Abstract
There is a need for refinement of the current behavioral phenotyping methods for mouse models of genetic disorders. The current approach is to perform a behavioral screen using standardized tasks to define a broad phenotype of the model. This phenotype is then compared to what is known concerning the disorder being modeled. The weakness inherent in this approach is twofold: First, the tasks that make up these standard behavioral screens do not model specific behaviors associated with a given genetic mutation but rather phenotypes affected in various genetic disorders; secondly, these behavioral tasks are insufficiently sensitive to identify subtle phenotypes. An alternate phenotyping strategy is to determine the core behavioral phenotypes of the genetic disorder being studied and develop behavioral tasks to evaluate specific hypotheses concerning the behavioral consequences of the genetic mutation. This approach emphasizes direct comparisons between the mouse and human that facilitate the development of neurobehavioral biomarkers or quantitative outcome measures for studies of genetic disorders across species.
Keywords: Behavioral Endophenotype, Fragile X Premutation, Fragile X Syndrome, 22q11.2 Deletion Syndrome, Transgenic Mouse
I. Introduction
With the increasing sophistication of the genetic techniques used to develop mouse models of genetic disorders, it is imperative that the techniques used to elucidate the behavioral phenotype of these models evolve just as rapidly. Although there is a movement toward adopting standardized behavioral phenotyping protocols, to a large part neuroscientists evaluating mouse models of genetic disorders still lack the sensitive behavioral assays that are required to evaluate the core cognitive deficits present in genetic disorders. At present, mouse models, particularly those developed to study neurodevelopmental or other genetic disorders, demonstrate inconsistent phenotypes or lack behavioral phenotypes when tested using the most common behavioral tasks, including the water maze or fear conditioning (Baker et al., 2010; Bohlen et al., 2009; Cannon and Keller, 2006; Hasler et al., 2006; Kendler and Neale, 2010; Long et al., 2006; Manji et al., 2003; Paylor and Lindsay 2006; Rustay et al., 2003; Spencer et al., 2011; ; Weiser et al., 2005; Yan et al., 2004). Furthermore, it has been shown that minute differences in the protocols used for these common tasks across labs result in altered phenotypes as well (e.g., morris water maze, rotarod, etc; Crabbe et al., 1999; Crabbe and Wahlsten, 2003; Wahlsten, 1972a, 2001; Wahlsten et al., 2003a, 2003b, 2003d, 2003e, 2006).
Additionally, mouse models often demonstrate phenotypes that are not specifically associated with any genetic disorder in particular, but are more aptly described as shared clinical phenotypes similarly present across a wide array of disorders (e.g., general memory deficits, fear conditioning deficits). The interpretation of such inconclusive findings is often that the mouse model fails to recapitulate the phenotypes observed in patients (cf., Gottesman and Gould, 2003; Gould and Gottesman, 2006; Weiser et al., 2005). I propose that inconsistent behavioral results observed in mouse models do not infer the lack of cognitive impairments, but rather these “null” data reflect the insensitivity of the behavioral tasks commonly employed.
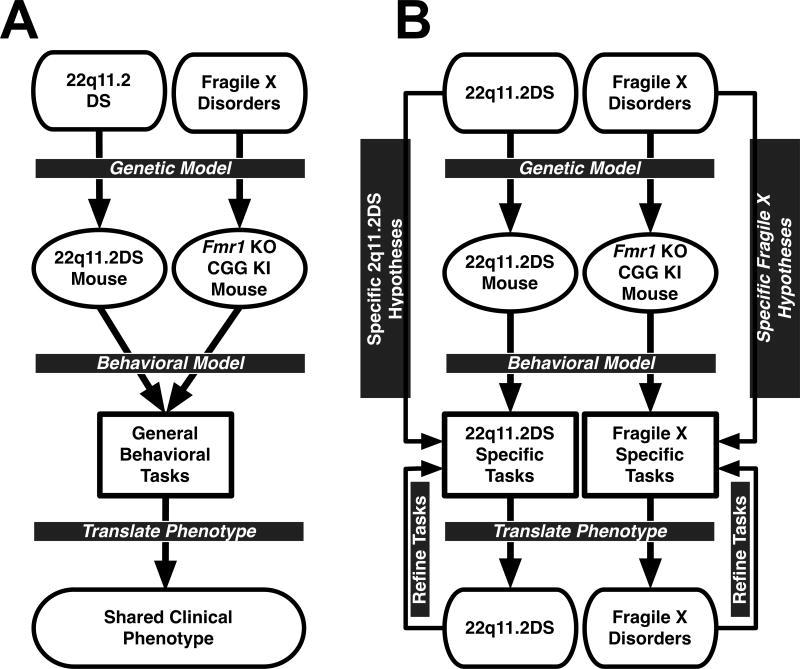
In situations where, based on standardized behavioral tasks, mouse models do not appear to specifically model clinical phenotypes observed in patient populations, one strategy is to evaluate intermediate- or endophenotypes associated specifically with the genetic mutation and subserved by neuroanatomical structures disrupted by the mutation (Figure 1; Karayiorgou et al., 2010; Simon, 2008, 2011). Endophenotypes are collections of quantitative traits hypothesized to represent risk for genetic disorders at more biologically (and empirically) tractable levels than the full clinical phenotype which often contains more profound deficits shared across numerous genetic disorders (Gould and Einat, 2007). This behavioral endophenotyping approach facilitates the identification of behavioral deficits that are specifically associated with both the specific genetic mutation and the pathological features observed in the clinical populations being modeled. When designed to evaluate specific disease related hypotheses, behavioral endophenotypes model quantitative patterns of behavioral deficits that scale with the size and/or severity of the genetic mutation (Gottesman and Gould, 2003; Gould and Gottesman, 2006; Hasler et al., 2006; Weiser et al., 2005).
Figure 1.
A. Diagram of standard behavioral phenotyping process in which different mouse models are given the same battery of tasks to define a behavioral phenotype. The outcome of the behavioral tasks are compared to the full clinical phenotype of the genetic disorders being modeled. This approach lacks the specificity and selectivity to identify phenotypes unique to a single disorder. B. Diagram of behavioral endophenotyping process in which disorder-specific hypotheses are used to develop unique batteries of behavioral tasks that directly translate to the phenotype of the clinical disorder. This approach does not model the general deficits seen across genetic disorders, but rather specifically identifies phenotypes known to be unique to the genetic disorder being modeled. Parallel examples of mice with fragile X-associated disorders and 22q11.2 deletion syndrome are given.
The behavioral endophenotyping process deviates from the current method for determining behavioral phenotypes. The present method (using behavioral tasks chosen from collections of common tasks designed without prior consideration of the observed human clinical phenotype) relies on behavioral tasks that are not sufficiently sensitive to characterize gene-brain-behavior interactions (Amann et al., 2010; Gur et al., 2007; Karayiorgou et al., 2010; Simon, 2007, 2008, 2011; cf., Figure 1A). In contrast, behavioral endophenotyping emphasizes the use of behavioral paradigms developed to specifically evaluate a priori hypotheses concerning the gene-brain-behavior interactions using carefully selected tasks to identify unique phenotypes within each model; and thus are more capable of characterizing the neurocognitive consequences of the specific gene mutations underlying the genetic disorder (Gould and Gottesman, 2006; cf., Figure 1B).
In addition to evaluating behavioral endophenotypes of mouse models, it is critical to evaluate neuroanatomical phenotypes and endophenotypes with equal sophistication. If a genetic mutation disrupts one neural network but spares another, then the identification of analogous neuroanatomical alterations in the mouse model may guide the selection or development of behavioral tasks to specifically evaluate the function of the affected system.
In this review I will evaluate advances in neurobehavioral endophenotyping, and will propose a clear strategy to efficiently and comprehensively characterize neurobehavioral deficits in mouse models of genetic disorders. This approach uses neurocognitive theory to design and select behavioral tasks that test specific hypotheses concerning the genetic disorder being studied. I propose this novel approach will extend the utility of mouse models by integrating the expertise of clinical neurology and cognitive neuroscience into the mouse behavioral laboratory. Further, I propose that directly emphasizing the reciprocal translation of research between human disease states and the associated mouse models is essential for both groups to mutually inform each other's research to more efficiently generate hypotheses and elucidate treatment strategies.
II. Behavioral Phenotyping Strategies
Any discussion concerning the behavioral phenotyping of mouse models of genetic disorders must necessarily begin with a description of what a behavioral phenotype is and what assumptions underly tasks used to evaluate them. In short, behavioral phenotyping quantifies performance of mutant mice across behavioral experiments; and the behavioral performance is related to the clinical population to identify parallels that may exist. The analogy between the phenotype of human genetic disorder and the behavioral phenotype of the mouse model can be expressed as a combination of three factors: face validity, construct (or content) validity, and predictive validity (Crawley, 2004; Guion, 1977).
Face validity is the surface similarity between the behavior of the mouse model and the patient on analogous tasks (i.e., does the performance of the mouse and human resemble each other at face value). In other words, if a mouse has to perform a similar response during a task as the patient makes during performance of a similar task, the task shows face validity. Similarly, if the mouse and human behavioral tasks can be intuitively interpreted as being similar, the task shows face validity.
Construct (or content) validity, so far as the development of behavioral experiments is concerned, refers to the similarity between the behavioral or cognitive domains being tested by a given task in the mouse model and human patient. This means that for tests to show construct validity, the tasks must be designed to directly model specific aspects of the genetic disorder and additionally that performance be subserved by similar neural substrates and/or cognitive process across species. More specifically, the tasks need to be developed to explicitly model the human disorder, not solely rely on creative post hoc interpretations of behavioral performance on general behavioral tasks. One necessity of construct validity is that a basic understanding of the disorder being modeled is required, such that the research is into translating a behavioral phenotype across species, not providing the primary elucidation of any phenotype at all in a model.
Predictive validity refers to the utility of a mouse model as a proxy for the patient in studies of disease progression or therapeutic intervention--this can refer to either the endpoints of a behavioral study or the physiology of the model. Although predictive validity is commonly thought of as a characteristic of phenotyping approaches, it is more accurate to state that predictive validity is the quantified endpoint of an adequately designed behavioral phenotyping experiment--that is, to define some behavior or set of behaviors that serve as valid outcome measures for later studies (Berge, 2011; Greene-Schloesser et al., 2011). In other words, predictive validity is only present when behavioral performance of the model during a given experiment proves useful for inferring or correlating dosage of a given mutation, disease progression, or treatment outcomes in not only the model, but also the clinical population.
II.I. Common behavioral approach
Commonly, the selection of behavioral tasks to evaluate a behavioral phenotype emphasizes either a high-throughput battery tasks to determine gross deficits for cognitive function or a limited selection of tasks that roughly assay cognitive processing. There are definite advantages to this approach as it provides a rich array of information from commonly implemented, easily interpreted tasks, but this approach does not explicitly model the behavioral phenotypes of the human disorder being modeled. When a behavioral screening approach becomes essential is for the primary screen for phenotypes in novel mouse disease models. For example, in cases where the mouse model has not been evaluated for gross cognitive function, this process is analogous to initial neuropsychological screens given in the clinic prior to more in depth neurocognitive testing.
II.II. Endophenotyping approach
Endophenotypes are collections of quantitative traits hypothesized to represent risk for genetic disorders at more biologically (and empirically) tractable levels than the full clinical phenotype which often contains profound deficits shared across numerous genetic disorders (e.g., memory loss; Gottesman and Gould, 2003; Gould and Einat, 2007; Gould and Gottesman, 2006; Hasler et al., 2006). The overall goal of developing a behavioral test battery to evaluate a behavioral endophenotype is to define a pattern of behavioral strengths and weaknesses in a mouse model comparable with the pattern of deficits observed in clinical populations that can be used as a behavioral biomarker to predict symptom onset or progression, or serve as an outcome measure in studies of intervention or treatment. Optimally, endophenotypes are designed such that observed behavioral deficits will scale with the dosage or severity of the genetic mutation, thus allowing the researcher to more directly evaluate specific roles of the mutation in cognitive deficits associated with the genetic disease (Gould and Gottesman, 2006; Simon, 2010, 2011). Importantly, endophenotypes are made up from a spectrum of tasks covering a broad pattern of deficits and strengths observed in the clinical population, which assists in differentiating similar models.
The weakness in the endophenotyping approach is twofold: when the mouse model has not been previously characterized, and when there may be gross cognitive deficits that overshadow the proposed endophenotype. In both cases, if one would only use the endophenotyping approach, then the true nature of the underlying phenotype would be overlooked. As a concrete example, if one hopes to study schizophrenia in a mouse model and begins behavioral studies using specific schizophrenia related tasks based on clinical research (e.g., gamma oscillation impairments, working memory impairments, etc.; Carter and Barch, 2007) and fails to find the hypothesized deficits in the mouse model, the mouse line may be prematurely abandoned. However, for such a model a more general screening approach would potentially uncover phenotypes resembling mood disorders that could potentially be investigated further to uncover aspects of known schizophrenia phenotypes. An endophenotyping approach allows researchers to test hypotheses from a number of disorders showing partially overlapping phenotypes to hone in on unique patterns of quantifiable behavioral deficits shared among the clinical population and the mouse model.
In recent years, transgenic mice with targeted deletions or over expression of genes have become important tools for evaluating cognitive processes. These experiments provide behavioral scientists with an invaluable tool to unravel the molecular mechanisms through which genetic and neural networks may affect brain function (Chen and Tonegawa, 1997; Nakazawa et al., 2003, 2004). In addition to advancing the genetic tools being used to dissect cognitive processes in mice, this research has led to an acceleration in the development of sophisticated behavioral tasks that have been shown to be exquisitely sensitive for evaluating dysfunction to known neural networks and behavioral processes. This means that performance on the behavioral tasks designed for these studies can be used to elucidate relatively subtle consequences of disruptions to specific anatomical or genetic loci in mouse disease models. For the most part, the tasks developed to evaluate these hypotheses have not been extended to the study of human genetic disorders.
II.III. Comparison of the Common and Endophenotyping Approaches
To compare the more common phenotyping approach with the endophenotyping approach, the tasks and underlying hypotheses from each approach will be compared and contrasted based on the cognitive domains being evaluated. In no cases are the tasks described under the common or endophenotyping approaches sections intended to be exhaustive, but rather represent a representative sampling of tasks chosen to demonstrate the level of domain specificity that can be achieved in modeling behavioral endophenotypes. Table 1 contains behavioral tasks organized by the component attribute or process being tested and by phenotyping approach, as well as a collection of references that emphasize the methods for each behavioral protocol.
Table 1.
Summary of behavioral tasks commonly used in behavioral phenotyping strategies organized by general domain. Also summarized are behavioral tasks proposed to be useful for behavioral endophenotyping organized by component attributes. Also included are references for each task that emphasize the methods for each paradigm.
| Attribute Tested | Behavioral Phenotyping Tasks | Behavioral Endophenotyping Tasks | Reference(s) |
|---|---|---|---|
| Memory | Water Maze | Babovic et al., 2008; Bainbridge et al., 2008; Corwin et al., 1994; Ellis and Kesner, 1983; Gleason et al., 1999; Holmes et al., 2002; Llano Lopez et al., 2010; Paylor et al., 2001; Sigurdsson et al., 2010; Whishaw and Tomie, 1996 | |
| Radial Arm Maze | |||
| Barnes Maze | |||
| Active/Passive Avoidance | |||
| Contextual Fear Conditioning | |||
| Spatial Processing | Categorical (Metric) Processing | Bartko et al., 2011; Clelland et al., 2009; Creer et al., 2010; Goodrich-Hunsaker et al., 2005, 2008b; Kesner et al., 2001; Kirwan et al., 2005; McTighe et al., 2009; Talpos et al., 2010 | |
| Coordinate (Topological) Processing | |||
| Touchscreen Pattern Separation | |||
| Delay Match to Place with Variable Interference | |||
| Delay Match to Place with Variable Cues | |||
| Temporal Processing | Trace Fear Conditioning | Balci et al., 2008; Balsam and Gallistel, 2009; Chiba et al., 2002; Cordes and Gallistel, 2008; Cordes et al., 2007a, 2007b; Devito and Eichenbaum, 2011; DeVito et al., 2009; Eichenbaum and Fortin, 2009; Fortin et al., 2002; Gallistel et al., 2010; Hunsaker et al., 2010; Kesner and Hunsaker, 2010; Jackson et al., 1998; | |
| Temporal Ordering of Stimuli | |||
| Sequence Learning Tasks | |||
| Sequence Completion Tasks | |||
| Duration Discrimination | |||
| Associative Memory | Biconditional Discrimination | Bannerman, 2009; Bussey et al., 2011; Gilbert and Kesner, 2003; Kesner et al., 2008; Poirier et al., 2010 | |
| Cued-Recall Task for Trial Unique Associations | |||
|
| |||
| Affect | Classical Fear Conditioning | Cain and LeDoux, 2007; Crawley, 2004, 2007; Debiec et al., 2010; Kopec et al., 2007; Porsolt et al., 1977; Wahlsten et al., 2006 | |
| Open Field | |||
| Elevated Plus Maze | |||
| Porsolt Test | |||
| Emotional Valence | Reward Contrast with Variable Reward Value | Gilbert and Kesner, 2002; Gilbert et al., 2003 | |
| Anhedonia | Anticipatory Contrast Task | Carola et al., 2008; Kesner and Gilbert, 2007; Maasberg et al., 2011; Malkesman et al. 2009 | |
| Species Relevant Sexual Behaviors | |||
| Approach-Avoidance | Hyponeophagia | Bannerman et al., 2002, 2003; Deacon, 2011; Meert and Colpaert, 1986 | |
| Defensive Burial | |||
| Fear Processing | Defensive Test Battery | Blanchard et al., 1993; Ferris et al., 2008; Luisa-Scattoni et al., 2011; Velez et al., 2010 | |
| Classical, Contextual, Trace Fear Conditioning | |||
|
| |||
| Motor | Rotarod | Ryan et al, 2008; Zeyda et al., 2001 | |
| Visuomotor | Skilled Forelimb Reaching | Allred et al., 2008; Ballermann et al., 2000, 2001; Bury and Jones, 2002; Farr et al., 2006; Kamens and Crabbe, 2007; Kamens et al., 2005; Tennant et al., 2010; Tennant and Jones, 2009; Whishaw and Coles, 1996; Whishaw et al., 2008 | |
| Capellini Handling Task | |||
| Seed Shelling Tasks | |||
| Parallel Beam or Ladder Walking Tasks | |||
| Motor Learning | Acquisition of Skilled Reaching | Diep et al., 2011; Hunsaker et al., 2011; Kesner and Gilbert, 2006 | |
| Acquisition of Rotarod (initial training) | |||
| Working Memory for Motor Movements | |||
| Sensory | Prepulse Inhibition | Prepulse Inhibition | Crawley, 2004, 2007; Dulawa and Geyer, 1996 Noble et al., 1964 |
| Acoustic Startle | Acoustic Startle | ||
| Hot Plate Analgesia | Psychonomic Threshold | ||
| Social | Three Chamber Social Novelty | Social Dyadic Behavior | Defensor et al., 2011; Moretti et al., 2005; Nadler et al., 2004; Pearson et al., 2010; Pobbe et al., 2011; Spencer et al., 2008; Uchida et al., 2005; Yang et al., 2011 |
| Resident Intruder Tests | |||
| Social Transmission of Food Preference | |||
| Social Dominance | |||
|
| |||
| Executive Function | Operant Conditioning | Crawley, 2007; Spencer et al., 2011; Thomas et al., 2009 | |
| Holeboard Exploration | |||
| Reversal Learning | |||
| Cognitive Control | Contextually Cued Biconditional Discrimination | Amodeo et al., 201; Casten et al., 2011; Endo et al., 2011; Garner et al., 2006; Haddon et al., 2008; Haddon and Killcross, 2005, 2006, 2007; Kesner and Ragozzino, 1998 | |
| Serial Reversal Learning | |||
| Stop Signal task Probabalistic | |||
| (80/20) Reversal learning | |||
| Attention | 5 Choice Serial Reaction Time task | Loos et al., 2010; Ward and Brown, 1996, 1997; Ward et al., 1998 | |
| Covert Attention Tasks | |||
II.III.I. Memory
II.III.I.I. Common Approach
In the memory domain, the most common behavioral paradigms are the Morris water maze, the water radial arm maze, the Barnes maze, and active/passive avoidance of foot shock. What these tasks have in common is that they are spatial memory tasks subserved by a wide number of different neural networks. Furthermore, it is difficult to identify common memory tasks in humans that are directly modeled by these murine tasks, though some research has been done using virtual navigation in humans, but rarely in the context of genetic disease (cf., Goodrich-Hunsaker et al., 2010; MacLeod et al., 2010).
One problematical factor shared among these tasks is the use of negative reinforcement motivating task performance. For the water mazes, the mouse is placed in a pool of cool (usually 24-28°C) water and is required to swim to locate a platform to escape the water--something that mice do not do as well as rats (cf., Whishaw and Tomie, 1996). In the Barnes maze, the mouse is placed on a round tabletop with a number of equally-spaced holes along the periphery of the maze and is required to find a hidden goal box placed under one of the holes to escape a bright light and/or loud noise aversive stimulus (Paylor et al., 2001). For the active or passive avoidance tasks, the mouse is required to avoid receiving a foot shock by either actively exiting or passively not entering into a predefined area of space (Ellis and Kesner, 1983). This negative reinforcement approach is particularly troublesome for comparison with clinical populations, which do not regularly receive negative reinforcement such as cold or applied electrical stimuli to motivate task performance. Non-aversive versions of a number of the water-based tasks are available, but are not common in mouse behavioral phenotyping screens (e.g., dry land water maze and radial arm maze; Corwin et al., 1994; Llano Lopez et al., 2010).
II.III.I.II. Endophenotyping Approach
For memory processes, an endophenotyping approach tries to get to the core cognitive deficits underlying memory, not just memory itself. As such, memory is separated into a number of component attributes that can be tested individually and more directly evaluate the cognitive processes commonly evaluates in clinical populations. The primary benefit over the more common phenotyping approach is that the tasks presented below were developed to evaluate specific attributes of memory processing and are designed to evaluate the differential roles for cognitive domains in task performance. Furthermore, these tasks were developed to not only evaluate cognitive domains affected in human disease, but also were designed to mimic the behavioral tasks used in human clinical populations as much as possible.
II.III.I.II.I. Spatial Processing
II.III.I.II.I.I. Coordinate Spatial Relationships
For evaluating the spatial attributes of memory, it is important to determine which of the core spatial processes are disrupted by the mutation. One type of spatial processing is coordinate processing, also called metric processing (Gallistel, 1989, 1990). This type of processing refers to the role of the brain in determining the locations of objects within spatial with mathematical precision (i.e., precise angles and distances among objects in space, as well as how the individual fits into that “cognitive map”). There are a number of tasks that probe this type of spatial processing, particularly those evaluating spatial pattern separation (Bartko et al., 2011; Hunsaker et al., 2009), a process proposed to be disrupted in a number of genetic disorders (cf., Hanson and Madison, 2010). These tasks evaluate the ability of mice to specifically determine spatial relationships among stimuli in ways similar to studies in humans (cf., Goodrich-Hunsaker et al., 2011a; Kessels et al., 2010; Kosslyn, 2006; Kosslyn et al., 1989, 1992).
II.III.I.II.I.I. Categorical Spatial Relationships
Another type of spatial processing is categorical or topological processing, which evaluates the relationships among stimuli in a somewhat less precise manner (connectedness, enclosure, etc.). This type of processing is best conceptualized as using prepositions to describe the relationships among objects in the environment (e.g., behind, next to, etc), but lacking the mathematical precision required by coordinate processing. Tasks evaluating these processes are available in the literature, but are not widely utilized in mouse behavioral studies (Goodrich-Hunsaker et al., 2008a; cf., Robertson et al., 1997; Kessels et al., 2010).
II.III.I.II.II. Temporal Processing
Another critical aspect of memory is knowledge of the temporal relationships among stimuli. To evaluate this attribute of memory, simple sequence learning tasks can be used (Devito and Eichenbaum, 2011; Kesner and Hunsaker, 2010), tasks evaluating recency judgments (Eichenbaum and Fortin, 2009), or tasks evaluating discrimination of duration information (Chiba et al., 2002; Jackson et al., 1998). Processes such as these have been shown to be impaired in a number of genetic disorders but has not been widely applied in research into mouse models of these disorders (Allman et al., 2011; Hampstead et al., 2010; Johnson and Kesner, 1997; Pirogovsky et al., 2009; Schwartz et al., 1991; Shipley et al., 2002; Vriezen and Moscovitch 1990).
II.III.I.II.III. Associative Learning
Associative memory is disrupted in a number of disorders such as Alzheimer's disease and Parkinson's disease (Dierckx et al., 2009; Saka and Eilbol 2009; Vriezen and Moscovitch 1990), so it is critical to specifically evaluate associative learning in mice. Simple stimulus-stimulus or stimulus-spatial location tasks are commonly used for these types of experiments (Bannerman, 2009; Bussey et al., 2011). Cued recall tasks that serve as useful analogs for list learning tasks used in clinical testing have also been developed for rats, but are not yet prevalent in the mouse literature (Kesner et al., 2008).
II.III.I.II. Affect Processing
II.III.I.II.I. Common Approach
To evaluate affective or emotional processing in mice, typically variations on conditioned fear are used. Classical fear conditioning pairs an auditory cue and/or a context with a foot shock, and the ability of the mouse to learn or remember this conditioned fear (as measured by freezing behaviors) is used to index emotional learning (Cain and LeDoux, 2007). Again, it is not common in clinical research to perform tasks requiring aversive reactions to physical discomfort similar to these fear conditioning tasks. Furthermore, the response measured in mice (i.e., freezing) appears to be more related to panic states than fearful or phobia-related states and may not be effective measures for emotion-related processing (cf., Gray and McNaughton, 1996).
To evaluate anxiety processes, the elevated plus or elevated zero mazes are typically used, which require the mouse to explore open or enclosed areas, using the tendency of the mouse to explore the environment while preferring enclosed over exposed spaces to serve as a proxy for anxiety (Moy et al., 2007). Alternately, a mouse can be placed in a large box and the relative time the mouse spends near the edges/corners of the box compared to time spent in the center--more exposed--region of the box is used as a proxy measure of anxiety (Crawley, 2004).
To evaluate depression, the most widely used test is the Porsolt test, a test of helplessness behavior seen when mice give up after placed in an unescapable bucket of cool water (Porsolt et al., 1977). What these affect tasks share in common is that they emphasize negative affect by quantifying punishment or the valuation of negative valence, without regard to positive affect or reward processing. Additionally, the direct comparison between these tasks evaluating affect and measures used in clinical populations are not easily reconcilable (Gray and McNaughton, 1996, 2000).
II.III.I.II.II. Endophenotyping
To evaluate the processing of affect, one has to dissect out different types of affective or emotional processing into attributes, as fear and anxiety are only components of affect, not affect in themselves (cf., Gray and McNaughton, 1996). Furthermore, it is useful to evaluate both positive and negative affect in mouse models, as well as more directly modeling the paradigms used to evaluate anxiety in clinical populations, an approach more in line with studies into phobia, anxiety, and depressive states.
II.III.I.II.II.I. Reward Valence
To evaluate the ability of mice to properly process affect information, one relatively simple task that can be used is to measure the conditioned flavor preference for different flavors of liquid containing different concentrations of sucrose reward (Gilbert and Kesner, 2002). One can get at the nature or severity of any impairments by making the sweetness levels of each of the conditioning flavors increasingly similar and looking for differences in discrimination functions among groups (e.g., 16% vs 2% sucrose is easier to discriminate than 16% sucrose vs. 8% sucrose; cf., Gilbert et al., 2003).
II.III.I.II.II.II. Anhedonia
To evaluate anhedonia or depression-related symptoms, on can use an anticipatory contrast task, which evaluates the tendency of animals to reduce consumption of a given reward if there will be a greater reward in the near future--a direct measure of cognitive processing the differences among rewards (Kesner and Gilbert, 2007). Anhedonia results in mice acting as if both rewards were equivalent, despite being able to discriminate the relative sweetness levels. A simple version of this task is to give mice free access to 2% sucrose for 15 min and then follow that 30 min minutes later with 32% sucrose for 15 min every day for a seven days and determine if the mice consume less and less of the 2% sucrose each subsequent day in anticipation of the 32% sucrose presented later (Maasberg et al., 2011).
The presence or lack of species specific sexual behavior can also be used to quantify anhedonia or anhedonic-like processes (e.g., latency and duration of sniffing female scents for male mice or mating behaviors; Carola et al., 2008; Malkesman et al. 2009).
II.III.I.II.II.III. Approach-Avoidance Conflict
To better dissect processes underlying anxiety, Gray and McNaughton have conceptualized anxiety as a unique form of approach-avoidance conflict (Gray and McNaughton, 1996; McNaughton and Gray, 2000). To evaluate approach-avoidance processes, one can quantify the hesitancy of hungry mice to eat in a novel environment or situation (i.e., hyponeophagia). These protocols have been parameterized in rats to include tests of hyponeophagia in environments across differing levels of perceived insecurity (Bannerman et al., 2002, 2003), and adapted for mice (Deacon, 2011). Various shock probe burial tasks are also classic tests of this model as a test of a mouse's desire to approach the probe to bury it in bedding despite simultaneous fear of the shock (Meert and Colpaert, 1986). The probe burial task has fallen out of favor in recent years, appearing only sporadically in the literature (Chee and Menard, 2011; Saldivar-Gonzalez et al., 2003; Shah and Treit, 2003; Sikiric et al., 2001; Treit and Fundytus, 1988). Conceptually similar approach-avoidance conflict paradigms have been used applied in research of human genetic disease (Drago et al., 2008).
II.III.I.II.II.IV. Fear-Related Processes
To dissect fear more precisely than by using classical conditioning, one can use a collection of tasks evaluating defensive behavior (Blanchard et al., 2003a, 2003b, 2005, 2008; Ribeiro-Barbosa et al., 2005; Yang et al., 2004), wherein mice are exposed to different levels of predator stress or aggressive conspecifics and are monitored for their responses using standardized criteria (Blanchard et al., 1993). This type of task as a measure of fear is more relevant to human fearful/phobia states; commonly expressed as a heightened vigilance and concern over one's safety and less as a panic state induced by the inescapability present in classical fear conditioning paradigms (Gray and McNaughton, 1996).
II.III.I.III. Motor Function
II.III.I.III.I. Common Approach
To evaluate motor function and coordination in mice, the accelerating rotarod is the most common apparatus. In this task mice are placed on a slowly accelerating rod and are required to not fall off. To test motor learning the rotational speed of the rod is increased until the mouse falls (Zeyda et al., 2001). For the most part, any data during the acquisition and pretraining are not collected or remain unreported, and as such potential differences among groups of mice may be overcome during this training phase prior to testing. As such, any resulting “lack” of a motor phenotype in mice does not necessarily mean there will not be motor phenotypes uncovered using more sensitive tests or analyses of the acquisition phase.
II.III.I.III.II. Endophenotyping
To get at motor function and motor learning, one has to go beyond the rotarod, as it has been shown that mice and rats can develop strategies to perform supranominally on the rotarod, which may over shadow motor impairments (cf., Wahlsten et al., 2003). Importantly, a number of cognitive processes underlie potential motor deficits, so it bears dissecting motor function into motor attributes and testing each in turn and not lumping all motor deficits into a single category.
II.III.I.III.II.I. Visuomotor Processing
To evaluate the ability of mice to perform voluntary, skilled movements, one can use skilled forelimb reaching or performance tasks. Such tasks include skilled forelimb reaching for pellet tasks, tasks requiring shelling of seeds, and capellini handling tasks that require skillful limb and digit usage (Allred et al., 2008; Ballermann et al., 2000; Whishaw and Coles, 1996). Furthermore, skilled walking can be evaluated using horizontal ladder walking tasks adapted to quantify stroke models (Farr et al., 2006) or parallel beam tasks originally designed to evaluate ethanol-induced ataxia (Kamens and Crabbe, 2007; Kamens et al., 2005).
II.III.I.III.II.II. Motor Learning
To evaluate motor learning, mice can be tested using modified versions of the tasks mentioned above, but quantifying the learning curve during acquisition or training or by requiring the mouse to learn and perform increasingly skilled movements for reward (Hunsaker et al., 2011; Diep et al., 2011). Additionally, mice can be tested for memory for motor movements (Kesner and Gilbert, 2006).
II.III.I.IV. Sensory Gating
II.III.I.IV.I. Common Approach
To evaluate sensory gating phenomena in mice, a prepulse inhibition (PPI) protocol is commonly used, a modification of the acoustic startle paradigm (cf., Dulawa and Geyer, 1996). In this task, the natural startle response to a sudden, loud auditory cue is reduced by presenting a slightly softer priming cue. Any attenuated responsively on the part of the animal is taken as a measure of PPI impairment. Intriguingly, sensory gating is a case where the same task used for the common and endophenotyping is the same, PPI.
II.III.I.IV.II. Endophenotyping
Prior to evaluating sensory gating phenomena in mice, it is important for mice to be evaluated for the ability to discriminate stimuli. To evaluate these sensory and perceptual attributes, mice can be tests using simple psychonomic threshold tasks based on protocols used for discrimination tasks (Noble et al., 1964). Such measures can be evaluated across all sensory domains. For the endophenotying approach, the nature of PPI impairment is evaluated and directly compared to the clinical disorder to identify parallels.
II.III.I.V. Social Behavior
II.III.I.V.I. Common Approach
To evaluate social behavior or social preferences, the three chamber test is used. In this task, mice are placed in a box with three chambers. In one chamber there is a mouse in a small cage, in a second chamber, there is a small cage without a mouse, and an empty chamber separating the two (Nadler et al., 2004). The preference for a study mouse to spend time in the chamber with the caged mouse is taken as a measure of social behavior. A modification can be used to evaluate social novelty preferences, which involves adding a novel mouse to the previously empty chamber and the preference of the study mouse to explore this novel caged mouse compared to the familiar mouse is used as a dependent measure. The construct validity of this procedure has been recently brought into question as it appears equally likely that mice respond to spatial novelty as much as to social novelty in this task (i.e., a familiar mouse in a previously unoccupied spatial location is just as, if not more, interesting than a new mouse in a previously occupied location), which is a significant confound that needs to be controlled to clearly interpret the results of this task.
II.III.I.V.II. Endophenotyping
To evaluate social behaviors, one can use tasks evaluating social dyadic behavior (Defensor et al., 2011). One can record the behavior of a cage or cages of mice overnight and evaluate the behavior using manual, semiautomated, or automated methods (Pobbe et al., 2011). A modification of this task places two mice in the same box but separated by a grid barrier and quantifies both the time and nature of reciprocal interactions (Moretti et al., 2005; Spencer et al., 2008). Additionally, to evaluate the role of social hierarchy in behavioral testing, one can place a mouse in a cage or tube and after a set amount of time introduce a second mouse as an intruder and quantify the level of aggressive behaviors associated with social dominance (Uchida et al., 2005). These tasks emphasize the ethological behavior of the mice more so than the three chambered task.
II.III.I.VI. Executive Function
II.III.I.VI.I. Common Approach
Although not completely ignored in the common screening approach to phenotyping mice, executive function is not included in most batteries of behavioral tasks, mostly due to the fairly extensive training (and thus time) required to assay executive function in mice. For the most part, acquisition of simple operant conditioning is used as the measure of executive function in the screening approach. Although this type of task does assess executive function, simple operant conditioning does not directly test specific aspects of executive function shown to be disrupted in human clinical populations. Recently, creative, but strained, reinterpretations of tasks used in phenotyping screens have come into the literature, including holeboard exploration (Crawley, 2007; Spencer et al., 2011) and marble burying, but these tasks have a number of contradictory interpretations in the literature (Thomas et al., 2009).
II.III.I.VI.II. Endophenotyping Approach
An often ignored aspect of mouse models genetic disorders are the profound effects of genetic mutation on executive function, particularly for cognitive control and attentional deficits (cf., Haddon and Killcross, 2005; Simon, 2007, 2008, 2011). This is critical as a number of disorders have executive dysfunction as a core component of the diagnostic tests.
II.III.I.VI.II.I. Cognitive Control
To evaluate cognitive control in mice, one may use a cued context biconditional discrimination task that has face and construct validity with the Stroop task (Haddon and Killcross, 2005), or else a serial reversal learning task that requires the mouse to explicitly reverse established rules or else to learn to change rules based on changes to presented stimulus sets (Endo et al., 2011). Tasks evaluating intra and extra-dimensional attentional set shifting have also been developed in mice (Casten et al., 2011; Garner et al., 2006). In all cases, perseverative behavior can be explicitly quantified, as well as the ability of mice to learn, apply, and reverse rules (Kesner and Ragozzino, 1998). Additionally, probabilistic reversal learning tasks have been used to model tasks used in humans that rely on an 80/20 reward contingency to guide reversing behavior (Amodeo et al., 2011). This modification was critical as it has been demonstrated that individuals with genetic disorders have a greater difficulty reversing learned rules under 80/20 reward contingencies than 100% reward contingencies. Stop signal tasks can also be used to evaluate the ability of mice to inhibit either prepotent or highly trained responses in a manner both methodologically and cognitively similar to procedures used in clinical populations to evaluate cognitive control (Eagle et al., 2007).
II.III.I.VI.II.II. Attentional Processes
In mice, it has been difficult to explicitly assess attention as mice do not stay “on task” as well as human subjects and are notoriously slow to learn reward contingency rules, but a number of sufficiently simple paradigms have emerged. The 3 or 5 choice serial reaction time tasks and simple reaction time tasks can be used to evaluate sustained attentional processes in mice (Loos et al., 2010). Furthermore, exogenous and endogenous cueing tasks have been developed for mice that probe covert attentional processes in mice (Ward and Brown, 1996) in a manner similar to the spatial cueing task introduced by Posner (Posner et al., 1987) that is an invaluable tool used to evaluate attentional deficits across wide arrays of clinical disorders.
II.IV. Evaluating Neuropathological Features
II.IV.I. Neuroanatomical Phenotypes
It is critical to identify and characterize any pathologic neuroanatomical features that result from the genetic mutation as precisely as possible. In clinical populations, such neuropathology are principally characterized through magnetic resonance imaging (MRI); optimally followed by thorough post mortem histopathological analysis. In mouse models, such neuroanatomic sequelae are characterized primarily through gross histological studies.
Unfortunately, subtle pathological anatomical features are often very easily overshadowed by histological artifacts and differences in techniques that render comparisons among (and within) labs difficult (cf., Simmons and Swanson, 2009; Swanson, 1995 for a consideration of these challenges; cf., Wenzel et al., 2010; Willemsen et al., 2003 for a specific example of seemingly contradictory findings in the same mouse model explained by subtle methodological differences). Recently, in vivo MRI analyses of brain in mouse models of genetic disorders have been developed with variable levels of success (Ellegood et al., 2010; Kooy et al., 1999; Kovacevic et al., 2005). Increasingly sophisticated analysis techniques and imaging technologies are necessary to allow true cross-species comparison of neuropathological features in vivo.
II.IV.II. Neuroanatomical Endophenotypes
Neurological phenotypes that appear at more of a functional, rather than grossly anatomic, level have emerged in clinical research. Much of this work has been advanced by improvements in diffusion weighted imaging quantifying white matter in the brain as well as functional MRI (fMRI) that can evaluate regional brain activity in response to cognitive or behavioral tasks (Adamczak et al., 2010; Rivera et al., 2002). Abnormal patterns of brain activation during a task (i.e., reduced or enhanced task-related signal relative a comparison group) may also inform further investigations into microscopic anatomical changes or altered connectivity that remain undetected using traditional MRI analyses focusing on gross brain structure.
Patterns of brain responses triggered by stimuli called event related potentials (ERPs) can be measured on the scalp electroencephalogram in both humans and mice and serve as a neurophysiological outcome measure for abnormal information processing (Choi et al., 2010; He et al., 2001; Olichney et al., 2010). Direct in vivo neurophysiolgoical recording has become more readily accessible in mice to evaluate the firing patterns of cells across brain regions during task performance (cf., Sigurdsson et al., 2010). Also, evaluating task related gene transcription of so called immediate early genes (IEGs) has also proven a useful took for evaluating the role of different brain regions for cognitive processing during behavioral tasks in mouse models of genetic disorders (Drew et al., 2011; Guzowski and Worley, 2001; Krueger et al., 2011).
II.V Proposed Comprehensive Behavioral Phenotyping Approaches
Currently implemented behavioral screens have the benefit of clear face validity as the implications of behavioral deficits on a task or collection of tasks are intuitively applicable in the context of the clinical phenotype, but often these tasks lack construct validity (cf., Chadman et al., 2009; Crawley, 1985; Humby et al., 2005; McFarlane et al., 2008; Moy et al., 2008a, 2008b; Nadler et al., 2006; Ricceri et al., 2007; Ryan et al., 2009; Silverman et al., 2009, 2010; Yang and Crawley, 2009). The behavioral endophenotyping process I am proposing emphasizes clearly defined construct validity across paradigms designed to test specific disease or mutation-related hypotheses.
An optimal, comprehensive behavioral phenotyping strategy integrates common behavioral tasks as well as endophenotyping approaches performed across the lifespan. Such an approach is important because a number of genetic disorders show distinct early and late manifestations of disease that bear independent scrutiny. Often times, carriers of genetic mutations show few or at most subtle characteristics of later clinical disease early in life, but with increasing age these symptomatology emerge and the individuals receive a clinical diagnosis (Chonchaiya et al., 2009a, 2009b; Pirogovsky et al., 2009; Rupp et al., 2009). This does not infer, however, that early in life these individuals are unaffected by the mutation; more likely the consequences of the mutation are present early in life, but require more sophisticated analyses to identify patterns of behavioral abnormalities (cf., Goodrich-Hunsaker et al., 2011a, 2011b, 2011c).
In cases of genetic disorders, it is useful to evaluate the cognitive domains that underly later clinical phenotypes early in life to determine if there are markers that can quantify or predict disease progression (Devanand et al., 2000; Pirogovsky et al., 2009; Salomonczyk et al., 2010; Yong-Kee et al., 2010). Research into a number of neurodegenerative disorders have been able to characterize subclinical endophenotypes early in the disease process that seem to predict the severity of disease progression (Gilbert and Murphy, 2004a, 2004b; Karayiorgou et al., 2010; Salomonczyk et al., 2010; Xu et al., 2010; Yavich et al., 2007; Yong-Kee et al., 2010).
Similar strategies in neuroimaging can dissect alterations to the trajectories of brain growth and development across the lifespan and how these neuroanatomical factors relate to cognitive development (Carrion et al., 2009; Gothelf et al., 2010b; Hall et al., 2009; Hazlett et al., 2009; Hoeft et al., 2010; Lightbody and Reiss, 2009; Reiss, 2009; Walter et al., 2009). These approaches will illuminate not only the genetic contributions to behavioral phenotypes, but also the neurocognitive substrates underlying the observed behavioral phenotypes.
III. Specific Examples
In the evaluation of mouse models of neurodevelopmental disorders, there is not yet an organized movement toward synthesizing the traditional behavioral phenotyping with emerging endophenotyping approaches. The examples below are provided to illustrate the advances in determining the clinical phenotypes and endophenotypes of patient populations and the need for rapid advancement in techniques used for behavioral analysis in mouse models.
The specific examples covered in this review will include disorders among the spectrum of fragile X-associated disorders caused by a polymorphic expansion of CGG trinucleotide repeats in the FMR1 gene: 55-200 CGG repeats is the fragile X premutation, and >200 CGG repeats is the fragile X full mutation that results in fragile X syndrome (FXS; Hagerman and Hagerman, 2004). An analysis of the behavioral phenotypes and endophenotypes associated with fragile X-associated disorders allows researchers to elucidate the role of the dosage of a single-gene mutation in brain function (i.e., expanded CGG repeat length parametrically modulates behavioral phenotypes).
Also evaluated in this review will be the 22q11.2 deletion syndrome (22q11.2DS; historically referred to as DiGeorge Syndrome or velo-cardio-facial syndrome (VCFS)) which is the result of a spontaneous deletion of a variable number of genes on the 22q11.2 locus. These mutations range in size from deletions of virtually the complete 22q11.2 locus (∼60% of cases) to various single or multiple gene deletions within the 22q11.2 locus (remaining ∼40%; Karayiorgou and Gogos, 2004; Karayiorgou et al., 1996; Kiehl et al., 2009; Kimber et al., 1999; Liu et al., 2002a, 2002b; Long et al., 2006; Meechan et al., 2006; Mukai et al., 2004; Paylor et al., 2006; Sporn et al., 2004; Uliana et al., 2008; Walter et al., 2009; Yamagishi and Srivastava, 2003)--thus providing a metric by which to evaluate neurobehavioral disruptions, the dosage of 22q11.2 deletion per individual or mouse model. Importantly, upwards of 50% of individuals with 22q11.2DS develop schizophrenia, so the need for identifying a risk prodrome is critical to identify at risk populations prior to the onset of schizophrenic symptomatology (Karayiorgou et al., 2010).
The present analysis of the neurobehavioral endophenotypes of fragile X-associated disorders and 22q11.2DS will focus on a theory suggesting that a number of neurodevelopmental disorders, including 22q11.2DS and fragile X-associated disorders, show nonverbal learning impairments: particularly reduced resolution of spatial and temporal attention (Johnson-Glenberg, 2008; Simon, 2007, 2008). By no means is this theory all inclusive to the potential deficits present in these populations, but this spatiotemporal processing theory provides a useful scaffold upon which to design and evaluate behavioral research into one of many potential endophenotypes.
III.I. Fragile X-Associated Disorders -- Fragile X Premutation and CGG KI Mouse
The fragile X premutation underlying results in a different phenotype than the full mutation, that of increased (2-8 fold) FMR1 mRNA and concomitant slight reductions to FMR1 protein (FMRP) levels (i.e., 10-25% reductions; Garcia-Arocena and Hagerman, 2010; Raske and Hagerman, 2009;Tassone and Hagerman, 2003; Tassone et al., 2000, 2007). The CGG KI mouse model of the fragile X premutation was developed by selectively inserting a human premutation CGG repeat (99 CGG repeats) into the mouse Fmr1 gene by homologous recombination (Willemsen et al., 2003) and models the molecular phenotypes of the human premutation (e.g., elevated Fmr1 mRNA and reduced Fmrp levels; Brouwer et al., 2007, 2008a, 2008b). A second mouse, a CGG-CCG KI mouse has also been evaluated by Entezam et al. (2007) and shows similar, albeit more profound, molecular phenotypes (>70% reduction in Fmrp levels; Qin et al., 2011).
III.I.I. Human Fragile X Premutation Neurobehavioral Phenotype
Fragile X premutation carriers have been long considered largely cognitively unaffected by the premutation in carriers under the age of 50 (Hunter et al., 2008, 2010), after which premutation carriers are prone to a neurodegenerative course that results in late onset neurodegenerative states characterized by cerebellar gait ataxia and intention tremor (Hagerman and Hagerman, 2004; Yachnis et al., 2010). Once the carriers demonstrate neurodegeneration, the premutation is associated with profound memory deficits, visuomotor performance, and deficient executive function (Berry-Kravis et al., 2007; Bourgeois et al., 2006, 2007; Grigsby et al., 2006a, 2006b, 2008).
The neuropathological hallmarks seen in premutation carriers presenting with late onset neurodegeneration are cortical atrophy, white matter disease (particularly in subcortical and ponto-cerebellar white matter tracts) as well as the presence of intranuclear inclusions in neurons and astrocytes in brain (Greco et al., 2006). There have been reports of altered neuroanatomical volumes in carriers of the premutation prior to the onset of neurodegeneration, but these reports appear incomplete and contradictory, suggesting more rigorous studies are needed to characterize any true brain phenotype of young premutation carriers (cf., Moore et al., 2004a, 2004b; Murphy et al., 1999).
III.I.II. CGG KI Mouse Neurobehavioral Phenotype
Only two studies to date have evaluated the behavioral phenotype of the CGG KI and CGG-CCG mouse using a targeted screening process. van Dam et al. (2005) reported age related worsening of memory using the water maze and declining motor function using the accelerating rotarod in CGG KI mice with age (though the studies were carried out using separate groups of mice so no causative relationship of age could be elucidated). A study evaluating CGG-CCG mice demonstrated very subtle abnormalities for social behavior using the three chambered apparatus, reductions in levels of anxiety in the open field, and a subtle memory deficit evaluated by performance on a passive avoidance paradigm (Qin et al., 2011). Importantly, no cerebellar ataxia or tremor-like phenotypes have been described in either mouse model.
To date, the presence of intranuclear inclusions in neurons and astrocytes have been identified in CGG KI mice (Brouwer et al., 2008a, 2008b; Hunsaker et al., 2009; Wenzel et al., 2010; Willemsen et al., 2003), and inclusions in brain cells in addition to Purkinje cell pathology (axonal torpedos) have been identified in the CGG-CCG mouse (Entezam et al., 2007). No gross histopathological features have been reported in either mouse model.
III.I.III. Human Fragile X Premutation Neurobehavioral Endophenotype
It has been shown that there are visual processing deficits in premutation carriers selective to the magnocellular but not the parvocellular visual streams, specifically as relating to biological and mechanical motion processing (Keri and Benedek, 2009, 2010). It has been suggested that this dorsal visual stream-specific deficit reflects (or causes) impairments for spatial and temporal attention. In a spatial magnitude comparison task, it has been demonstrated that female premutation carriers show performance for discriminating small differences in magnitude that appears to be modulated by both CGG repeat length and age (i.e., task performance shows a negative association with CGG repeat length), this despite female premutation carriers showing enhanced reaction times on a simple reaction time task run during the same behavioral session (Goodrich-Hunsaker et al., 2011a, 2011b). Similar dosage effects have been demonstrated in an enumeration task that requires sequential shifting of spatial attention (Goodrich-Hunsaker et al., 2011c). In addition to these effects, arithmetic processing deficits have been reported in the female premutation carriers (Lachiewicz et al., 2006), a further indication that a fundamental spatiotemporal attention deficit is present in the fragile X premutation (cf., Simon, 1999).
In studies using fMRI, it has been demonstrated that both the amygdala and hippocampus in premutation carriers show less task related neural activity than control participants (Hessl et al., 2007, 2011; Koldewyn et al., 2008), and ERP studies have revealed abnormal cortical function during a semantic oddball detection paradigm (Olichney et al., 2010). Furthermore, there are evidence for reduced task-related activation of the dorsal and ventral inferior frontal cortex during working memory tasks (Hashimoto et al., 2010). It has also been reported that there is reduced white matter integrity in ponto-cerebellar white matter tracts, the fornix, and the stria terminalis in premutation carriers (Hashimoto et al., 2011).
III.I.IV. CGG KI Mouse Neurobehavioral Endophenotype
In evaluating the endophenotype of the CGG KI mouse model of the premutation, the focus has been on directly evaluating spatiotemporal processing and visuomotor function. Hunsaker et al. (Hunsaker et al., 2009), using a coordinate spatial processing task, demonstrated that CGG KI mice showed a CGG repeat length dependent impairment for processing the distance between objects, a task analogous to the spatial magnitude comparison tasks reported in female premutation carriers (Goodrich-Hunsaker et al., 2011a). Furthermore, CGG KI mice also demonstrated a CGG repeat dependent deficit for temporal processing in a simple sequence learning task for visual objects (Hunsaker et al., 2010). It has also been demonstrated that CGG KI mice show impaired visuomotor function, even at ages as young as 2 months of age on a skilled walking task (Hunsaker et al., 2011). This visuomotor deficit is interpreted as a selective impairment to spatiotemporal coordination as relating to motor control. Similar effects were seen in a skilled forelimb reaching task (Diep et al., 2011). What is unique about this overall pattern of deficits in the CGG KI mouse is that there is a clear negative association between task performance and the dosage of the gene mutation (e.g., increasing CGG repeat length on the Fmr1 gene), such that performance deteriorates as CGG repeat length increases across spatiotemporal and visuomotor domains. Furthermore, these deficits arise relatively early in life (2-3 months of age), and thus may provide behavioral biomarkers prior to the onset of any neurodegenerative features associated with the premutation.
The CGG-CCG mouse model of the premutation has altered dendritic morphology throughout the brain, suggesting altered brain function. The CGG KI mouse has recently been evaluated for the presence of these same neuropathological features, and reduced dendritic complexity in basal, but not apical dendrites in visual cortex layer 3 pyramidal neurons was observed, but no alteration in the pattern of dendritic spine morphology was detected (unpublished observations). There also appears to be reduced protein synthesis in the cortex of the CGG-CCG mouse (Qin et al., 2011). To date, protein synthesis levels have not been evaluated in CGG KI mouse brain.
III.II. Fragile X-Associated Disorders -- Fragile X Full Mutation and Fmr1 KO mouse
The full mutation underlying fragile X syndrome results in a molecular null phenotype for FMR1 mRNA and FMR1 protein (FMRP) levels. The Fmr1 KO mouse was developed by selectively knocking out function of the Fmr1 gene (Bakker and Oostra, 2003; Dutch-Belgian Fragile-X Consortium, 1994). Although the Fmr1 KO mouse does not directly model the genetics of the fragile X full mutation, it does model the molecular consequences of the full mutation, that of virtual absence of Fmr1 mRNA and Fmrp, and is thus comparable with the full mutation molecular phenotype (Tassone et al., 1999; but cf., Yan et al., 2004).
III.II.I. Human Fragile X Full Mutation Neurobehavioral Phenotype
The cognitive or behavioral deficits identified in carriers of the fragile X full mutation underlying Fragile X Syndrome (FXS) include heightened anxiety levels, reduced Full Scale IQ (FSIQ), poor sensorimotor gating, and poor visuomotor function (Kemper et al., 1986, 1988). Heightened anxiety reported in FXS includes social and nonsocial anxiety, with particular elevations in anxiety to novelty in either situations or objects (i.e., neophobia). It has been reported that the FSIQ of male FXS patients is typically below 50, with pronounced verbal IQ (VIQ) and nonverbal IQ (performance--PIQ) deficits and mild to severe memory problems. FXS patients also often demonstrate an attenuated prepulse inhibition (PPI) response, meaning the natural startle response to a loud auditory stimulus is not reduced when a quieter priming stimulus is presented 50-200 ms beforehand. Attenuated PPI responses are cited as evidence for sensorimotor gating abnormalities in FXS, though there are also reports of abnormal sensorimotor integration, similar to that reported in autism (Hessl et al., 2008, 2009; McConkie-Rosell et al., 2007; Utari et al., 2010; Yuhas et al., 2010). Poor visuomotor function has also been reported in FXS, as has general clumsiness and awkward movements (Bennetto et al., 2001; Mazzocco et al., 1993).
Based on structural MRI and limited post mortem histological analyses of FXS brain, a consistent pattern has emerged pointing to specific neuropathological features in FXS. It has been reported that the cerebellar vermis, specifically the superior lobe, is reduced in volume, and the caudate is larger than normal--even when corrected for brain volume, as are the hippocampus and several thalamic nuclei (Hessl et al., 2004, 2008, 2010; Mostofsky et al., 1998; Reiss et al., 1988, 1991). Interestingly, the superior temporal sulcus, an area hypothesized to be critical for social cognition is reduced in volume in FXS (Gothelf et al., 2008). Post mortem analyses have confirmed a number of these radiological findings and further identified histopathological features in both the cerebellar vermis and hippocampal formation (Greco et al., 2011). It has further been demonstrated that the neuronal architecture in FXS is disrupted, with dendritic spines appearing more thin and immature in FXS brain than in non FXS brain throughout the hippocampus and neocortex (Irwin et al., 2001; Rudelli et al., 1985).
III.II.II. Fmr1 KO Mouse Neurobehavioral Phenotype
In evaluating the mouse model(s) for FXS, primarily the Fmr1 knockout (KO) mouse has been studied (and will be the focus of discussion here as the other FXS models demonstrate a very similar phenotypes; cf., I304N point mutation model; Zang et al., 2009). There has been a wide discrepancy in the behavioral findings that appear to be related to background strain of the specific mice in the lab, as well as with different lab procedures. There have been reports of Fmr1 KO mice having alternately elevated or reduced anxiety levels measured in the open field and elevated plus maze tests of anxiety (Eadie et al., 2009; Yan et al., 2004; Zang et al., 2009), and abnormal marble burying phenotypes that are suggested to measure general activity, repetitive behaviors, and anxiety levels (Spencer et al., 2005; Thomas et al., 2009); however, these phenotypes are highly background strain dependent (Baker et al., 2010; Moy et al., 2007, 2008a). There have been tests of working memory and general memory using the Morris water maze, Barnes maze, and water radial arm mazes that demonstrate marginal effects at times, but these effects have been difficult to consistently replicate among labs, with even reports of Fmr1 KO mice performing better than wildtype mice during different aspects of task performance relatively prevalent in the literature (Baker et al., 2010; Hayashi et al., 2007; Larson et al., 2008; Mineur et al., 2002, 2006; Mineur and Crusio, 2002; Yan et al., 2004). Attenuated PPI responses in Fmr1 KO mice also vary among strains and among labs, as does a susceptibility to audiogenic seizures (Spencer et al., 2005; Zang et al., 2009).
Recently, to mitigate complications arising from intra-strain differences, it has been proposed that an albino C57BL/6J Tyrc-Brd background strain be used for behavioral and pharmacological evaluation of FXS in Fmr1 KO mice as both male and female Fmr1 KO mice bred onto this background strain show profound behavioral deficits (Baker et al., 2010). It has also been proposed that F1 hybrid between C57BL/6J Fmr1 KO mice and DBA/2J wildtype mice are a preferable model for FXS, as the resulting F1 hybrid mice show a greater number of autism-like phenotypes that other strains lack (Spencer et al., 2011). Though efficacious for modeling various aspects of disease pathogenesis, the initial choice of a background strain based solely upon a desire that the mouse model fulfill or demonstrate a particular phenotype is not in itself a valid rationale to develop or choose a mouse disease model; however, breeding a model onto a different background strain with the intent to further characterize or understand deficits across strains could serve to provide useful information to better understand a given phenotype.
Structural MRI analyses of Fmr1 KO mouse brains report that there are no major volumetric or morphological differences between wildtype mice and Fmr1 KO mice for any of the neuroanatomical structures identified in human studies as being abnormal, but those negative findings likely result from insufficiently sensitive MRI techniques rather than provide evidence for a definitive lack of a neuroanatomical phenotype (cf., Kooy et al., 1999). More recently, volumetric reductions in the deep cerebellar nuclei have been identified by MRI in Fmr1 KO mice, which may prove to be somewhat analogous to the cerebellar pathology identified in FXS cases (Ellegood et al., 2010). At an histological level, dendritic abnormalities identified in human FXS have been confirmed in Fmr1 KO mice using both Golgi-Cox/Golgi-Kopsch staining techniques and more advanced genetic techniques that allow in vivo analysis of neuronal architecture (Grossman et al., 2006; Kao et al., 2010; Pan et al., 2010).
III.II.II. Human Fragile X Full Mutation Neurobehavioral Endophenotype
Work identifying endophenotypes in FXS has demonstrated behavioral deficits across the domains of spatiotemporal processing, numerical or arithmetic processing, to some extent executive function, and specific visual biological motion processing abnormalities (Bregman et al., 1987; Curfs et al., 1989a, 1989b, 1989c; Dykens et al., 1987; Mazzocco et al., 1993; Van der Molen et al., 2010). In the spatiotemporal domain, there have been reports of specific deficits in spatial and temporal memory performance in FXS, even in cases where FSIQ is controlled as a covariate and verbal performance is equal to or surpasses that of controls (cf., Farzin and Rivera, 2010; Farzin et al., 2008, 2009; Johnson-Glenberg, 2008; Kemper et al., 1986, 1988; Mazzocco et al., 1992a, 1993; Simon, 2007). Executive function as defined as poor performance on the Stroop task as well as other tests of attentional and executive function is impaired in FXS (Cornish et al., 1997, 1998, 1999, 2004a, 2004b, 2004c, 2008, 2009; Loesch et al., 2003; Schneider et al., 2008, 2009; Tamm et al., 2002). Even in cases of females with FXS that have normal FSIQ, there appear to be robust behavioral deficits when visuospatial attention is required for task performance (Steyaert et al., 1992, 1994).
Furthermore, there have been numerous reports in behavioral and functional MRI studies demonstrating arithmetic/numerical processing deficits in both males and females with FXS (Bennetto et al., 2001; Mazzocco, 1992a, 1992b, 1993, 1994, 1998, 2000, 2001, 2008; Murphy et al., 2007; Rivera et al., 2002). It has also been shown that a specific visual motion processing deficit is present in both male and female FXS that reflects visual motion processing deficits in FXS and spatiotemporal processing abnormalities that implicate the dorsal visual stream and parietal lobe function (Bertone et al., 2010; Keri and Benedek, 2009, 2010; Kogan et al., 2004a, 2004b, 2008, 2009; MacLeod et al., 2010). Intriguingly, this visual deficit is similar, but more profound than, similar deficits reported in the fragile X premutation using identical tasks (Keri and Benedek, 2009, 2010), suggesting a potential role for the dosage of the CGG repeat on the FMR1 gene for visual processing in fragile X-associated disorders. Recent work bears out this hypothesis in control subjects, demonstrating a positive association between peripheral (e.g., leukocyte) FMRP levels and performance for this task (i.e., higher FMRP levels are associated with better performance; Keri and Benedek, 2011).
Advanced measures of functional activation during cognitive tasks as well as diffusion weighted imaging have revealed a clear pattern of abnormalities in FXS. It has been shown that while performing simple arithmetic tasks individuals with FXS do not show typical increases in neural activity in the intraparietal sulcus that scale with task difficulty, suggesting abnormal cortical function in FXS (Rivera et al., 2002). There are also reduced levels of task-related hippocampus and basal forebrain activation during memory tasks (Greicius, 2008; Greicius et al., 2003, 2004), and reduced amygdala activation to emotionally salient stimuli (Watson et al., 2008). There have also been reports of abnormal fronto-striatal white matter pathways that may participate in cognitive control processes as well as abnormal white matter pathways in the parietal-sensory-motor tracts (Barnea-Goraly et al., 2003; Haas et al., 2009). Additionally, brain region-specific altered neurodevelopmental trajectories have been identified in 1-3 year old FXS children, providing an invaluable insight into the development of neuroanatomical abnormalities reported in FXS adults (Hoeft et al., 2008, 2010). These altered fiber pathways and reduced functional activation are candidate neuroanatomical loci that are specifically affected by the FMR1 mutation and underlie the neurocognitive deficits present in FXS.
III.II.III. Mouse FXS Neurobehavioral Endophenotype
There has not been a systematic effort to model the behavioral endophenotypes described in human FXS in the Fmr1 KO mouse. The current focus is to optimize behavioral tasks to better capitulate the behavioral phenotypes of clinical FXS for use in interventional studies and drug testing. Tasks measuring social deficits and attenuated PPI responses have been proposed to serve as primary screens for therapeutic studies (Crawley et al., 1997; Crawley and Paylor, 1997; Paylor and Crawley, 1997; Spencer et al., 2005, 2011; Zang et al., 2009). The paradigms evaluating categorical and coordinate spatial processing and temporal attention used in the CGG KI mouse have not yet been applied to Fmr1 KO mice (Hunsaker et al., 2009, 2010).
One study evaluating a behavioral parallel between the Fmr1 KO mouse and FXS using analogous behavioral tasks suggests that both FXS and Fmr1 KO mice are impaired on a spatial reasoning task, the modified Hebb Williams maze for mice and a computerized Hebb Williams Maze for humans (MacLeod et al., 2010). Though cross-species comparisons are difficult to interpret for this particular behavioral paradigm emphasizing spatial navigation, the data provide compelling rationale to pursue cross species studies using tasks with both face and construct validity.
III.III. 22q11.2 Deletion Syndrome in Humans and Mice
The 22q11.2DS results from a number of spontaneous mutations along the 22q11.2 locus, ranging from deletion of the whole locus to single or multiple gene mutations along the 22q11.2 locus. A number of different mutations encompassing the 22q11.2DS have been modeled in a number of mouse models. For the present review, the following models are included and collectively referred to as 22q11.2DS mouse models: Lgdel, Del(Dgcr2-Hira)1Ra, DF(16)A, Del(Dgcr2-Hira)2Aam, Df1, Del(16Es2d-Ufd1l)2l7Bld, Smdel, Tbx1, Dgcr8, and Del(16Zpf520-Slc25a1)1Awb. All 22q11.2DS mouse models were generated via targeted deletions of the analogous portions of mouse chromosome 16 covering the same complement of genes deleted across the different 22q11.2 deletions characterized in human 22q11.2DS (Arguello and Gogos, 2010; Babovic et al., 2007; Gogos, 2007; Gogos and Gerber, 2006; Gogos and Karayiorgou, 2001; Kaenmaki et al., 2010; Karayiorgou and Gogos, 2004, 2006; Karayiorgou et al., 1996, 2010; Long et al., 2006; Mukai et al., 2004, 2008; Paterlini et al., 2005; Paylor and Lindsay, 2006; Stark et al., 2009).
III.III.I. Human 22q11.2DS Neurobehavioral Phenotype
22q11.2DS patients demonstrate behavioral phenotypes ranging from learning disabilities to mild–moderate intellectual disability, but severe intellectual disability is present only in rare instances (Karayiorgou et al., 2010). Full Scale IQ (FSIQ) is usually around 75, but discrepancies between a typically average to high VIQ and typically low PIQ have led to the hypothesis that individuals with the 22q11.2DS have a nonverbal learning disability (similar to that reported in fragile X-associated disorders). Behavioral and psychiatric disorders are common in children with 22q11.2DS and include emotional instability, social withdrawal, attention-deficit/hyperactivity disorder, anxiety disorders, and depression (Bearden et al., 2001, 2004a, 2005; Bish et al., 2005, 2007; Gothelf et al., 2010a; Karayiorgou et al., 2010; Kiehl et al., 2009; Simon, 2008; Simon et al., 2005a, 2005b, 2008a, 2008b; Stoddard et al., 2010; Takarae et al., 2009; Xu et al., 2010; Yamagishi and Srivastava, 2003).
In the 22q11.2DS, there have been a number of neuroanatomical phenotypes identified that appear to result from altered developmental trajectories as the phenotypic pattern in adults and children differs. There is the occasional presence of a large cavum septum pellucidum reflecting incomplete subcortical midline development (Beaton et al., 2010), polymicrogyria has been reported (Gerkes et al., 2010; Kiehl et al., 2009; Sztriha et al., 2004), and white matter hyperintensities are often reported throughout the brain (Bearden et al., 2004b; Kiehl et al., 2009). In children, there is increased ventricular volume, reduced parietal lobe volume (but with normal frontal and temporal lobe volumes), reduced cortical thickness, developmental anomalies along the subcortical midline, and reduced cerebellar volumes (Beaton et al., 2010; Bish et al., 2004, 2006; Campbell et al., 2006; Gerkes et al., 2010; Karayiorgou et al., 2010; Machado et al., 2007; Schaer et al., 2006; Simon, 2008; Simon et al., 2005c; Sporn et al., 2004). Interestingly, this pattern of anatomical sequelae suggest that in the 22q11.2DS there is a specific reduction or delayed development of posterior neocortical structures, whereas anterior structures appear normal, at least in children.
In adults with 22q11.2DS, there is a general reduction in brain volume, reduced frontal and temporal lobe volumes (the opposite pattern to that observed in children), increased ventricular volume, reduced cerebellar volume, and diffuse white matter abnormalities seen on diffusion weighted imaging in parietal-parietal and frontal-frontal white matter projections as well as frontal-temporal white matter tracts (Bearden et al., 2004b; Chow et al., 1999; Connor et al., 2010; Drew et al., 2010; Eliez et al., 2001, 2002; Gothelf et al., 2007b, 2010b; Karayiorgou et al., 2010; Kiehl et al., 2009; Machado et al., 2007; Madan et al., 2010; Schaer et al., 2006, 2009; Simon et al., 2005b; Sundram et al., 2010). The altered developmental trajectories underlying distinct patterns of neuropathology in children and adults are presently under investigation.
III.III.II. Mouse 22q11.2DS Neurobehavioral Phenotype
In different mouse models of the 22q11.2DS there are distinct patterns of behavioral phenotypes (Drew et al., 2010; Karayiorgou et al., 2010; Long et al., 2006; Paylor and Lindsay, 2006; Sigurdsson et al., 2010; Stark et al., 2008b, 2009). Behaviorally, poor working memory has been reported in a 22q11.2DS mouse model, but the task used in those studies was actually a test of short-term spatial memory (Lee and Kesner, 2003a; Sigurdsson et al., 2010; Stark et al., 2008a) rather than working memory as evaluated in human populations (Baddeley et al., 2009). There have been reports of impaired sensorimotor gating, reduced grip strength, reduced nociception, and impaired movement initiation in 22q11.2DS mice; but phenotypes depend upon the specific mouse model and background strain used in the studies (Drew et al., 2010; Long et al., 2006; Paylor and Lindsay, 2006). A robust behavioral measure identified in a number of studies has demonstrated impaired classical fear conditioning in 22q11.2DS mouse models, but the relative contributions of the hippocampus, rostral cortex, and the amygdala have yet to be elucidated (Drew et al., 2010; Karayiorgou et al., 2010; Sigurdsson et al., 2010; Stark et al., 2008a).
It has been demonstrated that haploinsufficiency of a number of different genes deleted in the human 22q11.2DS leads to abnormal cortical development in a 22q11.2DS mouse model (Meechan et al., 2009). The 22q11.2DS mouse model has immature dendritic structures that appear similar to what is observed in FXS and the Fmr1 KO mouse model; to date, whether such neuropathology is present in the human 22q11.2DS remains unknown (Drew et al., 2010; Karayiorgou et al., 2010; Mukai et al., 2008).
III.III.III Human 22q11.2DS Neurobehavioral Endophenotype
It has been demonstrated that a number of neurodevelopmental disorders, such as fragile X-associated disorders and 22q11.2DS, share common nonverbal learning impairments. These impairments involve reduced resolution of spatial and temporal attention, as well as poor executive function. Collectively, these impairments result in, among other measures, an impairment in arithmetic processing (Simon, 1999, 2008). Recent work has quantified nonverbal learning impairments in the 22q11.2DS by evaluating the behavioral endophenotypes predicted by the spatial and temporal attention model (Bearden et al., 2001, 2004a, 2005; Bish et al., 2005, 2007; Gothelf et al., 2010a; Karayiorgou et al., 2010; Kiehl et al., 2009; Simon, 2008; Simon et al., 2005a, 2005b, 2008a, 2008b; Stoddard et al., 2010; Takarae et al., 2009; Xu et al., 2010; Yamagishi and Srivastava, 2003). Individuals with the 22q11.2DS show impaired spatiotemporal processing, impaired executive function, and profound impairments in numerical and arithmetic processing. These findings have been placed into the context of a reduced resolution of spatial and temporal attention such that greater differences in space and time are needed to allow the individual to normally process stimuli, a process proposed to underly the nonverbal learning impairments characterized in the 22q11.2DS (Simon, 2008).
Counterintuitively, it has been shown the individuals with the 22q11.2DS show increased parietal cortex activation, but reduced frontal cortex activation, compared to a control population when confronted with a go/no-go response inhibition task that has been shown to depend upon the frontal, but not parietal, cortex (Gothelf et al., 2007a). Since the task performance did not differ between the two groups, it is suggested that adults with the 22q11.2DS recruit posterior cingulate and parietal cortices to assist a hypofunctional frontal cortex to perform attentionally demanding tasks. Reduced frontal cortex activity in 22q11.2DS was also reported by Kates et al. (Kates et al., 2007a, 2007b) who reported reduced task-related dorsolateral prefrontal cortex activity in 22q11.2DS compared to both sibling and non-sibling controls--without apparent behavioral differences on the working memory task presented. Together, these findings suggest that there are alterations to the fronto-parietal attentional network that fundamentally change the way individuals with the 22q11.2DS process information. Altered white matter pathways have been identified in diffusion weighted imaging studies of the 22q11.2DS that demonstrate abnormal connectivity patterns throughout the 22q11.2DS brain, including abnormal corpus callosum location, shape, and diffusivity, as well as abnormal long and short distance white matter projections throughout the neocortex (Aneja et al., 2007; Karayiorgou et al., 2010; Kiehl et al., 2009; Machado et al., 2007; Simon et al., 2005c, 2008b).
III.III.IV Mouse 22q11.2DS Neurobehavioral Endophenotype
At present, the phenotype that receives the most attention for the mouse models of 22q11.2DS is an attenuated PPI response, similar to the Fmr1 KO mouse model (Long et al., 2006; Paylor and Lindsay, 2006; Stark et al., 2009). Potential behavioral endophenotypes are not widely studied in 22q11.2DS mouse models as the focus has been on modeling the aspects of the 22q11.2DS phenotype associated with schizophrenia and not on elucidating the underlying cognitive deficits in the 22q11.2DS population (cf., Karayiorgou et al., 2010; Paylor and Lindsay, 2006). One study has evaluated a few endophenotypes in the mouse models of 22q11.2DS, which was the report from Sigurdsson et al. (Sigurdsson et al., 2010; Stark et al., 2008a) demonstrating altered fear processing, attenuated PPI response, and poor spatial memory retrieval in the 22q11.2DS mouse model. To the author's knowledge, current research into the 22q11.2DS mouse models have not evaluated any of the hypotheses from the spatiotemporal attention model tested in humans. This may be because these mouse models have been used to model schizophrenic symptomatology more than the 22q11.2DS disorder associated with the genetic mutation (Drew et al., 2010; Karayiorgou et al., 2010).
At present, the genetics of the 22q11.2DS mouse models and the downstream consequences of the genetic dysregulation have been elucidated (e.g., micro-RNA disruption), and those findings have been correlated with behavioral outcomes (Stark et al., 2008b). One study has evaluated abnormal temporo-ammonic connectivity in 22q11.2DS mice evaluated as an abnormal lack of synchrony between hippocampal and rostral cortical neural activity during performance of a behavioral task shown to involve the interaction between these structures (Sigurdsson et al., 2010). This finding supports the reports of abnormal temporal-frontal lobe connectivity as well as hypoactivation in the prefrontal cortex during frontal-dependent tasks reported in 22q11.2DS individuals (Gothelf et al., 2007a, 2007b, 2010a). Another task suggests impaired hippocampal area CA3 function using immediate early gene transcription in response to novelty as a measure (Drew et al., 2011).
I. Comprehensive Behavioral Phenotyping Strategy
As demonstrated above, the research into mouse models of fragile X-associated disorders and the 22q11.2DS show promise for the general behavioral phenotypes associated with the full clinical manifestation of the disorders, but the results are often mixed and difficult to interpret (Karayiorgou et al., 2010; van Dam et al., 2000, 2005). Additionally, the research into behavioral endophenotypes have been largely absent. To overcome these challenges, it is necessary to evaluate the general health and well being of a mouse model with a strategy similar to using behavioral phenotyping screens and common behavioral tasks while, in parallel, evaluating how well the mouse models the cognitive deficits associated with specific genetic disorder in humans by applying what is known about the disorder from clinical neurology and cognitive neuroscience to develop specific hypotheses concerning the core deficits underlying the disorders. These hypotheses can then be used to selectively choose tasks to model the disorder in such a way as to maximize the application of the behavioral phenotype.
IV.I. Behavioral Screening -- Evaluating Gross-Level Disruptions
Although the direction of this review has been an emphasis on the need for behavioral endophenotyping techniques in the study of mouse models of neurodevelopmental disorders, it does not mitigate the necessity for a thorough analysis of the gross behavioral phenotype of the model using a standardized behavioral task battery (Crawley, 2004; Wahlsten, 1974, 2001). Optimally, a wide reaching behavioral assessment should be performed, ranging from an analysis of basic sensory function to tasks evaluating general memory--a process analogous to neurological/neuropsychological testing in patient populations (e.g., SHIRPA; Hatcher et al., 2001; Rogers et al., 1997, 1999, 2001; other protocols; Ahmed, 2010; Deo et al., 2010; Kendler and Neale, 2010; Kuang et al., 2010; Matsuo et al., 2010; Ryan et al., 2008, 2010; Takao et al., 2010; Taylor et al., 2010; Tennant and Jones, 2009; Wang et al., 2009; Zang et al., 2009). With such a battery, any confounding factors related to strain background may be identified and controlled for by comparison of the model with littermate or strain-related controls, as it is important to determine whether any major behavioral phenotypes are present in the mouse model at the outset of experimentation.
As neuroimaging techniques are being developed and applied to mouse models of genetic disorders, behavioral neuroscientists need to apply careful histological techniques to the study of neurodevelopmental and neurodegenerative disease. As a concrete example, in a number of disorders altered hippocampal volume has been reported (FXS patients show enlarged hippocampal volumes; Gothelf et al., 2008; Hallahan et al., 2010; Hessl et al., 2004; fragile X premutation carriers and individuals with 22q11.2DS show reductions in hippocampal volumes; Adams et al., 2010; Deboer et al. 2007; Jäkälä et al., 1997). It is important to evaluate whether analogous neuroanatomical features are present in the mouse model. One way to specifically evaluate these types of anatomical changes is to perform unbiased stereological estimates of not only regional volumes, but also of cellular number in regions of interest (cf., West et al., 1991). Stereological techniques involve relatively automated processes that requires a trained technician, but do not require advanced technologies beyond those available at most research institutions. The benefit of stereological methods over gross histological analysis is the ability to quantify anatomical features using a process that minimizes experimental bias and is significantly less time intensive as exhaustive counting procedures (Gatome et al., 2010; Simmons and Swanson, 2009; West et al., 1991). Similar techniques can be used not only using classic histological stains, but also can be used to quantify immunohistochemical staining or tissue processed for in situ hybridization (Jinno, 2010).
IV.II. Endophenotypes -- Uncovering Subtle, Disease Specific Effects
Once a behavioral phenotype (or lack of behavioral phenotype) is defined using common tasks, it is worthwhile to evaluate not only the disease being modeled by the mouse, but also the cognitive paradigms commonly administered to the population and the tasks used in the behavioral phenotyping strategy defined above. One concrete example within the fragile X-associated disorders and the 22q11.2DS, nonverbal learning impairments often manifest in clinical populations as difficulty in numerical or mathematical/arithmetic processing (Johnson-Glenberg, 2008; Simon, 2007, 2011). Reports such as these are problematic for the mouse researcher as no tasks clearly evaluating arithmetic or numerical processing have been undertaken in mice, and very little work has been undertaken evaluating spatial magnitude estimation. There have been limited studies into processes conceptualized as counting or enumeration in rats (Suzuki and Kobayashi, 2000), but nothing truly arithmetic (and the interpretation of rodent “counting” tasks remains controversial; cf., Gallistel and Gelman, 1992, 2000; Rayburn-Reeves et al., 2010; Simon, 1999). In contrast, if one approaches arithmetic deficits as the end behavioral outcome of a number of fundamental cognitive processes involving spatial ad temporal attention, a potential experimental battery becomes apparent (Gallistel, 1989, 1990; Simon, 1999, 2008).
Numerical processing is a high level cognitive process encompassing spatial and temporal attentional components that underlie judgment of temporal magnitude or duration (Cordes et al., 2007a; Gallistel, 1989, 1990; Gallistel and Gelman, 1990; Hunsaker et al., 2009, 2010; Johnson-Glenberg, 2008; Leslie et al., 2008). For example, one can evaluate mouse models for the ability to discriminate the temporal duration of tones to evaluate the ability of mice to judge temporal magnitude or evaluate the ability of mice to learn simple temporal sequences of stimuli (Balci et al., 2008; Balsam and Gallistel, 2009; Chiba et al., 2002; Cordes and Gallistel, 2008; Cordes et al., 2007b; Gallistel et al., 2010; Hunsaker et al., 2010; Jackson et al., 1998). One can further use tests evaluating the relative size of objects or absolute distance between objects compared to some standard to evaluate the ability of the mouse model to judge spatial magnitude (Clelland et al., 2009; Hunsaker et al., 2009; cf., Goodrich-Hunsaker et al., 2011a). None of these tasks evaluate numerical processing in themselves, but the processes being evaluated are the same as those shown to underly numerical cognition in humans (Gallistel and Gelman, 1990; Simon, 1999). The 22q11.2DS and FXS populations show deficits for spatial and temporal magnitude processing (Simon, 2007, 2008, 2011), and there are reports of the same in the fragile X premutation (Goodrich-Hunsaker et al., 2011a, 2011c), so if the corresponding mouse models show deficits for processing spatial or temporal magnitude (cf., Hunsaker et al., 2009, 2010), then inferences can be made concerning analogous processes underlying numerical processing in the mouse model and human disorder.
As functional imaging in rodents is in its infancy and used primarily in paralyzed preparations that do not allow for concurrent behavioral analysis (cf., Adamczak et al., 2010), it becomes important to use molecular tools to provide a functional snapshot of brain activity during task performance in mouse models of disease. Recently, in the learning and memory literature, early immediate genes such as Homer1a, Arc/Arg3.1, zif268, and c-Fos have been used as markers of neural activity and correlated with learning (Drew et al., 2011; Guzowski et al., 2005; Guzowski and Worley, 2001; Shepherd and Bear, 2011). Although such a technique (in situ hybridization) only allows for a snapshot of neural activity in response to very limited stimuli conditions, the technique can potentially be extended to include evaluation of regional differences in protein expression that may predict disease onset or severity prior to the emergence of disease-related pathological features (cf., Poirier et al., 2010).
Another functional assay is reported by Sigurdsson and colleagues (Sigurdsson et al., 2010) is that of in vivo neurophysiological recording to evaluate differences in either cellular spiking activity or extracellular rhythms that may be altered in disease--a process also being explored in human epilepsy (Jacobs et al., 2010). It has also been shown in mice that electrophysiological correlates of information processing used in humans, specifically event related potentials (ERP) measures on an electroencephalogram. It has further been suggested that the P300 and N400 potentials may show similar patterns in mice as humans when tasks are carefully designed (Choi et al., 2010; Olichney et al., 2010).
V. Conclusions
In recent years, there has been impetus placed on developing behavioral biomarkers that can be used to predict not only later disease onset or progression, but perhaps disease severity. These collections of intermediate or behavioral endophenotypes serve as outcome measures for pharmacological interventions (Almasy and Blangero, 2001; Amann et al., 2010; Burdick et al., 2006; Cannon and Keller, 2006; Castellanos and Tannock, 2002; Einat, 2007; Gottesman and Gould, 2003; Gould and Gottesman, 2006; Gur et al., 2007; Joo, 2008; Kendler and Neale, 2010; Keri and Benedek, 2009; Kuntsi et al., 2005; Saperstein et al., 2006). This search for behavioral biomarkers, however, has not consistently been extended into the mouse models of genetic disorders. To date, the closest research into mouse disease models comes to developing behavioral biomarkers is to thoroughly parameterize a single task (e.g., attenuated PPI response or audiogenic seizures for the Fmr1 KO mouse model) and apply the biomarker as a screen for various mouse models to choose candidates for drug studies (Long et al., 2006; Spencer et al., 2011; Zang et al., 2009). The strength of the standard approach is the ability to define a canon against which to gauge later models; however, the limitation of this approach is that it lacks the ability to evaluate complimentary models of a given disease to get at the fundamental processes disrupted in the human mutation.
This limitation occurs because a model may fail to model one phenotype, even though the mouse may model any number of other phenotypes that are not included in the standard behavioral screen. This lack of sensitivity is a major limitation as studies into the therapeutic effects of pharmacological agents will be incomplete in the absence of predefined behavioral biomarkers as outcome measures.
If the recent advances in the cognitive neuroscience of neurodevelopmental disorders are extended to their respective mouse models, perhaps the associated behavioral biomarkers of such disorders may not only be complimented by, but extended though use of mouse models studying the component processes underlying disease states. These well defined behavioral biomarkers can be used as correlates or covariates with molecular studies of underlying disease mechanisms in mice that cannot be directly studied in human patient populations.
Research Highlights.
Batteries of behavioral tasks often do not identify deficits in transgenic mice.
Hypothesis driven collections of tasks efficiently characterize subtle behavioral phenotypes
Behavioral Endophenotypes are quantitative traits modulated by dosage of gene mutations.
Behavioral endophenotypes can be used to define behavioral biomarkers for treatment studies.
Acknowledgments
The author would like to thank Dr. Tony J. Simon for helpful conversations concerning the direction of this manuscript, and Drs. Raymond P. Kesner, Ph.D. and Naomi J Goodrich-Hunsaker, Ph.D. for critical reading and discussions of preliminary versions of this manuscript.
Funding: MRH was supported by NINDS RL1 NS062411 awarded to Robert F. Berman. This work was also made possible by a Roadmap Initiative grant (UL1 DE019583) from the National Institute of Dental and Craniofacial Research (NIDCR) in support of the NeuroTherapeutics Research Institute (NTRI) consortium; and MRH was further supported by a training grant (UL1 RR024146) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this manuscript are solely the responsibility of the author and do not represent the official view of NCRR, NINDS, NIDCR, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VI. References
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M. High field BOLD response to forepaw stimulation in the mouse. NeuroImage. 2010;51:704–712. doi: 10.1016/j.neuroimage.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Adams PE, Adams JS, Nguyen DV, Hessl D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, Decarli C, Hagerman PJ, Hagerman RJ. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B. 2010;153B:775–785. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Pelphrey KA, Meck WH. Developmental Neuroscience of Time and Number: Implications for Autism and Other Neurodevelopmental Disabilities. Front Int Neurosci. 2011 doi: 10.3389/fnint.2012.00007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170:229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet B. 2001;105:42–44. [PubMed] [Google Scholar]
- Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, Siegel SJ. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;83(3-4):147–161. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2011;227(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja A, Fremont WP, Antshel KM, Faraone SV, AbdulSabur N, Higgins AM, Shprintzen R, Kates WR. Manic symptoms and behavioral dysregulation in youth with velocardiofacial syndrome (22q11.2 deletion syndrome) J Child Adolesc Psychopharmacol. 2007;17:105–114. doi: 10.1089/cap.2006.0023. [DOI] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull. 2010;36:289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babovic D, O'Tuathaigh CM, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Karayiorgou M, Gogos JA, Cotter D, Waddington JL. Exploratory and habituation phenotype of heterozygous and homozygous COMT knockout mice. Behav Brain Res. 2007;183:236–239. doi: 10.1016/j.bbr.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Bainbridge NK, Koselke LR, Jeon J, Bailey KR, Wess J, Crawley JN, Wrenn CC. Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav Brain Res. 2008;190:50–58. doi: 10.1016/j.bbr.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9(6):562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Oostra BA. Understanding fragile X syndrome: insights from animal models. Cytogenet Genome Res. 2003;100:111–123. doi: 10.1159/000072845. [DOI] [PubMed] [Google Scholar]
- Balci F, Papachristos EB, Gallistel CR, Brunner D, Gibson J, Shumyatsky GP. Interval timing in genetically modified mice: a simple paradigm. Genes Brain Behav. 2008;7:373–384. doi: 10.1111/j.1601-183X.2007.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Metz GA, McKenna JE, Klassen F, Whishaw IQ. The pasta matrix reaching task: a simple test for measuring skilled reaching distance, direction, and dexterity in rats. J Neurosci Methods. 2001;106:39–45. doi: 10.1016/s0165-0270(01)00326-0. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Tompkins G, Whishaw IQ. Skilled forelimb reaching for pasta guided by tactile input in the rat as measured by accuracy, spatial adjustments, and force. Behav Brain Res. 2000;109:49–57. doi: 10.1016/s0166-4328(99)00164-3. [DOI] [PubMed] [Google Scholar]
- Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM. Fractionating spatial memory with glutamate receptor subunit-knockout mice. Biochem Soc Transact. 2009;37:1323–1327. doi: 10.1042/BST0371323. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B. 2003;118B:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Vendrell I, Saksida LM, Bussey TJ. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine and improvements following donepezil. Psychopharmacology. 2011;214:537–548. doi: 10.1007/s00213-010-2050-1. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Monterossso JR, Sokol S, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, Zackai E, Emanuel BS, Simon TJ. Effects of COMT genotype on behavioral symptomatology in the 22q11.2 Deletion Syndrome. Child Neuropsychology. 2005;11:109–117. doi: 10.1080/09297040590911239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, Zackai E, Emanuel BS, Simon TJ. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry. 2004a;161:1700–1702. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Monterosso JR, Simon TJ, Glahn DC, Saleh PA, Hill NM, McDonald-McGinn DM, Zackai E, Emanuel BS, Cannon TD. Regional brain abnormalities in 22q11.2 deletion syndrome: association with cognitive abilities and behavioral symptoms. Neurocase. 2004b;10:198–206. doi: 10.1080/13554790490495519. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emannuel B, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychology. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Qin Y, Nguyen V, Johnson J, Pinter JD, Simon TJ. Increased incidence and size of cavum septum pellucidum in children with chromosome 22q11.2 deletion syndrome. Psychiatry Res. 2010;181:108–113. doi: 10.1016/j.pscychresns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15:290–299. [PubMed] [Google Scholar]
- Berge OG. Predictive validity of behavioral animal models for chronic pain. B J Pharmacology. 2011;164(4):1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22:2018–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- Bertone A, Hanck J, Kogan C, Chaudhuri A, Cornish K. Associating Neural Alterations and Genotype in Autism and Fragile X Syndrome: Incorporating Perceptual Phenotypes in Causal Modeling. J Autism Dev Disord. 2010;40(12):1541–1548. doi: 10.1007/s10803-010-1110-z. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Griebel G. Defensive responses to predator threat in the rat and mouse. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2005;Chapter 8 doi: 10.1002/0471142301.ns0819s30. Unit 8 19. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Rosen J. Olfaction and defense. Neurosci Biobehav Rev. 2008;32:1207–1208. doi: 10.1016/j.neubiorev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003a;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003b;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Blanchard DC. Alcohol, aggression and the stress of subordination. J Stud Alcohol. 1993;11:146–155. doi: 10.15288/jsas.1993.s11.146. [DOI] [PubMed] [Google Scholar]
- Bohlen M, Cameron A, Metten P, Crabbe JC, Wahlsten D. Calibration of rotational acceleration for the rotarod test of rodent motor coordination. J Neurosci Methods. 2009;178:10–14. doi: 10.1016/j.jneumeth.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JA, Cogswell JB, Hessl D, Zhang L, Ono MY, Tassone F, Farzin F, Brunberg JA, Grigsby J, Hagerman RJ. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen Hosp Psychiatry. 2007;29:349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JA, Farzin F, Brunberg JA, Tassone F, Hagerman P, Zhang L, Hessl D, Hagerman R. Dementia with mood symptoms in a fragile X premutation carrier with the fragile X-associated tremor/ataxia syndrome: clinical intervention with donepezil and venlafaxine. J Neuropsychiatry Clin Neurosci. 2006;18:171–177. doi: 10.1176/jnp.2006.18.2.171. [DOI] [PubMed] [Google Scholar]
- Bregman JD, Dykens E, Watson M, Ort SI, Leckman JF. Fragile-X syndrome: variability of phenotypic expression. J Am Acad Child Adolescent Psychiatry. 1987;26:463–471. doi: 10.1097/00004583-198707000-00001. [DOI] [PubMed] [Google Scholar]
- Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra BA, Willemsen R. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008a;107:1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313:244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008b;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis. 2006;194:255–260. doi: 10.1097/01.nmd.0000207360.70337.7e. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. Escape from fear: a detailed behavioral analysis of two atypical responses reinforced by CS termination. J Exp Psychol An Behav Process. 2007;33:451–463. doi: 10.1037/0097-7403.33.4.451. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, Murphy DG, Murphy KC. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Carola V, Scalera E, Brunamonti E, Gross C, D'Amato F. Mating-related interactions share common features with anxiety in the mouse. Behav Brain Res. 2008;186(2):185–90. doi: 10.1016/j.bbr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172:226–234. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131–7. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Casten KS, Gray AC, Burwell RD. Discrimination learning and attentional set formation in a mouse model of Fragile X. Behav Neurosci. 2011;125:473–479. doi: 10.1037/a0023561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee SS, Menard JL. Lesions of the dorsal lateral septum do not affect neophagia in the novelty induced suppression of feeding paradigm but reduce defensive behaviours in the elevated plus maze and shock probe burying tests. Behav Brain Res. 2011;220:362–366. doi: 10.1016/j.bbr.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Ann Rev Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Jackson PA. Two forms of spatial memory: a double dissociation between the parietal cortex and the hippocampus in the rat. Behav Neurosci. 2002;116:874–883. doi: 10.1037//0735-7044.116.5.874. [DOI] [PubMed] [Google Scholar]
- Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, Woo NH, Tranfaglia MR, Bear MF, Zukin RS, McDonald TV, Jongens TA, McBride SM. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Koch KP, Poppendieck W, Lee M, Shin HS. High resolution Electroencephalography in Freely Moving Mice. J Neurophysiol. 2010;104(3):182518–34. doi: 10.1152/jn.00188.2010. [DOI] [PubMed] [Google Scholar]
- Chonchaiya W, Schneider A, Hagerman RJ. Fragile X: a family of disorders. Adv Pediatr. 2009a;56:165–186. doi: 10.1016/j.yapd.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonchaiya W, Utari A, Pereira GM, Tassone F, Hessl D, Hagerman RJ. Broad clinical involvement in a family affected by the fragile X premutation. J Dev Behav Pediatrics. 2009b;30:544–551. doi: 10.1097/DBP.0b013e3181c35f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry. 1999;46:1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Crawford BC, Akbarian S. White matter neuron alterations in schizophrenia and related disorders. Int J Dev Neurosci. 2010;29(3):325–334. doi: 10.1016/j.ijdevneu.2010.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes S, Gallistel CR. Intact interval timing in circadian CLOCK mutants. Brain Res. 2008;1227:120–127. doi: 10.1016/j.brainres.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes S, Gallistel CR, Gelman R, Latham P. Nonverbal arithmetic in humans: light from noise. Percept Psychophys. 2007a;69:1185–1203. doi: 10.3758/bf03193955. [DOI] [PubMed] [Google Scholar]
- Cordes S, King AP, Gallistel CR. Time left in the mouse. Behav Process. 2007b;74:142–151. doi: 10.1016/j.beproc.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Ment Retard Dev Disabil Res Rev. 2004a;10:11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- Cornish K, Swainson R, Cunnington R, Wilding J, Morris P, Jackson G. Do women with fragile X syndrome have problems in switching attention: preliminary findings from ERP and fMRI. Brain Cogn. 2004b;54:235–239. doi: 10.1016/j.bandc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69:551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44:628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. The nature of the spatial deficit in young females with Fragile-X syndrome: a neuropsychological and molecular perspective. Neuropsychologia. 1998;36:1239–1246. doi: 10.1016/s0028-3932(97)00162-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with Fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex. 1999;35:263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Pilgram J, Shaw K. Do anomalies of handedness exist in children with Fragile-X syndrome? Laterality. 1997;2:91–101. doi: 10.1080/713754261. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R. Annotation: Deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. J Child Psychol Psychiatry. 2004c;45:1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- Corwin JV, Fussinger M, Meyer RC, King VR, Reep RL. Bilateral destruction of the ventrolateral orbital cortex produces allocentric but not egocentric spatial deficits in rats. Behav Brain Res. 1994;61:79–86. doi: 10.1016/0166-4328(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D. Of mice and their environments. Science. 2003;299:1313–1314. doi: 10.1126/science.299.5611.1313c. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Ret Dev Disabil Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci, USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curfs LM, Borghgraef M, Wiegers A, Schreppers-Tijdink GA, Fryns JP. Strengths and weaknesses in the cognitive profile of fra(X) patients. Clin Genet. 1989a;36:405–410. [PubMed] [Google Scholar]
- Curfs LM, Schreppers-Tijdink G, Wiegers A, Borghgraef M, Fryns JP. Intelligence and cognitive profile in the fra(X) syndrome: a longitudinal study in 18 fra(X) boys. J Med Genet. 1989b;26:443–446. doi: 10.1136/jmg.26.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curfs PM, Schreppers-Tijdink GA, Wiegers AM, van Velzen W, Fryns JP. Adaptive behavior in the fra(X) syndrome: a longitudinal study in eight patients. Am J Med Genet. 1989c;34:502–505. doi: 10.1002/ajmg.1320340409. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Hyponeophagia: a measure of anxiety in the mouse. J Vis Exp. 2011;17(51):2613. doi: 10.3791/2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13:536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Wu Z, Lee A, Simon TJ. Hippocampal volume reduction in children with chromosome 22q11.2 deletion syndrome is associated with cognitive impairment. Behav Brain Funct. 2007;3:54. doi: 10.1186/1744-9081-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo AJ, Costa R, DeLisi LE, DeSalle R, Haghighi F. A novel analytical framework for dissecting the genetic architecture of behavioral symptoms in neuropsychiatric disorders. PLoS ONE. 2010;5:e9714. doi: 10.1371/journal.pone.0009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J Neurosci. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, 3rd, Eichenbaum H. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx E, Engelborghs S, De Raedt R, Van Buggenhout M, De Deyn PP, Verté D, Ponjaert-Kristoffersen I. Verbal cued recall as a predictor of conversion to Alzheimer's disease in Mild Cognitive Impairment. Int J Geriatr Psychiatry. 2009;24(10):1094–100. doi: 10.1002/gps.2228. [DOI] [PubMed] [Google Scholar]
- Drago V, Foster PS, Skidmore F, Trifiletti D, Heilman KM. Spatial emotional akinesia in Parkinson disease. Cogn Behav Neurol. 2008;21(2):92–7. doi: 10.1097/WNN.0b013e31816bdfb2. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2010;29(3):259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Stark KL, Fenelon K, Karayiorgou M, Macdermott AB, Gogos JA. Evidence for altered hippocampal function in a mouse model of the human 22q11.2 microdeletion. Mol Cell Neurosci. 2011;47(4):293–305. doi: 10.1016/j.mcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Psychopharmacology of prepulse inhibition in mice. Chin J Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Leckman JF. Strengths and weaknesses in the intellectual functioning of males with fragile X syndrome. Am J Ment Defic. 1987;92:234–236. [PubMed] [Google Scholar]
- Eadie BD, Zhang, Boehme F, Gil-Mohapel J, Kainer L, Simpson JM, Christie BR. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis. 2009;36:361–373. doi: 10.1016/j.nbd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MR, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ. The neurobiology of memory based predictions. Phil Transact R Soc Lond B. 2009;364:1183–1191. doi: 10.1098/rstb.2008.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet. 2007;37:244–255. doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. NeuroImage. 2010;53:1023–1029. doi: 10.1016/j.neuroimage.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Ellis ME, Kesner RP. The noradrenergic system of the amygdala and aversive information processing. Behav Neurosci. 1983;97:399–415. doi: 10.1037//0735-7044.97.3.399. [DOI] [PubMed] [Google Scholar]
- Endo T, Maekawa F, Võikar V, Haijima A, Uemura Y, Zhang Y, Miyazaki W, Suyama S, Shimazaki K, Wolfer DP, Yada T, Tohyama C, Lipp HP, Kakeyama M. Automated test of behavioral flexibility in mice using a behavioral sequencing task in IntelliCage. Behav Brain Res. 2011;221(1):172–81. doi: 10.1016/j.bbr.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395:125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Liu L, Colwell KL, Whishaw IQ, Metz GA. Bilateral alteration in stepping pattern after unilateral motor cortex injury: a new test strategy for analysis of skilled limb movements in neurological mouse models. J Neurosci Methods. 2006;153:104–113. doi: 10.1016/j.jneumeth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Farzin F, Rivera SM. Dynamic Object Representations in Infants with and without Fragile X Syndrome. Front Hum Neurosci. 2010;4:12. doi: 10.3389/neuro.09.012.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Hessl D. Brief report: Visual processing of faces in individuals with fragile X syndrome: an eye tracking study. J Autism Dev Disord. 2009;39:946–952. doi: 10.1007/s10803-009-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Whitney D, Hagerman RJ, Rivera SM. Contrast detection in infants with fragile X syndrome. Vision Res. 2008;48:1471–1478. doi: 10.1016/j.visres.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM, Machado-Vieira R, Graybeal C, Sharp, Zarate CA, Harvey-White J, Du J, Sprengel R, Gass P, Bannerman DM, Holmes Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis. 2010;40(3):608–621. doi: 10.1016/j.nbd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. Animal cognition: The representation of space, time and number. Ann Rev Psychol. 1989;40:155–189. doi: 10.1146/annurev.ps.40.020189.001103. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. Representations in animal cognition: an introduction. Cognition. 1990;37:1–22. doi: 10.1016/0010-0277(90)90016-d. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gelman I. Non-verbal numerical cognition: from reals to integers. Trends Cog Sci. 2000;4:59–65. doi: 10.1016/s1364-6613(99)01424-2. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gelman R. The what and how of counting. Cognition. 1990;34:197–199. doi: 10.1016/0010-0277(90)90043-j. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gelman R. Preverbal and verbal counting and computation. Cognition. 1992;44:43–74. doi: 10.1016/0010-0277(92)90050-r. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, King AP, Daniel AM, Freestone D, Papachristos EB, Balci F, Kheifets A, Zhang J, Su X, Schiff G, Kourtev H. Screening for Learning and Memory Mutations: A New Approach. Xin Li Xue Bao. 2010;42:138–158. doi: 10.3724/SP.J.1041.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gatome CW, Slomianka L, Lipp HP, Amrein I. Number estimates of neuronal phenotypes in layer ll of the medial entorhinal cortex of rat and mouse. Neuroscience. 2010;170(1):156–165. doi: 10.1016/j.neuroscience.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Gerkes EH, Hordijk R, Dijkhuizen T, Sival DA, Meiners LC, Sikkema-Raddatz B, van Ravenswaaij-Arts CM. Bilateral polymicrogyria as the indicative feature in a child with a 22q11.2 deletion. Eur J Med Genet. 2010;53:344–346. doi: 10.1016/j.ejmg.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Campbell A, Kesner RP. The role of the amygdala in conditioned flavor preference. Neurobiol Learn Mem. 2003;79:118–121. doi: 10.1016/s1074-7427(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The amygdala but not the hippocampus is involved in pattern separation based on reward value. Neurobiol Learn Mem. 2002;77:338–353. doi: 10.1006/nlme.2001.4033. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer's disease. Exp Gerontol. 2004a;39:433–441. doi: 10.1016/j.exger.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer's disease, probable Alzheimer's disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004b;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Gleason TC, Dreiling JL, Crawley JN. Rat strain differences in response to galanin on the Morris water task. Neuropeptides. 1999;33:265–270. doi: 10.1054/npep.1999.0044. [DOI] [PubMed] [Google Scholar]
- Gogos JA. Schizophrenia susceptibility genes: in search of a molecular logic and novel drug targets for a devastating disorder. Int Rev Neurobiol. 2007;78:397–422. doi: 10.1016/S0074-7742(06)78013-8. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Karayiorgou M. “Targeting” schizophrenia in mice. Am J Med Genet. 2001;105:50–52. [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Howard BP, Hunsaker MR, Kesner RP. Human topological task adapted for rats: Spatial information processes of the parietal cortex. Neurobiol Learn Mem. 2008a;90:389–394. doi: 10.1016/j.nlm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008b;122:16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, Rivera SM, Simon TJ. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn. 2011a;75:255–260. doi: 10.1016/j.bandc.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Enhanced Manual and Oral Motor Reaction Time in Young Adult Female Fragile X Premutation Carriers. J Int Neuropsych Soc. 2011b doi: 10.1017/S1355617711000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Adult Female Fragile X Premutation Carriers Exhibit Age- and CGG Repeat Length-Related Impairments on an Attentionally Based Enumeration Task. Front Hum Neurosci. 2011c;5:63. doi: 10.3389/fnhum.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O'Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psych Res. 2010;45(3):322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–831. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Neb Symp Motiv. 1996;43:61–134. [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(Pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Navarro CS, Hunsaker MR, Maezawa I, Shuler JF, Tassone F, Delany M, Au JW, Berman RF, Jin LW, Schumann C, Hagerman PJ, Hagerman RJ. Neuropathologic features in the hippocampus and cerebellum of three older men with fragile X syndrome. Mol Autism. 2011;2:2. doi: 10.1186/2040-2392-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser DM, Van der Zee EA, Sheppard DK, Castillo MR, Gregg KA, Burrow T, Foltz H, Slater M, Bult-Ito A. Predictive validity of a non-induced mouse model of compulsive-like behavior. Behav Brain Res. 2011;221:55–62. doi: 10.1016/j.bbr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci, USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Boyett-Anderson JM, Menon V, Reiss AL. Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome. Neuroreport. 2004;15:1579–1583. doi: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, Jardini T, Tassone F, Hagerman PJ. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006a;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Leehey MA, Jacquemont S, Brunberg JA, Hagerman RJ, Wilson R, Epstein JH, Greco CM, Tassone F, Hagerman PJ. Cognitive impairment in a 65-year-old male with the fragile X-associated tremor-ataxia syndrome (FXTAS) Cogn Behav Neurol. 2006b;19:165–171. doi: 10.1097/01.wnn.0000213906.57148.01. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Guion RM. Content Validity--The Source of My Discontent. App Psychol Meas. 1977;1:1–10. [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schiz Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Guzowski, Worley PF. Cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2001;Chapter 1 doi: 10.1002/0471142301.ns0108s15. Unit 1.8. [DOI] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, George DN, Killcross S. Contextual control of biconditional task performance: evidence for cue and response competition in rats. Q J Exp Psychology. 2008;61:1307–1320. doi: 10.1080/17470210701515819. [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross S. Medial prefrontal cortex lesions abolish contextual control of competing responses. J Exp Anal Behav. 2005;84:485–504. doi: 10.1901/jeab.2005.81-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon JE, Killcross S. Both motivational and training factors affect response conflict choice performance in rats. Neural networks. 2006;19:1192–1202. doi: 10.1016/j.neunet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross S. Contextual control of choice performance: behavioral, neurobiological, and neurochemical influences. Ann NY Acad Sci. 2007;1104:250–269. doi: 10.1196/annals.1390.000. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal Daly, Moore CJ, Ambikapathy A, Robertson, Murphy KC, Murphy In vivo brain anatomy of adult males with Fragile X syndrome: An MRI study. NeuroImage. 2010;54(1):16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Libon DJ, Moelter ST, Swirsky-Sacchetti T, Scheffer L, Platek SM, Chute D. Temporal order memory differences in Alzheimer's disease and vascular dementia. J Clin Exp Neuropsychol. 2010;32(6):645–54. doi: 10.1080/13803390903418918. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Imbalanced pattern completion vs. separation in cognitive disease: network simulations of synaptic pathologies predict a personalized therapeutics strategy. BMC Neurosci. 2011;11:96. doi: 10.1186/1471-2202-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto RI, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2010;45(1):36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto RI, Srivastava S, Tassone F, Hagerman RJ, Rivera SM. Diffusion tensor imaging in male premutation carriers of the fragile x mental retardation gene. Mov Disord. 2011;26(7):1329–1336. doi: 10.1002/mds.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psych. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hatcher JP, Jones DN, Rogers DC, Hatcher PD, Reavill C, Hagan JJ, Hunter AJ. Development of SHIRPA to characterise the phenotype of gene-targeted mice. Behav Brain Res. 2001;125:43–47. doi: 10.1016/s0166-4328(01)00275-3. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci, USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Lian J, Spencer KM, Dien J, Donchin E. A cortical potential imaging analysis of the P300 and novelty P3 components. Hum Brain Map. 2001;12:120–130. doi: 10.1002/1097-0193(200102)12:2<120::AID-HBM1009>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, Senturk D, Schneider A, Lightbody A, Reiss AL, Hall S. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J Neurodev Disord. 2009;1:33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased Fragile X Mental Retardation Protein Expression Underlies Amygdala Dysfunction in Carriers of the Fragile X Premutation. Biol Psychiatry. 2011;70(9):859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Cordeiro L, Koldewyn K, McCormick C, Green C, Wegelin J, Yuhas J, Hagerman RJ. Brief report: aggression and stereotypic behavior in males with fragile X syndrome--moderating secondary genes in a “single gene” disorder. J Autism Dev Disord. 2008;38:184–189. doi: 10.1007/s10803-007-0365-5. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci, USA. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65:1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Humby T, Wilkinson L, Dawson G. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2005;Chapter 8 doi: 10.1002/0471142301.ns0805hs31. Unit 8.5H. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behav Brain Res. 2010;213:263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, von Leden RE, Ta BT, Goodrich-Hunsaker NJ, Arque G, Kim K, Willemsen R, Berman RF. Motor deficits on a ladder rung task in male and female adolescent and adult CGG knock-in mice. Behav Brain Res. 2011;222:117–121. doi: 10.1016/j.bbr.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123:1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behav Genet. 2008;38:493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, Sherman Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genetics. 2010;77(4):374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Christmon CA, Grossman AW, Galvez R, Kim SH, DeGrush BJ, Weiler IJ, Greenough WT. Fragile X mental retardation protein levels increase following complex environment exposure in rat brain regions undergoing active synaptogenesis. Neurobiol Learn Mem. 2005;83:180–187. doi: 10.1016/j.nlm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jackson PA, Kesner RP, Amann K. Memory for duration: role of hippocampus and medial prefrontal cortex. Neurobiol Learn Mem. 1998;70:328–348. doi: 10.1006/nlme.1998.3859. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Korolev IO, Caplan JB, Ekstrom AD, Litt B, Baltuch G, Fried I, Schulze-Bonhage A, Madsen JR, Kahana MJ. Right-lateralized brain oscillations in human spatial navigation. J Cog Neurosci. 2010;22:824–836. doi: 10.1162/jocn.2009.21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkälä P, Hänninen T, Ryynänen M, Laakso M, Partanen K, Mannermaa A, Soininen H. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J Clin Invest. 1997;100(2):331–8. doi: 10.1172/JCI119538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S. Topographic differences in adult neurogenesis in the mouse hippocampus: A stereology-based study using endogenous markers. Hippocampus. 2010;21(5):467–480. doi: 10.1002/hipo.20762. [DOI] [PubMed] [Google Scholar]
- Johnson DL, Kesner RP. Comparison of temporal order memory in early and middle stage Alzheimer's disease. J Clin Exp Neuropsychol. 1997;19(1):83–100. doi: 10.1080/01688639708403839. [DOI] [PubMed] [Google Scholar]
- Johnson-Glenberg MC. Fragile X syndrome: Neural network models of sequencing and memory. Cog Systems Res. 2008;9:274–292. doi: 10.1016/j.cogsys.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo YH. Neurophyisological and neurocognitive endophenotypes for schizophrenia genetics research. Psych Invest. 2008;5:199–202. doi: 10.4306/pi.2008.5.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nat Protocols. 2007;2:277–281. doi: 10.1038/nprot.2007.19. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ, Holstein SE, Crabbe JC. Characterization of the parallel rod floor apparatus to test motor incoordination in mice. Genes Brain Behav. 2005;4:253–266. doi: 10.1111/j.1601-183X.2004.00100.x. [DOI] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Mannisto PT. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114:1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Kao DI, Aldridge GM, Weiler IJ, Greenough WT. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc Natl Acad Sci, USA. 2010;107:15601–15606. doi: 10.1073/pnas.1010564107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res Mol Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA, Galke BL, Jeffery JA, Nestadt G, Wolyniec PS, Antonarakis SE, Kazazian HH, Housman DE, Driscoll DA, Pulver AE. Genotype and phenotype analysis at the 22q11 schizophrenia susceptibility locus. Cold Spring Harb Symp Quant Biol. 1996;61:835–843. [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, Higgins AM. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007a;143A:2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- Kates WR, Krauss BR, Abdulsabur N, Colgan D, Antshel KM, Higgins AM, Shprintzen RJ. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007b;45:2863–2873. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper MB, Hagerman RJ, Altshul S. Cognitive profiles of boys with the fragile X syndrome. Am J med Genet B. 1988;30:191–200. doi: 10.1002/ajmg.1320300118. [DOI] [PubMed] [Google Scholar]
- Kemper MB, Hagerman RJ, Ahmad RS, Mariner R. Cognitive profiles and the spectrum of clinical manifestations in heterozygous fra (X) females. Am J med Genet B. 1986;23:139–156. doi: 10.1002/ajmg.1320230109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K, Hess K, Tonegawa S, Small SA. CA3 NMDA receptors are required for experience-dependent shifts in hippocampal activity. Hippocampus. 2007;17:1003–1011. doi: 10.1002/hipo.20332. [DOI] [PubMed] [Google Scholar]
- Keri S, Benedek G. Visual pathway deficit in female fragile X premutation carriers: a potential endophenotype. Brain Cogn. 2009;69:291–295. doi: 10.1016/j.bandc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain Cogn. 2010;72:197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Keri S, Benedek G. Fragile X protein expression is linked to visual functions in healthy male volunteers. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.06.074. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the medial caudate nucleus, but not the hippocampus, in a matching-to sample task for a motor response. Eur J Neurosci. 2006;23:1888–1894. doi: 10.1111/j.1460-9568.2006.04709.x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR. The temporal attributes of episodic memory. Behav Brain Res. 2010;215:299–309. doi: 10.1016/j.bbr.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci. 2008;122:1217–1225. doi: 10.1037/a0013592. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Ragozzino M. Pharmacological approaches to animal models of human working memory and shifting attentional set. Commentary on Robbins' homology in behavioural pharmacology: an approach to animal models of human cognition. Behav Pharmacol. 1998;9:521–524. doi: 10.1097/00008877-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Rijken S, Joosten-Weyn Banningh LW, Van Schuylenborgh-VAN Es N, Olde Rikkert MG. Categorical spatial memory in patients with mild cognitive impairment and Alzheimer dementia: positional versus object-location recall. J Int Neuropsychol Soc. 2010;16(1):200–4. doi: 10.1017/S1355617709990944. [DOI] [PubMed] [Google Scholar]
- Kiehl TR, Chow EW, Mikulis DJ, George SR, Bassett AS. Neuropathologic features in adults with 22q11.2 deletion syndrome. Cerebral Cortex. 2009;19:153–164. doi: 10.1093/cercor/bhn066. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. J Neurosci. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL, Finn DA, Crabbe JC. Validation of a modified mirrored chamber sensitive to anxiolytics and anxiogenics in mice. Psychopharmacology. 2003;169:190–197. doi: 10.1007/s00213-003-1493-z. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Bertone A, Cornish K, Boutet I, Der Kaloustian VM, Andermann E, Faubert J, Chaudhuri A. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004a;63:1634–1639. doi: 10.1212/01.wnl.0000142987.44035.3b. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Graham GE, Berry-Kravis E, Drouin A, Milgram NW. A comparative neuropsychological test battery differentiates cognitive signatures of Fragile X and Down syndrome. J Intellect Disabil Res. 2009;53:125–142. doi: 10.1111/j.1365-2788.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish KM, Zangenehpour S, Mullen KT, Holden JJ, Der Kaloustian VM, Andermann E, Chaudhuri A. Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain. 2004b;127:591–601. doi: 10.1093/brain/awh069. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: a controlled study. Am J Med Genet B. 2008;147B:859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- Koldewyn K, Hessl D, Adams J, Tassone F, Hagerman PJ, Hagerman RJ, Rivera SM. Reduced Hippocampal Activation During Recall is Associated with Elevated FMR1 mRNA and Psychiatric Symptoms in Men with the Fragile X Premutation. Brain Imaging Behav. 2008;2:105–116. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy RF, Reyniers E, Verhoye M, Sijbers J, Bakker CE, Oostra BA, Willems PJ, Van Der Linden A. Neuroanatomy of the fragile X knockout mouse brain studied using in vivo high resolution magnetic resonance imaging. Eur J Hum Genet. 1999;7:526–532. doi: 10.1038/sj.ejhg.5200348. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Kessels HW, Bush DE, Cain CK, LeDoux JE, Malinow R. A robust automated method to analyze rodent motion during fear conditioning. Neuropharmacology. 2007;52:228–233. doi: 10.1016/j.neuropharm.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. You can play 20 questions with nature and win: categorical versus coordinate spatial relations as a case study. Neuropsychologia. 2006;44:1519–1523. doi: 10.1016/j.neuropsychologia.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Chabris CF, Marsolek CJ, Koenig O. Categorical versus coordinate spatial relations: computational analyses and computer simulations. J Exp Psychol Hum Percept Perf. 1992;18:562–577. doi: 10.1037//0096-1523.18.2.562. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Koenig O, Barrett A, Cave CB, Tang J, Gabrieli JD. Evidence for two types of spatial representations: hemispheric specialization for categorical and coordinate relations. J Exp Psychol Hum Percept Perf. 1989;15:723–735. doi: 10.1037//0096-1523.15.4.723. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Henderson JT, Chan E, Lifshitz N, Bishop J, Evans AC, Henkelman RM, Chen XJ. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cerebral cortex. 2005;15:639–645. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci, USA. 2011;108:2587–2592. doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang H, Mei B, Cui Z, Lin L, Tsien JZ. A novel behavioral paradigm for assessing concept of nests in mice. J Neurosci Methods. 2010;189(2):169–175. doi: 10.1016/j.jneumeth.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Borger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz AM, Dawson DV, Spiridigliozzi GA, McConkie-Rosell A. Arithmetic difficulties in females with the fragile X premutation. Am J Med Genet A. 2006;140:665–672. doi: 10.1002/ajmg.a.31082. [DOI] [PubMed] [Google Scholar]
- Larson J, Kim D, Patel RC, Floreani C. Olfactory discrimination learning in mice lacking the fragile X mental retardation protein. Neurobiol Learn Mem. 2008;90:90–102. doi: 10.1016/j.nlm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AM, Gelman R, Gallistel CR. The generative basis of natural number concepts. Trends Cog Sci. 2008;12:213–218. doi: 10.1016/j.tics.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Reiss AL. Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev Disabil Res Rev. 2009;15:343–352. doi: 10.1002/ddrr.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropharmacol. 2011;14:618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano Lopez L, Hauser J, Feldon J, Gargiulo PA, Yee BK. Evaluating spatial memory function in mice: a within-subjects comparison between the water maze test and its adaptation to dry land. Behav Brain Res. 2010;209:85–92. doi: 10.1016/j.bbr.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Grigsby J, Butler E, Epstein J, Huggins RM, Taylor AK, Hagerman RJ. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17:646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- Long JM, LaPorte P, Merscher S, Funke B, Saint-Jore B, Puech A, Kucherlapati R, Morrow BE, Skoultchi AI, Wynshaw-Boris A. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 2006;7:247–257. doi: 10.1007/s10048-006-0054-0. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Luisa-Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisa-Scattoni M, Ricceri L, Crawley JN. Unusual Repertoire of Vocalizations in Adult BTBR T+tf/J Mice During Three Types of Social Encounters. Genes Brain Behav. 2011;10(1):44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maasberg DW, Shelley LE, Gracian EI, Gilbert PE. Age-related differences in the anticipation of future rewards. Behav Brain Res. 2011;223:371–375. doi: 10.1016/j.bbr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AM, Simon TJ, Nguyen V, McDonald-McGinn DM, Zackai EH, Gee JC. Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Res. 2007;1131:197–210. doi: 10.1016/j.brainres.2006.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod LS, Kogan CS, Collin CA, Berry-Kravis E, Messier C, Gandhi R. A comparative study of the performance of individuals with fragile X syndrome and Fmr1 knockout mice on Hebb-Williams mazes. Genes Brain Behav. 2010;9:53–64. doi: 10.1111/j.1601-183X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- Madan S, Madan-Khetarpal S, Park SC, Surti U, Bailey AL, McConnell J, Tadros SS. Left ventricular non-compaction on MRI in a patient with 22q11.2 distal deletion. Am J Med Genet A. 2010;152A:1295–1299. doi: 10.1002/ajmg.a.33367. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni L, Paredes D, Tragon T, Pearson B, Shaltiel G, Chen G, Crawley JN, Manji HK. The Female Urine Sniffing Test: A Novel Approach for Assessing Reward-Seeking Behavior in Rodents. Biol Psych. 2009;67(9):864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Gottesman II, Gould TD. Signal transduction and genes-to-behaviors pathways in psychiatric diseases. Sci STKE. 2003;2003:pe49. doi: 10.1126/stke.2003.207.pe49. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocco MM. Advances in research on the fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM. Math learning disability and math LD subtypes: evidence from studies of Turner syndrome, fragile X syndrome, and neurofibromatosis type 1. J Learn Disabil. 2001;34:520–533. doi: 10.1177/002221940103400605. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Baumgardner T, Freund LS, Reiss AL. Social functioning among girls with fragile X or Turner syndrome and their sisters. J Autism Dev Disord. 1998;28:509–517. doi: 10.1023/a:1026000111467. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Devlin KT, McKenney SJ. Is it a fact? Timed arithmetic performance of children with mathematical learning disabilities (MLD) varies as a function of how MLD is defined. Dev Neuropsychol. 2008;33:318–344. doi: 10.1080/87565640801982403. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Hagerman RJ, Cronister-Silverman A, Pennington BF. Specific frontal lobe deficits among women with the fragile X gene. J Am Acad Child Adolesc Psychiatry. 1992a;31:1141–1148. doi: 10.1097/00004583-199211000-00025. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Hagerman RJ, Pennington BF. Problem solving limitations among cytogenetically expressing fragile X women. Am J Med Genet. 1992b;43:78–86. doi: 10.1002/ajmg.1320430112. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J Dev Behav Pediatrics. 1993;14:328–335. [PubMed] [Google Scholar]
- Mazzocco MM, Pennington BF, Hagerman RJ. Social cognition skills among females with fragile X. J Autism Dev Disord. 1994;24:473–485. doi: 10.1007/BF02172129. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Abrams L, Finucane B, Cronister A, Gane LW, Coffey SM, Sherman S, Nelson LM, Berry-Kravis E, Hessl D, Chiu S, Street N, Vatave A, Hagerman RJ. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J Genet Couns. 2007;16:593–606. doi: 10.1007/s10897-007-9099-y. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affective Disord. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meert TF, Colpaert FC. The shock probe conflict procedure. A new assay responsive to benzodiazepines, barbiturates and related compounds. Psychopharmacology. 1986;88:445–450. doi: 10.1007/BF00178505. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Crusio WE. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res Bull. 2002;57:41–47. doi: 10.1016/s0361-9230(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Daly EM, Schmitz N, Tassone F, Tysoe C, Hagerman RJ, Hagerman PJ, Morris RG, Murphy KC, Murphy DG. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004a;42:1934–1947. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Daly EM, Tassone F, Tysoe C, Schmitz N, Ng V, Chitnis X, McGuire P, Suckling J, Davies KE, Hagerman RJ, Hagerman PJ, Murphy KC, Murphy DG. The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain. 2004b;127:2672–2681. doi: 10.1093/brain/awh256. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav Brain Res. 2008a;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008b;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Mentis MJ, Pietrini P, Grady CL, Moore CJ, Horwitz B, Hinton V, Dobkin CS, Schapiro MB, Rapoport SI. Premutation female carriers of fragile X syndrome: a pilot study on brain anatomy and metabolism. J Am Acad Child Adolesc Psychiatry. 1999;38:1294–1301. doi: 10.1097/00004583-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Mazzocco MM, Hanich LB, Early MC. Cognitive characteristics of children with mathematics learning disability (MLD) vary as a function of the cutoff criterion used to define MLD. J Learn Disabil. 2007;40:458–478. doi: 10.1177/00222194070400050901. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Zou F, Huang H, Moy SS, Lauder J, Crawley JN, Threadgill DW, Wright FA, Magnuson TR. Large-scale gene expression differences across brain regions and inbred strains correlate with a behavioral phenotype. Genetics. 2006;174:1229–1236. doi: 10.1534/genetics.106.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res. 2002;927:8–17. doi: 10.1016/s0006-8993(01)03309-1. [DOI] [PubMed] [Google Scholar]
- Noble C, Baker BL, Jones TA. Age and Sex Parameters in Psychomotor Learning. Percept Motor Skills. 1964;19:935–945. doi: 10.2466/pms.1964.19.3.935. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Chan S, Wong LM, Schneider A, Seritan A, Niese A, Yang JC, Laird K, Teichholtz S, Khan S, Tassone F, Hagerman R. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci, USA. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Iwunze M, Davis SF, Malekiani SA, Hanania T. Comparison of the predictive validity of the mirror chamber and elevated plus maze tests in mice. J Neurosci Methods. 2010;188:62–70. doi: 10.1016/j.jneumeth.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Paylor R, Lindsay E. Mouse models of 22q11 deletion syndrome. Biol Psychiatry. 2006;59:1172–1179. doi: 10.1016/j.biopsych.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacol. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi CH, Oghalai J, Curran, Murphy KC, Monks S, Williams N, O'Donovan MC, Owen MJ, Scambler PJ, Lindsay E. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Zhao Y, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. 2001;73:781–792. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. C57BL/6J mice fail to exhibit preference for social novelty in the three-chamber apparatus. Behav Brain Res. 2010;213:189–194. doi: 10.1016/j.bbr.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky E, Goldstein J, Peavy G, Jacobson MW, Corey-Bloom J, Gilbert PE. Temporal order memory deficits prior to clinical diagnosis in Huntington's disease. J Int Neuroopsych Soc. 2009;15:662–670. doi: 10.1017/S1355617709990427. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier GL, Amin E, Good MA, Aggleton JP. Early-onset dysfunction of retrosplenial cortex precedes overt amyloid plaque formation in Tg2576 mice. Neuroscience. 2010;174:71–83. doi: 10.1016/j.neuroscience.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2001;Chapter 8 doi: 10.1002/0471142301.ns0810as14. Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FA, Rafal RD. How do the parietal lobes direct covert attention? Neuropsychologia. 1987;25(1A):135–45. doi: 10.1016/0028-3932(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42:85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Raske C, Hagerman PJ. Molecular Pathogenesis of Fragile X-Associated Tremor/Ataxia Syndrome. J Invest Med. 2009;57(8):825–829. doi: 10.231/JIM.0b013e3181be329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, Miller HC, Zentall TR. “Counting” by pigeons: Discrimination of the number of biologically relevant sequential events. Learn Behav. 2010;38:169–176. doi: 10.3758/LB.38.2.169. [DOI] [PubMed] [Google Scholar]
- Reiss AL. Childhood developmental disorders: an academic and clinical convergence point for psychiatry, neurology, psychology and pediatrics. J Child Psychol Psychiatry. 2009;50:87–98. doi: 10.1111/j.1469-7610.2008.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Aylward E, Freund LS, Joshi PK, Bryan RN. Neuroanatomy of fragile X syndrome: the posterior fossa. Ann Neurol. 1991a;29:26–32. doi: 10.1002/ana.410290107. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Freund L, Tseng JE, Joshi PK. Neuroanatomy in fragile X females: the posterior fossa. Am J Hum Genet. 1991b;49:279–288. [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Patel S, Kumar AJ, Freund L. Preliminary communication: neuroanatomical variations of the posterior fossa in men with the fragile X (Martin-Bell) syndrome. Am J Med Genet. 1988;31:407–414. doi: 10.1002/ajmg.1320310220. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Barbosa ER, Canteras NS, Cezario AF, Blanchard RJ, Blanchard DC. An alternative experimental procedure for studying predator-related defensive responses. Neurosci Biobehav Rev. 2005;29:1255–1263. doi: 10.1016/j.neubiorev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley JN. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Menon V, White CD, Glaser B, Reiss AL. Functional brain activation during arithmetic processing in females with fragile X Syndrome is related to FMR1 protein expression. Human Brain Map. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L, Triesman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: evidence from Balint's Syndrome. J Cogn Neurosc. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilter CA, Fisher EM. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett. 2001;306:89–92. doi: 10.1016/s0304-3940(01)01885-7. [DOI] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathologica. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Rupp J, Blekher T, Jackson J, Beristain X, Marshall J, Hui S, Wojcieszek J, Foroud T. Progression in Prediagnostic Huntington Disease. J Neurol Neurosurg Psychiatry. 2009;81(4):379–384. doi: 10.1136/jnnp.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Influence of task parameters on rotarod performance and sensitivity to ethanol in mice. Behav Brain Res. 2003;141:237–249. doi: 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka E, Elibol B. Enhanced cued recall and clock drawing test performances differ in Parkinson's and Alzheimer's disease-related cognitive dysfunction. Parkinsonism Relat Disord. 2009;15(9):688–91. doi: 10.1016/j.parkreldis.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Saldivar-Gonzalez JA, Posadas-Andrews A, Rodriguez R, Gomez C, Hernandez-Manjarrez ME, Ortiz-Leon S, Martinez-Pineda A, Gomez-Laguna D, Salgado V, Manjarrez J, Alvarado R. Effect of electrical stimulation of the baso-lateral amygdala nucleus on defensive burying shock probe test and elevated plus maze in rats. Life Sci. 2003;72:819–829. doi: 10.1016/s0024-3205(02)02335-4. [DOI] [PubMed] [Google Scholar]
- Salomonczyk D, Panzera R, Pirogovosky E, Goldstein J, Corey-Bloom J, Simmons R, Gilbert PE. Impaired postural stability as a marker of premanifest Huntington's disease. Mov Disord. 2010;25(14):2428–2433. doi: 10.1002/mds.23309. [DOI] [PubMed] [Google Scholar]
- Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, Gold JM. Spatial working memory as a cognitive endophenotype of schizophrenia: assessing risk for pathophysiological dysfunction. Schiz Bull. 2006;32:498–506. doi: 10.1093/schbul/sbj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Debbane M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, Eliez S. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res. 2009;115:182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Schaer M, Schmitt JE, Glaser B, Lazeyras F, Delavelle J, Eliez S. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11.2): an MRI study. Psychiatry Res. 2006;146:1–11. doi: 10.1016/j.pscychresns.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, Hessl D. Fragile X syndrome -- from genes to cognition. Dev Disabil Res Rev. 2009;15:333–342. doi: 10.1002/ddrr.80. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Robertson MM, Rizzo R, Turk J, Bhatia KP, Orth M. Fragile X syndrome associated with tic disorders. Mov Disord. 2008;23:1108–1112. doi: 10.1002/mds.21995. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Deutsch LH, Cohen C, Warden D, Deutsch SI. Memory for temporal order in schizophrenia. Biol Psychiatry. 1991;29(4):329–39. doi: 10.1016/0006-3223(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley BA, Deary IJ, Tan J, Christie G, Starr JM. Efficiency of temporal order discrimination as an indicator of bradyphrenia in Parkinson's disease: the inspection time loop task. Neuropsychologia. 2002;40(8):1488–93. doi: 10.1016/s0028-3932(01)00195-6. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikiric P, Jelovac N, Jelovac-Gjeldum A, Dodig G, Staresinic M, Anic T, Zoricic I, Ferovic D, Aralica G, Buljat G, Prkacin I, Lovric-Bencic M, Separovic J, Seiwerth S, Rucman R, Petek M, Turkovic B, Ziger T. Anxiolytic effect of BPC-157, a gastric pentadecapeptide: shock probe/burying test and light/dark test. Acta Pharmacol Sinica. 2001;22:225–230. [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology. 2009;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley Sociability and Motor Functions in Shank1 Mutant Mice. Brain Res. 2010;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparing histological data from different brains: sources of error and strategies for minimizing them. Brain Res Rev. 2009;60:349–367. doi: 10.1016/j.brainresrev.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ. The foundations of numerical thinking in a brain without numbers. Trends Cogn Sci. 1999;3:363–365. doi: 10.1016/s1364-6613(99)01383-2. [DOI] [PubMed] [Google Scholar]
- Simon TJ. Cognitive characteristics of children with genetic syndromes. Child Adolesc Psychiatr Clin N Am. 2007;16:599–616. doi: 10.1016/j.chc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ. A new account of the neurocognitive foundations of impairments in space, time and number processing in children with chromosome 22q11.2 deletion syndrome. Dev Disabil Res Rev. 2008;14:52–58. doi: 10.1002/ddrr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ. Rewards and challenges of cognitive neuroscience studies of persons with intellectual and developmental disabilities. Am J Intellect Disabilities. 2010;115:79–82. doi: 10.1352/1944-7558-115.2.79. [DOI] [PubMed] [Google Scholar]
- Simon TJ. Clues to the foundation of numerical cognitive impairments: evidence from genetic disorders. Dev Neuropsychol. 2011;36(6):788–805. doi: 10.1080/87565641.2010.549879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005a;41:145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bish JP, Bearden CE, Ding L, Ferrante S, Nguyen V, Gee JC, McDonald-McGinn DM, Zackai EH, Emanuel BS. A multilevel analysis of cognitive dysfunction and psychopathology associated with chromosome 22q11.2 deletion syndrome in children. Dev Psychopathol. 2005b;17:753–784. doi: 10.1017/S0954579405050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. NeuroImage. 2005c;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Takarae Y, DeBoer T, McDonald-McGinn DM, Zackai EH, Ross JL. Overlapping numerical cognition impairments in children with chromosome 22q11.2 deletion or Turner syndromes. Neuropsychologia. 2008a;46:82–94. doi: 10.1016/j.neuropsychologia.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Wu Z, Avants B, Zhang H, Gee JC, Stebbins GT. Atypical cortical connectivity and visuospatial cognitive impairments are related in children with chromosome 22q11.2 deletion syndrome. Behav Brain Funct. 2008b;4:25. doi: 10.1186/1744-9081-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1 KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Sporn A, Addington A, Reiss AL, Dean M, Gogtay N, Potocnik U, Greenstein D, Hallmayer J, Gochman P, Lenane M, Baker N, Tossell J, Rapoport JL. 22q11 deletion syndrome in childhood onset schizophrenia: an update. Mol Psychiatry. 2004;9:225–226. doi: 10.1038/sj.mp.4001477. [DOI] [PubMed] [Google Scholar]
- Stark KL, Burt RA, Gogos JA, Karayiorgou M. Analysis of prepulse inhibition in mouse lines overexpressing 22q11.2 orthologues. Int J Neuropsychopharmacol. 2009;12(7):1–7. doi: 10.1017/S1461145709000492. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genetics. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophrenia research. 2010;118:118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert J, Borghgraef M, Fryns JP. Apparently enhanced visual information processing in female fragile X carriers: preliminary findings. Am J Med Genet B. 1994;51:374–377. doi: 10.1002/ajmg.1320510415. [DOI] [PubMed] [Google Scholar]
- Steyaert J, Borghgraef M, Gaulthier C, Fryns JP, Van den Berghe H. Cognitive profile in adult, normal intelligent female fragile X carriers. Am J Med Genet B. 1992;43:116–119. doi: 10.1002/ajmg.1320430117. [DOI] [PubMed] [Google Scholar]
- Sundram F, Campbell L, Azuma R, Daly E, Bloemen O, Barker G, Chitnis X, Jones D, van Amelsvoort T, Murphy K. White matter microstructure in 22q11 deletion syndrome: a pilot diffusion tensor imaging and voxel-based morphometry study of children and adolescents. J Neurodevel Disorders. 2010:1–16. doi: 10.1007/s11689-010-9043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kobayashi T. Numerical competence in rats (Rattus norvegicus): Davis and Bradford (1986) extended. J Comp Psych. 2000;114(1):73–85. doi: 10.1037/0735-7036.114.1.73. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Mapping the human brain: past, present, and future. Trends Neurosci. 1995;18:471–474. doi: 10.1016/0166-2236(95)92766-j. [DOI] [PubMed] [Google Scholar]
- Sztriha L, Guerrini R, Harding B, Stewart F, Chelloug N, Johansen JG. Clinical, MRI, and pathological features of polymicrogyria in chromosome 22q11 deletion syndrome. Am J Med Genet A. 2004;127A:313–317. doi: 10.1002/ajmg.a.30014. [DOI] [PubMed] [Google Scholar]
- Takao K, Tanda K, Nakamura K, Kasahara J, Nakao K, Katsuki M, Nakanishi K, Yamasaki N, Toyama K, Adachi M, Umeda M, Araki T, Fukunaga K, Kondo H, Sakagami H, Miyakawa T. Comprehensive Behavioral Analysis of Calcium/Calmodulin-Dependent Protein Kinase IV Knockout Mice. PloS One. 2010;5:e9460. doi: 10.1371/journal.pone.0009460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Schmidt L, Tassone F, Simon TJ. Catechol-O-methyltransferase polymorphism modulates cognitive control in children with chromosome 22q11.2 deletion syndrome. Cog Affect Behav Neurosci. 2009;9:83–90. doi: 10.3758/CABN.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL. fMRI study of cognitive interference processing in females with fragile X syndrome. J Cogn Neurosci. 2002;14:160–171. doi: 10.1162/089892902317236812. [DOI] [PubMed] [Google Scholar]
- Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman P. Expression of the FMR1 gene. Cytogenet Genome Res. 2003;100:124–128. doi: 10.1159/000072846. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet. 2000;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84:250–261. [PubMed] [Google Scholar]
- Taylor TN, Greene JG, Miller GW. Behavioral phenotyping of mouse models of Parkinson's Disease. Behav Brain Res. 2010;211(1):1–10. doi: 10.1016/j.bbr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Asay AL, Allred RP, Ozburn AR, Kleim JA, Jones TA. The vermicelli and capellini handling tests: simple quantitative measures of dexterous forepaw function in rats and mice. J Vis Exp. 2010;41:2076. doi: 10.3791/2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J Neurosci Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol (Berl) 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. A comparison of buspirone and chlordiazepoxide in the shock-probe/burying test for anxiolytics. Pharmacol Biochem Behav. 1988;30:1071–1075. doi: 10.1016/0091-3057(88)90141-4. [DOI] [PubMed] [Google Scholar]
- Uchida S, Kitamoto A, Umeeda H, Nakagawa N, Masushige S, Kida S. Chronic reduction in dietary tryptophan leads to changes in the emotional response to stress in mice. J Nutr Sci Vitaminol (Tokyo) 2005;51(3):175–81. doi: 10.3177/jnsv.51.175. [DOI] [PubMed] [Google Scholar]
- Utari A, Adams E, Berry-Kravis E, Chavez A, Scaggs F, Ngotran L, Boyd A, Hessl D, Gane LW, Tassone F, Tartaglia N, Leehey MA, Hagerman RJ. Aging in fragile X syndrome. J Neurodev Disord. 2010;2:70–76. doi: 10.1007/s11689-010-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, D'Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117:127–136. [Google Scholar]
- Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJ, Curfs LM, Ramakers GJ. Profiling Fragile X Syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabilities. 2010;31:426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Velez L, Sokoloff G, Miczek KA, Palmer AA, Dulawa SC. Differences in aggressive behavior and DNA copy number variants between BALB/cJ and BALB/cByJ substrains. Behav Genet. 2010;40:201–210. doi: 10.1007/s10519-009-9325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen ER, Moscovitch M. Memory for temporal order and conditional associative-learning in patients with Parkinson's disease. Neuropsychologia. 1990;28(12):1283–93. doi: 10.1016/0028-3932(90)90044-o. [DOI] [PubMed] [Google Scholar]
- Wahlsten A developmental time scale for postnatal changes in brain and behavior of B6D2F2 mice. Brain Res. 1974;72:251–264. doi: 10.1016/0006-8993(74)90863-4. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Standardizing tests of mouse behavior: reasons, recommendations, and reality. Physiol Behav. 2001;73:695–704. doi: 10.1016/s0031-9384(01)00527-3. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Genetic experiments with animal learning: a critical review. Behav Biol. 1972a;7:143–182. doi: 10.1016/s0091-6773(72)80197-4. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Standardizing tests of mouse behavior: reasons, recommendations, and reality. Physiol Behav. 2001;73:695–704. doi: 10.1016/s0031-9384(01)00527-3. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten, Phillips TJ, Boehm SL, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003a;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Rustay NR, Metten P, Crabbe JC. In search of a better mouse test. Trends Neurosci. 2003b;26:132–136. doi: 10.1016/S0166-2236(03)00033-X. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003c;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci, USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Walter E, Mazaika PK, Reiss AL. Insights into brain development from neurogenetic syndromes: evidence from fragile X syndrome, Williams syndrome, Turner syndrome and velocardiofacial syndrome. Neuroscience. 2009;164:257–271. doi: 10.1016/j.neuroscience.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Simpson HB, Dulawa SC. Assessing the validity of current mouse genetic models of obsessive-compulsive disorder. Behav Pharmacol. 2009;20:119–133. doi: 10.1097/FBP.0b013e32832a80ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NM, Brown VJ. Covert orienting of attention in the rat and the role of striatal dopamine. J Neurosci. 1996;16:3082–3088. doi: 10.1523/JNEUROSCI.16-09-03082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NM, Brown VJ. Deficits in response initiation, but not attention, following excitotoxic lesions of posterior parietal cortex in the rat. Brain Res. 1997;775:81–90. doi: 10.1016/s0006-8993(97)00915-3. [DOI] [PubMed] [Google Scholar]
- Ward NM, Sharkey J, Marston HM, Brown VJ. Simple and choice reaction-time performance following occlusion of the anterior cerebral arteries in the rat. Exp Bran Res. 1998;123:269–281. doi: 10.1007/s002210050569. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Van Os J, Davidson M. Time for a shift in focus in schizophrenia: from narrow phenotypes to broad endophenotypes. B J Psychiatry. 2005;187:203–205. doi: 10.1192/bjp.187.3.203. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010;1318:155–66. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Coles BL. Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav Brain Res. 1996;77:135–148. doi: 10.1016/0166-4328(95)00209-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996;60:1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Whishaw P, Gorny B. The structure of skilled forelimb reaching in the rat: a movement rating scale. J Vis Exp. 2008;18:816. doi: 10.3791/816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, van Unen L, Tassone F, Hoogeveen AT, Hagerman PJ, Mientjes EJ, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- Xu B, Karayiorgou M, Gogos JA. microRNAs in Psychiatric and Neurodevelopmental Disorders. Brain Res. 2010;1338:78–88. doi: 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis AT, Roth HL, Heilman KM. Fragile X dementia Parkinsonism Syndrome (FXDPS) Cogn Behav Neurol. 2010;23:39–43. doi: 10.1097/WNN.0b013e3181b6e1b9. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Srivastava D. Unraveling the genetic and developmental mysteries of 22q11 deletion syndrome. Trends Mol Medicine. 2003;9:383–389. doi: 10.1016/s1471-4914(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav. 2004;81:465–473. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2009;Chapter 8 doi: 10.1002/0471142301.ns0824s48. Unit 8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Kee CJ, Salomonczyk D, Nash JE. Development and Validation of a Screening Assay for the Evaluation of Putative Neuroprotective Agents in the Treatment of Parkinson's Disease. Neurotox Res. 2010;19(4):519–526. doi: 10.1007/s12640-010-9174-2. [DOI] [PubMed] [Google Scholar]
- Yuhas J, Cordeiro L, Tassone F, Ballinger E, Schneider A, Long JM, Ornitz EM, Hessl D. Brief Report: Sensorimotor Gating in Idiopathic Autism and Autism Associated with Fragile X Syndrome. J Autism Dev Disord. 2010;41(2):248–53. doi: 10.1007/s10803-010-1040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang JB, Nosyreva Spencer, Volk LJ, Musunuru K, Zhong R, Stone EF, Yuva-Paylor LA, Huber KM, Paylor, Darnell JC, Darnell RB. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genetics. 2009;5:e1000758. doi: 10.1371/journal.pgen.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906:107–114. doi: 10.1016/s0006-8993(01)02563-x. [DOI] [PubMed] [Google Scholar]