Abstract
A core component of cognitive control – the ability to regulate thoughts and actions in accordance with internally represented behavioral goals – might be its intrinsic variability. In this article, I describe the dual-mechanisms of control (DMC) framework, which postulates that this variability might arise from qualitative distinctions in temporal dynamics between proactive and reactive modes of control. Proactive control reflects the sustained and anticipatory maintenance of goal-relevant information within lateral prefrontal cortex (PFC) to enable optimal cognitive performance, whereas reactive control reflects transient, stimulus-driven goal reactivation that recruits lateral PFC (plus a wider brain network) based on interference demands or episodic associations. I summarize recent research that demonstrates how the DMC framework provides a coherent explanation of three sources of cognitive control variation – intra-individual, inter-individual, and between-groups – in terms of proactive vs. reactive control biases.
Shifting the emphasis to variability in cognitive control
One of the most fascinating mysteries of human cognition is the capacity for cognitive control: the ability to regulate, coordinate, and sequence thoughts and actions in accordance with internally maintained behavioral goals. Although it is clearly the case that substantial theoretical and experimental progress has occurred in the past 20 years regarding the mechanisms that enable cognitive control [1-7], there is still a great deal that remains poorly understood, subject to debate, and without clear consensus among investigators working in this field
The majority of research efforts in this field have focused on accounting for the diversity, scope and range of cognitive control functions in terms of an ever-expanding conceptual taxonomy or fine-grained anatomically-oriented fractionation scheme [8-15]. In this article, instead, I discuss the Dual Mechanisms of Control (DMC) framework [16, 17], which shifts the emphasis towards an exploration and appreciation of the intrinsic variability that may in fact be a core component of cognitive control, and a means of potentially capturing and explaining this variability in terms of the temporal dynamics of control processes.
The main tenet of the DMC account, first described in detail in [16], posits variation between two qualitatively distinct control modes. In the sections that follow, I lay out the DMC account, and draw upon recent research to demonstrate how it provides a coherent explanation of three empirically observed sources of variation in cognitive control function: intra-individual (i.e., state or task-related), inter-individual (i.e., trait-related), and between-groups (i.e., related to changes in brain function or integrity in different populations).
The Dual Mechanisms of Control Framework
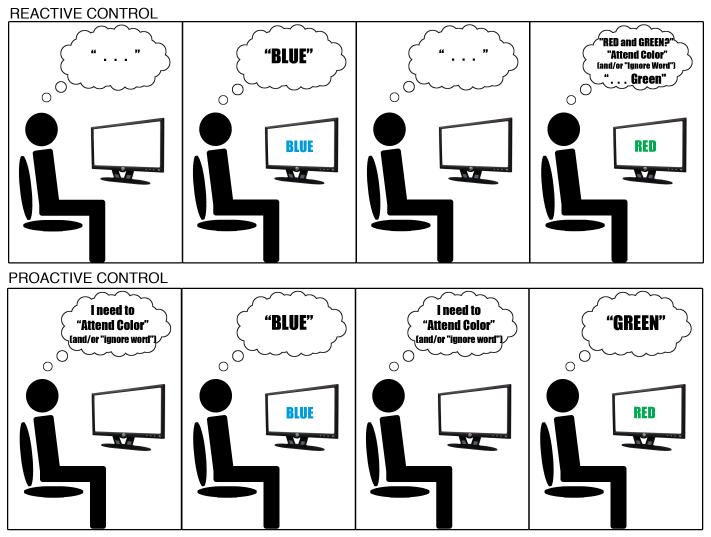
The central hypothesis of the DMC framework is that cognitive control operates via two distinct operating modes – proactive control and reactive control. The proactive control mode can be conceptualized as a form of “early selection,” in which goal-relevant information is actively maintained in a sustained manner, prior to the occurrence of cognitively demanding events, in order to optimally bias attention, perception and action systems in a goal-driven manner [1]. In contrast, in reactive control, attention is recruited as a “late correction” mechanism that is mobilized only as needed in a just-in-time manner, such as after a high interference event is detected [18]. Thus, proactive control relies upon the anticipation and prevention of interference before it occurs, whereas reactive control relies upon the detection and resolution of interference after its onset (see Figure 1).
Figure 1.
Conceptual distinction between reactive and proactive control, as illustrated in the classic Stroop color-naming task. Upper panel illustrates reactive control, which relies upon detection of interference (occurring in the last panel following presentation of an incongruent stimulus), to drive reactivation of task-goals and enable successful responding (albeit with slower responding). In this control mode, task goals are not actively maintained during inter-trial periods (first and third panels), and may not be triggered following presentation of congruent stimuli. Lower panel illustrates proactive control, which does involve sustained active maintenance of task goals during inter-trial intervals (first and third panels), and results in less conflict experienced during presentation of incongruent stimuli (last panel). It is important to note, that the representation of task goals is illustrated in this manner purely for ease of description. The DMC framework makes no claims about whether these involve verbal coding or are consciously accessible.
The DMC account provides a strong prediction about the dynamics and location of brain activity under proactive versus reactive control. Proactive control should be associated with sustained and/or anticipatory activation of lateral prefrontal cortex (PFC), which reflects the active maintenance of task goals. This goal maintenance activity serves as a source of top-down bias that can facilitate processing of expected upcoming events that have a high cognitive demand. In contrast, reactive control should be reflected in transient activation of lateral PFC, along with a wider network of additional brain regions. This transient activity might reflect the bottom-up reactivation of task goals, mediated either via the detection of interference (e.g., through the engagement of conflict monitoring regions such as the anterior cingulate cortex (ACC); [5]) or via associative and episodic associations (as might occur through posterior cortical or medial temporal lobe regions). Additionally, the two control mechanisms should differ in terms of the involvement of the dopaminergic (DA) system. The ability to actively sustain inputs in PFC requires a phasic DA-mediated gating signal occurring at the time when contextual cues are presented [19, 20]. Without such a gating signal, PFC can only be transiently activated.
Because both reactive and proactive cognitive control are postulated to be associated with complementary advantages and limitations (see Box 1), successful cognition likely depends upon some mixture of both strategies. Indeed, it may be the case that the two systems are at least semi-independent, and thus may be both engaged simultaneously. Nevertheless, there is likely to be some bias favoring one type of control strategy over the other. These factors can be characteristics of the task situation, but may also be characteristics of the individual. Indeed, the central aspect of the DMC account is that it provides a unifying framework for understanding both intra-individual and inter-individual variability in cognitive control function, as well as the changes in cognitive control that may be present in different populations, such as children and older adults, and groups with specific neuropsychiatric disorders.
BOX 1: Why dual mechanisms?
A key question that arises when considering these alternative modes of control, is what is the advantage of having a dual-mode system, given that it is less parsimonious than a single-mode system? This question can be answered by considering that there may be both costs and benefits associated with proactive and reactive control, such that a computational tradeoff exists. Thus, on purely computational grounds, it is sensible to argue that in the face of such trade-offs, a dual-process control mechanism is one that best optimizes information processing across the widest range of situations. Consider the following trade-offs.
Under proactive control, goal representations are triggered in advance of their implementation, and maintained continuously during periods in which they are required, thus optimizing preparation while minimizing interference from internal or external sources of distraction. Consequently, the advantage of proactive control is that plans and behaviors can be continually adjusted to facilitate successful completion of the goal. However, the disadvantage of a proactive control strategy is that it is strongly resource consuming, requiring continuous goal maintenance. Given the clear and strong capacity limitations of goal representation in the focus of attention [48-50], the engagement of proactive control will substantially reduce available capacity for maintenance of other information that could be held in working memory. In contrast, under reactive control, goal representations are only activated (or re-accessed) at the time in which they are needed. The advantage of this control strategy is that it is computationally efficient, such that during the interval between when the intention is formed and completed, resources are freed up, such that other tasks and goals can be carried out more effectively. However, the disadvantage of this strategy is that it requires repeated re-activation of the goal, rather than continuous maintenance. Thus, there is greater dependence on the trigger events themselves, since if these are insufficiently salient or discriminative they will not drive re-activation.
A second form of tradeoff between proactive and reactive control is the attentional commitment required. The continuous maintenance of internal goals implements a form of sustained mental set that makes cognitive processing more brittle, and hence less sensitive to other unexpected but potentially relevant sources of bottom-up information (e.g., changing environmental contingencies). Relatedly, the engagement of proactive control is dependent on the availability of contextual cues that are strong and reliable enough to trigger goal activation and maintenance in advance of the time when those goals are needed. In contrast, because reactive control is stimulus-driven and transient, it is by definition not dependent on advance contextual cues, and makes greatly reduced demands on attentional resources and commitment. However, because this form of control is stimulus-dependent and late-acting, it will be much more vulnerable to transient attentional capture or orienting effects that may disrupt the ability to trigger goal re-activation when necessary. In additional, reactive control will be reliant on strong bottom-up associative cues that enable stored goals to be retrieved and re-accessed, or on conflict detection mechanisms that signal when control needs to be rapidly mobilized.
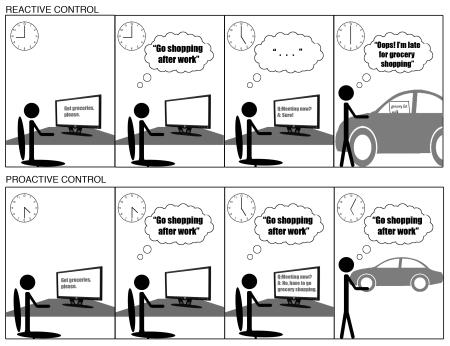
A concrete example may make these contrasts more clear. Imagine a typical prospective memory situation [51], in which an intention is formed about a behavioral goal to be completed at some later point, such as stopping at the grocery store to go shopping before going home from work (see Box 1 Figure). A proactive control strategy would require the goal information to be actively sustained from the time the intention is formed until the goal is satisfied (e.g., the end of the day). In contrast, with a reactive control strategy the shopping goal would only be transiently activated at the time of intention (e.g., earlier in the day), and then be re-activated again by an appropriate trigger event (e.g., noticing the shopping list left on the car seat). In this example, a situational factor, such as the expected duration over which the intention would need to be actively maintained, might be important in determining which is the most useful control strategy. If the duration is short (e.g., the intention is formed close to the end of the day), continuous maintenance of the cognitive goal could be achieved and might be used to strongly constrain behavior during that period (e.g., not scheduling a late meeting; ensuring that the route to the store, rather than home, is followed). In contrast, if the delay is long, continuous goal maintenance may be impractical and too consuming of cognitive resources that could be deployed elsewhere. Because of the complementary computational tradeoffs between proactive and reactive control, a range of variables and factors could bias which strategy is preferred in different task situations, and for different individuals.
Box 1 Figure. Tradeoffs between reactive and proactive control in everyday situations. Illustration of a prospective memory situation, in which an individual forms an intention to go grocery shopping after work. Top panel indicates a reactive control strategy that involves representation and then storage of the intention after it is first formed (here, in the morning). As a consequence, the intention may not be accessible when scheduling other activities (e.g., a late meeting), and would only be retrieved by a salient trigger event (e.g., when the grocery list is noticed in the car). Lower panel indicates proactive control strategy, which may only be feasible when there is a short delay between intention formation and implementation (i.e., the intention is formed in the late afternoon). However, the advantage of proactive control is that continuous access to task goals may bias the scheduling of activities (i.e., avoiding late meetings), so as to facilitate successful task completion.
Intra-individual variation
A central assumption of the DMC framework is that a change in situational factors will result in alteration of the weighting between proactive and reactive control strategies. Thus, the DMC account naturally leads to the idea that potentially subtle differences between otherwise similar tasks might lead to significant changes in an individual’s preferred cognitive control strategy. These control mode differences would be expected to result in shifts in both behavioral performance characteristics and in brain activation profiles. Thus, we have utilized a research strategy of directly manipulating factors expected to influence the preferred mode of cognitive control during tasks with high control demands.
As an example of this approach, in one recent study, Burgess and Braver [21] focused on shifts in cognitive control mode that might be utilized to deal with interference during working memory, according to whether such interference can be anticipated or not. This issue was investigated by using a recently popularized working memory paradigm, known as the recent probes task [22], since prior studies with this paradigm have reliably observed activity in left inferior PFC occurring selectively following the presentation of high interference probes (recent negatives), suggesting the presence of a reactive control mechanism [23]. Participants performed the recent probe task under conditions of high and low interference expectancy. In the low expectancy condition, recent negative probes occurred only rarely, while in the high expectancy condition they were frequent (note that other aspects of trials were also varied across conditions to compensate for the recent probe manipulation and actually served to make the expectancy effect very subtle). The expectancy manipulation led to a shift in the temporal dynamics and specificity of lateral PFC activation, in accordance with predictions of the DMC framework. Specifically, in the low expectancy condition, we replicated prior results by demonstrating that left inferior PFC (as well as other medial and lateral PFC regions), exhibited a probe-triggered increase in activity, specifically on recent negative probe trials, consistent with recruitment of reactive control (Figure 2A). In contrast, in the high expectancy condition, lateral PFC activity (in adjacent regions) increased during the delay period, prior to probe onset, with this effect occurring globally, i.e., on all trials (Figure 2A). In other words, when expectancy is high, proactive control is recruited instead.
Figure 2.
Shifts in temporal dynamics of lateral PFC activity reflecting associated with different sources of variation. A. Intra-individual (state-related) variation due to manipulation of interference expectancy during the recent probes working memory interference task. Low interference expectancy conditions were primarily associated with interference-effects at the time of the probe (blue region and blue solid bar), reflecting reactive control. However, in high expectancy conditions, probe-related activation was decreased (blue hatched bar), while in adjacent regions delay-related activation increased (orange region and bars), indicating an anticipatory and global (i.e., present on all trials) proactive control effect. Adapted from Burgess & Braver (2010). B. Inter-individual variation due to trait reward sensitivity during working memory. Task performance under reward motivation conditions was associated with an increase in sustained and early-trial early-trial transient activity (potentially reflecting across-trial maintenance of task goals and encoding/updating of working memory information), but a decrease in late-trial transient activity (potentially reflecting probe-related processing), consistent with a shift towards proactive control. The effects were observed much more prominently in highly reward sensitive individuals. Adapted from Jimura et al (2010). C. Between-groups variation and training effects observed in schizophrenia patients on the AX-CPT context processing task. Prior to cognitive training, schizophrenia patients showed reduced cue-related activity, but increased probe-related activity, indicating a differential reliance on reactive control. However, following extensive strategy training with the task, normalization of activation dynamics (and task performance) was observed. Adapted from Edwards et al (2010).
Other studies have shown similar findings when using distinct manipulations to induce shifts in cognitive control mode, or when exploring such effects within different task domains. For example, in another study of working memory, expected working memory load was manipulated across conditions, rather than interference expectancy [24]. The hypothesis was that when the expected load was low participants would be biased to adopt a proactive control strategy, using the items maintained in working memory to prepare for the upcoming probe. In contrast, when load was expected to be high (and beyond working memory capacity) participants would instead utilize the probe as a retrieval cue from which to query memory. Indeed, a distinct set of brain regions and activity dynamics were observed across conditions, even when considering trials that were matched on actual load. Specifically, in the low load condition, an anticipatory, proactive pattern was observed, with activity increasing during the delay period; in the high load condition, the pattern was more reactive, with downward ramping delay activity, but increased activation when the probe was presented. In studies of cued task-switching, activity dynamics within the same lateral PFC region has been found to shift on a trial-by-trial basis along with presumed shifts from reactive to proactive control. For example, on trials emphasizing high accuracy and speed (through motivational incentives) cue-related activation of a region of left dorsolateral PFC was increased, relative to intermixed low-incentive trials [25]. Such trial-by-trial shifts in control mode might even occur spontaneously: in another study, it was found that task-switching trials associated with fast performance were marked by increased cue-related and reduced probe-related activity within left lateral PFC regions compared to intermixed trials associated with slow performance [26]. In all of these studies, the DMC framework helps provide a unifying explanation, by interpreting the effects of subtle experimental manipulations on brain activation dynamics in terms of a shift in the relative utilization of proactive versus reactive control mechanisms.
Inter-individual variation
A second assumption of the DMC framework is that there may be stable individual difference factors that lead to biases in whether proactive or reactive control is the preferred mode in performing tasks with high cognitive control demands. The key insight underlying this assumption is that the utilization of proactive control will be related to cost/benefit tradeoffs that relate both to the efficacy or ease of actively maintaining goal representations in advance of their utilization, as well as to internal estimates of how beneficial or valuable are the consequences of such a control strategy for task performance. Thus, cognitive individual differences such as working memory capacity and fluid intelligence should impact the utilization of proactive control, potentially because they reflect the ease or efficacy of active goal maintenance in working memory, as has been suggested by previous investigators [27, 28]. Consistent with this hypothesis, in the recent probes study described above [21], individuals with higher fluid intelligence showed increased evidence of PFC activation dynamics associated with proactive control (i.e., delay-related activation), while individuals with low fluid intelligence showed a contrasting pattern of increased reactive control (i.e., probe-triggered activation on interference trials).
Perhaps more surprisingly, the DMC framework suggests that affect-related traits (e.g., personality factors) could also influence which cognitive control mode is preferred. These traits are not postulated to impact the efficacy of goal maintenance, but rather may impact estimates of the value of relative costs and benefits that proactive vs. reactive strategies have for on-going behavioral performance. To investigate this hypothesis, we have also directly examined the role of personality-related individual differences in explaining between-subject variation in neural and behavioral signatures of proactive vs. reactive control. As an example, Jimura et al [30] recently examined whether the personality trait of reward sensitivity [29] might explain individual differences in the utilization of proactive control, when performing difficult cognitive tasks in rewarding motivational contexts. The authors hypothesized that highly reward sensitive individuals might estimate successful behavioral performance to be especially valuable in contexts in which it is associated with reward attainment. Thus, in these contexts they would be expected to be preferentially motivated to adopt a proactive cognitive control strategy, in order to optimize their performance.
Participants were asked to perform a high-load working memory task both under baseline conditions and conditions in which performance-contingent monetary rewards were offered on a subset of trials [30]. In all participants, task performance was improved in the reward context (even on the non-rewarded trials) relative to baseline, but the largest effects were observed in individuals showing high reward sensitivity. These behavioral effects were also reflected in terms of context-related shifts in lateral PFC activation dynamics. Consistent with the hypothesis that the reward context was associated with a shift towards proactive control, there was an increase in both sustained (i.e., persisting across trials) and anticipatory (encoding and delay-related) activation within right dorsolateral PFC in this condition, but a decrease in activity during the probe period, when reactive control processes might occur (Figure 2B). More importantly, this PFC activation shift was most prominent in highly reward sensitive individuals (Figure 2B), and was found to statistically mediate the relationship between trait reward sensitivity and reward-related improvements in performance. Interestingly, trait reward sensitivity has been found to be associated with behavioral and neural signatures of proactive control in similar studies manipulating reward contexts but involving different tasks and domains, such as the AX-CPT [31] and cued task-switching [32]. Together, the results suggest that individual differences in trait reward sensitivity help explain between-individual variation in the tendency to adopt a proactive control strategy, but particularly under cognitive task conditions having high reward motivational value.
Trait reward sensitivity is not the only affect-related individual difference factor that has been found to explain between-individual variation in reactive vs. proactive control. For example, threat sensitivity also appears to predict behavioral signatures of proactive control under conditions in punishment-oriented motivational contexts [32]. In contrast, trait (and state) anxiety was associated with a neural signature of increased reactive control during the N-back working memory task, i.e., reduced sustained but increased transient activity (particularly on high interference lure trials) in lateral PFC and the rest of the brain cognitive control network [33]. The relationship between anxiety and reactive control makes sense from the DMC perspective, if anxiety is associated with a reduction in the capacity to actively maintain cognitive task goals in working memory because this capacity is taken up with a sustained internal attentional focus towards task unrelated thoughts (i.e., worries and rumination) or an external focus towards unpredictable threats in the environment.
Between-Group Variation
A final assumption of the DMC framework is that the differences between proactive and reactive control modes might be important for understanding the variation in cognitive control functions observed in different clinical and developmental populations or groups (e.g., individuals with schizophrenia, older adults, etc). In particular, rather than making the simpler hypothesis that these populations have global impairments in cognitive control, we instead suggest that they might show differential reliance on reactive versus proactive control. This hypothesis motivates a more nuanced and fine-grained analysis of cognitive control function in these different groups, and further, may provide more appropriate targets for cognitive intervention.
The AX-CPT context processing task has become a popular paradigm for examining changes in the use of proactive and reactive control in different populations. In the AX-CPT, certain probe trials (termed BX) evoke dominant, but inappropriate response tendencies that may require reactive control to over-ride. In contrast, preceding contextual cues produce expectancies regarding the upcoming probes that can be used for proactive control. Proactive control is beneficial for BX probes, but is actually detrimental to performance on another probe type (AY), because on these the cue-triggered expectancy is invalid. In studies conducted in a variety of populations, including older adults, young children, and individuals with schizophrenia, a similar pattern of impaired BX performance, but relative sparing on AY trials is observed [34-36]. This suggests an impairment in the use of proactive control in these groups. Interestingly, however, in some groups (e.g., older adults), the BX impairment is expressed primarily in terms of response slowing rather than elevated errors [37], suggesting that reactive control may be relatively intact. This hypothesis has been supported by brain imaging studies in both older adults and individuals with schizophrenia, that have observed reduced cue-related activation of lateral PFC but at the same time increased probe-related activation, particularly for BX probes [38, 39] (see Figure 2C). These changes in both cue and probe-related activity have been observed within the same lateral PFC regions – consistent with a shift towards more reactive control. The within-region shift in activation dynamics is also critical because it rules out a simple hypothesis that cognitive control is generically impaired, as well as alternative methodologically-based interpretations (e.g., reduced hemodynamic response, increased variability in neural activity, or other sources).
The DMC framework has proved useful for exploring cognitive control changes, not just in older adults and individuals with schizophrenia, but in a range of other populations as well, including children [35, 40] and adolescents [41, 42], expert video game players [43], ADHD [44], and individuals with depressed mood [45]. Although many of these studies have also used AX-CPT to investigate proactive and reactive control, other studies have employed different tasks and paradigms, including the Stroop and n-back [33, 44, 45], and also have used ERP in addition to behavioral or fMRI methods [43, 46]. The range of populations and research approaches used in conjunction with the DMC framework suggests that it may have wide applicability for understanding the diversity of cognitive control processes. Moreover, the more nuanced conceptualization of cognitive control provided by the DMC framework may provide a clearer target for intervention strategies. Specifically, it suggests that proactive control will only be utilized if the cost/benefit tradeoff is favorable, and if the salience and efficacy of this control mode is appreciated. Likewise, the DMC framework makes clear that increased utilization of proactive control will not only result in performance enhancements, but could also result in some types of decrements as well (e.g., when preparation is based on misleading contextual expectancies, such as on AY trials in the AX-CPT). Finally, it is clear that a shift towards proactive control might result in a reduced need to utilize reactive control processes, suggesting that interventions aimed at enhancing proactive control might find other-wise counter-intuitive evidence of reduced control engagement in situations typically dominated by high reactive control.
These intervention-related components of the DMC framework have recently been tested in studies aimed at increasing proactive control in the AX-CPT task [17, 39]. Here, proactive control was explicitly trained by calling attention to the importance of contextual cue information, and by highlighting how such information could be used to generate proactive expectancies regarding the responses to upcoming probes. In one study with older adults, progressive training and practice with these strategies led to a shift in AX-CPT performance with performance improvements observed on BX trials, but actually worse (but theoretically predicted) performance on AY trials [47]. This behavioral shift was accompanied by (and statistically associated with) a shift in lateral PFC activity dynamics, in which cue-related activation was increased following training, while probe-related activity actually decreased. A very similar pattern of results was also observed in a study conducted in individuals with schizophrenia [39] (Figure 2C). The findings from this work can be easily interpreted within the DMC framework as reflecting a shift from reactive to proactive control. However, without the benefit of the framework, the results might have otherwise been puzzling and hard to interpret.
Concluding remarks
In this article, I have attempted to lay out a potentially effective framework for understanding the variable nature of cognitive control mechanism. The critical insight of the framework is an appreciation of the fact that variability might be an intrinsic component of the mechanisms of cognitive control, that increases its effectiveness and applicability in dealing with the fluctuating and dynamic nature of both internal physiological states and external environmental constraints. The DMC framework may provide a unifying explanation of how even subtle experimental manipulations can have potentially strong influences on the deployment of cognitive control, and the dynamics of brain regions engaged. Additionally, I have suggested that the framework generates specific intuitions and predictions regarding the central nature of trait-like individual differences in modulating cognitive control function, not only standardly accepted cognitive traits, such as working memory capacity and fluid intelligence, but also personality traits that are typically thought of as “non-cognitive”, such as reward and threat sensitivity. Finally, the framework has also proved to be useful in reconceptualizing the nature of cognitive impairment found in different populations – such as older adults and individuals with schizophrenia – not just as generic deficit in control function, but as a more specific shift from proactive to reactive control. This insight not only provides greater interpretational leverage regarding existing behavioral and neuroimaging findings, but it also creates new targets for intervention efforts to enhance cognitive control. Of course, there are currently limitations and central unresolved issues associated with the DMC framework that will need to be addressed in future investigations (see Box 2). I hope that other investigators interested in cognitive control will join in these research efforts.
BOX 2: Unresolved Issues and Future Directions.
As the prior sections illustrate, the DMC framework has proved to be a promising one for understanding intra-individual, inter-individual, and between-groups variation in the mechanisms of cognitive control. Nevertheless, there are a number of unresolved questions and issues regarding the framework that remain to be addressed:
Independent Mechanisms? The DMC framework postulates that proactive and reactive control may involve potentially independent mechanisms, though this has not been directly confirmed. A key question is whether there are experimental factors (or individual-difference factors) associated with an increased utilization of reactive control, without finding a linked decrease in proactive control. Examination of the Stroop task to address this theoretical question might be useful, since recent studies have found evidence of experimental manipulations that appear to selectively engage reactive control [52].
Neural Architecture. A primary claim of the DMC framework is that many PFC regions can show both proactive (sustained) and reactive (transient) dynamics depending on the specifics of task demands. Nevertheless, there may be anatomical constraints, given prior findings and theoretical models of functional specialization within lateral PFC [14, 15, 53], including accounts that postulate a posterior-anterior gradient that seems to align well with reactive-proactive distinctions (i.e., temporally extended and sustained representation in anterior PFC, more transient engagement in posterior PFC, [7]). Regions outside of lateral PFC may also play key roles that will need to be further clarified, such as that postulated for ACC in conflict detection process that initiate reactive control, but which might also facilitate a reactive-to-proactive shift [54, 55], or for associative and episodic retrieval mechanisms in cortical (e.g., parietal) and subcortical (e.g., hippocampal) brain regions that may provide another route for bottom-up triggering of reactive control [24].
Formal Mechanistic Models. There is strong need to formalize the DMC account within explicit computational models. A key question is to understand the mechanisms by which various task and individual difference factors can lead to shifts or fluctuations in control state, without recourse to a homunculus, that mysteriously “detects” demands for reactive or proactive control. We have made initial attempts in this regard, but these have been limited to specific task domains (e.g., task-switching; [56]) and experimental effects (e.g., Stroop proportion congruence; [55]); thus, more comprehensive and rigorous modeling efforts are needed.
Behavioral Markers. A key goal for future research will be to establish behavioral markers that provide robust and independent indices of both proactive or reactive control. In prior work, we have used a variety of markers including: AY vs. BX trials in the AX-CPT [17, 37], switch-costs in cued task-switching [26, 32], proportion congruence and related effects in the Stroop [55, 57], and “recent negative” interference in item recognition working memory [21]. However, given that no task or measure is “process pure”, a latent variable approach might be the most fruitful, involving multiple behavioral indices, along with correlational or more advanced statistical techniques (e.g. structural equation modeling; e.g., [9]) to establish that these indices tap into shared and dissociable variance components associated with proactive and reactive control.
Sources of Individual Difference. A tantalizing possibility, hinted at by the DMC framework, is that there may be an individual difference dimension that reflects a stable trait-like tendency to prefer reactive or proactive control. One promising route may be to look for this individual difference factor might in terms of normal genetic variation, reflecting the fact that the cost-benefit tradeoffs associated with proactive and reactive might be adaptively optimized to certain environmental niches or have other selection advantages [58]. The COMT allele is a promising candidate genetic mechanism of this sort, since prior work suggest COMT variation might relate to variation in phasic vs. tonic dopamine actions in PFC that align well to computational distinctions we have posited between proactive and reactive control [59].
Acknowledgements
This work was supported by NIH R01 MH66078. Thanks to Greg Burgess, Koji Jimura and Bethany Edwards for their contributions to the work described here, and to the rest of the Cognitive Control and Psychopathology lab for many fruitful discussions. Thanks to Carol Cox for assistance with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of Learning and Motivation. 2003;44:145–199. [Google Scholar]
- 3.Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The prefrontal cortex: Executive and cognitive functions. Oxford University Press; Oxford: 1996. pp. 87–103. [DOI] [PubMed] [Google Scholar]
- 4.O’Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314(5796):91–4. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- 5.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 6.Monsell S, Driver J. Attention and Performance. XVIII. MIT Press; Cambridge, MA: 2000. Control of cognitive processes. [Google Scholar]
- 7.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11(6):229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 9.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 10.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 11.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):901. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1456):781. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- 14.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Braver TS, Ruge H. Functional neuroimaging of executive functions. In: Cabeza R, Kingstone A, editors. Functional neuroimaging of cognition. MIT Press; Cambridge, MA: 2006. pp. 307–347. [Google Scholar]
- 16.Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway A, et al., editors. Variation in Working Memory. Oxford University Press; Oxford: 2007. pp. 76–106. [Google Scholar]
- 17.Braver TS, et al. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106(18):7351–6. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby LL, Kelley CM, McElree BD. The role of cognitive control: Early selection versus late correction. In: Chaiken S, Trope E, editors. Dual Process Theories in Social Psychology. Guildford; NY: 1999. pp. 383–400. [Google Scholar]
- 19.Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: The gating model. Progress in Brain Research. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- 20.Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and Performance XVIII. MIT Press; Cambridge, MA: 2000. pp. 713–738. [Google Scholar]
- 21.Burgess GC, Braver TS. Neural Mechanisms of Interference Control in Working Memory: Effects of Interference Expectancy and Fluid Intelligence. PLoS One. 2010;5(9):e12861. doi: 10.1371/journal.pone.0012861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139(1):181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 23.D’Esposito M, et al. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proceedings of the National Academy of Sciences USA. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speer NK, Jacoby LL, Braver TS. Strategy-dependent changes in memory: Effects on brain activity and behavior. Cogn Affect Behav Neurosci. 2003;3:155–167. doi: 10.3758/cabn.3.3.155. [DOI] [PubMed] [Google Scholar]
- 25.Savine AC, Braver TS. Motivated cognitive control: reward incentives modulate preparatory neural activity during task-switching. J Neurosci. 2010;30(31):10294–305. doi: 10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39(4):713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 27.Duncan J, et al. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 28.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin and Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 29.Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22(3):491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 30.Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proc Natl Acad Sci U S A. 2010;107(19):8871–6. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- 32.Savine AC, et al. Enhancement of cognitive control by approach and avoidance motivational states. Cogn Emot. 2010;24(2):338–356. doi: 10.1080/02699930903381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fales CL, et al. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 34.Braver TS, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 2001;130:746–763. [PubMed] [Google Scholar]
- 35.Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proceedings of the National Academy of Sciences. 2009;106(14):5529. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barch DM, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58(3):280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 37.Braver TS, et al. Context processing and context maintenance in healthy aging and early-stage dementia of the Alzheimer’s type. Psychology & Aging. 2005;20:33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Paxton JL, et al. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front Hum Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorsbach TC, Reimer JF. Developmental differences in cognitive control: Goal representation and maintenance during a continuous performance task. Journal of Cognition and Development. 2010 [Google Scholar]
- 41.Iselin AMR, DeCoster J. Reactive and proactive control in incarcerated and community adolescents and young adults. Cognitive development. 2009;24(2):192–206. doi: 10.1016/j.cogdev.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews-Hanna JR, et al. Cognitive Control in Adolescence: Neural Underpinnings and Relation to Self-Report Behaviors. PLoS One. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey K, West R, Anderson CA. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2010;47(1):34–42. doi: 10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 44.Burgess GC, et al. Attentional control activation relates to working memory in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67(7):632–640. doi: 10.1016/j.biopsych.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West R, Choi P, Travers S. The influence of negative affect on the neural correlates of cognitive control. International Journal of Psychophysiology. 2010;76(2):107–117. doi: 10.1016/j.ijpsycho.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Czernochowski D, Nessler D, Friedman D. On why not to rush older adultsórelying on reactive cognitive control can effectively reduce errors at the expense of slowed responses. Psychophysiology. 2010;47(4):637–646. doi: 10.1111/j.1469-8986.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paxton JL, et al. Effects of environmental support and strategy training on older adults’ use of context. Psychol Aging. 2006;21(3):499–509. doi: 10.1037/0882-7974.21.3.499. [DOI] [PubMed] [Google Scholar]
- 48.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 49.McElree B. Working memory and focal attention. Journal of Experimental Psychology: Learning, Memory and Cognition. 2001;27(3):817–835. [PMC free article] [PubMed] [Google Scholar]
- 50.Oberauer K. Access to information in working memory: exploring the focus of attention. J Exp Psychol Learn Mem Cogn. 2002;28(3):411–21. [PubMed] [Google Scholar]
- 51.Kliegel M, McDaniel MA. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives2008. Lawrence Erlbaum; [Google Scholar]
- 52.Bugg JM, Jacoby LL, Chanani S. Why it is too early to lose control in accounts of item-specific proportion congruency effects. Journal of Experimental Psychology: Human Perception and Performance. 2011;37(3):844. doi: 10.1037/a0019957. [DOI] [PubMed] [Google Scholar]
- 53.De Pisapia N, Slomski JA, Braver TS. Functional Specializations in Lateral Prefrontal Cortex Associated with the Integration and Segregation of Information in Working Memory. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- 54.Ullsperger M, King JA. Proactive and reactive recruitment of cognitive control: Comment on Hikosaka and Isoda. Trends Cogn Sci. 2010;14(5):191. doi: 10.1016/j.tics.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 55.De Pisapia N, Braver TS. A model of dual control mechanisms through anterior cingulate and prefrontal cortex interactions. Neurocomputing. 2006;69:1322–1326. [Google Scholar]
- 56.Reynolds JR, et al. Computational and neural mechanisms of task switching. Neurocomputing. 2006;69:1332–1336. [Google Scholar]
- 57.Bugg JM, et al. Revealing list-level control in the Stroop task by uncovering its benefits and a cost. 2011 doi: 10.1037/a0024670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braver TS, Cole MW, Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Current opinion in neurobiology. 2010;20(2):242–250. doi: 10.1016/j.conb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilder RM, et al. The Catechol-O-Methyltransferase Polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]