Abstract
In this review we discuss recent research in the field of human skeletal muscle protein metabolism characterizing the acute regulation of mammalian target of rapamycin complex (mTORC) 1 signaling and muscle protein synthesis (MPS) by exercise, amino acid nutrition and aging. Resistance exercise performed in the fasted state stimulates mixed MPS within 1 h post-exercise, which can remain elevated for 48 h. We demonstrate that the activation of mTORC1 signaling (and subsequently enhanced translation initiation) is required for the contraction-induced increase in MPS. In comparison, low-intensity blood flow restriction (BFR) exercise stimulates MPS and mTORC1 signaling to an extent similar to traditional, high-intensity resistance exercise. We also show that mTORC1 signaling is required for the essential amino acid (EAA) induced increase in MPS. Ingestion of EAAs (or protein) shortly following resistance exercise enhances MPS and mTORC1 signaling as compared to resistance exercise or EAAs alone. In older adults, the ability of skeletal muscle to respond to anabolic stimuli is impaired. For example, in response to an acute bout of resistance exercise, older adults are less able to activate mTORC1 or increase MPS during the first 24h of post-exercise recovery. However, BFR exercise can overcome this impairment. Aging is not associated with a reduced response to EAAs provided the EAA content is sufficient. Therefore, we propose that exercise combined with EAA should be effective not only in improving muscle repair and growth in response to training in athletes, but that strategies such as EAA combined with resistance exercise (or BFR exercise) may be very useful as a countermeasure for sarcopenia and other clinical conditions associated with muscle wasting.
Keywords: sarcopenia, protein turnover, mTORC1, essential amino acids, leucine
Introduction
Skeletal muscle represents 50 to 75% of all body proteins and approximately 40% of total body weight (72). In addition to sheer volume, muscle possesses numerous vital functions such as force generation, temperature regulation, energy metabolism, amino acid reserves, immune function and the ability to grow and regenerate (68). Consequently, decrements in skeletal muscle mass and function can introduce complications, which become especially apparent during treatment and rehabilitation for various clinical conditions such as cancer cachexia, chronic heart failure, forced inactivity (i.e., bed rest), AIDS, etc. Additionally, the loss of muscle mass with advancing age (sarcopenia) is quickly becoming recognized as a major health concern as it has been linked to increased functional disability, loss of independence, and decreased life expectancy (12, 22, 76). Considering this link to various debilitating clinical conditions, strategies are needed to counteract the loss of muscle mass and function to improve quality of life. The purpose of this review is to summarize recent research on the role of exercise and nutrition in human muscle protein metabolism. Such research elucidating the cellular mechanisms regulating muscle mass seek the development of evidence-based interventions to prevent muscle wasting in aging and other clinical conditions.
Resistance Exercise and the Regulation of Muscle Protein Synthesis
Resistance exercise stimulates an increase in the rate of skeletal MPS (17, 24, 82). The increase in MPS occurs within the first hour following exercise (24) and can persist for 24 to ~48 hours (82). Concomitant with the increase in protein synthesis, resistance exercise performed in the fasted state also elicits an increase in muscle protein breakdown (MPB) (67, 82). However, changes in MPS appear to be much more responsive to an exercise stimulus (82). Consequently, skeletal muscle protein turnover is increased and net protein balance (difference between protein synthesis and protein breakdown) becomes less negative following an acute bout of resistance exercise, and the accumulation of these acute changes in protein metabolism are believed to provide the foundation for increased muscle mass and strength following resistance exercise training.
The molecular mechanisms that lead to acute increases in MPS following resistance exercise have been linked to enhanced mRNA translation. Studies in rodent and cell models (6, 8, 89) have identified the mammalian target of rapamycin complex (mTORC) 1 pathway as a critical regulator of mRNA translation and MPS. This pathway is described in Figure 1, showing a simplified diagram of the key signal transduction steps leading to mTORC1 activation and, subsequently, enhanced mRNA translation. Other reviews are available for a more comprehensive description of the regulation of mRNA translation (59, 85).
Figure 1.
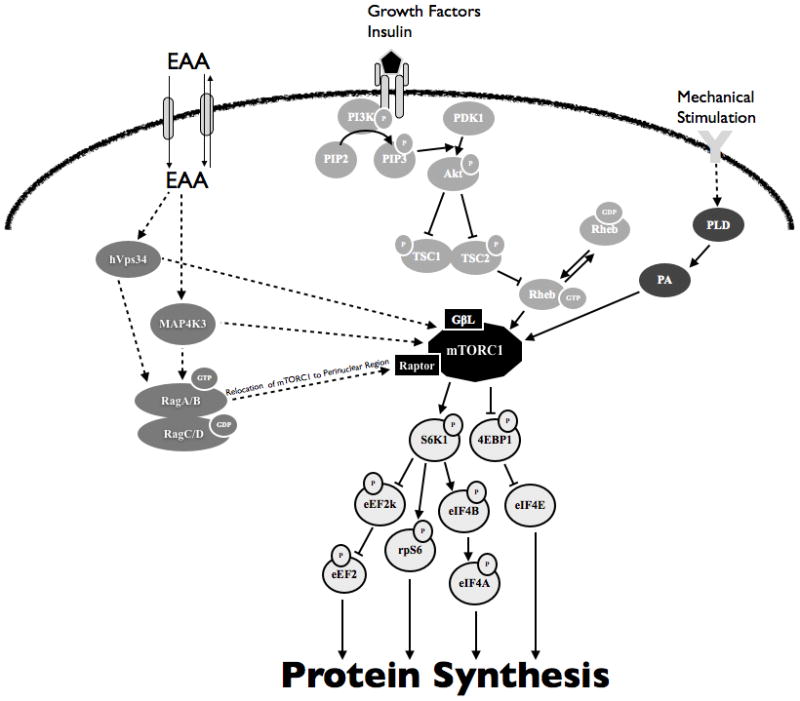
A simplified diagram illustrating the upstream and downstream mammalian target of rapamaycin complex 1 (mTORC1) signaling and regulation of protein synthesis by essential amino acids, hormones and growth factors, and mechanical stimulation. Signaling proteins are labeled in different shades of grey to indicate positive regulation of mTORC1 by essential amino acids, hormones and growth factors, and mechanical stimulation. mTORC1 and associated proteins are labeled black and downstream mTORC1 signaling proteins are outlined in black. Solid lines indicate defined interactions between molecules whereas dotted arrows indicate suggested interactions. EAA, essential amino acids; hVps34, human vacuolar protein sorting-34; MAP4K3, mitogen activated protein kinase kinase kinase kinase-3; RAG, ras-related GTPase; PI3K, phosphatidylinositol 3-kinases; PIP2, phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, PIP3,phosphatidylinositol (3,4,5)-trisphosphate; PDK1, 3-phosphoinositide-dependent protein kinase-1; Akt, protein kinase B; TSC1, tuberous sclerosis complex 1; TSC2, tuberous sclerosis complex 2; Rheb, ras-homologue enriched in brain; PLD, phospholipase D; PA, phosphotidic acid; mTORC1, mammalian target of rapamycin complex 1; GβL, G-protein β-subunit like protein; LST8, 4E-BP1, 4E binding protein 1; eIF4F, eukaryotic initiation factor 4F; S6K1, p70 ribosomal S6 kinase 1; eIF4B, eukaryotic initiation factor 4B; eIF4A, eukaryotic initiation factor 4A; rpS6, ribosomal protein S6; eEF2k, eukaryotic elongation factor 2 kinase; eEF2, eukaryotic elongation factor 2.
Table 1 provides a review of the literature examining the post-exercise mTORC1 signaling responses in fasted, untrained humans in response to an acute bout of resistance exercise. The variability in responses is likely due to different exercise protocols, time of measurement, and intra-subject variability. However, the one consistent theme is that an acute resistance exercise-induced increase in MPS is associated with enhanced mTORC1 signaling (13, 20, 24, 25, 34, 40, 42, 50, 52, 54, 64, 66, 73, 88, 100). Similarly, in trained individuals, a single bout of resistance exercise increases the protein anabolic response, but not to the same magnitude as in untrained individuals (18, 58, 83, 98). To determine whether mTORC1 signaling was required for the contraction-induced increase in MPS, we performed a study utilizing rapamycin (a specific mTOR inhibitor). We found that rapamycin administration (using a dose much smaller than typically used in rodents) prevented the increase in MPS (28) while partially blocking mTORC1 and it downstream effectors, S6 kinase 1 (S6K1), ribosomal protein S6 (rpS6) and eukaryotic elongation factor 2 (eEF2) during early post-exercise recovery in young men. Although a positive correlation between S6K1 phosphorylation and resistance exercise-induced muscle hypertrophy in humans has been demonstrated (99), it remains to be determined whether mTORC1 signaling and enhanced mRNA translation is directly responsible for changes in muscle growth following resistance exercise training.
Table 1.
| Reference | SetsxReps; Mode | Intensity | Time course (post- exercise) | AMPK (Thr172) | TSC2 (Thr1462) | Akt (Ser473) | Akt (Thr308) | mTOR (Ser2448) | S6K1 (Thr389) | S6K1 (Thr421/Ser424) | 4E-BP1 (Thr37/46) | eEF2 (Thr56) | rpS6 (Ser240/244) | rpS6 (Ser235/236) | eIF2Bε(Ser539) | GSK-3β (Ser9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apro and Blomstrand (4) | 4×10 (80%), 4×15 (65%); LP | 80% and 65% 1RM | 0 and 1h | ↔ | - | ↔ | - | ↑ | ↔ | - | - | ↓ | - | ↑ | - | - |
| Burd et al. (13) | 1 or 3 sets LE to volitional fatique | 70% 1RM | 24h | - | - | - | - | - | ↔ | - | - | - | ↔ | - | ↔ | - |
| Camera et al. (14) | 8×5; LE | 80% 1RM | 0, 15, 30 and 60 min | ↔ | ↔ | ↑ | ↑ | ↑ | ↑ | - | ↔ | - | - | - | ↔ | ↔ |
| Deldicque et al. (19) | 10×10; LE | 80% 1RM | 30s, 24 and 72h | - | - | ↔ | ↓ | - | ↔ | - | ↓ | - | - | - | - | - |
| Dreyer et al. (22) | 11×10; KE | 70% 1RM | 0, 1 and 2h | - | ↓ | ↑ | - | ↑ | ↑ | - | ↓ | ↓ | - | - | - | - |
| Dreyer et al. (23) | 11×10; KE | 70% 1RM | 1 and 2h | - | - | ↑ | - | ↑ | ↑ | - | ↔ | ↓ | - | - | - | - |
| Eliasson et al. (30) | 4×6; Con LP | Maximal | 0, 1 and 2h | - | - | ↔ | - | ↔ | ↔ | ↔ | - | - | - | ↔ | - | - |
| Eliasson et al. (30) | 4×6; Ecc LP | Maximal | 0, 1 and 2h | - | - | ↔ | - | ↔ | ↑ | ↑ | - | - | - | ↑ | - | - |
| Fry et al. (32) | 8×10; KE | 70% 1RM | 3, 6 and 24h | - | - | ↑ | - | ↑ | ↑ | - | ↑ | - | - | ↑ | - | - |
| Fujita et al. (36) | 11×10; KE | 70% 1RM | 0, 1 and 2h | - | - | ↔ | - | ↑ | ↑ | - | ↓ | ↓ | - | - | - | - |
| Glover et al. (38) | 4×10; LP 4×10; KE | 10 RM | 6h | - | - | ↑ | - | ↔ | ↑ | - | - | - | ↑ | ↑ | ↓ | ↔ |
| Holm et al. (46) | 10×36; KE or 10×8; KE | 16% or 70% 1RM | 30 min, 3 and 5.5h | ↑ | - | ↑ | - | - | ↔ | - | ↔, ↑ | ↑ | - | - | - | - |
| Hulmi et al. (48) | 5×10; LP | 10RM (~75% 1RM) | 1 and 48h | - | - | ↔ | - | ↔ | ↔ | - | ↓ | ↔ | - | ↑ | - | - |
| Karlsson et al. (50) | 4×10; LP | 80% 1RM | 0, 1 and 2h | - | - | - | - | - | ↔ | ↑ | - | - | - | ↔ | - | - |
| Koopman et al (58) | 8×10; LP 8×10; LE | 75% 1RM | 0, 30min and 2h | ↑ | - | - | - | - | - | ↑ | ↓ | - | - | ↔ | - | - |
| Kumar et al (60) | 3×9 (60%), 3×8 (75%), or 6×3 (90%); LE | 60–90% 1RM (combined groups) | 10min, 1, 2 and 4h | - | - | - | - | - | ↑ | - | ↑ | ↔ | - | - | - | - |
|
|
|
|||||||||||||||
| Mayhew et al. (66) | 3sets each LP, KE, S | 8–12 RM | 24h | - | - | ↑ | - | ↔ | ↔ | ↑ | ↑ | - | ↔ | - | - | - |
| Reitelseder et al. (77) | 10×8; LE | 80% 1RM | 1, 3.5 and 6h | - | - | ↔ | ↔ | - | ↔ | - | ↑ | - | - | - | - | ↔ |
| Terzis et al. (89) | 1×6, 3×6 or 5×6; LP | 6RM | 30 min | ↔ | - | ↔ | - | ↑ | ↑ | - | - | - | - | ↑ | - | - |
Note: Signaling molecules were recorded above if included within two or more studies. Arrows denote direction of phosphorylation. ↑, significantly increased; ↓, significantly decreased; , no change. RM, repetition maximum; LP, leg press; KE, knee extensions; S, squats; LE, leg extensions; Ecc, eccentric; Con, concentric.
Despite the link between mTORC1 signaling and MPS, it is still unclear how muscle contraction stimulates mTORC1 signaling. Recent attention has been drawn to a phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) independent mechanism involving mechanical activation of phospholipase D1 (PLD1) and the production of phosphatidic acid, which can directly activate mTOR (78). In addition, the early activation of mTORC1 in skeletal muscle in response to mechanical overload is independent of PI3K/Akt signaling (75). Furthermore, the importance of amino acid availability through the activation of amino acid transporters (i.e., LAT1/SLC7A5, SNAT2/SLC38A2, PAT1/SLC36A1) (53) and upstream nutrient sensors such as class III PI3K, human vacuolar protein sorting (hVps)-34 (69) and perhaps the Rag proteins (90) may also play a synergistic role in maximal activation of mTOR signaling following resistance exercise. These mechanisms have yet to be extensively examined in human models of resistance exercise, however we have recently found that human skeletal muscle amino acid transporter expression is upregulated following an acute bout of high-intensity resistance exercise (29).
Blood Flow Restriction Exercise and the Regulation of Muscle Protein Synthesis
As previously mentioned, high intensity resistance exercise (typically greater than 70% 1 RM) is a potent stimulus of MPS and hypertrophy (17, 65, 82, 108). However, recent studies have shown that low intensity (20–50% 1RM) resistance exercise, in combination with blood flow restriction (BFR) to the working muscles, produce similar increases in muscle size and strength as traditional, high-intensity resistance exercise (1, 92, 97). To determine the effects of BFR exercise on the anabolic response of muscle, we performed an acute study in young adults and observed a 46% increase in MPS, similar to what is observed with traditional, high intensity resistance exercise (38). The increase in MPS was also associated with the activation of the mTORC1 signaling pathway (37, 38). Although several hypotheses have been proposed regarding the mechanism(s) of muscle protein accretion due to BFR exercise, the current literature encompasses primarily descriptive studies. BFR exercise increases limb blood flow, strength, and MVC following 4 weeks of BFR training (80) and it has been reported that motor unit activity during the second, third, and fourth sets of BFR exercise were greater than in non-occluded exercise (70). Additionally, the latter study showed expression of the proteolytic genes, FOXO3A, atrogin, and MuRF-1 to be downregulated 8 hours following BFR exercise. In contrast, we reported no differences at 3 hours post-exercise in growth related or proteolytic genes between BFR exercise and non-restricted blow flow exercise (30). A potential mechanism for the muscle growth promoting effects of BFR exercise is that during exercise venous return is occluded resulting in the build up of metabolic end products. Perhaps this altered metabolic milieu plays an important role in motor unit recruitment and subsequent activation of mTORC signaling. At this point, the cellular mechanisms responsible for the BFR exercise-induced increase in muscle growth are unclear, however, it is apparent that both high-intensity resistance exercise and BFR exercise stimulate mTORC1 signaling to a similar extent (38).
Aerobic Exercise and the Regulation of Muscle Protein Synthesis
The impact of aerobic exercise on human skeletal muscle protein metabolism has received significant attention in recent years. Acute aerobic exercise has been shown to stimulate MPS in both the fasted (16, 48, 91) and fed (46, 48, 51) state, while chronic aerobic exercise appears to elicit an increase in MPS rate at rest (84, 93). Examination of various muscle protein subfractions in the fed state suggests that acute aerobic exercise may primarily stimulate mitochondrial protein synthesis while having a minimal influence on myofibrillar protein synthesis (106). However, fed state myofibrillar protein synthesis has been reported to increase following an acute bout of prolonged one-legged kicking exercise (74), indicating the necessity to more clearly define the response of various protein subfractions to aerobic exercise. Interestingly, aerobic exercise training has recently been reported to elicit a considerable increase in muscle size and strength in older women (47), suggesting that aerobic exercise training can produce a chronic net positive muscle protein balance and may provide a novel countermeasure to sarcopenia. The ability for aerobic exercise to increase muscle mass in the elderly may be due in part to its ability to sensitize the muscle to the anabolic effects of insulin (41). Despite limited data describing the cellular mechanisms contributing to the increase in MPS, the mTORC1 pathway does appear to have a role in the regulation of muscle protein metabolism following aerobic exercise, as mTORC1 phosphorylation (Ser2448) has been shown to be upregulated in response to acute aerobic exercise (7, 14, 71). However, more research is needed to characterize the cellular mechanisms responsible for the regulation of muscle protein turnover following aerobic exercise in humans, especially in the context of the ability of aerobic exercise to preserve or restore muscle mass and/or function in conditions of muscle wasting.
Aging and Resistance Exercise
The loss of muscle mass associated with aging cannot be explained by detectable age related differences in post-absorptive skeletal muscle protein metabolism as healthy, young and older adults appear to have similar resting rates of MPS and MPB (79, 86, 103). Rather, the muscle loss observed with aging may be, in part, related to the observations that older individuals do not appear to have the same magnitude of anabolic response as younger individuals to an anabolic stimulus. For instance, an acute bout of resistance exercise, which is a very robust anabolic stimulus, has been shown to increase MPS to a greater magnitude in young than in older subjects (27, 105). Although some researchers have reported no age-related difference in the anabolic response to resistance exercise (49, 108). Nonetheless, older men demonstrate a smaller anabolic response to a range of resistance exercise intensities compared to young men (66). In our recent study of young and older adults, we found that aging is associated with an impaired ability to activate mTORC1 signaling and MPS over a 24 h post exercise time course (36). Similar to acute studies, Kosek et al. discovered that four months of resistance exercise training (3d/wk) resulted in significantly greater skeletal muscle hypertrophy in young as compared to older men and women (65). Collectively, these data show that both young and older adults can benefit from resistance exercise training. However, aging may result in decreased anabolic responsiveness to resistance exercise, and thus, potentially contribute to age-related muscle loss.
Recently, we examined whether a novel rehabilitation tool (BFR exercise) would be effective in restoring the contraction-induced increase in MPS in older adults. We found that MPS increased 56% following an acute bout of BFR exercise (37), indicating that this novel treatment was capable of overcoming the impaired MPS response seen with aging in response to traditional resistance exercise. Additionally, mTORC1 signaling, as indicated by S6K1 phosphorylation increased after BFR exercise compared to non-restricted blood flow exercise. Keeping in mind that BFR exercise increases muscle fiber recruitment (70), it is conceivable that increased muscle fiber recruitment would coincide with greater mTORC1 activation and, subsequently, elevated MPS. These data suggest that BFR exercise could be a potential countermeasure in the treatment in sarcopenia. Furthermore, BFR applications could be extended to other clinical populations who are unable to withstand high resistance exercise such as conditions of arthritis, osteoporosis, ligament injuries or post operation rehabilitation.
Essential Amino Acids and the Regulation of Muscle Protein Synthesis
Amino acids have been shown to stimulate a muscle protein anabolic response (39, 79, 101). Ingestion of essential and nonessential amino acids significantly increases plasma amino acid concentrations for up to 3 hours post-ingestion; however, the availability of essential amino acids is the primary stimulator of MPS (101). Regardless of the time course of elevated plasma amino acids, the stimulation of MPS is short-lived, lasting 1–2 hours post EAA ingestion (9, 44). Of the essential amino acids, leucine has received considerable attention due to it’s ability to independently stimulate MPS (2, 3, 94). In some human studies, ingestion of a high quality protein or amino acid solution with extra leucine does not further increase MPS rates (44, 56, 62). However, the added leucine may promote a greater overall anabolic response through a decrease in muscle protein breakdown (44) potentially attenuating muscle loss.
Amino acid availability stimulates muscle protein synthesis partly through activation of mTORC1 signaling (3, 5). Our understanding of the exact mechanism(s) of amino acid induced stimulation of mTORC1 is limited, however, recent findings in animal and cell models have indicated some potential upstream nutrient sensors. Amino acid availability can likely stimulate mTORC1 by activation of hVps34 in a calcium dependent manner (45, 77). Additionally, mitogen-activated protein kinase kinase kinase kinase (MAP4K) 3 is activated upon amino acid availability and results in mTORC1 dependent phosphorylation of S6K1 (35). Rag small GTPases may be required for the amino acid induced upregulation of mTORC1 activity by mediating relocalization of mTORC1 within the cell (57, 90). More recent data suggests that activation of Rag proteins by MAP4K3 (11) may be required for subsequent mTORC1 activation. Relative to the events upstream of mTORC1 activation, downstream events of mTORC1 are well defined. Upon activation, mTORC1 enhances phosphorylation of downstream targets such as S6K1 and 4E binding protein 1 (4EBP1) leading to translational initiation (3, 104). These findings in cell and rodent models have been confirmed in human skeletal muscle as we have recently shown that rapamycin administration to humans blocks the EAA induced stimulation of MPS indicating that mTORC1 activation is required for EAA activation of MPS (21). Stimulation of mTORC1 by EAA was previously shown in our lab to increase mRNA expression of the amino acid transporters LAT1, CD98, SNAT2, and PAT1 and protein expression of LAT1 and SNAT2 1 to 3 hours post-ingestion (32). The latter suggests that an increase in amino acid transporters may sensitize muscle to an ensuing increase in EAA availability. Additionally, the regulation of gene expression by amino acids could be mediated by miRNAs (31). Although potential mechanisms are starting to emerge as described in Figure 1, a better understanding of the mechanisms involved in EAA induced muscle protein anabolism in humans is needed.
Aging and Essential Amino Acids
In healthy, young and older adults, the ingestion of both intact protein and EAA has been shown to increase MPS and amino acid net balance (79, 96, 102). Despite similar (63, 81) or increased (102) splanchnic extraction of amino acids in older subjects relative to younger subjects, there does not appear to be a significant age- related difference in the muscle protein anabolic response to amino acids, provided that the composition and/or dose are adequate (55, 56). For example, MPS is stimulated in older adults following the ingestion of a leucine-enriched supplement (6.7 g of essential amino acids, 41% Leu), but when EAA (6.7g of essential amino acids, 26% Leu) was ingested, no change in MPS was observed (56), whereas young adults experienced a significant increase in MPS with both supplements. Older and younger adults experience a dose-dependent response to essential amino acids below 10 grams, such that MPS plateaus upon increasing levels of amino acid ingestion in young and older subjects alike (19). By contrast, following the ingestion of a small bolus of amino acids (7g of essential amino acids), older subjects had a smaller muscle protein anabolic response than young subjects (55). A plateau also appears to exist for the anabolic effect of intact protein, as the consumption of a higher protein meal (340g lean beef) did not further stimulate MPS, whereas ingestion of a moderately sized protein meal (113g lean beef) stimulated muscle protein synthesis equally in young and older adults (95). This suggests that the regular consumption of meals containing moderate amounts of protein would support the maintenance of lean tissue better than ingestion of a single high protein meal. Overall, recent evidence seems to imply that older adults retain the ability to respond to amino acid and protein ingestion, assuming moderate consumption of high-quality protein. Given that older adults are at increased risk for protein malnutrition (15) this may play a more pivotal role in the development of sarcopenia than any age-related differences in amino acid sensitivity.
Combining Resistance Exercise with EAA Ingestion and the Regulation of Muscle Protein Synthesis
As mentioned previously, resistance exercise and amino acid ingestion independently stimulate MPS; however, net muscle protein balance remains negative when resistance exercise is performed in the fasted state. It has been demonstrated that EAA ingestion following resistance exercise results in greater increases in MPS rates than when EAA are ingested at rest or when resistance exercise is performed in the fasted state (26, 107). Based on these data, supplying EAA following resistance exercise creates a larger positive protein balance by increasing the difference between the rates of MPS and MPB. For instance, ingestion of 6 g of EAA 1 hour after resistance exercise dramatically increased MPS, with minor increases in MPB up to 3 hours post-ingestion leading to an overall large positive net protein balance (10). Several other studies have demonstrated similar effects of EAA ingestion on MPS during post resistance exercise recovery (23, 27, 43, 87).
Recent studies (4, 23, 40, 43) have investigated the mechanisms behind the increase in MPS when EAA are ingested following resistance exercise. The maximal MPS response with EAA given after resistance exercise is attributed to increases in intracellular availability of amino acids, particularly leucine, and subsequently, the activation of mTORC1 signaling (23). For example, ingestion of leucine-enriched EAA and carbohydrate (4, 23) and EAA only (27) following a bout of resistance exercise increased phosphorylation of Akt, mTOR, S6K1, and 4E-BP1 and decreased phosphorylation of eEF2, reflecting improved translation initiation and elongation, respectively. However, studies examining changes in gene expression following resistance exercise and EAA ingestion found no differences in the mRNA abundance of translationally controlled tumour protein (TCTP), mTORC1, and S6K1 or the nutrient sensors, hVPS34 and MAP4K3 (33). However, we have found that EAA provided following resistance exercise increased the mRNA expression of Rheb and cMyc and decreased the mRNA expression of REDD2, which may also contribute to the regulation of mTORC1 activity (33). It is apparent that ingestion of EAA following a bout of resistance exercise can enhance the muscle protein anabolic response as compared to resistance exercise alone or EAA ingestion at rest.
Aging and Essential Amino Acids Combined with Resistance Exercise
Muscle loss that accompanies aging has been reported extensively, but the associated physiological mechanisms are still not entirely clear. As previously mentioned, resistance exercise increases MPS in older adults, but to a lesser degree than in young individuals. Nonetheless, ingestion of EAA or protein following a bout of resistance exercise has demonstrated additive effects on MPS. Koopman et al. examined the potential age-related differences in the response to combined resistance exercise and carbohydrate + protein + leucine ingestion and reported that MPS and whole-body protein balance were increased in both old and young subjects with no age-related differences (60, 61). Similarly, we have shown that EAA ingestion (20 g) given 1-hr after resistance exercise resulted in a similar overall increase in MPS in both young and older adults (27). More recently, Pennings et al. (81) demonstrated that ingesting 20 g of intact protein following a bout of resistance exercise resulted in a similar MPS response between younger and older men. Taken together, these studies seem to indicate that young and older subjects demonstrate a similar protein anabolic response to the combined influence of resistance exercise and EAA/protein. More research is needed to determine whether repeated bouts of resistance exercise and EAA ingestion will be an effective countermeasure for sarcopenia.
Conclusions
In summary, both resistance and aerobic exercise increase human skeletal muscle protein synthesis. Additionally, when resistance exercise is performed at lower intensities and blood flow is occluded, a muscle protein anabolic response is achieved similar to that of typical high-intensity resistance exercise. Following ingestion of EAA, muscle protein synthesis and mTORC1 signaling is enhanced; however, the muscle protein anabolic response is increased to a greater extent when EAA are ingested after resistance exercise. Current research suggests that the anabolic signaling and changes in expression of growth-related genes in response to EAA and/or resistance exercise is mediated through mTORC1 signaling in human skeletal muscle. Moreover, the increase in MPS is blunted in older adults in response to an acute bout of resistance exercise. The age-related differences in the protein anabolic response to ingestion of EAA is unclear, but some data indicate that older individuals may have a blunted response to lower doses of EAA compared to young individuals. However, when ingestion of EAAs is combined with resistance exercise, the age-related differences in muscle protein synthesis and anabolic signaling are less apparent. We conclude that, in humans, resistance exercise with EAA ingestion maximally stimulates MPS, primarily via regulation by mTORC1 signaling. Therefore, we propose that BFR exercise or exercise combined with EAA should be effective, not only in improving muscle repair and growth in response to training in athletes, but may be a useful countermeasure to sarcopenia and other clinical conditions associated with muscle wasting.
Acknowledgments
Funding Disclosure: Supported by the U.S. NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR049877, NIH/National Institute on Aging P30 AG024832, NIH T32-HD07539, and 1UL1RR029876-01 from the NIH/National Center for Research Resources.
We thank Shelley Medina, Ming-Qian Zheng, and Junfung Hao for technical assistance, Dr. Sarah Toombs-Smith for editing the manuscript, and Drs. Shaheen Dhanani and Elena Volpi for clinical and medical support. The authors declare no conflict of interest. The experiments described in this review that were performed in the authors laboratory were supported by NIH grants R01 AR049877, P30 AG024832, NIH T32-HD07539, and 1UL1RR029876 01. The results from our laboratory do not constitute endorsement by the American College of Sports and Medicine.
Bibliography
- 1.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100(5):1460–6. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130(2):139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 4.Apro W, Blomstrand E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol (Oxf) 2010;200(3):237–48. doi: 10.1111/j.1748-1708.2010.02151.x. [DOI] [PubMed] [Google Scholar]
- 5.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–9. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 6.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1 Pt 1):C120–7. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 7.Benziane B, Burton TJ, Scanlan B, Galuska D, Canny BJ, Chibalin AV, Zierath JR, Stepto NK. Divergent cell signaling after short-term intensified endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295(6):E1427–38. doi: 10.1152/ajpendo.90428.2008. [DOI] [PubMed] [Google Scholar]
- 8.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 9.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532(Pt 2):575–9. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283(4):E648–57. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 11.Bryk B, Hahn K, Cohen SM, Teleman AA. MAP4K3 regulates body size and metabolism in Drosophila. Dev Biol. 2010;344(1):150–7. doi: 10.1016/j.ydbio.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–83. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588(Pt 16):3119–30. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc. 2010;42(10):1843–52. doi: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- 15.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56(6):M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 16.Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol. 1990;259(4 Pt 1):E470–6. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- 17.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73(4):1383–8. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- 18.Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20(1):190–2. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 20.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104(2):371–8. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141(5):856–62. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–27. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 23.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294(2):E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(Pt 2):613–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199(1):71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106(4):1374–84. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–61. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011 doi: 10.1152/japplphysiol.01408.2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40(4):691–8. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond MJ, Glynn EL, Fry CS, Dhanani S, Volpi E, Rasmussen BB. Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr. 2009;139(12):2279–84. doi: 10.3945/jn.109.112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(5):E1011–8. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106(4):1403–11. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291(6):E1197–205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- 35.Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403(1):13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108(5):1199–209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103(3):903–10. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 39.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582(Pt 2):813–23. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106(5):1730–9. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56(6):1615–22. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2Bepsilon phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R604–10. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- 43.Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R533–40. doi: 10.1152/ajpregu.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140(11):1970–6. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7(5):456–65. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R708–14. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 47.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452–9. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1254–62. doi: 10.1152/ajpregu.00348.2010. [DOI] [PubMed] [Google Scholar]
- 49.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278(4):E620–6. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 50.Holm L, Hall GV, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E257–E69. doi: 10.1152/ajpendo.00609.2009. [DOI] [PubMed] [Google Scholar]
- 51.Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Co-ingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2008;106(4):1394–402. doi: 10.1152/japplphysiol.90333.2008. [DOI] [PubMed] [Google Scholar]
- 52.Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA. The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur J Appl Physiol. 2008;102(2):205–13. doi: 10.1007/s00421-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 53.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296(4):E603–13. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287(1):E1–7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- 55.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 56.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 57.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10(8):935–45. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568(Pt 1):283–90. doi: 10.1113/jphysiol.2005.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem. 2010;285(38):29027–32. doi: 10.1074/jbc.R110.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84(3):623–32. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 61.Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99(3):571–80. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- 62.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288(4):E645–53. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 63.Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139(9):1707–13. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- 64.Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290(6):E1245–52. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- 65.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101(2):531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 66.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(Pt 1):211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103(5):1744–51. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 68.MacIntosh B, Gardiner P, McComas A. Muscle Architecture and Muscle Fiber Anatomy. In: Robertson L, editor. Skeletal Muscle: Form and Function. Champaign, IL: Human Kinetics; 2006. pp. 3–21. [Google Scholar]
- 69.MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol. 2009;587(Pt 1):253–60. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 2011;201(2):255–63. doi: 10.1111/j.1748-1716.2010.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E. Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 2007;191(1):67–75. doi: 10.1111/j.1748-1716.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 72.Matthews D. Protein and Amino Acids. In: Shils M, Olson J, Shike M, Ross A, editors. Modern Nutrition and Health and Disease. Baltimore, MD: Williams & Wilkins; 1999. pp. 11–48. [Google Scholar]
- 73.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107(5):1655–62. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567(Pt 3):1021–33. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol. 2011;589(Pt 7):1831–46. doi: 10.1113/jphysiol.2011.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81(5):953–63. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 77.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102(40):14238–43. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587(Pt 14):3691–701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 80.Patterson SD, Ferguson RA. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur J Appl Physiol. 2010;108(5):1025–33. doi: 10.1007/s00421-009-1309-x. [DOI] [PubMed] [Google Scholar]
- 81.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–31. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 82.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 83.Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276(1 Pt 1):E118–24. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- 84.Pikosky MA, Gaine PC, Martin WF, Grabarz KC, Ferrando AA, Wolfe RR, Rodriguez NR. Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J Nutr. 2006;136(2):379–83. doi: 10.1093/jn/136.2.379. [DOI] [PubMed] [Google Scholar]
- 85.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403(2):217–34. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 86.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88(2):386–92. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 88.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, Kjaer M, Holm L. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab. 2011;300(1):E231–42. doi: 10.1152/ajpendo.00513.2010. [DOI] [PubMed] [Google Scholar]
- 89.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 90.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287(3):E513–22. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 92.Shinohara M, Kouzaki M, Yoshihisa T, Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur J Appl Physiol Occup Physiol. 1998;77(1–2):189–91. doi: 10.1007/s004210050319. [DOI] [PubMed] [Google Scholar]
- 93.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 94.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol. 1992;262(3 Pt 1):E372–6. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 95.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86(2):451–6. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 96.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109(9):1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 98.Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R172–8. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- 99.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2008;102(2):145–52. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- 100.Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E. The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol. 2010;110(4):835–43. doi: 10.1007/s00421-010-1527-2. [DOI] [PubMed] [Google Scholar]
- 101.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277(3 Pt 1):E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 103.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–9. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 105.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol. 1995;268(3 Pt 1):E422–7. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- 106.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586(Pt 15):3701–17. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72(2 Suppl):551S–7S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 108.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265(2 Pt 1):E210–4. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]


