Abstract
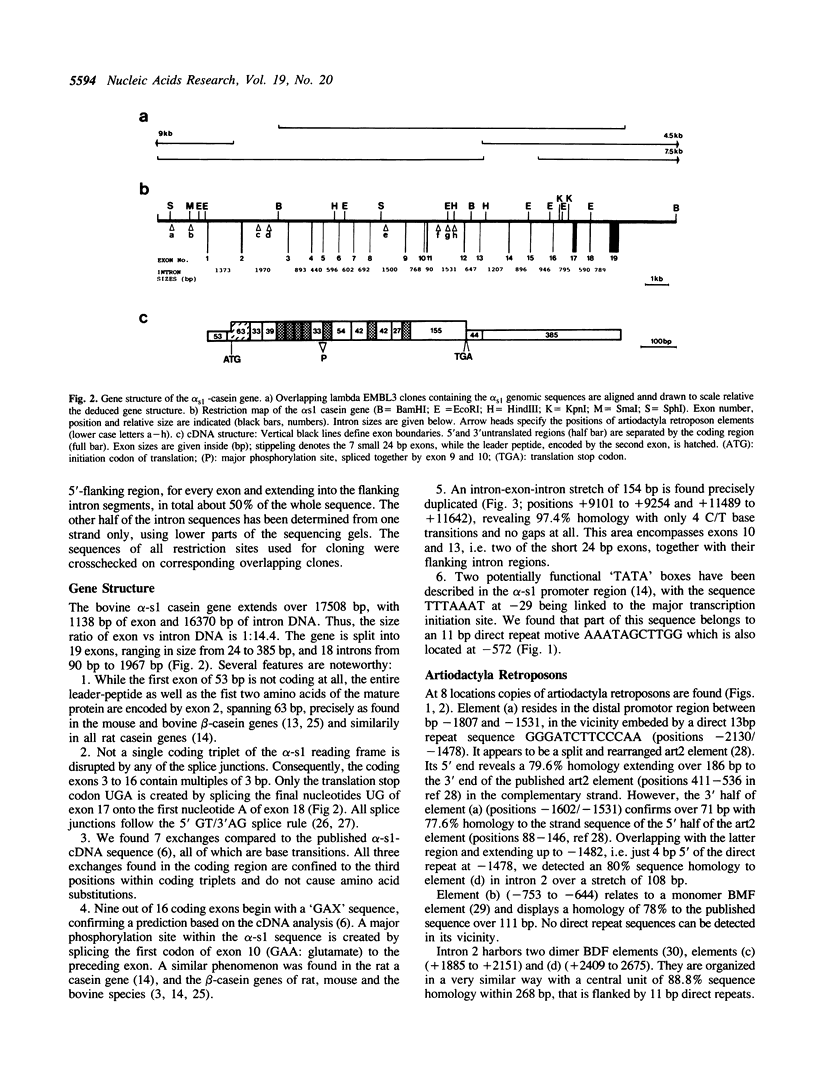
We report the sequence of the complete bovine alpha-s1 casein gene eludicating for the first time the genomic organization of an alpha-s type casein gene. Extending over 17508 bp the gene is split into 19 exons, ranging in size from 24 bp to 385 bp. Except for the translational stop codon not a single coding triplet of the alpha-s1 reading frame is disrupted by any of the splice junctions, which all confirm to known splice consensus sequences. Nine out of 16 coding exons begin with a 'GAX' codon, specific for glutamate. Splicing of this codon from exon 10 to the preceding exon creates a major phosphorylation site. An intron-exon-intron stretch of 154 bp comprising exons 10 and 13 is found precisely duplicated. Associated with the gene, copies of 8 atriodactyla retroposons are found, 6 of which are interspersed into the sequences of the three longest introns. We discuss the possibility that three functional parts of the gene have been recruited and evolutionary conserved at a time before gene diversification gave rise to the separate evolution of alpha- and beta-type casein-genes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander L. J., Stewart A. F., Mackinlay A. G., Kapelinskaya T. V., Tkach T. M., Gorodetsky S. I. Isolation and characterization of the bovine kappa-casein gene. Eur J Biochem. 1988 Dec 15;178(2):395–401. doi: 10.1111/j.1432-1033.1988.tb14463.x. [DOI] [PubMed] [Google Scholar]
- Archibald A. L., McClenaghan M., Hornsey V., Simons J. P., Clark A. J. High-level expression of biologically active human alpha 1-antitrypsin in the milk of transgenic mice. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5178–5182. doi: 10.1073/pnas.87.13.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly A., Le Page C., Rauch M., Milgrom E. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, antihormone and receptor phosphorylation. EMBO J. 1986 Dec 1;5(12):3235–3241. doi: 10.1002/j.1460-2075.1986.tb04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Induction of transcription by steroid hormones. Biochim Biophys Acta. 1987 Nov 20;910(2):95–102. doi: 10.1016/0167-4781(87)90060-1. [DOI] [PubMed] [Google Scholar]
- Blackburn D. E., Hobbs A. A., Rosen J. M. Rat beta casein cDNA: sequence analysis and evolutionary comparisons. Nucleic Acids Res. 1982 Apr 10;10(7):2295–2307. doi: 10.1093/nar/10.7.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Retroviral integration: structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2525–2529. doi: 10.1073/pnas.86.8.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler T. A., Bruyère T., Went D. F., Stranzinger G., Bürki K. Rabbit beta-casein promoter directs secretion of human interleukin-2 into the milk of transgenic rabbits. Biotechnology (N Y) 1990 Feb;8(2):140–143. doi: 10.1038/nbt0290-140. [DOI] [PubMed] [Google Scholar]
- Dean D. C., Knoll B. J., Riser M. E., O'Malley B. W. A 5'-flanking sequence essential for progesterone regulation of an ovalbumin fusion gene. Nature. 1983 Oct 6;305(5934):551–554. doi: 10.1038/305551a0. [DOI] [PubMed] [Google Scholar]
- Devinoy E., Schaerer E., Jolivet G., Fontaine M. L., Kraehenbuhl J. P., Houdebine L. M. Sequence of the rabbit alpha S1-casein cDNA. Nucleic Acids Res. 1988 Dec 23;16(24):11813–11813. doi: 10.1093/nar/16.24.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H. Novel Alu-type repeat in artiodactyls. Nucleic Acids Res. 1987 Feb 11;15(3):1340–1340. doi: 10.1093/nar/15.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti L., Leone P., Sgaramella V. Long range restriction analysis of the bovine casein genes. Nucleic Acids Res. 1990 Dec 11;18(23):6829–6833. doi: 10.1093/nar/18.23.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky S. I., Tkach T. M., Kapelinskaya T. V. Isolation and characterization of the Bos taurus beta-casein gene. Gene. 1988 Jun 15;66(1):87–96. doi: 10.1016/0378-1119(88)90227-2. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Steudle A., Sippel A. E. Nucleotide sequence of cloned cDNA coding for mouse epsilon casein. Eur J Biochem. 1982 Sep 1;126(3):569–572. doi: 10.1111/j.1432-1033.1982.tb06818.x. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Rosen J. M. Sequence of rat alpha- and gamma-casein mRNAs: evolutionary comparison of the calcium-dependent rat casein multigene family. Nucleic Acids Res. 1982 Dec 20;10(24):8079–8098. doi: 10.1093/nar/10.24.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Revision of consensus sequence of human Alu repeats--a review. Gene. 1987;53(1):1–10. doi: 10.1016/0378-1119(87)90087-4. [DOI] [PubMed] [Google Scholar]
- Meade H., Gates L., Lacy E., Lonberg N. Bovine alpha S1-casein gene sequences direct high level expression of active human urokinase in mouse milk. Biotechnology (N Y) 1990 May;8(5):443–446. doi: 10.1038/nbt0590-443. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Matusik R. J., Richards D. A., Gupta P., Rodgers J. R. Multihormonal regulation of casein gene expression at the transcriptional and posttransciptional levels in the mammary gland. Recent Prog Horm Res. 1980;36:157–193. doi: 10.1016/b978-0-12-571136-4.50011-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer E., Devinoy E., Kraehenbuhl J. P., Houdebine L. M. Sequence of the rabbit beta-casein cDNA: comparison with other casein cDNA sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11814–11814. doi: 10.1093/nar/16.24.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Plucienniczak A., Bednarek A., Jaworski J. Bovine 1.709 satellite. Recombination hotspots and dispersed repeated sequences. J Mol Biol. 1984 Aug 15;177(3):399–416. doi: 10.1016/0022-2836(84)90292-4. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Bonsing J., Beattie C. W., Shah F., Willis I. M., Mackinlay A. G. Complete nucleotide sequences of bovine alpha S2- and beta-casein cDNAs: comparisons with related sequences in other species. Mol Biol Evol. 1987 May;4(3):231–241. doi: 10.1093/oxfordjournals.molbev.a040437. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Willis I. M., Mackinlay A. G. Nucleotide sequences of bovine alpha S1- and kappa-casein cDNAs. Nucleic Acids Res. 1984 May 11;12(9):3895–3907. doi: 10.1093/nar/12.9.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgill D. W., Womack J. E. Genomic analysis of the major bovine milk protein genes. Nucleic Acids Res. 1990 Dec 11;18(23):6935–6942. doi: 10.1093/nar/18.23.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Tsukada T., Notake M., Nakanishi S., Numa S. Structural analysis of repetitive DNA sequences in the bovine corticotropin-beta-lipotropin precursor gene region. Nucleic Acids Res. 1982 Mar 11;10(5):1459–1469. doi: 10.1093/nar/10.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Richter-Mann L., Couch C. H., Stewart A. F., Mackinlay A. G., Rosen J. M. Evolution of the casein multigene family: conserved sequences in the 5' flanking and exon regions. Nucleic Acids Res. 1986 Feb 25;14(4):1883–1902. doi: 10.1093/nar/14.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Mitchell D. L., Venema J., van Hoffen A., van Zeeland A. A., Mullenders L. H., de Wit J., Simons J. W. DNA repair characteristics and mutability of the UV-sensitive V79 Chinese hamster cell mutant V-B11 (complementation group 7). Mutagenesis. 1991 May;6(3):179–183. doi: 10.1093/mutage/6.3.179. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]


