Abstract
The advent of the postgenomics era has led to increased interest in exploring the role of gene networks and signaling pathways in controlling plant development. The last two decades have seen a particular increase in the number of studies focusing on the development of the Arabidopsis thaliana root system. However, the investigation of such a seemingly simple system as an Arabidopsis root can lead to problems in quantification and errors in interpretation if knowledge of root organization is lacking. In this article, we identify a number of these problems and give examples of potentially erroneous and correct determinations of lateral root parameters. Our aim is to bring this important issue to the attention of the plant science community and to suggest ways in which the problems inherent in quantifying the process of lateral root development can be avoided.
The importance of root development in plant biology arises not only from the crucial role that roots play in supporting plant growth and crop productivity but also because the root is a convenient model for studies of plant development. Root system architecture is an integrative result of lateral root (LR) initiation, morphogenesis, emergence, and growth. Thus LR development is fundamental to the way in which a plant elaborates its root system to explore the soil volume. Despite recent progress in this area (Péret et al., 2009; Fukaki and Tasaka, 2009; Benková and Bielach, 2010; Ingram and Malamy, 2010), we are far from understanding how the multistage process of LR development is controlled during plant ontogenesis and how its complex responses to multiple intrinsic and extrinsic factors are integrated. It is axiomatic that improving our understanding of these problems depends on the proper quantitative analysis of the process of LR development. In this Commentary, we address the problems of LR quantification solely from a developmental perspective, considering how LR formation is evaluated in an individual parent root. We do not consider studies that approach root system development from ecological, root-soil-continuum, or high-throughput phenotyping perspectives (for example, Dupuy et al., 2005, 2010; Trachsel et al., 2011).
We have seen greatly increasing attention directed toward root development over the past 20 years. A bibliographic search on the term “lateral roots” in Web of Science from Thomson Reuters during the period 1990 to 2010 produced a total of 2619 documents and showed a 12-fold increase in publications in the last 21 years (19 publications in 1990 compared with 238 in 2010). This enhanced number of studies of root development has involved many plant biologists whose field of expertise is not root biology or plant development. As a consequence, it is our observation that many studies suffer from one or a number of inaccuracies or elementary errors in quantification of LR formation. This prompted us to write this Commentary with the aim of illustrating how an elementary error can lead to uncertain or misleading conclusions about root development. Our intention is to draw the attention of plant scientists to some critical factors that should be taken into account when quantifying the process of root development.
THE BASICS: THE IMPORTANCE OF GROWTH CONDITIONS
If LR formation is to be quantified, it seems obvious that the plants should be grown under conditions that do not limit or inhibit root growth in unintended ways. This is because the rate of root growth reflects root apical meristem activity and cell production (Ivanov and Dubrovsky, 1997; Baskin, 2000) and anything that perturbs these activities in the parent root is almost certain to affect the process of LR formation that begins close behind the root apex (Dubrovsky et al., 2011). In young seedlings, root growth accelerates as the plant develops (Beemster and Baskin, 1998), and growth rates also differ between Arabidopsis thaliana accessions (Armengaud et al., 2009). Thus, when comparing the rate of root growth, the age and genotype of the plants must be taken into account. Various environmental factors, such as growth medium, temperature, light, and aeration, also affect root growth (McMichael and Burke, 1998; Kiss et al., 2003; López-Bucio et al., 2003; MacGregor et al., 2008; Ingram and Malamy, 2010). There is currently no agreed standard medium for growing seedlings for root studies, but many labs use complete or half-strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962). This is a medium designed for tissue culture that contains very high and nonphysiological concentrations of mineral nutrients: For example, the N supply in full-strength MS consists of 21 mM NH4+ and 40 mM NO3−. It is therefore not surprising that full-strength MS medium was found to inhibit root growth (Dubrovsky et al., 2009). As alternatives, other groups have used diluted media based on MS (Dubrovsky et al., 2006; Monshausen et al., 2009; Peer et al., 2009; Morquecho-Contreras et al., 2010), Gamborg's B5 (Zhang and Forde, 1998), Hoagland (Werner et al., 2010), or other media (Remans et al., 2006). An additional issue is that when seedlings are grown on vertical agar plates, contact between the aerial parts and Suc in the medium affects LR formation (MacGregor et al., 2008). To avoid this problem, the shoot can be isolated from the agar using a strip of Parafilm (MacGregor et al., 2008) or by excising the segment of agar from the top of the plate (Figure 1; see also Figure 1A in Walch-Liu and Forde, 2008). The study by MacGregor et al. (2008) suggests that good aeration in the Petri dish is also important for normal root growth. Recent data showed that in Columbia-0 (Col-0) plants grown in Petri dishes wrapped with gas-permeable 3M Micropore tape, the mean rate of root growth between days 5 and 6 after germination was 394 μm h−1 (Tapia-López et al., 2008, Supplemental Table 1). In plants of the same age grown under the same conditions but in Petri dishes wrapped with a plastic gas-impermeable film, the mean rate of growth was 271μm h−1 (Dubrovsky et al., 2006, Figure 4). Based on the range of root growth rates observed for Col-0 in published studies (Table 1), it seems that the very low growth rates reported in some cases for seedlings of similar age may be due to suboptimal growth conditions.
Figure 1.
A Petri Dish System with the Upper Segment of Agar Excised to Avoid Direct Contact of Shoot Tissues with the Nutrient Medium.
Arabidopsis seeds were germinated on the surface of vertical agar nursery plates and after 4 to 5 d transferred to treatment plates that had the upper segment of agar removed. By positioning the bases of the hypocotyls in line with the cut edge of the agar, the shoots are able to develop without coming into contact with the agar surface.
[See online article for color version of this figure.]
Table 1.
Examples from the Literature Showing the Ranges of Seedling Root Growth Rates and LR Densities Reported in the Col-0 Accession of Arabidopsis
| Plant Age | Parameters and Their Values | Reference |
| Root Growth Rate (μm h−1) | ||
| 6 to 11 d old, average | 448 | Ditengou et al. (2008), Supplemental Table 1 |
| 10 d old | 445 | Beemster and Baskin (1998), Figure 2 |
| 11 d old | 375 | Armengaud et al. (2009), Supplemental Table 2 |
| 8 d old | ∼320 | Al-Ghazi et al. (2003), Figure 4A |
| 6 d old | 241 | Beemster and Baskin (1998), Figure 2 |
| 8 d old | 170 | Zhou et al. (2010), Figure 1B |
| 13 d old | 80 | Yazdanbakhsh and Fisahn (2010), Figure 6C |
| LR Density (LR mm−1), Based on Total Root Length | ||
| 6 d old | ∼0.02 | Sanz et al. (2011), Figure 3B |
| 12 d old | ∼0.09 | Pelagio-Flores et al. (2011), Figure 13 |
| 12 d old | ∼0.1 | Kapulnik et al. (2011), Figure 1B |
| 12 d old | 0.16 | Fukaki et al. (2006), Figure 6B |
| 12 to 14 d old | 0.1–0.2 | Swarup et al. (2008), Supplemental Figures 1C, S2D, S3I |
| Not reported | ∼0.2 | De Smet et al. (2008), Figure 2C |
| 10 d old | 0.3 | Nodzon et al. (2004), Figure 8 |
| 10 d old | 0.27 | De Smet et al. (2007), Table 2 |
| 10 d old | ∼0.35 | De Rybel et al. (2010), Figure 3A |
| 13 d old | ∼0.6 | Marchant et al. (2002), Figure 1 |
| 11 d old | 0.8 | Coates et al. (2006), Figure 3B |
ESTIMATING LR DENSITIES
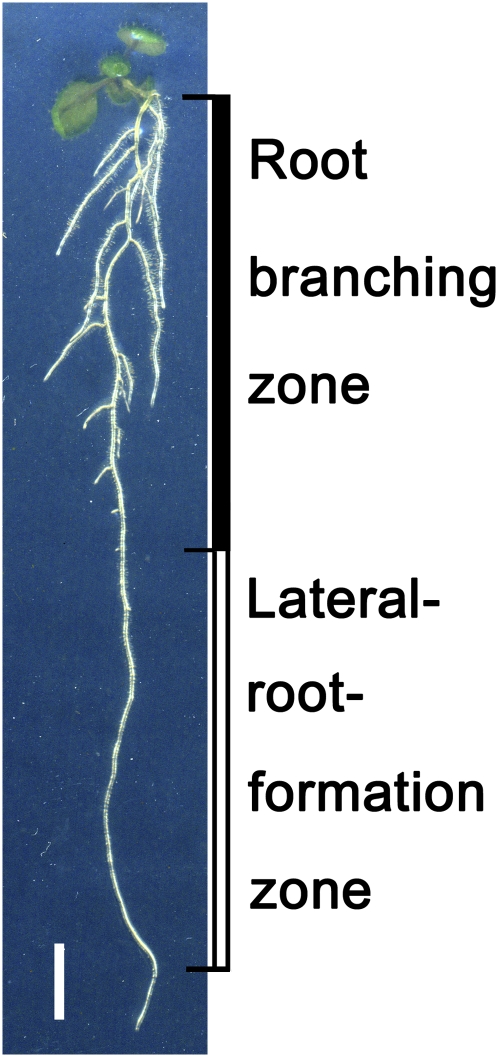
The process of LR initiation takes place in the pericycle a few millimeters behind the root apical meristem. It begins with priming of some pericycle cells within the root elongation zone, including the transition zone (De Smet et al., 2007; Moreno-Risueno et al., 2010; De Rybel et al., 2010) and subsequent specification of founder cells followed by the first cell divisions leading to formation of a lateral root primordium (LRP) (Dubrovsky et al., 2008, 2011). In wild-type Arabidopsis, LRPs are always initiated acropetally (i.e., new initiation events take place rootward, in the direction of the root apex). However, initiated primordia do not develop at uniform rates, and some arrested or delayed LRPs are found between emerged LRs (Dubrovsky et al., 2006; Moreno-Risueno et al., 2010, Figure 1D). Therefore, in a growing root, we can define two zones related to LR development: a root branching zone that extends rootward from the shoot base to the youngest emerged LR and an LR formation zone that spreads from below the youngest emerged LR to the youngest and most rootward LRP, which is normally just 2 to 6 mm from the root apex (Dubrovsky et al., 2011). These zones are illustrated in Figure 2. As founder cell priming takes place rootward of the Stage I LRP (primordium formation per se), the root portion between the priming location and Stage I LRP is excluded from the LR formation zone. LRP developmental stages in Arabidopsis are defined in the classic work of Malamy and Benfey (1997). The short (2 to 3 mm) zone near the shoot base where, in young plants, LRs have not yet emerged has been termed the “basal zone” (Armengaud et al., 2009). However, since this zone contains primordia (Figure 3) that eventually emerge, we consider this region to be part of the root branching zone. If, in a developmental study the aim is to determine the density of visible LRs on the parent root (omitting unemerged LRPs), it is most logical to estimate this parameter only within the branching zone. In this case, the parameter is termed the “branching density” and is the number of emerged LRs divided by the length of the branching zone (Figure 3, Table 2). Branching density defined in this manner is a developmentally meaningful parameter as it reflects average spacing between emerged LRs, even though some LRPs may emerge later. It should be mentioned that the term “branching density” used here in a developmental context is very different from the same term used in an ecological context as there it refers to number, length, and/or biomass of roots per certain soil volume (Dupuy et al., 2005). In Arabidopsis, the branching density varies between ecotypes: In Col-0 seedlings (6 to 9 d old), when evaluated correctly, the branching density is normally ∼0.4 mm−1 (Dubrovsky et al., 2006; Armengaud et al., 2009; Ikeyama et al., 2010), although higher densities (0.5 to 0.8 mm−1) were observed in older (11 to 14 d old) seedlings (Dubrovsky et al., 2006; Hermans et al., 2010).
Figure 2.
Root Branching Zone and LR Formation Zone in Arabidopsis.
A 10-d-old Col-0 seedling showing the root branching zone (black bar), from the shoot base to the most rootward emerged LR, and the LR formation zone (white bar), from the most rootward emerged LR to the most rootward primordium as detected on preparations cleared as described by Malamy and Benfey (1997). Bar = 5 mm.
[See online article for color version of this figure.]
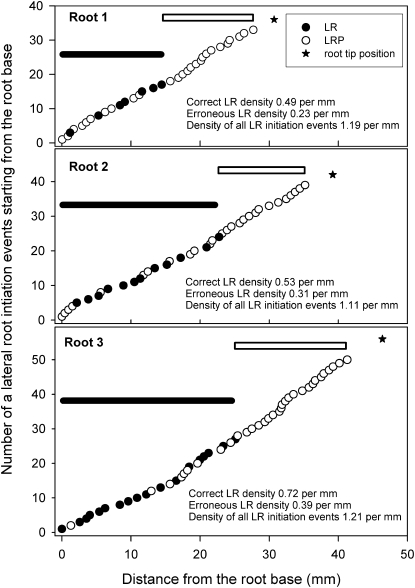
Figure 3.
Distribution of LRs and LRPs in the Primary Root and Density Evaluations.
Roots of three different 8-d-old Col-0 plants are shown. Roots were grown under conditions described by Dubrovsky et al. (2009) and were cleared using the method of Malamy and Benfey (1997). Distances between each subsequent LRP and/or LR were measured with an ocular micrometer under a microscope equipped with Nomarski optics. Note the high variability in these individual distances: Minimum and maximum distances were 266 and 1355 μm (root 1), 278 and 2242 μm (root 2), and 121 and 2145 μm (root 3). The black bar indicates the length of the root portion corresponding to the branching zone (from the shoot base to the most rootward emerged LR), and the white bar indicates the LR formation zone (from the most rootward emerged LR to the most rootward LRP). Note that in each root, primordia are present within the branching root portion. For illustration purposes, “correct” and “erroneous” estimations of LR density are shown. Correct LR density was estimated using the length of the branching zone as the denominator, and erroneous LR density was estimated using the total length of the primary root as the denominator. Density of all LR initiation events was estimated using the sum of the length of the branching zone and LR formation zone as the denominator.
Table 2.
Definition of Terms and Parameters Used in Studies of LR Development
| Term or Parameter | Definition |
| Terms | |
| LR initiation | A complex developmental process that includes priming of founder cells, their subsequent specification in the pericycle, and the first anticlinal divisions of these cells leading to early-stage primordium formation. |
| LRP | After the first anticlinal division of founder cells and before the first periclinal divisions, a Stage I primordium is formed. This is followed by patterned division leading to a dome-shaped primordium, which is considered a primordium while it is inside the parent root. In general terms, a primordium is a presumptive lateral organ within a root system. |
| LR | When the primordium protrudes through the epidermis of a parent root, it becomes an emerged LR (this process is mainly driven by cell elongation). Soon after emergence, the LR becomes mature and begins to grow due to both cell proliferation in the newly activated apical meristem and cell elongation. |
| LR formation zone | The zone of the parent root that extends from the most rootward (closest to the root tip) LRP to the most rootward emerged LR. This zone of the parent root contains only LRPs. Length of the zone = LP (mm). |
| Root branching zone | The zone of the parent root that extends from the most rootward emerged LR to the shoot base. Length of the zone = LB (mm) |
| Parameters | |
| LR branching density or LR density | A parameter that quantifies the average spacing of LRs specifically within the root branching zone, defined as the number of (emerged) LRs per unit length of the root branching zone, NoRLB−1 (mm−1). |
| LRP density | A parameter that quantifies the average spacing of LRPs, usually within the LR formation zone, defined as the number of all LRPs per unit length of the LR formation zone, NoPLP−1 (mm−1). To estimate this parameter, cleared root preparations are made and LRPs are detected, usually using differential interferential contrast (Nomarski) microscopy. |
| Density of all LR initiation events | A parameter that quantifies the average spacing of both LRs and LRPs within the root portion where LRs and LRPs are present, defined as the combined number of all LRs and all LRPs per unit length comprising the LR branching zone and the LR formation zone of the parent root, (NoR + NoP) (LB + LP)−1 (mm−1). |
| LR initiation index | A parameter that quantifies the number of LRs distributed along the primary root length comprising 100 cortical cells. If a genotype or a treatment affects the length of elongated cells, then simple density measurements may not be sufficient to reflect differences in LR initiation. The parameter can be evaluated for the whole root or for the LR formation zone only. In the latter case, it is estimated as 100 times the LRP density in this zone multiplied by the average elongated cortical length (l, mm) [i.e., 100 (NoPLP−1) l.] |
Despite the simplicity of the branching density parameter, we can find many cases in the literature of alternative measures of LR density that can be misleading. The most common problem is that the number of emerged roots is divided by the total primary root length, including both branching and LR formation zones. When estimated in this way, the values can lead to erroneous conclusions because of variations in the proportion of the primary root that is branching. The ratio between the lengths of the branching and LR formation zones increases naturally as the primary root grows (simply because, with time, an increasing proportion of the root carries LRs). As a result, LR density estimated as a function of total primary root length will always be lower than the one correctly estimated and will gradually increase as the seedling develops, even though no developmental changes in the spacing of the LRs are actually occurring. The LR density values estimated in this way are meaningless in terms of developmental biology as they do not reflect average spacing between emerged LRs. When calculated on the basis of total primary root length, estimates of LR density in Col-0 have varied from 0.02 to 0.8 mm−1 across numerous publications (Table 1). Thus, 40-fold differences are recorded in the same genotype if the LR density is estimated in this manner, whereas only twofold differences are recorded when estimated (correctly in our view) as branching density (see above).
Examples of apparent age-dependent changes in estimated LR density can be found in a number of published studies (Marchant et al., 2002 [Figure 1, LR density of ∼0.2 in 7-d-old and ∼0.6 mm−1 in 13-d-old Col-0 plants]; Uehara et al., 2008 [Figure 2, LR density of ∼0.05 mm−1 in 6-d-old and ∼0.2 mm−1 in 8-d-old Col-0 plants]; Lucas et al., 2011 [Supplemental Figure 5, in the wild type, LR density of ∼0.05 mm−1 in 7-d-old and ∼0.3 in 10-d-old Col-0 plants]; Sanz et al., 2011 [Figure 3B, LR density of ∼0.02 mm−1 in 6-d-old and ∼0.13 mm−1 in 10-d-old Col-0 plants]). However, as explained above, this apparent increase is a result of a progressive increase in the length of the branching zone with time and does not reflect actual changes in spacing between LRs. As shown previously in a number of species (MacLeod and Thompson, 1979), including Arabidopsis (Dubrovsky et al., 2009), the combined density of both LRs and LRPs does not change with plant age when estimated within the zone where LRs and LRP are present. However, branching density correctly estimated can increase slightly with age because LRPs present between LRs eventually emerge (Dubrovsky et al., 2006). An illustration of arrested or delayed LRPs located between already emerged LRs in wild type Col-0 plants is seen in Figure 3.
The term “LR formation” often is used interchangeably with “LR production,” and both terms are sometimes used to describe only the production of visible LRs (Nodzon et al., 2004). In a developmental sense, both terms incorporate the process of “LR initiation” because roots are formed from primordia. In fact, “LR formation” is a more general term that refers to all processes subsequent to pericycle cell priming, including LRP initiation, LRP morphogenesis, and LR emergence. Therefore, if one is referring only to the production of emerged LRs, “LR formation” would be an inappropriate term. As an alternative, the term “root branching” (or “LR root emergence”) can be used as it refers only to emerged roots (Table 2). LR density, even if evaluated correctly, cannot be used as a parameter to judge whether LR formation is affected in a genotype or by a treatment. This is because LR density, being based on counting visible LRs, is dependent on the process of LR emergence, which is highly susceptible to genetic and environmental factors that are distinct from those affecting LR initiation. It is because these factors operate at the level of the individual LR that some unemerged LRPs are often found between LRs (MacLeod and Thompson, 1979; Dubrovsky et al., 2006, 2009). Nevertheless, in the literature, it is not uncommon to find conclusions about the effect of a certain factor on LR initiation or LR formation based entirely on measurements of LR density (or LR number) without consideration of LRPs. We argue that such conclusions cannot be considered substantiated and final.
In summary, if LR density is evaluated per total length of the parent root, the results are highly variable and do not adequately reflect the average distance between emerged LRs. These erroneous LR density estimations can be up to 40 times lower than correctly estimated LR density and thus do not give any meaningful information. Furthermore, even if evaluated correctly as branching density (Table 2), the counting of visible LRs cannot be used to establish whether LR formation or initiation is affected in a mutant or by a particular treatment; LRPs must be taken into account. Pragmatically, if an initial screen of seedlings analyzed at later stages, when delayed LRPs between LRs have emerged, shows no effects (of a genotype or a treatment) on branching density, then in most cases it is unlikely that LR initiation has been affected; however, where differences are seen, then further investigation is required to establish which stage(s) of LR formation is affected.
ESTIMATING THE DENSITY OF ALL LR INITIATION EVENTS
As already stated, if LR initiation is a main point of interest in a study, we must evaluate the density of all initiation events, including both LRs and LRPs (Figure 3). To estimate this density, the combined numbers of LRs and LRPs detected in a root are divided by the length of the parent root from the shoot base to the most rootward LRP (Table 2). This calculation will give the most reliable results (Method 1). Sometimes, when the volume of samples is very high, to decrease time for data collection, it would be acceptable to divide the combined number of LRs and LRPs by the total length of root or root portion analyzed (Method 2) without subtracting the distance from the tip to the most rootward LRP (Ivanchenko et al., 2008, 2010). In this case, density is slightly underestimated (because of the small distance between the tip and the youngest LRP), and it is therefore important to estimate the extent of the experimental error. To do this, the density should initially be evaluated by both Methods 1 and 2 for a representative sample of roots. If, under the conditions studied, the estimated experimental errors, are similar (e.g., across different genotypes or treatments), then Method 2 is acceptable. However, Method 1 is always preferable.
When the density of all LR initiation events is to be evaluated, the roots are usually cleared with some chemical treatments (Malamy and Benfey, 1997; Dubrovsky et al., 2009) and LRPs are analyzed with differential interferential contrast (Nomarski) optics starting from Stage I (the earliest stage at which LR initiation is detectable). As priming is a preinitiation event taking place in protoxylem and not pericycle cells (De Smet et al., 2007), it does not affect the number of LR initiation events and the most rootward LRP should first be detected. Good quality clearing is critical for quantification of LRP as early stages cannot be detected without it. Thus, if clearing is not performed, the number of LRP can be underestimated (Lee et al., 2009, Figure 4A, LRP density in 8-d-old plants is ∼0.1 mm−1). Simple use of marker lines without detailed microscopy analysis may not be sufficient. For example, Sun et al. (2009) used a CYCB1;1:β-glucuronidase (GUS) line (Colón-Carmona et al., 1999) and reported that in 7-d-old Col-0 plants there are six LR initiation events (LRs and LRP; Figure 1B in Sun et al., 2009); considering that at this age the primary root is ∼37-mm long (Figure 1C in Sun et al., 2009), the density of all LR initiation events based on these counts would appear to be ∼0.16 (6/37) mm−1, which is about 5 times lower than expected (see below). This type of error can arise because the CYCB1;1 gene is a marker for dividing cells, so that de-layed or arrested primordia may not express it. Use of the auxin-responsive DR5:GUS marker (Ulmasov et al., 1997) may also be problematic as an aid to estimating LRP densities because it has been found that not all LRPs in an Arabidopsis line expressing the DR5:GUS marker are GUS positive (J.G. Dubrovsky, unpublished data; J.C. Del Pozo, personal communication).
Estimating the density of all LR initiation events is a demanding task, particularly for a long root. In our experience, it is easier to evaluate this parameter in Arabidopsis seedlings 5 to 6 d after germination. To simplify data collection, the LRP density specifically in the LR formation zone can be evaluated. As mentioned above, already initiated LRP develop asynchronously, and the density of all initiation events does not depend on plant age. Also, analysis of our own data (Dubrovsky et al., 2006; J.G. Dubrovsky, unpublished data) showed that the density of all LR initiation events along the root gives statistically the same values as LRP density evaluated in the LR formation zone alone. For example, for Col-0, 8-d-old plants (6 d after germination) grown in Petri dishes wrapped with a plastic film (which limits gas exchange), the mean LRP density evaluated in the LR formation zone was 0.63 ± 0.14 mm−1, and the mean density of all initiation events in the same root samples was 0.67 ± 0.11 mm−1 (combined data of two independent experiments, ± sd, n = 35, P = 0.247, Student's t test). In plants of the same age grown under the same conditions but wrapped with gas-permeable 3M Micropore tape, the mean LRP density in the LR formation zone was 0.86 ± 0.19 mm−1 (combined data of five independent experiments, ± sd, n = 54), the mean density of all initiation events in some of the these root samples was 0.87 ± 0.15 mm−1 (combined data of two independent experiments, ± sd, n = 21), and there were no differences between these values (P = 0.744, Student's t test). LRP density values estimated in the LR formation zone in the above example are within normal distribution (Kolmogorov-Smirnov Normality test, P > 0.200). Therefore, it is practical to evaluate LRP density in the LR formation zone only. This parameter is evaluated by dividing the number of LRPs in the LR formation zone by the length of the zone (Table 2). In developmental terms, this will reveal whether a treatment or a genotype affects LR initiation. As can be seen from the above examples, in Col-0, the density of all initiation events is between 0.7 and 0.9 mm−1. Similar values are published (Dubrovsky et al., 2009, 2011; Ivanchenko et al., 2008, 2010; Moreno-Risueno et al., 2010 [0.77 mm−1, estimated from Figure 4G]). Some variations in this parameter may be expected. However, the average number of LR initiation events is one or close to one event per millimeter, which might be considered as a rule of thumb (or “rule of one”) for quantification of LR formation in Arabidopsis. In different accessions, it can be a greater or a lesser value (Dubrovsky et al., 2006, 2009), but this gives an idea of the approximate average potential branching density. Estimates of LRP density leading to false impression of the phenotype and misleading conclusions can occur if the number of LRPs is divided by the full length of the primary root rather than by the length of the LR formation zone (or a zone where they were counted) (Coates et al., 2006 [Figure 3C, ∼0.25 LRP mm−1 in 11-d-old Col-0 plants]; Vellosillo et al., 2007 [Figure 4D, total number of LRPs was divided by primary root length, LRP density in 10-d-old Col-0 plants 0.66 mm−1]; De Smet et al., 2008 [Figure 2A, LRP density in Col-0 plants ∼0.22 mm−1]). Also, when low density of LR initiation events is reported (Shkolnik-Inbar and Bar-Zvi, 2010 [Figure 1E, ∼0.3 mm−1, LR + LRP density in 12-d-old Col-0 plants]), this can be related either to underscoring of LRPs or to nonphysiological conditions used for growth.
The eventual emergence of delayed or quiescent LRPs between emerged LRs (Figure 3) means that the branching density in older roots becomes closer to the density of all LR initiation events. Nevertheless, when evaluated in mutants or under a treatment, we do not know how the LR emergence process is affected and that is why the density of all LR initiation events or the LRP density evaluated in the LR formation zone are explicit parameters when one wants to establish whether a mutation or a treatment affects LR formation. To obtain information on whether a treatment affects LR emergence, a percentage of emerged LRs could be a useful parameter (Ivanchenko et al., 2008, Figure 3B). However, this parameter can be misleading if emergence is compared between a mutant and a wild type because, if primary roots are of significantly different length (for example, in the mutant being much shorter), then the percentage of emerged LRs in the wild type will be different from the mutant merely because of the differences in growth. In such cases, the percentage of LRPs within the LR-branching zone (Table 3, section 2f) could aid in understanding whether LR emergence is affected in a mutant.
Table 3.
A Possible Scenario for Data Collection and Analysis When LR Development in an Arabidopsis Mutant Is Studied
| Questions to Be Answered and Parameters and Data to Be Collected and Reported | Description |
| 1. Questions to be answered before the beginning of the analysis | (a) What is the primary root growth capacity in 5- to 10-d-old seedlings? If the primary root is longer than 10 mm →go to 2a and 2b (optionally to 2c), if it is 10 mm or shorter →go to 2c. |
| (b) Is the LR formation zone at least 5- to 7-mm long in seedlings 5 to 6 d after germination ? If yes →go to 2a and 2b (optionally to 2c), if no →go to 2c. | |
| (c) Is the average cortical cell length in the one-third of the proximal (shootward) root portion the same as within the one-third of the distal (rootward) root portion? Lengths should be compared in at least five roots, 10 cortical cells in each. If cell length is the same →go to 2d, if cell length is different →go to 2d’ and 2d’’. | |
| (d) Are some LRP stages delayed? →go to 2e. | |
| (e) Is LR emergence altered? →go to 2f. | |
| 2. Parameters to be evaluated | (a) LR branching density →go to 3a. |
| (b) LRP density in the LR formation zone →go to 3d. | |
| (c) Density of all LR initiation events →go to 3a and 3d. | |
| (d) LR initiation index within the primary root →go to 3c and 3d. | |
| (d’) LR initiation index within the LR-formation zone →go to 3c and 3d. | |
| (d’’) LR initiation index within the LR-branching zone →go to 3b and 3d. | |
| (e) Distribution of different LRP developmental stages within the LR formation zone (expressed in percentage of total number) →go 3e. | |
| (f) Percentage of LRP of the total LR initiation events within the LR branching zone →go to 3a. | |
| 3. Data to be collected | (a) Length of the branching zone; number of LR within the branching zone; number of LRP within the branching zone. |
| (b) Cortical cell length within the LR branching zone. | |
| (c) Cortical cell length within the LR formation zone. | |
| (d) Distance from the most rootward LRP to the root tip and the length of the LR formation zone; number of LRP within the LR branching zone (when the primordium protrudes through the epidermis of the parent root it is considered to be an emerged LR). | |
| (e) Number (or proportion) of LRP of each developmental stage found within the LR formation zone. | |
| 4. Variables and conditions to be reported | Plant age at the time of the analysis, growth conditions (light, temperature, and photoperiod), medium composition, plate sealing type, how plates were oriented (vertically, at an angle, etc.), whether shoots were in contact with the agar, how LR or LRP density was evaluated (per total parent root length or per specific root portion), in what root portion developmental stages were evaluated. |
Counting of LRPs is performed on cleared root preparations under a microscope.
The progression from one developmental stage to another during LRP development is an important part of LR formation. If, in a mutant or under a treatment, this progression is affected, this information is valuable toward understanding the role of a gene or the effect of a treatment in this process. Changes from Stage I LRP to LR emergence take place within the LR formation zone. These data are frequently presented either as number (Zhang et al., 1999; De Smet et al., 2007; Vellosillo et al., 2007; Ikeyama et al., 2010; Pelagio-Floreset al., 2011) or as a density (De Rybel et al., 2010; Morquecho-Contreras et al., 2010) of LRPs found at each stage. If the length of the LR formation zone varies between genotypes, which is often the case, then reporting only the number of LRPs present at each developmental stage may be inadequate. Rather, reporting the density of each developmental stage would be more meaningful even though it will not reflect inter-LRP distance. However, frequently, reports do not indicate whether the density was evaluated per LR formation zone (an adequate method) or per other root portion. Therefore, comparisons between the mutant and wild type or between treated and untreated roots represent a considerable challenge. In our view, estimation of the percentage of LRPs found at each developmental stage out of all LRPs within the LR formation zone would be most useful. This approach is infrequently used to date (Krouk et al., 2010); hopefully, it will become more common when LRP developmental stages are evaluated. It is useful then, as discussed above, to know if LR emergence is affected by estimating the percentage of LRPs in the root branching zone. Alternatively, if the primary root length in the mutant and wild type is of similar length, LRPs from both the root branching zone and the LR formation zone can be counted. Then, reporting the number of each developmental stage could be sufficiently informative. Therefore, it is critical to report per what root portion the analysis of LRP developmental stages is performed (see section 4 of Table 3).
Since in mutants, or in the wild type under treatments, elongated cells may become shorter, normalization for cell length is helpful to better understand the effect. For this, a parameter called the “LR initiation index” can be evaluated that considers how many LR initiation events take place along the parent root portion comprising 100 elongated cells (Ivanchenko et al., 2008, 2010; Dubrovsky et al., 2009). The LR initiation index is a parameter that reveals how LR initiation is affected in a genotype or by a treatment (Table 2). If, for example, under a treatment, cell length decreases by 50%, the root will become shorter and so the density of LRP will increase by 50% compared with untreated roots. However, estimating the LRP frequency per 100 cells may show that each new initiation event took place on average per the same number of cells along the root. More details on this parameter can be found in Dubrovsky et al. (2009).
ARE NUMBERS OF LRs OR LRPs PER PARENT ROOT USEFUL PARAMETERS?
LR number can be a useful parameter for the analysis of LR development in certain contexts, for example, when the LR number is evaluated as a way of illustrating qualitative differences under treatments (LRs absent under one treatment and present under another treatment) (Dubrovsky et al., 2011, Figure 5A). Also, when an increase in the number of LRs with time is evaluated, it can give valuable information on the rate of LR formation (MacLeod and Thompson, 1979; Chevalier et al., 2003, Figure 8; Al-Ghazi et al., 2003, Figure 5A; Brun et al., 2010, Figure 8). It can also be useful to present data on LR number together with the LR density data (Ivanchenko et al., 2010). However, to present data on LR number without density data is insufficient for a complete understanding of what is happening to LR formation because, as already pointed out, root length and branching zone length vary to different extents in different genotypes or under different conditions. Examples of the usage of the LR number without density information can be found (Dubrovsky et al., 2008, Figure 2C; Swarup et al., 2008, Figure 1F; Pérez-Torres et al., 2008, Figure 5I; Li et al., 2009, Figure 2G; Effendi et al., 2011, Figures 2A and 2B; Prasad et al., 2010, Figures 1A and 3). Even when both the data on primary root length and LR (and/or LRP) number or data on LR and LRP numbers are given (Swarup et al., 2008, Supplemental Figures 1D and 1E; Li et al., 2009, Figures 2F and 2G; Jiang et al., 2010, Figure 4B; Moreno-Risueno et al., 2010, Figure 4B; Ortiz-Castro et al., 2011, Figure 1E), one cannot explicitly understand whether a mutation or a treatment affects LR initiation when the data on LRP density and on the length of branching zone are not present. For example, if a mutant has a branching zone that is half the length of that in the wild type and both the numbers of LR and LRP per root are half those in the wild type, the density of LR initiation events can be the same. This explains why LRP density or density of all LR initiation events is needed to make conclusions about the effect of a gene on root development. If the length of fully elongated cells is changed in a genotype or a treatment compared with the wild type or a control treatment, then a supplementary useful parameter can be the LR initiation index discussed above.
ANALYSIS OF ROOT SYSTEM FORMATION IN A MUTANT OR IN THE WILD TYPE UNDER A TREATMENT
Here, we consider what would be the minimal list of parameters to be evaluated for a quantitative description of root system formation that permit an understanding of whether LR initiation and formation are affected in a mutant or under a treatment. These parameters are outlined in the Table 3. Many more parameters could be proposed depending on the specific research focus. The aim of this Commentary is not to provide a specific protocol but rather to suggest the most appropriate way to address the problem of quantitative analysis of LR development and, particularly, of LR initiation. Therefore, we only briefly consider here how the analysis of root system formation could be performed. If a mutant is to be analyzed, a few questions should be answered before beginning the analysis. For example, is the primary root length ≤10 mm by 10 d after germination? If so, then the density of all LR initiation events should be evaluated (Table 3; 10-mm root length is chosen arbitrarily, as in our experience the shortest LR formation zone in the wild type typically is 5 to 7 mm). Also, can the LR formation zone be clearly defined in seedlings 5 to 6 d after germination? If so, the LRP density in the LR formation zone and branching density might be evaluated. The parameters that can be evaluated are described in Table 3 and discussed above.
If the effect of a treatment is to be evaluated in the wild type, a possible scenario for data collection and analysis can be similar to that outlined in Table 3. However, we must consider that some parameters should be evaluated differently when comparing treatments rather than comparing a mutant with its wild type (see an example discussed above related to a percentage of emerged LRs in the section “Estimating the Density of All LR Initiation Events”). Frequently, mutant and wild-type plants are subjected to a treatment. In each of these situations, the value of analyzing each parameter must be assessed. Response to a treatment can be studied using different experimental designs. A typical design is based on seed germinated from the outset in the medium supplemented or not with a compound of interest (for example, Laplaze et al., 2007). Alternatively, seedlings can be grown initially on a control medium and then transferred to a medium supplemented with the compound of interest (for example, Walch-Liu and Forde, 2008; Ikeyama et al., 2010). If the compound is expected to have a complex mode of action, we have found that more information can be extracted from an experiment wherein seedlings are transferred to a medium supplemented with the compound and the analysis then performed separately on the root portion formed before treatment and during treatment (within a newly grown portion of primary root). Treatments may have differential and sometimes opposite effects on the root portions formed before and during the treatment (Ivanchenko et al., 2008, 2010).
CONCLUSIONS
In this Commentary, we reviewed the ways in which it is common to make quantitative evaluations of LR formation that can lead to unsubstantiated, misleading, or even incorrect conclusions. The main errors can be summarized as follows: (1) LR or LRP density is evaluated per total length of the primary root; when LR density is evaluated in this way, some false changes with plant age are observed; (2) conclusions about LR initiation or LR formation are based on data about LR density or LR number without taking LRPs into account; (3) LR or LRP numbers are given without density data and misleading conclusions are drawn; (4) the number of LRPs is underestimated due to estimation of LRP without clearing roots, without using differential interferential contrast microscopy or due to overreliance on marker genes. Analysis of the qualitative changes in LR formation, such as abnormal or fused primordia, is beyond the scope of this Commentary.
We illustrated these problems only using examples from Arabidopsis research. However, the types of errors identified can cause uncertainty in data interpretation in any studied species, and we recommend avoiding them for any study performed in a developmental context. Our comments are not intended to be seen as criticism of any of the studies cited herein, but rather to draw the attention of researchers to unnoticed problems that have become embedded in contemporary research on root development. Therefore, we also attempted to present a general outline of considerations that should be taken into account when a quantitative analysis of the process of LR formation is required. Due to limitations of space, we could not present an exhaustive review of the literature, and we focused on providing examples of problems in the quantification of LR initiation and branching in the recent literature. We sincerely hope that the various issues we identify here will be taken into account by the plant science community to improve our understanding of the complex processes that regulate LR formation.
Acknowledgments
We thank Selene Napsucialy-Mendivil and Orlando Trujillo-Dominguez for technical help and Shirley Ainsworth for collection of bibliometric information. Research in J.G.D.’s laboratory is supported by the Dirección General de Asuntos del Personal Académico–Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México (Grant IN212009) and Consejo Nacional de Ciencia y Technología, Mexico (Grant 127957). B.G.F. acknowledges Science Bridges funding from Research Councils UK.
AUTHOR CONTRIBUTIONS
J.G.D. initiated literature analysis and planned and wrote the article. B.G.F. critically reviewed the literature and wrote the article.
References
- Al-Ghazi Y., Muller B., Pinloche S., Tranbarger T.J., Nacry P., Rossingol M., Tardieu F., Doumas P. (2003). Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant Cell Environ. 26: 1053–1066 [Google Scholar]
- Armengaud P., Zambaux K., Hills A., Sulpice R., Pattison R.J., Blatt M.R., Amtmann A. (2009). EZ-Rhizo: Integrated software for the fast and accurate measurement of root system architecture. Plant J. 57: 945–956 [DOI] [PubMed] [Google Scholar]
- Baskin T.I. (2000). On the constancy of cell division rate in the root meristem. Plant Mol. Biol. 43: 545–554 [DOI] [PubMed] [Google Scholar]
- Beemster G.T.S., Baskin T.I. (1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116: 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Bielach A. (2010). Lateral root organogenesis - From cell to organ. Curr. Opin. Plant Biol. 13: 677–683 [DOI] [PubMed] [Google Scholar]
- Brun F., Richard-Molard C., Pagès L., Chelle M., Ney B. (2010). To what extent may changes in the root system architecture of Arabidopsis thaliana grown under contrasted homogenous nitrogen regimes be explained by changes in carbon supply? A modelling approach. J. Exp. Bot. 61: 2157–2169 [DOI] [PubMed] [Google Scholar]
- Chevalier F., Pata M., Nacry P., Doumas P., Rossingol M. (2003). Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 26: 1839–1850 [Google Scholar]
- Coates J.C., Laplaze L., Haseloff J. (2006). Armadillo-related proteins promote lateral root development in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Carmona A., You R., Haimovitch-Gal T., Doerner P. (1999). Technical advance: Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20: 503–508 [DOI] [PubMed] [Google Scholar]
- De Rybel B., et al. (2010). A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I., et al. (2007). Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- De Smet I., et al. (2008). Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- Ditengou F.A., Teale W.D., Kochersperger P., Flittner K.A., Kneuper I., van der Graaff E., Nziengui H., Pinosa F., Li X., Nitschke R., Laux T., Palme K. (2008). Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 18818–18823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Gambetta G.A., Hernández-Barrera A., Shishkova S., González I. (2006). Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann. Bot. (Lond.) 97: 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Napsucialy-Mendivil S., Duclercq J., Cheng Y., Shishkova S., Ivanchenko M.G., Friml J., Murphy A.S., Benková E. (2011). Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 191: 970–983 [DOI] [PubMed] [Google Scholar]
- Dubrovsky J.G., Sauer M., Napsucialy-Mendivil S., Ivanchenko M.G., Friml J., Shishkova S., Celenza J., Benková E. (2008). Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky J.G., Soukup A., Napsucialy-Mendivil S., Jeknic Z., Ivanchenko M.G. (2009). The lateral root initiation index: An integrative measure of primordium formation. Ann. Bot. (Lond.) 103: 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy L., Fourcaud T., Stokes A., Danjon F. (2005). A density-based approach for the modelling of root architecture: application to Maritime pine (Pinus pinaster Ait.) root systems. J. Theor. Biol. 236: 323–334 [DOI] [PubMed] [Google Scholar]
- Dupuy L., Gregory P.J., Bengough A.G. (2010). Root growth models: Towards a new generation of continuous approaches. J. Exp. Bot. 61: 2131–2143 [DOI] [PubMed] [Google Scholar]
- Effendi Y., Rietz S., Fischer U., Scherer G.F.E. (2011). The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J. 65: 282–294 [DOI] [PubMed] [Google Scholar]
- Fukaki H., Taniguchi N., Tasaka M. (2006). PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J. 48: 380–389 [DOI] [PubMed] [Google Scholar]
- Fukaki H., Tasaka M. (2009). Hormone interactions during lateral root formation. Plant Mol. Biol. 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Hermans C., Porco S., Verbruggen N., Bush D.R. (2010). Chitinase-like protein CTL1 plays a role in altering root system architecture in response to multiple environmental conditions. Plant Physiol. 152: 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama Y., Tasaka M., Fukaki H. (2010). RLF, a cytochrome b(5)-like heme/steroid binding domain protein, controls lateral root formation independently of ARF7/19-mediated auxin signaling in Arabidopsis thaliana. Plant J. 62: 865–875 [DOI] [PubMed] [Google Scholar]
- Ingram P.A., Malamy J.E. (2010). Root system architecture. Advances in Botanical Research, J.-C. Kader, and M. Delseny, eds (New York: Academic Press/Elsevier), pp. 75–117 [Google Scholar]
- Ivanchenko M.G., Muday G.K., Dubrovsky J.G. (2008). Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 55: 335–347 [DOI] [PubMed] [Google Scholar]
- Ivanchenko M.G., Napsucialy-Mendivil S., Dubrovsky J.G. (2010). Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant J. 64: 740–752 [DOI] [PubMed] [Google Scholar]
- Ivanov V.B., Dubrovsky J.G. (1997). Estimation of the cell-cycle duration in the root meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. Int. J. Plant Sci. 158: 757–763 [Google Scholar]
- Jiang H.W., Liu M.J., Chen I.C., Huang C.H., Chao L.Y., Hsieh H.L. (2010). A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 154: 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y., et al. (2011). Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233: 209–216 [DOI] [PubMed] [Google Scholar]
- Kiss J.Z., Mullen J.L., Correll M.J., Hangarter R.P. (2003). Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 131: 1411–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G., et al. (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18: 927–937 [DOI] [PubMed] [Google Scholar]
- Laplaze L., et al. (2007). Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.W., Kim N.Y., Lee D.J., Kim J. (2009). LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Mo X., Wang J., Chen N., Fan H., Dai C., Wu P. (2009). BREVIS RADIX is involved in cytokinin-mediated inhibition of lateral root initiation in Arabidopsis. Planta 229: 593–603 [DOI] [PubMed] [Google Scholar]
- López-Bucio J., Cruz-Ramírez A., Herrera-Estrella L. (2003). The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6: 280–287 [DOI] [PubMed] [Google Scholar]
- Lucas M., et al. (2011). SHORT-ROOT regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol. 155: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor D.R., Deak K.I., Ingram P.A., Malamy J.E. (2008). Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell 20: 2643–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod R.D., Thompson A. (1979). Development of lateral root primordia in Vicia faba, Pisum sativum, Zea mays and Phaseolus vulgaris: Rates of primordium formation and cell doubling times. Ann. Bot. (Lond.) 44: 435–449 [Google Scholar]
- Malamy J.E., Benfey P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marchant A., Bhalerao R., Casimiro I., Eklöf J., Casero P.J., Bennett M., Sandberg G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael B.L., Burke J.J. (1998). Soil temperature and root growth. HortScience 33: 947–951 [Google Scholar]
- Monshausen G.B., Bibikova T.N., Weisenseel M.H., Gilroy S. (2009). Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21: 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno M.A., Van Norman J.M., Moreno A., Zhang J., Ahnert S.E., Benfey P.N. (2010). Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morquecho-Contreras A., Méndez-Bravo A., Pelagio-Flores R., Raya-González J., Ortíz-Castro R., López-Bucio J. (2010). Characterization of drr1, an alkamide-resistant mutant of Arabidopsis, reveals an important role for small lipid amides in lateral root development and plant senescence. Plant Physiol. 152: 1659–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497 [Google Scholar]
- Nodzon L.A., Xu W.H., Wang Y., Pi L.Y., Chakrabarty P.K., Song W.Y. (2004). The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J. 40: 996–1006 [DOI] [PubMed] [Google Scholar]
- Ortiz-Castro R., Díaz-Pérez C., Martínez-Trujillo M., del Río R.E., Campos-García J., López-Bucio J. (2011). Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 108: 7253–7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer W.A., Hosein F.N., Bandyopadhyay A., Makam S.N., Otegui M.S., Lee G.J., Blakeslee J.J., Cheng Y., Titapiwatanakun B., Yakubov B., Bangari B., Murphy A.S. (2009). Mutation of the membrane-associated M1 protease APM1 results in distinct embryonic and seedling developmental defects in Arabidopsis. Plant Cell 21: 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelagio-Flores R., Ortíz-Castro R., Méndez-Bravo A., Macías-Rodríguez L., López-Bucio J. (2011). Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana. Plant Cell Physiol. 52: 490–508 [DOI] [PubMed] [Google Scholar]
- Péret B., De Rybel B., Casimiro I., Benková E., Swarup R., Laplaze L., Beeckman T., Bennett M.J. (2009). Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Pérez-Torres C.A., López-Bucio J., Cruz-Ramírez A., Ibarra-Laclette E., Dharmasiri S., Estelle M., Herrera-Estrella L. (2008). Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M.E., Schofield A., Lyzenga W., Liu H., Stone S.L. (2010). Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiol. 153: 1587–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T., Nacry P., Pervent M., Girin T., Tillard P., Lepetit M., Gojon A. (2006). A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 140: 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L., et al. (2011). The Arabidopsis D-type cyclin CYCD2;1 and the inhibitor ICK2/KRP2 modulate auxin-induced lateral root formation. Plant Cell 23: 641–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik-Inbar D., Bar-Zvi D. (2010). ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22: 3560–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., et al. (2009). Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21: 1495–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Tapia-López R., García-Ponce B., Dubrovsky J.G., Garay-Arroyo A., Pérez-Ruíz R.V., Kim S.H., Acevedo F., Pelaz S., Alvarez-Buylla E.R. (2008). An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 146: 1182–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel S., Kaeppler S., Brown K., Lynch J. (2011). Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341: 75–87 [Google Scholar]
- Uehara T., Okushima Y., Mimura T., Tasaka M., Fukaki H. (2008). Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol. 49: 1025–1038 [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellosillo T., Martínez M., López M.A., Vicente J., Cascón T., Dolan L., Hamberg M., Castresana C. (2007). Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 19: 831–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Liu P., Forde B.G. (2008). Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J. 54: 820–828 [DOI] [PubMed] [Google Scholar]
- Werner T., Nehnevajova E., Köllmer I., Novák O., Strnad M., Krämer U., Schmülling T. (2010). Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22: 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh N., Fisahn J. (2010). Analysis of Arabidopsis thaliana root growth kinetics with high temporal and spatial resolution. Ann. Bot. (Lond.) 105: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Forde B.G. (1998). An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409 [DOI] [PubMed] [Google Scholar]
- Zhang H., Jennings A., Barlow P.W., Forde B.G. (1999). Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wei L., Xu J., Zhai Q., Jiang H., Chen R., Chen Q., Sun J., Chu J., Zhu L., Liu C.M., Li C. (2010). Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell 22: 3692–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]