In plants, the directional flow and sites of hormone accumulation facilitate organ development. Identifying leaves as the source of a mobile signal that induces gibberellin accumulation and signaling revealed the hormone’s roles in plant primary and secondary growth. It is demonstrated that leaf-induced gibberellin is required for stem elongation, cambial activity, and fiber formation.
Abstract
The gibberellins (GAs) are a group of endogenous compounds that promote the growth of most plant organs, including stem internodes. We show that in tobacco (Nicotiana tabacum) the presence of leaves is essential for the accumulation of bioactive GAs and their immediate precursors in the stem and consequently for normal stem elongation, cambial proliferation, and xylem fiber differentiation. These processes do not occur in the absence of maturing leaves but can be restored by application of C19-GAs, identifying the presence of leaves as a requirement for GA signaling in stems and revealing the fundamental role of GAs in secondary growth regulation. The use of reporter genes for GA activity and GA-directed DELLA protein degradation in Arabidopsis thaliana confirms the presence of a mobile signal from leaves to the stem that induces GA signaling.
INTRODUCTION
The gibberellins (GAs) are a well-studied class of growth regulators that participate in many developmental processes in plants (Richards et al., 2001; Yamaguchi, 2008). Since their discovery in plants in the 1950s, understanding of the molecular mechanisms by which GAs promote growth has advanced impressively (Harberd et al., 2009). However, while the role of GA in facilitating the degradation of the DELLA growth repressors is known in considerable detail (Silverstone et al., 2001; Ueguchi-Tanaka et al., 2005; Murase et al., 2008; Shimada et al., 2008), knowledge of the downstream signaling events that mediate most GA-induced developmental processes is only beginning to emerge. Furthermore, there is limited information on GA transport and its relevance to GA function. Many contradictory results have been published concerning the origin, transport, and perception of GAs. In rice (Oryza sativa), it was found that sites of bioactive GA synthesis and signaling overlap in most cases, an exception being the aleurone cells of the endosperm that are incapable of GA synthesis and are dependent on the scutellum as a GA source (Lovegrove and Hooley, 2000; Kaneko et al., 2003). In tobacco (Nicotiana tabacum), bioactive GAs are produced in dividing and elongating cells through the temporal and spatial regulation of the expression of the GA 3-oxidases (Itoh et al., 1999), which encode the last enzyme in the biosynthetic pathway (MacMillan, 1997; Hedden and Proebsting, 1999). Nevertheless, translocation of the hormone was not ruled out for the unexplained expansion of epidermal cells, which do not express this gene (Itoh et al., 1999). It was also shown that local de novo synthesis of bioactive GAs is necessary for stamen development and that their short-distance transport is required to support petal growth (Hu et al., 2008). Although GA feeding studies have demonstrated long-distance movement of bioactive GAs and some precursors (Katsumi et al., 1983; Reid et al., 1983; Proebsting et al., 1992; Gallego-Giraldo et al., 2007), the importance of this transport is unclear. Expression of GA1, encoding ent-copalyl diphosphate synthase, which catalyzes the first committed step of GA biosynthesis, was localized to the vascular tissues of some nongrowing organs, such as expanded leaves, suggesting that they may be sites of GA biosynthesis for transport to other organs (Silverstone et al., 1997). Additionally, GAs have been detected in the phloem, consistent with their origin in vegetative tissues (Garcia-Martinez et al., 1991; Hoad et al., 1993), while labeled GA4 applied to an Arabidopsis thaliana rosette leaf was detected in the shoot apex (Eriksson et al., 2006). These findings all indicate transport of GAs from leaves to sink organs via the phloem. Recently, using grafting of GA biosynthesis and signaling mutants, it was demonstrated that GA is a mobile signal from the shoot that triggers xylem expansion in wild-type Arabidopsis hypocotyls (Ragni et al., 2011).
In this study, we investigated the physiological importance of leaves as a potential source of remote GA signaling and the consequences for stem development following their removal. We show that the presence of leaves is essential for normal internode elongation, cambial activity, and fiber differentiation along the stem and that these processes can be rescued after leaf removal by exogenous GA.
As plants populated land, they developed organs and tissues to overcome their immobility and acclimate to their new terrestrial habitats. Initial growth of a plant is therefore focused on its anchoring to the ground, via roots, and supporting their photosynthetic organs, via the stem. The apical meristems in these organs provide the plant with the primary tissues to support its elongation and structure, but in dicots these are usually not sufficient for continuous growth and maintenance of the plant. The cambium, a lateral meristem, provides plants with the ability to grow thicker stems by producing the secondary vascular tissues in both the xylem and phloem. Cambial derivatives in the xylem have the potential to differentiate to tracheary elements, fiber or parenchyma cells if they originate from fusiform initials, or to ray cells if originating from the ray initials. Each cambial initial produces radial files of cells by periclinal divisions (Evert, 2006). These additive divisions along with the cambial anticlinal divisions (which enlarge its circumference) account for the plant’s secondary growth. The vascular tissues enable water transpiration and assimilate transport throughout the whole plant as well as provide the structural support for the elongating body. As a result, cell production by the cambium determines root and shoot thickening. Consequently, the cambium also impacts the amount of incorporated carbon in the walls of vascular cells. Therefore, secondary growth is of great economic importance as it results in the production of wood, which is a valuable renewable source of energy and is a raw material for pulping and construction purposes.
As in all developmental processes, cambial activity is tightly regulated by hormonal signaling. Phytohormones, namely auxin (indole-3-acetic acid [IAA]), GA, cytokinin, abscisic acid, ethylene, and brassinosteroids, have been implicated in the integration of environmental signals to regulate cambial activity (Aloni, 1987; Yamamoto et al., 1997; Helariutta and Bhalerao, 2003; Israelsson et al., 2005; Aloni et al., 2006). In this respect, auxin has been shown to be the major regulator for cambial proliferation and derivation. Half a century ago, IAA was determined as the primary regulator for cambial division and derivative differentiation (Digby and Wareing, 1966), while GA has also been implicated in these processes (Digby and Wareing, 1966). It was determined that the relative levels of applied IAA and GA determine whether xylem or phloem tissues are produced: high IAA:GA ratios favor xylem formation, while low IAA:GA ratios favor phloem production (Digby and Wareing, 1966). More recent molecular and biochemical approaches show that auxin maxima are generated in the cambial region, where it regulates secondary xylem development (Nilsson et al., 2008). Genetic enhancement of GA content by overexpressing GA biosynthesis genes or silencing GA deactivation produced a substantial promotion of stem growth and xylem development, with higher fiber yields (Eriksson et al., 2000; Biemelt et al., 2004; Dayan et al., 2010). However, in contrast with auxin and cytokinin, GA was not depicted as an essential signal for cambial activity.
Here, by separating GA signaling from auxin flow, we demonstrate their unique roles in cambial regulation and, specifically, in xylem fiber (libriform fibers) and vessel differentiation.
RESULTS
Leaves Regulate GA-Dependent Internode Elongation and Secondary Growth
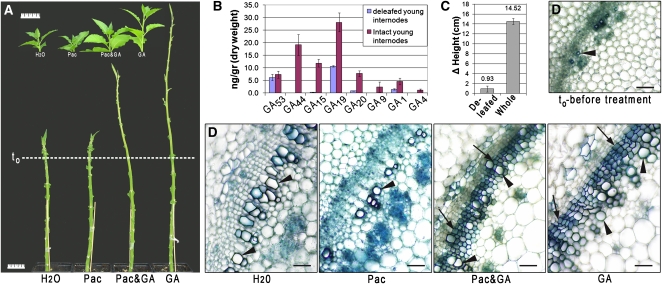
We investigated the physiological relevance of leaf-derived GAs by excising developing and mature leaves from 5-week-old tobacco plants, leaving the youngest apical leaf and leaf primordia intact to maintain a natural auxin source. Defoliation resulted in the development of extremely short (shorter than 3 mm) internodes at the apex, resembling GA-deficient phenotypes (Koornneef et al., 1990). The dwarfing effect was severe to the extent that treatment with paclobutrazol, a GA synthesis inhibitor (Hedden and Graebe, 1985), had no additional impact. As for intact plants treated with paclobutrazol, internode growth was restored by application of GA3 to the base of the stem, well below the shoot apex (Figure 1A). GA application to paclobutrazol-treated defoliated plants did not restore growth to the same extent. The GA3 concentration used in these highly reproducible studies (over six independent biological repeats totaling over 120 plants) was similar to the minimal dose found to enhance secondary growth in decapitated but leafy plants. This GA concentration also promoted stem elongation in intact leafy plants (Figure 1A), but to a lesser extent than for defoliated stems, indicating that lack of GA is a major factor for the loss of internode elongation. Quantification of GAs in the young internodes revealed that the concentration of the bioactive GA1 in deleafed plants was reduced by 60% compared with internodes from intact plants, while GA4 was not detected in the deleafed internodes (Figure 1B; see Supplemental Table 1 online), which failed to elongate following leaf excision (Figure 1C). Comparison of cross sections of internodes from deleafed and intact plants revealed that plants stripped of their developing leaves (excluding the youngest leaves surrounding the apex) lacked cambial proliferation and secondary vascular development, with only primary vessels present. The application of GA3 restored normal cambial development and xylem fiber differentiation (Figure 1D; macerations and quantification are shown in Supplemental Figure 1 online). We repeated this treatment, but left one developed leaf intact (below the sixth internode). As in the application of GA, the leaf maintained normal secondary growth, which was not observed in its absence (see Supplemental Figure 2A online). Additionally, we observed a decrease in the rate of development of new internodes in defoliated plants (see Supplemental Figure 2B online). In contrast with the immediate halt in elongation, the continued development of internodes and the rescued elongation subsequent to GA application indicate GA regulates internode elongation. Although it is known to enhance fiber-to-vessel ratio in the xylem (Digby and Wareing, 1966), GA has not been previously acknowledged as a key cambium regulator in young internodes above source leaves. These results show that the leaf-derived signal is necessary for cambial activity and differentiation; in the absence of leaves, xylem fibers do not develop.
Figure 1.
Developed Leaves Act as a Source of the Mobile Signal, Inducing GA-Dependent Developmental Processes throughout the Shoot.
(A) Tobacco plants after leaf excision were treated with water, paclobutrazol (Pac; 50 μM, sprayed once every 3 d), paclobutrazol and 0.8% GA3(lanolin), or 0.8% GA3(lanolin) alone. Leaf initials developed normally, while differences were observed in internode length (line indicates plant height at the time of leaf excision and first treatments). The phenotype of water-treated plants resembled that of plants treated with paclobutrazol. Application of GA3 alone restored internode elongation. At the top, control whole plants, treated as deleafed plants. Bars = 5 cm.
(B) Concentration of GAs in young internodes of whole tobacco plants (red) compared with deleafed plants (blue). The data shown represent the average of three and four independent biological replicates for deleafed and intact plants, respectively. Each replicate combined ≥15 plants for deleafed and >5 for whole plants. Bars represent sd.
(C) Comparison of height change between intact and deleafed plants. The data shown represent the average of three biological repeats. For deleafed plants, n = 13 to 15, and for whole plants n = 6 to 8. Bars indicate sd. Results are highly significant (t test, P < 0.005).
(D) Internode cross sections prepared at the end of the experiment showing secondary growth development in what was the uppermost internode at the time of the initial treatments. Plants were treated as in (A). Only GA3-treated leafless plants had developed fibers (lower lumen to cell wall ratio compared with the hollow vessels) and exhibited an active cambium (arrows mark xylem fibers; arrowheads mark vessels). Bars = 50 μm.
[See online article for color version of this figure.]
C19-GAs Possess the Characteristics for the Leaf-Derived Mobile Signal
While our results indicate that internode elongation and vascular differentiation are dependent on a leaf-derived signal that causes GA to accumulate in the stem, the mobile signal is unknown. Candidates include bioactive GAs, biosynthetic precursors or a secondary messenger that promotes local GA synthesis. To address this issue, we compared the efficacy of the bioactive GA1 with its precursors GA20 and GA19 in promoting elongation of deleafed stems when injected at physiological concentrations below the elongating internodes. Both C19-GAs, namely, GA1 and GA20, but not the C20 precursor GA19, promoted stem elongation (Figure 2A). Furthermore, it is evident that the apical intact leaves in the mock- and GA19-treated plants are epinastic and that their petioles are compact compared with the plants injected with the C19-GAs. Thus, in deleafed tobacco stems, the C19-GAs are mobile and, in the case of GA20, converted to a bioactive form in the stem, whereas GA19 is immobile and/or is not converted to biologically active GAs. Since conversion of GA19 requires GA 20-oxidase activity and GA 3-oxidase catalyzes the oxidation of GA20 to the bioactive GA1, we analyzed their relative expression in the apical internodes of deleafed compared with intact plants. We found that although GA20ox1 transcript abundance is elevated in the deleafed internodes, GA20ox2 transcript level is reduced. On the other hand, highly elevated levels of two tobacco GA 3-oxidase transcripts were detected in the same internodes, presumably due to relief of feedback inhibition in the low GA environment (Yamaguchi, 2008) (Figure 2B). These results indicate that the elongating internodes are potentially capable of converting GA19 to GA20 and subsequently to GA1. However, the low levels of C19-GAs relative to that of GA19 in the mature internodes (see Supplemental Table 1 online) suggest that GA19 is unlikely to be converted to C19-GAs at the site of injection and that its failure to promote growth was due to immobility. Both GA1 and GA20 are present at relatively high levels in expanding leaves (see Supplemental Table 1 online) and are therefore candidates for the mobile signal.
Figure 2.
C19-GAs Rescue Internode Elongation in Defoliated Plants.
(A) GAs were injected beneath the last nonelongated internode of deleafed plants at physiological concentrations, as determined by gas chromatography–mass spectrometry in the corresponding tissues of intact tobacco plants (Figure 1). Values are the mean increase in height during 3.5 weeks of treatment. Bars = 2 cm. The data shown represent the average of three biological repeats; n (total) ≥ 9 for each of the treatments; sd depicted below the averages. Asterisk indicates the results are highly significant compared with the mock treatment (t test, P < 0.0001).
(B) Change in the relative GA 20-oxidase (Top) and GA 3-oxidase (Bottom) transcription levels in the apical nonelongated internodes, induced by leaf excision. Results show the minimal change measured for two independent biological repeats (n ≥ 11 for deleafed plants, and n = 5 for each of the intact repeats). Error bars represent sd.
[See online article for color version of this figure.]
GA and Auxin Are Each Essential for Cambial Activity
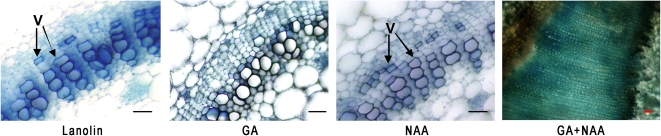
Thus far, we have shown that deleafing plants depletes their endogenous GA concentrations, which impairs both shoot elongation and cambial activity (Figure 1). Since auxin is a major regulator of secondary growth and GA was thought only to enhance auxin activity, we repeated the leaf excision study in decapitated plants (eliminating endogenous auxin). Performing the experiment on 6-week-old plants that exhibited secondary growth, we validated our findings on the distinctive roles of GA and auxin. To differentiate between the effects of auxin and GA, we applied the hormones separately and combined onto the apical cut surface. As in the control lanolin application, each of these hormones applied separately resulted in an undeveloped vasculature. The xylem in the cross sections of the uppermost internode beneath the cut consisted of primary vessels (in rare cases, secondary vessels that formed prior to treatment were detected) and a region of undifferentiated cells formed between the outer phloem and the xylem (Figure 3; see Supplemental Figures 3 and 4 online). We did not detect any new derivation by the cambium under these conditions. The small cells with only primary walls between the phloem and xylem did not proliferate or differentiate and were thus more parenchyma-like than the cambial cells that would be anticipated in the same region. The only noticeable vessel differentiation occurred under the auxin treatment, where sporadic short and narrow vessels developed from these parenchyma-like cells (Figure 3; see macerations depicting the cells in Supplemental Figure 4 online). This induction is not surprising since the cambial zone is the preferred route for auxin transport (Tuominen et al., 1997). As described for wound repair, auxin flow induces the redifferentiation of parenchyma cells to vessel elements (Aloni, 2010). In the absence of auxin, plants treated only with GA did not exhibit vascular development. However, the simultaneous application of both auxin and GA stimulated cambial activity, characterized by a high proliferation rate and the formation of an overwhelming thick, fiber-rich xylem (Figure 3; see Supplemental Figure 4 online). These results imply that GA and auxin are each essential for cambial activity and that they regulate distinctive pathways that act synergistically to promote secondary growth.
Figure 3.
GA and Auxin Are Each Essential for Cambial Activity.
Tobacco plants treated with lanolin (control), GA3, auxin, and GA3 and auxin. Hormones were applied to the apical cut end after decapitation and leaf excision. Cambial activity and fiber formation are only detected in internodes of plants treated with the combination of GA and 1-naphthalene acetic acid (NAA). V, vessels. Bars = 50 μm. Microphotographs in the same scale can be found in Supplemental Figure 3 online.
[See online article for color version of this figure.]
GA Is the Specific Signal for Xylem Fiber Differentiation
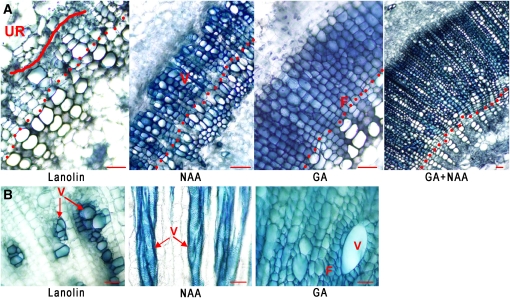
Since the proliferation and differentiation of initials are separate processes, we searched for a GA-specific differentiation process. While it is known (Aloni, 2010) that auxin induces vessel formation (also shown in Figure 3), GA has not been shown to induce the differentiation of certain cell types specifically. To examine if there are differentiation processes that can be attributed to GA, we induced the formation of undifferentiated cells in the cambial region. Plants from which the shoot apex and mature leaves had been excised were maintained for 2 weeks before hormonal application, during which time an undifferentiated region was induced (Figure 4A). Subsequently, application of auxin alone induced their differentiation to vessels, while GA stimulated mostly fiber formation (Figure 4A). We have verified the identity of these cells by longitudinal sectioning, which enabled specific characterization of all the cells in situ (see Supplemental Figure 5 online). Shown in Figure 3, the simultaneous application of both hormones resulted in prolific xylem formation with an active cambium (Figure 4A). We also show the same GA induction of fibers in petioles (Figure 4B). In decapitated tobacco plants, the petiole of a solitary young leaf is composed of many undifferentiated cells surrounding only a few vessels (Figure 4B). The application of auxin had no effect on fiber formation, while supplying the tissue with GA swiftly resulted in their differentiation to fiber cells (Figure 4B). We conclude from these results that GA is the specific signal that induces secondary xylem fiber differentiation.
Figure 4.
GA Is the Specific Signal for Fiber Differentiation.
Tobacco plants were decapitated and deleafed.
(A) After maintaining the plants for 2 weeks with lanolin applied to the apical cut end, they were treated with GA3, NAA, and the combination of the two hormones. Vessels (hollow cells with a higher lumen–to–cell wall ratio compared with fibers) are mainly detected subsequent to NAA application, while fibers form in response to GA3 treatment. Dots indicate the area in the beginning of the xylem phenotype induced by the respective treatments. Curved line indicates the area of the undifferentiated region.
(B) Plants were treated as in (A) with the exception of an apical leaf that was kept intact. Cross sections were performed at the bottom of the leaf’s petiole. F, fibers; UR, undifferentiated region; V, vessels. Bars = 55 μm.
[See online article for color version of this figure.]
GA Induces Fiber Formation in Both the Acro- and Basipetal Directions
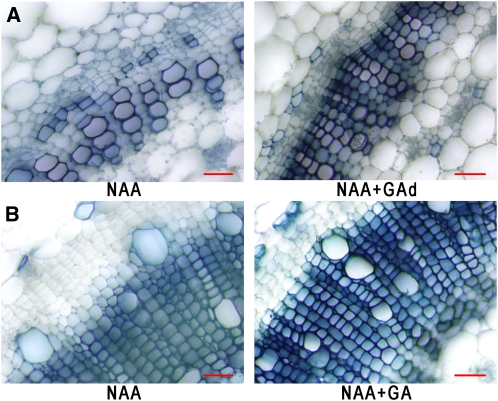
Earlier we showed that GA application to the bottom of defoliated stems restores internode elongation and secondary growth at the apex (Figure 1), implying that the GA signal flows acropetally. Moreover, we found that auxin applied to an apical cut does not restore secondary growth, which is rescued by the additional application of GA to the base of the stem, affirming that the acropetal movement is significant for fiber formation (Figure 5A). On the other hand, we found that applying GA along with auxin at the decapitation site of leafless plants restores cambial proliferation several internodes below that point (Figure 5B). These results indicate that the impact of the GA signal is nonpolar along the stem, inducing secondary growth above and below its application site.
Figure 5.
Exogenous GA Restores Secondary Growth to GA-Deficient Plants Irrespective of the Point of Application.
Decapitated plants treated with NAA at the apical cut. GAd, GA applied at the bottom of the stem. Bars = 50 μm.
(A) Cross sections in the second internode from the apex. NAA induces vessel development in the area where the cambium is anticipated to be situated in intact plants. GA3 applied to the bottom of the stem restores cambial activity and fiber formation.
(B) Cross sections in the sixth internode below the apex exhibiting GA3 signaling in the basipetal orientation. In the absence of exogenous GA, the last developed xylem cell lines exhibit larger vessels and fiber differentiation is not complete (i.e., cell wall staining diminishes; see longitudinal section in Supplemental Figure 5C online).
[See online article for color version of this figure.]
The Direction of a GA-Like Signal within and from Developing Arabidopsis Leaves
In Arabidopsis, GA signaling and local bioactive GA movement are necessary for petal development and flower maturation (Hu et al., 2008). Therefore, we used the polar acropetal movement of paclobutrazol (Rademacher, 2000) in Arabidopsis to assay the competency of rosette leaves to promote normal floral development. Spraying the whole plant with paclobutrazol prevented sepals from opening (Figure 6, left), while daily submergence of the whole inflorescence stems, but not the rosette, in paclobutrazol did not impair floral development to the same extent, enabling petal development (Figure 6, center). Submerging the inflorescence in paclobutrazol interfered with normal stem elongation and flower development (some sterile flowers compared with mock treatment), although both developmental processes were less severely affected than after spraying the whole plant, producing fertile flowers and longer inflorescence stems. These results indicate that normal GA signaling in the Arabidopsis inflorescence requires the presence of rosette leaves.
Figure 6.
Arabidopsis Rosette Leaves Facilitate Flower Development.
GA deficiency induced by spraying paclobutrazol (Pac) on the whole shoot halts normal flower development in Arabidopsis, and flowers remain closed. Application of the inhibitor only to the inflorescence, by its daily dipping in a paclobutrazol solution, resulted in normal flower development. Bar in main figure = 5 cm.
[See online article for color version of this figure.]
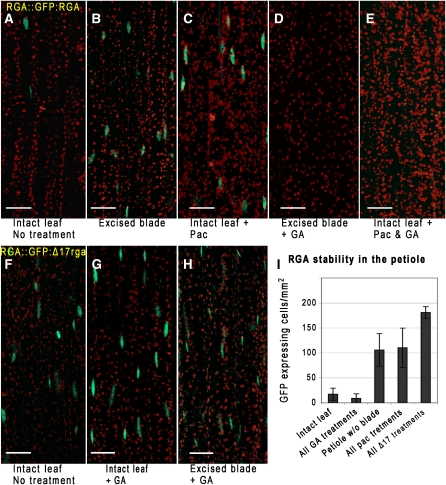
To obtain further evidence that developing rosette leaves are a source for long-distance GA signaling, we examined the GA-dependant stability of a DELLA protein, Repressor of ga1-3 (RGA), fused to green fluorescent protein (GFP) (Silverstone et al., 2001). Mediated by the GA-receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1), GA induces RGA degradation by the 26S proteasome via interaction with an SCF E3 ligase and polyubiquitination (Murase et al., 2008). Using transgenic Arabidopsis plants harboring ProRGA:GFP-RGA, we determined the effect of excising the leaf blade on GFP-RGA stability in the petiole. GFP-RGA accumulation in petioles after blade excision was substantially enhanced compared with petioles from intact leaves (Figures 7A and 7B). Paclobutrazol application to intact leaves also stabilized GFP-RGA (Figure 7C), while exogenous GA4 caused increased RGA degradation in debladed petioles and paclobutrazol-treated leaves (Figures 7D and 7E). To assess the specificity of the response to deblading and to exclude any indirect effects due to wounding, the same treatments were applied to transgenic Arabidopsis plants harboring a similar construct, but with a 17–amino acid deletion in RGA (ProRGA:GFP-rga-Δ17). This mutation prevents GA-dependent DELLA degradation (Dill et al., 2001). Indeed, plants harboring this construct have substantially reduced RGA degradation in the petiole (Figures 7F to 7I), consistent with the GA sensitivity of the observed response to deblading. Additionally, partial incisions were made in leaf blades to assess the potential influence of wound-generated ethylene on RGA stability (Achard et al., 2006). Apart from full blade, we did not notice any effect on RGA stability (fluorescence was not detected in the vicinity of the incisions).
Figure 7.
DELLA Protein Stability in the Petiole Depends on a GA Signal from the Blade.
Longitudinal images of Arabidopsis petioles expressing ProRGA:GFP-RGA, demonstrating the requirement of the leaf blade for GA-induced DELLA degradation. The chimeric proteins localize to the nuclei (stained green). Bars = 50 μm.
(A) GFP-RGA accumulation is low in the petiole of untreated plants.
(B) Excising leaf blades stabilized the protein (green nuclei).
(C) Paclobutrazol (Pac) treatment stabilizes the protein.
(D) GA application to petioles following blade excision reduced GFP-RGA content to wild-type levels (A), as did GA treatment of paclobutrazol sprayed leaves (E).
(F) to (H) GFP fluorescence remains high in plants harboring ProRGA:GFP-rga-Δ17, which is resistant to GA-induced degradation, after treatments as in (A), (D), and (E), respectively. All images are projections of multiple slices along the depth of the vasculature. Red exhibits the autofluorescence of the chloroplasts.
(I) Chart representing the number of GFP expressing nuclei per mm2 in petiole depth confocal projections (n = 5, 11, 10, 9, or 6 for treatments as presented in the figure from left to right). Results are mean ± sd. P < 0.0001 for intact blades compared with excised blades and for all paclobutrazol treatments compared with all GA treatments; P = 0.001 for all treatments of ProRGA:GFP-rga-Δ17–expressing plants compared with all paclobutrazol treatments; two-tailed unpaired Student’s t test.
To further analyze the flow characteristics of the leaf generated signal, we analyzed expression of three reporter genes in which the GA-inducible promoters EXP1, MYB34, and GA2OX2 were fused to the β-glucuronidase (GUS) coding sequence (see Supplemental Appendix 1 online). In addition, we produced a reporter gene with a synthetic minimal promoter consisting of GA responsive motifs. The expression profile of the synthetic GA-responsive promoter corresponds to the tissues and cells we show are GA regulated, particularly the fibers in the vascular bundle and interfasicular region (see Supplemental Figure 6 online). Arabidopsis plants harboring these constructs exhibited GUS activity (Figures 8A to 8D) that was abolished by treatment with paclobutrazol. Interestingly, elevated levels of GUS expression were detected in the petiole-stem junctions (Figures 8A and 8C; see Supplemental Figure 7 online). The GA-inducible gene LEAFY was also shown to be highly expressed in this region (Blázquez and Weigel, 2000). These junctions have a distinct anatomy, characterized by short cells in both the ground and vascular tissues (illustrated in Figures 8E to 8G), which will at the appropriate time participate in leaf abscission (Raven et al., 2005). We propose that the elevation in GUS expression is caused by a local decreased cell size that slows the flow of the mobile signal, thereby causing its local accumulation (Figure 8). We cut leaf blades and petioles in different locations to produce artificial barriers that would simulate the hypothesized role of the abscission zone. Indeed, the incisions caused GUS accumulation in their vicinity (Figures 9A to 9E). Furthermore, the observed pattern of GUS staining indicates that the flow in the leaf blade is nonpolar and associated with the vascular veins, primarily in the phloem and bundle sheath cells (Figures 9F and 9G). This localization is consistent with previous findings localizing the primary GA responsive tissue in Arabidopsis roots to the endodermis (Ubeda-Tomás et al., 2008) (both the bundle sheath, in leaves, and the endodermis, in roots, surround the vascular tissues). Only when approaching the major leaf veins at the leaf base and petiole does the expression pattern become polar, oriented toward the stem (Figures 9A to 9E). This local basipetal polarity is consistent along the petiole (Figures 8 and 9E). Since GA is probably not the only signal that induces these genes, we tested their responses to auxin transport inhibitors. This treatment did not affect the expression patterns in the same manner as paclobutrazol or GA application (see Supplemental Figures 8A and 8B online). As a control, ProDR5:GUS (an artificial auxin responsive promoter [Ulmasov et al., 1999] fused to GUS) expression displayed a strict polar pattern in the cut blades away from the mid-vein, in contrast with ProGA2ox2:GUS expression (see Supplemental Figure 8C online), indicating that auxin signaling did not influence our results. The enhanced GUS expression in the petiole may be functionally related to the fact that the majority of leaf elongation occurs at its bottom third (Poethig and Sussex, 1985), regardless of leaf developmental stage (see Supplemental Table 2 online). A comparison of bioactive GA content in the upper and lower halves of the petiole did not confirm an accumulation of GA at the petiole base (see Supplemental Table 1 online), which could imply that the enhanced GA signal transduction at this site is due to a different signal. However, it is possible that only a few cells accumulate the GA signal and much more precise resolution would be necessary to detect it. Moreover, enhanced GUS expression above the disturbance to the flow in the petiole (Figure 9E) suggests that these cells have the capacity to perceive the GA signal. Taken together, these results indicate that the orientation of GA signaling is nonpolar in leaf blades and through the stem, while directionality was seen in the petioles, consistent with the involvement of leaves in the production of the mobile signal (summarized in Figure 10).
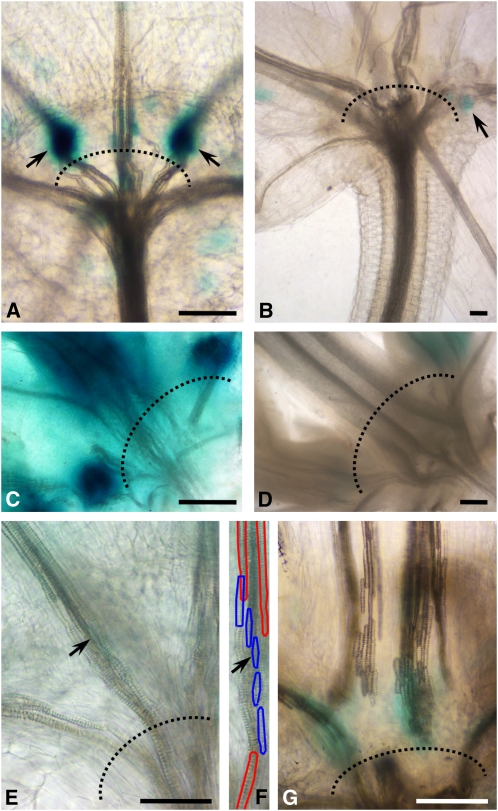
Figure 8.
GA Signaling at Petiole-Stem Junctions.
Longitudinal images of cleared Arabidopsis plants with rosette leaves. Dotted lines mark petiole-stem junctions. Bars = 55 μm in (A), 80 μm in (C), and 275 μm in (D).
(A) Accumulation of ProGA2ox2:GUS expression is observed just above the petiole-stem junctions. Arrows point to site of GUS accumulation.
(B) Paclobutrazol treatment abolishes the GUS expression.
(C) Pro70Fk:GUS (artificial GA-responsive marker) is highly expressed just above the petiole-stem junctions.
(D) Expression of Pro70Fk:GUS is abolished by paclobutrazol treatment. Similar expression profiles of other GA-responsive promoters are shown in Supplemental Figure 7 online.
(E) to (G) The leaf abscission region at the junction with the stem is characterized by short cells. Short tracheary elements (marked with blue in [F]) differentiated between the long vessels (marked with red in [F]) of the petiole and stem. An identical vessel element is marked by arrows in (E) and (F).
(F) The cell walls of some of the short vessels between the long ones are outlined in blue and red, respectively.
(G) Longitudinal section through the base of the petiole, showing short vessel elements with high ProGA2ox2:GUS expression.
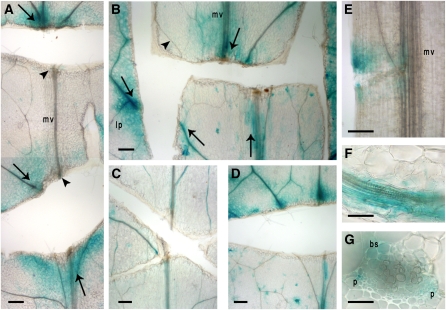
Figure 9.
Direction of the Signal Translocation.
Various incisions in rosette leaves and petioles of ProGA2ox2:GUS-expressing Arabidopsis plants (top part of images are closest to the leaf tip). Bars = 210 μm in (A) to (E) and 55 μm in (F) and (G). Arrows mark GUS activity and flow orientation, arrowheads mark lack of GUS activity in cut veins. bs, bundle sheath; lp, leaf periphery; mv, midvein; p, phloem.
(A) Two parallel horizontal cuts (perpendicular to the midvein axis) in the blade caused GUS accumulation (arrows, marking signal flow orientation) that is nonpolar in the leaf periphery with high activity above the upper cut of the midvein. Lack of GUS activity is indicated between the cuts (arrowheads).
(B) An “H” shape cut displaying the same peripheral nonpolar nature, as in (A), and emphasizing a preferable transport along the midvein.
(C) Paclobutrazol substantially decreases GUS expression.
(D) GA restores expression in paclobutrazol-treated leaves.
(E) GUS expression above the cut, indicating basipetal polar flow along the petiole.
(F) and (G) High magnification of leaf vascular bundles showing longitudinal and cross-section views, respectively. GUS expression localizes in the bundle sheath and phloem cells. See Figure 10 for a summary illustration.
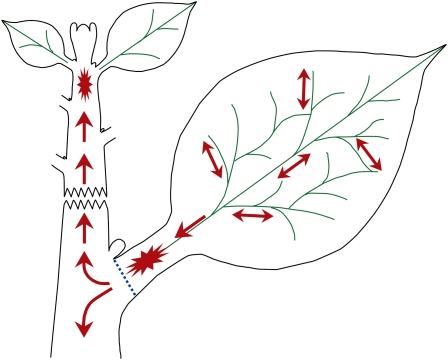
Figure 10.
The Source and Translocation of the Leaf-Derived Signal.
An illustration modeling the translocation of the leaf-derived mobile signal. The signal originates in developing leaves (longer than 3 cm, but not leaves that are emerging at the shoot apex). Its flow is nonpolar in the leaf blade and becomes polar only in the lower midvein toward the stem (arrows mark flow orientation). The unique anatomy at the base of the petiole (blue dotted line) potentially retards the flow, which induces a local maximum (star), thereby acting as the leaf's elongation driving force. The signal flows in both directions along the stem; its upward movement from developing leaves reaches the young internodes (star) and induces stem elongation at the shoot apex. Throughout the flow along the stem, the signal results in bioactive GA signaling that controls cambial activity and fiber differentiation.
[See online article for color version of this figure.]
DISCUSSION
Our results demonstrate that GA accumulation in the apical internodes is regulated by developing leaves (excluding the youngest leaves surrounding the shoot apical meristem), which thereby regulate stem elongation and secondary growth, specifically inducing fiber formation. Thus, defoliation caused growth retardation, for which depletion of GA in the apical internodes is a major factor, since application of GA to defoliated stems restored internode growth and development.
We provide evidence to support the movement of a GA-like signal from the blades of unfolded leaves by demonstrating the accumulation of GFP-DELLA proteins in petioles after blade removal in Arabidopsis (Figure 7). DELLA degradation is a direct result of GA signaling due to the molecular interaction of DELLA with the GA receptor GID1 in the presence of GA (Murase et al., 2008); thus, GFP-DELLA accumulation indicates a reduction in GA signaling in these petioles. Creating flow barriers in the form of incisions, we found, on the basis of reporter activity of GA-responsive genes, that there was a nonpolar distribution of the GA-like signal in the leaf veins, while basipetal polarity was detected in the petiole, oriented in the direction of the stem (Figure 9). This was reinforced by the accumulation of reporter gene activity at the leaf-stem junction (Figure 8), where the unique anatomy of the tissue creates a bottleneck for substances flowing out of the leaf. In tobacco stems, GA application restored cambial activity when applied either below or above the internodes, demonstrating that GA movement through the stem is nonpolar (Figures 5 and 10).
There is general agreement that auxin transport induces the continuity of vascular strand formation (Berleth et al., 2000; Aloni, 2010), occurring along the route of its polar basipetal transport from the shoot apex. The cells perceiving the auxin differentiate in a continuous file of interconnected vascular cells (Sachs, 1981). Our results show that the continuity of fibers also requires leaf-derived GA signaling (Figures 1 to 5). As in the case of auxin, the cell layer in which GA acts to promote fiber formation is narrow and restricted to the vascular region. We therefore postulate that GA transport would be restricted to this tissue.
It has been shown that auxin regulates GA biosynthesis in young stems by inducing GA 3-oxidase expression in pea (Pisum sativum; Ross et al., 2000), while in tobacco, auxin was found to promote the activity of GA 20-oxidases (Wolbang and Ross, 2001). According to our results, auxin could not restore secondary growth in defoliated stems (Figure 3), which would be expected if GA synthesis was promoted. In fact, rescue of cambial activity required exogenous GA, which was applied via injection of C19-GAs at physiological concentrations (Figure 2). The reduced transcript abundance of GA20ox2 in defoliated tobacco stems is consistent with this gene being regulated by leaf-derived factors. By contrast, the highly elevated transcript levels of GA20ox1 and both tobacco GA 3-oxidase genes after defoliation (Figure 2B) may result from relief of feedback repression in the low GA environment (Yamaguchi, 2008) and be independent of the leaves. On the other hand, the abundance of the C19-GAs, GA20 and GA1, in maturing leaves (see Supplemental Table 1 online) and their proven ability to rescue internode growth following injection into the base of the stem (Figure 2A) promote their candidacy for the mobile leaf-derived signal. In addition, the observed localization of ProGA2ox2:GUS expression in the phloem (Figures 9F and 9G) is consistent with GA transport through this route, which is supported by the measurements of Garcia-Martinez et al. (1991), who found relatively high levels of GA20 in the phloem of pea plants. It was also shown previously that applied radiolabeled C19-GAs can translocate through the stem (Proebsting et al., 1992). Finally, Ragni et al. (2011) found that in Arabidopsis GA may move from the shoot to the hypocotyl to promote xylem expansion. Although it is unclear whether the signal from the leaves is a C19-GA or a different signal that stimulates GA production in the stem, our results show that by promoting GA signaling, the leaves induce stem elongation as well as secondary growth and fiber differentiation.
Understanding the stimuli that drive secondary growth is of considerable importance for the study of meristematic cell regulation, cell differentiation, and for future biotechnological approaches to induce fiber formation as renewable resources. To assess auxin- and GA-dependent regulation of secondary growth, we first analyzed their action in wild-type tobacco plants, using the physical elimination of their source. This was feasible only after establishing that GA signaling required the presence of expanding leaves. As a consequence, we were able to distinguish between GA and auxin regulation of cambial-directed growth. These results can now be integrated into the larger context of hormonal regulation of secondary growth, as recently described for cytokinin (Matsumoto-Kitano et al., 2008), ethylene (Love et al., 2009), and jasmonic acid (Sehr et al., 2010). We show that both auxin and GA are required for cambium proliferation, but each induces different derivatives: Auxin promotes the development of vessel elements only below the site of application, while GA induces xylem fiber formation both above and below the source. Since it is known that cytokinin-regulated xylogenesis is necessary for fiber development (Aloni, 1982; Saks et al., 1984; Matsumoto-Kitano et al., 2008), we assume that both GA and cytokinin signaling are required for the initiation and maturation of fiber cells.
The requirement of mature leaves for xylogenesis was suggested by Hess and Sachs (1972), who showed that a mature leaf in 3-week-old Phaseolus vulgaris plants provided, directly or indirectly, both auxin and GA signals that induce xylem development in the internode beneath the studied leaf. Although they recognized that the GA signal may be nonpolar, they concluded that its role in cambial derivation proceeds in the same polar manner as auxin (i.e., toward the root). They also showed that in the deleafed plants the application of auxin alone could restore normal cambial activity and that GA application enhanced this activity. In these experiments, it is evident that they had left two photosynthetically active leaves in place to eliminate the influence of assimilate depletion. Doing so, according to our results, they potentially left a major source of GA signaling intact. Their conclusions were later questioned by a study that indicated that GA is of limited importance for cambial development, whereas its major function during wood development is to stimulate fiber elongation (Israelsson et al., 2005). This was concluded from GA measurements showing low levels of active GAs in the cambial zone compared with the developing xylem. Our results indicate that in tobacco, GA signaling modulated by maturing leaves regulates auxin-dependent cambial activity both acro- and basipetally and that in the absence of GA the tissue remains dormant (Figures 1, 2, and 5).
Our demonstration that leaves are required for stem growth and vascular development through provision of a GA signal is consistent with the report of Wang et al. (1997), who found that removal of needles, but not apex excision (decapitation), abolished elongation and reduced the GA content of current-year terminal shoots of Pinus sylvestris, whereas both treatments impaired vascular development. However, only partial recovery of shoot growth could be obtained by exogenous GA or IAA, with best results obtained when both hormones were applied to the defoliated shoot. By contrast, we were able to restore internode elongation after defoliation (maintaining the apical auxin source) by applying GA (Figure 1), while exogenous IAA was ineffective in restoring secondary growth in the absence of GA (Figure 3).
Evidence from work with woody species has indicated that auxin, rather than GA, was the predominant factor regulating cambium formation, although, in the presence of auxin, exogenous GA or enhanced GA production gives some stimulation of cambium proliferation (Elo et al., 2009). The presence of leaves in these studies would have enabled GA signaling, as demonstrated in our work. In most studies, the developing leaves were maintained as a source of carbohydrates, while decapitation and bud removal were regarded as a means to deplete hormonal signals (mainly auxin). Therefore, the importance of GA for cambial activity and derivation was overshadowed by the influence of auxin, and the stimulatory effect of GA could be detected only by the addition of exogenous hormone or enhanced GA production. From our results, it could be postulated that in woody species, cambial dormancy, subsequent to the shedding of leaves, is dependent on the loss of GA signaling. Moreover, the spatial separation of hormone sources in plants, shoot apices, and young leaves for auxin (Ljung et al., 2001), root apices for cytokinin (Aloni et al., 2005; Matsumoto-Kitano et al., 2008), and maturing leaves for GA, either directly or indirectly, may be a means to signal the developmental status of these organs for coordination of growth processes. According to our results, maturing leaves may signal their presence to the shoot apices by releasing a signal that enables GA-dependent internode elongation. In GA-depleted plants, growth will cease until newly developed leaves signal their integrity. These processes are further modulated by other hormones, such as ethylene (Love et al., 2009) and jasmonic acid (Sehr et al., 2010). Given the specific significance of auxin, cytokinin, and GA for secondary growth (Elo et al., 2009), we can propose that only by the integration of their signaling (and the integrity of its organs) will the plant proceed with using its resources for this purpose.
METHODS
Plant Material
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used for transformation with the GUS-expressing vectors. Arabidopsis ecotype Landsberg erecta expressing the ProRGA:GFP-RGA and ProRGA:GFP-rga-Δ17 constructs was described previously (Dill et al., 2001; Silverstone et al., 2001). PCR analyses were performed to validate transgenic line integrity. Seeds were sterilized with chlorine gas and stratified at 4°C for 2 d in the dark. Seedlings were grown on half-strength Murashige and Skoog medium (Duchefa Biochemie). Plants were grown under long-day (16 h light/8 h dark, irradiance 100 μmol s−1 m−2) conditions at 20 to 22°C, except for the GFP-RGA–expressing plants, which were grown under short-day conditions (8 h light/16 h dark, irradiance 120 μmol s−1 m−2). We used Nicotiana tabacum cv Samsun NN for the physiological experiments. Plants were cultivated in soil in a growth chamber at 25°C under a 16-h-light/8-h-dark regime.
Confocal Imaging
GFP-RGA–expressing tissues were analyzed by confocal imaging, performed on a Zeiss LSM 510 confocal laser scanning microscope without fixation, and figures represent projections (excitation wavelength 488 nm, pinhole 200 μm) of the entire focused depth of the petiole (n ≥ 3, for each of three independent experiments). Images were processed using a Zeiss LSM image browser (Carl Zeiss Microimaging) and Adobe Photoshop software (Adobe Systems). Statistical analyses were conducted using Student's two-way t test using data from three independent experiments.
GA Quantification
GA quantification was performed as described by Griffiths et al. (2006).
Constructs and Generation of Transgenic Plants
Promoters from three GA-inducible genes were used to prepare reporters for GA signaling: MYB34 (Gubler et al., 1995; Ogawa et al., 2003), GA2ox2 (Thomas et al., 1999; Hedden and Phillips, 2000), and EXP1 (Lee and Kende, 2002; Ogawa et al., 2003; Xu et al., 2007). Genomic fragments ~2-kb long upstream of each of these genes were amplified using the primers listed in Supplemental Table 3 online. Using the restriction sites HindIII(GA2ox2)/BamHI(EXP1)/HindIII(MYB34) and XbaI(GA2ox2)/SmaI(EXP1)/XbaI(MYB34), the promoters were cloned in their original orientation into the pBI101 binary plasmid (Clontech) (see Supplemental Figure 9A online), 5′ to the GUS reporter gene (Jefferson et al., 1987). The resulting constructs were transformed into Agrobacterium tumefaciens strain GV3101 and through the usual floral dip method transformed to Arabidopsis ecotype Col-0 plants (Clough and Bent, 1998).
Our artificial putative GA-sensitive promoter (FK) design was based on known GA response cis-elements found in promoters of α-amylase genes from the rice (Oryza sativa), barley (Hordeum vulgare), and wheat (Triticum aestivum) genomes, including Amy32b (Lanahan et al., 1992; Rogers et al., 1994; Gómez-Cadenas et al., 2001), Amy pHVl9 (Gubler and Jacobsen, 1992), Amy6-4 (Rogers et al., 1994), REP-1 (Sutoh and Yamauchi, 2003), Amy1/6-4 (Skriver et al., 1991), aAmy3 (Chen et al., 2002, 2006), aAmy8 (Chen et al., 2002), and Amy2 (Peng et al., 2004). Motifs are described in Supplemental Appendix 2 online. Eight assembly oligonucleotides were designed as described (Wu et al., 2006) to construct the 218-bp promoter (i.e., EfrnB, Ofrn, Gfrn, Hfrn, Kfrn, Lfrn, Mfrn, and Nfrn, which are listed in Supplemental Appendix 2 online; see Supplemental Figure 9B online for complete sequence and motifs). Each oligonucleotide was 40 to 60 bases long and possessed a 20- to 25-base overlapping region.
The PCR-amplified artificial FK promoter was cloned into a modified pUC 57 plasmid (GenScript) upstream to the 35S minimal promoter. The plasmid was previously modified by an insertion of the cauliflower mosaic virus 35S minimal promoters (−46 bp/−64 bp/−90 bp) fused to the GUS reporter gene (Jefferson et al., 1987).
The DNA fragments containing the FK promoter, 35S minimal promoter, and the GUS reporter gene (Jefferson et al., 1987) were extracted from pUC57 by enzymatic HindIII/SalI (Biolabs) digestions. These fragments were inserted into the pBINPLUS binary vector (van Engelen et al., 1995) (see Supplemental Figure 9C online), which was transformed into Arabidopsis ecotype Col-0 plants, as described above. Selection for all transformants was performed on seeds using 50 μg/mL kanamycin (Sigma-Aldrich). Transformed plants that grew on kanamycin were also confirmed by PCR (primers listed in Supplemental Table 3 online).
Hormone and Inhibitor Treatments
Lanolin paste was used as the carrier for exogenous application of GA3 (0.8% GA3 [w/w]) to tobacco stems. Analyses of GA competency to rescue plant growth in deleafed plants were performed by the injection of GA19, GA20, or GA1 below elongating internodes in physiological concentrations, according to the GA quantification results (see Supplemental Appendix 3 online). On the morphologic assays conducted on Arabidopsis plants, a solution of the relevant substance was administered by spraying or inflorescence dipping (concentrations are listed in Supplemental Appendix 3 online). Plants grown on Murashige and Skoog medium were supplemented with the described substance. In relevant experiments, plants were pretreated with paclobutrazol (for 1 week) before additional treatments or analyses were performed. All hormones and inhibitors were purchased from Duchefa Biochemie.
GUS Staining and Analysis
Plant scissions were hand-cut with razor blades under a Nikon type 102 stereoscope. Qualitative GUS assays with X-Gluc were performed as described (Blázquez et al., 1997). Incubation with GUS staining buffer at 37°C varied from 2 h to 2 d, depending on the transgenic line. Following staining, tissues were bleached by consecutive 70% ethanol washes followed by a 100% ethanol wash and preserved in 85% lactic acid (for clearing purposes). The localization and level of staining in independent transformants was determined visually on 2- to 3-week-old plants, according to treatments. Leaf blades that were cut to simulate flow disturbances were kept for an extra week before staining.
Histochemical Visualization
Hand-cut transverse sections from the middle of the respective internodes were analyzed. Prior to preparation of cross sections, plants were dehydrated in 70% alcohol. Lacmoid staining procedures were essentially performed as previously described (Aloni, 1979). Tissue and cross section imaging was performed with an Olympus DP 71 camera mounted on an Olympus BH-2 light microscope.
Quantitative Real-Time RT-PCR Analyses
Total RNA was extracted from ~100 mg of tissues using the SV Total RNA isolation kit (Promega). RNA samples (1.5 μg) were reverse transcribed. Transcription levels of tobacco GA3ox1, GA3ox2, GA20ox1, and GA20ox2 were analyzed by quantitative real-time PCR as previously described (Gallego-Giraldo et al., 2008). PCRs were performed in an MX3000P system (Stratagene) using SYBR FAST (Kapa Biosystems) to monitor double-stranded DNA synthesis. All PCRs were performed using three technical replicates. Biological repeats were performed as described in the figure legends.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: GA3ox1 (Nty) AB032198; GA3ox2, EF471116; GA20ox1 (Ntc12), AB012856.1; and GA20ox2, (Ntc16) AB016084.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Fiber and Vessel Isolation and Quantification.
Supplemental Figure 2. The Effects of Leaves on Internode Development and Cambial Activity.
Supplemental Figure 3. GA and Auxin Are Each Essential for Cambial Activity.
Supplemental Figure 4. Comparison of Cell Composition in Tobacco Stems.
Supplemental Figure 5. Longitudinal Sections of GA- and NAA-Treated Decapitated Stems.
Supplemental Figure 6. ProFK:GUS Expression Profile in the Stem.
Supplemental Figure 7. Bottleneck Region Induces GA-Sensitive Gene Expression in Its Locality.
Supplemental Figure 8. Application of an Auxin Transport Inhibitor Does Not Affect the Reporter Gene Expression.
Supplemental Figure 9. Constructs for GA-Inducible GUS Reporter Genes.
Supplemental Table 1. GA Quantification Results.
Supplemental Table 2. Leaf Elongation Occurs Predominantly at the Basal Third of the Leaf.
Supplemental Table 3. Primers Used for Amplification, Sequencing, and Promoter Assembly.
Supplemental Appendix 1. Description of GA-Sensitive Genes.
Supplemental Appendix 2. Motif Description of Gibberellin Response cis-Elements.
Supplemental Appendix 3. Hormone and Hormone Inhibitor Concentrations Used in Application Experiments.
Acknowledgments
We thank Tom J. Guilfoyle for providing the ProDR5:GUS seeds. We also thank the Manna Foundation for partially funding the travel costs to complete the quantification study at Rothamsted Research, Guido Sessa for sharing real-time equipment, and Philip Benfey for sharing microscopy facilities. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the UK. This work was partially supported by the U.S. National Science Foundation (IOS-0641548 and MCB-0923723 to T.-p.S.).
AUTHOR CONTRIBUTIONS
J.D., N.V., and R.A. conceived and designed the project. N.V. was responsible for plasmid construction and plant transformations, directed by H.F. and R.A. Tissue preparation and physiological experiments were performed by J.D. and N.V., directed by R.A. and H.F. Light microscopy and tissue staining were conducted by J.D. and R.A. Macerations and longitudinal sectioning were performed by J.D. under the supervision of T.-p.S. J.D. was responsible for confocal microscopy. F.G. and J.D. performed the GA quantification under the supervision of P.H. J.D., P.H., and R.A. wrote the article with the help and critique of T.-p.S., and H.F.
References
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Aloni R. (1979). Role of auxin and gibberellin in differentiation of primary phloem fibers. Plant Physiol. 63: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. (1982). Role of cytokinin in differentiation of secondary xylem fibers. Plant Physiol. 70: 1631–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. (1987). Differentiation of vascular tissues. Annu. Rev. Plant Physiol. 38: 179–204 [Google Scholar]
- Aloni R. (2010). The induction of vascular tissues by auxin. In Plant Hormones: Biosynthesis, Signal Transduction, Action! P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic), pp. 471–492
- Aloni R., Aloni E., Langhans M., Ullrich C.I. (2006). Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. (Lond.) 97: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R., Langhans M., Aloni E., Dreieicher E., Ullrich C.I. (2005). Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 56: 1535–1544 [DOI] [PubMed] [Google Scholar]
- Berleth T., Mattsson J., Hardtke C.S. (2000). Vascular continuity and auxin signals. Trends Plant Sci. 5: 387–393 [DOI] [PubMed] [Google Scholar]
- Biemelt S., Tschiersch H., Sonnewald U. (2004). Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol. 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez M.A., Soowal L.N., Lee I., Weigel D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124: 3835–3844 [DOI] [PubMed] [Google Scholar]
- Blázquez M.A., Weigel D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404: 889–892 [DOI] [PubMed] [Google Scholar]
- Chen P.W., Chiang C.M., Tseng T.H., Yu S.M. (2006). Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of α-amylase genes. Plant Cell 18: 2326–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.W., Lu C.A., Yu T.S., Tseng T.H., Wang C.S., Yu S.M. (2002). Rice α-amylase transcriptional enhancers direct multiple mode regulation of promoters in transgenic rice. J. Biol. Chem. 277: 13641–13649 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dayan J., Schwarzkopf M., Avni A., Aloni R. (2010). Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol. J. 8: 425–435 [DOI] [PubMed] [Google Scholar]
- Digby J., Wareing P.F. (1966). Effect of applied growth hormones on cambial division and differentiation of cambial derivatives. Ann. Bot. (Lond.) 30: 539–548 [Google Scholar]
- Dill A., Jung H.-S., Sun T.P. (2001). The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA 98: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo A., Immanen J., Nieminen K., Helariutta Y. (2009). Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 20: 1097–1106 [DOI] [PubMed] [Google Scholar]
- Eriksson M.E., Israelsson M., Olsson O., Moritz T. (2000). Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 18: 784–788 [DOI] [PubMed] [Google Scholar]
- Eriksson S., Böhlenius H., Moritz T., Nilsson O. (2006). GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18: 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert F.R. (2006). Vascular cambium. In Esau's Plant Anatomy, 3rd ed. (Hoboken, New Jersey: John Wiley & Sons), pp. 323–355
- Gallego-Giraldo L., García-Martínez J.L., Moritz T., López-Díaz I. (2007). Flowering in tobacco needs gibberellins but is not promoted by the levels of active GA1 and GA4 in the apical shoot. Plant Cell Physiol. 48: 615–625 [DOI] [PubMed] [Google Scholar]
- Gallego-Giraldo L., Ubeda-Tomás S., Gisbert C., García-Martínez J.L., Moritz T., López-Díaz I. (2008). Gibberellin homeostasis in tobacco is regulated by gibberellin metabolism genes with different gibberellin sensitivity. Plant Cell Physiol. 49: 679–690 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez J.L., Santes C., Croker S.J., Hedden P. (1991). Identification, quantitation and distribution of gibberellins in fruits of Pisum sativum L. cv. Alaska during pod development. Planta 184: 53–60 [DOI] [PubMed] [Google Scholar]
- Gómez-Cadenas A., Zentella R., Sutliff T.D., Ho T.H. (2001). Involvement of multiple cis-elements in the regulation of GA responsive promoters: Definition of a new cis-element in the Amy32b gene promoter of barley (Hordeum vulgare). Physiol. Plant. 112: 211–216 [DOI] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z.L., Powers S.J., Gong F., Phillips A.L., Hedden P., Sun T.P., Thomas S.G. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F., Jacobsen J.V. (1992). Gibberellin-responsive elements in the promoter of a barley high-pI α-amylase gene. Plant Cell 4: 1435–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F., Kalla R., Roberts J.K., Jacobsen J.V. (1995). Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI α-amylase gene promoter. Plant Cell 7: 1879–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd N.P., Belfield E., Yasumura Y. (2009). The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21: 1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P., Graebe J.E. (1985). Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J. Plant Growth Regul. 4: 111–122 [Google Scholar]
- Hedden P., Phillips A.L. (2000). Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 5: 523–530 [DOI] [PubMed] [Google Scholar]
- Hedden P., Proebsting W.M. (1999). Genetic analysis of gibberellin biosynthesis. Plant Physiol. 119: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y., Bhalerao R. (2003). Between xylem and phloem: The genetic control of cambial activity in plants. Plant Biol. 5: 465–472 [Google Scholar]
- Hess T., Sachs T. (1972). Influence of a mature leaf on xylem differentiation. New Phytol. 71: 903–914 [Google Scholar]
- Hoad G.V., Retamales J.A., Whiteside R.J., Lewis M.J. (1993). Phloem translocation of gibberellins in three species of higher plants. J. Plant Growth Regul. 13: 85–88 [Google Scholar]
- Hu J., et al. (2008). Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20: 320–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M., Sundberg B., Moritz T. (2005). Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. Plant J. 44: 494–504 [DOI] [PubMed] [Google Scholar]
- Itoh H., Tanaka-Ueguchi M., Kawaide H., Chen X.B., Kamiya Y., Matsuoka M. (1999). The gene encoding tobacco gibberellin 3β-hydroxylase is expressed at the site of GA action during stem elongation and flower organ development. Plant J. 20: 15–24 [DOI] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Itoh H., Inukai Y., Sakamoto T., Ueguchi-Tanaka M., Ashikari M., Matsuoka M. (2003). Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J. 35: 104–115 [DOI] [PubMed] [Google Scholar]
- Katsumi M., Foard D.E., Phinney B.O. (1983). Evidence for the translocation of gibberellin A3 and gibberellin-like substances in grafts between normal, dwarf1 and dwarf5 seedlings of Zea mays L. Plant Cell Physiol. 24: 379–388 [Google Scholar]
- Koornneef M., Bosma T.D.G., Hanhart C.J., Vanderveen J.H., Zeevaart J.A.D. (1990). The isolation and characterization of gibberellin-deficient mutants in tomato. Theor. Appl. Genet. 80: 852–857 [DOI] [PubMed] [Google Scholar]
- Lanahan M.B., Ho T.H., Rogers S.W., Rogers J.C. (1992). A gibberellin response complex in cereal α-amylase gene promoters. Plant Cell 4: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kende H. (2002). Expression of α-expansin and expansin-like genes in deepwater rice. Plant Physiol. 130: 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K., Bhalerao R.P., Sandberg G. (2001). Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Love J., Björklund S., Vahala J., Hertzberg M., Kangasjärvi J., Sundberg B. (2009). Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc. Natl. Acad. Sci. USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove A., Hooley R. (2000). Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci. 5: 102–110 [DOI] [PubMed] [Google Scholar]
- MacMillan J. (1997). Biosynthesis of the gibberellin plant hormones. Nat. Prod. Rep. 14: 221–243 [Google Scholar]
- Matsumoto-Kitano M., Kusumoto T., Tarkowski P., Kinoshita-Tsujimura K., Václavíková K., Miyawaki K., Kakimoto T. (2008). Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA 105: 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Hirano Y., Sun T.P., Hakoshima T. (2008). Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456: 459–463 [DOI] [PubMed] [Google Scholar]
- Nilsson J., Karlberg A., Antti H., Lopez-Vernaza M., Mellerowicz E., Perrot-Rechenmann C., Sandberg G., Bhalerao R.P. (2008). Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R., Yao Q., Xiong A., Fan H., Li X., Peng Y., Cheng Z.M., Li Y. (2004). A new rice zinc-finger protein binds to the O2S box of the α-amylase gene promoter. Eur. J. Biochem. 271: 2949–2955 [DOI] [PubMed] [Google Scholar]
- Poethig R.S., Sussex I.M. (1985). The developmental morphology and growth dynamics of the tobacco leaf. Planta 165: 158–169 [DOI] [PubMed] [Google Scholar]
- Proebsting W.M., Hedden P., Lewis M.J., Croker S.J., Proebsting L.N. (1992). Gibberellin concentration and transport in genetic lines of pea: Effects of grafting. Plant Physiol. 100: 1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher W. (2000). Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51: 501–531 [DOI] [PubMed] [Google Scholar]
- Ragni L., Nieminen K., Pacheco-Villalobos D., Sibout R., Schwechheimer C., Hardtke C.S. (2011). Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 23: 1322–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven P.H., Evert R.F., Eichhorn S.E. (2005). Leaf abscission. In Biology of Plants. (New York: W.H. Freeman), p. 570
- Reid J.B., Murfet I.C., Potts W.C. (1983). Internode length in Pisum. II. Additional information on the relationship and action of loci Le, La, Cry, Na and Lm. J. Exp. Bot. 34: 349–364 [Google Scholar]
- Richards D.E., King K.E., Ait-Ali T., Harberd N.P. (2001). How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 67–88 [DOI] [PubMed] [Google Scholar]
- Rogers J.C., Lanahan M.B., Rogers S.W. (1994). The cis-acting gibberellin response complex in high pI α-amylase gene promoters. Requirement of a coupling element for high-level transcription. Plant Physiol. 105: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.J., O’Neill D.P., Smith J.J., Kerckhoffs L.H.J., Elliott R.C. (2000). Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 21: 547–552 [DOI] [PubMed] [Google Scholar]
- Sachs T. (1981). The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 9: 151–262 [Google Scholar]
- Saks Y., Feigenbaum P., Aloni R. (1984). Regulatory effect of cytokinin on secondary xylem fiber formation in an in vivo system. Plant Physiol. 76: 638–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehr E.M., Agusti J., Lehner R., Farmer E.E., Schwarz M., Greb T. (2010). Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 63: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A., Ueguchi-Tanaka M., Nakatsu T., Nakajima M., Naoe Y., Ohmiya H., Kato H., Matsuoka M. (2008). Structural basis for gibberellin recognition by its receptor GID1. Nature 456: 520–523 [DOI] [PubMed] [Google Scholar]
- Silverstone A.L., Chang C., Krol E., Sun T.P. (1997). Developmental regulation of the gibberellin biosynthetic gene GA1 in Arabidopsis thaliana. Plant J. 12: 9–19 [DOI] [PubMed] [Google Scholar]
- Silverstone A.L., Jung H.S., Dill A., Kawaide H., Kamiya Y., Sun T.P. (2001). Repressing a repressor: Gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Olsen F.L., Rogers J.C., Mundy J. (1991). cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc. Natl. Acad. Sci. USA 88: 7266–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K., Yamauchi D. (2003). Two cis-acting elements necessary and sufficient for gibberellin-upregulated proteinase expression in rice seeds. Plant J. 34: 635–645 [DOI] [PubMed] [Google Scholar]
- Thomas S.G., Phillips A.L., Hedden P. (1999). Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 96: 4698–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H., Puech L., Fink S., Sundberg B. (1997). A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol. 115: 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomás S., Swarup R., Coates J., Swarup K., Laplaze L., Beemster G.T.S., Hedden P., Bhalerao R., Bennett M.J. (2008). Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat. Cell Biol. 10: 625–628 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Ashikari M., Nakajima M., Itoh H., Katoh E., Kobayashi M., Chow T.Y., Hsing Y.I., Kitano H., Yamaguchi I., Matsuoka M. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437: 693–698 [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Hagen G., Guilfoyle T.J. (1999). Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 96: 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engelen F.A., Molthoff J.W., Conner A.J., Nap J.P., Pereira A., Stiekema W.J. (1995). pBINPLUS: An improved plant transformation vector based on pBIN19. Transgenic Res. 4: 288–290 [DOI] [PubMed] [Google Scholar]
- Wang Q., Little C.H.A., Odén P.C. (1997). Control of longitudinal and cambial growth by gibberellins and indole-3-acetic acid in current-year shoots of Pinus sylvestris. Tree Physiol. 17: 715–721 [DOI] [PubMed] [Google Scholar]
- Wolbang C.M., Ross J.J. (2001). Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214: 153–157 [DOI] [PubMed] [Google Scholar]
- Wu G., Wolf J.B., Ibrahim A.F., Vadasz S., Gunasinghe M., Freeland S.J. (2006). Simplified gene synthesis: A one-step approach to PCR-based gene construction. J. Biotechnol. 124: 496–503 [DOI] [PubMed] [Google Scholar]
- Xu J.C., Tian J., Belanger F.C., Huang B.R. (2007). Identification and characterization of an expansin gene AsEXP1 associated with heat tolerance in C3 Agrostis grass species. J. Exp. Bot. 58: 3789–3796 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. (2008). Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Demura T., Fukuda H. (1997). Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol. 38: 980–983 [DOI] [PubMed] [Google Scholar]