Abstract
OBJECTIVES:
Pulmonary hypertension is associated with bronchopulmonary dysplasia in extremely low birth weight (ELBW) infants and contributes to morbidity and mortality. The objective was to determine the prevalence of pulmonary hypertension among ELBW infants by screening echocardiography and evaluate subsequent outcomes.
METHODS:
All ELBW infants admitted to a regional perinatal center were evaluated for pulmonary hypertension with echocardiography at 4 weeks of age and subsequently if clinical signs suggestive of right-sided heart failure or severe lung disease were evident. Management was at discretion of the clinician, and infants were evaluated until discharge from the hospital or pre-discharge death occurred.
RESULTS:
One hundred forty-five ELBW infants (birth weight: 755 ± 144 g; median gestational age: 26 weeks [interquartile range: 24–27]) were screened from December 2008 to February 2011. Overall, 26 (17.9%) were diagnosed with pulmonary hypertension at any time during hospitalization (birth weight: 665 ± 140 g; median gestational age: 26 weeks [interquartile range: 24–27]): 9 (6.2%) by initial screening (early pulmonary hypertension) and 17 (11.7%) who were identified later (late pulmonary hypertension). Infants with pulmonary hypertension were more likely to receive oxygen treatment on day 28 compared with those without pulmonary hypertension (96% vs 75%, P < .05). Of the 26 infants, 3 died (all in the late group because of cor pulmonale) before being discharged from the hospital.
CONCLUSIONS:
Pulmonary hypertension is relatively common, affecting at least 1 in 6 ELBW infants, and persists to discharge in most survivors. Routine screening of ELBW infants with echocardiography at 4 weeks of age identifies only one-third of the infants diagnosed with pulmonary hypertension. Further research is required to determine optimal detection and intervention strategies.
KEY WORDS: premature infant, bronchopulmonary dysplasia, pulmonary hypertension
What's Known on This Subject:
Pulmonary hypertension is associated with bronchopulmonary dysplasia in extremely low birth weight infants and contributes to morbidity and mortality.
What This Study Adds:
Pulmonary hypertension affects at least 1 in 6 extremely low birth weight infants and persists to discharge in most survivors. Routine screening of these infants with echocardiography at 4 weeks of age identifies only one-third of those affected.
Bronchopulmonary dysplasia (BPD) continues to be a major cause of morbidity and late mortality in extremely preterm infants.1 Pulmonary hypertension is a cardiovascular complication that is associated with increased morbidity and mortality in infants with BPD.2,3 Reduction of cross-sectional perfusion area and abnormal muscularization of peripheral pulmonary vessels are important in the pathogenesis of pulmonary hypertension in BPD,4 and abnormal vascular development also may contribute to the development of BPD.5,6 Previous studies from the presurfactant era7,8 and more recent studies9,10 have used retrospective designs, which make it difficult to determine the prevalence and outcomes of pulmonary hypertension in BPD. There are no clear guidelines for assessment and monitoring, and case series and cross- sectional studies may identify only infants with more severe BPD and pulmonary hypertension,11 because pulmonary hypertension may be silent initially and become evident only after the onset of right-sided heart failure. After the diagnosis of right-sided heart failure in a series of infants, routine echocardiographic screening of extremely low birth weight (ELBW) infants and tracking of subsequent outcome were implemented in our neonatal intensive care unit to determine if we could diagnose and manage pulmonary hypertension before it became severe and possibly irreversible. We hypothesized that routine screening of extremely premature infants would lead to diagnosis of pulmonary hypertension in infants without overt signs and determine the prevalence of this complication.
METHODS
Over a 2-year period (December 2008–February 2011), ELBW infants (birth weight ≤1000 g) who survived to 28 days of age at the NICU of the University of Alabama at Birmingham Hospital were evaluated soon thereafter (before 6 weeks of age) with echocardiography to determine the presence or absence of pulmonary hypertension. Infants with structural heart disease other than persistent ductus arteriosus or patent foramen ovale and those with multiple congenital anomalies were excluded. Infants who were screened but transferred to other hospitals before 36 weeks’ corrected postmenstrual age (PMA) also were excluded, because outcomes by discharge were not always available. Subsequent echocardiography was done if severe lung disease or clinical signs suggestive of right-sided heart failure were present. Publication of this study was approved by the University of Alabama at Birmingham institutional review board, with waiver of informed consent.
Infants were diagnosed with pulmonary hypertension if at least 1 of the following echocardiographic findings was present: (1) right ventricular hypertrophy, (2) flattening of interventricular septum, (3) presence of tricuspid regurgitation in the absence of pulmonary stenosis, and (4) elevated right ventricular pressures as estimated by Doppler studies of tricuspid regurgitation jet. Management of pulmonary hypertension was at the discretion of the clinician, and infants were managed to discharge from the hospital or death. We defined early pulmonary hypertension as pulmonary hypertension detected during the initial screening. Late pulmonary hypertension was defined as pulmonary hypertension diagnosed after a negative initial screening (after 6 weeks of age). All infants diagnosed with pulmonary hypertension had a predischarge echocardiogram. All echocardiograms were interpreted and reported by pediatric cardiologists.
Patient characteristics and outcomes were collected prospectively until death or discharge (Tables 1 and 2). BPD was diagnosed and graded at 36 weeks’ PMA12 with the physiologic definition used for oxygen requirement.13 Pulmonary hypertension was considered to be treated if supplemental oxygen with higher oxygen targets, sildenafil, inhaled nitric oxide, bosentan, and/or intravenous prostacyclin was used. Pulmonary hypertension was considered to be persistent if the predischarge echocardiogram showed any evidence of pulmonary hypertension. Pulmonary hypertension was considered to have resolved if the last echocardiogram before discharge showed no evidence of pulmonary hypertension. Baseline characteristics and outcomes were compared between infants who developed pulmonary hypertension and those who did not.
TABLE 1.
Characteristics and Morbidity/Mortality of Infants Screened for Pulmonary Hypertension
| Total (n = 145) | |
|---|---|
| Characteristics | |
| Birth wt, mean ± SD; g | 755 ± 144 |
| Gender, M/F; % | 42.8/57.2 |
| Gestational age, wk; median (IQR) | 26 (24–27) |
| Small for gestational age, n (%) | 36 (24.8) |
| Age at first echocardiographic screening, d; median (IQR) | 31 (28–40) |
| Morbidity and mortality | |
| BPD, n (%) | |
| None | 33 (22.8) |
| Mild | 58 (40.7) |
| Moderate | 22 (15.9) |
| Severe | 32 (22.1) |
| Severe intraventricular hemorrhage (grade 3 or 4), n (%) | 34 (23.4) |
| Proven necrotizing enterocolitis (stage 2 or 3), n (%) | 6 (4.1) |
| Patent ductus arteriosus requiring medical or surgical treatment, n (%) | 20 (13.8) |
| Retinopathy of prematurity (stage 2 or greater), n (%) | 13 (9) |
| Length of stay, d; median (IQR) | 72 (3–112) |
| Mortality, n (%) | 6 (4.1) |
IQR indicates interquartile range; M/F, male/female.
TABLE 2.
Characteristics of Infants With and Without Pulmonary Hypertension
| Infants Without Pulmonary Hypertension (n = 119) | Infants With Pulmonary Hypertension (n = 26) | P | |
|---|---|---|---|
| Demographics | |||
| Birth wt, g (mean ± SD) | 775 ± 137 | 665 ± 140 | <.001 |
| Gender, M/F (%) | 51/68 (43/57) | 11/15 (42/58) | .95 |
| Gestational age, wk; median (IQR) | 26 (24–27) | 26 (24–27) | .79 |
| Small for gestational age, n (%) | 24 (20.2) | 12 (46.2) | .01 |
| Age at first echocardiographic screening, d; median (IQR) | 31 (27–40) | 31 (29–41) | .66 |
| Respiratory support on postnatal day 28 | |||
| Mechanical ventilation, n (%) | 21 (17.8) | 9 (34.6) | .06 |
| CPAP, n (%) | 32 (27.1) | 7 (26.9) | .98 |
| Mechanical ventilation or CPAP, n (%) | 53 (44.9) | 16 (61.5) | .12 |
| O2 supplementation, n (%) | 89 (74.8) | 25 (96.2) | .01 |
| Fraction of inspired O2, median (IQR); (%) | 30 (21–40) | 40 (30–50) | <.01 |
| Morbidity and mortality | |||
| O2 supplementation at 36 wk, n (%) | 30 (25.6) | 25 (96.2) | <.0001 |
| BPD, n (%) | |||
| No | 29 (27.0) | 1 (3.8) | <.0001 |
| Mild | 57 (49.1) | 1 (3.8) | |
| Moderate | 14 (12.1) | 8 (30.8) | |
| Severe | 16 (13.8) | 16 (61.5) | |
| Severe intraventricular hemorrhage (grade 3 or 4), n (%) | 26 (21.8) | 8 (30.7) | .33 |
| Proven necrotizing enterocolitis (stage 2 or 3), n (%) | 5 (4.2) | 1 (3.8) | .93 |
| Patent ductus arteriosus requiring treatment treated, n (%) | 17 (14.3) | 3 (11.5) | .65 |
| Length of stay, median IQR; d | 91 (77–108) | 150 (120–189) | <.0001 |
| Retinopathy of prematurity (stage 2 or greater), n (%) | 10 (8.4) | 3 (11.5) | .49 |
| Mortality, n (%) | 3 (2.5) | 3 (11.5) | <.05 |
CPAP indicates continuous positive airway pressure; IQR, interquartile range; O2, oxygen; M/F, male/female.
Descriptive statistics were used to evaluate the study population. Continuous variables were compared by using either the t test or the Wilcoxon rank test/Mann–Whitney U test for normal or skewed distributions, respectively. Proportions were compared by χ2/Fisher’s exact test. Risk factors were evaluated by odds ratios and confidence intervals by using contingency tables. Adjusted odd ratios were determined by multiple logistic regression, to control for possible confounding effects of other variables. In the multivariable model, pulmonary hypertension at or after initial screening was considered the dependent variable, and risk factors significant at an α cutoff of P < .20 were analyzed as independent variables. All data were analyzed with SPSS 17.0 for Windows (SPSS Inc, Chicago, IL). All statistical tests were 2-tailed, and P values < .05 were considered statistically significant.
RESULTS
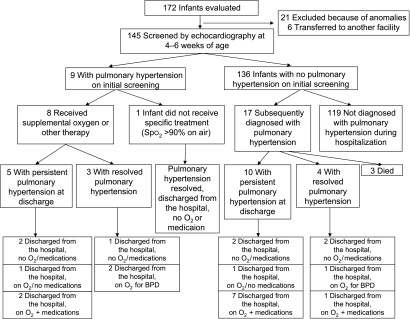
During the study period, 172 ELBW infants who survived to postnatal day 28 were evaluated for echocardiographic screening. Twenty-one infants were excluded because of congenital heart disease or other anomalies, and 6 were excluded because of transfer to another facility (Fig 1). The remaining 145 infants had an echocardiogram at 4 to 6 weeks of age. The characteristics of this study population are shown in Table 1.
FIGURE 1.
Flowchart of infants evaluated in the study. O2, oxygen; Spo2, pulse oximeter oxygen saturation.
Overall, 26 (17.9%) were diagnosed with pulmonary hypertension by at least 1 echocardiogram at any time during hospitalization: 9 (6.2%) were identified by initial screening (early pulmonary hypertension), and 17 (11.7%) were identified later (late pulmonary hypertension) (Fig 1). The differences in characteristics between the infants with and without pulmonary hypertension are shown in Table 2. In brief, infants with pulmonary hypertension had lower birth weight but similar gestational age, which was consistent with the observation that more infants with pulmonary hypertension were identified as “small for gestational age.” Infants with pulmonary hypertension received higher concentrations of oxygen (P < .01) and were more frequently (P = .06) on mechanical ventilation on postnatal day 28 (Table 2). One of 31 infants on room air at postnatal day 28 was diagnosed with early pulmonary hypertension. None of the infants on room air on day 28 were later diagnosed with pulmonary hypertension. Of 75 infants who were receiving neither continuous positive airway pressure nor intermittent mandatory ventilation (IMV) on postnatal day 28, 10 infants (13.3%) developed pulmonary hypertension. At 36 weeks’ PMA, infants with pulmonary hypertension were more likely to be on oxygen supplementation or mechanical ventilation and to be diagnosed with moderate or severe BPD. Four of 9 infants with early pulmonary hypertension and 12 of 17 infants with late pulmonary hypertension were classified as having severe BPD. Mortality and length of stay also were increased in infants with pulmonary hypertension (Table 2). The multivariable logistic regression model identified lower birth weight as being associated with pulmonary hypertension after adjustment for the other variables (gestational age, male gender, fraction of inspired oxygen on day 28, and IMV on day 28) included in the model (Table 3).
TABLE 3.
Risk Factors for Pulmonary Hypertension Among ELBW Infants Surviving to Postnatal Day 28
| Crude OR (95% CI) | Adjusted OR | 95% CI | P | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Birth wt, ga | 0.53 (0.37–0.76) | 0.63 | 0.41 | 0.99 | .03 |
| Small for gestational age | 3.39 (1.39–8.28) | 2.55 | 0.89 | 7.30 | .08 |
| Fraction of inspired oxygen on postnatal day 28 | |||||
| Room air | Reference | Reference | — | — | — |
| 0.22–0.40 | 3.97 (0.85–18.5) | 3.81 | 0.71 | 20.47 | .33 |
| >0.40 | 7.73 (1.54–38.66) | 5.11 | 0.74 | 35.17 | .16 |
| IMV on postnatal day 28 | |||||
| No | Reference | Reference | — | — | — |
| CPAP | 1.42 (0.50–4.08) | 0.64 | 0.18 | 2.32 | .37 |
| IMV | 2.79 (1.00–7.77) | 1.08 | 0.26 | 4.56 | .62 |
Adjusted for gestational age, gender, fraction of inspired oxygen, and IMV on day 28. CI, confidence interval; CPAP, continuous positive airway pressure; OR, odds ratio; IMV, intermittent mandatory ventilation.
Per additional 100-g increment in birth wt.
Details of the management of pulmonary hypertension are shown in Table 4, Table 5, and Fig 1. Respiratory support on postnatal day 28 was similar between early and late pulmonary hypertension groups (Table 4). No significant difference was noted between management of early or late pulmonary hypertension, although the limitations of small sample size and low power must be considered. Clinical practice generally involved sequential or additive use of higher oxygen saturation limits (91%–95% vs 85%–95%), inhaled nitric oxide, sildenafil, bosentan, and epoprostenol (prostacyclin or Flolan) (Tables 4 and 5). Eight of the 9 infants diagnosed with early pulmonary hypertension were managed with supplemental oxygen: 1 infant with pulse oximeter oxygen saturation>90% on room air did not receive any additional therapy, and pulmonary hypertension had resolved by the time this infant was discharged from the hospital. Of the 26 infants with pulmonary hypertension, 3 died in the late pulmonary hypertension group because of right ventricular failure (cor pulmonale). The median age at pulmonary hypertension diagnosis was 31 days (interquartile range: 29–41) in the early pulmonary hypertension group and 112 days (interquartile range: 93–122) in the late pulmonary hypertension group (P < .01). Fifteen infants had persistence of pulmonary hypertension at time of discharge (5 of 9 with early pulmonary hypertension, 10 of 17 with late pulmonary hypertension). Three of the 5 infants with persistent pulmonary hypertension in the early pulmonary hypertension group and 8 of the 10 infants with persistent pulmonary hypertension in the late pulmonary hypertension group were discharged from the hospital on oxygen therapy. Ten infants also received home medication at the discretion of the treating clinician, mainly from the late pulmonary hypertension group (8 of 10). Two of 4 infants with resolved pulmonary hypertension in the early pulmonary hypertension group and 2 of 4 infants with resolved pulmonary hypertension in the late group were discharged on supplemental oxygen therapy for BPD.
TABLE 4.
Comparison of Echocardiographic Findings, Respiratory Support on Postnatal Day 28, Management, and Outcomes Between Early and Late Pulmonary Hypertension Groups
| Total (n = 26) | Early Pulmonary Hypertension (n = 9) | Late Pulmonary Hypertension (n = 17) | P | |
|---|---|---|---|---|
| Echocardiographic findings | ||||
| Tricuspid regurgitation, n (%) | 15 (58) | 4 (44) | 11 (65) | .42 |
| Right ventricular hypertrophy, n (%) | 11 (42) | 1 (11) | 10 (59) | .04 |
| Septal flattening, n (%) | 14 (54) | 6 (67) | 8 (47) | .43 |
| Elevated right ventricular systolic pressure, n (%) | 18 (69) | 5 (55) | 13 (76) | .38 |
| Respiratory support on postnatal day 28 | ||||
| Mechanical ventilation, n (%) | 9 (35) | 4 (44) | 5 (29) | .67 |
| CPAP, n (%) | 7 (27) | 1 (11) | 6 (35) | .36 |
| Mechanical ventilation or CPAP, n (%) | 16 (62) | 5 (55) | 11 (65) | .92 |
| Oxygen supplementation, n (%) | 25 (96) | 8 (89) | 17 (100) | .35 |
| Fraction of inspired oxygen, median (IQR); (%) | 40 (30–50) | 35 (30–40) | 40 (35–50) | .11 |
| Management | ||||
| Oxygen supplementation and higher pulse oximeter oxygen saturation targets, n (%) | 25 (96) | 8 (89) | 17 (100) | .35 |
| Inhaled nitric oxide, n (%) | 14 (54) | 4 (44) | 10 (59) | .68 |
| Sildenafil, n (%) | 20 (77) | 6 (67) | 14 (82) | .63 |
| Bosentan, n (%) | 6 (23) | 1 (11) | 5 (29) | .38 |
| Epoprostenol, n (%) | 4 (15.4) | 1 (11) | 3 (18) | .90 |
| Outcomes | ||||
| Oxygen at discharge, n (%) | 15 (58) | 5 (56) | 10 (59) | .87 |
| Medication at discharge, n (%) | 10 (44) | 2 (22) | 8 (47) | .40 |
| Persistence of pulmonary hypertension at discharge, n (%) | 15 (58) | 5 (56) | 10 (59) | .87 |
| Mortality, n (%) | 3 (12) | 0 (0) | 3 (18) | .53 |
CPAP, continuous positive airway pressure; IQR, interquartile range.
TABLE 5.
Management of Pulmonary Hypertension at Any Time During Hospitalization
| Total (n = 26) | Early Pulmonary Hypertension (n = 9) | Late Pulmonary Hypertension (n = 17) | |
|---|---|---|---|
| No medication, n | 1 | 1 (Outcome: discharged from the hospital; no medications or supplemental oxygen.) | 0 |
| Oxygen alone , n | 5 | 2 (Outcome: 1 with persistent pulmonary hypertension and 1 resolved. Both discharged from the hospital; no medications or supplemental oxygen.) | 3 (Outcome: 2 with persistent pulmonary hypertension; 1 discharged from the hospital on oxygen supplementation; none with medications.) |
| Oxygen + sildenafil, n | 6 | 2 (Outcome: both with persistent pulmonary hypertension, discharged from the hospital on supplemental oxygen; 1 taking medication at discharge.) | 4 (Outcome: 3 of 4 with persistent pulmonary hypertension; 2 discharged from the hospital on supplemental oxygen and medication.) |
| Oxygen + sildenafil + inhaled nitric oxide, n | 8 | 3 (Outcome: 1 with persistent pulmonary hypertension, discharged from the hospital taking no medications or supplemental oxygen; 2 with resolved pulmonary hypertension discharged from the hospital on supplemental oxygen for BPD.) | 5 (Outcome: 1 died, 2 had persistent pulmonary hypertension requiring supplemental oxygen and medication at discharge; 2 with resolved pulmonary hypertension discharged from the hospital on supplemental oxygen for BPD; 1 taking medication.) |
| Oxygen + sildenafil + inhaled nitric oxide + bosentan, n | 2 | 0 | 2 (Outcome: 1 died, 1 with persistent pulmonary hypertension, discharged from the hospital on supplemental oxygen and medication.) |
| Oxygen + sildenafil + inhaled nitric oxide + bosentan + epoprostenol, n | 4 | 1 (Outcome: with persistent pulmonary hypertension, discharged from the hospital on supplemental oxygen and medication.) | 3 (Outcome: 1 died, 2 with persistent pulmonary hypertension, discharged from the hospital on supplemental oxygen and medication.) |
Discussion
The major findings of our study are that 18% or ∼1 in 6 extremely premature infants develop pulmonary hypertension. Preterm infants with growth restriction and higher oxygen requirements at 4 weeks of age and those with severe BPD were at higher risk for the development of pulmonary hypertension. Prospective screening by echocardiography at 4 to 6 weeks of age identified only one-third of the ELBW infants who developed pulmonary hypertension, because the remaining two-thirds of infants were diagnosed with pulmonary hypertension at ∼3 to 4 months of age.
The strengths of this study are its prospective nature, with well-defined echocardiographic criteria and clinical variables, in a reasonably large recent cohort from a tertiary care NICU. It has been noted by other investigators that the prevalence of pulmonary hypertension complicating chronic neonatal lung disease is unknown because it is not routinely assessed,11 and case series and retrospective analyses may be biased toward identification of pulmonary hypertension in more severe BPD.11 Limitations of our study include that although the initial echocardiogram was performed as a screening test, subsequent echocardiography was done only at the discretion of the clinician, which may underestimate the true incidence of pulmonary hypertension because mild forms of late-onset pulmonary hypertension may have been missed. We also excluded 6 infants who were transferred from our institution before 36 weeks’ PMA. Although these infants did not have early pulmonary hypertension on initial screening, it is possible they may have had late pulmonary hypertension. Inclusion of these 6 infants in the analysis does not noticeably change the incidence of early pulmonary hypertension (from 6.2% to 6.0%). Another limitation is that it may be difficult to extrapolate findings from a single-center study to other neonatal intensive care units, because clinical practices (eg, mechanical ventilation management, oxygen saturation targets, and resuscitation practices of infants at the borderline of viability) may alter the incidence of pulmonary hypertension. A more subtle limitation is that although echocardiographic estimates of systolic pulmonary artery pressure are relatively sensitive in identification of pulmonary hypertension, the severity of pulmonary hypertension is difficult to determine, and qualitative echocardiographic findings may be even less accurate.14 Because this study was observational, there was no attempt to regulate therapeutic interventions in infants diagnosed with pulmonary hypertension, and the sample size is too small to permit determination of therapeutic effectiveness of the different interventions. Data from this study have enabled the design of a randomized controlled trial for the management of pulmonary hypertension in ELBW infants, however.
We observed that 18% of ELBW infants developed pulmonary hypertension. Pulmonary hypertension persisted to time of discharge in most infants (58% of those with pulmonary hypertension), and the 3 infants who died did so with cor pulmonale. The relatively high incidence of pulmonary hypertension is concerning, because this condition is more common than other well-known morbidities in this population, such as early-onset sepsis, periventricular leukomalacia, and necrotizing enterocolitis.1 An et al,9 in a retrospective analysis of 116 preterm infants with BPD, observed that 25% had pulmonary hypertension (9.5% and 58.0% of infants with moderate and severe BPD, respectively) at a median postnatal age of 65 days, and that it was improved in 76% of infants after a median of 85 days, whereas 14% of infants died. Slaughter et al,10 in a retrospective cohort study of 1156 ELBW infants, identified 216 infants with BPD who required IMV, CPAP, or nasal cannula (flow >2 liters per minute (LPM)) at the postnatal age of 28 days. Forty-one percent of these infants were evaluated with echocardiography, with 37% having evidence of pulmonary hypertension.10 Thirty-eight percent of the 29 infants with pulmonary hypertension died, as compared with 14% of the 49 infants without pulmonary hypertension.10 Khemani et al15 studied 42 infants with BPD and pulmonary hypertension at a median age of 4.8 months and noted that 38% of these infants died during follow-up. Multivariate analysis indicated that severe pulmonary hypertension and being small for gestational age were associated with higher mortality15; however, it is difficult to compare the results from these studies with our results, because they deal with selected populations of infants with BPD, only some of whom were evaluated by using echocardiography. Our results indicate that although a substantial number of infants who will develop pulmonary hypertension are identified by 4 to 6 weeks of age, many infants who go on to develop pulmonary hypertension do so at 3 to 4 months of age, and repeated evaluation with echocardiography may be necessary in infants at higher risk (higher oxygen requirement or severe BPD). The later detection of pulmonary hypertension in these infants may possibly be secondary to ongoing deterioration of lung function and vascular remodeling associated with intermittent or persistent episodes of hypoxemia.16–18 It is possible that intercurrent infections, such as urinary tract infections, may exacerbate or present as pulmonary hypertension in such infants, but because many sick infants in the intensive care unit have a fluctuating course, it is difficult to assign causality to changes over time in the clinical course. Alternatively, it is possible that infants with absence of a tricuspid regurgitation jet may not have had detection of elevated pulmonary pressures on the initial echocardiogram.14 This pilot observational study does not have the statistical power to demonstrate that earlier detection of pulmonary hypertension (and earlier therapy) is associated with better outcomes, although it must be noted that all the infants who died were in the late pulmonary hypertension group.
Preterm infants who were growth-restricted at birth were at greater risk for development of pulmonary hypertension. The increased risk may be possibly secondary to in utero inhibition of lung development caused by nutritional or placental insufficiency, with a reduction in cross-sectional vascular area, resulting in elevated pulmonary pressures and a greater probability of severe BPD due to pre-existing impaired development. It must be noted that almost one-fourth of our evaluated infants were small for gestational age (SGA), which may have influenced the prevalence of pulmonary hypertension. Acute episodes of pulmonary hypertension also have been reported in growth-restricted preterm infants soon after birth.19 As expected, infants with pulmonary hypertension were more likely to receive higher fraction of inspired oxygen at 4 weeks of age and have severe BPD at 36 weeks’ PMA. Although these variables identified infants at higher risk, use of these risk factors as sole screening criteria would have led to a missed diagnosis in several infants.
The optimal management of premature infants with pulmonary hypertension is not clear. Multiple therapies, including inhaled nitric oxide,20 sildenafil,21,22 and endothelin blockers such as bosentan22 and epoprostenol23 have been evaluated only in retrospective studies that indicate preliminary efficacy and possible safety for the management of pulmonary hypertension in premature infants with chronic lung disease. In our center, if pulmonary hypertension appeared to be resolved on subsequent echocardiograms, we attempted to wean and discontinue the therapy before discharge. Our study provides data for the calculation of sample size and design of prospective randomized controlled trials to rigorously evaluate these unproven and sometimes expensive therapies for which the risk-benefit ratio has not yet been determined.
CONCLUSIONS
Pulmonary hypertension is a relatively common problem, affecting ∼1 in 6 ELBW infants, and it persists to discharge in most affected infants. Routine screening of ELBW infants with echocardiography at 4 to 6 weeks of age identifies only one-third of these infants, so frequent follow-up evaluations may be required to determine later onset of pulmonary hypertension. Further research is required to determine optimal detection and intervention strategies (eg, higher oxygen saturation targets, inhaled nitric oxide, and sildenafil) and timing of treatment. The longer-term benefits and risks of medications such as sildenafil and bosentan in this population need to be evaluated.
Glossary
- BPD
bronchopulmonary dysplasia
- ELBW
extremely low birth weight
- PMA
postmenstrual age
Footnotes
FINANCIAL DISCLOSURE: Dr Ambalavanan received funding from and is on the external advisory board of IKARIA; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by grant R01 HL092906. Funded by the National Institutes of Health (NIH).
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subhedar NV. Recent advances in diagnosis and management of pulmonary hypertension in chronic lung disease. Acta Paediatr Suppl. 2004;93(444):29–32 [DOI] [PubMed] [Google Scholar]
- 3.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2002;87(1):F15–F18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorenflo M, Vogel M, Obladen M. Pulmonary vascular changes in bronchopulmonary dysplasia: a clinicopathologic correlation in short- and long-term survivors. Pediatr Pathol. 1991;11(6):851–866 [DOI] [PubMed] [Google Scholar]
- 5.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661 [DOI] [PubMed] [Google Scholar]
- 6.Thébaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology. 2007;91(4):291–297 [DOI] [PubMed] [Google Scholar]
- 7.Fouron JC, Le Guennec JC, Villemant D, Perreault G, Davignon A. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatrics. 1980;65(3):529–535 [PubMed] [Google Scholar]
- 8.Halliday HL, Dumpit FM, Brady JP. Effects of inspired oxygen on echocardiographic assessment of pulmonary vascular resistance and myocardial contractility in bronchopulmonary dysplasia. Pediatrics. 1980;65(3):536–540 [PubMed] [Google Scholar]
- 9.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40(3):131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011; 31(10):635–640 [DOI] [PubMed]
- 11.Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010;11(3):149–153 [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 13.Walsh MC, Yao Q, Gettner P, et al. National Institute of Child Health and Human Development Neonatal Research Network Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311 [DOI] [PubMed] [Google Scholar]
- 14.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121(2):317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269 [DOI] [PubMed] [Google Scholar]
- 16.Poets CF, Stebbens VA, Richard D, Southall DP. Prolonged episodes of hypoxemia in preterm infants undetectable by cardiorespiratory monitors. Pediatrics. 1995;95(6):860–863 [PubMed] [Google Scholar]
- 17.Bolivar JM, Gerhardt T, Gonzalez A, et al. Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127(5):767–773 [DOI] [PubMed] [Google Scholar]
- 18.Dimaguila MA, Di Fiore JM, Martin RJ, Miller MJ. Characteristics of hypoxemic episodes in very low birth weight infants on ventilatory support. J Pediatr. 1997;130(4):577–583 [DOI] [PubMed] [Google Scholar]
- 19.Danhaive O, Margossian R, Geva T, Kourembanas S. Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol. 2005;25(7):495–499 [DOI] [PubMed] [Google Scholar]
- 20.Banks BA, Seri I, Ischiropoulos H, Merrill J, Rychik J, Ballard RA. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics. 1999;103(3):610–618 [DOI] [PubMed] [Google Scholar]
- 21.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 2009;154(3):379–384 [DOI] [PMC free article] [PubMed]
- 22.Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatr Cardiol. 2008;29(6):1082–1086 [DOI] [PubMed] [Google Scholar]
- 23.Rugolotto S, Errico G, Beghini R, Ilic S, Richelli C, Padovani EM. Weaning of epoprostenol in a small infant receiving concomitant bosentan for severe pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Minerva Pediatr. 2006;58(5):491–494 [PubMed] [Google Scholar]