SUMMARY
Current anti-angiogenic agents used to treat cancer only partially inhibit neovascularization and cause normal tissue toxicities, fueling the need to identify therapeutic agents that are more selective for pathological angiogenesis. Tumor Endothelial Marker 8 (TEM8), also known as anthrax toxin receptor 1 (ANTXR1), is a highly conserved cell-surface protein overexpressed on tumor-infiltrating vasculature. Here, we show that genetic disruption of Tem8 results in impaired growth of human tumor xenografts of diverse origin including melanoma, breast, colon, and lung cancer. Furthermore, antibodies developed against the TEM8 extracellular domain blocked anthrax intoxication, inhibited tumor-induced angiogenesis, displayed broad anti-tumor activity and augmented the activity of clinically approved anti-cancer agents without added toxicity. Thus, TEM8 targeting may allow selective inhibition of pathological angiogenesis.
INTRODUCTION
Solid tumors have an insidious ability to nourish their own expansive growth by evoking the sprouting of new blood vessels, or angiogenesis, from nearby vessels of neighboring non-malignant tissues. Upon vascularization, tumor blood vessels supply tumor cells with vital oxygen and nutrients needed to support their continued growth, and provide a key escape route for metastasis. Due to their critical role in promoting tumor growth and metastasis, tumor blood vessels have become a major target of current anti-cancer therapy (Kerbel, 2008). Vascular Endothelial Growth Factor (VEGF) and its receptor, VEGFR2, represent the most advanced targets of current anti-angiogenic therapy, and agents that target the VEGF/VEGFR2 axis have been clinically approved to treat patients with colon, lung, brain and kidney cancer (Brastianos and Batchelor, 2010; Kerbel, 2008). Although therapies targeting VEGF/VEGFR2 have improved the efficacy of current anti-cancer treatment strategies, angiogenesis is seldom completely halted, and both angiogenesis and tumor growth inevitably progress in the face of continued therapy. Furthermore, in addition to its well known role in physiological angiogenesis of the adult, for example, during menstruation, ovulation and wound healing, VEGF is also widely expressed in non-angiogenic normal adult tissues where it plays critical roles in normal adult physiology (Maharaj and D'Amore, 2007). For example, it is required for normal kidney filtration (Eremina et al., 2006), for preventing neural degeneration (Oosthuyse et al., 2001), and for maintaining functional hematopoietic, endocrine, and skeletal systems (Sung et al., 2010). Given the pleiotropic activities of the VEGF pathway, it is not surprising that anti-VEGF/VEGFR2 therapies are associated with a number of toxicities, such as hypertension, proteinuria, hypothyroidism, diarrhea, deep vein thromboses, fatigue, and surgical wound healing complications (Verheul and Pinedo, 2007). VEGF blocking agents have also been associated with some rare, more serious side effects, including life-threatening thromboembolic events and severe bleeding complications (Chen and Cleck, 2009; Verheul and Pinedo, 2007). Anti-angiogenic therapies need to be administered for months to years and may eventually prove useful in long term adjuvant therapy for the prevention of recurrent disease, raising further concerns about long term toxicities. Thus, drugs that can selectively target pathological host vasculature with minimal side effects are urgently needed.
TEM8 is a highly-conserved single-pass cell-surface glycoprotein that was originally identified based on its overexpression in the endothelial cells (ECs) that line the tumor vasculature of human colorectal cancer (St Croix et al., 2000). Although our understanding of its physiological function is limited, TEM8 has been found to bind to collagens and promote migration of ECs in vitro (Nanda et al., 2004; Werner et al., 2006). TEM8 was also identified as an anthrax toxin receptor (ANTXR1) (Bradley et al., 2001), and it shares 58% amino acid identify with CMG2, a second receptor for anthrax toxin protein (ANTXR2) (Scobie et al., 2003). TEM8 is upregulated on tumor vessels of various tumor types in both mice and humans (Carson-Walter et al., 2001; Fernando and Fletcher, 2009; Nanda et al., 2004), and in some tumors is also expressed by the tumor cells themselves (Carson-Walter et al., 2001; Jinnin et al., 2008; Yang et al., 2011b). TEM8 was unique among the original TEMs identified in that it could not be detected in the angiogenic corpus luteum of human ovaries (Nanda et al., 2004; St Croix et al., 2000), and developmental angiogenesis and wound healing are unperturbed in Tem8 knockout (KO) mice (Cullen et al., 2009). Indeed, aside from misaligned incisors, adult Tem8 KO mice are overtly normal in appearance. However, murine B16 melanoma tumor growth was impaired in Tem8 KO versus wildtype (WT) mice demonstrating that host-derived TEM8 can promote tumor growth on an immunocompetent background (Cullen et al., 2009). Furthermore, previous studies have shown that a soluble TEM8-Fc trap, TEM8 vaccines or sublethal doses of anthrax toxin can inhibit angiogenesis, slow tumor growth, and prolong survival (Duan et al., 2007; Felicetti et al., 2007; Liu et al., 2008; Rouleau et al., 2008; Ruan et al., 2009; Yang et al., 2010). Taken together, these studies suggest that TEM8 may be required for tumor angiogenesis, but not physiological angiogenesis. Here, we sought to develop anti-TEM8 antibodies that can block TEM8 function in an effort to selectively block pathological angiogenesis.
RESULTS
TEM8 functions in pathological but not physiological angiogenesis
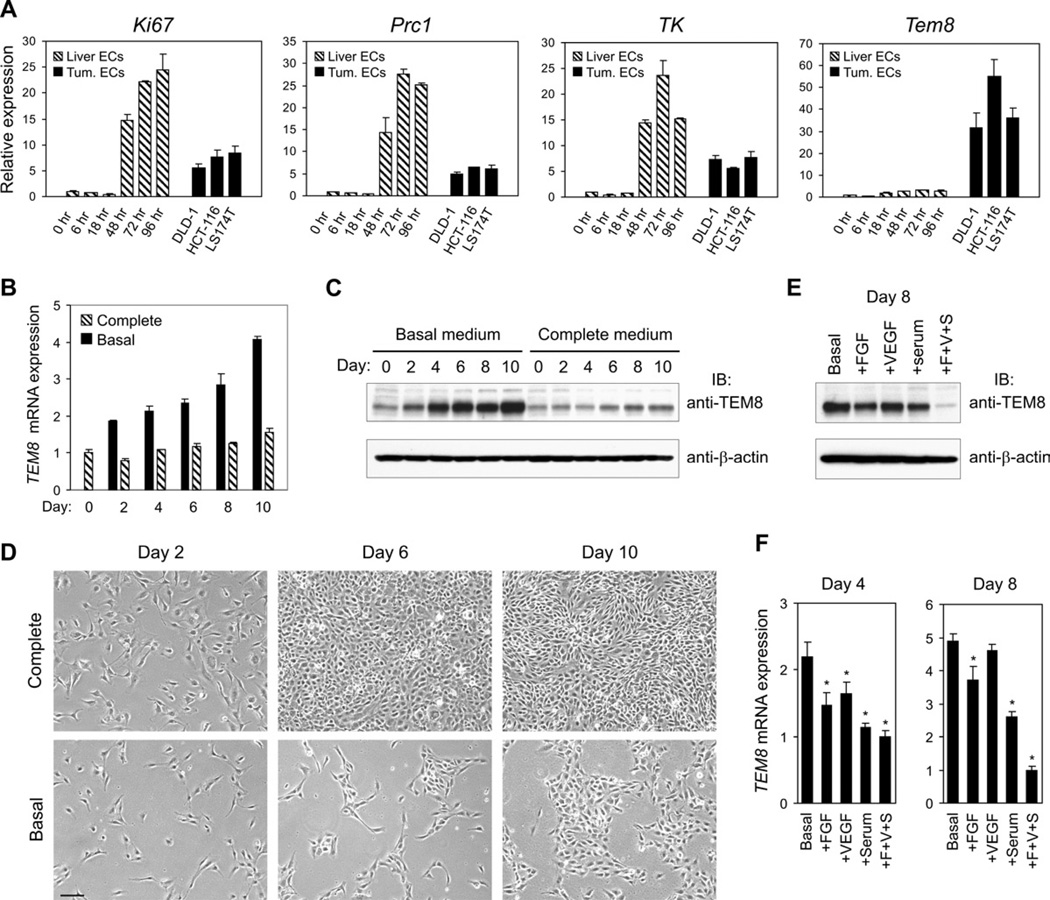
To obtain further evidence that TEM8 is selectively associated with pathological angiogenesis, we compared the Tem8 expression pattern between tumor ECs and adult regenerating liver ECs. Following 70% partial hepatectomy the remaining liver grows rapidly in a highly regulated angiogenesis-dependent process (Drixler et al., 2002; Seaman et al., 2007). In this model, quiescent ECs enter the cell cycle synchronously at around 24 hours post surgery and cease proliferation about 72 hours later. To examine gene expression, we performed quantitative RT-PCR (QPCR) on ECs purified from tumor xenografts derived from DLD1, HCT116 or LS174T cells, or ECs isolated from quiescent resting liver (0 hr) or regenerating liver taken at various post-surgical time-points (6 hr, 18 hr, 48 hr, 72 hr, or 96 hr). Although markers of proliferation, such as Ki67, protein regulator of cytokinesis 1 (Prc1), and thymidine kinase (TK) were highly induced in liver ECs by 48 hours post-partial hepatectomy, Tem8 expression levels remained baseline in regenerating liver ECs. In contrast, Tem8 was expressed 32 to 55-fold higher in each of the tumor EC fractions compared to resting liver ECs (Figure 1A). The peak expression levels of the cell cycle genes in regenerating liver ECs were higher than that in tumor ECs presumably because of the synchronous nature of the proliferating liver EC population.
Figure 1. TEM8 is selectively upregulated on tumor vasculature and is elevated in cultured HMECs in response to growth factor deprivation.
(A) QPCR was used to evaluate the expression of the indicated genes in ECs isolated from resting adult liver (0 hr), regenerating liver taken 6 hr, 18 hr, 48 hr, 72 hr, or 96 hr following 70% partial hepatectomy, or from DLD1, HCT116 or LS174T colon cancer xenografts.
(B) TEM8 mRNA levels over the course of 10 days in HMECs grown in endothelial basal medium (EBM-2) or in complete medium [EBM-2 supplemented with FGF (F), VEGF (V) and 5% fetal bovine serum (S)].
(C) TEM8 protein levels over the course of 10 days in HMECs grown in basal medium or in complete medium. The media are the same as in (B).
(D) The appearance of the cells used in (B) and (C) is shown. Note that HMECs became confluent by day 6 in complete medium but formed only small colonies by day 10 in basal medium. The media are the same as in (B). Bar = 100µm.
(E, F) Effect of supplementation of basal growth medium with FGF, VEGF, or serum alone or all three together on the expression of TEM8 protein (E) and mRNA (F). (*p<0.05). Values in (A),(B) and (F) represent mean ± SD. See also Figure S1.
To investigate whether Tem8 is expressed by tumor associated inflammatory cells, such as CD11b+ myeloid cells or other bone marrow derived cells that have been shown to promote tumor angiogenesis and may be involved in the refractoriness of tumors to VEGF inhibition (Du et al., 2008; Shojaei et al., 2007), we examined its expression in CD45+ (pan hematopoietic), CD11b+ (myeloid), and CD105+ (endothelial) cells isolated from tumors. Tem8 was highly expressed only in the endothelial fraction (Figure S1A). To determine potential tumor microenvironmental factors that induce TEM8 expression on tumor vasculature, we examined cultured human microvascular endothelial cells (HMECs) in response to several conditions. Neither co-culture with tumor cells nor exposure to hypoxia induced TEM8 (Figure S1B and S1C). However, upon serum starvation, TEM8 levels steadily increased in these cells which normally express low endogenous TEM8 levels, resulting in a 4-fold increase in TEM8 mRNA (Figure 1B) and a 5-fold increase in TEM8 protein (Figure 1C) by day 10. In contrast, TEM8 levels remained low in cells maintained in complete medium, and the slight increase in TEM8 expression noted at later time points (Figure 1B and 1C) may have been due to rapid growth factor depletion caused by increasing cell numbers (Figure 1D, top panel). The increase in TEM8 expression upon growth factor starvation was not influenced by the amount of cell-cell contact based on comparisons of sparse versus confluent cells wherein the cell numbers were held constant but surface area altered (data not shown). Importantly, TEM8 elevation in growth factor starved cells could be inhibited by FGF, VEGF or serum treatment, and the combination of all three resulted in the lowest TEM8 levels (Figure 1E and 1F and Figure S1D). Thus, TEM8 may be part of a compensatory angiogenic or survival pathway that is activated, at least in part, by insufficient local angiogenic growth factors.
Host-derived TEM8 promotes the growth of human tumor xenografts
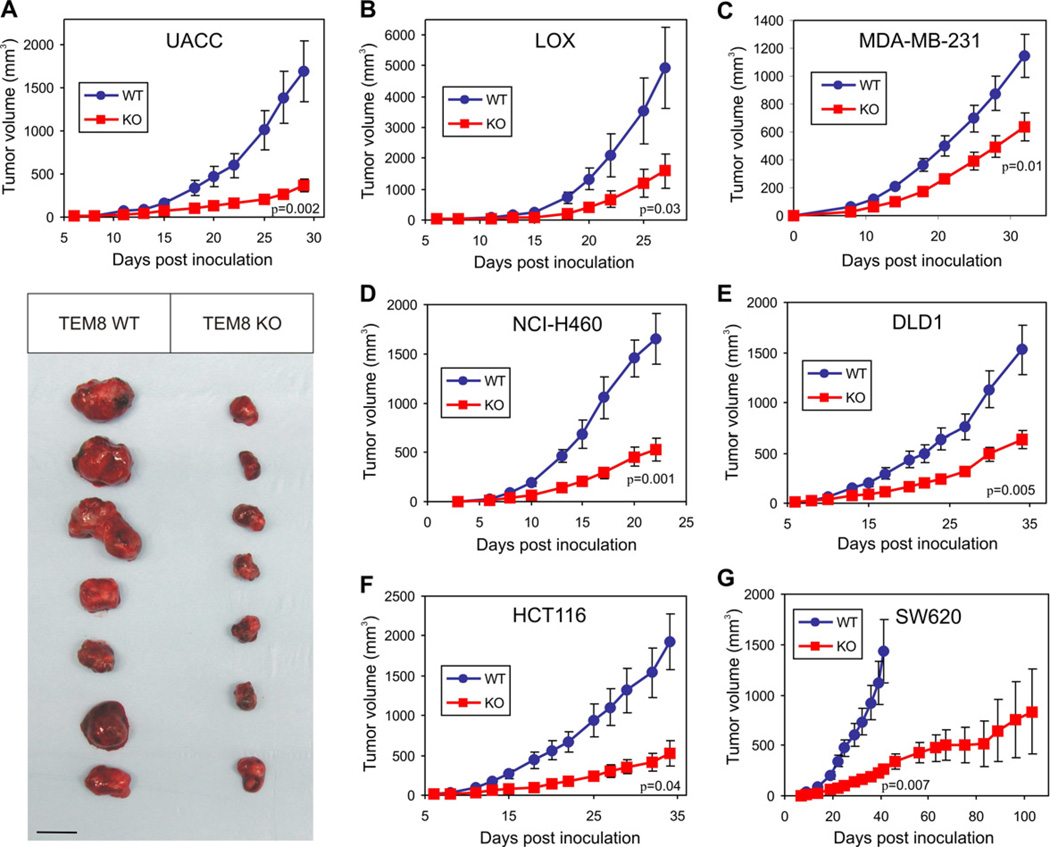
To determine if TEM8 could promote the growth of human tumor xenografts, we generated Tem8 KO mice on an immunocompromised athymic nude background. Tumor growth was inhibited in the Tem8 KOmice compared to WT littermate controls when challenged with various tumor types including melanoma (UACC and LOX), breast (MDA-MB-231), lung (NCI-H460), and colon cancer (SW620, HCT116 and DLD1) (Figure 2). The MDA-MB-231 breast tumors were grown orthotopically in the mammary fat pad, while the melanoma and other tumor types were grown subcutaneously. Tumor growth was consistently slower in Tem8 KO versus WT mice, and SW620 tumors required over 100 days to reach an average size of 800mm3, compared to only 35 days for WT littermates (Figure 2G). Thus, host-derived TEM8 functions to promote the subcutaneous and orthotopic growth of human tumor xenografts of diverse origin.
Figure 2. The Growth of Human Tumor Xenografts is Impaired in Tem8 KO Mice.
(A–G) Melanoma (A–B), breast (C), lung (D), and colon (E–G) cancer cell lines were injected into Tem8 wildtype (WT, blue) or knockout (KO, red) mice and tumor volume monitored over time. The physical appearance of the resected UACC tumors is shown in (A). p-values were calculated from the final tumor measurement (A–F) or at day 41 (G) when the WT group reached its maximum size and had to be euthanized (Students t-test). n = 7 to 15 mice/group. Values in (A–G) represent mean ± SE. Bar = 10mm.
Development of anti-TEM8 IgGs
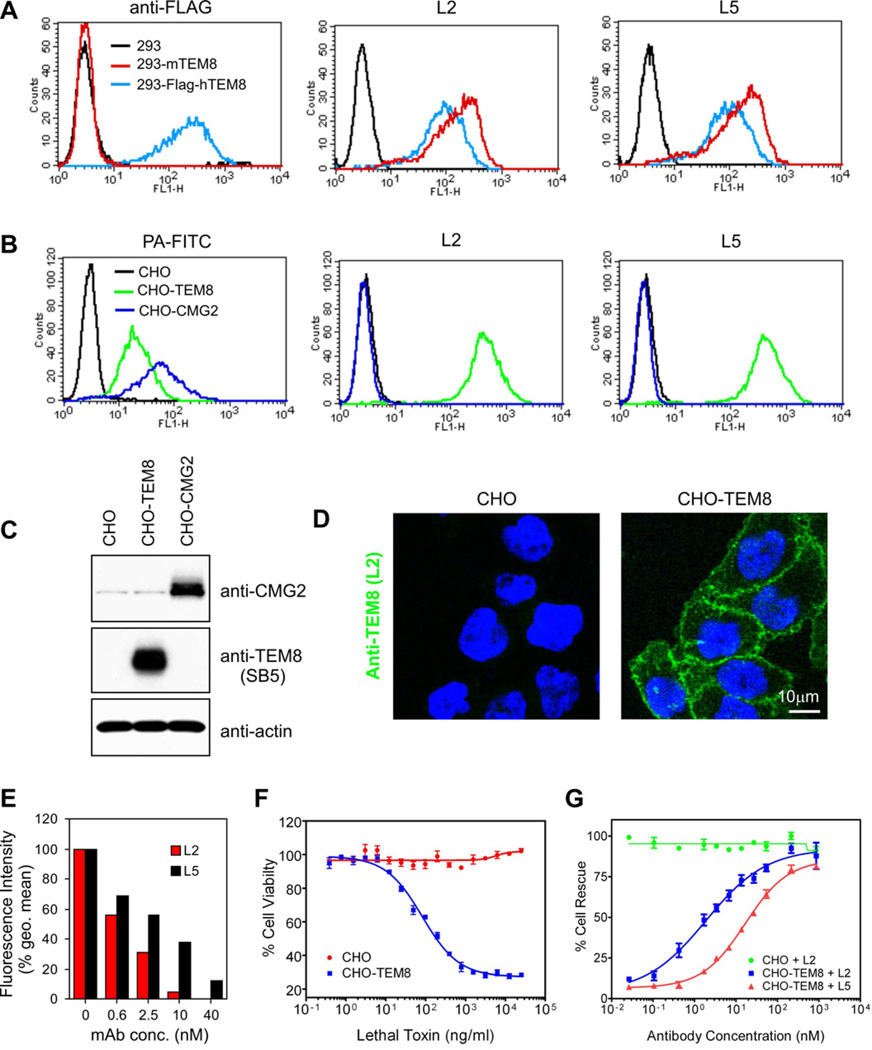
Based on the functional importance of TEM8 in tumor growth promotion, we sought to develop therapeutic anti-TEM8 antibodies that could block TEM8 function in vivo. We had previously generated the SB series of anti-TEM8 antibodies (Nanda et al., 2004). However, these antibodies were murine derived and none of these could bind the predominant native form of TEM8 on the cell surface (Nanda et al., 2004; Yang et al., 2011b). To overcome these obstacles and circumvent potential difficulties associated with breaking tolerance, we developed another panel of fully human anti-TEM8 antibodies in vitro using antibody phage display. The selection strategy, which involved panning of Fab libraries on Tem8-transfected mammalian cells and purified recombinant mammalian-derived TEM8-ED, resulted in the identification of 5 independent Fabs, L1, L2, L3, L5 and ID2. Each of the Fabs were found to react with both mouse and human TEM8 in an ELISA and on the surface of live TEM8-positve cells by immunofluorescence and flow cytometry (Figure S2A–S2C). Although the physiologic ligand(s) of TEM8 in vivo are unclear, the TEM8 extracellular region contains a single structural motif, that is, a von Willebrand factor type A (VWA) domain, where physiologic TEM8 ligand(s) are most likely to bind. VWA domains are found in many extracellular eukaryotic proteins including integrins, and are known to mediate adhesion to other proteins via metal ion-dependent adhesion sites (MIDAS). We reasoned that the protective antigen (PA) subunit of anthrax toxin, which binds the exposed VWA domain of TEM8 in a metal ion-dependent manner, may usurp the physiologic binding site of a natural ligand. Thus, we screened the Fabs for the ability to block FITC-labeled anthrax toxin PA binding to the surface of cells expressing TEM8. Each of the Fabs blocked FITC-PA binding in a dose-dependent manner (Figure S2D). Thus, we identified five human anti-TEM8 Fabs that were positive in all screens and that contained a unique variable domain.
Fabs have a relatively short half-life in vivo (several hours) compared to full IgGs (several days). To enhance their stability in vivo, two of the Fabs, L2 and L5, were selected for reformatting to full IgG. For preclinical testing in mice, the constant domains of mouse IgG2a (CH1, CH2, CH3 and CL) were fused to the human variable domains (VH and VL) in order to minimize immunogenicity, resulting in human-mouse chimeric antibodies. After reformatting, both L2 and L5 IgGs maintained their activity against TEM8 in the same screens used to test the Fabs, and were specific because they failed to react with mouse or human CMG2, the closest homologue of TEM8 (Figure 3A–3D and data not shown). Upon titration and comparison at non-saturating concentrations, L2 bound TEM8 expressing cells with 7-fold higher affinity than L5 (EC50 0.4 nM and 2.8 nM, respectively, see Figure S2E). Similarly, L2 was ~4-fold more potent at blocking the binding of FITC-labeled protective antigen and ~ 9-fold more potent at preventing cytotoxicity caused by anthrax lethal toxin (Figures 3E–3G).
Figure 3. The L2 and L5 IgGs React Selectively with TEM8 and Block Binding and Toxicity of Anthrax Toxin Proteins.
(A) L2 and L5 antibodies were used for flow cytometry staining of 293 cells stably transfected with mouse Tem8 (293-mTEM8) or a FLAG-tagged human TEM8 (293-Flag-hTEM8).
(B) L2 and L5 were used for flow cytometry staining of CHO/PR230 (CHO) cells (an anthrax toxin receptor-deficient cell line) that had been stably transfected with human TEM8 (CHO-TEM8) or human CMG2 (CHO-CMG2). FITC-labeled protective antigen (PA-FITC), which binds both TEM8 and CMG2, was used as a positive control.
(C) Western blot analysis was used to evaluate the expression of TEM8 and CMG2 in stably transfected CHO-TEM8 and CHO-CMG2 cells.
(D) L2 antibodies were used for cell-surface Immunofluorescence labeling of CHO and CHO-TEM8 cells.
(E) The ability of L2 and L5 antibodies to block binding of PA-FITC to CHO-TEM8 cells was measured by flow cytometry.
(F) The viability of CHO and CHO-TEM8 cells was evaluated 48 hours post-treatment with lethal toxin.
(G) The ability of L2 and L5 antibodies to protect cells from toxicity following treatment with 1µg of lethal toxin was evaluated. In this assay the EC50 for L2 and L5 were 1.9nM and 16.6nM respectively. Values in (F) and (G) represent mean ± SE. See also Figure S2.
Anti-TEM8 IgGs inhibit tumor growth but do not delay wound healing
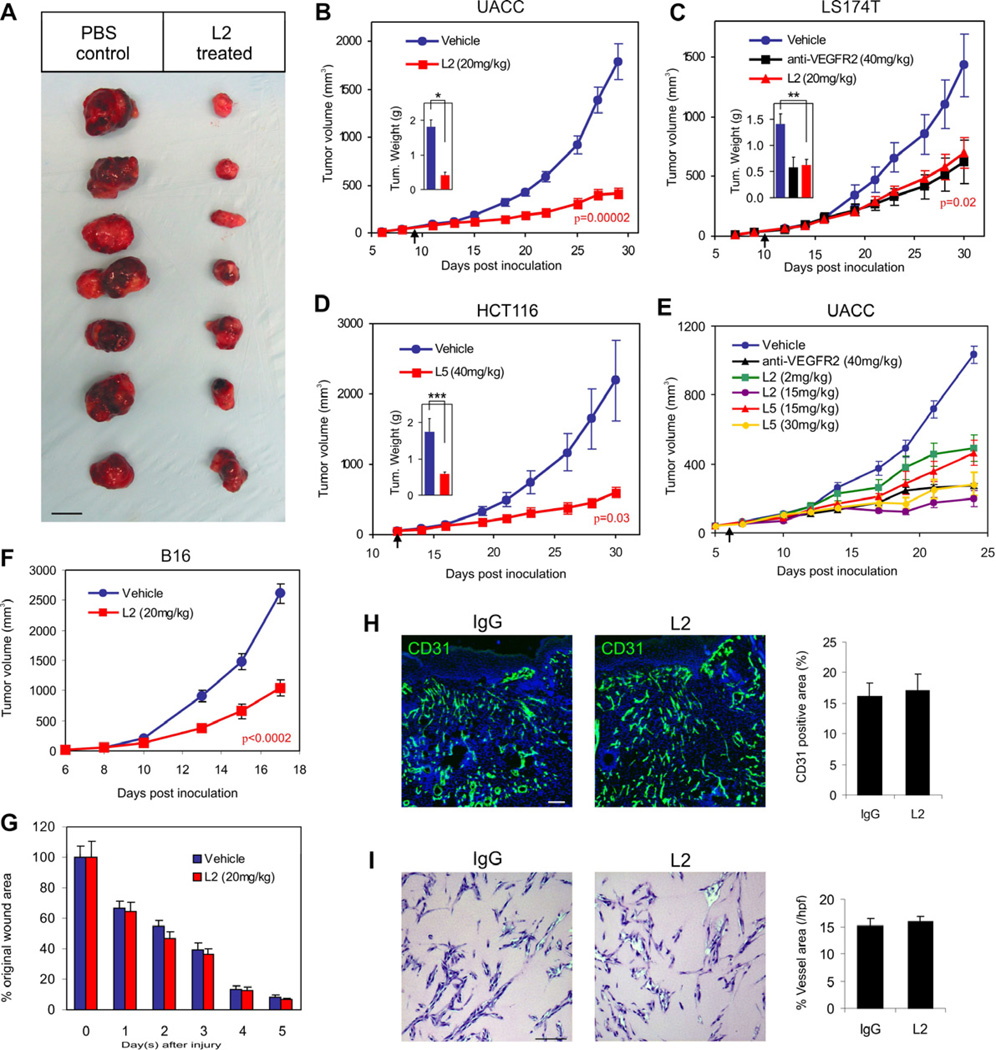
We tested the L2 and L5 antibodies for their activity against UACC, HCT116, and DLD1 colon tumor xenografts in athymic nude mice. In these studies, mice were treated with L2 or L5 once tumors reached an average size of 50mm3. For each tumor type analyzed a marked tumor growth inhibition was observed in each of the treated groups compared to vehicle (PBS) alone (Figure 4A–4E). The antitumor activity was comparable to that of anti-VEGFR2 antibodies (Figure 4C). When L2 and L5 were compared in a dose-escalation study to determine the amount of antibody required for optimal tumor growth inhibition, L2 showed superior activity. A partial growth inhibition was observed when mice were given 2mg/kg of L2, while maximum growth inhibition was observed with 15mg/kg (Figure 4E). L5, on the other hand, only showed partial growth inhibition at 15mg/kg, similar to that observed in the 2mg/kg L2 treatment group, and in each tumor study required 30 to 40 mg/kg to achieve its optimal biologic dose (OBD). Although L5 required a higher dose than L2 to achieve maximum efficacy, at their OBD both antibodies showed similar anti-tumor activity. Taken together, these studies demonstrate a marked in vivo anti-tumor activity of two independent anti-TEM8 antibodies. Because the full IgG of L2 appeared more potent than L5 both in vitro and in vivo, we focused on L2 for the remainder of our studies.
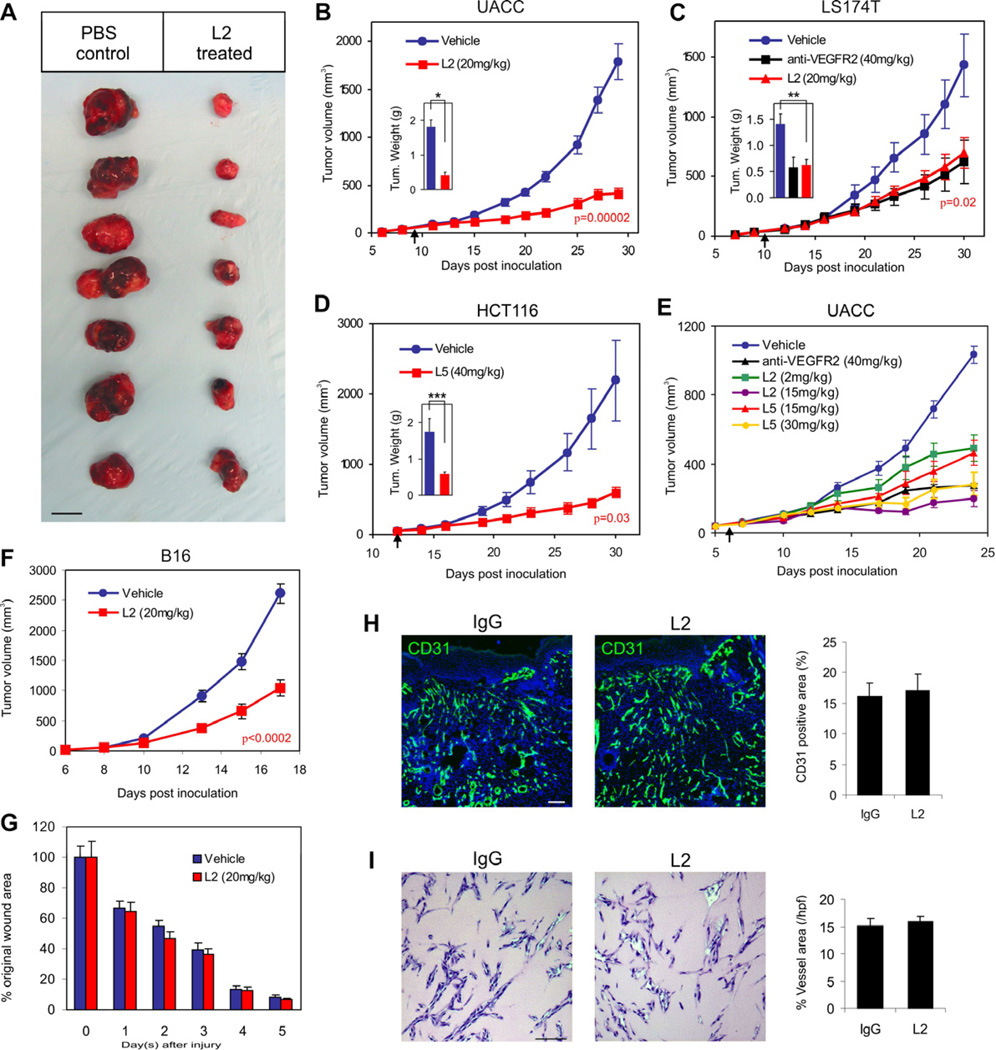
Figure 4. Anti-TEM8 Antibodies Inhibit Tumor Growth but do not Delay Wound Healing.
(A–F) Melanoma (A, B and E; UACC, F; B16) or colon cancer (C; LS174T, D; HCT116) tumor cells were inoculated subcutaneously into athymic nude (UACC, LS174T, HCT116) or C57BL/6 (B16) mice and tumor growth monitored. Treatments with PBS (vehicle), anti-VEGFR2 antibodies, or anti-TEM8 antibodies (L2 or L5) were administered 3 times per week and were initiated when tumors reached a size of 50mm3 (arrow). A Students t-test was used to calculate p-values between the vehicle and L2 treatment groups at the final tumor measurement. Tumors were excised at the end of the study to calculate final tumor weights (Insets in B, C and D) *p=0.00005, **p=0.002, ****p=0.02.
(A) The physical appearance of the UACC melanoma tumors at the end of the study following surgical excision. Bar = 10mm.
(B) L2 inhibition of UACC melanoma tumor growth.
(C) L2 and DC101 (anti-VEGFR2) inhibition of LS174T tumor growth.
(D) L5 inhibition of HCT116 tumor growth.
(E) L2 and L5 dose-dependent inhibition of UACC tumor growth.
(F) L2 inhibition of B16 melanoma tumor growth.
(G) Wound closure rates following treatment with L2 or vehicle (PBS) alone. In this experiment, wounds were generated in the same tumor-bearing mice as shown in (F).
(H) CD31-immunofluorescence staining of granulation tissue vasculature in control and L2 treated groups. Control mice received non-specific IgG in this experiment (n=6 wounds per group).
(I) Matrigel Plug vascularization was assessed following treatment with non-specific IgG or L2 anti-TEM8 antibodies. Vessels areas were calculated from 6 plugs per group. Values in (B) to (I) represent mean ± SE. Bar in (H) and (I): 100µm.
The aforementioned studies were conducted in immune compromised mice. To determine if L2 could suppress tumor growth in the presence of an intact immune system, we injected murine B16 melanoma cells into syngeneic C57BL6 mice and began treating mice with L2 at a tumor size of 50mm3. The L2-treated group had a 60% reduction in tumor growth by the end of the study (Figure 4F). Midway through the therapeutic course we also inflicted 6mm diameter wounds into each of the tumor bearing mice to determine if L2 treatment would interfere with wound healing. Wound closure rates were not significantly altered by L2 (Figure 4G), despite its clear anti-tumor activity in the same mice. Immunofluorescence staining for CD31 showed no alteration in the amount of vasculature present within the healing wound granulation tissue (Figure 4H). Matrigel-induced vascularization was also unaffected by L2 treatment (Figure 4I). Thus, L2 antibodies inhibited chronic pathological tumor growth while not interfering with normal healing processes dependent on physiological angiogenesis.
L2 has no detectable toxicity
Two types of toxicology studies were conducted to determine how well the L2 anti-TEM8 antibody was tolerated. The first study involved dose-escalation, wherein mice were administered 20, 50 or 100mg/kg of L2 every other day for a total of three treatments, and then analyzed twenty-four hours later. All serum chemistry and blood cell counts in the group treated with 100 mg/kg L2 were similar to that of the control group, and no dose-dependent alterations were observed (Table 1; data not shown). Treated mice consumed food and socialized similarly to control animals, and both body and organ weights were unchanged (Figure S3A and S3B). A comprehensive histopathologic analysis of 44 organs or tissues derived from 6 mice/group failed to reveal any abnormalities (data not shown). The second toxicology study involved treatment of mice with 20mg/kg of L2 3× per week for up to 6 weeks, followed by an analysis of the same toxicology parameters. Again, no abnormalities were noted (Figure S3C; data not shown).
Table 1.
Selected Toxicological Results and Organ Weights
| Control | 100mg/kg L2 Anti-TEM8 |
|
|---|---|---|
| Selected Parametersa | ||
| WBC (K/µL) | 5.9 ∀ 2.5 | 6.3 ∀ 2.4 |
| RBC (M/µL) | 9.5 ∀ 0.4 | 9.6 ∀ 0.4 |
| Albumin (g/dL) | 4.0 ∀ 0.4 | 3.9 ∀ 0.1 |
| ALT (U/L) | 52.7 ∀ 10.1 | 51.9 ∀ 27.4 |
| Total Bilirubin (mg/dL) | # 0.2 | # 0.2 |
| Creatine (mg/dL) | # 0.2 | # 0.2 |
| Hemoglobin (g/dL) | 13.9 ∀ 0.6 | 13.9 ∀ 0.4 |
| Total Protein (g/dL) | 5.6 ∀ 0.2 | 5.7 ∀ 0.4 |
| BUN (mg/dL) | 19.7 ∀ 2.0 | 17.5 ∀ 3.4 |
| Selected Organ Weightsb (mg) | ||
| Brain | 462 ∀ 19 | 465 ∀ 15 |
| Heart | 137 ∀ 15 | 155 ∀ 21 |
| Kidney | 312 ∀ 58 | 308 ∀ 63 |
| Liver | 1173 ∀ 180 | 1152 ∀ 271 |
| Lung | 153 ∀ 20 | 168 ∀ 34 |
| Spleen | 80 ∀ 13 | 83 ∀ 15 |
Represented toxicological data and
organ weights from mice (n=6/group) dosed i.p. every second day with saline (control) or 100 mg/kg anti-TEM8 mAb. Values are means ± SD. See also Figure S3.
L2 targets tumor vasculature in vivo
To determine the specificity of L2 for TEM8 in vivo, we decided to treat tumor-bearing Tem8 WT and KO mice with L2 reasoning that L2 should only have activity against tumors in Tem8 WT mice if the tumor cells employed do not themselves express endogenous TEM8. TEM8 expression varied among cultured tumor cell lines, among which DLD1 tumor cells expressed undetectable TEM8 both in cell culture and following purification from established tumors in vivo (Figures S4A and S4B). Therefore, to test the specificity of the L2 antibody in vivo, Tem8 WT and KO mice were challenged with DLD1 cells and treated with L2 or control IgG (Figure 5A). As expected, tumors grew slower in Tem8 KO compared to Tem8 WT mice treated with control IgG. When the L2 antibody was administered to Tem8 WT mice, tumor growth was inhibited relative to the IgG control group, but was indistinguishable from that in the Tem8 KO group. Importantly, L2 treatment of TEM8 KO tumor-bearing mice did not result in any further tumor growth inhibition. Taken together, these results indicate that TEM8 is the sole target of L2 in vivo, and supports the hypothesis that L2 is a function-blocking monoclonal antibody.
Figure 5. L2 Targets Tumor Vasculature In Vivo and Engages ADCC and CDC In Vitro.
(A) Non-specific antibodies (IgG control) or L2 anti-TEM8 antibodies were administered to Tem8 wildtype (T8-WT) or Tem8 knockout (T8-KO) mice at 20mg/kg mice 3 times per week beginning one day post subcutaneous inoculation of TEM8-negative DLD1 tumor cells. *At day 30 the tumors in the T8-WT + IgG control group were significantly larger than those in each of the other three groups (p<0.0001), but there was no differences in tumor size between the T8-KO + IgG, T8-WT + L2, and T8-KO + L2 groups (one-way ANOVA with Bonferroni post-test). n=12 mice/group.
(B) Immunofluorescence vessel staining of Tem8 WT and KO mice following treatment with L2 or IgG. Right panel, quantification of CD31 positive vessel area. *p<0.0001 between each of the groups and the IgG WT control group (one-way ANOVA). Bar = 200µm.
(C) Flow cytometry staining of dispersed tumor tissues was used to determine the percent of CD31-positive cells in L2-treated tumors from Tem8 WT mice (WT-L2), IgG treated tumors from Tem8 KO mice (KO-IgG) and IgG treated tumors from Tem8 wildtype mice (WT-IgG). The dot plots show representative data for each of the groups and the bar graph displays the average percent CD31-positive cells (n=6 per group). Both the WT-L2 and KO-IgG groups had significantly fewer cells than the WT-IgG group as determined by a one-way ANOVA.
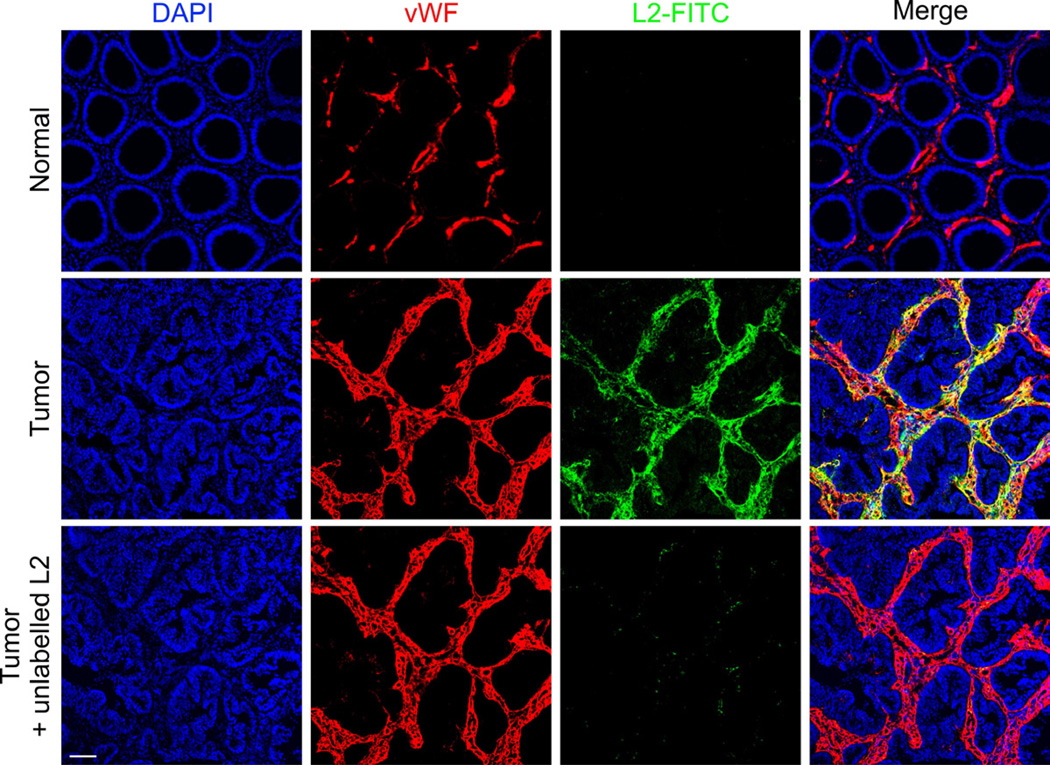
(D) L2 localization in vivo was assessed by immunofluorescence staining of various tissues following i.v. injection of FITC-labeled L2 into DLD-1 tumor bearing mice. An overlay of the L2 image (green) with the endothelial marker image (Meca-32 or CD31, red) was used to asses co-localization with vasculature (yellow, merge). Bar = 50µm.
(E) The specificity of L2-FITC for TEM8 in vivo was assessed by comparing the staining of tumor stroma from Tem8 wildtype (WT) and Tem8 knockout (KO) mice. Bar = 50µm.
(F) NK-mediated toxicity against TEM8 expressing target cells was measured in the presence of L2 or control IgG. The effector:target (E:T) cell ratio in this experiment was 25:1.
(G) The impact of increasing E:T cell ratios on L2-mediated ADCC was evaluated.
(H) Complement-dependent cytotoxicity (CDC) was assessed with varying amounts of L2 or control IgG.
(I) To evaluate complement dependency, variable amounts of complement (compl.) were added to the CDC assay. Values in (A–C) and (F–I) represent mean ± SE. See also Figure S4.
The previously described expression of TEM8 in tumor endothelium (Fernando and Fletcher, 2009; Nanda et al., 2004; St Croix et al., 2000) suggests that the target tissue of L2 in vivo may be the tumor associated vasculature. To assess this, we performed CD31 vessel staining of the human DLD1 colon cancer xenografts, and found a reduced number of vessels in tumors derived from Tem8 KO or L2-treated mice (Figure 5B). Quantification of the number of CD31-positive ECs in tumors using flow cytometry revealed significantly lower EC numbers following both pharmacologic and genetic ablation of TEM8 (Figure 5C). We reasoned that TEM8 may promote proliferation of tumor ECs based on previous studies that showed a role for CMG2 in promoting endothelial proliferation (Reeves et al., 2010). However, endothelial proliferation in DLD1 tumors was not altered in response to L2 treatment (Figure S4C), although the number of apoptotic ECs was significantly increased (p<0.02, Figure S4D). To further assess the specificity of L2 in vivo, L2 was labeled with FITC and then intravenously injected into DLD1 tumor-bearing mice. Immunofluorescence analysis revealed localization of TEM8 selectively in tumor associated vasculature, but not in any of the normal control tissues analyzed including brain, heart, intestine, liver, muscle, spleen and stomach (Figure 5D). Some tumor associated perivascular stromal cells, including pericytes based on their adjacent proximity to endothelium, were also positive. However, stromal cell staining was confined to the tumor region in Tem8 WT mice and was absent from the tumors in TEM8 KO mice, confirming the specificity of antibody staining (Figure 5E).
L2 can elicit NK-mediated and complement-mediated cytotoxicity
We reasoned that the anti-tumor activity of L2 in vivo may involve multiple mechanisms, and that antibody-dependent cellular cytotoxicity (ADCC) and/or complement dependent cytotoxicity (CDC) could contribute to this activity. To determine if TEM8 could potentially function as a target of ADCC, we mixed effector NK cells with TEM8-expressing 293 target cells at various ratios, and found that L2, but not control IgG, was able to elicit cytotoxicity that was dependent on both the antibody and effector cell concentration (Figure 5F and 5G). Similarly, L2 elicited CDC in both an antibody and complement-dependent manner (Figure 5H and 5I). Although these in vitro studies support a role for ADCC and CDC, further work is required to determine if these mechanisms contribute to the anti-tumor activity of L2 in vivo.
L2 potentiates tumoricidal responses
The delayed tumor growth in Tem8 KO mice and the encouraging anti-tumor activity of L2 against relatively small established 50mm3 tumors prompted us to explore the activity of L2 against larger tumors. Importantly, even tumors that were 200mm3 in size prior to L2 treatment showed a significant response to the antibody such that when the control tumors reached an average size of 2000 mm3, treated tumors had an average size of 1288mm3 (Figure 6A). However, because the L2-mediated growth inhibition was less effective against relatively large (200mm3) pre-established tumors compared to small (50mm3) tumors (compare L2 treated group in Figure 6A with that in Figure 4B), we determined whether the combination of L2 with other types of anticancer agents would result in enhanced antitumor efficacy. When L2 treatment was combined with the anti-VEGFR2 antibody DC101, which prevents VEGF from binding VEGFR2, L2 significantly enhanced the activity of DC101 against UACC melanoma (p<0.05; Figure 6A). Furthermore, the combination of L2 with DMXAA (ASA404), a vascular targeting agent that has shown promising activity in early clinical trials against lung cancer (Baguley and McKeage, 2010), proved highly effective against NCI-H460 lung cancer xenografts (Figure 6B). Both L2 and DMXAA significantly delayed tumor growth, but the combination was even more efficacious than either treatment alone (p<0.001; DMXAA + L2 versus DMXAA alone). Finally, when L2 was combined with 5-fluorouracil (5FU) and irinotecan (IRT), chemotherapeutic agents that are currently used to treat patients with colorectal cancer, L2 significantly enhanced their efficacy against HCT116 tumors (p<0.0001; 5FU + L2 versus 5FU, p<0.02; IRT + L2 versus IRT, see Figures 6C and 6D). L2 also enhanced the efficacy of irinotecan against SW620 (p<0.02; IRT + L2 versus IRT, Figure 6E), another colon cancer tumor model, demonstrating the generality of this response. Combination of L2 with IRT was highly efficacious, such that tumors in 5 of 11 mice in the HCT116 study and 4 of 11 mice in the SW620 study had completely regressed by 100 days post-inoculation, and these mice remained tumor-free for the duration of the study- an additional 7 months (Figures 6D and 6E). No complete tumor responses were observed in any of the monotherapy treatment arms. To further assess the inhibitory activity of L2 + IRT following long term therapy, treatment was discontinued after 100 days, which resulted in rapid expansion of the remaining tumors that had not completely regressed. Analysis of body weights, food consumption, serum chemistry and hematological profiles in these combination drug trials failed to reveal a change in toxicity caused by the addition of L2 to the chemotherapeutic agent (Figure 6F and Table S1). Taken together, these studies demonstrate that L2 treatment can enhance the anti-tumor responses of a wide variety of anti-cancer agents without added toxicity.
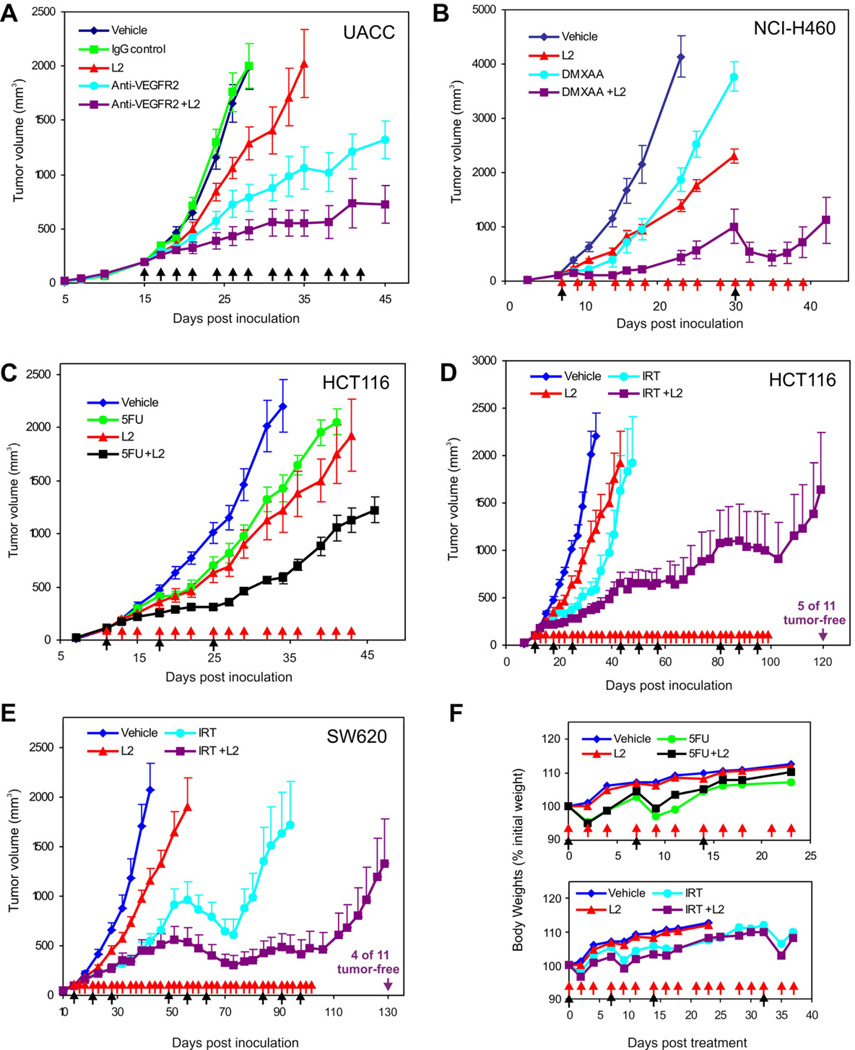
Figure 6. L2 Anti-TEM8 Antibodies Augment the Efficacy of Various Classes of Anti-Cancer Agents.
(A) UACC tumor growth was compared following treatment with vehicle (PBS), non-specific mouse IgG (20mg/kg), L2 anti-TEM8 (20mg/kg), anti-VEGFR2 (40mg/kg) or a combination of L2 (20mg/kg) and anti-VEGFR2 (40mg/kg). Treatments were administered 3 times per week (arrows) beginning 15 days post tumor cell inoculation when tumors reached a size of 200mm3.
(B) NCI-H460 tumor growth was compared following treatment with vehicle, L2 anti-TEM8, DMXAA or a combination of L2 and DMXAA beginning 15 days post tumor inoculation when tumors reached an average size of 100mm3. In this experiment L2 (20mg/kg) was administered 3 times per week (red arrows) for the duration of the study whereas DMXAA was administered at a high dose of 25mg/kg (black arrows), followed by 5mg/kg/day the following two days.
(C) HCT116 tumor growth was compared following treatment with vehicle, L2 anti-TEM8 (20mg/kg), 5-fluorouracil (5FU) (100 mg/kg) or a combination of L2 and 5-FU beginning 11 days post tumor inoculation when tumors reached an average size of 100mm3 in volume. In this experiment L2 was administered 3 times per week (red arrows) whereas 5-FU was administered once/week for three weeks (black arrows).
(D) HCT116 tumor growth was compared following treatment with vehicle, L2 anti-TEM8 (20mg/kg), irinotecan (IRT) (80 mg/kg) or a combination of L2 and irinotecan beginning 11 days post tumor inoculation when tumors reached a size of 100mm3. In this experiment L2 was administered 3 times per week (red arrows) until 100 days post-inoculation. To maximize efficacy without excessive toxicity and mimic the clinical situation, IRT was administered in three cycles (where 1 cycle = 1 treatment per week × 3weeks; black arrows) that were separated by a two week rest period to allow recovery. The HCT116 tumor studies in C and D were conducted simultaneously and contain the same vehicle and L2 groups which were duplicated for ease of comparison.
(E) SW620 tumor growth was compared following treatment with vehicle, L2 anti-TEM8 (20mg/kg), irinotecan (80 mg/kg) or a combination of L2 and irinotecan beginning 14 days post tumor inoculation when tumors reached a size of 100mm3. The treatments in this study were the same as those described for HCT116 above. IRT caused tumor regression in many of the treated mice. In (E), a decrease in tumor size caused by IRT is readily observed in both IRT arms (IRT and IRT+L2) during the second cycle of IRT treatment. During the subsequent two-week rest period the tumors rapidly rebounded. Following the last cycle of IRT, many of the tumors in the combination group (IRT + L2) regressed again, while the larger tumors in the control group did not respond. For ease of comparison, only half of the error bars are shown in D and E. Data in A–E represent mean ± SE.
(F) Body weights in HCT116 tumor-bearing mice from the 5FU study [upper panel, corresponding to (C)] or the irinotecan study [lower panel, corresponding to (D)] were monitored from the start of therapy until the tumors in the control groups reached their maximum allowable size and mice had to be euthanized. Data in (F) represent mean values. The SD ranged from 2 to 8% and error bars were omitted for clarity. The apparent reduction in mean body weight observed following 5-fluorouracil and irinotecan treatments (black arrows) were non-significant and were not altered by L2 treatment (red arrows). Values in (A) to (E) represent mean ± SE. See also Figure S5.
L2 binds human tumor vasculature
To examine the specificity of L2 binding in human tumors, in situ immunofluorescence staining with L2 was performed on colorectal tumors or adjacent normal colonic mucosa derived from 6 cases of late-stage colorectal cancer, 4 of which were patient-matched. Although staining was undetectable in all cases of normal colonic mucosa, in each of the tumor samples L2-FITC strongly labeled the tumor stroma, including von Willebrand factor (vWF) positive ECs as well as some perivascular stromal cells that, based on morphology, appeared to include pericytes and possibly fibroblasts (Figure 7). Although the intensity of stromal staining was variable in different regions of the tumor, the staining was considered specific because it was completely blocked by the addition of unlabelled L2 but not isotype-matched control IgG. Thus, in tumors derived from both patients and mouse xenografts, TEM8 is found in tumor-associated vasculature and tumor-associated perivascular stromal cells.
Figure 7. L2 Anti-TEM8 Antibodies Bind to the Vasculature of Human Colorectal Cancer.
FITC-conjugated L2 (green) was used for immunofluorescence labeling of human colorectal tumors and normal colonic mucosa. The vasculature was co-stained with antibodies against von Willebrand factor (vWF; red), a pan endothelial marker, and overlapping immunofluorescence is shown in the merged image (yellow). The normal and tumor samples shown were patient-matched and processed in parallel. To prevent non-specific binding, immunofluorescence staining was performed in the presence of a 50-fold excess of isotype-matched (human-mouse chimeric) control IgG that was generated against a foreign antigen (cyclosporine A). The staining was abolished by blocking the samples with unlabelled L2 (bottom panel) prior to adding L2-FITC. The middle and bottom panel were taken from serial sections. Bar = 100µm.
DISCUSSION
These studies demonstrate that TEM8 is critical for promoting pathological angiogenesis evoked by a variety of tumor types, and that antibody-mediated targeting of TEM8 provides a rational strategy for combating cancer. Most angiogenesis regulators that have been discovered to date cannot distinguish physiological and pathological angiogenesis. In immunocompetent mice L2 inhibited tumor growth but had no effect on wound healing in the same mice, consistent with earlier studies demonstrating no difference in wound healing between Tem8 WT and KO mice (Cullen et al., 2009). TEM8 was also dispensable for developmental angiogenesis and normal physiological angiogenesis of the corpus luteum (Cullen et al., 2009; Nanda et al., 2004; St Croix et al., 2000). A function for TEM8 in these normal physiological processes could potentially be masked through compensation by another molecule. However, CMG2 is the only other protein that shares significant amino acid identity with TEM8 and, aside from misaligned incisors, Cmg2/Tem8 double mutant mice, like Tem8 KO mice, appear to develop normally (Liu et al., 2009). These results support the conclusion that physiological and pathological angiogenesis are distinct and that antibody mediated targeting of TEM8 can selectively inhibit pathological tumor growth, while sparing normal healing processes that also require vascularization.
Based on our results, we propose that TEM8 overexpression in tumor vasculature may be caused, at least in part, by local decreases in the availability of stromal growth factors, such as VEGF and FGF. At first this might seem counterintuitive given the overall proangiogenic nature of tumors. However, blood flow through the tortuous vessels in tumors is known to be slow, erratic, and often static, which could contribute to the rapid local depletion of angiogenic growth factors. Tumor ECs may also have to compete for growth factors with tumor cells that often express VEGF and/or FGF receptors themselves and can sometimes utilize angiogenic growth factors for their own growth (Dallas et al., 2007; Masood et al., 2001). Finally, hypoxia, a well known inducer of VEGF gene transcription, may also lead to overexpression of the high affinity VEGFR1 (Gerber et al., 1997) or its soluble splice variant sVEGFR1 that can act as decoy receptors, limiting VEGF bioavailability (Lamszus et al., 2003, Yamaguchi et al., 2007, Yang et al., 2011a). Elevated TEM8 expression in tumor ECs in response to growth factor deprivation and possibly other unidentified microenvironmental stressors may be part of a survival pathway that helps ECs cope during suboptimal growth conditions.
Tem8 KO mice provide a valuable tool for assessing the role of TEM8 in pathological angiogenesis. For most pharmacological angiogenesis inhibitors, it is difficult to find animal models completely lacking the drug target, because the target proteins are usually required for developmental angiogenesis and temporally induced deletion of a conditional “floxed” target gene in adult mice using cre-lox technology is often incomplete. Importantly, by treating Tem8 WT or KO mice with L2 anti-TEM8 antibody we could verify that TEM8 is the target of this antibody in vivo, and that L2 treatment inhibits tumor growth to a level similar to complete genetic ablation. Further evidence for antibody specificity was obtained using FITC-labeled L2 that selectively reacted with the tumor vessels in Tem8 WT but not KO mice.
Multiple mechanisms could potentially contribute to the anti-tumor activity of L2 in vivo, but so far the evidence suggests that the antibodies work primarily by blocking TEM8 function and that ADCC and CDC may play a more limited role. The extent of tumor growth delay observed in the Tem8 KO was found to vary depending on the tumor type employed, but the same tumor type-dependent responses were observed following L2 blockade. For example, UACC tumors consistently displayed the most pronounced growth delay in Tem8 KO versus WT mice, and were also the most responsive to L2. Indeed, the tumor growth patterns observed in the Tem8 KO mice were found to be indistinguishable from those observed in the L2-treated Tem8 WT mice provided that L2 treatment began immediately following tumor cell inoculation (for example, see Figure 5A). It is currently unclear why some tumor types rely more on host-derived TEM8 than others, but the degree of TEM8 dependence does not appear to correlate with the tumor cells ability to evoke TEM8 expression in nearby tumor-associated ECs. For example, in Tem8 WT mice tumor ECs isolated from LLC tumors expressed 4-times more Tem8 than those isolated from B16 tumors, yet comparisons of tumor growth in Tem8 WT and KO mice revealed that B16 tumors are more dependent on host-derived TEM8 than LLC cells (Cullen et al., 2009). We expect that if ADCC and CDC were the major mechanisms governing tumor responses in vivo then tumor responsiveness would have correlated with TEM8 expression levels in tumor ECs, because ADCC and CDC both depend on target antigen expression levels. Therefore, further studies are required to establish if ADCC and CDC contribute significantly to L2’s activity in vivo. Nevertheless, affinity maturation of the variable domain and modifications to the Fc domain that enhance ADCC and CDC activity (Natsume et al., 2009) could lead to further enhancement of anti-tumor efficacy.
While anti-TEM8 antibodies inhibited tumor growth as a monotherapy, based on our results we predict that TEM8 antibodies may be most useful in combination with other agents. TEM8 antibodies were completely non-toxic and displayed efficacy when combined with various classes of anti-cancer agents. That anti-TEM8 antibodies augment the activity of VEGFR2 neutralizing antibodies suggests that signaling pathways involving TEM8 may be responsible, at least in part, for angiogenesis that persists following VEGF/VEGFR2 inhibition. Although human-mouse chimeric antibodies were employed in the preclinical studies described here, reengineering of the Fc domain can be used to make the IgG fully human for future clinical development.
In summary, we report the development of anti-TEM8 antibodies that retard tumor growth by inhibiting tumor angiogenesis. Anti-TEM8 antibodies were non-toxic and maintained efficacy in combination with various classes of anti-cancer agents. Thus, anti-TEM8 antibodies provide a rationally designed tool for selectively inhibiting pathological angiogenesis with important ramifications for the management of angiogenesis-dependent diseases.
EXPERIMENTAL PROCEDURES
Antibody Production and Purification
In vitro selection of the Morphosys HuCAL Gold phage library involved two rounds of sequential panning on biotinylated, purified recombinant TEM8(ED)-Fc fusion proteins, prepared as described in the Supplemental Information, and one round of panning on HEK293 cells transfected with human TEM8 (293/Flagh-TEM8). DNA inserts for the Fab heavy and light chains were subcloned, expressed, and bivalent Fabs evaluated for TEM8-binding by ELISA (see Supplemental Information). Two of the TEM8-binding clones (L2 and L5) were reformatted to generate mouse/human chimeric full IgGs. Anti-TEM8 antibodies were collected from HEK293T culture supernatants and purified by Protein A and size exclusion chromatography.
Western Blotting
Western blotting was performed using antibodies against TEM8 (clone SB5; Nanda et al., 2004), CMG2 (a kind gift from Dr. Steve Leppla), actin (Chemicon) or HIF-1α (Novus Biologicals) as previously described (Cullen et al., 2011).
Animal and Tumor Studies
To derive Tem8 KO mice on an immunodeficient background, Tem8 KO mice on a C57BL/6 background (Cullen et al., 2009) were crossed with athymic NCr-nu/nu mice and only Tem8 WT and KO littermates derived from Tem8 heterozygous intercrosses were used for comparison. Tumors were measured with a caliper, and tumor volumes calculated using the formula LxW2×0.5 and presented as the mean ±SE. All animal studies were carried out in accordance with protocols approved by the NCI animal care and use committee.
Immunofluorescence
For in vivo target identification, FITC-labeled L2 was co-injected with non-specific mouse IgG intraperitoneally into DLD1 tumor-bearing Tem8 WT and KO mice. Frozen sections were labeled with rat anti-PV-1 (Meca-32) or rat anti-CD31 (BD) antibodies. For immunofluorescence staining of human normal colonic mucosa or colorectal cancer, frozen tissue sections were blocked with non-specific mouse/human chimeric IgG antibodies (mouse Fc / human-Fab) generated against cyclosporine A and detected with L2-labeled FITC. The anonymized human colon tissue samples were obtained from the Cooperative Human Tissue Network (CHTN), with approval from the NIH Office of Human Subject Research. Further details regarding the immunofluorescence staining can be found in the Supplemental Information.
Statistical Analysis
A Students t-test was used to calculate differences in tumor volumes or weights between two groups (for example, Tem8 WT and KO mice) at the time when the WT (or control) group reached its maximum size and had to be euthanized. For comparisons between multiple tumor groups, a one-way ANOVA was used with a Bonferroni post-test. A one-way ANOVA was used for comparisons of microvascular densities and the fraction of CD31-positive cells by flow cytometry. p-values <0.05 were considered significant.
Significance.
Inhibiting angiogenesis has become an important adjunct to traditional anti-cancer therapy, but current anti-angiogenic agents, including VEGF/VEGFR2 pathway inhibitors, disrupt normal physiological processes and are associated with an increasing number of adverse side effects. TEM8 is an appealing target for selective inhibition of tumor angiogenesis because it is functionally required for optimal tumor angiogenesis and growth, but dispensable for normal development and physiological angiogenesis. Function blocking antibodies specific to the extracellular domain of TEM8 blocked pathological angiogenesis and tumor growth, and augmented the activity of various classes of anti-cancer agents, including VEGFR inhibitors. Thus, targeting TEM8 on tumor vasculature may provide opportunities for the selective blockade of cancer and other diseases dependent on pathological angiogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Bert Vogelstein, Terry Van Dyke, Lino Tessarollo and Isaiah J. Fidler for helpful suggestions. We thank Rou-Fun Kwong for help with the initial selection and characterization of anti-TEM8 antibodies. We thank Dr. Stephan H. Leppla, NIAID, NIH, for the CHO, CHO/TEM8 cells and CHO/CMG2 cells, and recombinant PA and lethal factor, and Dr. Arthur E. Frankel for the AF334 hybridoma. This work was supported in part by a Cooperative Research and Development Agreement (CRADA) between the Novartis Institutes for BioMedical Research and the intramural research program of the National Cancer Institute (NCI), National Institutes of Health, Department of Health and Social Services (DHSS), and with federal funds from the NCI under Contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the DHSS nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information Includes Supplemental Experimental Procedures, 4 figures and one table, and can be found with this article online.
REFERENCES
- Baguley BC, McKeage MJ. ASA404: a tumor vascular-disrupting agent with broad potential for cancer therapy. Future Oncol. 2010;6:1537–1543. doi: 10.2217/fon.10.122. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Batchelor TT. Vascular endothelial growth factor inhibitors in malignant gliomas. Target Oncol. 2010;5:167–174. doi: 10.1007/s11523-010-0158-1. [DOI] [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas NA, Fan F, Gray MJ, Van Buren G, 2nd, Lim SJ, Xia L, Ellis LM. Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 2007;26:433–441. doi: 10.1007/s10555-007-9070-2. [DOI] [PubMed] [Google Scholar]
- Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, Borel Rinkes IH. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002;236:703–711. doi: 10.1097/00000658-200212000-00002. discussion 711-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan HF, Hu XW, Chen JL, Gao LH, Xi YY, Lu Y, Li JF, Zhao SR, Xu JJ, Chen HP, et al. Antitumor activities of TEM8-Fc: an engineered antibody-like molecule targeting tumor endothelial marker 8. J Natl Cancer Inst. 2007;99:1551–1555. doi: 10.1093/jnci/djm132. [DOI] [PubMed] [Google Scholar]
- Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17:724–735. doi: 10.1681/ASN.2005080810. [DOI] [PubMed] [Google Scholar]
- Felicetti P, Mennecozzi M, Barucca A, Montgomery S, Orlandi F, Manova K, Houghton AN, Gregor PD, Concetti A, Venanzi FM. Tumor endothelial marker 8 enhances tumor immunity in conjunction with immunization against differentiation Ag. Cytotherapy. 2007;9:23–34. doi: 10.1080/14653240601048369. [DOI] [PubMed] [Google Scholar]
- Fernando S, Fletcher BS. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer Res. 2009;69:5126–5132. doi: 10.1158/0008-5472.CAN-09-0725. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamszus K, Ulbricht U, Matschke J, Brockmann MA, Fillbrandt R, Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, Moayeri M, Basile JR, Alfano RW, Gutkind JS, Frankel AE, et al. Matrix Metalloproteinase-activated Anthrax Lethal Toxin Demonstrates High Potency in Targeting Tumor Vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj AS, D'Amore PA. Roles for VEGF in the adult. Microvasc Res. 2007;74:100–113. doi: 10.1016/j.mvr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Reeves CV, Dufraine J, Young JA, Kitajewski J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene. 2010;29:789–801. doi: 10.1038/onc.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau C, Menon K, Boutin P, Guyre C, Yoshida H, Kataoka S, Perricone M, Shankara S, Frankel AE, Duesbery NS, et al. The systemic administration of lethal toxin achieves a growth delay of human melanoma and neuroblastoma xenografts: assessment of receptor contribution. Int J Oncol. 2008;32:739–748. [PubMed] [Google Scholar]
- Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, Guo S, Han J, Liang H, Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Sung HK, Michael IP, Nagy A. Multifaceted role of vascular endothelial growth factor signaling in adult tissue physiology: an emerging concept with clinical implications. Curr Opin Hematol. 2010;17:206–212. doi: 10.1097/MOH.0b013e32833865e6. [DOI] [PubMed] [Google Scholar]
- Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Bando H, Mori T, Takahashi K, Matsumoto H, Yasutome M, Weich H, Toi M. Overexpression of soluble vascular endothelial growth factor receptor 1 in colorectal cancer: Association with progression and prognosis. Cancer Sci. 2007;98:405–410. doi: 10.1111/j.1349-7006.2007.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Jin C, Jiang YJ, Li J, Di Y, Fu DL. Potential role of soluble VEGFR-1 in antiangiogenesis therapy for cancer. Expert Rev Anticancer Ther. 2011a;11:541–549. doi: 10.1586/era.10.171. [DOI] [PubMed] [Google Scholar]
- Yang MY, Chaudhary A, Seaman S, Dunty J, Stevens J, Elzarrad MK, Frankel AE, St Croix B. The cell surface structure of tumor endothelial marker 8 (TEM8) is regulated by the actin cytoskeleton. Biochim Biophys Acta. 2011b;1813:39–49. doi: 10.1016/j.bbamcr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhu H, Hu Z. Dendritic cells transduced with TEM8 recombinant adenovirus prevents hepatocellular carcinoma angiogenesis and inhibits cells growth. Vaccine. 2010;28:7130–7135. doi: 10.1016/j.vaccine.2010.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.