Abstract
Our objective was to determine whether atrial fibrillation (AF) results from excessive activation of intrinsic cardiac neurons (ICNs) and, if so, whether select subpopulations of neurons therein represent therapeutic targets for suppression of this arrhythmogenic potential. Trains of five electrical stimuli (0.3–1.2 mA, 1 ms) were delivered during the atrial refractory period to mediastinal nerves (MSN) on the superior vena cava to evoke AF. Neuroanatomical studies were performed by injecting the neuronal tracer DiI into MSN sites that induced AF. Functional studies involved recording of neuronal activity in situ from the right atrial ganglionated plexus (RAGP) in response to MSN stimulation (MSNS) prior to and following neuromodulation involving either preemptive spinal cord stimulation (SCS; T1–T3, 50 Hz, 200-ms duration) or ganglionic blockade (hexamethonium, 5 mg/kg). The tetramethylindocarbocyanine perchlorate (DiI) neuronal tracer labeled a subset (13.2%) of RAGP neurons, which also colocalized with cholinergic or adrenergic markers. A subset of DiI-labeled RAGP neurons were noncholinergic/nonadrenergic. MSNS evoked an ∼4-fold increase in RAGP neuronal activity from baseline, which SCS reduced by 43%. Hexamethonium blocked MSNS-evoked increases in neuronal activity. MSNS evoked AF in 78% of right-sided MSN sites, which SCS reduced to 33% and hexamethonium reduced to 7%. MSNS-induced bradycardia was maintained with SCS but was mitigated by hexamethonium. We conclude that MSNS activates subpopulations of intrinsic cardiac neurons, thereby resulting in the formation of atrial arrhythmias leading to atrial fibrillation. Stabilization of ICN local circuit neurons by SCS or the local circuit and autonomic efferent neurons with hexamethonium reduces the arrhythmogenic potential.
Keywords: neurocardiology, atrial fibrillation, cardiac nervous system, spinal cord stimulation, ganglionic blockade
excessive activation of select inputs to the intrinsic cardiac nervous system (ICN) are known to elicit atrial arrhythmias in normal (9, 22, 28) and pathophysiological states (11, 30). Discrete activation of the axons in select mediastinal nerves can reproducibly elicit self-terminating periods of atrial tachyarrhythmias/fibrillation (ATF) (9). Mediastinal nerves are made up of sympathetic and parasympathetic efferent neuronal inputs into the intrinsic cardiac nervous system, as well as afferent axons arising from cardiac tissues (9). They likewise contain interganglionic connections mediated via local circuit neuronal projections (15), which subserve, in part, to coordinate peripheral reflex function (7, 34). Because excessive activation of the axons in select mediastinal nerves reproducibly elicits self-terminating periods of ATF, this animal model has been employed to study the neuropharmacological basis of neurally evoked atrial arrhythmias (9, 28).
The extent of involvement of various ICN neuronal populations in mediating atrial fibrillation (AF) has yet to be determined. Secondly, whether neuronally induced atrial arrhythmias involve excessive activation of select populations within the intrinsic cardiac neurons remains unknown. Since neuromodulation therapy has been shown to suppress the ability of mediastinal nerves to induce AF (10), it has been postulated that spinal cord stimulation (SCS) acts to suppress the responsiveness of the ICN to excessive sensory inputs arising from the diseased myocardium in the induction of such arrhythmias (10, 13). Likewise, pharmacological neuromodulation that blocks neuronal transmission within the ICN has been shown to decrease the propensity of ATF formation secondary to mediastinal nerve stimulation (9, 28). While these neuromodulation therapies are known to act upon the peripheral autonomic nervous system (4, 13), how they target the ICN to reduce ATF formation initiated by excessive mediastinal nerve stimulation has yet to be determined.
To understand how neurons within the ICN respond to excessive inputs from extracardiac sources (as mimicked by mediastinal nerve stimulation) in the induction of ATF, the present study was designed to test the following hypotheses: 1) mediastinal nerve stimulation activates select neuronal populations within the ICN in the induction of ATF; and 2) stabilization of those populations within the intrinsic cardiac nervous system via neuromodulation (electrical or pharmacological) blunts the capacity of the ICN to respond to excessive inputs and thereby suppresses its potential to induce atrial tachyarrhythmias. This study identified the fact that excessive inputs to the ICN in the induction of ATF does, indeed, primarily involve excessive activation of local circuit neurons therein, sparing for the most part direct efferent outflows to the heart.
METHODS
Animal preparation.
Thirty mongrel dogs (either sex), weighing 18.6–26.9 kg, were used in this study. All experiments were performed in accordance with the guidelines for animal experimentation described in the “Guiding Principles for Research Involving Animal and Human Beings” (1). The Institutional Animal Care and Use Committee of the East Tennessee State University approved these experiments.
Neuronal tracer injection.
Animals (n = 2) were premedicated with sodium thiopental (15 mg/kg iv), intubated, and anesthetized using 2% isoflurane. Heart rate and blood pressure were continuously monitored (Surgivet Advisor Monitor, Smiths Medical) with depth of anesthesia determined by corneal reflex, jaw tone, and hemodynamic parameters. Body temperature was maintained via a circulating water heating pad (Gaymar T/Pump, Gaymar Industries, Orchard Park, NY). Using aseptic techniques, we performed a right thoracotomy at the 4th intercostal space; an incision was made into the pericardial sac to expose the superior vena cava at the pericardial reflection into the right atrium, and a pericardial cradle formed. Using techniques described previously (9, 28), we identified mediastinal nerve projection sites on the superior vena cava at the pericardial reflection, in which train electrical stimuli delivered during the atrial refractory period reproducibly induced transient periods of atrial fibrillation. Ten-microliter boluses of 0.1 M solution of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) were then injected via a 1 cc Hamilton syringe into that site. Following tracer injection, the pericardium and thoracic cavity were closed in layers, and residual air was removed from the chest via a chest tube. Buprenorphine (0.01 mg/kg im or iv) was administered preoperatively and postoperatively for pain management. Antibiotic therapy (intramuscular cefazolin or oral cephalexin, 22 mg/kg) was administered twice daily prior to surgery and continued for 2 days postoperatively. Animals were recovered for 2 days following surgery to allow for transport of the tracer. The animals were reanesthetized in the manner described above, and a thoracotomy was performed to collect the right atrial ganglionated plexus (RAGP), an aggregate of intrinsic cardiac neurons associated with control of atrial electrical and mechanical function (5, 27).
Immunohistochemistry.
The RAGP cardiac ganglia were briefly washed in saline and then transferred to a 4°C fixative solution containing 4% paraformaldehyde and 0.2% picric acid in PBS (pH 7.3). Tissues were fixed at 4°C for 24 h. They were then transferred to 20% sucrose in PBS (4°C, 4 days) for cryoprotection, frozen on powdered dry ice, and stored in sealed tubes at −80°C until processed. The ganglia were sectioned at a thickness of 16 μm using a Leica CM 3050S cryostat (Leica Microsystems, Bannockburn, IL). Adjacent sections were collected on separate chrome-alum gelatin-coated slides, such that six representative sets of tissue sections were obtained from each tissue sample. Slide-mounted sections were stored in closed slide boxes at −20°C prior to immunohistochemistry. Single- and double-labeling of sections were studied at room temperature using standard methods previously described (19). Digitonin was used in solution instead of Triton X-100 to minimize loss of DiI during processing (24). Briefly, slides were rinsed with 0.1 M PBS (pH 7.3), permeabilized in PBS containing 1,000 μg/ml digitonin (cat. no. 300411, EMD Biosciences, San Diego, CA) and 0.5% BSA, and blocked for 2 h in PBS containing 10% normal donkey serum (cat. no. S30; Millipore, Billerica, MA), 1% BSA, and 1,000 μg/ml digitonin. Tissues were then incubated overnight in buffer containing either rabbit anti-Protein Gene Product 9.5 (PGP 9.5, AB1761; Millipore, Billerica, MA), or various combinations of sheep anti-tyrosine hydroxylase (anti-TH; AB1542, Millipore, Billerica, MA), goat/rabbit anticholine acetyltransferase (ChAT, AB144P; Millipore and AB-N34, Advanced Targeting Systems, San Diego, CA) or guinea pig antivesicular ACh transporter (VAChT, AB1588; Millipore). After being washed with PBS, tissues were incubated for 2 h with donkey anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 and donkey anti-sheep secondary antibodies conjugated to Alexa Fluor 647 (A-21206, A-21448; Invitrogen, Carlsbad, CA) and washed with PBS. Coverglasses were attached with Citifluor mounting medium (Ted Pella, Redding, CA) and sealed with clear nail polish. Negative control slides were processed similarly without using primary antibodies. The selectivity of fluorescence filters and the presence of colocalized markers were confirmed in several sections obtained from each animal by sequential scanning with a Leica TCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL). The software used to discriminate DiI-positive neuronal somata from background was ImageJ. This was performed on images obtained by confocal microscopy. The neurochemical phenotype of DiI-labeled neurons was identified on the basis of overlay confocal images.
Hemodynamic recording.
In the remaining canines (n = 28), animals were anesthetized and instrumented for recording of neuronal activity from the RAGP ganglia. Responses (neural and atrial electrical activity) to mediastinal nerve stimulation were evaluated prior to and following neuromodulation using either ganglionic blockade (n = 12) or spinal cord stimulation (n = 16) (see below). Anesthesia was maintained during surgery using 2% isoflurane with depth of anesthesia determined by corneal reflex, jaw tone, and hemodynamic parameters. Following completion of the surgery, anesthesia was changed to α-chloralose (75 mg/kg iv bolus), with repeat doses (12.5 mg/kg iv) administered as required throughout the duration of each study. Body temperature was maintained via a circulating water heating pad (Gaymar T/Pump, Gaymar Industries, Orchard Park, NY). The left femoral artery and vein were catheterized to record arterial blood pressure and to allow for anesthetic, fluid replacement, and pharmacological agent delivery, respectively. The right femoral artery was catheterized, and left ventricular pressure was measured via a Mikro-Tip Pressure Transducer Catheter (Millar Instruments, Houston, TX). Heart rate was monitored via a Lead II electrocardiogram.
Spinal cord stimulation.
For the spinal cord stimulation (SCS) treatment group, each animal was placed in the prone position, and the spinal epidural space was penetrated percutaneously with a Touhy needle through a small skin incision in the caudal dorsal thorax, as detailed previously (4). An eight-pole lead (Octrode, Advanced Neuromodulation Systems, Plano, TX) was advanced rostrally in the epidural space to the T1–T3 spinal cord level. The tip of the lead was positioned slightly to the left of midline using anterior-posterior fluoroscopy. The rostral pole was positioned at T1, while the caudal pole was positioned at T3 level. Proper electrode placement was determined via electrical current delivered to the rostral and caudal poles using a stimulus isolation unit and constant current generator (PSIU6; Grass Instruments, Quincy, MA) connected to a stimulator (S88, Grass Instruments). SCS was delivered at 50-Hz and 200-μs duration, stimulus parameters that have previously been shown to induce neuromodulation of extracardiac, intrathoracic, and intrinsic cardiac ganglia neurons during periods of acute cardiac stress (4, 13). Motor threshold (MT) intensity was determined as the lowest current that induced muscle contractions in the proximal forepaw and shoulder. Animals were rotated to the supine position for the experimental protocols with MT rechecked in this position. MT was checked periodically during the experiments and did not vary significantly from initial levels (0.25 ± 0.02 mAmp).
Mediastinal nerve stimulation.
Following thoracotomy, an incision was made into the pericardial sac, and a pericardial cradle formed. A bipolar electrode was affixed to the right atrium, 1 cm dorsal to the SA node to record atrial electrograms. Right-sided mediastinal nerves were identified visually on the ventral and ventrolateral surface of the intrapericardial aspects of the superior vena cava and then stimulated using techniques detailed elsewhere (9, 28). Briefly, trains of five electrical stimuli (0.3–1.2 mA, 1-ms duration, 5-ms pulse interval) were delivered for up to 20 s to select mediastinal sites during the refractory period on each atrial beat, as triggered off the reference atrial electrogram. This was done to avoid direct atrial capture. Electrical stimuli were delivered via a roving bipolar probe electrode (1.5-mm spacing) connected to a constant current generator (PSIU6, Grass Instruments, Quincy, MA) affixed to a Grass S88 stimulator (S88, Grass Instruments). The stimulator was externally controlled by a computer running LabChart5 (ADInstruments, Colorado Springs, CO) interfacing with a PowerLab 4/30 (ADInstruments) and triggered by atrial wavefront detections. Active sites were identified by the creation of atrial tachyarrhythmias (including atrial fibrillation) when exposed to focal electrical stimuli. Each active site was marked with India ink for repeat stimulation. Two to four active sites were identified in each animal. Contact between the bipolar electrodes and tissue was discontinued immediately after the onset of the atrial tachyarrhythmia.
Neuronal recording.
Activity generated by neurons in the RAGP was recorded in situ, as reported previously (3, 14). Briefly, to decrease epicardial motion during each cardiac cycle, a circular ring of stiff wire was placed gently on the epicardial fat on the ventral surface of the right atrium—a region known to contain the RAGP. A tungsten microelectrode (250-μm diameter and exposed tip of 1 μm; impedance of 9–11 MΩ at 1,000 Hz), mounted on a micromanipulator, was lowered into this fat using a microdrive. The ganglionated plexus therein was explored, and electrical signals so derived were led into a differential preamplifier (BMA-831, CWE, Ardmore, PA) with a high-impedance head stage (bandwidth set at 300 to 10 kHz). Signals were further amplified by a battery-driven preamplifier (5113 Pre-Amp, Signal Recovery, Oak Ridge, TN) (bandwidth 100 Hz to 2 kHz). Amplified neuronal signals, together with recorded cardiovascular indices, were digitized (Cambridge Electronics Design, power 1401 data acquisition system) and analyzed using the Spike 2 software package (Cambridge Electronics Design).
Neuronal activity was identified as action potentials with signal-to-noise ratios greater than 3:1. The activity generated by individual neuronal somata was identified by the amplitude and configuration (waveform recognition) of these recorded action potentials, using the Spike 2 program. By using these techniques and criteria, action potentials generated by individual somata and/or dendrites, rather than axons of passage, can be recorded for extended periods of time (3, 4, 14).
Treatment groups.
Animals were divided randomly into two neuromodulation treatment groups, one that had SCS electrode implantation (n = 16) and another that evaluated the effects of ganglionic blockade on arrhythmia responses evoked by mediastinal nerve stimulation (MSNS; n = 12). For each of these animals, 2 or 3 mediastinal sites were first identified that reproducibly evoked atrial arrhythmias. In 8 of these animals (4 from each group), time controls were done to verify reproducibility of evoked responses to MSNS over time. For the SCS treatment group, electrical stimuli (50 Hz, 200-μs duration, 90% motor threshold) were delivered to the T1–T3 spinal level for 20 min. Responses to MSNS stimulation were evaluated prior to and 5–30 min following SCS. For the hexamethonium treatment group, responses to MSNS stimulation were evaluated prior to and 15 min after hexamethonium administration (5 mg/kg iv).
Data analysis.
Action potentials arising from a site within each RAGP studied were characterized by means of their differing amplitudes and configurations (waveform recognition via the Spike2 program: Cambridge Electronic Design, Cambridge, England). Neuronal activity responses elicited by MSNS were evaluated by comparing activity generated immediately before stimulation with data obtained during nerve stimulation; neuronal activity data are presented as impulses per second (imp/s). Characterization of atrial rhythms evoked by MSNS included latency and duration of atrial arrhythmias; this analysis was performed offline using Spike 2. Data are expressed as means ± SE. Sigmastat 3.1 (Systat software) was used to test for differences between groups by ANOVA with post hoc comparisons (Holm-Sidak or Tukey method for comparisons, depending on results of normality tests). A significance of P < 0.05 was used for these experiments.
RESULTS
Neuroanatomical data.
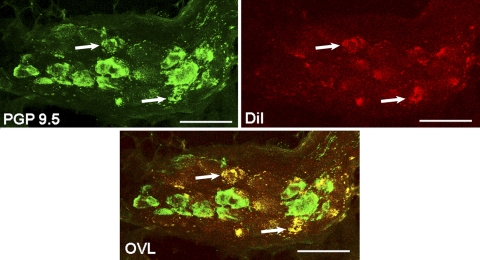
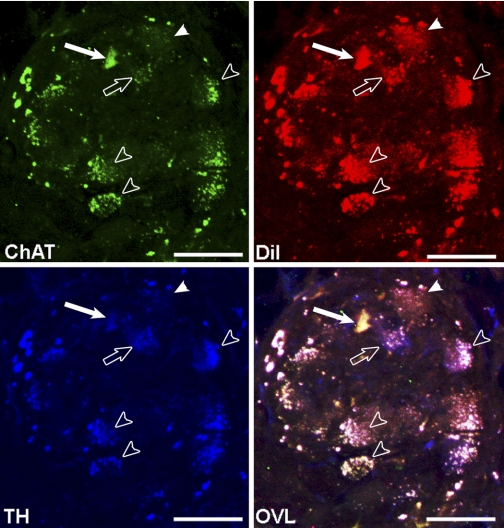
Select mediastinal nerve projections to the right atrial ganglionated plexus were identified that, when subjected to trains of focal electrical stimuli delivered during the atrial refractory period, reproducibly induce transient periods of atrial fibrillation. DiI was then microinjected into the wall of the superior vena cava at that nerve site. RAGP tissues harvested 2 days after application of DiI to that mediastinal nerve site identified 13.2% of RAGP neuronal soma labeled. PGP 9.5 (green) labeled all neuron soma in these RAGP. Some neuronal somata demonstrated colocalization of PGP 9.5 and DiI (Fig. 1). The neurochemical profile of DiI-labeled neurons within these RAGP demonstrated that 16.7% of DiI-labeled neurons also labeled for cholinergic marker choline acetyltransferase (ChAT) (Fig. 2). 22.2% of DiI-labeled neurons labeled for the noradrenergic marker tyrosine hydroxylase (TH); 55.6% of DiI-labeled neurons colocalized with markers for ChAT and TH. The remaining 5.5% of DiI-labeled neurons did not exhibit either noradrenergic or cholinergic markers.
Fig. 1.
Characterization of neuronal cell bodies in the right atrial ganglionated plexus (RAGP), labeled with the tracer (DiI) injected into right-sided mediastinal nerve sites, where electrical stimulation (mediastinal nerve stimulation, or MSNS) induced atrial fibrillation (AF). PGP 9.5 labeled all intrinsic cardiac neurons (ICN) green and DiI-labeled neurons projecting from MSNS-responsive sites in red. Colocalization of PGP 9.5 and DiI is indicated by yellow (arrows indicate double-labeled soma) in the overlap figure (OVL). 13.2% of PGP 9.5-labeled neurons within the RAGP colocalized with DiI. Scale bar = 75 μm.
Fig. 2.
Neurochemical profile of RAGP neurons labeled with the tracer (DiI) injected into right-sided mediastinal nerve sites, where electrical stimulation (MSNS) induced AF. Neurons were immunolabeled for choline acetyltransferase (ChAT; green label), tyrosine hydroxylase (TH; blue label), and DiI (red label). Solid arrow indicates colocalization of ChAT and DiI (16.7% of neurons). Open arrow indicates colocalization of TH and DiI (22% of neurons). Open arrowheads indicate colocalization of DiI with both ChAT and TH (55.6% of neurons). Solid arrowhead indicates a DiI neuron that did not label with either TH or ChAT markers (5.5% of neurons). Scale bar = 40 μm.
Intrinsic cardiac neuronal activity.
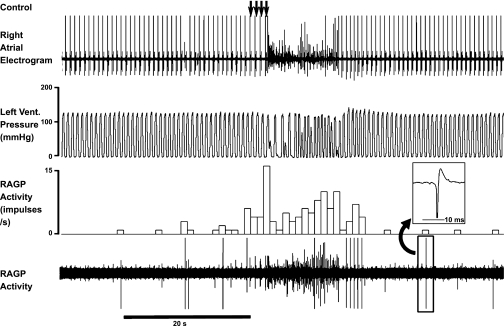
The activity generated by RAGP neurons increased in response to MSNS (Fig. 3). Mediastinal nerve stimuli induced an initial bradycardia (average 23.7 ± 3.5% change from baseline cycle length) that transitioned rapidly to atrial fibrillation/flutter (AF/AFl). The average latency of such responses was 1.3 ± 0.2 s from stimulus onset; the average duration of AF/AFl was 26.4 ± 6.0 s. Intrinsic cardiac neuronal activity increased in association with stimulus onset; neuronal activity increased further during the induction of AF. This enhancement of activity persisted during the initial period following sinus rhythm restoration (Fig. 3). On average, MSNS evoked ∼4-fold increase in neuronal activity (Fig. 5, left panels; 0.29 ± 0.08 imp/s to 1.20 ± 0.16 imp/s, P < 0.001).
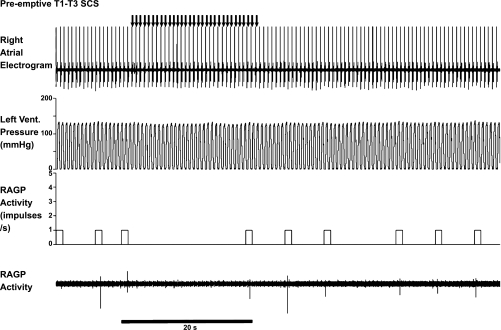
Fig. 3.
RAGP neuronal activity and corresponding cardiac electrical activity in response to MSNS. Arrows indicate delivery of four successive burst stimuli to a right-sided mediastinal nerve located on the superior vena cava at the level of the pericardial reflection. MSNS induced an increase in RAGP neuronal activity, which persisted after stimulus cessation. Neuronal activity was further enhanced once the arrhythmia was induced. Vent., ventricle; RAGP activity, right atrial ganglionated plexus neuronal activity. Trace three (activity in impulses per second) indicates ongoing 1-s bin summed activities. Note that during burst stimuli, heart rate was reduced from 83 to 76 beats per minute (bpm) immediately prior to initiation of atrial fibrillation. Inset: expanded trace for one of the RAGP neurons recorded.
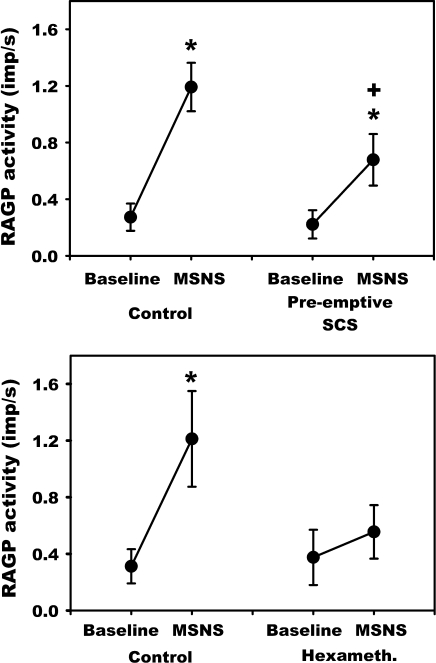
Fig. 5.
Effects of MSNS on RAGP neuronal activity recorded prior to and following preemptive SCS (top) or hexamethonium administration (bottom). MSNS initiated a ∼4-fold increase in neuronal activity. Preemptive SCS blunted the ability of MSNS to increase neuronal activity. Hexamethonium blocked MSNS evoked increase in neuronal activity. *P < 0.002 vs. baseline; +P < 0.001 vs. control.
Neuronal responses evoked by MSNS were altered by both preemptive SCS and nicotinic receptor blockade. While, SCS did not decrease basal neuronal activity (Fig. 4 and Fig. 5, top; 0.27 ± 0.10 imp/s vs. 0.22 ± 0.10 imp/s), MSNS-induced changes in RAGP activity was suppressed significantly after 20 min of preemptive SCS (1.19 ± 0.17 to 0.68 ± 0.18 imp/s, P < 0.001) (Fig. 5, top). Hexamethonium administration likewise did not affect basal neuronal activity (Fig. 5, bottom) yet completely suppressed the MSNS-evoked increase in RAGP neuronal activity (0.38 ± 0.20 to 0.55 ± 0.19 imp/s; P = 0.143) (Fig. 5, bottom).
Fig. 4.
Neuronal activity recorded from the same RAGP site depicted in Fig. 3 demonstrating the effects of MSNS repeated 5 min after 20-min preemptive spinal cord stimulation (SCS). Note that following SCS, MSNS was ineffectual in evoking atrial arrhythmias.
MSNS-induced bradycardia.
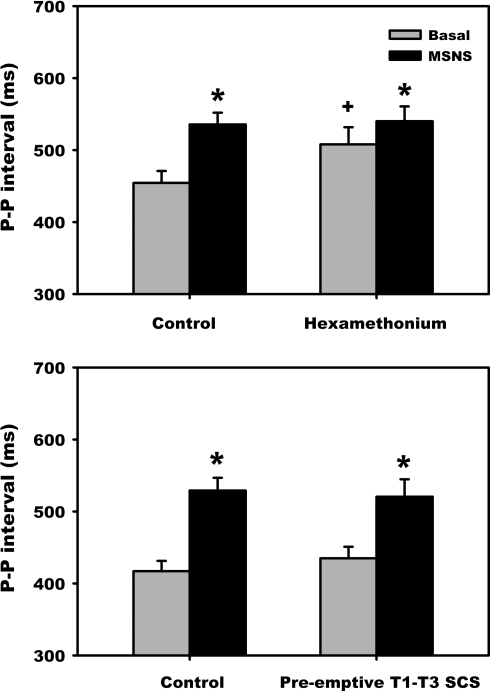
Figure 6 summarizes the effects of hexamethonium (top) or preemptive SCS (bottom) on the magnitude of the initial MSNS-evoked bradycardia. In the hexamethonium-treated group, while basal cardiac cycle length increased (454.3 ± 16.6 vs. 507.9 ± 20.5 ms, P = 0.009), the absolute MSNS-evoked bradycardia was similar over time (535.6 ± 16.3 vs. 540.1 ± 20.5 ms, P = 0.78). Moreover, even following nicotinic receptor blockade, MSNS continued to increase cardiac cycle length (507.9 ± 23.8 baseline to 540.1 ± 20.5 MSNS, P < .001), albeit at a lesser magnitude compared with control. In contrast, preemptive SCS did not affect the MSNS-evoked bradycardia (23.7 ± 3.5%: 433.6 ± 11.4 to 532.0 ± 11.9 ms, P < 0.001).
Fig. 6.
Mediastinal nerve stimulation-induced an initial atrial bradycardia before and following hexamethonium administration (top) or preemptive SCS (bottom). *P < 0.001 vs. basal; +P < 0.01 vs. control.
Time controls.
Time controls for both treatment groups exhibited similar MSNS evoked changes in RAGP neuronal activity (0.21 ± 0.05 to 1.00 ± 0.40 imp/s vs. 0.15 ± 0.04 to 1.02 ± 0.11 imp/s, basal to MSNS). Time controls also demonstrated consistent responses in basal cycle length (420.3 ± 9.6 ms vs. 415.8 ± 7.9 ms, P = 0.99) and equivalent evoked changes in cardiac cycle length with MSNS onset (526.1 ± 22.2 ms vs. 507.3 ± 20.9 ms; P = 0.70).
Atrial rhythms.
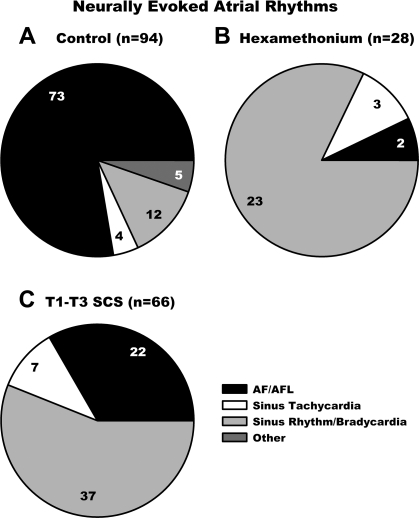
Atrial rhythm responses to MSNS were studied before (A: control 94 active sites) and following either hexamethonium administration (B: 28 of the 94 sites) or preemptive SCS (C: 66 of the 94 sites). In control states, the predominant atrial rhythm evoked by right-sided MSNS was AF/AFl (77.7%); sinus bradycardia (12.8%), sinus tachycardia (4.2%), and other rhythms (5.3%) were induced less frequently. Time controls demonstrated no significant change in these response characteristics. Following ganglionic blockade, MSNS evoked AF/AFl from only 7.1% of the nerve sites stimulated (Fig. 7B); sinus rhythm/bradycardia was the predominant rhythm (82.2% of sites).
Fig. 7.
Types of atrial rhythms evoked during mediastinal nerve stimulation prior to (A, control) and following treatment with hexamethonium (B) or preemptive T1-T3 SCS (C). The number of stimulation sites in each group is indicated above each chart; the number of arrhythmias elicited in each subgrouping is also indicated. AF/AFL, atrial fibrillation/atrial flutter.
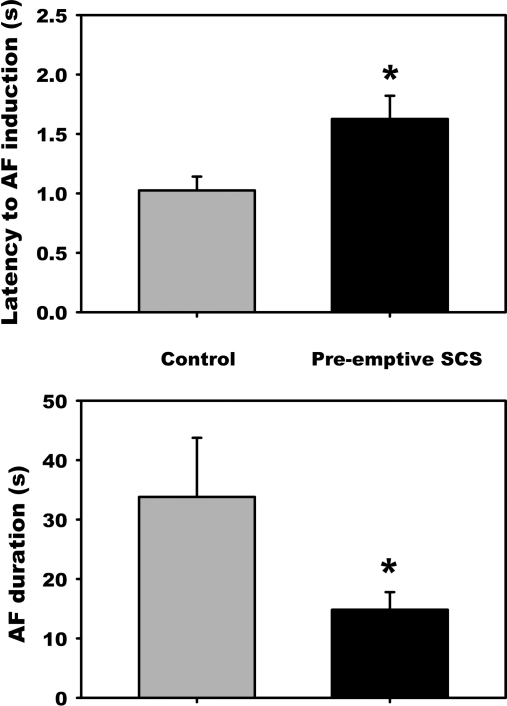
Following preemptive SCS, the predominant atrial rhythm evoked by MSNS shifted to sinus bradycardia (56.2%). Not only did preemptive SCS reduce the number of mediastinal sites that evoked AF (77.7% to 33.3%, Fig. 7), but for the residual active sites, the latency to evoke such AF increased (1.03 ± 0.12 to 1.63 ± 0.20 s; P = 0.004), and duration of such evoked AF decreased (33.8 ± 9.9 to 14.8 ± 3.0 s; P = 0.047) (Fig. 8). Time controls exhibited no change in the latency to AF onset (1.26 ± 0.29 to 1.15 ± 0.25 s; ns) or the duration of MSNS-evoked AF (21.5 ± 13.9 to 22.6 ± 6.8 s; not significant).
Fig. 8.
SCS-induced changes in AF characteristics in SCS-resistant stimulation sites. Even when atrial fibrillation persisted from 22 sites following preemptive SCS, its latency increased and duration decreased. *P < 0.05 from control.
DISCUSSION
Excessive inputs to the intrinsic cardiac nervous system via, for instance, activating select mediastinal nerve inputs to that system are known to initiate ectopic atrial activation that can lead to alterations in atrial rhythms (9, 10, 28). As such, it has been proposed that selective activation of inputs to the ICN leads to excessive and heterogeneous activation within that nervous system to induce atrial arrhythmias (9).
In fact, select neuronal receptors (specifically nicotinic and α-adrenergic ones) have been implicated in mediating neuronally induced atrial arrhythmias (9, 28). In agreement with that concept, systemic administration of hexamethonium or α-adrenergic receptor blocking agent obtunds the ability of MSNS to generate atrial dysrhythmias (9, 28). In addition to such pharmacological neuromodulation, neuromodulation involving electrical stimulation of the dorsal, thoracic spinal cord can similarly decrease the ability of the intrinsic cardiac nervous system to generate atrial arrhythmias when excessively activated (10). While previous studies have inferred the involvement of specific subpopulations of intrinsic cardiac neurons in neural mechanisms associated with atrial arrhythmias, this study demonstrates the specifics of the neural substrate so involved and the potential to target it therapeutically to manage atrial arrhythmias.
The ICN has been traditionally thought to represent a simple relay station for parasympathetic efferent innervation of the heart (21). However, recent research has altered this view by elucidating the presence of a variety of intrinsic cardiac neurochemical phenotypes in conjunction with different functional classes of neurons, including afferent, efferent, and local circuit neurons that interact reflexly to modulate regional cardiac electrical and mechanical activities (6, 16, 26). Most intrinsic cardiac neurons display immunoreactivity to cholinergic markers, some without cholinergic inputs (18). Other intrinsic cardiac neurons, including human intrinsic cardiac ganglia, express an adrenergic phenotype (17, 18, 32). Moreover, various neurons are also innervated by peptidergic, nitrergic, and noradrenergic fibers (16, 18, 29). In accord with such anatomy, in this study, immunohistochemistry analysis demonstrated that the populations of ICN neuronal somata receiving inputs from mediastinal nerves that induce arrhythmogenic activity express cholinergic and/or adrenergic markers. In addition, there is another population of intrinsic cardiac neurons that appear to be involved in the genesis of atrial tachyarrhythmias that are neither cholinergic nor adrenergic in function.
It has been reported that the largest population of intrinsic cardiac neurons, represented by local circuit neurons, has the ability to process afferent and efferent inputs, thereby contributing to efferent neuronal control over regional cardiac function (6). As such, this population receives not only cardiac sensory inputs (3), but also efferent inputs from both limbs of the autonomic nervous system (8, 14). The integration of afferent and efferent inputs, combined with the capacity of intrinsic cardiac local circuit neurons to interact with neurons within other intrathoracic ganglia, comprises organ-level neuronal processing for functional control (7, 15, 34, 36).
The present study demonstrates that MSN activation in the induction of atrial arrhythmias is associated with alterations in the activity generated by populations of intrinsic cardiac neurons that represent different phenotypical subtypes (Fig. 3). Furthermore, these data demonstrate that excessive inputs to the ICN can indeed result in activation of neuronal populations therein—such neurons responding with activity rates considerably above control levels. As such, these data confirm the fact that atrial electrical instability can be related to excessive activation of select populations of ICN neurons. When synaptic efficacy within the ICN is compromised by nicotinic blockade, this arrhythmogenic potential is reduced in response to nerve imbalances within the cardiac nervous system. However, such ganglionic blockade is associated with major deficits in efferent outflow to the periphery (33). On the other hand, SCS apparently imparts an obtunding effect on such excessive excitation of intrinsic cardiac neurons, such that in many instances excessive mediastinal nerve inputs failed to enhance intrinsic cardiac neuronal activity (Fig. 4). Yet efferent function to the heart is maintained following SCS (Fig. 6). This, in turn, may explain why SCS appears to influence cardiac indices (12, 23), albeit in a relatively minor fashion (13, 20). It does so by restraining reflex pathways within intrathoracic autonomic ganglia associated with cardiac control (4, 13). SCS likewise does not blunt the chronotropic or dromotropic responses associated with extracardiac stimulation of principal parasympathetic or sympathetic projections to the heart (25). On the other hand, the reduction in ICN neuronal activation induced by excessive mediastinal nerve inputs presumably was the result of SCS modulation/suppression of local circuit neurons (cf. Fig. 3). In fact, that population represents the largest target population within the ICN (8, 14). That such neuronal responsiveness to excessive inputs was obtunded is further demonstrated by the fact that more than half of the previously responsive neurons identified were no longer activated by MSNS following SCS.
Perspectives and Significance
It is becoming apparent that neuronal control of the heart is significantly more complex than previously thought (6). Multiple tiers of neuronal processing occur within the cardiac neuronal hierarchy that ultimately acts to coordinate neuronal inputs to the heart (4, 7, 13). Stresses, either pathological or physiological, may disrupt the balance of efferent neuronal inputs that can lead to altered cardiac function (6, 22). As such, novel therapies that overcome any such imbalance in cardiac control, as represented by neuromodulation of the ICN via SCS, may moderate excessive activation of the ICN in response to transduction of pathological cardiac stressors. Other electrical neuromodulation-based therapies, such as low-level vagosympathetic stimulation may target peripheral aspects of the cardiac nervous system to reduce the arrhythmogenic potential (31, 35), but their respective neuronal targets have yet to be established (2).
Indeed, data derived from this study indicate that SCS can 1) stabilize intrinsic cardiac local circuit neurons in the presence of excessive extracardiac inputs, thereby obtunding any capacity to induce atrial arrhythmias, and 2) that such therapy does so without affecting direct autonomic efferent neuronal outflows to the heart to compromise cardiac function. In contrast, nicotinic receptor blockade suppressed not only the capacity of local circuit neurons to become excited but also autonomic efferent projections that influence cardiac indices. Such data delineate the possibility of targeting local, as opposed to central derived inputs, to the heart. They also support the thesis that SCS may represent a novel therapeutic strategy to counteract pathologically induced excessive activation of the ICN that would otherwise lead to atrial arrhythmia induction.
GRANTS
This work was supported by National Institutes of Health HL71830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.D.G., D.B.H., J.A.A., and J.L.A. conception and design of research; D.D.G., E.M.S., E.B., J.A.A., and J.L.A. performed experiments; D.D.G., E.M.S., D.B.H., E.B., J.A.A., and J.L.A. analyzed data; D.D.G., E.M.S., D.B.H., E.B., J.A.A., and J.L.A. interpreted results of experiments; D.D.G., D.B.H., and J.L.A. prepared figures; D.D.G. and J.L.A. drafted manuscript; J.L.A. edited and revised manuscript; J.L.A. approved final version of manuscript.
REFERENCES
- 1.American Physiological Society Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Ardell JL. The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm 8: 590–591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronic decentralized canine hearts. Am J Physiol Heart Circ Physiol 260: H713–H721, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Ardell JL, Cardinal R, Vermeulen M, Armour JA. Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia. Am J Physiol Regul Integr Comp Physiol 297: R470–R477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol Heart Circ Physiol 251: H764–H773, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Armour JA. Potential clincial relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Armour JA, Collier K, Kember G, Ardell JL. Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am J Physiol Regul Integr Comp Physiol 274: R939–R949, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Armour JA, Hopkins DA. Activity of canine in situ left atrial ganglion neurons. Am J Physiol Heart Circ Physiol 259: H1207–H1215, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Armour JA, Richer LP, Pagé PL, Vinet A, Kus T, Vermeulen M, Nadeau R, Cardinal R. Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci 118: 68–78, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cardinal R, Pagé PL, Vermeulen M, Bouchard C, Ardell JL, Foreman RD, Armour JA. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol 291: R1369–R1375, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paraoxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 121: 2615–2623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eliasson T, Augustinsson LE, Mannheimer C. Spinal cord stimulation in severe angina pectoris: Presentation of current studies, indications and clinical experience. Pain 65: 169–179, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, TerHorst GJ, DeJongste MJL, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for therapeutic use in angina pectoris. Cardiovasc Res 47: 367–375, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Gagliardi M, Randall WC, Bieger D, Wurster RD, Hopkins DA, Armour JA. Activity of in vivo canine cardiac plexus neurons. Am J Physiol Heart Circ Physiol 255: H789–H800, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Gray AL, Johnson CI, Ardell JL, Massari VJ. Parasympathetic control of the heart. A novel interganglionic intrinsic cardiac circuit mediates neural control of the heart. J Appl Physiol 96: 2273–2278, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol 94: 46–53, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norephinephrine transporters, and the neurotropin receptors tropomyosin-related kinase A and p75. Neuroscience 156: 129–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Hoover DB, Shepherd AV, Southerland EM, Armour JA, Ardell JL. Neurochemical diversity of afferent neurons that transduce sensory signals from dog ventricular myocardium. Auton Neurosci 141: 38–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kingma JG, Linderoth B, Ardell JL, Armour JA, DeJongste MJL, Foreman RD. Neuromodulation therapy does not influence blood flow distribution or left-ventricular dynamics during acute myocardial ischemia. Auton Neurosci 91: 47–54, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Langley G. The Autonomic Nervous System. Cambridge, UK: Cambridge University Press, 1921 [Google Scholar]
- 22. Lu Z, Scherlag BJ, Lin J, Yu L, Guo JH, Niu G, Jackman WM, Lazzara R, Jiang H, Po SS. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res 84: 245–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mannheimer C, Camici P, Chester MR, Collins A, DeJongste MJL, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, Pasic M, Thelle D. The problem of chronic refractory angina. Report from the ESC joint study group on the treatment of refractory angina. Eur Heart J 23: 355–370, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Matsubayashi Y, Iwai L, Kawasaki H. Fluorescent double-labeing with carbocyanine neuronal tracing and immunohistochemistry using a chloesterol-specific deterent digitonin. J Neurosci Methods 174: 71–81, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Olgin JE, Takahashi T, Wilson E, Vereckei A, Steinberg H, Zipes DP. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiovasc Electrophysiol 13: 475–481, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Parsons RL. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston: Kluwer Academic Publishers, 2004 [Google Scholar]
- 27. Randall DC, Brown DR, McGuirt AS, Thompson G, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285: R1066–R1075, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL, Armour JA. α-Adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 295: R1175–R1180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rysevaite K, Saburkina I, Pauziene N, Vaitkevicius R, Noujaim SF, Jalife J, Pauza DH. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm 8: 731–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and development of pacing-induced atrial fibrillation. Heart Rhythm 8: 583–589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Chen P-S. Continuous low-level vagus stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 123: 2204–2212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh S, Johnson PI, Javed A, Gray TS, Lonchyna VA, Wurster RD. Monamine- and histamine-synthesizing enzymes and neurotransmitters within neurons of adult human cardiac ganglia. Circulation 99: 411–419, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Taylor P. Agents acting at the neuromuscular junction and autonomic ganglia. In: Goodman & Gilman's: The Pharmacological Basis of Therapeutics, edited by Brunton LL, Lazo JS, Parker KL. New York: McGraw-Hill, 2006 [Google Scholar]
- 34. Waldmann M, Thompson GW, Kember G, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: Direct evidence by neural recording from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol 22: 455–463, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec 239: 75–87, 1994 [DOI] [PubMed] [Google Scholar]