Abstract
Despite a lack of consistent diagnostic criteria, the metabolic syndrome (MetS) is increasingly evident in children and adolescents, portending a tsunami of chronic disease and mortality as this generation ages. The diagnostic criteria for MetS apply absolute cutoffs to continuous variables and fail to take into account aging, pubertal changes, and race/ethnicity. We attempt to define MetS mechanistically to determine its specific etiologies and to identify targets for therapy. Whereas the majority of studies document a relationship of visceral fat to insulin resistance, ectopic liver fat correlates better with dysfunctional insulin dynamics from which the rest of MetS derives. In contrast to the systemic metabolism of glucose, the liver is the primary metabolic clearinghouse for 4 specific foodstuffs that have been associated with the development of MetS: trans-fats, branched-chain amino acids, ethanol, and fructose. These 4 substrates (1) are not insulin regulated and (2) deliver metabolic intermediates to hepatic mitochondria without an appropriate “pop-off” mechanism for excess substrate, enhancing lipogenesis and ectopic adipose storage. Excessive fatty acid derivatives interfere with hepatic insulin signal transduction. Reactive oxygen species accumulate, which cannot be quenched by adjacent peroxisomes; these reactive oxygen species reach the endoplasmic reticulum, leading to a compensatory process termed the “unfolded protein response,” driving further insulin resistance and eventually insulin deficiency. No obvious drug target exists in this pathway; thus, the only rational therapeutic approaches remain (1) altering hepatic substrate availability (dietary modification), (2) reducing hepatic substrate flux (high fiber), or (3) increasing mitochondrial efficiency (exercise).
KEY WORDS: metabolic syndrome, insulin resistance, dyslipidemia, hypertension, diabetes, pediatrics
A recent documentary entitled Fat, Sick, and Nearly Dead (a Joe Cross film; Reboot Media, 2011) resembles Supersize Me (a Morgan Spurlock film; Roadside Attractions, 2004), in reverse. But “fat” does not automatically mean “sick” or “nearly dead.” Absolute mass of adiposity and ensuing metabolic complications overlap but are distinct phenomena. For instance, ∼30% of obese adults are metabolically normal,1,2 whereas 5% to 45% of normal-weight people exhibit the same metabolic perturbations as seen in the obese.3,4 BMI, a calculation (kg/m2) based on weight and height used to define “overweight” and “obesity,” thus clearly does not account for all the variance in cardiometabolic risk.5
The metabolic syndrome (MetS) is a clinical condition composed of anthropometric, physiologic, and biochemical abnormalities predisposing affected individuals to the development of type 2 diabetes (T2DM) and cardiovascular disease (CVD). Rather than total adiposity, the core clinical component of the syndrome is visceral6–10 and/or ectopic fat (ie, fat in organs not designed for fat storage),11,12 whereas the principal metabolic abnormality is insulin resistance (IR).13–15
The concept that cardiovascular risk factors “cluster” in certain individuals has been known for nearly a century.16 However, it was not until the early 1980s that the relationship between obesity, dyslipidemia (particularly hypertriglyceridemia), and hypertension was recognized17 and not until the late 1980s that the central roles of IR and abdominal adiposity in the pathogenesis of T2DM became apparent.13,18 In 1988, Reaven19 described the central role of IR in human disease and its interrelationship in adults with obesity, hypertension, T2DM, dyslipidemia, and CVD. Although modulation of these MetS components by race, ethnicity, and age complicates the assignment of clear thresholds in the pediatric population, the same “clustering” of risk factors is increasingly apparent in obese children.20,21 Thus, MetS knows no age limit.
With heritability estimates for BMI ranging between 40% and 70%,22 extensive searches for the causative gene(s) have been undertaken. Both candidate gene analyses23 and genome-wide association scans24 have hinted at complex gene networks with pleiotropic effects but modest effect sizes. In fact, only ∼10% of the variance of MetS appears to be explained by genetic susceptibility,25,26 leaving ∼90% to changes in the environment and/or to epigenetic interactions with potential for hereditary imprinting.27 Yet, despite this, certain components of MetS, including nonalcoholic fatty liver disease (NAFLD), have preferential racial/ethnic variations in prevalence.28 One potential explanation for this phenomenon is the preferential presence of the rs738409 allele of the patatin-like phospholipase domain-containing protein 3 (PNPLA3), which encodes a protein under nutritional regulation.29 Although the exact role of PNPLA3 in lipid processing has yet to be elucidated, the rs738409 allele has been associated with the accumulation of intrahepatic lipid and is particularly frequent in Hispanic individuals, who have the highest prevalence of NAFLD in the United States.28 Moreover, another allele in the same gene is associated with low hepatic fat content in African Americans, who have the lowest risk of developing NAFLD. PNPLA3 may also affect other ectopic lipid depots because visceral adipose tissue is related to intrahepatic lipid accumulation, irrespective of race or ethnicity.30
Rather than debate the role of genetics versus environment, or the veracity, validity, or utility of MetS screening criteria in children, our aim is to elucidate the most current understanding of the syndrome’s etiology and pathogenesis to provide a construct for future clinical and research endeavors.
IR
Conceptually, individuals can be classified as being “insulin sensitive” or “insulin resistant” on the basis of their response to an oral glucose challenge31; specifically, how well a glucose load stimulates insulin release from the pancreas and the uptake of glucose by peripheral tissues.32 Whereas “insulin-sensitive” individuals have normal insulin secretion and rapid glucose clearance, “insulin-resistant” individuals manifest some degree of compensatory hyperinsulinemia to force glucose into peripheral tissues. Diabetes ensues when β-cells cannot compensate further to maintain euglycemia. However, it is important to not simply dichotomize an individual as being completely insulin sensitive or completely insulin resistant; rather, insulin sensitivity exists along a continuum and is modified by a host of factors.33,34 Furthermore, various cells and tissues have differential sensitivities to insulin,35–37 contributing to the variability of MetS expression and its phenotype. In fact, although multiple tissues are affected, IR in the liver is emerging as the likely primary lesion in the syndrome’s pathogenesis.
Hepatic IR
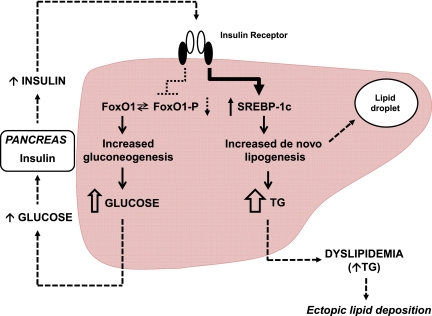
The liver plays a major role in substrate metabolism and is a primary target of insulin action. Thus, it is at the crossroads of metabolism and disease. After insulin’s release from the β-cell following a glucose load, it travels directly to the liver via the portal vein, where it binds to the insulin receptor and elicits 2 key actions at the level of gene transcription. First, insulin stimulates the phosphorylation of forkhead box protein O1 (FoxO1), which prevents it from entering the nucleus38 and thus diminishes the expression of genes required for gluconeogenesis, mainly phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. The net effect is diminished hepatic glucose output. Second, insulin activates the transcription factor sterol regulatory element-binding protein (SREBP)-1c, which in turn increases the transcription of genes required for fatty acid and triglyceride (TG) biosynthesis, most notably adenosine triphosphate-citrate lyase, acetyl-coenzyme A carboxylase, and fatty acid synthase.39,40 These 3 enzymes constitute the process of de novo lipogenesis (DNL). TGs synthesized by DNL are then packaged with apolipoprotein B (apoB) into very low-density lipoproteins (VLDL) for export to the periphery for storage or utilization by reciprocal activation of lipoprotein lipase on the surfaces of endothelial cells in adipose or muscle tissues.41,42
For reasons that remain unclear, insulin-resistant subjects typically have “selective” or “dissociated” hepatic IR; that is, they have impaired glucose homeostasis (mediated by the FoxO1 pathway) but enhanced insulin-mediated hepatic DNL (mediated by the SREBP-1c pathway)37 (Fig 1). The increase in free fatty acid (FFA) flux within the liver, either by DNL or FFA delivery via the portal vein, impairs hepatic insulin action,43 leading to increases in hepatic glucose output, the synthesis of proinflammatory cytokines, and major changes in lipoprotein metabolism. Specifically, hepatic DNL limits fatty acid β-oxidation via production of the intermediary malonyl coenzyme A (CoA), which inhibits carnitine palmitoyltransferase-1 (CPT-1) and thus reduces the regeneration of carnitine, which normally shuttles fatty acids into the mitochondria.44–46 Thus, in the liver of insulin-resistant individuals, FFA flux is high, TG synthesis and intrahepatic lipid storage are increased, and excess TG is released with apoB as VLDL. This excess VLDL-TG secretion by the liver is considered the primary cause for MetS-associated dyslipidemia, characterized by elevated TG, low HDL cholesterol levels, and an elevated number of relatively cholesterol-depleted LDL particles.47
FIGURE 1.
Selective IR in the liver. Under normal conditions, dietary glucose stimulates insulin secretion from the pancreatic β-cells. Insulin then travels directly to the liver via the portal vein, where it binds to the insulin receptor and elicits 2 key actions at the level of gene transcription. First, insulin stimulates the phosphorylation of FoxO1, which prevents it from entering the nucleus and thus diminishes the expression of genes required for gluconeogenesis; the net effect is diminished hepatic glucose output. Second, insulin activates the transcription factor SREBP-1c, which in turn increases the transcription of genes required for fatty acid and TG biosynthesis; the net effect is DNL. In the liver of individuals with MetS, insulin fails to decrease gluconeogenesis because of decreased signaling through the FoxO1 pathway but continues to stimulate the production of fatty acids and TGs through the SREBP-1c pathway. Resistance to the FoxO1 pathway leads to dysglycemia and subsequent compensatory hyperinsulinemia, whereas sensitivity to the SREBP-1c pathway leads to DNL, intrahepatic lipid deposition, and dyslipidemia/hypertriglyceridemia, enhancing ectopic lipid deposition. (Adapted from Brown and Goldstein37.)
IR and MetS are also associated with intrahepatic lipid accumulation, oxidative stress, lipid peroxidation, and proinflammatory cytokine production.34 The intrahepatic accumulation of FFA and lipid are also detrimental to liver insulin sensitivity because they lead to the generation of toxic lipid-derived metabolites, such as diacyglycerol, fatty acyl CoA, and ceramides. These in turn trigger activation of protein kinase C-ε (PKCε), and serine/threonine phosphorylation of insulin receptor substrate-1 (IRS-1), which attenuates hepatic insulin signal transduction.48–50
Adipose Tissue IR
Although alterations in hepatic insulin signaling may be a cardinal feature of MetS, adipose tissue IR also appears to be important.19,36,51,52 The expanded adipose tissue mass that accompanies obesity often leads to increased lipolysis and FFA turnover.53,54 Normally, insulin inhibits adipose tissue lipolysis; however, in the insulin-resistant state, the process is accelerated,51,55 leading to increased FFA release into the circulation.34,52 Moreover, visceral adipocytes and macrophage foam cells are more sensitive to catecholamine-stimulated lipolysis than subcutaneous adipocytes,56 further increasing FFA flux. Because FFAs released from the visceral adipose tissue drain directly into the liver via the portal system, 1 potential mechanism for increased hepatic FFA accumulation and subsequent hepatic IR is increased delivery from the visceral fat depot (the “portal theory” of hepatic IR).43 Increasing evidence suggests that macrophages also infiltrate into adipose tissue and contribute substantively to both adipocyte hypertrophy and cytokine release,57–59 with larger fat cells producing larger amounts.60 These circulating cytokines not only affect insulin action in other tissues, such as liver and muscle, but may also have paracrine effects locally in fat.61,62
Skeletal Muscle IR
Downstream of an insulin-resistant liver, increased plasma FFA levels disrupt the glucose–fatty acid or “Randle” cycle53,63 and insulin-mediated glucose transport in skeletal muscle,64–66 facilitating the development of hyperglycemia. The ectopic deposition in skeletal muscle of fat as intramyocellular lipid may also play a direct role in the pathogenesis of IR and MetS via lipid metabolite-induced activation of protein PKCε with subsequent impairment of insulin signaling.36,50,67,68
Dietary Factors
The incidence of obesity, diabetes, and MetS has increased over the past few decades in conjunction with the rise in daily caloric intake.69 The continuous provision of energy via dietary carbohydrate, lipid, and protein fuels, unmatched by physical activity/energy demand, arguably creates a backlog of the products of mitochondrial oxidation,70 a process associated with progressive mitochondrial dysfunction and IR.71
In the context of overconsumption, and in a glycogen-replete and nonanabolic/nonmuscle building state (which pertains to the vast majority of the population), specific macronutrients of the “Western diet” may differentially contribute to the pathogenesis of IR and MetS.72,73 Historically, 1 of the most common food constituents associated with MetS has been dietary fat. However, the absolute consumption of total dietary fat has not changed over the past 30 years; in fact, the percentage of calories ingested from saturated fats has decreased from 40% to 30%.74 The finding that high-fat, low-carbohydrate diets are protective against MetS75 further confounds the conventional wisdom that simple fat restriction is beneficial. In experimental models, saturated fats are robustly proinflammatory, omega-6 polyunsaturated fatty acids are weakly reactive, monounsaturated fatty acids are neutral, and omega-3 fatty acids have antiinflammatory properties.76 In humans, the story is much more complex because most fatty foods are composed of many types of fats, and cardiometabolic risk relates more to the balance of saturated versus unsaturated fats than to the total amount ingested.77 It may thus be the underrepresentation of unsaturated fatty acids in the typical American diet78 that gives saturated fat such a bad reputation. Studies suggest that monounsaturated fats such as oleic acid (found in olive oil)79 and polyunsaturated linoleic acid80 or omega-3 fatty acids81 all decrease inflammation and intrahepatic lipid deposition and improve postprandial TG levels, possibly by increasing peroxisomal activity and thereby limiting damage by reactive oxygen species (ROS).82
Trans-unsaturated Fatty Acids (Trans-fats)
Trans-unsaturated fats in processed foods have been a staple in the Western diet since the early 20th century. This is because the trans-isomerization of the double bond prevents fatty acid breakdown by bacteria, prolonging the shelf life of foods. Like their bacterial predecessors, human mitochondria cannot subject trans-fats to β-oxidation in the liver,83 contributing to ectopic intrahepatic lipid accumulation.84 Fortunately, because of the recognized association between trans-fat consumption and cardiovascular disease in the mid-1980s and more stringent labeling requirements since 2006, the percent of calories from trans-fats consumed in the “Western diet” has been gradually declining.74 Trans-fats have no health benefit and cause hepatic steatosis and IR85; however, their current consumption trends are temporally disparate with the current increasing prevalence of MetS,34 suggesting that other factors are involved.
Branched-Chain Amino Acids
Branched-chain amino acids (BCAAs: valine, leucine, and isoleucine) are essential amino acids that account for >20% of the amino acids in the typical “Western diet.”86 Although normally used for protein biosynthesis and cell growth, when provided in excess, they are diverted away from protein synthesis and toward energy utilization.87
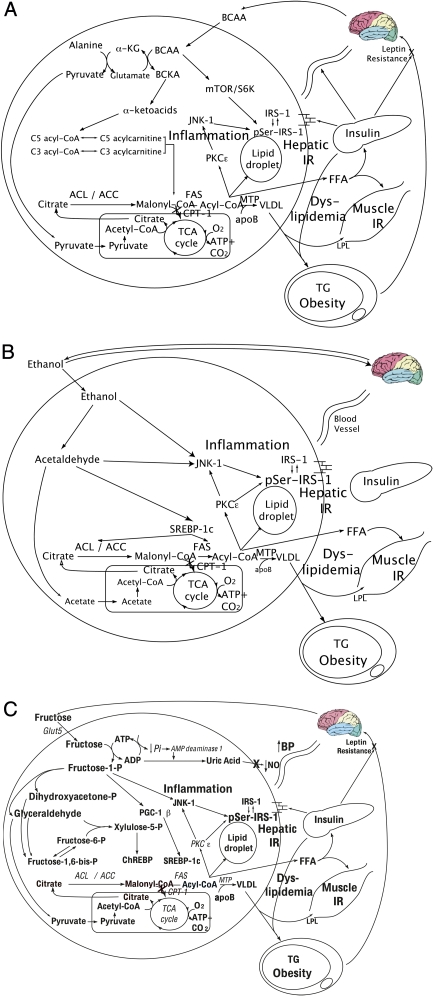
In the liver, BCAAs increase transcription of carbohydrate regulatory element-binding protein and SREBP-1c,88 facilitating DNL. Furthermore, BCAAs limit insulin-induced phosphoinositide 3-kinase (PI3-K) signaling89,90 and stimulate the activation of the mammalian target of rapamycin (mTOR),91,92 promoting the serine phosphorylation of IRS-1 and impairment of insulin signaling.87,89 In addition, just as there are obesity-related changes in adipokines and cardiovascular risk markers, there also appears to be obesity-associated changes in BCAA metabolism and subsequent serum levels. In particular, valine and leucine/isoleucine levels have been reported to be 20% and 14% higher, respectively, in obese compared with lean subjects.87 Mechanistically, this appears to be accounted for by a high rate of flux through the BCAA catabolic pathway (Fig 2A), resulting in the increased production of alanine. Because alanine is a highly gluconeogenic amino acid, increased BCAA catabolism may thus contribute to increased hepatic glucose output.87 Furthermore, the increased α-ketoacids generated by increased flux of the BCAAs through their catabolic pathways also potentially suppress mitochondrial β-oxidation.93
FIGURE 2.
A, Hepatic BCAA metabolism. BCAAs induce: excess formation of alanine, which serves as a substrate for gluconeogenesis, contributing to hyperglycemia; excess formation of α-ketoacids, leading to increased levels of C5 and C3 acylcarnitine levels and subsequently excess formation of malonyl-CoA, which inhibits β-oxidation; hepatic lipid droplet formation and steatosis; activation of mTOR and its downstream target S6K, which contributes to serine phosphorylation of IRS-1 and hepatic IR, which in turn promotes hyperinsulinemia and influences substrate deposition into fat; and export of free fatty acids, which leads to VLDL formation and muscle IR. α-KG, α-ketoglutarate; BCKA, branched-chain keto acid; pSer-IRS-1, serine phosphorylated IRS-1; S6K, S6 kinase. B, Hepatic ethanol metabolism. Ethanol induces: DNL and dyslipidemia; JNK-1 activation, which serine phosphorylates hepatic IRS-1, rendering it inactive, and contributing to hepatic IR, which promotes hyperinsulinemia and influences substrate deposition into fat; hepatic lipid droplet formation, leading to steatosis; and stimulation of the reward pathway, promoting continuous consumption. (Reproduced from Lustig107.) ACC, acetyl CoA carboxylase; ACL, adenosine triphosphate citrate lyase; FAS, fatty acid synthase; MTP, microsomal transfer protein. C, Hepatic fructose metabolism. Fructose induces substrate-dependent phosphate depletion, which increases uric acid and contributes to hypertension through inhibition of endothelial nitric oxide synthase and reduction of NO; DNL and dyslipidemia; hepatic lipid droplet formation and steatosis; muscle IR; JNK-1 activation, contributing to hepatic IR, which promotes hyperinsulinemia and influences substrate deposition into fat; and CNS hyperinsulinemia, which antagonizes central leptin signaling and promotes continued energy intake. (Reproduced from Lustig107.) ACC, acetyl CoA carboxylase; ACL, adenosine triphosphate citrate lyase; ACSS2, acyl-CoA synthetase short-chain family member 2; AMP, adenosine monophosphate; BP, blood pressure; CNS, central nervous system; FAS, fatty acid synthase; Glut5, glucose transporter 5; MTP, microsomal transfer protein; NO, nitric oxide; pSer-IRS-1, serine phosphorylated IRS-1.
Because of the liver’s relatively low activity of the branched-chain aminotransferase enzyme, dietary BCAAs also reach the systemic circulation in levels proportional to dietary intake,86,94 increasing their exposure to peripheral tissues. This increased peripheral delivery of BCAAs when combined with a high-fat diet may promote IR by causing the accumulation of lipid-derived metabolites such as diacylglycerols and ceramides and/or the activation of the mTOR pathway along with serine phosphorylation of IRS-1.87,89,95,96 In addition, in the setting of a high-fat diet, BCAAs cause overaccumulation of C3 and C5 acylcarnitines, which may saturate capacity for mitochondrial β-oxidation, leading to the accumulation of incompletely oxidized lipid-derived metabolites.87 Alterations in adipose tissue BCAA metabolism also appear to influence circulating BCAA levels,97,98 and the exaggerated insulin secretion response to glucose that is often observed in obese individuals may be due to potentiation of glucose-induced insulin secretion by BCAAs,87,99 potentially facilitating subsequent β-cell failure.
Furthermore, chronic BCAA elevation impairs the transport of aromatic amino acids into the brain; the reduced production of serotonin (derived from tryptophan) and catecholamines (derived from phenylalanine and tyrosine) may drive hunger.87 The “BCAA overload” hypothesis suggests that in the context of a dietary pattern that includes high fat consumption, BCAAs may make an independent contribution to the development of IR,87 a hypothesis supported by metabolomics studies demonstrating high BCAA levels in normoglycemic individuals who subsequently develop IR and diabetes.100,101
Ethanol
Although adult epidemiologic studies associate light to moderate ethanol consumption with improved insulin sensitivity102 and wine consumption with reduced cardiovascular risk,103 other cross-sectional104,105 and prospective studies106 implicate a dose-dependent effect of alcohol in MetS and suggest that chronic consumption of large amounts of ethanol worsens insulin sensitivity. Furthermore, its metabolism (Fig 2B) bears important similarities to fructose (see below).107
Ethanol neither elicits an insulin response nor requires a transporter to enter the liver. Once inside the hepatocyte, it bypasses glycolysis and is converted by alcohol dehydrogenase-1B to form acetaldehyde, which promotes ROS formation and toxic damage to the liver if not quenched by hepatic antioxidants such as glutathione or ascorbic acid.108 Acetaldehyde is then metabolized by the enzyme aldehyde dehydrogenase-2 to acetic acid, which in turn is metabolized by the enzyme acyl-CoA synthetase short-chain family member 2 to form acetyl-CoA. The acetyl-CoA can then enter the mitochondrial tricarboxylic acid cycle (as per acetyl-CoA derived from glucose metabolism); however, in the presence of other caloric substrates, it is preferentially used for the synthesis of fatty acids through DNL (as per acetyl-CoA derived from fructose metabolism). The excess malonyl-CoA produced from ethanol metabolism inhibits CPT-1, thereby limiting mitochondrial fatty acid β-oxidation. Ethanol also blocks fatty acid β-oxidation by inhibiting both peroxisome proliferator-activated receptor (PPAR)-α and adenosine monophosphate-activated protein kinase, which lead to increased activity of acetyl-CoA carboxylase and increased levels of malonyl-CoA.109 PPAR-α is expressed mainly in the liver, kidney, and heart and stimulates the transcription of genes involved in fatty acid uptake and both mitochondrial and peroxisomal fatty acid β-oxidation.110 The ethanol-induced suppression of PPAR-α also suppresses microsomal triglyceride transfer protein,111 thereby altering the liver’s lipid export machinery.112–114 Buildup of intrahepatic lipid metabolites leads to subsequent activation of the enzyme c-jun N-terminal kinase 1 (JNK-1)115 and serine-phosphorylation of the IRS-1, driving further hepatic IR. Thus, ethanol metabolism results in intrahepatic lipid accumulation and liver injury,116–118 driving hepatic IR119,120 and promoting MetS.
Fructose
Another dietary component that has been clearly implicated in the pathogenesis of MetS is the monosaccharide fructose. Fructose is typically consumed either as sucrose (50% fructose) or as high-fructose corn syrup (HFCS; 42% or 55% fructose). Unlike those of trans-fats or ethanol, the secular consumption trends for fructose have paralleled the rise of obesity and MetS, especially in children. Before World War II, Americans consumed ∼24 g per day of fructose; by the mid-1970s, it had increased to ∼37 g per day; and by the mid-1990s, to ∼55 g per day (a progressive increase from ∼5% to 7% to 10% of total calories). In fact, recent NHANES data suggest that ∼15% of the US population consumes ≥25% of energy from added sugars.121 Adolescents are by far the highest fructose consumers, consuming >70 g per day (∼12% of total calories); and more than 20% of adolescents consume ≥25% of their total calories as fructose.121,122
Although considerable debate exists as to whether HFCS is different than sucrose, examination of the data suggests that HFCS and sucrose have similar endocrine and metabolic effects.123 Unlike the hepatic metabolism of glucose, which principally leads to glycogen synthesis, the hepatic metabolism of fructose (Fig 2C) results in sustained elevations in postprandial TG levels.124–128 Importantly, increased fructose consumption, particularly in the form of sugar-sweetened beverages, has been implicated in promoting weight gain,129–131 visceral adiposity,132,133 dyslipidemia,133,134 and IR/glucose intolerance,133,135,136 as well as hepatic steatosis.137–140
Fructose in the gut is transported into the enterocyte via the fructose transporter Glut5, independent of adenosine triphosphate hydrolysis and sodium absorption.141 Once inside in the enterocyte, a small portion of the fructose load is converted to lactic acid and released in the portal circulation142; another small portion may also be converted to glucose.142 However, the majority of ingested fructose is secreted into the portal circulation and delivered to the liver. There, fructose is rapidly metabolized to fructose-1-phosphate (F1P) via fructokinase,141 an insulin-independent process which also bypasses the negative feedback regulation of phosphofructokinase in the glycolytic pathway. Thus, fructose metabolism generates lipogenic substrates (eg, glyceraldehyde-3-phosphate and acetyl-CoA), which are delivered straight to the mitochondria, in an unregulated fashion. This excessive mitochondrial substrate then drives hepatic DNL, which can then overwhelm apoB and the lipid export machinery, leading to intrahepatic lipid deposition and steatosis.140 Hepatic DNL also limits further fatty acid oxidation in the liver via excess production of malonyl-CoA, which reduces entry of fatty acids into the mitochondria by inhibiting CPT-1.44–46 F1P also stimulates SREBP-1c via peroxisome proliferator-activated receptor-γ coactivator-1β143 independently of insulin, which activates the genes involved in DNL144–146; moreover, fructose has been shown to induce activation of carbohydrate regulatory element-binding protein and increase the expression of all the enzymes of DNL.
Furthermore, F1P activates dual-specificity mitogen-activated protein kinase 7,147 which subsequently stimulates JNK-1,148 a hepatic enzyme considered to act as a bridge between hepatic metabolism and inflammation.149 In addition, the lipogenic intermediate diacyglycerol (formed during fructose metabolism in the liver) activates PKCε.150 Both of these events stimulate serine phosphorylation of IRS-1, leading to hepatic IR. This impairs insulin-mediated phosphorylation of FoxO1, leading to increased expression of the genes required for gluconeogenesis and promoting increased hepatic glucose output, possibly contributing to hyperglycemia and the development of T2DM. The excess TGs secreted from the liver into the circulation as fat-laden VLDL particles after the ingestion of fructose may couple with a fructose-induced reduction in lipoprotein lipase activity to cause sustained postprandial dyslipidemia, thereby augmenting the risk for CVD.133,151
How are these 4 dietary foodstuffs similar? Each share 3 biochemical properties: (1) they are metabolized for energy primarily within the liver; (2) they are not insulin regulated; and (3) they do not have a “pop-off” mechanism to form glycogen for storage. So, although the kinetics of their metabolism may differ, virtually all their intermediates are delivered directly to the mitochondria, which cannot process the volume of substrate, resulting in a backlog of metabolic intermediates, ROS generation, excessive DNL, and impaired β-oxidation, driving IR and the downstream comorbidities of MetS.
Although the data supporting a role of BCAAs in the development of MetS components are currently only associative in nature, the “BCAA overload” hypothesis is intriguing and relevant. Alternatively, data supporting the role of the other 3 dietary foodstuffs (ie, trans-fats, ethanol, and fructose) in the development of MetS components are more definitive and demonstrate causation.
Subcellular ROS Metabolism
Mitochondria and ROS Formation
The “free radical theory” holds that imbalance between ROS generation and antioxidant defenses is a major factor in the determination of lipid peroxidation and protein misfolding, with resultant DNA and cellular damage.152 Excessive intracellular ROS formation occurs via 3 pathways: (1) inflammatory cytokines derived from visceral fat accumulation153,154; (2) dysfunctional mitochondrial energetics155; and (3) glycation (see below). Excessive nutrient processing by mitochondria can result in uncoupling of oxidative phosphorylation and increased generation of ROS; this, in turn, leads to altered mitochondrial function and further ROS generation.156 ROS accumulation can also impair endoplasmic reticulum (ER) function, causing ER stress and the compensatory unfolded protein response (UPR). The UPR can itself be overwhelmed by persistent excessive nutrient processing and ROS generation, leading to cellular shutdown, defective insulin secretion, and T2DM.157,158
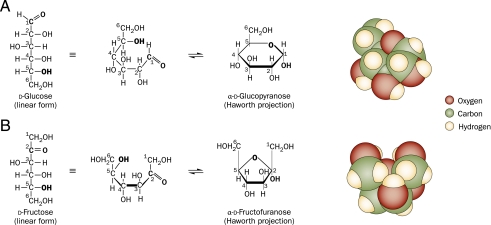
Fructose is a well-known driver of excessive ROS formation. Molecularly, glucose is found in 2 steroisomeric forms (Fig 3): the majority in the glucopyranose (6-membered ring) form and the minority in the linear aldehyde form, which is highly reactive with ε-amino groups of lysine, generating a ROS with each reaction, known as the Maillard or “browning” reaction. Fructose is also found in 2 stereoisomeric forms: the majority in the linear ketone form (also highly reactive) and the minority in the fructofuranose (5-membered ring) form. The latter has 2 axial (abutting) hydroxymethyl groups, which exert allosteric and ionic forces to the unstable furanose ring and drive it toward the linear form. This difference explains why nonenzymatic fructosylation is 7 times more rapid than protein glycation and why fructose generates 100 times more ROS than glucose.159
FIGURE 3.
Molecular renditions of (A) glucose and (B) fructose, in the linear, chair, and space-occupying projections. In the linear form, both glucose and fructose possess a reactive aldehyde or one ketone moiety, which can bind nonenzymatically to freely available amino groups of proteins. At normal body temperature and pH, the chair form of glucose predominates. This conformation is a glucopyranose (6-membered ring), with equatorial hydroxyl groups and is molecularly stable, which limits its protein reactivity. However, the chair form of fructose is a fructofuranose (5-membered ring) with 2 axial hydroxymethyl groups that exert allosteric and ionic forces on the unstable furanose ring, which favors the linear form. Thus, at normal body temperature and pH, the majority of fructose exists in the linear form and is more reactive with proteins than is glucose. (Reproduced from Lim et al140.)
Peroxisomes and ROS Quenching
Because ROS are inherent by-products of cellular metabolism, endogenous cellular antioxidants (eg, catalase and glutathione) quench the ROS before they have a chance to promote peroxidation. These antioxidants are found primarily in peroxisomes, which abut the mitochondria, and act as “support staff” for ROS processing.160 Mouse models of peroxisomal disorders result in mitochondrial and ER dysfunction.161 Furthermore, cytokines such as tumor necrosis factor-α can reduce peroxisomal number and function, rendering cells even more vulnerable.162
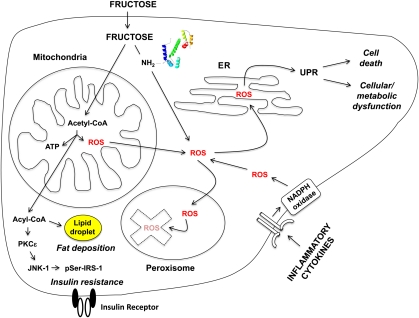
In the absence of antioxidant quenching, fructose can cause cellular damage. In an in vitro study, incubation of hepatocytes with fructose yielded no direct damage; however, when these hepatocytes were preincubated with sublethal doses of hydrogen peroxide to reduce their peroxisomal ROS-quenching ability, fructose was as hepatotoxic as other organic aldehydes.163 Furthermore, an in vivo study in antioxidant-deficient mice demonstrated that intrahepatic lipid toxicity and hepatocellular death occurred after sucrose administration.164 These data thus suggest that excessive ROS, in combination with micronutrient insufficiencies which impair antioxidant reserves, can lead to cellular damage (Fig 4).
FIGURE 4.
Intracellular ROS formation and consequences of fructose metabolism. Dietary fructose, because of its metabolic processing in the mitochondria and the fructosylation of protein ε-amino groups via the Maillard reaction, and circulating inflammatory cytokines, because of their receptor-mediated activation of NADPH oxidase, increase intracellular levels of ROS. In the absence of sufficient peroxisomal quenching and degradation, the ROS moieties lead to the UPR response, causing either cell death (apoptosis) or cellular/metabolic dysfunction. The formation of acetyl-CoA also leads to lipid deposition and IR through the activation of inflammatory pathways as described in the text. (Adapted from Parola and Marra153.) NADPH, nicotinamide adenine dinucleotide phosphate; pSer-IRS-1, serine phosphorylated IRS-1.
ER, ROS, and UPR
Excessive ROS that are not quenched by peroxisomes find their way to the adjacent ER, where they alter the redox environment crucial for proper protein folding.165 Accumulation of ROS and misfolded proteins within the ER activates the UPR,166 designed to decrease protein synthesis to allow for their clearance.167 However, excessive ROS impairs the ability to clear misfolded proteins and activates the enzyme caspase-3, leading to even further ROS generation, apoptosis, and cellular demise.168 ER stress in the liver is a specific mechanism of hepatic injury in NAFLD,169 and ER stress in the pancreas reduces β-cell number and promotes diabetes.170
Thus, dietary foodstuff–induced increases in intracellular ROS formation in conjunction with hepatic DNL and ectopic fat deposition (see above) lead to IR and metabolic dysfunction, increasing an individual’s risk for MetS and its associated comorbidities.
Prevention of MetS
Unfortunately, there is no actionable drug target in either the lipogenesis or ROS pathways. Currently, the only options to prevent MetS are to reduce mitochondrial substrate metabolism to prevent these 2 phenomena. This can be accomplished in 3 ways, as follows.
Reduction in substrate availability: caloric restriction, particularly of lipogenic substrates, improves insulin sensitivity and reduces liver fat accumulation in humans, thus reducing risk for MetS.171
Reduction in hepatic substrate flux: by reducing the rate of substrate absorption and resultant liver metabolic capacity, the serum glucose rise and subsequent insulin response can be attenuated. This can be accomplished by reducing glycemic load, which is most easily accomplished by increasing the fiber content of food.172 Furthermore, lipogenesis and hepatic lipid export can also be reduced with improved dietary fiber intake.173
Increase in substrate clearance: by increasing hepatic mitochondrial substrate metabolism, the availability of substrate for lipogenesis will be reduced, and hepatic IR can be mitigated. This can be directly accomplished by exercise. By stimulating the sympathetic nervous system and inducing the transcription factor peroxisome proliferator-activated receptor γ coactivator-1α, exercise causes mitochondrial biogenesis in liver and muscles.174 The age of the mitochondria is relevant because new mitochondria are more efficient, generating fewer ROS.175 Exercise also burns acetyl-CoA and prevents the buildup of fatty acids, which improves insulin sensitivity in the liver and muscles.176
CONCLUSIONS
The pathways delineated in this article make it clear how our current food environment is mismatched to our biochemistry. Although we have drugs that can treat individual cardiometabolic complications such as dyslipidemia, hypertension, and IR, there is no clear drug target that can mitigate the underlying cellular damage wrought by dysfunctional mitochondrial ROS and protein misfolding. The only rational approaches to reduce mitochondrial ROS formation and toxicity are preventive, by limiting specific substrate availability (dietary modification), reducing hepatic substrate flux (high fiber), and/or increasing hepatic mitochondrial biogenesis to improve mitochondrial capacity and efficiency (exercise). Dietary recommendations to restrict trans-fats, refined carbohydrates (including fructose), and excessive protein loads can be bolstered by policy changes. The food industry has been increasingly successful in reintroducing whole grains and adding fiber back to processed foods, but dietary intake of fiber continues to fall short, and policies for removal of added sugar are currently nonexistent. Improved access to healthful unprocessed foods, and to regular physical activity in schools and communities, has been a policy priority for the current administration. Although such fundamental goals of lifestyle modification are laudable, their implementation is easier said than done.
Glossary
- apoB
apolipoprotein B
- BCAA
branched-chain amino acid
- CoA
coenzyme A
- CPT-1
carnitine palmitoyltransferase-1
- CVD
cardiovascular disease
- DNL
de novo lipogenesis
- ER
endoplasmic reticulum
- F1P
fructose-1-phosphate
- FFA
free fatty acid
- FoxO1
forkhead box protein O1
- HFCS
high-fructose corn syrup
- IR
insulin resistance
- IRS-1
insulin receptor substrate-1
- JNK-1
c-jun N-terminal kinase 1
- MetS
metabolic syndrome
- mTOR
mammalian target of rapamycin
- NAFLD
nonalcoholic fatty liver disease
- PKCε
protein kinase C-ε
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- SREBP
sterol regulatory element-binding protein
- T2DM
type 2 diabetes
- TG
triglyceride
- UPR
unfolded protein response
- VLDL
very low-density lipoprotein
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health (NIH).
References
- 1.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–969 [DOI] [PubMed] [Google Scholar]
- 2.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care. 2009;12(4):438–443 [DOI] [PubMed] [Google Scholar]
- 3.Conus F, Rabasa-Lhoret R, Péronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32(1):4–12 [DOI] [PubMed] [Google Scholar]
- 4.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699–713 [DOI] [PubMed] [Google Scholar]
- 5.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40(5):937–943 [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Lemieux S, Lamarche B, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19(suppl 1):S76–S86 [PubMed] [Google Scholar]
- 7.Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38(1):52–63 [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48 [DOI] [PubMed] [Google Scholar]
- 9.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087–2094 [DOI] [PubMed] [Google Scholar]
- 11.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49(6):1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194 [DOI] [PubMed] [Google Scholar]
- 14.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. 1992;41(6):715–722 [DOI] [PubMed] [Google Scholar]
- 15.Balkau B, Charles MA, European Group for the Study of Insulin Resistance (EGIR) Comment on the provisional report from the WHO consultation. Diabet Med. 1999;16(5):442–443 [DOI] [PubMed] [Google Scholar]
- 16.Kylin E. Studien uber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zentralbl Inn Med. 1923;44:105–127 [Google Scholar]
- 17.Albrink MJ, Krauss RM, Lindgrem FT, von der Groeben J, Pan S, Wood PD. Intercorrelations among plasma high density lipoprotein, obesity and triglycerides in a normal population. Lipids. 1980;15(9):668–676 [DOI] [PubMed] [Google Scholar]
- 18.Després JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9(5):452–459 [PubMed] [Google Scholar]
- 19.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607 [DOI] [PubMed] [Google Scholar]
- 20.Luciano A, Zoppi G. Blood insulin values after the oral glucose tolerance test (OGTT) and the body composition in 30 obese children. Pediatr Med Chir. 1994;16(5):471–473 [PubMed] [Google Scholar]
- 21.Biltoft CA, Muir A. The metabolic syndrome in children and adolescents: a clinician’s guide. Adolesc Med State Art Rev. 2009;20(1):109–120 [PubMed] [Google Scholar]
- 22.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord. 1996;20(6):501–506 [PubMed] [Google Scholar]
- 23.Povel CM, Boer JM, Reiling E, Feskens EJ. Genetic variants and the metabolic syndrome: a systematic review. Obes Rev. 2011;12(11):952–967 [DOI] [PubMed] [Google Scholar]
- 24.Monda KL, North KE, Hunt SC, Rao DC, Province MA, Kraja AT. The genetics of obesity and the metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2010;10(2):86–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Zhu J, Lum PY, et al. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452(7186):429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebert S, Sharkey D, Budge H, Symonds ME. The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr. 2011;94(suppl 6):1953S–1958S [DOI] [PubMed] [Google Scholar]
- 28.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, He S, Li JZ, et al. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107(17):7892–7897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantartzis K, Peter A, Machicao F, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58(11):2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacini G. The hyperbolic equilibrium between insulin sensitivity and secretion. Nutr Metab Cardiovasc Dis. 2006;16(suppl 1):S22–S27 [DOI] [PubMed] [Google Scholar]
- 32.Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol. 2008;159(suppl 1):S67–S74 [DOI] [PubMed] [Google Scholar]
- 33.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38(12):1512–1527 [DOI] [PubMed] [Google Scholar]
- 34.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991;12(1):3–13 [DOI] [PubMed] [Google Scholar]
- 36.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18 [DOI] [PubMed] [Google Scholar]
- 37.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95–96 [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116(9):2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340 [DOI] [PubMed] [Google Scholar]
- 40.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37(4):693–707 [PubMed] [Google Scholar]
- 42.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42(6):833–842 [DOI] [PubMed] [Google Scholar]
- 43.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007;120(2 suppl 1):S3–S8 [DOI] [PubMed] [Google Scholar]
- 44.McGarry JD. Malonyl-CoA and carnitine palmitoyltransferase I: an expanding partnership. Biochem Soc Trans. 1995;23(3):481–485 [DOI] [PubMed] [Google Scholar]
- 45.McGarry JD. The mitochondrial carnitine palmitoyltransferase system: its broadening role in fuel homoeostasis and new insights into its molecular features. Biochem Soc Trans. 1995;23(2):321–324 [DOI] [PubMed] [Google Scholar]
- 46.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244(1):1–14 [DOI] [PubMed] [Google Scholar]
- 47.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36(3):232–240 [DOI] [PubMed] [Google Scholar]
- 48.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 suppl 1):S10–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428 [DOI] [PubMed] [Google Scholar]
- 53.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789 [DOI] [PubMed] [Google Scholar]
- 54.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14(4):263–283 [DOI] [PubMed] [Google Scholar]
- 55.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab. 1998;24(5):409–418 [PubMed] [Google Scholar]
- 57.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(11):2276–2283 [DOI] [PubMed] [Google Scholar]
- 58.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14(12):1225–1230 [DOI] [PubMed] [Google Scholar]
- 59.Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(11):E1782–E1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033 [DOI] [PubMed] [Google Scholar]
- 61.Sopasakis VR, Sandqvist M, Gustafson B, et al. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes Res. 2004;12(3):454–460 [DOI] [PubMed] [Google Scholar]
- 62.Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res. 1999;31(12):626–631 [DOI] [PubMed] [Google Scholar]
- 63.Schalch DS, Kipnis DM. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965;44(12):2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93(6):2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roden M, Krssak M, Stingl H, et al. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes. 1999;48(2):358–364 [DOI] [PubMed] [Google Scholar]
- 67.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116 [DOI] [PubMed] [Google Scholar]
- 68.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104(31):12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control Trends in intake of energy and macronutrients - United States. Morb Mortal Wkly Rep. 2004;53(4):80–82 [PubMed] [Google Scholar]
- 70.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401 [DOI] [PubMed] [Google Scholar]
- 71.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118(2):789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86(2):285–300 [DOI] [PubMed] [Google Scholar]
- 73.Cave M, Deaciuc I, Mendez C, et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18(3):184–195 [DOI] [PubMed] [Google Scholar]
- 74.Chanmugam P, Guthrie JF, Cecilio S, Morton JF, Basiotis PP, Anand R. Did fat intake in the United States really decline between 1989-1991 and 1994-1996? J Am Diet Assoc. 2003;103(7):867–872 [DOI] [PubMed] [Google Scholar]
- 75.York LW, Puthalapattu S, Wu GY. Nonalcoholic fatty liver disease and low-carbohydrate diets. Annu Rev Nutr. 2009;29:365–379 [DOI] [PubMed] [Google Scholar]
- 76.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–16689 [DOI] [PubMed] [Google Scholar]
- 77.Willett WC. The great fat debate: total fat and health. J Am Diet Assoc. 2011;111(5):660–662 [DOI] [PubMed] [Google Scholar]
- 78.Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757 [DOI] [PubMed] [Google Scholar]
- 79.Assy N, Nassar F, Nasser G, Grosovski M. Olive oil consumption and non-alcoholic fatty liver disease. World J Gastroenterol. 2009;15(15):1809–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagao K, Inoue N, Wang YM, Shirouchi B, Yanagita T. Dietary conjugated linoleic acid alleviates nonalcoholic fatty liver disease in Zucker (fa/fa) rats. J Nutr. 2005;135(1):9–13 [DOI] [PubMed] [Google Scholar]
- 81.Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94(10):3842–3848 [DOI] [PubMed] [Google Scholar]
- 82.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771(8):972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rasooly R, Kelley DS, Greg J, Mackey BE. Dietary trans 10, cis 12-conjugated linoleic acid reduces the expression of fatty acid oxidation and drug detoxification enzymes in mouse liver. Br J Nutr. 2007;97(1):58–66 [DOI] [PubMed] [Google Scholar]
- 84.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G987–G995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorfman SE, Laurent D, Gounarides JS, et al. Metabolic implications of dietary trans-fatty acids. Obesity (Silver Spring). 2009;17(6):1200–1207 [DOI] [PubMed] [Google Scholar]
- 86.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136(suppl 1):319S–323S [DOI] [PubMed] [Google Scholar]
- 87.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higuchi N, Kato M, Miyazaki M, et al. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem. 2011;112(1):30–38 [DOI] [PubMed] [Google Scholar]
- 89.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101(7):1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baum JI, O’Connor JC, Seyler JE, Anthony TG, Freund GG, Layman DK. Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288(1):E86–E91 [DOI] [PubMed] [Google Scholar]
- 91.Chotechuang N, Azzout-Marniche D, Bos C, et al. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297(6):E1313–E1323 [DOI] [PubMed] [Google Scholar]
- 92.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3(6):393–402 [DOI] [PubMed] [Google Scholar]
- 93.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41(3):197–239 [DOI] [PubMed] [Google Scholar]
- 94.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454 [DOI] [PubMed] [Google Scholar]
- 95.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51(3):599–605 [DOI] [PubMed] [Google Scholar]
- 96.Krebs M, Brehm A, Krssak M, et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 2003;46(7):917–925 [DOI] [PubMed] [Google Scholar]
- 97.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293(6):E1552–E1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285(15):11348–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45(9):1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care. 1994;17(2):115–119 [DOI] [PubMed] [Google Scholar]
- 103.Di Castelnuovo A, Costanzo S, di Giuseppe R, de Gaetano G, Iacoviello L. Alcohol consumption and cardiovascular risk: mechanisms of action and epidemiologic perspectives. Future Cardiol. 2009;5(5):467–477 [DOI] [PubMed] [Google Scholar]
- 104.Athyros VG, Liberopoulos EN, Mikhailidis DP, et al. Association of drinking pattern and alcohol beverage type with the prevalence of metabolic syndrome, diabetes, coronary heart disease, stroke, and peripheral arterial disease in a Mediterranean cohort. Angiology. 2007;58(6):689–697 [DOI] [PubMed] [Google Scholar]
- 105.Sakurai Y, Umeda T, Shinchi K, et al. Relation of total and beverage-specific alcohol intake to body mass index and waist-to-hip ratio: a study of self-defense officials in Japan. Eur J Epidemiol. 1997;13(8):893–898 [DOI] [PubMed] [Google Scholar]
- 106.Baik I, Shin C. Prospective study of alcohol consumption and metabolic syndrome. Am J Clin Nutr. 2008;87(5):1455–1463 [DOI] [PubMed] [Google Scholar]
- 107.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110(9):1307–1321 [DOI] [PubMed] [Google Scholar]
- 108.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 suppl 1):S63–S74 [DOI] [PubMed] [Google Scholar]
- 109.García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90(3):460–466 [DOI] [PubMed] [Google Scholar]
- 110.Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR). Biol Cell. 1993;77(1):67–76 [DOI] [PubMed] [Google Scholar]
- 111.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E10–E16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinberg D, Pearson TA, Kuller LH. Alcohol and atherosclerosis. Ann Intern Med. 1991;114(11):967–976 [DOI] [PubMed] [Google Scholar]
- 113.Suter PM, Schutz Y. The effect of exercise, alcohol or both combined on health and physical performance. Int J Obes (Lond). 2008;32(suppl 6):S48–S52 [DOI] [PubMed] [Google Scholar]
- 114.Schneider J, Tesdorfpf M, Kaffarnik H, Hausmann L, Zöfel P, Zilliken F. Alteration of plasma lipids and intermediates of lipid metabolism in healthy fasting volunteers by ethanol and fructose. Res Exp Med (Berl). 1976;167(2):159–170 [DOI] [PubMed] [Google Scholar]
- 115.Lee YJ, Aroor AR, Shukla SD. Temporal activation of p42/44 mitogen-activated protein kinase and c-Jun N-terminal kinase by acetaldehyde in rat hepatocytes and its loss after chronic ethanol exposure. J Pharmacol Exp Ther. 2002;301(3):908–914 [DOI] [PubMed] [Google Scholar]
- 116.You M, Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34(1):39–43 [DOI] [PubMed] [Google Scholar]
- 117.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34(1):35–38 [DOI] [PubMed] [Google Scholar]
- 118.Guzmán M, Castro J. Alterations in the regulatory properties of hepatic fatty acid oxidation and carnitine palmitoyltransferase I activity after ethanol feeding and withdrawal. Alcohol Clin Exp Res. 1990;14(3):472–477 [DOI] [PubMed] [Google Scholar]
- 119.Yokoyama H, Hiroshi H, Ohgo H, Hibi T, Saito I. Effects of excessive ethanol consumption on the diagnosis of the metabolic syndrome using its clinical diagnostic criteria. Intern Med. 2007;46(17):1345–1352 [DOI] [PubMed] [Google Scholar]
- 120.Onishi Y, Honda M, Ogihara T, et al. Ethanol feeding induces insulin resistance with enhanced PI 3-kinase activation. Biochem Biophys Res Commun. 2003;303(3):788–794 [DOI] [PubMed] [Google Scholar]
- 121.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit Rev Food Sci Nutr. 2010;50(3):228–258 [DOI] [PubMed] [Google Scholar]
- 122.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 123.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88(6):1733S–1737S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abraha A, Humphreys SM, Clark ML, Matthews DR, Frayn KN. Acute effect of fructose on postprandial lipaemia in diabetic and non-diabetic subjects. Br J Nutr. 1998;80(2):169–175 [PubMed] [Google Scholar]
- 125.Cohen JC, Schall R. Reassessing the effects of simple carbohydrates on the serum triglyceride responses to fat meals. Am J Clin Nutr. 1988;48(4):1031–1034 [DOI] [PubMed] [Google Scholar]
- 126.Teff KL, Elliott SS, Tschöp M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89(6):2963–2972 [DOI] [PubMed] [Google Scholar]
- 127.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87(5):1194–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Swarbrick MM, Stanhope KL, Elliott SS, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100(5):947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63(5):133–157 [DOI] [PubMed] [Google Scholar]
- 130.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543 [DOI] [PubMed] [Google Scholar]
- 131.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol. 2008;19(1):16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010;303(15):1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999-2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2009;163(4):328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138(8):1452–1455 [DOI] [PubMed] [Google Scholar]
- 138.Collison KS, Saleh SM, Bakheet RH, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (Silver Spring). 2009;17(11):2003–2013 [DOI] [PubMed] [Google Scholar]
- 139.Lê KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89(6):1760–1765 [DOI] [PubMed] [Google Scholar]
- 140.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264 [DOI] [PubMed] [Google Scholar]
- 141.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46 [DOI] [PubMed] [Google Scholar]
- 142.Bjorkman O, Crump M, Phillips RW. Intestinal metabolism of orally administered glucose and fructose in Yucatan miniature swine. J Nutr. 1984;114(8):1413–1420 [DOI] [PubMed] [Google Scholar]
- 143.Nagai Y, Yonemitsu S, Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9(3):252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54(7):1907–1913 [DOI] [PubMed] [Google Scholar]
- 145.Matsuzaka T, Shimano H, Yahagi N, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53(3):560–569 [DOI] [PubMed] [Google Scholar]
- 146.Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282(5):E1180–E1190 [DOI] [PubMed] [Google Scholar]
- 147.Wei Y, Wang D, Pagliassotti MJ. Fructose selectively modulates c-jun N-terminal kinase activity and insulin signaling in rat primary hepatocytes. J Nutr. 2005;135(7):1642–1646 [DOI] [PubMed] [Google Scholar]
- 148.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem. 2007;18(1):1–9 [DOI] [PubMed] [Google Scholar]
- 149.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336 [DOI] [PubMed] [Google Scholar]
- 150.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Civitarese AE, Ravussin E. Mitochondrial energetics and insulin resistance. Endocrinology. 2008;149(3):950–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parola M, Marra F. Adipokines and redox signaling: impact on fatty liver disease. Antioxid Redox Signal. 2011;15(2):461–483 [DOI] [PubMed] [Google Scholar]
- 154.Subasinghe W, Syed I, Kowluru A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic β-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol. 2011;300(1):R12–R20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(pt 2):335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med. 2010;49(11):1646–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9(12):2277–2293 [DOI] [PubMed] [Google Scholar]
- 158.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–955 [DOI] [PubMed] [Google Scholar]
- 159.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213(4504):222–224 [DOI] [PubMed] [Google Scholar]
- 160.Thoms S, Grønborg S, Gärtner J. Organelle interplay in peroxisomal disorders. Trends Mol Med. 2009;15(7):293–302 [DOI] [PubMed] [Google Scholar]
- 161.Dirkx R, Vanhorebeek I, Martens K, et al. Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology. 2005;41(4):868–878 [DOI] [PubMed] [Google Scholar]
- 162.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763(12):1755–1766 [DOI] [PubMed] [Google Scholar]
- 163.Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O’Brien PJ. Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact. 2009;178(1-3):332–339 [DOI] [PubMed] [Google Scholar]
- 164.Pickens MK, Yan JS, Ng RK, et al. Dietary sucrose is essential to the development of liver injury in the methionine-choline-deficient model of steatohepatitis. J Lipid Res. 2009;50(10):2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13(4):581–590 [DOI] [PubMed] [Google Scholar]
- 166.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135(5):933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15(5):767–776 [DOI] [PubMed] [Google Scholar]
- 168.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11(10):2409–2427 [DOI] [PubMed] [Google Scholar]
- 169.Mollica MP, Lionetti L, Putti R, Cavaliere G, Gaita M, Barletta A. From chronic overfeeding to hepatic injury: role of endoplasmic reticulum stress and inflammation. Nutr Metab Cardiovasc Dis. 2011;21(3):222–230 [DOI] [PubMed] [Google Scholar]
- 170.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107(5):579–591 [DOI] [PubMed] [Google Scholar]
- 171.Yki-Järvinen H. Nutritional modulation of nonalcoholic fatty liver disease and insulin resistance: human data. Curr Opin Clin Nutr Metab Care. 2010;13(6):709–714 [DOI] [PubMed] [Google Scholar]
- 172.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342(19):1392–1398 [DOI] [PubMed] [Google Scholar]
- 173.Brynes AE, Mark Edwards C, Ghatei MA, et al. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br J Nutr. 2003;89(2):207–218 [DOI] [PubMed] [Google Scholar]
- 174.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299(2):E145–E161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hannukainen JC, Borra R, Linderborg K, et al. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol. 2011;54(3):545–552 [DOI] [PubMed] [Google Scholar]