Abstract
Associations between early deprivation/neglect in the form of institutional care with the cortisol awakening response (CAR) were examined as a function of pubertal status among 12- and 13-year-old post-institutionalized youth. CARs indexed hypothalamic-pituitary-adrenocortical reactivity. Post-institutionalized youth were compared to youth adopted internationally from foster care (adoption control) and to nonadopted youth reared in families comparable in parental education and income to the adoptive families. Post-institutionalized youth exhibited a blunted CAR if they were at earlier but not if they were at later stages of puberty. Similarly, for both groups of internationally adopted youth combined, earlier but not later stages of puberty were associated with more blunted CARs at higher but not lower levels of parent-reported pre-adoption physical and social neglect.
Keywords: physical neglect, emotional neglect, institutional care, cortisol awakening response, HPA, puberty
The regulation of physiological and behavioral arousal is an early developmental task that takes place within responsive caregiver-infant relationships (Calkins, Graziano, Berdan, Keane, & Degnan, 2008). These early relationships help calibrate the developing hypothalamic-pituitary-adrenal (HPA) axis (Sanchez, Ladd, & Plotsky, 2001). The HPA axis undergoes a second period of reorganization during puberty in reciprocal transactions with the hypothalamic-gonadal axis (Romeo, 2010). The novel goal of this study was to examine whether deprivation/neglect in the form of institutional or orphanage rearing early in life was associated with altered activity of the HPA axis in youth adopted into stable families, and notably whether associations with early institutional care observed prior to early puberty were enhanced or diminished with increasing stages of puberty.
The HPA axis plays multiple roles in adaptation via the impact of its hormonal product (cortisol in humans) on basic vegetative functions and behavioral, cognitive and emotional reactions to challenge and threat. The cortisol awakening response (CAR) is a sharp increase in cortisol production about 30 minutes post-awakening above the already high levels at awakening (Chida & Steptoe, 2009). The CAR is hypothesized to play a role in preparing organisms for the challenges of the upcoming day (Adam, 2006; Fries, Dettenborn, & Kirschbaum, 2009), activating prospective memory and aiding in time/space orientation (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2009; Fries, et al., 2009). Atypically large CARs are associated with high current anticipatory stress, while atypically small CARs (i.e., blunted) are associated with chronic stress, post-traumatic stress disorder, burnout, fatigue (Chida & Steptoe, 2009; Wessa, Rohleder, Kirschbaum, & Flor, 2006), subclinical depressive symptoms (Dedovic et al., 2010) and childhood trauma (Mangold, Wand, Javors, & Mintz, 2010).
Research shows that adverse early care can have a long-term impact on the function and regulation of the HPA axis (e.g. Shonkoff, Boyce, & McEwen, 2009). In rodents, disrupted early care is associated with increased HPA and behavioral reactivity to acute stressors in adulthood mediated by epigenetic changes on the HPA axis’ receptor system (Meaney & Szyf, 2005). Among non-human primates, repeated maternal separations and peer-only rearing are associated with atypical diurnal rhythms of the HPA axis and dysregulated HPA responses to threat (Sanchez, et al., 2001). In humans, patterns of HPA axis functioning following adverse early care vary with the type of adverse care (neglect/deprivation versus physical or sexual abuse) and the period of development, pre-adolescence or adulthood, when effects are assessed (Gunnar & Quevedo, 2007).
Children who have spent their early years in institutional (e.g., orphanage) care provide a model of adverse early care. Most institutions lack the quality of socio-emotional and cognitive stimulation experienced by children reared in families (Gunnar, 2001; Zeanah, Smyke, & Settles, 2006). When cortisol is assessed while toddlers are still in institutional care or soon after adoption, studies of HPA axis activity have shown a blunted diurnal rhythm in cortisol due to low early morning levels (Carlson & Earls, 1997; Gunnar, Bruce, & Grotevant, 2000). Similarly, children in the U.S. foster care system who experienced severe physical neglect show abnormally low morning cortisol levels (Bruce, Fisher, Pears, & Levine, 2009). Lower morning cortisol concentrations have also been reported in adults who were adopted internationally as children and in these individuals, cortisol levels were negatively associated with both severity of early maltreatment and psychopathology (van der Vegt, van der Ende, Huizink, Verhulst, & Tiemeier, 2010). Because most of the studies examining early morning cortisol levels in institutionalized and post-institutionalized individuals have sampled at about 30 minutes post awakening, these data may reflect a blunting of the magnitude of the CAR.
There have now been a number of studies of HPA axis activity in individuals who experienced neglect and other forms of maltreatment early in life. Notably, patterns of findings observed during childhood sometimes differ from those observed among adults who experienced similar types of adversity in childhood (Gunnar & Quevedo, 2007; Hankin, Badanes, Abela, & Watamura, 2010). Discrepancies between child and adult findings have led to questions about whether the neurobiological changes associated with puberty might alter the activity of HPA axis in relation to early life stressors. Indeed, it is possible that puberty constitutes a sensitive period for reorganizing the axis in relation to current life conditions.
In animal research, there is now good evidence that puberty is a sensitive period during which rising levels of gonadal steroids reprogram the responsiveness of the HPA axis to stressors (Romeo, 2010). In addition, pubertal changes influence emotion-processing brain areas including those that regulate the HPA axis (Cameron, 2004). In research on children and youth, the onset of puberty has been associated with increases in both basal and stress reactivity of the HPA axis as well as increased activity in defensive biological systems (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Quevedo, Benning, Gunnar, & Dahl, 2009). Basal cortisol levels increase from late childhood through early adolescence, perhaps peaking around Tanner Stage 3 (see review, Gunnar & Vazquez, 2006). Importantly, adolescents at higher stages of pubertal development show a larger CAR, steeper diurnal decline, more elevated daytime cortisol and increased cortisol responses to stressors (Adam, 2006; Oskis, Loveday, Hucklebridge, Thorn, & Clow, 2009). To date, however, it is unknown how adverse early care and puberty interact to influence activity of the HPA axis.
In this study we examined the CAR among 12- and 13-year-old post-institutionalized internationally adopted youth who were at differing stages of pubertal development. We chose two comparison groups for the post-institutionalized children. To equate for post-adoption conditions, we included nonadopted youth who were reared in families similar to those who adopt internationally with regards to parental education and income. To equate for the experiences common to being internationally adopted, we examined youth adopted internationally who had not experienced much if any institutional care prior to adoption. These youth had spent the majority of their pre-adoption lives in foster care overseas and were adopted at younger ages. Based on prior research, we anticipated that the number of stressful life events post adoption would be low in both adoption groups and comparable to levels experienced by youth reared in families of high parental education and income (Loman, Wiik, Frenn, Pollak, & Gunnar, 2009). Thus, we hypothesized that if institutional care was associated with changes in the CAR, then youth adopted early from foster care overseas would not differ from nonadopted youth, but post-institutionalized youth would differ. In addition, we hypothesized that if puberty allowed for reorganization of the HPA axis to reflect concurrent levels of stress and adversity, the CAR for post-institutionalized children might shift towards that of the comparison groups at higher stages of puberty. Yet it was also possible that with increasing pubertal development the CAR would become atypically large (Pruessner, Hellhammer, Pruessner, & Lupien, 2003), due to higher risks for depression among youth with institutional care histories (Sonuga-Barke, Schlotz, & Kreppner, 2010).
Post-institutionalized children and youth are at increased risk for a variety of behavioral and emotional problems; still, most post-institutionalized children do not develop clinical disorders (Juffer & van Ijzendoorn, 2005). Because patterns of HPA axis functioning, including the CAR, may vary as a function of psychopathology (Dedovic, et al., 2010), and because non-clinically disordered youth of the ages we studied constitute the majority of the post-institutionalized, internationally adopted population, in the following study we chose to exclude youth with clinical disorders from the sample and focus on youth with behavioral and emotional issues within the non-disordered range.
Method
Participants
The study involved 159 early adolescent participants (12.0 to 14.0 years; M = 12.93, SD = 0.70). Two groups of internationally adopted children were examined: post-institutionalized youth adopted at 8 months or older (M = 25.3 mos) after having spent at least 75% of their pre-adoptive life, and thus most of their infancy, in institutional care (n = 55, 30 female); post-foster care youth adopted between 2 and 8 months (M = 4.2 months) having spent at least 75% of their preadoption lives in foster care and no more than 2 months in institutional care (n = 44, 25 female). These groups, and their selection criteria, were similar to previous studies involving internationally adopted children among which lower performance on visual memory, attention and inhibitory control, and higher average daily cortisol levels distinguished post-institutionalized versus post-foster care children (Gunnar, Morison, Chisholm, & Schuder, 2001; Pollak et al., 2010; van den Dries, Juffer, van Ijzendoorn, & Bakermans-Kranenburg, 2010). We also recruited a group of nonadopted children reared in families of similar education and income to the other two groups (n = 60, 28 female). Demographic information and youth psychopathology symptom scores are provided in Table 1. Groups did not significantly differ in parental education level, family income, internalizing or externalizing symptoms. Fewer parents of post-institutionalized youth were living with a spouse or partner than in the other groups, χ2 (2, N = 156) = 12.92, p < 0.05. This likely reflects the greater opportunity of single parents to adopt from countries using institutions rather than those using foster homes (Hellerstedt et al., 2008).
Table 1. Descriptive Statistics for Participant Characteristics and Family Characteristics.
| Post- Institutionalized n = 55 M (SD) |
Post-Foster Care n = 44 M (SD) |
Nonadopted n = 60 M (SD) |
|
|---|---|---|---|
| Participant Characteristics | |||
| Age (years) | 12.9 (0.6) | 12.8 (0.7) | 13.1 (0.8) |
| Months at adoption** | 25.3 (19.5) | 4.2 (1.7) | - |
| % time institutional care** | 91.7 (1.4) | 26.5 (40.2) | - |
| % time foster care** | 1.7 (6.9) | 67.9 (40.4) | - |
| Region of origin (n) | |||
| Eastern Europe | 26 | 0 | - |
| Asia | 22 | 30 | - |
| Central & South America | 4 | 14 | - |
| Africa | 1 | 0 | - |
| Missing | 2 | 0 | - |
| Behavior Problem T-Scores | |||
| Internalizing | 44.7 (8.0) | 45.0 (8.5) | 45.1 (7.1) |
| Externalizing | 47.6 (6.5) | 45.0 (4.9) | 45.8 (7.3) |
| Attention** | 49.5 (9.1) | 43.1 (7.3) | 44.3 (7.1) |
|
| |||
| Family Characteristics | |||
| % 4-year College Degree or higher | |||
| Respondent | 64.9 | 77.3 | 66.7 |
| Partner | 42.6 | 69.0 | 62.7 |
| Family income (% > $75,000) | 72.5 | 81.8 | 67.3 |
| % Married or with partner* | 74.1 | 95.5 | 93.1 |
p < .05
p<.001
Procedures
Recruitment
Internationally adopted children were recruited from a registry of families interested in being contacted about research on internationally adopted children. The registry was built in 2001 from families who responded to a survey sent to all families who adopted internationally through Minnesota agencies between 1990 and 1998; 63% responded and joined the registry (Hellerstedt, et al., 2008). Analyses of families who did and did not join the survey and registry indicated a slight bias towards better-educated parents and families who had adopted later in the sampled period. Otherwise, the registry sample appeared to be representative of the children adopted in Minnesota during the sampling period (1990-1998), (Hellerstedt, et al., 2008). Over half (53%) of internationally adopted youth contacted agreed to participate in the current study.
Children in the nonadopted group were recruited from a registry of families who indicated interest in being contacted about child development research upon receipt of a letter sent to all families with live births in the counties surrounding the university. Only about 10% of families respond to the request. Previous analyses of this registry indicate that parents who join have higher education and income than the general population. Phone calls were placed to 102 families from this registry and of those reached, 58% were recruited. Thus approximately equal response rates to recruiting were observed between the adopted and nonadopted youth. In all groups, the most common reason for not participating was that the child was in so many extra-curricular activities that s/he did not have time to take part in the study. The adopted youth were fairly representative of children adopted internationally during the time period in which these children were adopted; while the nonadopted children reflected a more highly educated and wealthy segment of the general population. That segment of the population, however, typically is of similar education and income to families who adopt internationally.
Recruiters contacted the parents by phone and screened for pubertal development using the PDS to avoid confounding age, sex and group with pubertal stage (PDS, Petersen, Crockett, Richards, & Boxer, 1988). Participants with PDS score ≥ 2.5 were recruited as mid/late pubertal and those < 2.5 as pre/early pubertal. Participants with a psychiatric diagnosis, a chronic physical illnesses/disability, or on medication that would affect cortisol levels were screened out. Nineteen individuals were excluded due to reported medical diagnosis of at least one of the following conditions: fetal alcohol syndrome, ADHD, autism spectrum disorders, bipolar disorder, severe learning disability, severe hearing impairment, and cerebral palsy.
Data Collection
The present study is part of a larger project that included assessments of executive function, psychophysiology and risk-taking that are not considered in this report. Data collection included materials to be completed at home (morning cortisol samples, self- and parent-report questionnaires) and an experimental laboratory session. During the laboratory session parents completed background questionnaires and child behavior problem scales and their youth completed pubertal development scales.
Measures
Home Salivary Cortisol
Prior to the laboratory session, participants were mailed written instructions and supplies for saliva collection on at three times of day over four days: wakeup (M = 7:56 a.m., SD = 3.5 min), 30 min after wakening (M = 8:27 a.m., SD = 3.75 min), and 60 min after wakening (M = 9:00 a.m., SD = 3.75 min). It was not required to collect the saliva in consecutive days. Reported sampling times did not differ by group, sex, or pubertal score. Youth expelled saliva through a straw into pre-labeled vials. They were instructed to refrain from eating, drinking, or brushing their teeth before taking each sample and from taking samples on days they were ill or within two days after having a fever. A daily log of wakeup time and sampling time was provided. The samples were kept in the family refrigerator and brought to the laboratory session. They were then stored at −20° C until being assayed using a time-resolved fluorescence immunoassay (DELFIA; intra-assay CV < 6, inter-assay CV < 10). All of the samples from a child were included in the same assay batch, and the assay batches were balanced by group, puberty, and sex. Samples were assayed in duplicate and averaged. Cortisol values were log transformed to normalize the distributions.
Adverse Early Care
Adoptive parents reported the quality of their child’s postnatal care prior to adoption via a questionnaire. In addition to information on type (i.e., parent/relative, institution, foster care, unknown) and duration of each type of care, adoptive parents rated forms of early care adversity: known or suspected physical and sexual abuse, neglect of social needs, neglect of physical needs, and overall quality of care. All these indexes of early adversity ranged from 1 (low) to 5 (high) and were weighted equally. For only 3 youth (4%) was abuse (physical, sexual or both) known or suspected, thus this was not analyzed further. Reports of physical (r = 0.53, p < 0.01) and social neglect (r = 0.63, p < 0.01) correlated highly with the overall parent report rating of preadoption care. Physical neglect indexed neglect of basic physical needs (i.e., food, clothing, medical care), whereas social neglect indexed suspected neglect of social care (i.e., lack of love, affection, cuddling). The latter form of neglect is perhaps the more predominant in the context of institutional care for example these children experience (McCall, van IJzendoorn, Juffer, Groark, & Groza, in press).
Thus, the parent’s summary score, which reflected their overall impression of preadoption quality of care, was employed as an index of adverse early care (AEC). This 5-point rating —from low (1) to high AEC (5)— contained no scores of 5 and few 4’s in this sample. Ratings of 3 and 4 were combined to yield a 3-point scale of Adverse Early Care (AEC: l = low, 2 = medium, 3 = high). AEC scores were unrelated to sex or pubertal stage but, as expected, were related to group, χ2 (3) = 30.86, p < .001. Post-institutionalized youth received higher AEC scores than post-foster care youth, although low scores (1) were noted in both groups (14 post-institutionalized, 35 post-foster care).
Behavior Problem Scores
For descriptive purposes, parents completed the parent version of the Behavior Assessment System for Children, Second Edition (BASC-2; (Reynolds & Kamphaus, 2004). Internalizing, externalizing and attention problem symptoms scores were derived (see Table 1). Groups did not differ on these scores nor were there interactions of group with sex or pubertal scores for internalizing or externalizing, but post-institutionalized youth were described as exhibiting more attention problem symptoms, F(2, 149) = 8.50, p < .001. Overall, BASC-2 scores indicated low levels of behavior problems in all groups.
Pubertal Scores
Pubertal development was verified by adolescent report on the PDS (Petersen, et al., 1988), which correlates with Tanner Stages on physical exam and predicts basal hormones related to pubertal development (Shirtcliff, Dahl, & Pollak, 2009). We used only questions reflecting the main axes of puberty: growth (item 1), adrenal (items 2, 3), and gonadal (males: items 4 and 5, females: item 6), and averaged these and subtracted 1 to yield a meaningful 0 for HLM analyses (range 0-3). There were significant age differences when pubertal scores were dichotomized, F(2,143) = 19.66, p < 0.01, Mpre/early puberty = 12.57 (SD = 0.58); Mmid/late puberty = 13.24 (SD = 0.65) and females had higher pubertal scores than males but there were no group differences in this measure. The PDS scores, not our initial phone selection criteria, were used in the pubertal analyses.
Adolescent and Family-Level Life Events
A variation of the Adolescence Life Experience Survey (Swearingen & Cohen, 1985) was employed to measure the number of positive and negative events as well as the total number of events in the last six months. Family-level life events were also gathered via questionnaire and the number of family-level negative life events since the birth or adoption of the child were totaled.
Missing Data
All salivary cortisol data for 15 youth were missing, thus the sample size for cortisol analysis was 144. In addition, 10 youth had one or several samples (8 to 16%) that were more than 4 SDs above the mean; these samples were removed from the analyses. Missing cortisol data did not follow a pattern as indicated by the Little’s MCAR test: χ2 (31, 144) = 8.39, ns and participants with missing cortisol data did not differ by sex, group, or PDS scores from those with complete cortisol data. No other data were missing.
Cortisol Data Analysis Plan
Hierarchical Linear Models (HLM) were fitted employing the PROC MIXED procedure in SAS 9.2 to model the CAR. The observed data were modeled (See Equations 1 and 2, unconditioned model) to predict individual intercepts and slope parameters (β0i day and β1i day) distributed around mean intercept (γ00) and slopes (γ01and γ02). Hypotheses pertained to both mean intercept (γ00) and CAR (mean linear slope = γ01). Variance/covariance structures were tested for the observed data: compound symmetry (CS, AIC=2637.5), autocorrelated (AR1, ACI=2644.3), random effects (RE, AIC=2615), and unspecified (UN, AIC=2580.2). UN was the best fitting structure. Wake-up time and time since wakeup to first saliva collection were included in all the models.
| Equation 1 |
| Equation 2 |
Note: δ = error, linear = 0, 1, 2, quadratic = 0, 1, 4, i = subject
Models were compared via a χ2 test of the −2LL fit difference. Sex did not influence cortisol intercept or CAR and including sex did not yield a better fit: −2LLsmaller model - 2LLlarger model = 3.5, df = 2 (NS), critical χ2(2) = 5.99, thus sex was excluded. By contrast, models that included only Puberty or Group did not fit the data better than the models including both. Solution for estimated parameters are reported in tables but their type 3 F tests of fixed effects are reported in the text as are specific estimated γ01 parameters resulting from planned contrast to follow up significant interactions. Figures show observed data, not estimated values.
Results
The Cortisol Awakening Response
Cortisol increased from wake-up to 30 minutes followed by a decrease to 60 minutes (see Table 2), as shown by the significant positive linear and negative quadratic slopes (γ01 = 1.62, F(1,551) = 41.57, p < .01, γ02 = −0.52, F(1,551) = 21.02, p < 0.01). Not shown in the table, but included in all models were the following effects: larger time lapse between wake-up and first saliva collection was associated with a slightly flatter CAR (γΔ-time*linear slope= −0.0001, F(1,551) = 16.03, p < 0.01) and a faster drop in cortisol values 60 minutes after wake-up, (γΔ-time*quadratic slope = 0.00002, F(1,551) = 9.86, p < 0.01). Time lapse, however, did not influence initial cortisol values. Waking up later was associated with slightly lower initial cortisol values, flatter CAR and slightly more pronounced drop in cortisol values 60 minutes after wake-up (γwake-up = −8.75E-06, F(1,551) = 4.67, p < 0.05; γwake-up*linear slope= −0.00004, F(1,551) = 20.99, p < 0.01; γwake-up*quadratic slope = 8.628E-6, F(1,551) = 9.86, p < 0.01). Age was included in all analyses except for the internationally adopted grouping due to lack of significance. Age was associated with higher cortisol initial values, γ00 age = 0.081, F(1,547) = 9.09, p < 0.01, but it did not influence the CAR.
Table 2. HLM Analysis of Cortisol Activity by Group and Pubertal Score, df=451.
| Effect | Estimate γ | SE γ | t | p |
|---|---|---|---|---|
| Intercept | 2.08 | 0.17 | 11.91 | <.001 |
| Linear Slope | 1.62 | 0.30 | 5.45 | <.001 |
| Quadratic Slope | −0.48 | 0.13 | −3.79 | <.001 |
| Group | ||||
| Post-Institut. | 0.08 | 0.15 | 0.55 | ns |
| Post-Foster C. | 0.10 | 0.16 | 0.67 | ns |
| Nonadopted | 0.00 | |||
| Puberty | 0.15 | 0.07 | 1.99 | <.05 |
| Group by Puberty | ||||
| Post-Institut. | −0.11 | 0.09 | −1.27 | ns |
| Post-Foster C. | −0.10 | 0.10 | −1.03 | ns |
| Nonadopted | 0.00 | |||
| Group by Linear Slope | ||||
| Post-Institut. | −0.29 | 0.11 | −2.66 | <.01 |
| Post-Foster C. | −0.04 | 0.11 | −0.37 | ns |
| Nonadopted | 0.00 | |||
| Linear Slope by Puberty | −0.06 | 0.05 | −1.08 | ns |
| Linear Slope by Puberty by Group |
||||
| Post-Institut. | 0.16 | 0.07 | 2.40 | <.05 |
| Post-Foster C. | 0.01 | 0.07 | 0.13 | ns |
| Nonadopted | 0.00 |
Early Experience and Puberty
Post-institutionalized youth had a blunted CAR compared to nonadopted adolescents as shown by a Group by Linear Slope interaction, F(2,451) = 5.38, p < 0.01. Table 2 in the Estimate γ column shows the relative estimated intercepts or slopes associated with each predictor variable. For example, the Group by Linear Slope weights represent the estimated difference of the slopes between post-institutionalized or post-foster care versus nonadopted adolescents. This also shows that post-foster care youth did not differ significantly from nonadopted youth in CAR. Puberty scores were positively associated with higher initial cortisol levels, F(1,451) = 4.22, p < 0.05 as shown in Table 2. These effects, however, were modified by a three-way interaction between Linear Slope (CAR), puberty score and group, F(2,451) = 4.55, p < 0.05 as indicated by a significant Linear Slope by Puberty by Group interaction (Table 2).
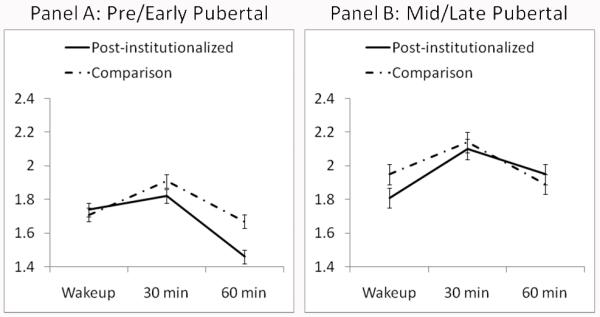
Because the post-foster care and nonadopted groups did not differ, to increase power for planned contrasts to unpack the 3-way interaction, youth in these two groups were combined into one comparison group. The HLM analysis was repeated to obtain the correct coefficients. Note: all effects reported above remained, including the 3-way interaction, F (1,338) = 5.23, p < .05. Additionally, pubertal scores were positively associated with the CAR, F(1,448) = 3.91, p < 0.05 as indicated by a puberty by linear slope interaction. Planned contrasts testing the differences in linear slope between the post-institutionalized youth and comparison youth during early puberty showed that the comparison group had a steeper CAR than the post-institutionalized youth (γearly puberty*EA/FC & NA *linear slope = 1.135, SE = 0.15; γearly puberty*LA/PI*linear slope = 1.014, SE = 0.05), t(548) = 2.58, p < 0.05), yet there was no CAR difference between the comparison group and the post-institutionalized group during mid/late puberty. Within groups, for post-institutionalized youth, a steeper CAR was associated positively with pubertal score (γLA/PI*advanced puberty*linear slope = 1.14, SE = 0.153); γLA/PI*early puberty*linear slope = 1.014, SE = 0.153), t (548) = 2.68, p < 0.01, but this was not true among the comparison youth. Figure 1 depicts these results. For the purpose of graphing, pubertal scores were grouped into pre/early (scores of 0-1) and mid/late (scores of 2-3) puberty. Specifically, during pre/early puberty, the CAR (linear slope) of the late adopted group is flatter than the CAR of the comparison group (Panel A), whereas during mid/late puberty there are no differences between the two groups CARs (Panel B). Figure 1 also shows that mid/late pubertal adolescents —averaging across post-institutionalized and comparison group— showed a steeper CAR than pre/early pubertal youth.
Figure 1. CAR and Group Status As a Function of Puberty.
Salivary cortisol (log 10 transformed nmol/L values) at wakeup, 30 minutes and 60 minutes with participants grouped into early/pre (Panel A) and mid/late (Panel B) pubertal groups. Because they did not differ in cortisol awakening response (linear slope), data from the two comparison groups (post-foster care and nonadopted) have been combined and labeled as comparison. Although depicted as two pubertal stage groups, the analyses were conducted using pubertal stage as a continuous variable. Bars are the standard errors of the mean.
Adverse Early Care
The model was repeated for only the internationally adopted youth (post-institutionalized and post-foster care combined). We examined whether AEC scores would yield similar interaction effects with puberty on cortisol awakening response and whether we could note a more robust CAR among youth with AEC scores of 1, even though this score was noted for many of the post-institutionalized as well as post-foster care youth.
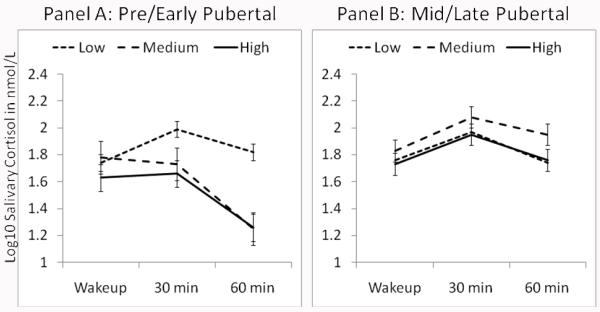
Indeed, lower AEC scores were associated with a robust CAR, while higher AEC scores were associated with a flatter CAR (see Table 3). Thus, the Linear Slope by AEC, F(1,325) = 6.48, p < 0.05, and the 3-way interaction was also significant, Linear Slope by Puberty by AEC, F(1,325) = 3.97, p < 0.05. Planned contrasts revealed that during early puberty, adopted youth with low AEC scores exhibited a significantly steeper CAR than those with medium or high AEC scores, Pre/Early Adolescence: γlow AEC linear slope = 1.11, SE = 0.20; γmedium AEC linear slope = 0.99, SE = 0.19; γhigh AEC linear slope = 0.87, SE = 0.20). During advanced puberty, however, there was no difference in the CAR by AEC level. Figure 2 depicts these results for internationally adopted youth. Specifically, during pre/early puberty, exposure to medium or high levels of AEC was associated with significantly flatter slopes than exposure to low levels of AEC (Panel A), t(325) lowAEC – mediumAEC = 2.59, p < 0.01, t(325)lowAEC- highAEC = 2.59, p < 0.01. By contrast, there were no differences in CAR due to exposure to low, medium or high levels of AEC during mid/late puberty (Panel B).
Table 3. HLM Analysis of Cortisol Activity by Adverse Early Care (AEC) Level and Puberty for Internationally Adopted Youth, df=325.
| Effect | Estimate γ | SE γ | t | p |
|---|---|---|---|---|
| Intercept | 2.29 | 0.20 | 11.24 | <.001 |
| Linear Slope | 1.60 | 0.35 | 4.85 | <.001 |
| Quadratic Slope | −0.43 | 0.15 | −2.90 | <.01 |
| Puberty | −0.01 | 0.09 | −0.07 | ns |
| Adverse Early Care | −0.05 | −0.05 | −0.70 | ns |
| Puberty by Adverse Early Care | −0.02 | 0.02 | 0.45 | ns |
| Linear Slope by Puberty | −0.08 | 0.07 | −1.15 | ns |
| Linear Slope by Adverse Early Care |
−0.14 | 0.05 | −2.54 | <.05 |
| Linear Slope by Puberty by Adverse Early Care |
0.07 | 0.03 | 1.99 | <.05 |
Figure 2. CAR as a Function of Parent-Reported Adverse Early Care for the Internationally Adopted Youth.
Salivary cortisol (log 10 transformed nmol/L values) at wakeup, 30 minutes and 60 minutes with participants grouped into early/pre (Panel A) and mid/late (Panel B) pubertal groups. Pubertal grouping was only for the purpose of the figure as puberty was a continuous variable in the analysis.
Life Events
None of the adolescent life event variables affected cortisol starting values or the CAR. The total number of family level negative life events, however, was associated with lower CAR in the comparison group versus the post-institutionalized group (linear slope * family negative life events * group: F (1,535) = 4.51, p < .05, but all other results remained similar. The best fitting model did not include adolescent life events as a variable, thus it is not included in the tables.
Discussion
To our knowledge this is the first report showing that the relation between social and physical deprivation in early in life and later activity of the HPA axis depends on stage of pubertal development during early adolescence. Many years post-adoption, youth adopted from institutional care showed a flatter CAR than youth reared in their families of origin. A similar pattern was noted among the combined group of post-institutionalized and post-foster care youth when parental report of adverse pre-adoption care was examined. Nonetheless, puberty moderated these associations, and the effects of early institutional rearing and level of adverse early care were no longer significant for mid/late pubertal youth, suggesting that puberty may open a window for reprogramming of the HPA axis. This exemplifies a key principle of developmental psychopathology: the distal impact of disturbed prior developmental tasks will emerge in the context of current environments and new developmental tasks (Sroufe & Rutter, 1984). Specifically, atypical HPA axis function associated with early institutional rearing and the social and physical neglect it entails is still evident in later years, but the new developmental tasks of puberty in the context of improved psychosocial circumstances modify the impact of early adverse care.
Puberty, Adverse Early History and CAR
The blunting of the CAR was specific to post-institutionalized youth and not associated more broadly with being an adopted youth. Importantly, the post-foster care and nonadopted youth did not differ on any measure in this report. This is important as youth in both of the adoption groups probably shared characteristics common to children who are orphaned or abandoned and become available for international adoption. Children who end up in orphanages in the countries represented in this report come from poorer segments of the population and have mothers who were likely exposed to stress and poor nutrition during pregnancy. Internationally adopted children, whether they come from countries that use foster care or institutions to care for orphaned or abandoned children, also share the experience of being abandoned by their birth families, transitions in care during early development and, for many, being raised post-adoption as a racial/ethnic minority child in a racial/ethnic majority family (Gunnar, et al., 2000). Thus, the failure to note significant differences in HPA axis activity between the post-foster care and nonadopted youth support the hypothesis that differences in the CAR noted in the post-institutionalized youth were due to their more adverse early care histories.
Of course, because children adopted from foster care overseas tend to join their adoptive parents earlier in life than children adopted from institutional care, it is also possible that the nature of the early care (family-like versus institutional) as critical as is age at adoption. Nonetheless, this would still implicate the duration of adverse early care as an important factor. It is also important that children adopted from foster care originated from different countries and therefore different gene pools from those adopted from institutions. Thus, it is possible that unmeasured genetic difference between post-institutionalized and post-foster care youth influenced the results. Finally, because we could not randomly assign children to different early rearing conditions, we do need to be cautious about concluding that it was the degree of early social ad physical deprivation or neglect that caused the differences in HPA axis we noted at earlier stages of pubertal development.
Keeping in mind these caveats, during pre/early puberty, post-institutionalized youth show a blunted CAR which was consistent with evidence of lower early morning cortisol levels for children (toddlers) still living in institutional care (Carlson & Earls, 1997) and with a blunted CAR for adults with PTSD (Wessa, et al., 2006) and subclinical depression (Dedovic, et al., 2010).That is, chronic stress exposure may down-regulate cortisol production as a counter-regulatory response to prolonged periods of elevated cortisol (Trickett, Noll, Susman, Shenk, & Putnam, 2010). However, post-institutionalized youth during mid/late puberty —even those that experienced moderate to high adverse early care— did not show a blunted CAR. Thus, puberty probably allows the reprogramming of the HPA axis (Romeo, 2010). If pubertal processes open the HPA axis to reprogramming, it is likely that current life circumstances will influence the patterns of altered activity observed. Thus it is important to note that adolescent life events were not a predictor of either CAR or initial cortisol values. Furthermore, family-level negative life events influenced the CAR of the comparison youth in the opposite direction to the difference observed between nonadopted and post-institutionalized youth. Additionally, in the present study, consistent with other reports (Loman, et al., 2009), negative life events post-adoption were generally quite low. We did not have a measure of early adversity in the nonadopted group and this was a limitation of this study; however, given the high parental education and income of the nonadopted children, we expect that as a group they too would have experienced relatively low levels of stressful life events and adequate early care.
In adolescence, however, emotional stressors related to peer acceptance/rejection and the onset of dating relationships may produce stressors of significant intensity to influence regulation of this stress-sensitive system. Thus, it would be important to examine a broader swath of the adolescent/pubertal period for individuals with adverse early life histories that may impact peer-related stressors during the teen years. Nonetheless, there is evidence in animal models that corrective social experiences in the pubertal period can reverse effects of early insults to the HPA axis (Twiggs, Popolow, & Gerall, 1978).
Puberty and the CAR
Initial levels of morning cortisol increased with age and higher pubertal stages were associated with both higher initial morning cortisol levels and larger CARs while controlling for age. These findings were consistent with and extend earlier reports in the literature. Oskis and colleagues (2009) noted an increase in the CAR with pubertal development, but they only included female adolescents. Additionally, studies of cortisol and puberty have primarily focused on Caucasian youth (for a review see, Gunnar & Vazquez, 2006) and here we demonstrate a similar finding for youth from many regions of the world. Thus, basal levels, the CAR, and cortisol reactivity to stressors all increase during adolescence with advancing pubertal development. These changes in HPA axis functioning with puberty raise a number of questions regarding the neurobiological processes involved. If, as hypothesized (Clow, et al., 2009; Fries, et al., 2009), the CAR reflects the integration of higher-order cortico-limbic input that allows anticipation of the challenges of the day, then an increasing CAR with puberty may reflect maturation of cortico-limbic structures and pathways and/or tighter integration of these pathways with basic HPA regulatory mechanisms in the hypothalamus (Giedd, 2004; Romeo, 2010).
Limitations and Future Directions
We have already noted several limitations in the present study. In addition to the limitations noted above, several more should be noted. First, this was a cross-sectional study with a narrow age group of 12- and 13-year old adolescents who were members of high-functioning families. To better assess the role of puberty and early adversity on the CAR, following children longitudinally from prior to puberty through the pubertal transition will be needed to verify that it was puberty and not some other difference between the these youth who were at various pubertal stages that produced the effects observed in the present study. Second, it will also be important to examine youth later in age/pubertal development than those in the present study to examine the trajectory of change over a wider swath of youth development. Third, all of the youth in the present study were living under conditions of relatively low stress (i.e., low negative life events) in families of high education and income. It is entirely possible that puberty may have quite different impacts on reactivity of the HPA axis for youth living under more adverse circumstances, perhaps particularly in interaction with adverse early life histories. Fourth, we chose to exclude youth with clinical diagnoses from the present study. This means that we cannot generalize to youth who are clinically anxious, depressed, who have clinical levels of conduct or antisocial problem behavior, or other clinical diagnoses. Because post-institutionalized youth can be at risk for these problems (Sonuga-Barke, et al., 2010), it will be important to examine early adversity and pubertal changes on the CAR and other stress-sensitive biological measures in the context of different clinical disorders.
Fifth, our results may not generalize to other types of adverse early care. The youth in the present study were reported to have experienced varying degrees of physical and social neglect as infants but physical and sexual abuse was infrequent. In a recent study of adults adopted internationally as children, a more blunted CAR was noted for those who experienced more severe physical and sexual abuse, but not for those experiencing more severe neglect (van der Vegt, van der Ende, Kirschbaum, Verhulst, & Tiemeier, 2009). If there is reprogramming of the impact of early adversity on HPA axis regulation with puberty, this may not extend to the impact of early physical and sexual abuse. Indeed, there is growing evidence that neglect/deprivation may have a different impact on the HPA axis than other types of maltreatment (Bruce, et al., 2009; Cicchetti, Rogosch, Gunnar, & Toth, 2010). Finally, the lack of any sex differences in the present report needs to be viewed with caution because of the relatively small sample sizes for examining sex differences in the two- and three-way interactions.
There are also future directions of note. It would be important to determine whether variations in the CAR among these youths are predictive of future emotional and behavioral functioning. There is emerging evidence that among youth at risk for depression that an atypically large CAR predicts increases in depressive symptoms with development in the adolescent years (Adam et al., 2010). In future work it will be important to examine whether variations in the CAR before and following the pubertal transition are predictive of pathways of adolescent adaptation for youth with adverse early life histories.
Conclusions
Despite these limitations, the results of the present study provide an impetus to examine the role of puberty as a window for reprogramming, not only of the HPA axis, but also of other neurobiological systems associated with adverse early care. Heightened neural plasticity with puberty might provide an opportunity for improving functioning following adverse early care for youth whose circumstances have improved markedly, and might, at the same time, increase vulnerability when concurrent experiences are stressful or traumatic. The current study highlights the importance of the pubertal period within research initiatives concerned with understanding long-term effects of adverse early experiences on human functioning.
Acknowledgements
We are grateful to the families and youth who volunteered for this study, to the Minnesota International Adoption Project who provided access to their registry of families and to the Center for Neurobehavioral Development, University of Minnesota for technical support. We are especially grateful to Yonghua Jiang for his programming expertise.
Funding Acknowledgement
Preparation of this manuscript was supported by the National Institute of Mental Health (T32 MH15755 and T32 HH01684 to the first author, T32 HD007151 to the second and third authors, R01 MH058857 to the last author) and a University of Minnesota Graduate School fellowship to the second author. No conflicts of interest reported.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. doi: S0306-4530(09)00369-2[pii]10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: differential effects of maltreatment type. Developmental Psychobiology. 2009;51(1):14–23. doi: 10.1002/dev.20333. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50(8):751–766. doi: 10.1002/dev.20344. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: Understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. In: Dahl RE, Spear LP, editors. Adolescent brain development. Vulnerabilities and opportunities. New York Academy of Sciences; New York, NY, US: 2004. p. 472. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychology. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. doi: S0301-0511(08)00220-2 [pii] 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth L. The differential Impacts of early physical and sexual abuse on internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.12.011. doi: S0149-7634(09)00208-5 [pii]10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, et al. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biological Psychiatry. 2010;68(9):847–853. doi: 10.1016/j.biopsych.2010.07.025. doi: S0006-3223(10)00811-5 [pii]10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. doi: S0167-8760(08)00794-0 [pii] 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology:Developmental Neuroscience. 2nd ed. Vol. 2. Wiley; New York: 2006. pp. 533–577. [Google Scholar]
- Gunnar MR. Effects of early deprivation: Findings from orphanage-reared infants and children. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. pp. 617–629. [Google Scholar]
- Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Development and Psychopathology. 2000;12(4):677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and Psychopathology. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. doi: S0954579409000054 [pii]10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. doi: S0006-3223(10)00348-3 [pii]10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally. Maternal and Child Health Journal. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behavior problems and mental health referrals of international adoptees: a meta-analysis. Jama. 2005;293(20):2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Loman MM, Wiik KL, Frenn KA, Pollak SD, Gunnar MR. Postinstitutionalized children’s development: growth, cognitive, and language outcomes. Journal of Developmental and Behavioral Pediatrics. 2009;30(5):426–434. doi: 10.1097/DBP.0b013e3181b1fd08. doi: 10.1097/DBP.0b013e3181b1fd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Hormones and Behavior. 2010;58(4):637–646. doi: 10.1016/j.yhbeh.2010.06.010. doi: S0018-506X(10)00170-4 [pii]10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, van IJzendoorn MH, Juffer F, Groark CJ, Groza VK. Children without Permanent Parental Care: Research, Practice, and Policy, Chapter 1: Children in Institutional Care: Delayed development and resilience. Monograph of the Society for Research in Child Development. in press. [Google Scholar]
- Meaney M, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34(3):307–316. doi: 10.1016/j.psyneuen.2008.09.009. doi: S0306-4530(08)00251-5 [pii]10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. doi: CDEV1391 [pii]10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosomatic Medicine. 2003;65(1):92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev Psychopathol. 2009;21(1):27–45. doi: 10.1017/S0954579409000030. doi: S0954579409000030 [pii]10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children - Second Edition manual. American Guidance Service Publishing; Circle Pines, MN: 2004. [Google Scholar]
- Romeo R. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Frontiers in Neuroendocrinology. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. doi: S0091-3022(10)00008-7 [pii]10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. doi: CDEV1263 [pii]10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. doi: 301/21/2252 [pii]10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Schlotz W, Kreppner J. Differentiating developmental trajectories for conduct, emotion, and peer problems following early deprivation. Monographs of the Society for Research in Child Development. 2010;75(1):102–124. doi: 10.1111/j.1540-5834.2010.00552.x. doi: MONO552 [pii]10.1111/j.1540-5834.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- Sroufe L, Rutter M. The domain of developmental psychopathology. Child Development. 1984;55(1):17–29. [PubMed] [Google Scholar]
- Swearingen EM, Cohen LH. Measurement of adolescents’ life events: The Junior High Life Experiences Survey. American Journal of Community Psychology. 1985;13(1):69–85. doi: 10.1007/BF00923260. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development & Psychopathology. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. doi: S0954579409990332 [pii]10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiggs DG, Popolow HB, Gerall AA. Medial preoptic lesions and male sexual behavior: age and environmental interactions. Science. 1978;200(4348):1414–1415. doi: 10.1126/science.663624. [DOI] [PubMed] [Google Scholar]
- van den Dries L, Juffer F, van Ijzendoorn MH, Bakermans-Kranenburg MJ. Infants’ physical and cognitive development after international adoption from foster care or institutions in China. Journal of Developmental and Behavioral Pediatrics. 2010;31(2):144–150. doi: 10.1097/DBP.0b013e3181cdaa3a. doi: 10.1097/DBP.0b013e3181cdaa3a. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJ, van der Ende J, Huizink AC, Verhulst FC, Tiemeier H. Childhood adversity modifies the relationship between anxiety disorders and cortisol secretion. Biological Psychiatry. 2010;68(11):1048–1054. doi: 10.1016/j.biopsych.2010.07.027. doi: S0006-3223(10)00814-0 [pii]10.1016/j.biopsych.2010.07.027. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults A study of international adoptees. Psychoneuroendocrinology. 2009;34(5):660–669. doi: 10.1016/j.psyneuen.2008.11.004. doi: S0306-4530(08)00303-X [pii]10.1016/j.psyneuen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31(2):209–215. doi: 10.1016/j.psyneuen.2005.06.010. doi: S0306-4530(05)00170-8 [pii]10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Settles L. Children in orphanages. In: McCartney K, Phillips D, editors. Handbook of early childhood development. Blackwell Publishing; Malden, MA: 2006. pp. 424–454. [Google Scholar]