Abstract
Learning increases neurogenesis by increasing the survival of new cells generated in the adult hippocampal formation [Shors, T. J. Saving new brain cells. Scientific American, 300, 46-52, 2009]. However, only some types of learning are effective. Recent studies demonstrate that animals that learn the conditioned response (CR) but require more trials to do so retain more new neurons than animals that quickly acquire the CR or that fail to acquire the CR. In these studies, task parameters were altered to modify the number of trials required to learn a CR. Here, we asked whether pharmacological manipulations that prevent or facilitate learning would decrease or increase, respectively, the number of cells that remain in the hippocampus after training. To answer this question, we first prevented learning with the competitive N-methyl-d-aspartate (NMDA) receptor antagonist (RS)-3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid. As a consequence, training did not increase cell survival. Second, we facilitated learning with the cognitive enhancer d-cycloserine, which increases NMDA receptor activity via its actions at the glycine binding site. Administration of d-cycloserine each day before training increased the number of learned responses and the number of cells that survived. All animals that learned the CR retained more of the new cells, but those that learned very quickly retained fewer than those that required more training trials to learn. Together, these results demonstrate that NMDA receptor activation modifies learning and as a consequence alters the number of surviving cells in the adult hippocampus.
INTRODUCTION
The dentate gyrus of the hippocampal formation generates new granule neurons throughout life (Kornack & Rakic, 1999; Eriksson et al., 1998; Kaplan & Hinds, 1977; Altman & Das, 1965). It is estimated that the adult dentate gyrus generates 5000–10,000 new cells per day (Cameron & McKay, 2001), the vast majority of which differentiate into neurons (Hastings & Gould, 1999; Markakis & Gage, 1999; Cameron, Woolley, McEwen, & Gould, 1993). However, over half of these cells die within a few weeks of their birth (Gould, Beylin, Tanapat, Reeves, & Shors, 1999). One week after they are born, these neurons display a remarkable sensitivity to various forms of learning that can prevent their death, thereby increasing the number of new neurons that survive in the adult brain (Shors, 2009; Waddell & Shors, 2008; Leuner et al., 2004; Dayer, Ford, Cleaver, Yassaee, & Cameron, 2003; Ambrogini et al., 2000; Gould et al., 1999).
The first study to report that learning increases neuronal survival indicated that tasks that depend on the hippocampus are the most effective (Gould et al., 1999). Since then, it has been determined that hippocampal dependence, per se, is not necessary to increase the number of surviving cells (reviewed in Shors, 2008). For example, animals that were trained with a hippocampal-independent form of trace conditioning, known as contiguous trace conditioning, possessed more new neurons after learning than naive controls (Dalla, Bangasser, Edgecomb, & Shors, 2007). That said, the most effective tasks tend to depend on the hippocampus for learning.
In general, tasks that are dependent on the hippocampus tend to be more difficult to learn than their non-hippocampal-dependent counterparts. Perhaps task difficulty is the critical factor. Indeed, this appears to be the case. Animals that learn, but do so after more training trials, rescue more newborn neurons than animals that quickly acquire the same learned response (Waddell & Shors, 2008; Dalla et al., 2007). Moreover, learning, not merely training, is likewise critical (Anderson, Sisti, Curlik, & Shors, 2010). Thus, those animals that are trained but do not learn do not retain any more cells than naive animals. Overall, these results indicate that animals that fail to acquire a learned response or do so very rapidly will have fewer surviving newborn neurons than animals that learn but require many trials of training to do so. These conclusions were drawn from studies in which task parameters were manipulated to alter acquisition and asymptotic performance. If these conclusions can be of general significance, then they should apply to other learning situations, including those in which learning is altered pharmacologically. In the current set of studies we pharmacologically manipulated acquisition of trace eyeblink conditioning because learning this task reliably increases cell survival. In the first experiment, we prevented learning with a competitive N-methyl-d-aspartate (NMDA) receptor antagonist, (RS)-3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid (CPP). In the second experiment, we enhanced learning with the partial glycine receptor agonist d-cycloserine (DCS). The effects of these agents on learning and the consequent effects on the number of surviving cells were determined.
METHODS
General Methods for All Experiments
Subjects
Adult male Sprague–Dawley rats ranging between 60 and 90 days of age were individually housed and given access to food and water ad libitum. The animals were maintained on a 12-hr light–dark cycle, with the lights turning on at 7:00 a.m. Rats were handled by the experimenter at least 1 week before surgery. The experiments were designed to fully comply with the rules and regulations set forth by the PHS Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals.
Surgical Procedures
Animals were anesthetized with sodium pentobarbital (15 mg/kg), which was supplemented with isoflurane gas. They then underwent stereotaxic surgery for the implantation of four periorbital electrodes, which were placed in the muscle of the right eyelid. The electrodes were composed of a stainless steel wire (0.005 in. diameter) that was inserted through the eyelid, under the skin of the scalp, to a headstage that was mounted on the skull. Dental cement secured the headstage to four small screws that were partially embedded within the skull (Servatius & Shors, 1996). All animals were given a minimum recovery period of 5 days before receiving one single intraperitoneal injection (200 mg/kg) of bromodeoxyuridine (BrdU), a thymidine analog that labels cells in the S-phase of the cell cycle (Cameron & McKay, 2001; Miller & Nowakowski, 1988).
Classical Eyeblink Conditioning
Six days after the BrdU injection, animals were acclimated to the conditioning chambers for 1 hr while spontaneous blinking activity was recorded. The next day (1 week after the BrdU injection), groups of animals began training with either paired trace or explicitly unpaired eyeblink conditioning. Training continued each day for 4 days (for the experimental time line, see Figure 1A). During each day of training, the animals were injected with either the drug or the physiological saline before or after the training session. All animals were trained with 200 trials per day. Each trial of trace conditioning consisted of an 82-dB white noise conditioned stimulus (CS), followed by a 500-msec trace interval, which was followed by a 100-msec unconditioned stimulus (US) of 0.65 mA of periorbital stimulation to the right eyelid. The intertrial interval was 25 ± 5 sec. Explicitly unpaired training consisted of the same number of CS and US presentations as trace conditioning; however, the CS and the US were presented in an explicitly unpaired manner.
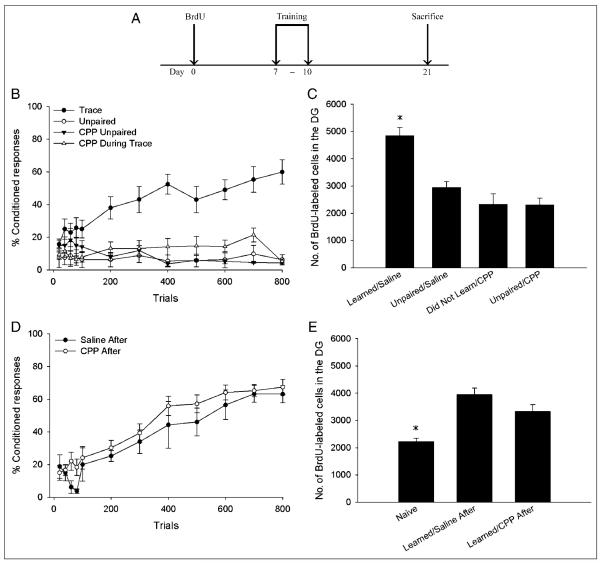
Figure 1.
(A) A diagram depicting the time line of both experiments. Animals underwent stereotaxic surgery at least 5 days before the BrdU injection. CPP, DCS, or saline was administered during the 4 days of training (days 7–10 post-BrdU). (B) Administration of the NMDA receptor antagonist CPP during trace eyeblink conditioning (CPP During Trace) prevented acquisition of the CR. CPP did not alter responding to unpaired stimuli (CPP Unpaired). Additional animals received saline during training with trace eyeblink conditioning (Trace) or explicitly unpaired stimuli (Unpaired). (C) Animals that received saline during training with trace conditioning that reached criterion (Learned/Saline) retained more BrdU-labeled cells than animals that received CPP during trace conditioning, none of which reached criterion (Did Not Learn/CPP). The saline-treated animals that learned retained more new cells than animals that received saline during unpaired training (Unpaired/Saline) and CPP during unpaired training (Unpaired/CPP). The number of BrdU-labeled cells in animals that received CPP during trace conditioning was similar to the number of cells in animals that received saline or CPP during unpaired training. (D) CPP did not alter acquisition or asymptotic performance of trace eyeblink conditioning when it was administered 6 hr after training. (E) CPP did not alter the number of BrdU-labeled cells when administered 6 hr after training, as animals that received saline or CPP after training that learned retained more newborn cells than experimentally naive animals.
The occurrence of an eyeblink was determined from EMG recording of the right eyelid muscle. During trace conditioning a baseline recording was taken 250 msec before every trial. A conditioned response (CR) was counted on that trial if a response occurred during the 500-msec trace interval and if that response had an amplitude greater than the maximum amplitude from the baseline recording for that trial plus four times the baseline recording’s standard deviation. A similar baseline recording was taken 250 msec before the onset of the CS during unpaired training. CRs during unpaired training were measured as responses that occurred up to 500 msec after the offset of the CS. Trials were divided into blocks of 100 consecutive trials. The first 100 trials were also examined in blocks of 20 trials. The number of trials required for an animal to reach a behavioral criterion of 60% CRs during any block of 100 trials was determined. Animals that reached this criterion were considered to have successfully learned the CR (Waddell & Shors, 2008; Dalla et al., 2007; Moyer, Thompson, & Disterhoft, 2000). In addition, we examined another common measure of acquisition of the CR: the number of trials an animal required to emit a CR on eight of nine consecutive trials during any one block of 100 trials (Dalla, Papachristos, Whetstone, & Shors, 2009; Nokia, Penttonen, Korhonen, & Wikgren, 2008).
Immunohistochemistry for BrdU
Twenty-one days after the BrdU injection, all animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with 4% paraformaldehyde. Brains were extracted and postfixed in 4% paraformaldehyde for a minimum of 48 hr before being sectioned. Coronal sections (40 μ M) were cut through the entire rostral-caudal extent of the dentate gyrus of one hemisphere with an oscillating tissue slicer. A 1:12 series of slices was mounted onto glass slides for BrdU immunohistochemistry. To stain for the presence of BrdU, these slices were heated in 0.1 M citric acid (pH 6.0), incubated in trypsin followed by 2N HCl, and then incubated over-night in primary antimouse BrdU (1:200; Becton Dickson, Franklin Lakes, NJ) and 0.5% Tween 20. The next day, the tissue was incubated in biotinylated antimouse antibody (1:200; Vector Laboratories, Burlingame, CA), followed by avidin–biotin–horseradish peroxidase (1:100; Vector Laboratories). The slices were then placed in diamino-benzidine, counterstained with cresyl violet, and cover-slipped. All slides were coded so that the experimenter was blind to the experimental condition while they counted the number of BrdU-labeled cells present in the entire dentate gyrus of each slice. To accurately estimate the number of BrdU-labeled cells throughout both hemispheres of the entire dentate gyrus of one animal, the number of BrdU-labeled cells in the slices from that animal was multiplied by 24.
Statistical Analysis
Performance during eyeblink conditioning was analyzed with repeated measures ANOVA, with trial blocks as the repeated measure and number of CRs as the dependent measure. Post hoc comparisons were made between groups using Tukey’s HSD test. The number of BrdU-labeled cell was analyzed with one-way and two-way ANOVAs. Independent samples t tests and Mann-Whitney U tests were performed as needed.
Experiment 1 Methods: Does the NMDA Receptor-dependent Blockade of Learning Prevent the Increase in Cell Survival?
The competitive NMDA receptor antagonist CPP (Tocris Bioscience) was dissolved in 0.9% saline and injected intraperitoneally (10 mg/kg) each day, 1 hr before training. Previous research has demonstrated that pretraining administration of this dose of CPP prevents acquisition of trace eyeblink conditioning (Leuner, Falduto, & Shors, 2003). The experiment consisted of seven groups. Group 1 (n = 7) received injections of CPP each day, 1 hr before training with trace eyeblink conditioning (7–10 days after the BrdU injection). Group 2 (n = 7) received saline injections at the same time before trace conditioning. Group 3 (n = 6) received injections of CPP 1 hr before explicitly unpaired training, whereas Group 4 (n = 4) received saline injections 1 hr before unpaired training. Comparison of these first four groups (Groups 1–4) allowed us to determine whether pretraining administration of CPP prevented trace eyeblink conditioning and the subsequent increase in cell survival that is normally seen after learning. Comparison of the BrdU-labeled cell counts in the two groups trained with unpaired stimuli (Groups 3 and 4) revealed whether CPP administration itself, irrespective of its effects on learning, increased the number of surviving cells.
Group 5 (n = 12) received injections of CPP every day, 6 hr after trace conditioning, when CPP administration did not interfere with acquisition of the CR. Group 6 (n = 4) received saline injections every day, 6 hr after trace conditioning. The remaining group, Group 7 (n = 4), received daily saline injections at the same time as Groups 5 and 6; however, this group was not trained with any form of eyeblink conditioning. The cell counts from Groups 5 to 7 were used to determine whether CPP decreased cell survival irrespective of its effects on learning.
Experiment 2 Methods: Does the NMDA Receptor Modulator DCS Facilitate Learning and Thereby Increase the Number of Cells That Survive?
DCS (Sigma-Aldrich, Atlanta, GA), a partial agonist of the strychnine-insensitive glycine binding site, was used to facilitate acquisition of the CR during trace eyeblink conditioning. The experiment consisted of five groups. Group 1 (n = 7) received intraperitoneal injections of DCS dissolved in 0.9% saline (15 mg/kg) 30 min before each session of trace conditioning. This dose of DCS has previously been shown to facilitate learning during trace eyeblink conditioning (Waddell, Mallimo, & Shors, 2010; Thompson & Disterhoft, 1997; Thompson, Moskal, & Disterhoft, 1992). Group 2 (n = 9) received daily injections of saline 30 min before trace conditioning. Comparisons between these two groups allowed us to determine whether DCS facilitated acquisition of the trace eyeblink response, and if so, if that facilitated learning resulted in an increase in the number of surviving cells. Two additional groups (Groups 3 and 4) received DCS (n = 7) or saline (n = 4) 30 min before training with explicitly unpaired stimuli. These two groups were examined to determine whether administration of DCS itself might increase cell survival, irrespective of its effects on learning. A fifth group of animals (n = 5) received daily injections of DCS 6 hr after trace conditioning, at a time point when administration of DCS did not alter acquisition of the CR. A comparison of the number of BrdU-labeled cells between this group and Group 1, which received saline before trace conditioning, allowed us to determine whether DCS decreased cell survival, irrespective of its effects on learning.
RESULTS
Experiment 1 Results: Administration of an NMDA Receptor Antagonist before but not after Training Prevents Learning and the Enhancement in Cell Survival Seen after Learning
Administration of the NMDA receptor antagonist CPP before training each day completely prevented acquisition of the CR. A repeated measures ANOVA using the drug condition (CPP vs. saline) and the training protocol (paired vs. unpaired) as the independent measures, the trial blocks (100 trial blocks, two blocks per day) as the repeated measure, and the percentage of CRs as the dependent measure revealed a significant interaction between the drug condition and the training protocol, F(1, 20) = 38.71, p < .01, indicating that CPP only prevented conditioned responding when it was administered before trace but not unpaired conditioning. Furthermore, a significant interaction was found between training blocks and drug condition, F(7, 140) = 2.40, p < .05, revealing that CPP prevented any increase in conditioned responding during trace conditioning (Figure 1B).
A two-way ANOVA was performed with drug condition (CPP vs. saline) and training protocol (paired vs. unpaired) as the independent measures and the percentage of CRs emitted during the last session as the dependent measure. Results revealed a significant effect of drug condition, F(1, 23) = 20.93, p < .01, and training protocol, F(1, 23) = 31.73, p < .01, with an interaction between drug condition and training protocol, F(1, 23) = 14.89, p < .01. Therefore, animals that received saline before trace conditioning emitted a greater percentage of CRs during the last day of training than either of the two groups that received unpaired training or the group that received CPP before trace conditioning. Furthermore, the percentage of CRs emitted by the group that received CPP before trace conditioning was not different than the percentages emitted by the two unpaired groups. Therefore, administration of CPP before trace eyeblink conditioning completely prevented acquisition of the CR. In addition, CPP administration did not alter responding to explicitly unpaired stimuli.
Nearly all (86%) of the animals that received saline during trace conditioning reached the 60% response criterion (Learned/Saline), whereas none of the animals that were trained with unpaired stimuli (Unpaired/Saline; Unpaired/CPP) or those that received CPP before trace conditioning did (Did Not Learn/CPP). To examine the difference in cell counts between animals that learned versus those that did not, a two-way ANOVA was conducted, with drug condition and training protocol as the independent measures and the number of BrdU-labeled cells as the dependent measure. Only saline-treated animals that reached the 60% criterion were included in this analysis. This was done to ensure that we compared the cell counts of one entire group of animals that successfully learned the task to one group that did not. Results revealed a significant interaction between drug condition and training protocol, F(1, 22) = 8.24, p < .05, indicating that only the animals that received saline before trace conditioning (and that reached criterion) displayed an increase in the number of surviving cells (Figure 1C).
To examine the possibility that CPP may decrease cell survival irrespective of its effects on learning, we examined the number of new cells in two additional groups that received saline (Saline After) or CPP (CPP After) 6 hr after each day of training, a time point when CPP administration did not interfere with acquisition of the CR. A repeated measures ANOVA was conducted, with drug condition as the independent measure, trial blocks as the repeated measure, and percentage of CRs as the dependent measure. The results revealed a significant main effect of trial block, F(7, 98) = 23.39, p < .01, indicating that the percentage of CRs emitted by these two groups increased over the course of training. There was no effect of drug condition, F(1, 14) = 1.00, p > .05, and no interaction between drug condition and trial block, F(7, 98) = .08, p > .05. Therefore, administration of the NMDA receptor antagonist 6 hr after every day of trace conditioning did not prevent acquisition of the CR (Figure 1D). Furthermore, administration of CPP 6 hr after training did not alter responding on the last session of training, t(14) = .38, p > .05 (Saline After vs. CPP After), nor did it change the number of trials required to reach the 60% criterion (U = 8.0, p > .05, Saline After vs. CPP After) or the number of trials required to emit eight of nine consecutive CRs (U = 19.0, p > .05).
An ANOVA was used to compare the number of BrdU-labeled cells in animals that received saline or CPP after training, which reached the 60% criterion, to the number of BrdU-labeled cells in experimentally naive animals, which received saline injections, but no training (naive). There was a difference in cell number among these groups, F(2, 17) = 7.12, p < .01. Post hoc Tukey comparisons indicated that animals that reached the 60% criterion despite being injected with saline or CPP each day after training possessed more BrdU-labeled cells than the naive controls (p values < .05; Figure 1E).
Thus, animals could still acquire the CR as long as the NMDA receptor antagonist was given after training. In fact, their performance was no different than that of animals injected with saline and trained with paired stimuli. Because the cell counts from these animals that received CPP after training were not different from those that were injected with saline and trained with paired stimuli, we can conclude that antagonism of NMDA receptors before but not 6 hr after training blocks learning of the CR and thereby prevents the increase in cell survival normally seen after learning. In other words, the effect of CPP on cell survival is via its effect on acquisition of the CR and not an effect of the drug alone.
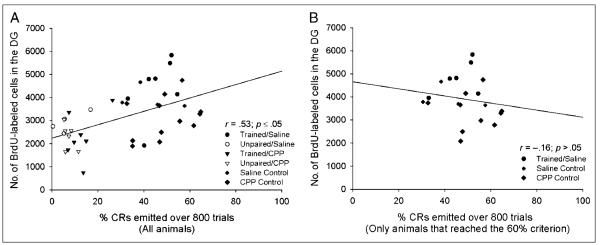
In the past, our laboratory has reported a positive correlation between the number of trials that an individual animal requires to learn a task and the number of surviving cells in that animal’s dentate gyrus (Waddell & Shors, 2008). When we examined the data from all animals in Experiment 1, including those animals that did not reach the 60% criterion, we observed a significant positive correlation between the percentage of CRs an animal emitted over all 800 trials and the number of surviving cells in that animal’s dentate gyrus (r = .53, p < .05; Figure 2A). This result confirms that animals that successfully learned the CR retained more new cells than animals that failed to acquire the CR. When we performed this analysis using only the data from animals that reached the 60% criterion, we observed no such correlation between the percentage of CRs emitted during training and the number of surviving cells (r =− .16, p > .05; Figure 2B).
Figure 2.
(A) When the data from all animals in the first experiment were examined, we observed a significant positive correlation between the percentage of CRs an individual animal emitted over the entire course of training and the number of BrdU-labeled cells in that animal’s dentate gyrus. (B) However, when we analyzed the data from only those animals that reached the 60% criterion during the first experiment, we did not observe a significant correlation.
Experiment 2 Results: DCS Facilitates Trace Conditioning and Neurogenesis
Pretraining administration of DCS has previously been demonstrated to facilitate learning of trace eyeblink conditioning (Waddell et al., 2010; Thompson & Disterhoft, 1997; Thompson et al., 1992). The current experiment was conducted to determine whether DCS-facilitated learning would result in an increase in the number of surviving newborn cells in the adult dentate gyrus. The experiment consisted of five groups. Groups 1 and 2 received DCS or saline before training with trace eyeblink conditioning. Groups 3 and 4 received DCS or saline before training with explicitly unpaired stimuli. Group 5 received DCS 6 hr after training with trace eyeblink conditioning. To determine whether DCS facilitated acquisition, we examined early acquisition, which was defined as the percentage of CRs emitted during the first day of training. An independent samples t test comparing the percentage of CRs emitted during the first day indicated that learning in the presence of DCS significantly enhanced early acquisition of the CR, t(14) = 2.63, p < .05 (Trace vs. DCS during Trace). A separate independent samples t test revealed that DCS did not alter responding to unpaired stimuli during the first day of training, t(9) = 0.85, p > .05 (Unpaired vs. DCS Unpaired).
The total number of CRs emitted during the entire 4 days of training differed between the five groups, F(4, 27) = 25.18, p < .05. Post hoc analysis indicated that pretraining administration of DCS did not alter the total number of CRs emitted during the 4 days of trace conditioning, (p > .05 Trace vs. DCS during Trace; Figure 3A). Therefore, although administration of DCS before trace conditioning facilitated early acquisition of the CR, it had no effect on overall responding during the remaining days of training. In addition, DCS did not alter the total number of CRs emitted during training when it was administered 6 hr after training (p > .05, Trace vs. DCS After, data not shown; Ledgerwood, Richardson, & Cranney, 2003) or when it was administered before training with explicitly unpaired stimuli (p > .05, Unpaired vs. DCS unpaired).
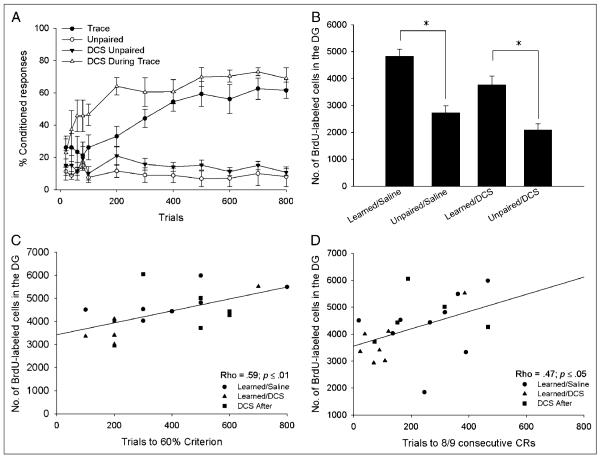
Figure 3.
(A) Intraperitoneal administration of DCS before training with trace eyeblink conditioning (DCS during Trace) facilitated acquisition of the CR, as evidenced by an increase in conditioned responding during the first day of training. DCS did not alter responding to explicitly unpaired stimuli (DCS Unpaired). For comparison, animals received saline during training with trace conditioning (Trace) or explicitly unpaired stimuli (Unpaired). (B) Animals treated with saline during trace conditioning that reached the 60% criterion (Learned/Saline) retained more BrdU-labeled cells than animals that received saline during unpaired training (Unpaired/Saline), or animals that received DCS during unpaired training (Unpaired/DCS). Those animals that received DCS during Trace conditioning (Learned/DCS) retained significantly more BrdU-labeled cells than animals that received DCS before unpaired training (Unpaired/DCS). (C) A significant positive correlation was observed between the number of trials an individual animal required to reach criterion and the number of BrdU-labeled cells in that animal’s dentate gyrus. Only animals that successfully reached the 60% criterion were included in this analysis. (D) The number of BrdU-labeled cells also positively correlated with the number of trials required to reach eight of nine consecutive CRs during any one block of trials.
Groups that were trained with trace conditioning (Trace, DCS during Trace, and DCS After) increased the percentage of CRs that they emitted across training blocks, F(7, 126) = 20.144, p < .05, with no interaction between group and training block, F(14, 126) = 1.25, p > .05, revealing that all trace conditioned groups successfully acquired the CR over the course of training. There was no increase in responding in animals exposed to unpaired stimuli, F(7, 63) = 1.18, p > .05, and no difference in overall responding between the two unpaired groups, F(1, 9) = 1.27, p > .05. Thus, only animals that were trained with trace conditioning successfully acquired the CR. DCS did not alter the number of responses emitted during explicitly unpaired training. These results suggest that DCS does not increase conditioning simply by increasing nonspecific responding to the CS.
There were no differences in responding during the last day of training among those groups exposed to trace conditioning, F(2, 18) = 0.82, p > .05. Together, these results indicate that the effects of DCS on trace eyeblink conditioning are limited to early acquisition of the CR. Moreover, DCS only facilitated acquisition when it was administered 30 min before but not 6 hr after training.
Most (78%) animals given saline during trace conditioning reached our 60% response criterion (Learned/Saline), whereas all animals (100%) given DCS during (Learned/DCS) or after trace conditioning (DCS After) reached this criterion. None of the animals that received unpaired training reached the criterion. Interestingly, all trace-conditioned and no unpaired animals reached the eight of nine response criterion. Furthermore, there was a strong positive correlation between these two criteria (Rho = 0.83, p < .01). However, animals tended to emit eight of nine consecutive CRs before they reached the 60% criterion.
The number of BrdU-labeled cells in the entire dentate gyrus was different between the trained groups, F(5, 27) = 13.27, p < .01. Post hoc comparisons revealed that saline-treated animals that reached the 60% response criterion retained more newborn cells than saline-treated animals that were trained with trace conditioning that did not reach criterion (p < .05, Learned/Saline vs. Did Not Learn/Saline). The saline-treated animals that reached this criterion also retained more cells than animals that received saline during unpaired training (p < .01, Learned/Saline vs. Unpaired/Saline) and DCS during unpaired training (p < .01, Learned/Saline vs. Unpaired/DCS).
Interestingly, although animals that received DCS during training expressed more CRs at the beginning of training, they did not retain more new neurons than animals that learned in the absence of the drug (p > .05, Learned/DCS vs. Learned/Saline; Figure 3B). In addition, administration of DCS by itself did not increase the survival of newborn neurons (p > .05, Unpaired/DCS vs. Unpaired/Saline). Furthermore, the cell counts from animals that received DCS 6 hr after training (which did not alter acquisition of the CR) were no different from those of animals that learned in the absence of the drug (p > .05, DCS After vs. Learned/Saline, data not shown), indicating that administration of DCS itself did not decrease the number of surviving cells.
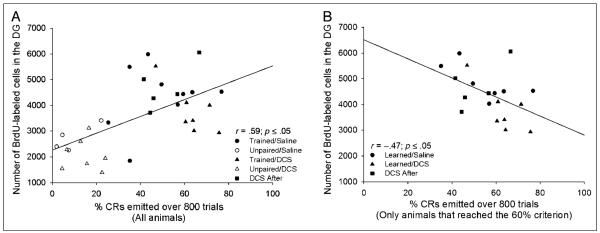
Consistent with previous results (Waddell & Shors, 2008), we observed a significant positive correlation between the number of trials that an individual animal required to reach the 60% response criterion and the number of BrdU-labeled cells in that animal’s dentate gyrus (Rho = .59, p < .05; Figure 3C). In addition, there was a positive correlation between the number of trials required to emit eight of nine consecutive CRs and the number of BrdU-labeled cells (Rho = .47, p < .05; Figure 3D). As in the first experiment, when we examined the data from all animals, including those that did not reach the 60% criterion, we observed a positive correlation between the percentage of CRs emitted over the entire course of training and the number of surviving BrdU-labeled cells (r = .59; Figure 4A). Furthermore, when we examined the data from animals that successfully learned the task (i.e., those that reached the 60% criterion), we found a strong negative correlation between the percentage of CRs an individual animal emitted during the 800 trials of trace conditioning and the number of surviving cells in the granule cell layer of that animal’s dentate gyrus (r =−.47, p < .05; Figure 4B). Thus, animals that successfully acquired the CR and required more trials to do so retained more of the newborn cells.
Figure 4.
(A) A significant positive correlation was observed between the percentage of CRs an individual animal emitted over the entire course of training (800 trials) and the number BrdU-labeled cells in that animal’s dentate gyrus. (B) After analyzing only data from animals that learned (i.e., reached the learning criterion), there was a significant negative correlation between the percentage of CRs an individual animal emitted during training and the number of BrdU-labeled cells in the granule cell layer of that animal’s dentate gyrus.
DISCUSSION
It is now well established that training with various learning tasks can increase the number of newly born cells that survive to become neurons in the adult hippocampus (Shors, 2009). The goal of these experiments was to assess the role of learning (per se) in this phenomenon. To do this, we manipulated performance of a learned response in two ways and directions. First, we completely prevented learning by using an NMDA receptor antagonist—which was injected each day before training with trace conditioning. Animals that received the antagonist before training did not learn, and they did not retain any more cells in their hippocampus than animals that were trained with unpaired stimuli, which also did not learn the CR. These effects were not due to adverse consequences of the drug itself because animals that were injected with the antagonist after training were able to learn the CR and retained as many new cells as the animals that were trained in the presence of saline. As noted, animals that learned the CR retained many more new cells than those trained with unpaired stimuli. Thus, preventing learning with an NMDA receptor antagonist prevents the increase in cell number that typically occurs after training. These effects are clear, albeit not necessarily surprising, because our previous studies have suggested that the effects of training on cell survival only occur in animals that actually learn (Dalla et al., 2007; Sisti, Glass, & Shors, 2007). However, these results extend our findings to demonstrate that pharmacological manipulations of learning determine how many cells ultimately survive to become neurons in the adult hippocampus. Because the cells that are rescued from death by learning remain in the hippocampus for months, at least (Leuner et al., 2004), these pharmacological effects of CPP presumably have similarly persistent effects on the circuitry of the adult hippocampal formation.
In the second experiment, we manipulated learning in the opposite way, again using a drug that modulates neuronal activity at the NMDA receptor. The drug, DCS, is a partial agonist of the strychnine-insensitive glycine binding site, which facilitates NMDA receptor-mediated excitatory transmission (Rouaud & Billard, 2003; Henderson, Johnson, & Ascher, 1990; Hood, Compton, & Monahan, 1989) and learning (Waddell et al., 2010; Thompson & Disterhoft, 1997; Thompson et al., 1992). Similarly, we observed an increase in performance during trace eyeblink conditioning in response to the drug. As a consequence, we report that the number of cells that were retained in the group that received DCS before trace conditioning was elevated when compared with the number of cells in animals that received unpaired training in the presence of the drug. However, DCS did not increase cell numbers beyond those observed in animals that learned in the presence of saline. Furthermore, those animals that naturally failed to acquire the CR did not retain any more of the new cells than those trained with unpaired stimuli. These data suggest that the overall increase in performance in the presence of the drug was not sufficient to increase the number of surviving cells beyond what learning without the drug would accomplish. This result is consistent with that of a previous study, where we found that learning, when it occurred, rescued nearly all, if not all, of the cells that were available to be rescued (Waddell & Shors, 2008). One might surmise that there are simply no more new cells left to rescue—at least not of this particular cohort. Despite a potential ceiling on the number of cells that can be rescued, animals that were trained in the presence of DCS tended to learn better and more of them did so. These results are consistent with recent findings, indicating that pretraining administration of DCS facilitates declarative learning in humans (Onur et al., 2010). Moreover, DCS is being used in conjunction with cognitive behavioral therapy to treat patients with various mental disorders (Davis, Ressler, Rothbaum, & Richardson, 2006; Hofmann et al., 2006). On the basis of the findings reported here, one might propose that the learning that occurs with DCS during cognitive behavioral therapy increases the number of new cells that survive to become mature neurons in these human subjects, a process that is likely to benefit them in the future.
It is noted that exposure to DCS alone did not seem to affect cell number. Animals that were injected with DCS before unpaired training did not possess any more or fewer cells than those injected with saline and exposed to unpaired training. Moreover, animals injected with DCS after paired training, which learned, retained as many cells as those trained with trace conditioning after an injection of saline. Because DCS is known to enhance excitability through facilitating activation of NMDA receptors, these data suggest that this activation is not sufficient to alter the survival of 1- to 2-week-old hippocampal cells.
The most intriguing results related to individual differences in learning and the number of surviving cells. Across both experiments, we observed a strong positive correlation between the percentage of learned responses that an individual animal emitted over the entire 800 trials of training and the number of surviving BrdU-labeled cells. Thus, animals that tended to learn better (i.e., emitted more CRs over the course of training) also tended to retain more of the new cells in their hippocampus. Because the animals were sacrificed 3 weeks after training, these cells would already be mature neurons. This relationship between learning and neurogenesis can be further parceled into those animals that learned quickly versus those that did not learn quickly but still did learn. In this case, we examined the data from animals that reached a criterion of 60% CRs in at least one block of training trials. This criterion is an established one in eyeblink conditioning because it tends to capture the vast majority of animals that will learn regardless of how many trials are given (Waddell & Shors, 2008; Dalla et al., 2007). Using this criterion, we correlated the number of trials an individual animal required to reach criterion with the number of BrdU-labeled cells in that animal’s dentate gyrus. There was a strong and positive correlation in the experiment with DCS (Rho = .59), irrespective of whether the animals received the drug or not. In other words, of the animals that learned those that required more trials to reach the 60% learning criterion tended to retain more of the new cells. These results were replicated with a second commonly used criterion; the number of trials required for an animal to emit a CR on eight of nine consecutive trials (Dalla et al., 2009; Nokia et al., 2008). Thus, in the DCS study, animals that learned well retained more new neurons than animals that did not learn. However, those animals that required more trials to do so retained more cells than those that learned with fewer training trials (Figure 5). These results are consistent with previous reports from our laboratory using task parameters to manipulate learning and rates of learning (Waddell & Shors, 2008). On the basis of these findings, we propose a model in which the number of trials required to learn a task and the relative difficulty of a task interact to determine the number of new neurons that survive after a learning experience (Figure 6). In animals that are able to master the behavioral response, those that require more trials to do so retain more new neurons. This effect is especially robust when animals are trained on tasks that are difficult to master.
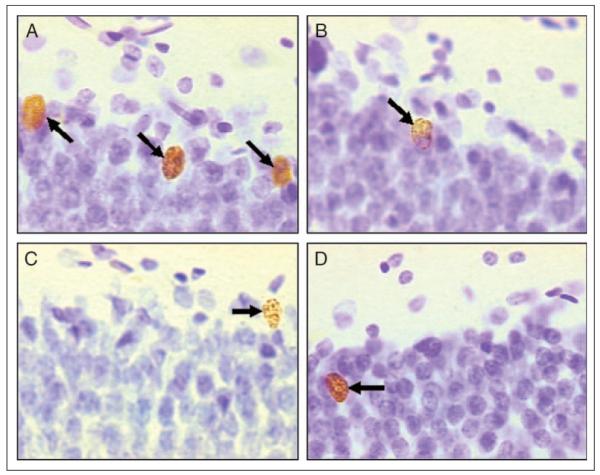
Figure 5.
Representative BrdU-labeled cells from animals that learned or did not learn in the presence of saline, DCS, or NMDA receptor antagonist: (A) Learned/Saline, (B) Unpaired/Saline, (C) Learned/DCS, and (D) Did Not Learn/CPP. Arrows indicate BrdU-labeled cells.
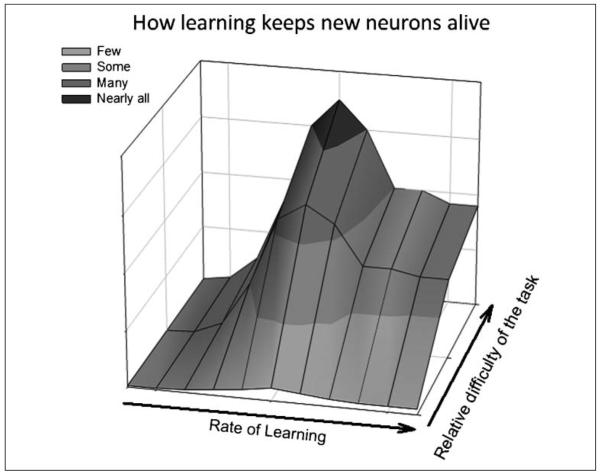
Figure 6.
A model of how learning influences the survival of adult-born hippocampal neurons. The relative difficulty of the task being learned and the rate at which an individual animal learns the task determine how many newborn neurons survive in the adult dentate gyrus. Successful learning of a relatively easy task rescues very few neurons, whereas learning a difficult task rescues many neurons. Of those animals that successfully learn, those that take many trials to do so retain more newborn neurons than those that rapidly learn.
It is noted that there are other explanations for the observed findings. For example, one injection of BrdU provides a “snapshot” of one population of cells—those that were dividing at the time of or shortly after the BrdU injection. It is possible that animals that rapidly acquire the CR, which possess fewer BrdU-labeled cells, may be rescuing cells that are slightly younger or slightly older than the population labeled with BrdU. Although this is a possibility, it does seem unlikely because we and others have found that there is a critical period during which the new cells can be rescued: Cells that are less than 3 days of age or greater than 3 weeks were not preferentially retained after learning (Anderson & Shors, submitted; Epp, Spritzer, & Galea, 2007).
A correlation between rate of acquisition and cell number did not exist for the data from the second experiment, in which an NMDA receptor antagonist was injected before and after training. There was much less variability among the individual animals in terms of learning, which likely explains the absence of a correlation. Alternatively, exposure to the antagonist may have decreased cell survival on its own. Indeed, we did observe a slight decrease in the number of surviving cells in animals that were given CPP after training (although they learned). This effect was not significant. However, others have reported that antagonism of NMDA receptors decreases cell survival (Tashiro, Zhao, & Gage, 2006). Still others report that NMDA receptor activation after the learning experience is necessary to retain some memories (Burgos-Robles, Vidal-Gonzalez, Santini, & Quirk, 2007). Perhaps if we had infused the antagonist persistently after training, more cells would have died as a consequence.
During training of the classically conditioned eyeblink response cells in area CA1 of the hippocampus become more excitable. This effect is most prevalent in those animals that successfully learn the CR (Moyer, Thompson, & Disterhoft, 1996). During trace fear conditioning, granule cells in the dentate also increase their responsiveness to the CS (Gilmartin & McEchron, 2005). Both of these effects do not appear to persist beyond the learning phase. Thus, it is possible that an increase in cell excitability in either CA1 or dentate gyrus contributes to the increase in cell survival. In other words, the new neurons in the DG of animals that require more trials (and time) to learn the CR would be exposed to a longer period of excitability when compared with the amount in animals that quickly learn the task or animals that fail to learn. How an increase in excitability would modulate neuronal survival is currently unknown, although one possibility may be through the activation of NMDA receptors in the hippocampal network. Regardless, the present findings indicate that learning will induce the survival of new neurons when that learning is both sufficiently difficult to achieve and successful (Figure 6).
Acknowledgments
This work was supported by the National Institutes of Health (grant nos. MH-59970 and ARRA-3R01MH059970-10S1) and the National Science Foundation (grant nos. IOB-0444364 and IOS-0914386) to T. J. S.
REFERENCES
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, et al. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neuroscience Letters. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Anderson M, Shors T. The effects of learning on neurogenesis: Survival versus proliferation. submitted. [Google Scholar]
- Anderson ML, Sisti HM, Curlik DM, Shors TJ. Associative learning increases adult neurogenesis during a critical period. European Journal of Neuroscience. 2010;33:175–181. doi: 10.1111/j.1460-9568.2010.07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiology of Learning and Memory. 2007;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proceedings of the National Academy of Sciences, U.S.A. 2009;106:2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: Translation from preclinical to clinical work. Biological Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. Journal of Comparative Neurology. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behavioral Neuroscience. 2005;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. Journal of Comparative Neurology. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Henderson G, Johnson JW, Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-d-aspartate receptor. Journal of Physiology. 1990;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. d-Cycloserine: A ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neuroscience Letters. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences, U.S.A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of d-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. Journal of Neuroscience. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. Journal of Neuroscience. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. Journal of Comparative Neurology. 1999;406:449–460. [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Research. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. Journal of Neuroscience. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. Journal of Neuroscience. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Penttonen M, Korhonen T, Wikgren J. Hippocampal theta (3–8 Hz) activity during classical eyeblink conditioning in rabbits. Neurobiology of Learning and Memory. 2008;90:62–70. doi: 10.1016/j.nlm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, et al. The N-methyl-d-aspartate receptor co-agonist d-cycloserine facilitates declarative learning and hippocampal activity in humans. Biological Psychiatry. 2010;67:1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rouaud E, Billard JM. d-Cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. British Journal of Pharmacology. 2003;140:1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Early acquisition, but not retention, of the classically conditioned eyeblink response is N-methyl-d-aspartate (NMDA) receptor dependent. Behavioral Neuroscience. 1996;110:1040–1048. doi: 10.1037//0735-7044.110.5.1040. [DOI] [PubMed] [Google Scholar]
- Shors TJ. From stem cells to grandmother cells: How neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–258. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Saving new brain cells. Scientific American. 2009;300:46–52. 54. doi: 10.1038/scientificamerican0309-46. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: Learning over time enhances memory and the survival of new neurons. Learning and Memory. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nature Protocols. 2006;1:3049–3055. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by d-cycloserine in rabbits. Behavioral Neuroscience. 1997;111:1303–1312. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or d-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- Waddell J, Mallimo E, Shors T. d-Cycloserine reverses the detrimental effects of stress on learning in females and enhances retention in males. Neurobiology of Learning and Memory. 2010;93:31–36. doi: 10.1016/j.nlm.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. European Journal of Neuroscience. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]