Abstract
This review provides an overview of Cryptococcus neoformans immunology and focuses on the pathogenesis of Cryptococcus-related paradoxical immune reconstitution inflammatory syndrome (IRIS). Cryptococcal IRIS has three phases: (1) before antiretroviral therapy (ART), with a paucity of cerebrospinal fluid (CSF) inflammation and defects in antigen clearance; (2) during initial ART immune recovery, with pro-inflammatory signaling by antigen-presenting cells without an effector response; and (3) at IRIS, a cytokine storm with a predominant type-1 helper T-cell (Th1) interferon-gamma (IFN-γ) response. Understanding IRIS pathogenesis allows for risk stratification and customization of HIV/AIDS care. In brief, persons at high IRIS risk may benefit from enhancing microbiologic clearance by use of adjunctive agents in combination with amphotericin, prolonging initial induction therapy, and/or increasing the initial consolidation antifungal therapy dose to at least 800 mg of fluconazole daily until the 2-week CSF culture is known to be sterile. Prophylactic anti-inflammatory therapies or undue delay of ART initiation in an attempt to prevent IRIS is unwarranted and may be dangerous.
Keywords: HIV, AIDS, Cryptococcal meningitis, CM-IRIS, Immune reconstitution inflammatory syndrome, Pathogenesis, Review, Antiretroviral therapy, Immunology, Risk stratification, Biomarkers, Antifungal therapy, Anti-inflammatory therapy
Introduction
The introduction of highly active antiretroviral therapy (ART) has lowered the incidence of opportunistic infections and death in HIV-infected persons, but ART use has also introduced new complications. A subset of patients will develop clinical deterioration with ART because as their immune system improves, they develop exaggerated inflammatory responses to persistent foreign antigens. This exaggerated inflammatory response is known as immune reconstitution inflammatory syndrome (IRIS). IRIS has emerged as a common complication of ART in sub-Saharan Africa, particularly as associated with cryptococcal meningitis or tuberculosis. The pathologic mechanisms that underlie the development of cryptococcal IRIS have only recently begun to be elucidated. This review will impart an overview of the normal immunology of cryptococcal infection and the aberrant pathogenesis of Cryptococcus-related paradoxical IRIS.
Cryptococcal Microbiology
Cryptococcus neoformans is a eukaryotic, unicellular organism that was serendipitously discovered more than 100 years ago. This fungus never achieved widespread notoriety as a human pathogen until the emergence of the HIV/AIDS epidemic, but C. neoformans has evolved to possess several unique virulence components [1]. Most notably, the polysaccharide capsule is critical to the yeast’s ability to survive within the environment and to cause disease in humans [2, 3]. The capsule consists of nearly 90% glucuronoxylomannan (GXM) polysaccharide, with about 9% galactoxylomannan (GalXM) and about 1% mannoprotein [4]. The capsule helps the organism evade various aspects of the innate and adaptive immune system [5]. As a result, antibody responses are believed to play a minor role in cryptococcal defense, whereas cell-mediated immunity is necessary and sufficient to protect most individuals from infection [6]. Clinically, this difference is most evident from the markedly increased risk of cryptococcosis in persons living with AIDS, compared with the relatively minor increased risk of cryptococcosis in persons with common variable immunodeficiency or other B-cell dyscrasias.
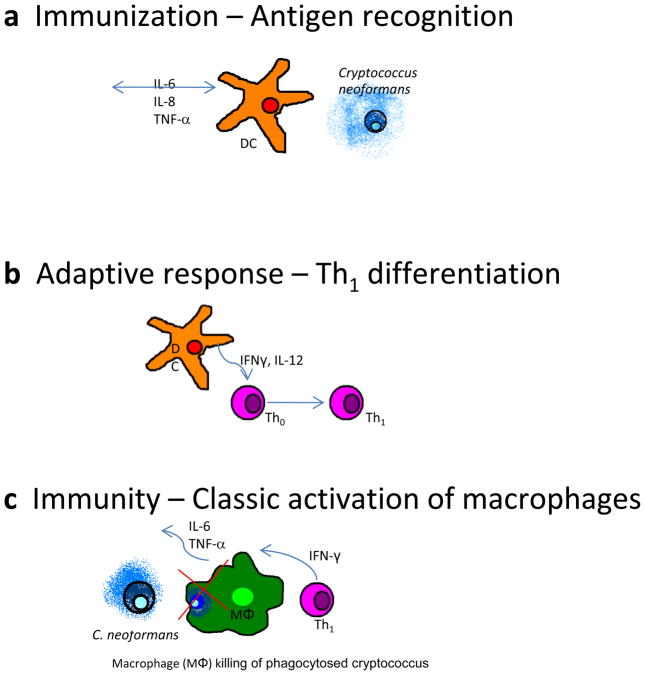
Capsular components are shed during C. neoformans replication and can be recovered in culture supernatants and serum of infected patients [3, 7]. Soluble forms of these molecules appear to elicit different responses [8]. GXM is recognized by pattern recognition receptors found on many innate immune cells, such as Toll-like receptors 2 and 4 on macrophages and dendritic cells (Fig. 1a) [9]. Conversely, mannoprotein is an immunodominant peptide that is recognized by antigen-specific T cells. Mannoprotein is implicated in type-II interferon (i.e., IFN-γ) and delayed-type hypersensitivity responses, both of which are critical to the classic activation of macrophages and fungal clearance [10–12]. These molecules are believed to be principal factors in cryptococcal immunity by influencing either a protective response via mannoprotein or a nonprotective immune response via GXM [3, 4, 12].
Figure 1.
Immune responses to Cryptococcus. a Myeloid dendritic cells (DC) recognize Cryptococcus neoformans pathogen-associated molecular patterns via Toll-like receptors 2 and 4 and pinocytosis of soluble antigen, initiating inflammatory signaling. b DCs communicate with naïve CD4+ T lymphocytes (Th0), leading to differentiation of type-1 helper T cells (Th1). c Th1 CD4+ lymphocytes classically activate macrophages (MΦ) via IFN-γ. Macrophages upregulate reactive oxygen species and kill C. neoformans within phagosomes. GM-CSF granulocyte-macrophage colony-stimulating factor; IL interleukin; TNF tumor necrosis factor.
Cryptococcal Host-Pathogen Interactions
Immunity and immune responses to Cryptococcus have been characterized through a combination of mouse and human studies. The innate defense begins in the submucosa through alternative complement opsonization of C. neoformans, allowing for efficient phagocytosis by neutrophils, macrophages, and dendritic cells [13–15]. Dendritic cells localize the pathogen to the endolysosome after phagocytosis, and the organism is efficiently degraded and processed, with antigenic peptides presented on surface MHC-II molecules (Fig. 1a) [15, 16]. While in the secondary lymphatic tissue, T cells receive the appropriate cytokine signals from dendritic cells, such as IL-12, to differentiate into effector T cells (Fig. 1b) [3]. Once activated by a subpopulation of effector T cells, macrophages have an essential function in fungal clearance (Fig. 1c). Macrophages are important for creating a pro-inflammatory signaling cascade via interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α to promote secondary lymphocyte recruitment. Macrophages require IFN-γ stimulation to upregulate antimicrobial peptides and reactive oxygen species sufficient to kill the phagocytosed, facultative intracellular parasites of Cryptococcus (Fig. 1c) [3]. The polysaccharide capsule renders humoral immunity less effective, but the adaptive arm of the immune system remains paramount to cryptococcal defense.
The paradigm of type-1 and type-2 helper T cells (Th1/Th2) provides a convenient context to understand cryptococcal immunity. Observation of delayed-type hypersensitivity, requirements for cell-mediated immunity, and dependence on IFN-γ all suggest that a Th1 response is essential to cryptococcal immunity [1–3]. The best evidence of the importance of Th1 responses in humans comes from observational data: IFN-γ levels in the cerebrospinal fluid (CSF) of persons with AIDS is positively correlated with increasing fungal clearance from the CSF [17•, 18]. This finding was experimentally validated by a randomized, controlled trial of adjunctive IFN-γ in combination with amphotericin and 5FC, which increased by the 30% the rate of fungal clearance of Cryptococcus, compared with amphotericin with flucytosine (5-FC) [19]. Conversely, Th2 responses (e.g., IL-4, IL-13) are not protective and result in disseminated cryptococcal infection [20, 21, 22•].
Since Th17 immune responses have recently been implicated in protection against fungal pathogens such as Candida and Aspergillus, one would assume that they could be similarly important for cryptococcal defense [23, 24]. However, the current evidence suggests that Th17 responses are not essential for protection and may in some cases be pathologic [25–27]. As our understanding of effector T lymphocytes and their role during cryptococcal infection develops, many ambiguities of human cryptococcosis are likely to become more transparent.
Pathogenesis of Cryptococcal IRIS
Underlying all processes of cryptococcal immunity is a complicated, but informative, network of immune signaling. Chemokines and cytokines can be measured in peripheral blood or CSF of patients with infection, and this information can then be used to characterize human immune responses. These approaches have been used in prospective cohorts of persons with recent cryptococcosis undergoing ART-associated immune reconstitution in an attempt to better understand the pathogenesis of IRIS following cryptococcal meningitis (CM-IRIS) [28•–30•]. Three sequential phases during immune recovery appear to be involved in Cryptococcus-related IRIS: baseline (pre-ART), early phase (pre-IRIS), and effector phase (IRIS event) (Table 1).
Table 1.
Summary of the theory of paradoxical cryptococcal immune reconstitution inflammatory syndrome (IRIS) pathogenesis
| Phase | Immunologic activity | Evidence in CM-IRIS patients |
|---|---|---|
| Baseline (before ART) | ||
| Early phase (after starting ART) |
|
|
| Effector phase (IRIS event) |
|
Whether higher dose consolidation therapy with fungicidal doses of fluconazole at ≥800 mg/day is a potential intervention to decrease the risk of IRIS is unknown. Standard consolidation therapy dosing of fluconazole at 400 mg/day is fungistatic [17•].
APCs antigen-presenting cells; ART antiretroviral therapy; CM cryptococcal meningitis; CrAg cryptococcal antigen; CRP C-reactive protein; CSF cerebrospinal fluid; FGF fibroblast growth factor; G-CSF granulocyte colony-stimulating factor; GM-CSF granulocyte-macrophage colony-stimulating factor; IFN interferon; IL interleukin; TNF tumor necrosis factor; VEGF vascular endothelial growth factor.
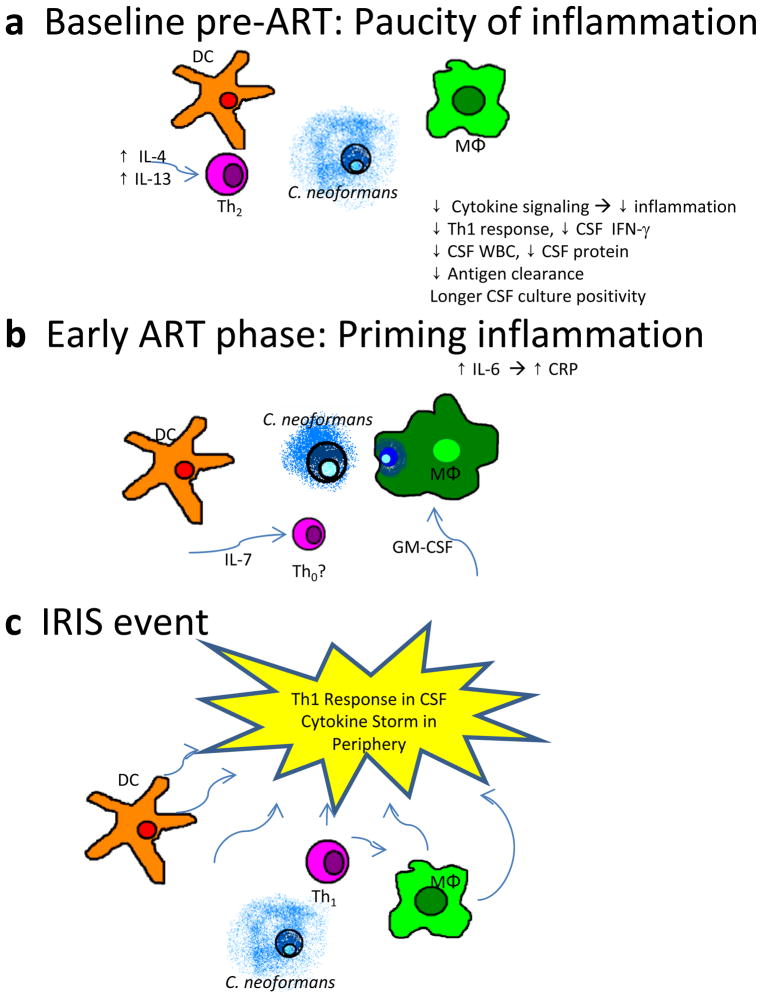
The baseline phase of IRIS pathogenesis begins before ART is even begun and reflects underlying damage and dysfunction to the immune system. This dysfunction is noted by a failure to generate appropriate effector responses and a paucity of pathogen recognition and antigen clearance. Clinically, normal CSF protein levels and/or low CSF white blood cell counts are seen in those who will go on to develop IRIS [29•]. Further investigation has revealed differences in cytokines and chemokines related to immune responses to Cryptococcus. Lower CSF levels of IFN-γ, TNF-α, IL-6, and IL-8 are present before ART in persons who later go on to develop IRIS, compared with patients who do not develop IRIS during ART [29•, 30•, 31]. This difference implies lower levels of pro-inflammatory signaling (e.g., IL-6, IL-8, TNF-α) and lower levels of effector responses with less IFN-γ. This trend is also apparent in patients’ serum immediately before starting ART (at a median of 5 weeks after the initial meningitis) [28•]. Protective innate signals are decreased: lower levels of TNF-α, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) were observed before ART in the serum of persons who later developed IRIS, compared with those who did not develop IRIS [28•]. Additionally, inappropriate responses (e.g., IL-4 and increased IL-17) are more frequent before ART in those going on to develop IRIS. In the Kampala cohort, initial CSF cryptococcal antigen (CrAg) levels did not differ, but those going on to develop IRIS had higher serum CrAg levels at ART initiation, suggestive of worse antigen clearance [28•, 29•]. In this pre-ART baseline phase of IRIS pathogenesis, persons who go on to develop IRIS have a more damaged immune system and are unable to mount appropriate or effective responses to antigens.
These cytokine and chemokine signals can be interpreted in the wider context of human immunology (Fig. 2a). IL-4 production by Th2 in response to CrAg may inhibit the differentiation of naïve CD4+ T cells (Th0) to Th1, and also may inhibit Th1 cell IFN-γ production normally required for the activation of macrophages [32–34]. IL-4, a classic Th2 cytokine, is nonprotective against Cryptococcus and results in disseminated infection [20, 21, 22•]. Additionally, insufficient GM-CSF with an overabundance of IL-4 may unbalance the differentiation of monocytes from helpful myeloid dendritic cells (mDC) to unhelpful plasmacytoid dendritic cells (pDC) [35]. mDCs are important for Th1 differentiation via IL-12 signaling and peptide–major histocompatibility complex II (MHC-II) antigen presentation to naïve T cells in secondary lymphoid organs [36]. Lastly, GM-CSF promotes macrophage phagocytosis, so decreases in GM-CSF may proportionally decrease macrophage phagocytosis of Cryptococcus [37–39]. These pathologic cytokine signals in response to foreign antigen may cause decreases in Th1 differentiation, macrophage classic activation, macrophage phagocytosis, and antigen presentation via mDC. The net result is ineffective responses that incompletely clear antigen, thereby setting the stage for the next phases of IRIS pathogenesis.
Figure 2.
Pathogenesis of cryptococcal immune reconstitution inflammatory syndrome (IRIS), which has three phases: a Before antiretroviral therapy (ART), inappropriate signals lead to a nonprotective type-2 helper T-cell (Th2) response and failure to clear the antigen. b During initial ART immune recovery, pro-inflammatory signaling by antigen-presenting cells does not produce an effector response. c During an IRIS event, a cytokine storm occurs in the periphery and a predominant type-1 helper T-cell (Th1) effector response is evident in the central nervous system, with both sites experiencing increased inflammation and possible absence of regulatory mechanisms. CSF cerebrospinal fluid; DC dendritic cells; GM-CSF granulocyte-macrophage colony-stimulating factor; IFN interferon; IL interleukin; MΦ macrophage; WBC white blood cells.
The second stage (the pre-IRIS phase), while the patient receives ART, is defined by a period of gradually increasing pro-inflammatory signaling without an effector response. During the pre-IRIS phase, there appears to be an inflection point at which the the immunodeficiency and anergy described in the preceding paragraph are reversed, with reconsititution of pro-inflammatory signaling by antigen-presenting cells, particularly macrophages. Acute phase signals (e.g., IL-6, C-reactive protein [CRP], and D-dimer), growth factors (G-CSF and IL-7), and Th2 lymphokines (IL-13) in the serum of patients with recent cryptococcal meningitis were associated with increased hazard of IRIS in time-to-event analyses (Fig. 2b) [28•]. Increased risk of developing IRIS was most notable for IL-6 produced by antigen-presenting cells and for downstream CRP, increasing in serum in the weeks prior to an IRIS event. For every twofold increase in IL-6 or CRP, the hazard of IRIS increased by 1.6 and 1.5 respectively [28•]. Reconstituting an immune system in the presence of copious antigen with accompanying IL-6 coupled with transforming growth factor beta (TGF-β) likely favors the restoration of Th17 cells and other effector cells and also favors the suppression of T regulatory (Treg) cells and/or other regulatory mechanisms [40, 41]. Similar increasing hazard was observed with IL-13 (HR= 1.5 per twofold increase), a Th2 lymphokine that induces alternative activation of macrophages. These alternatively activated macrophages can phagocytosize Cryptococcus, but the phagocytosized organisms are not killed; they can survive and propagate intracellularly within alternatively activated macrophages [21, 22•]. During this pre-IRIS phase of ART, cytokines reflective of effector function, such as IFN-γ or Th17, are not increased in peripheral blood, as they are in controls who do not develop IRIS [28•]. Thus, this second pre-IRIS phase is characterized by pro-inflammatory signaling (IL-6, CRP), growth factors (IL-7, G-CSF), and inappropriate responses (IL-13, alternatively activated macrophages), but without any appropriate effector responses that would efficiently clear antigen.
Upon sufficient immune restoration, anergy gives way to a physiologic immune response to persistant CrAg (Fig. 2c). Although clinically pathologic, the nature and magnitude of immune response is not particularly unusual, as it is predominantly a Th1 effector response. For instance, an IFN-γ response to CrAg is an essential component of normal cryptococcal defense, where by Th1 cells signal via IFN-γ to macrophages, resulting in classic macrophage activation, increased phagocytosis, and pathogen clearance [2]. During IRIS, at the site of inflammation in the CSF, numerous cytokines are increased, including IFN-γ (2.5-fold increase), TNF-α (threefold increase), and IL-6 (twofold increase). Although these increases in CSF cytokines between pre-ART cryptococcal meningitis and CM-IRIS are both substantial (and statistically significant), the actual magnitude of IFN-γ and other downstream cytokines and chemokines (CXCL10) is similar at the time of IRIS to the range observed before ART, at the time of the initial cryptococcal meningitis, among cohort controls who did not develop IRIS. The cytokine response in the periphery was a bit more dramatic: almost every assayed cytokine was significantly higher than in time-matched controls at the time of IRIS. Cytokine increases in the compartment (i.e., CSF) did not completely overlap with the cytokine increases observed in the serum [28•, 29•], so it is only partially correct to assume that what is occurring in peripheral blood is also happening at the site of inflammation. This may have implications for other non-cryptococcal IRIS research.
Previous IRIS Hypotheses
Though IRIS is an important clinical syndrome, it is incompletely understood. The increase in immune response and IFN-γ secretion seen in CM-IRIS could be caused by increased effector potential, decreased regulatory capacity to control the inflammatory response, or both. The predominant IRIS hypothesis has focused on potential pathologic deficits of the negative feedback mechanisms for controlling immune regulation during ART-associated immune restoration. The hypothesis has been that the immune dysregulation results in pathologic inflammation (i.e., a cytokine storm), which clinically manifests as a paradoxical IRIS event. Subsequently, as the effector and regulatory components of the immune system become unbalanced, the potential for boundless inflammation increases, thus setting the stage for IRIS. Though a sensible hypothesis, differences in regulatory T-cell populations have not been observed in other IRIS conditions, such as tuberculosis-related IRIS. Nor have marked increases in Th17 populations been observed. Though Th17 responses are present in the CSF at the time of cryptococcal IRIS events, with measurable IL-17, the relative magnitude of IL-17 increases is small at the time of IRIS. The median level of IL-17 increased from 5.2 pg/mL in pre-ART cryptococcal meningitis to 14.0 pg/mL at time of IRIS (P = 0.099) [29•]. In contrast, IFN-γ concentrations are nearly 10-fold higher than IL-17 at the time of IRIS, at a median of 124 pg/mL in CSF, having increased from a pre-ART level of 50 pg/mL (P = 0.043) [29•]. Thus, Th17:Treg axis at the site of inflammation in CM-IRIS does not appear to be a major influence.
Proposed IRIS Theory
The recent clinical and epidemiologic data on cryptococcal IRIS provide a basis for a slightly different intuitive theory, in which the failure to clear antigen during the early phase of immune recovery is followed by an unbounded response to this persistant antigen upon sufficient immune reconstitution. In this theory, the key defect is the failure to clear antigen because of severely damaged immune systems. In the normal physiologic feedback loop, pro-inflammatory signaling is abated when the foreign antigen is removed. If the antigen persists, the signaling persists. In the Ugandan cryptococcal cohort [28•, 29•], this effect was evident in the pre-ART phase, with decreased inflammation at the time of the initial infection coupled with more CrAg present when starting ART. With successful antifungal therapy and successful immune recovery by ART, CrAg is cleared slowly and pro-inflammatory immune signaling decreases over time. In contrast, in persons who will go on to develop IRIS, the pro-inflammatory signaling increases over time during the pre-IRIS phase on ART, in the absence of any measurable increased effector response. Many perturbations of the immune system can cause ineffective responses, and preexisting conditions (e.g., parasitic infections) may further contribute by skewing Th2 versus Th1 responses, but one issue is very clear: although IRIS is evident as an acute clinical condition, CSF and serum cytokine data from a prospective Ugandan cohort [28•] suggest that IRIS is a developing process with distinct immunologic phases that evolve pathologically long before an IRIS event clinically occurs. This theory reflects an overview of IRIS pathogenesis with the details becoming more complex.
Clinical Implications of IRIS Pathogenesis
Understanding IRIS pathogenesis may allow better medical care of patients. Based on current knowledge, the primary clinical lessons involve stratifying IRIS risk, customizing cryptococcal meningitis therapy, and optimizing the timing of ART initiation.
Risk Stratification
Risk stratification appears to be possible based on a prospective Ugandan cohort [28•, 29•]. A predictive algorithm based on seven pre-ART serum biomarkers was able to predict subsequent CM-IRIS, correctly classifying 82% of cases and controls. The IRIS algorithm could stratify persons into groups with high (82%) IRIS risk, moderate (47%) risk, and low (22%) risk. Although the biomarkers used may be considered research-only tests, there are also routine clinical tests that are informative of risk.
One major risk factor is the paucity of CSF inflammation at time of the initial cryptococcal meningitis diagnosis, based on CSF white blood cell (WBC) count and CSF protein levels, both of which are routine clinical tests. Persons with both a low WBC count (< 25 WBCs/μL) and low CSF protein (< 50 mg/dL) had sevenfold higher odds of subsequent IRIS [29•]. The combination of CSF WBC and CSF protein had a diagnostic sensitivity of 69%, specificity of 76%, positive likelihood ratio of 2.90, and negative likelihood ratio of 0.40. Patients with a paucity of inflammation were also less likely to have sterile CSF after 2 weeks of amphotericin induction therapy [42], as increasing CSF IFN-γ levels correspond to increasing 2-week CSF culture sterility [43]. It is unclear whether the IRIS risk results mainly from the paucity of an immune response evident by lack of CSF inflammation, from the failure to sterilize the CSF, or both. Similar findings were reported in a prospective study of a cryptococcal meningitis cohort conducted in Durban, South Africa: patients with a positive CSF culture when starting ART had a threefold higher risk of developing CM-IRIS [44•]. As well, in Cape Town, CSF cytokine analysis revealed that those with a paucity of CSF inflammation were more likely to develop IRIS, even in a trial of adjunctive IFN-γ, in which the majority of CSF cultures were sterile at 2 weeks [19, 30•]. Though very noteworthy, the incidence of IRIS was threefold lower in cross comparison between Cape Town and Kampala cohorts, suggesting that microbiologic clearance is important [28•, 30•]. Thus, based on the available prospective data, it appears that both a lack of CSF inflammation and CSF culture positivity at 2 weeks are significant risk factors for the subsequent development of CM-IRIS during ART.
Another common laboratory test that was a biomarker of poor outcome is CRP. Patients with a serum CRP level fourfold higher than normal (> 32 mg/L) on the day of starting ART had all-cause mortality eightfold higher than those with CRP less than 32 mg/L (P < 0.001) [28•]. The predominant cause of death during ART was CM-IRIS; however elevated CRP also may be a marker for other occult untreated opportunistic infections. The hypothesis is that CRP elevation, which is mediated by IL-6, reflected pro-inflammatory signaling and/or immune dysregulation of cryptococcosis that was not fully resolved immunologically or microbiologically by the time of starting ART. Further data are needed to understand this CRP association, but at present, a high pre-ART CRP level appears to be associated with poor outcome on ART.
Customization of Cryptococcal Meningitis Therapy
Predicting risk is helpful, but taking clinical action to alter the risk is more helpful. Clinicians can modify two interventions: They can customize the initial cryptococcal meningitis therapy, and they can change the timing of ART initiation. The first of these is perhaps the most important in ensuring that CSF cultures are sterile, by completing induction therapy before changing from fungicidal dosing of antifungal therapy to consolidation therapy. Standard cryptococcal meningitis consolidation therapy with 400 mg per day of fluconazole is recommended to be commenced after 2 weeks of amphotericin induction therapy [45], but fluconazole doses at this dosage is fungistatic [17•], so if the culture is not sterile before the switch to consolidation therapy, fungal clearance becomes dependent on the immune system. This is a vicious cycle, whereby persons who lack CSF inflammation are less likely to be able to clear Cryptococcus until immune reconstitution occurs, setting the stage for CM-IRIS to occur; the innate antigen-presenting cells are generating pro-inflammatory signaling without an effective immune response. The evidence of the benefit of culture sterility comes from the prospective Durban and Cape Town cohorts as well as cross-cohort comparisons. The incidence of CM-IRIS in patients achieving 2-week CSF culture sterility was 10% to 15% in the Durban and Cape Town cohorts [30•, 44•, 46], whereas the incidence in the Durban cohort among individuals with a positive CSF culture (and in a Kampala cohort) was 45% [28•, 44•]. Both in Durban and Cape Town, having a CSF culture which was sterile before starting ART was protective against IRIS [30•, 44•, 46].
Microbiologic clearance can be improved by prolonging induction therapy, using more microbiologically active induction therapy, or using higher-dose initial consolidation therapy. In developed countries, where lipid formulations of amphotericin and intensive electrolyte monitoring are available, prolonging induction therapy is a potential option, although expensive. In resource-limited areas, the toxicity of amphotericin deoxycholate, particularly with electrolyte abnormalities, becomes increasingly problematic with prolonged administration [47].
A second option is to improve the microbiologic activity of the induction therapy by using a concomitant adjunctive agent with amphotericin therapy, such as flucytosine (5-FC), fluconazole at doses ≥800mg/day, or recombinant IFN-γ [19, 43, 46]. Improved microbiologic clearance is associated with improved 2-week and 10-week survival [17•, 43], so adding adjunctive therapy is warranted even without respect to IRIS risk. In resource-limited areas, adding fluconazole at doses of 800 to 1200 mg per day in twice daily divided doses to amphotericin is an implementable option.
The third possible option is altering the initial phase of consolidation therapy. One clinical debate is the actual utility of a 2-week lumbar puncture in the management of cryptococcal meningitis. This lumbar puncture is critically important to verify CSF culture status, but the culture may take an additional 7 to 14 days to finalize as culture-negative. Although the initial diagnostic CSF cultures, which have a high burden of organisms, will often grow in ≤72 hours, the burden of viable infectious organisms in the follow-up 2-week cultures are typically several log10/mL lower and take longer to grow on culture. Thus, in real clinical practice (in both developed and resource-limited countries), the 2-week CSF culture status is unknown at the time of routine hospital discharge. With the new recognition that fluconazole at doses ≥800 mg per day is fungicidal [48], we recommend using 800 mg to 1200 mg per day of fluconazole in divided, twice-daily doses (i.e., 400–600 mg twice daily) until the CSF culture is known to be sterile. In an ongoing clinical trial (ClinicalTrials.gov, NCT01075152), we are routinely using 800 mg of fluconazole for an additional 3 weeks, until patients return to the outpatient clinic and the 2-week CSF culture results can be reviewed. If 2-week CSF cultures are positive, we recommend a repeat lumbar puncture to verify CSF culture status, and we continue 800 mg of fluconazole until the culture status is known to be sterile.
Optimizing ART Timing
Other potential clinical options to prevent CM-IRIS are either delaying ART initiation or using prophylactic anti-inflammatory medications. These options lack data to support them and could be dangerous. Delaying ART initiation is potentially hazardous, as patients with cryptococcal meningitis have severe immunosuppression and are at risk of other AIDS events and mortality in the absence of ART. In Kampala, 40% of patients died before starting ART, including approximately 25% after hospital discharge but before ART initiation [29•, 42]. In the AIDS Clinical Trials Group A5164 strategy trial to determine when to initiate ART, starting ART at 2 weeks versus 6 weeks did not alter the risk of cryptococcal IRIS among the 38 patients with cryptococcal meningitis [49]. The timing of ART initiation is the subject of an ongoing National Institutes of Health strategy trial (NCT01075152) testing whether early ART initiation (1–2 weeks after the diagnosis of cryptococcal meningitis) results in better survival than outpatient ART initiation at approximately 5 weeks after diagnosis. Based on the natural history of survival following cryptococcal meningitis without ART, delaying ART initiation beyond 6 to 8 weeks is probably not warranted as a preventive measure against CM-IRIS, particularly for those at low risk of CM-IRIS. Even in patients at high risk for IRIS, the onset of IRIS is often delayed, with a median time of onset of approximately 5 to 8 weeks after ART initiation (interquartile range, of 3–4 to 9–13 weeks) in three prospective African cohorts [28•, 44•, 50•]. Thus, enhanced antifungal therapy has potential time to be effective even within the normal timing of ART initiation.
Prophylactic Anti-inflammatory Therapy
A second option with unknown efficacy could be the use of prophylactic anti-inflammatory medications to prevent CM-IRIS. Such therapies are purely speculative. As a paucity of CSF inflammation was associated with IRIS [29•, 30•], there is a potential danger in suppressing early inflammation. Some inflammation is necessary to clear antigen, and one may inadvertently increase the risk of CM-IRIS by suppressing antigen clearance. Preventive anti-inflammatory therapy would require careful clinical trials to assess safety and efficacy.
Future Directions
The theory of IRIS pathogenesis presented here will require clinical validation in multicenter studies. Further investigation by interpreting cytokines and chemokines in the context of the cellular environment (i.e. flow cytometry experiments) and leukocyte gene expression would strengthen the present theory. More robust experimental methods will also be necessary to answer fundamental questions about IRIS. For example, what is the contribution to IRIS of exogenous factors (cryptococcal virulence traits and components) versus endogenous factors (immune abnormalities, host genetics)? Genotype and phenotype data from clinical cryptococcal isolates and ex vivo cryptococcal antigen stimulations from patients could help dissect these intricate processes. Although the current IRIS model assumes HIV infection to be a stochastic process, this is likely an oversimplification. IRIS is a clinical spectrum, and risk is influenced by multiple factors, which include the immune damage caused by HIV. Answering a question of this complexity will require subtantial effort and most likely animal models which are representative of human IRIS. In summary, observational cytokine data collected from a prospective cryptococcal cohort undergoing immune reconstitution offers the first immunologic link between cryptococcosis and cryptococcal IRIS. However, strong collaborations between basic science and clinical researchers will be necessary if our understanding of IRIS is to progress to the point of providing useful clinical methods of prevention or intervention.
Conclusions
ART-associated immune recovery of persons living with AIDS is often confounded by cryptococcal-related paradoxical IRIS. The etiology of IRIS is incompletely understood, yet several key clinical and epidemiological observations have allowed researchers to begin to elucidate IRIS pathogenesis. This article provides a brief review of Cryptococcus microbiology and immunology, as well as an extensive summary of recently published IRIS data, and this information was formulated into a confluent theory of IRIS pathogenesis. In short, we postulate that IRIS is the product of three distinct immunologic phases. First, during acute infection before starting ART, the periphery and site of disease (i.e. CNS) of persons who eventually develop IRIS is relatively lacking in the inflammatory cytokines one would expect to be necessary for efficient organism and antigen clearance. Second, as the immune system begins its restoration with ART, persons later developing IRIS appear to mount pro-inflammatory signaling responses to the persistent antigen, but these signals are not accompanied by appropriate effector responses to clear antigen. Third, the continued inability to clear antigen, the mounting inflammation, and the belated effector response eventually culminate in an acute, clinically apparent IRIS event. Thinking of IRIS pathogenesis as an immunologic and temporal spectrum also allows for practical clinical interventions, such as: risk stratification, customized cryptococcal meningitis therapy, optimizing timing of ART, and potentially anti-inflammatory prophylaxis. Despite the many questions of IRIS pathogenesis remaining unanswered, this review hopes to establish a foundation upon which clinical and basic science researchers can build upon.
Acknowledgments
Financial support is received from National Institutes of Health (K23AI073192-02: DRB; U01AI089244-01; DLW). Dr. Boulware thanks collaboration with Drs. Paul Bohjanen, David Meya, Andrew Kambugu, Edward Janoff, Tihana Bicanic, and the Infectious Disease Institute of Makerere University, Kampala, Uganda. We thank Dr. Bicanic for critical review of the manuscript.
Footnotes
Disclosure
Conflicts of Interest: D. Wiesner: none; D. Boulware: research support from GlaxoSmithKline’s HIV Collaborative Investigator Research Award and Merck’s Investigator-Initiated Studies Program; both of these firms manufacture HIV antiretroviral medications.
Contributor Information
Darin L Wiesner, Division of Infectious Disease & International Medicine, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
David R. Boulware, CTropMed, University of Minnesota, MTRF 3-222, 2001 6th Street SE, Minneapolis, MN 55455, USA.
References and Recommended Reading
Recently published papers of interest have been highlighted as
• Of importance
•• Of major importance
- 1.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507–44. v–vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Perfect JR. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol Med Microbiol. 2005;45:395–404. doi: 10.1016/j.femsim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–17. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 4.Chaka W, Verheul AF, Vaishnav VV, Cherniak R, Scharringa J, Verhoef J, et al. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997;65:272–8. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, Rhodes JC. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–23. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–12. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrella D, Cherniak R, Strappini C, Perito S, Mosci P, Bistoni F, et al. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect Immun. 2001;69:2808–14. doi: 10.1128/IAI.69.5.2808-2814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frases S, Nimrichter L, Viana NB, Nakouzi A, Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell. 2008;7:319–27. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yauch LE, Mansour MK, Shoham S, Rottman JB, Levitz SM. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect Immun. 2004;72:5373–82. doi: 10.1128/IAI.72.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yauch LE, Mansour MK, Levitz SM. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun. 2005;73:8429–32. doi: 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166:4620–6. doi: 10.4049/jimmunol.166.7.4620. [DOI] [PubMed] [Google Scholar]
- 12.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6:513–24. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 13.Kozel TR, Wilson MA, Pfrommer GS, Schlageter AM. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989;57:1922–7. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Asbeck EC, Hoepelman AI, Scharringa J, Herpers BL, Verhoef J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008;8:229. doi: 10.1186/1471-2180-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozniak KL, Levitz SM. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun. 2008;76:4764–71. doi: 10.1128/IAI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. Independent association between rate of clearance of infection and clinical outcome of HIV associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49:702–9. doi: 10.1086/604716. This article reports on the important association between survival and the quantitative rate of cryptococcal clearance from the CSF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–50. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis J, Meintjes G, Rebe K, Williams N, Bicanic T, Williams A, et al. Adjunctive IFN-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. Abstract 40. Presented at the Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA. Feb 28, 2011. [Google Scholar]
- 20.Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77:3450–7. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–77. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 22•.Stenzel W, Muller U, Kohler G, Heppner FL, Blessing M, McKenzie AN, et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol. 2009;174:486–96. doi: 10.2353/ajpath.2009.080598. Using an in vivo murine model, this article demonstrates that Th2 responses are nonprotective in cryptococcosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelante T, De Luca A, D’Angelo C, Moretti S, Romani L. IL-17/Th17 in anti-fungal immunity: what’s new? Eur J Immunol. 2009;39:645–8. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 25.Hardison SE, Wozniak KL, Kolls JK, Wormley FL., Jr Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun. 2010;78:5341–51. doi: 10.1128/IAI.00845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 28•.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. This article details a prospective Ugandan cohort and the evolving pattern of serum cytokines and chemokines over time that are associated with IRIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962–70. doi: 10.1086/655785. This article reports on the inflammatory cytokines and chemokines present in the CSF of persons with and without IRIS at the time of their initial infection and at the time of IRIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Jarvis J. CSF cytokine profiles in patients with HIV-associated cryptococcal meningitis: correlates with clinical outcome. Presented at the 8th International Conference on Cryptococcus and Cryptococcosis; Charleston, SC. May 4, 2011; This abstract presents confirmatory data on CSF cytokine profiles; an initial paucity of inflammation was associated with later IRIS. [Google Scholar]
- 31.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–7. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida GM, Andrade RM, Bento CA. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4(+) T cells in a dominant Th2 pattern. J Immunol. 2001;167:5845–51. doi: 10.4049/jimmunol.167.10.5845. [DOI] [PubMed] [Google Scholar]
- 34.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C. B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–9. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 35.Gilliet M, Liu YJ. Human plasmacytoid-derived dendritic cells and the induction of T-regulatory cells. Hum Immunol. 2002;63:1149–55. doi: 10.1016/s0198-8859(02)00753-x. [DOI] [PubMed] [Google Scholar]
- 36.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale DC, Liles WC, Llewellyn C, Price TH. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) on neutrophil kinetics and function in normal human volunteers. Am J Hematol. 1998;57:7–15. doi: 10.1002/(sici)1096-8652(199801)57:1<7::aid-ajh2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.van Pelt LJ, Huisman MV, Weening RS, von dem Borne AE, Roos D, van Oers RH. A single dose of granulocyte-macrophage colony-stimulating factor induces systemic interleukin-8 release and neutrophil activation in healthy volunteers. Blood. 1996;87:5305–13. [PubMed] [Google Scholar]
- 39.Chen G-H, Olszewski MA, McDonald RA, Wells JC, Paine R, III, Huffnagle GB, et al. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am J Pathol. 2007;170:1028–40. doi: 10.2353/ajpath.2007.060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 41.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 42.Kambugu A, Meya DB, Rhein J, O’Brien M, Janoff EN, Ronald AR, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 44•.Chang CC. Patients with cryptococcal meningitis who attain CSF sterility pre-ART commencement experience improved outcomes in the first 6 months. Presented at the 8th International Conference on Cryptococcus and Cryptococcosis; Charleston, SC. May 5, 2011; This abstract presents convincing data from a prospective cohort on the importance of CSF culture sterility before ART initiation and/or before fluconazole dosing is reduced to fungistatic levels. [Google Scholar]
- 45.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bicanic T, Jarvis J, Loyse A, Jackson A, Muzoora C, Wilson D, et al. Determinants of acute outcome and long-term survival in HIV-associated cryptococcal meningitis: Results from a combined cohort of 523 patients [Abstract 892]. Presented at the Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA. March 3, 2011. [Google Scholar]
- 47.Bahr N, Rolfes MAR, Musubire A, Nabeta H, Lo M, Meya DB, et al. The impact of routine electrolyte supplementation during amphotericin induction therapy in resource-limited settings. Presented at the 8th International Conference on Cryptococcus and Cryptococcosis; Charleston, SC. May 4, 2011. [Google Scholar]
- 48.Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–61. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 49.Grant PM, Komarow L, Andersen J, Sereti I, Pahwa S, Lederman MM, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS One. 2010;5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr. 2009;51:130–4. doi: 10.1097/QAI.0b013e3181a56f2e. This cohort is the first prospective study to detail the incidence of Cryptococcus-related IRIS in sub-Saharan Africa. [DOI] [PubMed] [Google Scholar]