Abstract
Though the role of the hippocampus in processes of learning and memory is well established, the role of new neurons generated there is less understood. Training on some associative learning tasks increases the likelihood that new cells in the subgranular zone of the dentate gyrus will survive. In the rat, an effective training procedure is trace eyeblink conditioning, in which a conditioned stimulus (CS) is paired with an aversive stimulation to the eyelid (unconditioned stimulus; US), but the stimuli are separated by a temporal gap. Here, we manipulated the asymptote or rate of acquisition during trace conditioning, and examined survival of cells generated 1 week before training. Acquisition was disrupted by decreasing associative strength by insertion of unpredicted USs or slowed with latent inhibition. The number of cells was increased in animals that were trained with trace conditioning, irrespective of the decrease in associative strength or slowed acquisition. Disrupting acquisition with unsignaled USs still increased cell numbers, suggesting that the learning effect on cell survival is not dependent on reliable expression of the conditioned response. Further, animals in the latent inhibition conditions that learned but required more trials also retained more of the new cells than animals requiring fewer trials. The number of cells that survived after the effective training procedures was similar to the number of cells that were available for rescue at the beginning of training. Thus, learning can rescue the majority of cells expressed at the beginning of training, and does so most effectively when acquisition requires many trials.
Keywords: associative strength, contiguity, dentate gyrus, memory, neurogenesis
Introduction
The dentate gyrus of the hippocampus produces thousands of new granule neurons each day, most of which differentiate into neurons (Altman & Das, 1965; Cameron & McKay, 2001; Cameron et al., 1993; Kaplan & Hinds, 1977; Markakis & Gage, 1999; Hastings & Gould, 1999; van Praag et al., 2002). Many die within weeks, but survival can be enhanced by exposure to hippocampal-dependent learning (Dayer et al., 2003; Dobrossy et al., 2003; Gould et al., 1999; Leuner et al., 2004). One task that rescues new neurons from death is trace eyeblink conditioning, in which the conditioned stimulus (CS) and unconditioned stimulus (US) are separated by an interval of time (e.g., Gould et al., 1999; Leuner et al., 2004). Hippocampal lesions disrupt trace but not delay eyeblink conditioning, in which the stimuli overlap in time (e.g., Solomon et al., 1986; Weiss et al., 1999). Trace eyeblink conditioning promotes long-term survival of adult-generated neurons beyond the time the hippocampus is necessary for retrieval of the association (Kim et al., 1995; Leuner et al., 2004). Delay conditioning with a brief CS does not enhance survival of new cells (Gould et al., 1999). However, training with a ‘long delay’ CS does rescue cells from death (Leuner et al., 2006), and this task is hippocampal-dependent (Beylin et al., 2001). Thus, temporal discontiguity is neither necessary nor sufficient to rescue new cells from death. The trace and ‘long delay’ procedures are slowly acquired, requiring more trials to achieve a reliable conditioned response (CR) relative to the shorter delay stimulus (Beylin et al., 2001; Leuner et al., 2006). It is possible that slow acquisition rather than hippocampal-dependent learning is sufficient for enhanced survival. Further, trace conditioning enhances survival only if the rat expresses evidence of learning (Dalla et al., 2007). The experiments here directly assessed the effect of disrupted acquisition of trace conditioning (Experiment 1) and slowed acquisition of both trace and delay conditioning (Experiment 2) on survival of adult-generated cells in the hippocampus.
If survival of new neurons is dependent upon acquisition of the association, then disrupting acquisition should fail to rescue new neurons from death. Experiment 1 decreased the contingency during trace conditioning by presenting the US with or without the CS with equal probability (Trace Unpredicted). This type of manipulation has been shown to elicit poor conditioned responding to the CS, presumably by decreasing the associative strength between the CS and the US (e.g., Rescorla, 1966, 1968; Murphy & Baker, 2004). If cell survival is dependent on associative strength, animals trained with unpredicted USs during paired training should possess fewer cells than those trained with typical trace conditioning.
Latent inhibition (LI) is the retarded acquisition of conditioning following multiple trials of the CS alone (Lubow, 1973; Lubow & Moore, 1959). To determine whether slowing acquisition would increase cell survival, we used LI of both delay and trace procedures. In eyeblink conditioning, LI manifests as an increase in the number of trials required to reach behavioral criterion (e.g., Katz et al., 2002; Shohamy et al., 2000; Solomon & Moore, 1975). Lesions of the hippocampus do not disrupt LI of delay conditioning (Shohamy et al., 2000). If slowed acquisition is sufficient to rescue new cells from death, animals trained with LI will possess more cells than their respective controls. If hippocampal dependence is important, then only rats trained with LI of trace conditioning (and trace conditioning alone) will possess more cells. Numbers of cells in the dentate gyrus available to rescue at the beginning of training as estimated in naïve rats killed at this time were compared to those remaining after training, in both a separate naïve group and animals receiving training.
General materials and methods
Subjects
Adult male Sprague–Dawley rats were bred at Rutgers University and housed in groups. At 60–80 days of age, they were housed individually with access to food and water ad libitum, and maintained on a 12 h light–dark cycle. All experiments were approved by the Rutgers University Animal Care and Use Committee and were conducted with full compliance with the rules and regulations specified by the PHS Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals.
Classical conditioning
Rats were anesthetized with sodium pentobarbital (40 mg/kg) supplemented with isoflurane inhalant. A headstage with four electrodes was secured to the skull. Electrodes consisted of stainless steel wire implanted subcutaneously to emerge through and around the eyelid. Two electrodes recorded electromyograph (EMG) activity for determination of the eyeblink and two delivered the periorbital stimulation to elicit the eyeblink reflex.
For eyeblink conditioning, headstages were connected to a cable that allowed free movement within the conditioning chamber. Twenty-four hours prior to any behavioral manipulation, rats were acclimated to the conditioning apparatus for 1 h. During this acclimation period, spontaneous blink rates for each rat were recorded. The following day, rats began conditioning. To detect the occurrence of an eyeblink, the maximum EMG response occurring during a 250 ms prestimulus baseline recording period was added to four times its SD. Responses that exceeded that value and were > 3 ms were considered eyeblinks. Eyeblinks were considered CRs if they began 500 ms prior to US onset. Eyeblink performance was calculated as the percentage of trials on which a CR was produced in response to a CS.
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (200 mg/kg) and transcardially perfused with 4% paraformaldehyde in 0.1 m phosphate buffer. Brains were dissected from the skulls and post-fixed for at least 2 days. Coronal sections (40 μm) throughout the entire rostrocaudal extent of the dentate gyrus were cut with a Vibratome from one hemisphere into a bath of 0.1 m PBS (pH 7.5). For bromodeoxyuridine (BrdU) peroxidase staining, a 1 : 12 series of sections were mounted onto glass slides, dried and pretreated by heating in 0.1 m citric acid (pH 6.0). Slides were then rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 m HCl in PBS for 30 min, rinsed and incubated with mouse antibodies to BrdU (diluted 1 : 250 with 0.5% Tween-20; Vector Laboratories, Burlingame, CA, USA). The next day, slides were rinsed, incubated with biotinylated antimouse (1 : 200; Vector) for 60 min, rinsed, incubated with avidin–biotin complex, rinsed and reacted in 0.01% diaminobenzidine with 0.003% H2O2. Slides were counterstained with cresyl violet, dehydrated, cleared and coverslipped under Permount (Fisher Scientific, Fair Lawn, NJ, USA).
For double labeling, free-floating sections were rinsed with Tris-buffered saline (TBS; 0.1 m at pH 7.5). DNA was denatured with 2N HCl in TBS. Sections were incubated in primary antibodies, goat antidoublecortin (DCX; 1 : 100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-BrdU (1 : 200; Becton-Dickinson, Franklin Lakes, NJ, USA), with 0.5% Tween-20 (Sigma–Aldrich, St. Louis, MO, USA) in TBS for 48 h at 4 °C. Sections were then incubated in secondary antibodies, Rhodamine Red-X antigoat (1 : 200; Jackson Immunoresearch, West Grove, PA, USA) and Fluro 488 anti-mouse (1 : 200, Molecular Probes, Carlsbad, CA, USA) in TBS for 30 min. Tissue was mounted onto slides and coverslipped with glycerol:TBS (1 : 3). The number of colocalized cells was determined with a Zeiss (Oberkochen, Germany) LSM 510 confocal laser scanning microscope. Sections were scanned using a Plan-Neofluar 25 × water-immersion objective and dual-channel excitation with argon (488 nm) and heliumneon (543 nm). Thirty cells per subject(n = 4)were counted on random sections throughout the hippocampus. Colocalization analysis included visual inspection of size and shape of the cells throughout a z-stack, orthogonal planes, and a profile of excitation intensity of the cells.
BrdU microscopic data analysis
Quantitative analysis was conducted blind to behavioral condition. For BrdU peroxidase, estimates of total numbers of BrdU-labeled cells were determined using an unbiased stereology protocol previously reported to successfully quantify BrdU labeling (West et al., 1991; Gould et al., 1999). BrdU-labeled cells in the subgranular zone (SGZ) and granule cell layer (GCL) on every twelfth unilateral section throughout the entire rostrocaudal extent of the dentate gyrus were counted at 1000 × (100 × objective with a 10 × ocular) on an Olympus BX-50 light microscope, avoiding cells in the outermost focal plane. The number of BrdU-labeled cells per dentate gyrus was then multiplied by 24 to obtain an estimate of the total number of BrdU-labeled cells throughout the rostrocaudal axis.
Experiment 1: decreasing the associative strength of the CS
Methods
Following surgery, rats were allowed 2–3 days of recovery. Rats were then administered a single dose of BrdU (200 mg/kg). Seven days following BrdU administration, rats were acclimated to the conditioning chamber. Twenty-four hours later, eyeblink conditioning began. In the Trace Predicted condition (n = 7), rats were presented with a 250-ms white noise CS (83 dB) and 100-ms periorbital stimulation separated by a 500-ms trace interval, during which no stimuli were delivered. Rats received 200 trials a day, at an intertrial interval (ITI) of 25 ± 5 s. Rats in the Trace Unpredicted group (n = 7) received paired CS–US trials intermixed with US-alone trials. Rats in this condition were trained with 200 paired trials a day, intermixed in a pseudorandom order with 200 US alone trials. All trials (i.e., both trial types) were presented at an ITI of 25 ± 5 s. A third group, Naïve (n = 6), was administered BrdU and killed at the same time points as rats in the Trace Predicted and Trace Unpredicted groups.
Results
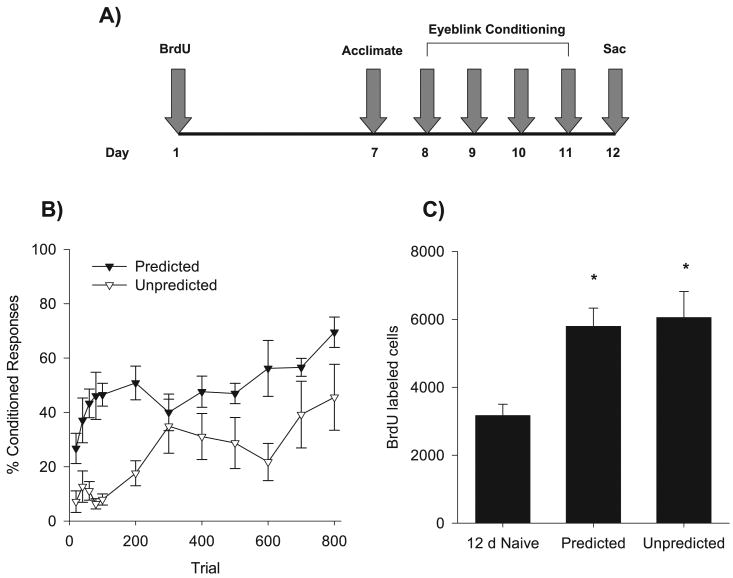
The experimental timeline is depicted in Fig. 1A. There was no difference in the numbers of spontaneous blinks between the groups before any training occurred (F1,12 < 1). Behavioral data are presented in blocks of 20 trials for the first 100 paired trials, and in blocks of 100 paired trials for the remaining 700 trials of conditioning (Fig. 1B). Repeated-measures anova revealed a significant effect of trials of training on the percentage of CRs that were emitted during the trace interval (F1,132 = 5.98, P < 0.0001). There was no interaction between trials of training and condition (Unpredicted vs. Predicted USs; F1,132 = 1.34, P > 0.05). However, there was a main effect of condition on performance of the CR (F1,12 = 26.52, P < 0.0001), reflecting the differences in performance between the two groups across trials. As shown in Fig. 1B, rats in the Trace Unpredicted group did not reach the same level of conditioned responding as rats in the Trace Predicted condition. However, rats in the Trace Unpredicted group did express evidence of conditioning, though performance was considerably more variable in this group.
Fig. 1.
(A) Experimental timeline. (B) Behavioral results from animals trained with only predicted USs (Predicted) and animals trained with the addition of Unpredicted USs. Data are presented as a mean ± SEM percentage of conditioned responses with the first 100 trials presented in 20-trial blocks, with the remaining 700 in 100-trial blocks. (C) Bars represent the mean ± SEM number of BrdU-labeled cells in the SGZ and GCL of the dentate gyrus of naïve animals and groups Predicted and Unpredicted. *P < 0.05, denotes groups which differ from Naïve controls.
Figure 1C displays the number of BrdU-labeled cells expressed in each group of animals after they were trained with eyeblink conditioning relative to the number of cells in a group of untrained naïves. anova revealed a significant effect of group on the number of BrdU-labeled cells in the dentate gyrus subgranular zone and granule cell layer (F2,17 = 6.83, P < 0.01). Using planned comparisons, isolation of the Trace group and naïve controls revealed a significant difference (F1,17 = 9.47, P < 0.006). Interestingly, rats that were trained with the Trace Unpredicted procedure also possessed more BrdU-labeled cells than naïve controls (F1,17 = 11.45, P < 0.003), and their numbers did not differ significantly from the numbers in animals trained with the standard trace conditioning procedure (Trace Predicted; F1,17 < 1). Thus, training with trace conditioning, irrespective of the additional unsignaled USs, resulted in a greater number of surviving cells than did no training at all.
Experiment 2: LI of delay and trace eyeblink conditioning
Methods
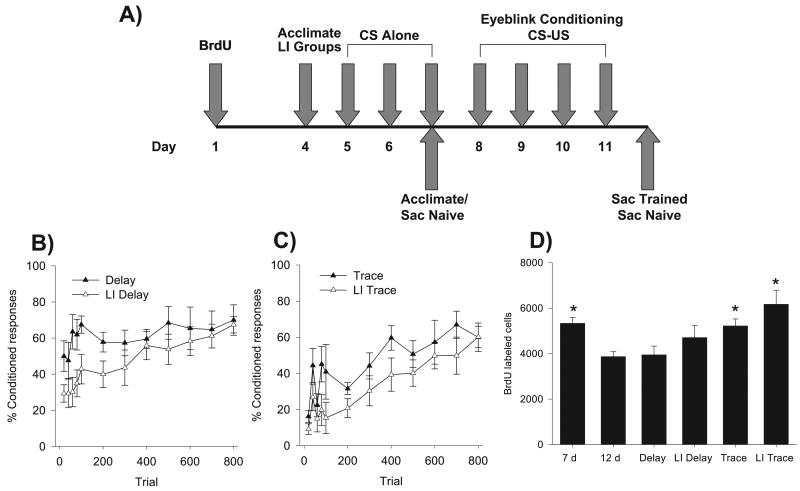
Following surgery, rats were allowed 2–3 days of recovery. Rats were then administered a single dose of BrdU (200 mg/kg). The experimental timeline is shown in Fig. 2A. Rats in the LI condition were acclimated to the eyeblink conditioning chambers 2 days following BrdU administration. Twenty-four hours later, rats in the LI groups began three consecutive days of CS-alone presentations. On each of these days, 200 trials of either the 850 ms (LI Delay condition) or the 250 ms (LI Trace condition) white noise was delivered at an ITI of 25 ± 5 s. Eyeblink responses to the stimulus were recorded. Rats in the Delay (n = 7) and Trace (n = 7) conditions were acclimated to the eyeblink chambers on the last day of CS-alone presentations for the LI groups. All groups began CS–US training 8 days following BrdU administration (Fig. 2A). Delay eyeblink conditioning proceeded for four consecutive days, with 200 trials each day. Conditioned responses were eyeblinks which occurred during the 500-ms period immediately prior to US delivery. Two additional untrained, naïve control groups were administered BrdU at the same time as those in the trained conditions, and killed either 7 days following BrdU administration (n = 12), the day prior to paired training in those rats exposed to eyeblink conditioning, or 12 days following BrdU administration (n = 10), the day following the end of training.
Fig. 2.
(A) Experimental timeline. (B) Behavioral results from animals trained with the Delay procedure. (C) Behavioral results from animals trained with the Trace procedure. Data are presented as a mean ± SEM percentage of conditioned responses with the first 100 trials presented in 20-trial blocks with the remaining 700 trials in 100-trial blocks. (D) Bars represent the mean ± SEM number of BrdU-labeled cells in the SGZ and GCL of the dentate gyrus of naïve animals and groups Delay, LI Delay, Trace and LI Trace conditions. *P < 0.05, denotes groups which differ from naïve controls.
Results
Behavioral data are presented in blocks of 20 trials for the first 100 paired trials, and in blocks of 100 trials for the remaining 700 paired trials of conditioning (Fig. 2B and C). A three-way repeated-measures anova across the 12-trial blocks of conditioning with training condition (LI or No LI) and task (Delay or Trace) as between-subject factors was conducted. This analysis confirmed a significant effect of trial (F11,264 = 11.48, P = 0.0001) as all conditions expressed an increase in conditioned responses across trials. The effect of trial did not interact with condition (F11,264 = 1.26, P = 0.25) or Task (F11,264 = 1.59, P = 0.59). The three-way interaction of trial × training condition × task did not reach significance (F11,264 < 1). The main effect of training condition (i.e., LI) was significant (F1,24 = 8.30, P = 0.008) as LI reduced conditioned responding. The main effect of task (i.e., Delay or Trace) was also significant (F1,24 = 8.35, P = 0.008). As expected, Delay conditioning proceeded quickly relative to Trace conditioning. There was no significant interaction between training condition and task (F1,24 < 1).
To confirm that LI was evident in the Delay task, repeated-measures anova was conducted across the 12-trial blocks of conditioning with condition as the between-subjects factor. This analysis (Fig. 2B) revealed a significant effect of trial (F11,132 = 5.95, P = 0.0001) as both groups expressed an increase in conditioned responses across training. The trial × condition interaction did not reach significance (F11,132 = 1.67, P = 0.08). The main effect of condition did not reach significance (F1,12 = 3.23, P > 0.05). Inspection of Fig. 2B suggests that LI was expressed only early during conditioning, as both groups performed similarly during the final four blocks of trials. To determine whether LI slowed at least early conditioning, the first 2 days of conditioning or the first eight blocks of conditioning (i.e., the first 400 trials) were analysed using repeated-measures anova. This analysis found a significant difference between LI Delay and Delay across trials of training (F7,84 = 2.89, P = 0.009), as well as a main effect of training condition (F1,12 = 5.81, P = 0.03). Thus, LI was expressed only during early training as both groups expressed similar rates of conditioned responding in late conditioning.
To confirm that LI was evident in the Trace task, repeated-measures anova was conducted across the 12-trial blocks of conditioning with Condition as the between-subjects factor. Isolation of groups LI Trace and Trace (Fig. 2C) revealed a significant effect of trial across the 12 blocks of training (F11,132 = 8.29, P < 0.001). The trial × training condition interaction was not significant (F11,132 = 0.53, P > 0.05). The main effect of training condition was significant (F1,12 = 6.82, P < 0.02), confirming that rats in the LI trace condition were slower to acquire trace eyeblink conditioning and thus expressed LI.
LI was used here to slow delay eyeblink conditioning to a rate similar to that of trace conditioning. To assess whether rates of acquisition were indeed similar as we predicted, the LI Delay and Trace groups were directly compared. Repeated-measures anova across the 12-trial blocks with condition as the between-subjects variable again found a significant effect of trial (F11,132 = 7.20, P < 0.0001). Neither the trial × condition (F11,132 = 0.69, P > 0.05) nor main effect of group was significant (F1,12 = 0.02, P > 0.05). Thus, LI slowed delay eyeblink conditioning to a rate similar to that of trace eyeblink conditioning, as these two groups did not differ in expression of conditioned responses across conditioning trials.
The number of BrdU-labeled cells detected in animals trained with delay and trace conditioning (with and without LI) were compared to the number of cells in animals that were injected with BrdU and left in their home cage until killing, either on the day before training would begin or when acclimation was conducted for the Delay and Trace conditions (7-day Naïve) or 1 day after training would end (12-day Naïve; Fig. 2D). One-way anova examining the number of BrdU-labeled cells across all six groups revealed a significant effect of group (F5,44 = 5.57, P < 0.0001). Using planned comparisons, groups were isolated and compared. As expected, the number of cells in the dentate gyrus 8 days after the injection was greater than that 12 days after the injection (F1,44 = 10.48, P < 0.002). This difference represents an approximation to the number of cells that do not survive during this period of time (see Dayer et al., 2003). The number of cells surviving 12 days after the injection were not different in the animals also exposed to 800 trials of delay conditioning (Delay; F1,44 = 0.02, P > 0.05). The group exposed to the CS before paired training also did not possess more cells than the naïve controls killed 12 days following BrdU administration (F1,44 = 2.91, P > 0.05). Though rats trained in the Delay condition expressed significantly fewer cells relative to 7-day naïves (F1,44 = 7.62, P < 0.008), rats in the LI Delay condition did not (F1,44 = 1.31, P > 0.05). Cell numbers in rats in the Delay and LI Delay conditions did not differ from each other (F1,44 = 2.06, P > 0.05). Together, these results suggest that slowed acquisition alone is not sufficient to significantly increase the survival of neurons generated one week before training relative to 12-day naïve controls. Rats trained in either Trace or LI Trace expressed cell numbers similar to those of naïves killed just prior to training (F1,44 = 2.81, P > 0.05). Rats in both Trace (F1,44 = 5.94, P < 0.02) and LI Trace (F1,44 = 19.62, P < 0.000001) conditions expressed significantly more cells than did 12-day naïve controls. Relative to rats trained in the Delay condition, rats in either the Trace (F1,44 = 4.45, P < 0.04) or LI Trace (F1,44 = 15.57, P < 0.0002) conditions expressed more cells. Cell numbers in rats in the Trace condition did not differ from rats trained in the LI Delay condition (F1,44 = 0.45, P > 0.05). Though rats in the Trace and LI Trace groups did not differ from each other (F1,44 = 3.37, P > 0.05), rats in the LI Trace condition did significantly differ from rats in the LI Delay condition (F1,44 = 6.30, P = 0.015). Figure 3 depicts examples BrdU labeling in each naïve condition (7-day and 12-day) and the Trace and Delay conditions. A subset of animals (n = 4) was assessed for the percentage of cells that differentiated into neurons at the time of killing. All rats analysed for co-expression of BrdU and DCX were trained and killed 12 days following BrdU administration (Nacher et al., 2001). Using confocal microscopy, a minimum of 30 cells expressing BrdU were determined to be co-labeled or not with DCX (Fig. 4). The average percentage of double-labeled cells was 82 ± 3.32%, similar to percentages at similar time points previously reported from our lab (e.g., Sisti et al., 2007). Thus, the majority of BrdU-labeled cells appeared to differentiate into neurons.
Fig. 3.
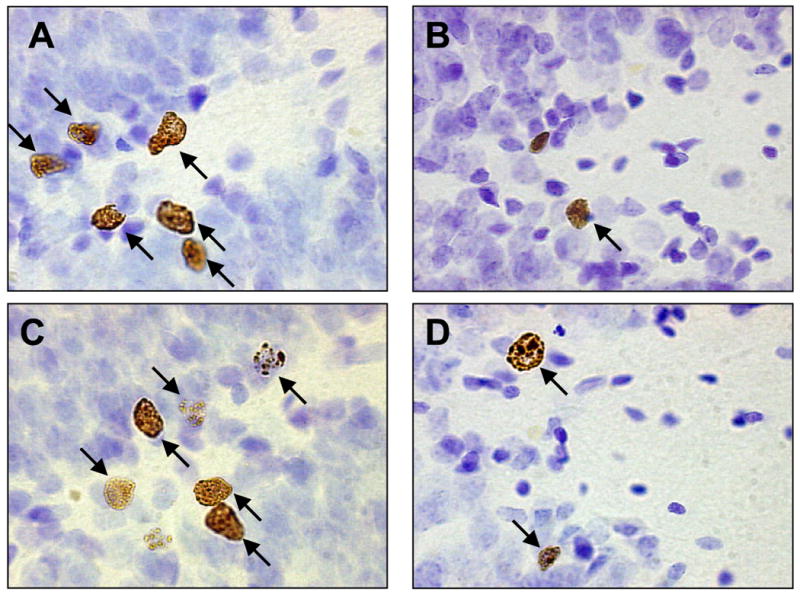
BrdU-labeled cells in the dentate gyrus of the hippocampus from similar sections of an animal in the (A) 7-day naïve condition, (B) 12-day naïve condition, (C) Trace condition and (D) Delay condition.
Fig. 4.
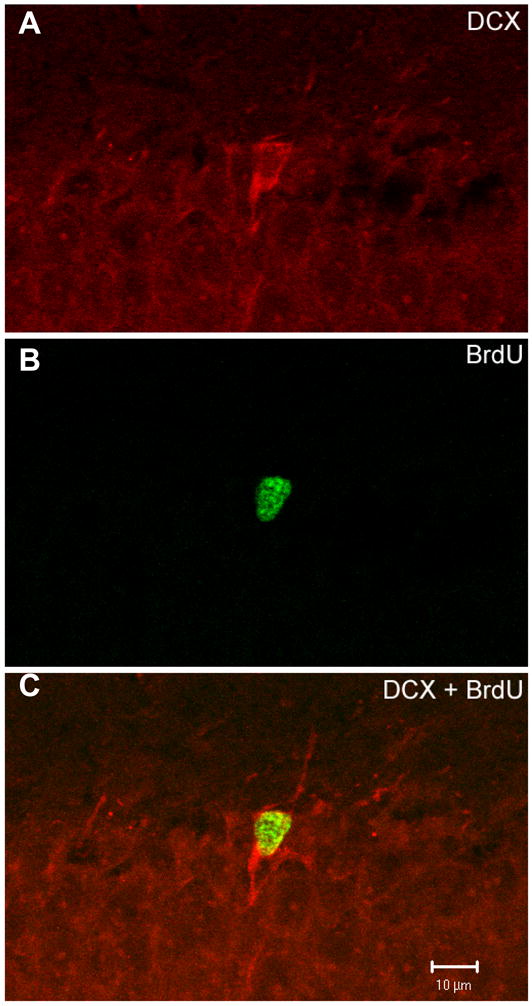
The majority of BrdU-labeled cells appear to differentiate into neurons. A representative cell from the dentate gyrus that expressed (A) DCX, (B) BrdU and (C) DCX is shown.
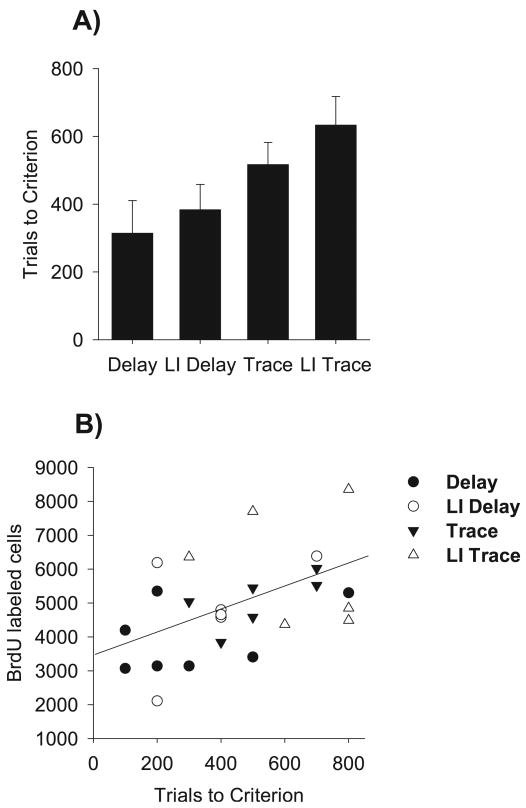
We hypothesized that slowed acquisition, regardless of contiguity of the CS and US, would rescue new neurons from death. To examine this relationship more fully, the number of training trials required for each rat to reach 60% conditioned responding within a 100-trial block of conditioning was determined (Fig. 5A). This criterion level was chosen to capture the majority of rats across all four training conditions. As shown in Fig. 2B and C, rats in the Trace and LI Trace conditions achieve an average of ∼60% responding by the end of training. Three rats failed to reach this criterion despite an apparent increase in conditioned responding across trials, and were therefore excluded from this analysis. The remaining rats included in this analysis were distributed across conditions as follows: Delay, n = 7; LI Delay, n = 6; Trace, n = 6; LI Trace, n = 6. The mean number of trials to reach the 60% performance criterion for each group is depicted in Fig. 5A. Non-parametric anova revealed an effect of group which just reached significance (P = 0.054, Kruskal–Wallis, df = 3). To further explore the relationship between rate of acquisition and cell survival, the trials to criterion value for each rat was correlated with the number of BrdU-labeled cells (Fig. 5B). The number of BrdU-labeled cells correlated with the number of trials required to reach criterion (Fig. 5B; Spearman's ρ = 0.435, P = 0.03). Overall, the results suggest that while rate of acquisition correlates with the number of cells expressed following training, this measure interacts with hippocampal dependence. However, slowed acquisition of delay eyeblink conditioning did not reliably enhance survival of new cells relative to naïve controls despite performance equivalent to rats in the Trace condition, further implicating the necessity of hippocampal dependence in the reliable increase in cell survival.
Fig. 5.
(A) Bars represent the average number of trials required for each behavioral condition in Experiments 2 and 3 to reach 100 trials with 60% conditioned responses. (B) The number of trials required to reach behavioral criterion correlates with the number of BrdU-labeled cells expressed in the dentate gyrus.
General discussion
The experiments presented here answer two basic questions about the survival and integration of newly generated cells from a learning-theoretical perspective. First, a decrement in the predictive value of the CS does not disrupt the enhancement of survival produced by hippocampal-dependent learning. Thus, the enhanced survival elicited by trace eyeblink conditioning is not specific to training procedures that promote simple yet gradual associations between the CS and US or reliable expression of the CR. Second, rate of acquisition does, to at least some degree, predict the number of surviving newly born neurons in the granule cell layer. Though LI of delay effectively slowed conditioning, this manipulation did not rescue a significantly higher number of neurons relative to untrained naïve controls. However, across groups receiving training in Experiment 2, the number of trials required to reach a behavioral criterion of 60% conditioned responding correlated with the number of cells expressed after training. This pattern of results tentatively suggests that rate of acquisition interacts with hippocampal dependence to influence survival of new cells.
Neurogenesis and decrements in associative strength
The results from Experiment 1 suggest that disrupted performance through presentations of unpredicted USs does not prevent the increase in cell survival. Therefore, reliable expression of a CR is not necessary for the protective effect of trace conditioning on neurogenesis. It is possible that presentation of unpredicted USs may have had an unanticipated effect on learning. Specifically, unsignaled presentations of the US may have resulted in conditioning of contextual cues making the discrimination of the trace interval and the ITI more difficult. Presentation of the US in the absence of the CS, intermixed with paired trials, does result in contextual conditioning at least in an appetitive preparation (Murphy & Baker, 2004). Importantly, training with the same number of trials in which the CS and US are explicitly unpaired does not rescue the new cells from death and does not produce excitatory responses to the CS (Gould et al., 1999; Leuner et al., 2004). Because the unpaired training procedure would presumably produce contextual conditioning, it does not seem that contextual conditioning is sufficient for the enhanced survival exhibited by rats in the Trace Unpredicted condition. Evidence exists that signaling the end of a trace trial with a different CS can facilitate acquisition of trace conditioning in a conditioned suppression preparation (Bolles et al., 1978). Further, it has been proposed that the dentate gyrus is involved in pattern separation and discrimination (e.g., Kesner et al., 2004; Lee & Kesner, 2004; Leutgeb et al., 2007; Rolls, 1996). Thus, trace conditioning may rely at least in part upon the accurate discrimination between the trace interval and the ITI. This interpretation implies that rats were disrupted in this discrimination by unsignaled US presentations, but the conditioning procedure still sufficiently engaged the hippocampus. Though rats trained with intermixed, unpredicted USs did express some evidence of learning as demonstrated by a significant increase in CRs across trials of training, the performance was variable and did not reach a stable criterion. Nonetheless, the number of cells in this group was significantly greater than the numbers in the naïve controls killed at the same time. Thus, the results from this study somewhat dissociate performance from the effect of training on survival.
Neurogenesis and slowed acquisition
In Experiment 2, animals were repeatedly exposed to the CS (600 trials) before the onset of paired training, a procedure that slowed acquisition, or LI. The animals that expressed LI in the delay arrangement did possess slightly more BrdU-labeled cells after training, but not significantly more than in the naïve controls. Thus the data indicate that LI, or slowed acquisition, is not sufficient to rescue the new cells from death. The slight increase in cell number may reflect a stimulatory effect on cell proliferation, as CS-alone exposure began just 3 days after the first cells were labeled, a time point when proliferation is still occurring (Cameron & McKay, 2001). A similar trend was observed in the animals that were trained with LI of trace conditioning as they also possessed, though not significantly, more cells than naïve controls killed 7 days following BrdU administration. Because cells are being generated from the initial pool of labeled cells while others are dying, it is not always possible to verify whether the manipulation in question is enhancing cell proliferation or reducing cell death. To address this issue, we included groups of animals that were injected with BrdU and killed just before training would have begun. This provided an estimate of the number of cells available to rescue at the beginning of training. From these numbers, it would appear that effective training rescued most of the cells that were available to rescue. Moreover, the number of BrdU-labeled cells expressed at the end of training correlated with the rate of acquisition across training conditions.
Neurogenesis and the hippocampal dependence of learning
The experiments presented here suggest that the rate of acquisition, perhaps an index of task difficulty, predicts the influence of learning on survival of new cells more accurately than hippocampal dependence of the task. The idea that level or duration of engagement during a learning experience, rather than presumed hippocampal-dependence alone, influences cells has been previously proposed (Greenough et al., 1999). This suggestion was an attempt to reconcile discrepant results between two experiments using the Morris water maze, a hippocampal-dependent task.
Gould et al. (1999) found that training with an invisible platform enhanced survival of new cells when training began 7 days following BrdU administration, while another report found no influence of water maze training on survival (van Praag et al., 1999). Though the experiments differ in many ways, the latter experiment reported asymptotic performance with fewer trials (van Praag et al., 1999). Greenough et al. (1999) suggested that perhaps only the more difficult task as indicated by slower acquisition reported by Gould et al. (1999) placed a sufficient ‘load’ on the hippocampus to effectively influence survival. Trace eyeblink conditioning may be particularly effective because it requires many trials to achieve reliable expression of the CR. Two recent reports found that rats expressing slower acquisition in a hippocampal-dependent water maze task expressed more neurons than did those who learned quickly (Dobrossy et al., 2003; Epp et al., 2007). These results, together with the experiments presented here, suggest that the duration of hippocampal activation is critical in determining the effect of learning on survival of adult-generated neurons.
The data presented here suggest that duration of activation as indexed by rate of acquisition may reliably predict the influence of learning on survival. Delay eyeblink conditioning elicited learning-related activity the hippocampus, despite its independence of the hippocampus, though excitation was slower to develop during trace conditioning (e.g., Katz et al., 2002; Green & Arenos, 2007). Similarly, trace fear conditioning elicited persistent learning-related increases in activity in the dentate gyrus, while CA1 expressed a transient increase (Gilmartin & McEchron, 2005). This relatively persistent increase in activity of mature cells during trace conditioning may be necessary for the increase in survival of immature cells. The experiments presented here examined the influence of training 1 week following BrdU administration. Adult-generated neurons are particularly sensitive to survival-enhancing manipulations at this time. At this age, immature cells are extending axons into the CA3 region of the hippocampus (Hastings & Gould, 1999; Zhao et al., 2006). Environmental enrichment promotes survival when the cells are up to 3 weeks old and does so maximally when enrichment begins approximately 1 week following BrdU administration (Tashiro et al., 2007). Similarly, hippocampal-dependent water maze training is most effective in promoting survival when initiated 6 days following BrdU administration (Epp et al., 2007). As new cells approach 6 weeks of age, they are selectively activated by retrieval of spatial learning relative to mature cells (Kee et al., 2007). Though adult-generated neurons are sensitive to experiential manipulations around 1 week of age for increased survival, they may not be involved in retrieval of memories formed at this early age. Indeed, trace eyeblink conditioning promotes increased survival for at least 2 months following BrdU administration, a time when the hippocampus is not necessary for retrieval or expression of trace eyeblink conditioning (Kim et al., 1995; Leuner et al., 2004).
In conclusion, the correlation between trials to criterion and cell number suggests that the duration of hippocampal activation, as indexed here by trials to criterion, contributes to cell survival, though this measure again appeared to interact with hippocampal dependence. Presumably, new cells in the dentate gyrus are responding differently to the stimulus configuration used during training with more difficult tasks such as trace conditioning than when trained with a short delay procedure. The results presented here add to the growing body of evidence suggesting that tasks which are difficult to learn, or at least slowly acquired, enhance the survival of new neurons generated in the deutate gyrus of the adult hippocampus.
Acknowledgments
This study was supported by NIH (NIMH59970) and NSF (IOB-0444364) to TJS and NIH-NIMH (MHO19957) to JW.
Abbreviations
- BrdU
bromodeoxyuridine
- CR
conditioned response
- CS
conditioned stimulus
- DCX
doublecortin
- EMG
electromyograph
- GCL
granule cell layer
- ITI
intertrial interval
- LI
latent inhibition
- SGZ
subgranular zone
- US
unconditioned stimulus
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in adult rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Collier AC, Bouton ME, Marlin NA. Some tricks for ameliorating the trace-conditioning deficit. Bull Psychon Soc. 1978;11:403–406. [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem. 2007;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LAM. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav Neurosci. 2005;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiol Learn Mem. 2007;87:269–284. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Cohen NJ, Juraska JM. New neurons in old brains: learning to survive? Nat Neurosci. 1999;2:203–205. doi: 10.1038/6300. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Katz DB, Rogers RF, Steinmetz JE. Novel factors contributing to the expression of latent inhibition. Behav Neurosci. 2002;116:824–836. doi: 10.1037//0735-7044.116.5.824. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on neurogenesis. J Neurosci. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition. Psychol Bull. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of non-reinforced preexposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Murphy RA, Baker AG. A role for CS-US contingency in Pavlovian conditioning. J Exp Psychol Anim Behav Process. 2004;30:229–239. doi: 10.1037/0097-7403.30.3.229. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Predictability and number of pairings in Pavlovian fear conditioning. Psychon Sci. 1966;4:383–384. [Google Scholar]
- Rescorla RA. Probability of shock in the presence or absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Allen MT, Gluck MA. Dissociating entorhinal and hippocampal involvement in latent inhibition. Behav Neurosci. 2000;114:867–874. [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol. 1975;89:1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz J. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. The Journal of Neuroscience. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming G, Gage F. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]