in experimental right tibia and contralateral left tibiae of the BTxA + OPG group compared with the same tibiae of the saline group. *P = 0.01, [212]P = 0.01 vs. corresponding saline group. B) Representative microCT cross-sectional images through the right tibia metaphysis of saline-, BTxA-, and BTxA+OPG-treated mice.
Mitigation of MPIBL via postnatal deletion of NFATc1
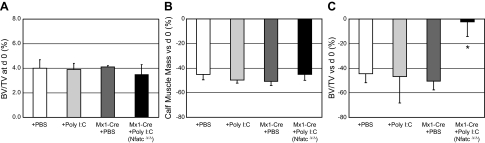
Postnatal conditional deletion of Nfatc1 abrogates osteoclastogenesis (15), facilitating the evaluation of the involvement of osteoclasts in a biological process in vivo without the need for pharmacologic manipulation. Initial microCT imaging at d 0 (4 wk after injection of saline or poly I:C) indicated that BV/TV in the proximal tibia metaphysis was not different for any of the 4 experimental groups (Nfatc1fl/fl with PBS or poly I:C and Nfatc1fl/fl,Mx1-Cre with PBS or poly I:C; Fig. 4A). Moreover, each experimental group demonstrated an equivalent reduction of calf muscle mass at 21 d following induction of BTxA-induced calf paralysis (range: −45.1 to −50.7% vs. contralateral limbs, all P<0.01; Fig. 4B).
Figure 4.
NFATc1 deficiency inhibits MPIBL. Tibial BV/TV at d 0 of BTxA treatment (A), percentage change in calf muscle mass at d 21 after BTxA treatment vs. d 0 (B), and percentage change in tibial BV/TV at d 21 after BTxA treatment vs. d 0 in right (BTxA) limbs (C). A) Nfatc1fl/fl or Nfatc1fl/fl,Mx1-Cre mice were injected with either PBS or poly I:C 4 wk before BTxA treatment to generate the 4 indicated groups. Mean ± se BV/TV at d 0 (4 wk following PBS or poly I:C injection) did not differ between 3 groups of control mice (+PBS, +poly I:C, Mx1-Cre+PBS) and the conditional deletion mice (Mx1-Cre+poly I:C). B) Likewise, calf muscle loss due to BTxA injection was equivalent across groups. C) While the control mice all lost the same proportion of trabecular BV/TV due to transient muscle paralysis, bone loss was ameliorated in the osteoclast deficient mice. *P < 0.01 vs. all other groups.
Nfatc1fl/fl mice demonstrated decreased BV/TV due to muscle paralysis regardless of whether they were treated with PBS (−44.5±7.2% vs. d 0, P<0.01) or with poly I:C 4 wk earlier (−46.7±21.7% vs. d 0, P=0.06). Furthermore, Nfatc1fl/fl,Mx1-Cre mice with PBS lost an equivalent amount of trabecular BV/TV as the Nfatc1fl/fl mice (−50.5±7.1% vs. d 0, P=0.002). In contrast, mice with conditional deletion of NFATc1 (i.e., Nfatc1fl/fl, Mx1-Cre mice that received poly I:C injection to generate Nfatc1Δ/Δ mice) demonstrated no change in trabecular BV/TV following transient paralysis of the calf muscles (−2.2±11.9%, P=0.30 vs. d 0, P<0.02 vs. other groups; Fig. 4C). The preservation BV/TV in mice with conditional deletion of NFATc1 following MPIBL was morphologically achieved by mitigating the trends toward decreased trabecular number and increased trabecular spacing observed in the other groups (Table 2).
Table 2.
Proximal metaphysis trabecular morphology, postnatal deletion of NFATc1
| Parameter | Nfatc1fl/fl + PBS | Nfatc1fl/fl + poly I:C | Nfatc1fl/fl,Mx1-Cre + PBS | Nfatc1fl/fl,Mx1-Cre + poly I:C |
|---|---|---|---|---|
| Trabecular number | −10.7 ± 3.8 | −12.4 ± 5.8 | −12.4 ± 2.1 | −4.1 ± 2.4 |
| Trabecular thickness | −6.6 ± 3.1 | −3.8 ± 9.6 | −14.0 ± 3.1 | 2.0 ± 5.7 |
| Trabecular spacing | 12.8 ± 5.3 | 16.9 ± 6.9 | 14.0 ± 3.2 | 4.1 ± 2.7 |
Values are mean ± se difference (%) vs. d 0.
DISCUSSION
We sought to identify the cellular mechanism underlying the profound loss of bone induced when an adjacent muscle group is paralyzed. The observation that transient muscle paralysis induced by BTxA leads to substantial bone loss has been observed by a number of groups, including ours, using a variety of animal models (29, 30, 35–39). Observed bone loss has varied in magnitude, extent, and time course with the BTxA dose, the muscle groups that are paralyzed, the site of analysis (trabecular vs. cortical, femur vs. tibia), and the resolution of technology used to asses bone quality (e.g., bone densitometry vs. microCT imaging). Interestingly, despite profound acute bone loss, this adaptive process is highly focal, with minimal systemic effects. The data in our study are consistent with these previous reports and, notably, serve to define how MPIBL is achieved.
In a series of complimentary studies, we demonstrated that acute muscle paralysis resulted in a rapid up-regulation of RANKL in the bone microenvironment, which was associated with an increase in osteoclast numbers. This increase in local osteoclastogenesis resulted in profound bone catabolism that was fully attenuated by pharmacologic treatment with the RANKL inhibitor OPG or genetic deficiency for NFATc1, the master transcriptional regulator of osteoclast differentiation. Thus, acute trabecular bone loss precipitated by BTxA-induced transient muscle paralysis is primarily achieved via RANKL-mediated NFATc1-dependent osteoclastogenesis. These data thereby provide a novel perspective to consider how muscle and bone cooperate to maintain skeletal homeostasis and highlight the essential coupling of muscle health with bone quality.
The data from these studies, in the context of our previous work, enable elucidation of how rapidly signaling for osteoclastogenesis occurs following muscle paralysis. We observed significantly elevated osteoclast numbers at 5 d and elevated RANKL by 7 d in tibiae adjacent to paralyzed calf muscles. We have previously noted significant trabecular bone loss in the proximal tibia metaphysis at 5 d following calf paralysis and have recently used a serial trabecular realignment imaging approach to detect initiation of trabecular resorption within 3 d of calf paralysis (30, 40, 41). Given the 3 to 5 d time course for activation of in vivo osteoclastogenesis (42), it follows that the initial signaling events underlying the observed catabolic response in our model were likely to occur within the first 24 h and or most certainly within the first 48 h following induced paralysis.
Although the observations reported here do not resolve how or why muscle function is essential to the maintenance of bone homeostasis, our findings, a consideration of the literature, and the neuromuscular mechanism of action of BTxA provide a framework to begin to explore this critical physiological relation. One clear possibility is that transient muscle paralysis may alter bone homeostasis via reductions in gait-induced mechanical loading, which, in a variety of conditions, has been associated with diminished bone morphology (43–45). Indeed, others have shown that both in vitro reduced gravity environments and unloading by hindlimb suspension trigger intracellular signaling in osteoblasts and osteoclast precursors leading to up-regulation of, and increased sensitivity to, proosteoclastogenic RANKL, respectively (46, 47). From a mechanical loading perspective, hindlimb suspension eliminates weight bearing and locomotion-induced bone deformation, but does not attenuate the ability of muscles to contract on their bony insertions (48). In contrast, transient muscle paralysis of the calf abolishes the muscle's contractile ability, with minimal effects on gait kinematics and small decreases in peak induced normal strain during free ambulation (<10% 12 d following calf paralysis; ref. 49). Intriguingly, despite these minimal alterations in the bone's loading environment, bone loss following BTxA-induced muscle paralysis is more rapid and significantly greater in extent than that observed following hindlimb suspension and even acutely exceeds that caused by sciatic neurectomy (30, 50, 51). At the least, it appears that the acute bone loss induced by muscle paralysis is not proportional with the relatively modest decline in skeletal loading induced by this intervention. We therefore speculate that the profound osteoclastogenesis following muscle paralysis is not primarily associated with the loss of mechanical stimuli derived from locomotion.
Given this observation, we have begun to consider alternative explanations for the profound bone loss induced by transient muscle paralysis. One possible explanation for our data is that the rapid activation of osteoclastic resorption following muscle paralysis arises due to a direct effect of BTxA on marrow cell populations. However, we believe 2 sets of data substantially diminish this possibility. First, if BTxA were to escape from the muscle (despite its very rapid uptake; ref. 52) and directly up-regulate RANKL or NFATc1 within the marrow, then we would anticipate systemic bone loss, rather than the highly localized bone loss observed in the model (30, 39, 40). Second, as a control experiment in a preliminary study in which we demonstrated that transient muscle paralysis inhibits periosteal callus formation following a uni-cortical tibia defect (53), we included an experimental group in which BTxA was injected through the defect directly into the marrow. Using the same high-resolution imaging as was used in this study, we did not observe elevated endocortical resorption or a significant change in healing of the defect compared with mice without BTxA. However, definitive experiments in this regard will require implementation of primary osteoclast culture studies.
Alternatively, in addition to inhibiting neuronal function when injected into a muscle, BTxA also impairs muscle proprioception and nociception (54–56). To our knowledge, these pathways have not been investigated in the context of bone homeostasis. However, trabecular bone is rich in nociceptive sensory neurons and sympathetic innervation, and nerve endings have been shown to be in direct contact with bone cells (57–59). Moreover, sensory and sympathetic innervation of bone share common axonal pathways with proprioceptive signals arising from skeletal muscles (60). Further, it is clear that neuropeptides (in particular CGRP and substance P) have the potential to modulate osteoclastogenesis (61–65). Finally, in a broader perspective, the sympathetic nervous system has been shown to modulate both formation and resorption of bone (66, 67). In combination, these studies suggest a potential role for sensory feedback between muscle and bone that functions to enable homeostasis under normal conditions but, when impaired pathologically (i.e., SCI and stroke) or experimentally (i.e., BTxA), rapidly removes signaling required to suppress bone catabolism in the adult skeleton.
Several general limitations with our experiments should be noted. The hrOPG intervention may have influenced osteoblast function as was demonstrated by increased trabecular BV/TV in contralateral tibiae of BTxA + OPG mice vs. saline control mice, an effect that has previously been reported (68, 69). However, when this limitation is considered in the context of the absence of bone loss following NFATc1 deletion at the time of muscle paralysis, the effect on our primary conclusions appears minimal. With regard to the NFATc1-deletion experiment, our conditional-knockout strategy used Mx1-Cre to delete NFATc1 in adult mice (15). Mx1-Cre is expressed broadly but deletes best in hematopoietic-derived cells, including osteoclasts. An alternative approach would have been to use an osteoclast specific Cre line, such as cathepsin K-Cre (70). However, this strategy results in severe baseline osteopetrosis with lethality around the time of weaning (data not shown), making it inappropriate for use in these studies. By deleting NFATc1 postnatally in adult mice (Nfatc1Δ/Δ), we generated a mouse model deficient in the ability to form new osteoclasts, but with a normal baseline skeleton. Although we cannot exclude that Nfatc1Δ/Δ mice fail to lose bone in the MPIBL model because of loss of NFATc1 in muscle or nonosteoclast bone cells, 2 observations make this unlikely. First, Mx1-Cre does not delete well in skeletal muscle (71). Second, there is not a baseline bone phenotype in mice where NFATc1 is conditionally deleted in osteoblasts (15).
Given the emerging understanding of how different tissues and organ systems cooperatively interact to regulate homeostasis, we believe our data also have significant relevance outside the bone biology field. For example, the observed osteoclastogenesis after muscle paralysis may be indicative of a relationship between muscle and bone established during early development. As such, studies of neuromuscular development could define the embryonic origin of the integration of muscle and bone and genetic manipulation could be used to explore specific neurological pathways that underlie this relationship (72). With regard to tissue engineering, our data suggest that optimal integration of synthetic bone with host bone will ultimately require osteogenic and neurogenic stem cells derived from adjacent musculature (73). Finally, BTxA is widely used both for cosmetic and off-label musculoskeletal treatments, some of which are adjacent to portions of the skeleton (such as the spine, joints, and tendons) that are predisposed for high risk of fracture in older adults (74). Although there are likely differences between our observations in a preclinical mouse model and use of BTxA in humans, the long-term skeletal health consequences of repeated Botox use have not been assessed. Our study strongly suggests vigilance is needed to identify untoward effects of BTxA on the skeletal system before clinically significant alterations in bone mass occur.
In summary, our complimentary pharmacological and genetic approaches substantiated our hypothesis that that transient loss of neuromuscular function of the calf muscle group rapidly leads to profound differentiation and activation of osteoclasts, the only cell capable of bone resorption, inside the adjacent tibia marrow space. This biological event was achieved via a RANKL-mediated process acting through an NFATc1 signaling pathway. A primary focus in the field of bone biology is to clarify how muscle and bone interact at a cellular level to assure the health and viability of both tissues. These data demonstrate a novel, but previously opaque, role for neuromuscular signaling as a critical mediator of bone homeostasis. Elucidation of the signaling pathways responsible for initiating the observed osteoclastogenesis may identify novel targets for inhibiting bone loss in conditions as diverse as spinal cord injury and age-related sarcopenia.
Acknowledgments
This work was supported, in part, by a Burroughs Wellcome Fund Career Award for Medical Scientists (to A.O.A.), U.S. National Institute of Arthritis and Metabolic Diseases grants K08-AR-54859 (to A.O.A.), AR-45665 (to T.S.G.), and AR-60304 (to T.S.G.); a grant from Amgen (to T.S.G.); and the Sigvard T. Hansen, Jr. Endowed Chair (T.S.G.). A.O.A., S.L.P., S.E.W., and S.D.B. report no conflicts of interest. T.S.G. received funding from Amgen that, in part, supported this work. M.S. and P.J.K. are employees of Amgen and have equity ownership in Amgen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Metabolic Diseases or the U.S. National Institutes of Health.
Footnotes
- BTxA
- botulinum toxin A
- BV/TV
- bone volume/tissue volume
- hrOPG
- human recombinant osteoprotegerin
- MPIBL
- muscle paralysis-induced bone loss
- NFATc1
- nuclear factor of activated T-cells c1
- OPG
- osteoprotegerin
- poly I:C
- polyinosinic:polycytidylic acid
- RANKL
- receptor activator for nuclear factor-κB ligand
- SCI
- spinal cord injury
- TRAP
- tartrate-resistant acid phosphatase.
REFERENCES
- 1. Garland D. E., Adkins R. H., Stewart C. A. (2008) Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J. Spinal Cord Med. 31, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cooper C. (2010) Osteoporosis: disease severity and consequent fracture management. Osteoporos. Int. 21(Suppl. 2), S425–S429 [DOI] [PubMed] [Google Scholar]
- 3. Gracia-Marco L., Vicente-Rodriguez G., Casajus J. A., Molnar D., Castillo M. J., Moreno L. A. (2011) Effect of fitness and physical activity on bone mass in adolescents: the HELENA study. Eur. J. Appl. Physiol. 111, 2671–2680 [DOI] [PubMed] [Google Scholar]
- 4. Rantalainen T., Nikander R., Daly R. M., Heinonen A., Sievanen H. Exercise loading and cortical bone distribution at the tibial shaft. Bone 48, 786–791 [DOI] [PubMed] [Google Scholar]
- 5. Sambrook P. N., Kotowicz M., Nash P., Styles C. B., Naganathan V., Henderson-Briffa K. N., Eisman J. A., Nicholson G. C. (2003) Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J. Bone Miner. Res. 18, 919–924 [DOI] [PubMed] [Google Scholar]
- 6. Geusens P. (2009) Emerging treatments for postmenopausal osteoporosis - focus on denosumab. Clin. Interv. Aging 4, 241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teitelbaum S. L., Ross F. P. (2003) Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 [DOI] [PubMed] [Google Scholar]
- 8. Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 9. Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 10. Bucay N., Sarosi I., Dunstan C. R., Morony S., Tarpley J., Capparelli C., Scully S., Tan H. L., Xu W., Lacey D. L., Boyle W. J., Simonet W. S. (1998) osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 12, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka S., Nakamura K., Takahasi N., Suda T. (2005) Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol. Rev. 208, 30–49 [DOI] [PubMed] [Google Scholar]
- 12. Min H., Morony S., Sarosi I., Dunstan C. R., Capparelli C., Scully S., Van G., Kaufman S., Kostenuik P. J., Lacey D. L., Boyle W. J., Simonet W. S. (2000) Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 192, 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Negishi-Koga T., Takayanagi H. (2009) Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231, 241–256 [DOI] [PubMed] [Google Scholar]
- 14. Sitara D., Aliprantis A. O. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol. Rev. 233, 286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aliprantis A. O., Ueki Y., Sulyanto R., Park A., Sigrist K. S., Sharma S. M., Ostrowski M. C., Olsen B. R., Glimcher L. H. (2008) NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Invest. 118, 3775–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asagiri M., Sato K., Usami T., Ochi S., Nishina H., Yoshida H., Morita I., Wagner E. F., Mak T. W., Serfling E., Takayanagi H. (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winslow M. M., Pan M., Starbuck M., Gallo E. M., Deng L., Karsenty G., Crabtree G. R. (2006) Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev. Cell 10, 771–782 [DOI] [PubMed] [Google Scholar]
- 18. Udagawa N., Takahashi N., Jimi E., Matsuzaki K., Tsurukai T., Itoh K., Nakagawa N., Yasuda H., Goto M., Tsuda E., Higashio K., Gillespie M. T., Martin T. J., Suda T. (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25, 517–523 [DOI] [PubMed] [Google Scholar]
- 19. Shiotani A., Shibasaki Y., Sasaki T. (2001) Localization of receptor activator of NFkappaB ligand, RANKL, in periodontal tissues during experimental movement of rat molars. J. Electron Microsc. (Tokyo) 50, 365–369 [DOI] [PubMed] [Google Scholar]
- 20. Kawai T., Matsuyama T., Hosokawa Y., Makihira S., Seki M., Karimbux N. Y., Goncalves R. B., Valverde P., Dibart S., Li Y. P., Miranda L. A., Ernst C. W., Izumi Y., Taubman M. A. (2006) B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 169, 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshinaga Y., Ukai T., Abe Y., Hara Y. (2007) Expression of receptor activator of nuclear factor kappa B ligand relates to inflammatory bone resorption, with or without occlusal trauma, in rats. J. Periodontal Res. 42, 402–409 [DOI] [PubMed] [Google Scholar]
- 22. Frost H. M. (1999) An approach to estimating bone and joint loads and muscle strength in living subjects and skeletal remains. Am. J. Hum. Biol. 11, 437–455 [DOI] [PubMed] [Google Scholar]
- 23. Zanchetta J. R., Plotkin H., Alvarez Filgueira M. L. (1995) Bone mass in children: normative values for the 2–20-year-old population. Bone 16, 393S–399S [DOI] [PubMed] [Google Scholar]
- 24. Rauch F., Bailey D. A., Baxter-Jones A., Mirwald R., Faulkner R. (2004) The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 34, 771–775 [DOI] [PubMed] [Google Scholar]
- 25. Lewis S. J., Smith P. E. (2001) Osteoporosis prevention in myasthenia gravis: a reminder. Acta Neurol. Scand. 103, 320–322 [DOI] [PubMed] [Google Scholar]
- 26. Sayer A. A., Syddall H., Martin H., Patel H., Baylis D., Cooper C. (2008) The developmental origins of sarcopenia. J. Nutr. Health Aging 12, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castro M. J., Apple D. F., Jr., Staron R. S., Campos G. E., Dudley G. A. (1999) Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J. Appl. Physiol. 86, 350–358 [DOI] [PubMed] [Google Scholar]
- 28. Hansen R. B., Biering-Sorensen F., Kristensen J. K. (2007) Urinary calculi following traumatic spinal cord injury. Scand. J. Urol. Nephrol. 41, 115–119 [DOI] [PubMed] [Google Scholar]
- 29. Warner S. E., Sanford D. A., Becker B. A., Bain S. D., Srinivasan S., Gross T. S. (2006) Botox induced muscle paralysis rapidly degrades bone. Bone 38, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poliachik S. L., Bain S. D., Threet D., Huber P., Gross T. S. (2010) Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 46, 18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stolina M., Adamu S., Ominsky M., Dwyer D., Asuncion F., Geng Z., Middleton S., Brown H., Pretorius J., Schett G., Bolon B., Feige U., Zack D., Kostenuik P. J. (2005) RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. J. Bone Miner. Res. 20, 1756–1765 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi N., Udagawa N., Tanaka S., Suda T. (2003) Generating murine osteoclasts from bone marrow. Methods Mol. Med. 80, 129–144 [DOI] [PubMed] [Google Scholar]
- 33. Parfitt A. M. (1983) Bone Histomorphometry: Techniques and Interpretation, CRC Press, Boca Raton, FL, USA [Google Scholar]
- 34. Bouxsein M. L., Boyd S. K., Christiansen B. A., Guldberg R. E., Jepsen K. J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 35. Rauch F., Hamdy R. (2006) Effect of a single botulinum toxin injection on bone development in growing rabbits. J. Musculoskelet. Neuronal Interact. 6, 264–268 [PubMed] [Google Scholar]
- 36. Blouin S., Gallois Y., Moreau M. F., Basle M. F., Chappard D. (2007) Disuse and orchidectomy have additional effects on bone loss in the aged male rat. Osteoporos. Int. 18, 85–92 [DOI] [PubMed] [Google Scholar]
- 37. Kwon T. G., Park H. S., Lee S. H., Park I. S., An C. H. (2007) Influence of unilateral masseter muscle atrophy on craniofacial morphology in growing rabbits. J. Oral Maxillofac. Surg. 65, 1530–1537 [DOI] [PubMed] [Google Scholar]
- 38. Libouban H., Blouin S., Moreau M. F., Basle M. F., Audran M., Chappard D. (2008) Effects of risedronate in a rat model of osteopenia due to orchidectomy and disuse: densitometric, histomorphometric and microtomographic studies. Micron 39, 998–1007 [DOI] [PubMed] [Google Scholar]
- 39. Manske S. L., Boyd S. K., Zernicke R. F. (2010) Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone 46, 24–31 [DOI] [PubMed] [Google Scholar]
- 40. Ausk B. J., Huber P., Poliachik S. L., Bain S. D., Srinivasan S., Gross T. S. (2012) Cortical bone resorption following muscle paralysis is spatially heterogeneous. Bone 50, 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ausk B. J., Huber P., Srinivasan S., Bain S. D., Gross T. S. (2011) Initiation of trabecular bone resorption following transient muscle paralysis is site specific. In 56th Annual Meeting of the Orthopaedic Research Society p. 2260, Long Beach, CA, USA [Google Scholar]
- 42. Jaworski Z. F., Duck B., Sekaly G. (1981) Kinetics of osteoclasts and their nuclei in evolving secondary haversian systems. J. Anat. 133, 397–405 [PMC free article] [PubMed] [Google Scholar]
- 43. Shaw N. J., White C. P., Fraser W. D., Rosenbloom L. (1994) Osteopenia in cerebral palsy. Arch. Dis. Child. 71, 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larson C. M., Henderson R. C. (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J. Pediatr. Orthop. 20, 71–74 [PubMed] [Google Scholar]
- 45. Sievanen H. Immobilization and bone structure in humans. Arch Biochem. Biophys 503, 146–152 [DOI] [PubMed] [Google Scholar]
- 46. Rucci N., Rufo A., Alamanou M., Teti A. (2007) Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J. Cell. Biochem. 100, 464–473 [DOI] [PubMed] [Google Scholar]
- 47. Saxena R., Pan G., Dohm E. D., McDonald J. M. (2011) Modeled microgravity and hindlimb unloading sensitize osteoclast precursors to RANKL-mediated osteoclastogenesis. J. Bone Miner. Metab. 29, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morey-Holton E. R., Globus R. K. (2002) Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 92, 1367–1377 [DOI] [PubMed] [Google Scholar]
- 49. Prasad J., Huber P., Bain S. D., Gross T. S. (2011) Diminished mechanical stimuli following transient muscle paralysis are not associated with acute trabecular bone loss. In 33rd American Society for Bone and Mineral Research p. SU0007, San Diego, CA, USA [Google Scholar]
- 50. Judex S., Garman R., Squire M., Busa B., Donahue L. R., Rubin C. (2004) Genetically linked site-specificity of disuse osteoporosis. J. Bone Miner. Res. 19, 607–613 [DOI] [PubMed] [Google Scholar]
- 51. Marenzana M., De Souza R. L., Chenu C. (2007) Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41, 206–215 [DOI] [PubMed] [Google Scholar]
- 52. Tang-Liu D. D., Aoki K. R., Dolly J. O., de Paiva A., Houchen T. L., Chasseaud L. F., Webber C. (2003) Intramuscular injection of 125I-botulinum neurotoxin-complex versus 125I-botulinum-free neurotoxin: time course of tissue distribution. Toxicon 42, 461–469 [DOI] [PubMed] [Google Scholar]
- 53. Bain S. D., Prasad J., Huber P., Nork S., Gross T. S. (2009) Transient muscle paralysis blocks the osteogenic response to skeletal injury. J. Bone Miner. Res. 24, S96 [Google Scholar]
- 54. Manni E., Bagolini B., Pettorossi V. E., Errico P. (1989) Effect of botulinum toxin on extraocular muscle proprioception. Doc. Ophthalmol. 72, 189–198 [DOI] [PubMed] [Google Scholar]
- 55. Hambleton P. (1992) Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. J. Neurol. 239, 16–20 [DOI] [PubMed] [Google Scholar]
- 56. Lang A. M. (2003) Botulinum toxin type A therapy in chronic pain disorders. Arch. Phys. Med. Rehabil. 84, S69–73; quiz S74–65 [DOI] [PubMed] [Google Scholar]
- 57. Imai S., Rauvala H., Konttinen Y. T., Tokunaga T., Maeda T., Hukuda S., Santavirta S. (1997) Efferent targets of osseous CGRP-immunoreactive nerve fiber before and after bone destruction in adjuvant arthritic rat: an ultramorphological study on their terminal-target relations. J. Bone Miner. Res. 12, 1018–1027 [DOI] [PubMed] [Google Scholar]
- 58. Serre C. M., Farlay D., Delmas P. D., Chenu C. (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25, 623–629 [DOI] [PubMed] [Google Scholar]
- 59. Mach D. B., Rogers S. D., Sabino M. C., Luger N. M., Schwei M. J., Pomonis J. D., Keyser C. P., Clohisy D. R., Adams D. J., O'Leary P., Mantyh P. W. (2002) Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113, 155–166 [DOI] [PubMed] [Google Scholar]
- 60. Wright D. E., Williams J. M., McDonald J. T., Carlsten J. A., Taylor M. D. (2002) Muscle-derived neurotrophin-3 reduces injury-induced proprioceptive degeneration in neonatal mice. J. Neurobiol. 50, 198–208 [DOI] [PubMed] [Google Scholar]
- 61. Zaidi M., Chambers T. J., Gaines Das R. E., Morris H. R., MacIntyre I. (1987) A direct action of human calcitonin gene-related peptide on isolated osteoclasts. J. Endocrinol. 115, 511–518 [DOI] [PubMed] [Google Scholar]
- 62. Vignery A., McCarthy T. L. (1996) The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 18, 331–335 [DOI] [PubMed] [Google Scholar]
- 63. Cornish J., Callon K. E., Lin C. Q., Xiao C. L., Gamble G. D., Cooper G. J., Reid I. R. (1999) Comparison of the effects of calcitonin gene-related peptide and amylin on osteoblasts. J. Bone Miner. Res. 14, 1302–1309 [DOI] [PubMed] [Google Scholar]
- 64. Mori T., Ogata T., Okumura H., Shibata T., Nakamura Y., Kataoka K. (1999) Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone resorption. Biochem. Biophys. Res. Commun. 262, 418–422 [DOI] [PubMed] [Google Scholar]
- 65. Goto T., Kido M. A., Yamaza T., Tanaka T. (2001) Substance P and substance P receptors in bone and gingival tissues. Med. Electron. Microsc. 34, 77–85 [DOI] [PubMed] [Google Scholar]
- 66. Togari A. (2002) Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc. Res. Tech. 58, 77–84 [DOI] [PubMed] [Google Scholar]
- 67. Chenu C., Marenzana M. (2005) Sympathetic nervous system and bone remodeling. Joint Bone Spine 72, 481–483 [DOI] [PubMed] [Google Scholar]
- 68. Kostenuik P. J., Bolon B., Morony S., Daris M., Geng Z., Carter C., Sheng J. (2004) Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone 34, 656–664 [DOI] [PubMed] [Google Scholar]
- 69. Ominsky M. S., Kostenuik P. J., Cranmer P., Smith S. Y., Atkinson J. E. (2007) The RANKL inhibitor OPG-Fc increases cortical and trabecular bone mass in young gonad-intact cynomolgus monkeys. Osteoporos. Int. 18, 1073–1082 [DOI] [PubMed] [Google Scholar]
- 70. Nakamura T., Imai Y., Matsumoto T., Sato S., Takeuchi K., Igarashi K., Harada Y., Azuma Y., Krust A., Yamamoto Y., Nishina H., Takeda S., Takayanagi H., Metzger D., Kanno J., Takaoka K., Martin T. J., Chambon P., Kato S. (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 [DOI] [PubMed] [Google Scholar]
- 71. Kuhn R., Schwenk F., Aguet M., Rajewsky K. (1995) Inducible gene targeting in mice. Science 269, 1427–1429 [DOI] [PubMed] [Google Scholar]
- 72. Vickerman L., Neufeld S., Cobb J. (2011) Shox2 function couples neural, muscular and skeletal development in the proximal forelimb. Dev. Biol. 350, 323–336 [DOI] [PubMed] [Google Scholar]
- 73. Usas A., Huard J. (2007) Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 28, 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh J. A. (2010) Botulinum toxin therapy for osteoarticular pain: an evidence-based review. Ther. Adv. Musculoskelet. Dis. 2, 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]