Abstract
Dicer is a cellular enzyme required for the processing of pre-miRNA molecules into mature miRNA, and Dicer and miRNA biogenesis have been found to play important roles in a variety of physiologic processes. Recently, reports of alterations in miRNA expression levels in cultured pre-adipogenic cell lines during differentiation and findings of differences between the miRNA expression signatures of white and brown adipose have suggested that miRNA molecules might regulate adipocyte differentiation and the formation of adipose tissue. However, direct evidence that miRNAs regulate adipogenesis is lacking. To determine if Dicer and mature miRNA govern adipocyte differentiation, we utilized primary cells isolated from mice bearing Dicer-conditional alleles to study adipogenesis in the presence or absence of miRNA biogenesis. Our results reveal that Dicer is required for adipogenic differentiation of mouse embryonic fibroblasts and primary cultures of pre-adipocytes. Furthermore, the requirement for Dicer in adipocyte differentiation is not due to miRNA-mediated alterations in cell proliferation, as deletion of the Ink4a locus and the prevention of premature cellular senescence normally induced in primary cells upon Dicer ablation fails to rescue adipogenic differentiation in fibroblasts and pre-adipocytes.
INTRODUCTION
Adipocytes store excess glucose in the form of triacylglycerol during times of nutritional abundance and release this energy source by converting the stored triacylglycerol into fatty acids during times of fasting. In addition to storing fat and regulating nutritional homeostasis, adipopcytes synthesize and release peptide hormones that help to regulate blood pressure, the immune response, bone density, and reproductive functions [Rosen and Spiegelman, 2006; Waki and Tontonoz, 2007].
Much of what is known about the molecular control of adipogenesis has been gleaned from differentiation studies using primary or immortalized fibroblasts or pre-adipocytes that were isolated from the vascular stroma of adipose tissue. These various precursor cells can be induced to differentiate into mature adipocytes in vitro, as scored by Oil Red O (ORO) staining of the large, numerous lipid deposits that form within these cells (lipogenesis), and by the induction of genes that are normally expressed in mature adipocytes [Smyth et al., 1993; Farmer, 2006; Rosen and MacDougald, 2006; Lefterova and Lazar, 2009].
Analysis of gene expression during adipocyte differentiation assays has indicated potential roles for microRNA (miRNA) molecules in this process. miRNAs are single-stranded, non-coding RNA molecules of approximately 22 nucleotides in length that function to regulate the expression of messenger RNA [Bartel, 2004]. miRNAs are encoded within the genome of the cell, and are transcribed as longer RNA molecules (pri-miRNA) that are processed into smaller precursor-miRNA (pre-miRNA) molecules and exported into the cytoplasm, where they undergo final cleavage by the ribonuclease III-like cellular enzyme Dicer. Once cleaved, the mature miRNAs are incorporated into the RNA-induced silencing complex (RISC), where they mediate either translational repression or cleavage of target messenger RNA. In mammalian cells, the differentiation of pre-adipocyte cells into adipocytes alters the expression level of many miRNA molecules. For example, miR-143 is upregulated in cultures of differentiating human preadipocytes, and reducing the level of miR-143 in these cells by addition of 2’-O-methyl-modified antisense oligonucleotides was found to inhibit triglycerol accumulation [Esau et al., 2004]. More recently, the miR-17-29 cluster was also found to accelerate adipogenic differentiation in cultured 3T3-L1 cells, by facilitating cell growth [Wang et al., 2008]. In addition, bioinformatic approaches indicate that nearly three quarters of the genes differentially expressed during the adipogenic differentiation of 3T3-L1 cells have the potential to be regulated by miRNA molecules [Hackl et al., 2005]. Collectively, these studies suggest that miRNAs may have important regulatory effects on adipocyte differentiation and adipogenesis. However, definitive proof for miRNA-mediated regulation of mammalian adipogenesis and adipose formation is lacking, and recent analysis of a subset of miRNAs whose expression levels were highly altered during adipogenic differentiation revealed that downregulation of these miRNAs did not affect differentiation of cultured pre-adipocytes [Kajimoto et al., 2006].
Similar to pre-adipocyte cell lines, mouse embryonic fibroblasts (MEFs) or preadipocytes isolated from the vascular stroma of adipose tissue can also be induced to differentiate into adipocytes ex vivo. To directly assess the requirement for miRNAs in adipocyte differentiation, we have utilized primary cells isolated from mice bearing a Dicer conditional allele [Mudhasani et al., 2008]. Loss of Dicer function inhibits miRNA biogenesis, and conditional ablation of Dicer in mice has been previously reported to alter numerous cellular and developmental processes [Andl et al., 2006; Chen et al., 2008; Harfe et al., 2005; Harris et al., 2006; Muljo et al., 2005; Yi et al., 2006]. Furthermore, we have previously demonstrated that conditional deletion of Dicer in primary fibroblasts results in the induction of Ink4a/Arf and p53-dependent cell senescence [Mudhasani et al., 2008]. In this present study, we have generated primary fibroblasts and pre-adipocytes from our Dicer-conditional mouse model, and examined adipocyte differentiation in vitro in the presence or absence of Dicer. Our results indicate that Dicer and miRNAs play an essential role in adipocyte differentiation distinct from the ability of miRNAs to regulate senescence in primary cells.
RESEARCH DESIGN and METHODS
Mice and cell culture
Dicer-conditional (Dicerc/c) mice, Dicerc/c × p53-null mice, and Dicerc/c × Ink4a-Arf mice have been described previously, as were Dicer-conditional mice bearing the CAG-Cre transgene [Mudhasani et al., 2008]. All mice were maintained and used in accordance with guidelines set by the University of Massachusetts Animal Care and Use Committee. Mouse embryonic fibroblasts (MEFs) were generated by harvesting embryos from pregnant female mice at E12.5 or E13.5, and treating the embryos with trypsin for 30 minutes at 37°C. Adherent cells were recovered from trypsinized embryos by placing the disassociated tissue in 10cm plates overnight in a 37°C, 5% CO2 incubator in Dulbecco’s modified Eagles medium (DMEM) supplemented with 15% fetal bovine serum, 48U/ml penicillin, and 50ug/ml streptomycin sulfate. All MEF studies were conducted using low passage (passage 0 or passage1) primary cells maintained in a 37°C, 5% CO2 incubator in DMEM supplemented with 10% fetal bovine serum. Dicer was deleted from the Dicerc/c, Cre-ER MEFs by addition of 1uM tamoxifen (Sigma, T5648) to the cell culture media. Pre-adipocytes were cultured as described [Daya et al., 2007] with minor modifications. Briefly, cells were extracted by isolating the deeper subcutaneous adipose tissue from the lateral regions in the lower abdomen of 12–14 day old mice, which was minced into small pieces and digested with type II collagenase (A, B & M, Roche, Cat #103578) at 1mg/ml in Hanks balanced salt solution [HBSS] with 10% Bovine serum albumin [Sigma-Aldrich] for 45min-1hr in a 37°C shaking water bath. Pre-adipocytes and other smaller cells in the mixed population were separated from the larger differentiated adipocytes and debris with a 100µm nylon filter. The filtered cells were plated on a 24 well plate and cultured in DMEM medium supplemented with 10% FBS and 140U/ml penicillin and 50ug/ml streptomycin for 48 hours. Cells were treated with 1µM Tamoxifen for three days. Dicerc/c cells and Dicerc/c, Ink/Arf-null cells were passaged for two or 4 times, respectively. MEFs or preadipocytes were then plated at 50–60% confluence and stimulated two days later (considered as day 0 [D0]) with a hormonal cocktail composed of 0.25mM 3-Isobutyl-1-methylxanthine (IBMX [Sigma, I5879]), 0.1µM Dexamethasone (Dex [Sigma, D4902]), Insulin (Sigma) at 10µg/ml and 1µM Rosiglitazone (Cayman, CAS 316371-84-3). Starting on day two (D2), the media was replaced every two days with fresh DMEM supplemented with Insulin (10µg /ml) and 1µM Rosiglitazone. Cells were harvested 7–9 days post-induction for RNA and genomic DNA isolation, or fixed in 10% formalin for Oil Red O (ORO) staining as described [Salma et al., 2004].
RNA Isolation and Analysis
Total RNA from cultured cells was isolated using the Trizol method (Invitrogen). For real-time PCR analysis, RNA was reverse transcribed using the Superscript II reverse transcriptase enzyme (Invitrogen) and used in quantitative PCR reactions (Qiagen master mix containing SYBR-green fluorescent dye [ABI]). Relative expression of mRNAs was determined after normalization with 36B4 levels using the ΔΔ-Ct method. Q-PCR was performed using the ABI-9300 PCR machine. Quantitative PCR (q-PCR) analysis for let-7a miRNA levels was performed using the mirVana qRT-PCR miRNA detection kit (Ambion), and data for let-7a miRNA was normalized with U6 expression levels. Primers used for PCR reactions are as follows: PPARγ2 (f) – gcatggtgccttcgctgatgc, PPARγ2 (r) – aggcctgttgtagagctgggt, aP2(f) – aaggtgaagagcatcatcaccct, aP2 (r) – tcacgcctttcataacacattcc, FAS (f) – ggaggtggtgatagccggtat, FAS (r) – tgggtaatccatagagcccag, Glut4 (f) – gtgactggaacactggtccta, Glut4 (r) – ccagccacgttgcattgtag.
RESULTS
Dicer is required for adipogenic differentiation of primary embryonic fibroblasts
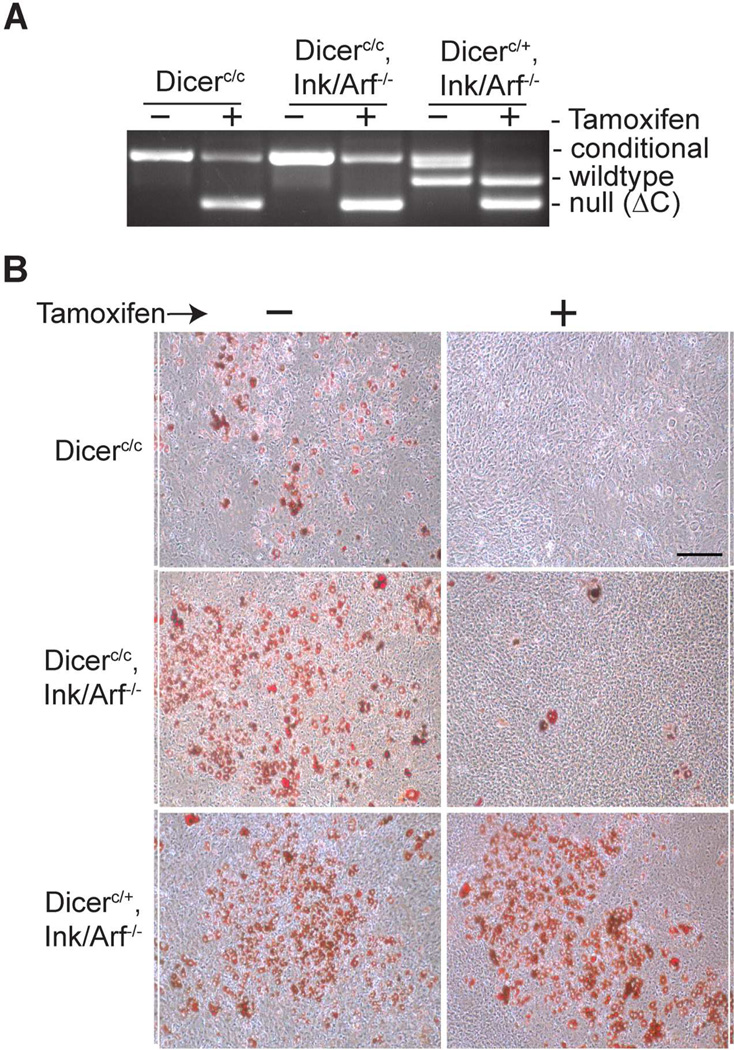
To explore a role for miRNA biogenesis in adipocyte differentiation, we examined the ability of primary MEFs to undergo adipogenesis after Dicer ablation. We previously reported that Dicer-ablated MEFs display reduced proliferation and premature cell senescence [Mudhasani et al., 2008]. Therefore, we analyzed the response of Dicer-conditional cells in the presence or absence of functional Ink4a alleles, as loss of the Ink4a (p16) and Arf (p19) genes encoded at this locus alters both Rb and p53 signaling and prevents premature senescence in Dicer-ablated MEF [Mudhasani et al., 2008]. Dicer-conditional (Dicerc/c) MEFs containing or lacking functional Ink4a/Arf alleles (Ink/Arf −/−) and control Ink/Arf−/− MEFs heterozygous for the Dicer-conditional allele (Dicerc/+) were generated from E12–E14 mouse embryos. All embryos also contained the CAG-CreER transgene, which encodes Cre activity that is positively regulated by the addition of tamoxifen [Hayashi and McMahon, 2002]. Low passage MEFs were plated on 10 cm dishes, and tamoxifen was added to the media to induce Cre activity and subsequent Dicer ablation. The cells were treated or mock-treated with tamoxifen for three days, passaged twice to promote loss of pre-existing mature miRNAs, then induced to differentiate into adipocytes. Cre-induced rearrangement of the Dicer conditional alleles was confirmed by PCR analysis of genomic DNA generated from a portion of the cells harvested after tamoxifen treatment (Figure 1A). Approximately 30% of the non-ablated Dicerc/c MEFs (no tamoxifen treatment) underwent differentiation and initiated lipogenesis, as determined by ORO staining (Figure 1B-top panels). In contrast, no differentiation was observed in these cells when treated with tamoxifen prior to induction of differentiation. To determine if the block in adipogenic differentiation in Dicer-ablated MEFs was caused by premature senescence of these cells, we repeated the experiment using Ink4a-null, Dicer-conditional MEFs, as Ink/Arf-null cells do not senesce upon Dicer ablation [Mudhasani et al., 2008]. Interestingly, a greater percentage (>50%) of Ink/Arf null MEFs underwent adipogenic differentiation when exposed to differentiation media, consistent with a positive role for cell proliferation in this process. However, Ink/Arf-null MEFs were severely compromised in their ability to differentiate into adipocytes following Dicer ablation (Figure 1B-middle panels). In contrast, control Dicer heterozygous, Ink/Arf-null cells displayed robust differentiation in the presence or absence of tamoxifen (Figure 1B-bottom panels). Therefore, tamoxifen treatment or expression of Cre by itself does not inhibit adipogenesis, but rather it is Dicer-ablation that correlates with a block to cell differentiation. Furthermore, premature cell senescence induced by Dicer deletion does not account for the block in adipogenic differentiation of MEFs.
Figure 1. Dicer is required for adipogenic differentiation of mouse embryonic fibroblasts.
(A) PCR analysis of Dicer alleles in MEFs after mock-treatment (−) or treatment (+) with tamoxifen to induce Dicer ablation. (B) ORO staining of R26-CreESR transgenic, Dicer-conditional MEFs in the presence (+) or absence (−) of tamoxifen (top panels), of CAG-CreESR transgenic, Dicer-conditional MEFs deleted for Ink4a locus (middle panels), and of control CAG-CreESR transgenic, Dicer-heterozygous MEFs deleted for Ink4a (bottom panels). Scale bar represents 250µm.
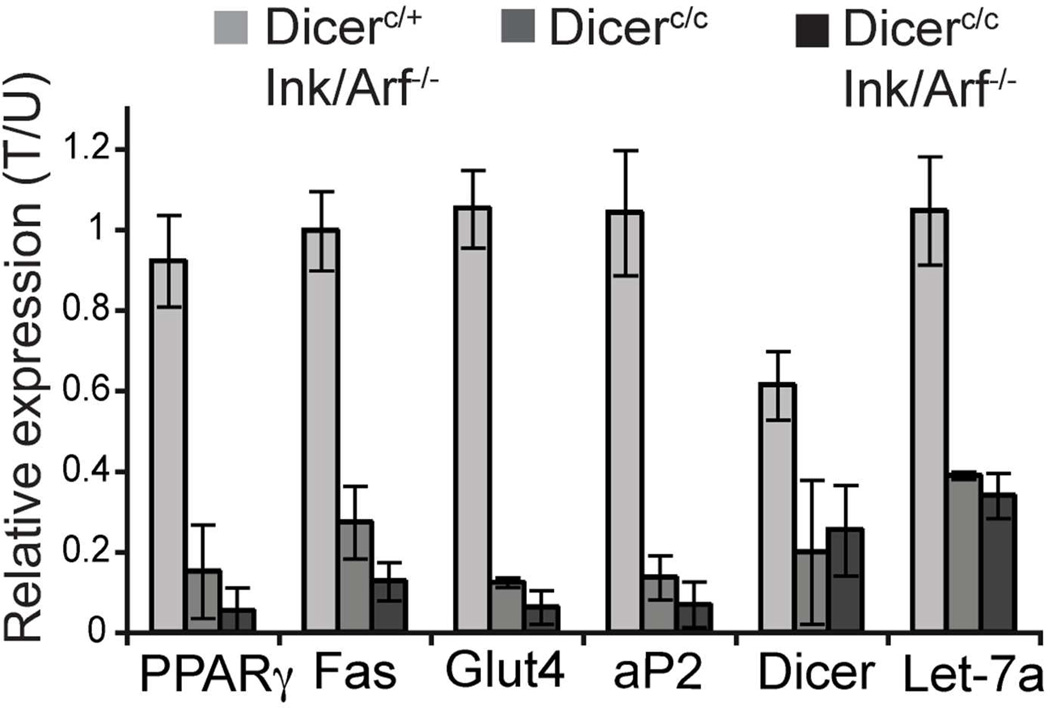
To confirm that loss of Dicer blocks adipogenic differentiation in MEFs, we analyzed the expression of various adipogenic marker genes before and after differentiation using quantitative PCR (Figure 2). Differentiation of wildtype (Dicer–retaining) MEFs strongly upregulated the expression of various adipogenic factors that regulate fat differentiation, fatty acid synthesis, and lipid and glucose transport, including Peroxisome Profilator Activator Receptorγ (PPARγ), Fatty acid synthase (Fas), fatty acid binding protein (aP2) and Glucose transporter 4 (Glut4) respectively. Induction of these adipogenic genes was greatly reduced in Dicer-ablated MEFs regardless of Ink/Arf status, whereas the expression of these adipogenic markers did not differ in non-treated versus tamoxifen-treated cells when at least one wild type allele of Dicer was present (Dicerc/+). Thus, in agreement with the ORO staining data in Figure 1, it is not the presence or absence of tamoxifen that alters adipogenic differentiation, but rather it the presence or absence (ablation) of Dicer. Analysis of gene expression levels in Dicer-ablated MEFs reveals that tamoxifen treatment of Dicer-conditional MEFs results in a 30–40% reduction of Dicer transcripts in Dicerc/+ MEFs, and an 80% reduction in Dicer message levels in Dicerc/c MEFs, consistent with ablation of the Dicer allele in most tamoxifen-treated Dicerc/c cells. In keeping with robust ablation of Dicer in these cells, there was a 75% reduction in the level of Let-7a (Figure 2), a representative miRNA species with an especially long half-life that is highly expressed in adipocyte precursors [Kajimoto et al., 2006].
Figure 2. Quantitative PCR analysis of transcripts present in differentiated MEFs.
Values are given as relative expression levels in tamoxifen treated (Dicer-ablated) cells compared to untreated (Dicer-wt) cells (T/U). Dicer PCR primers hybridize to sequences encoded by exons 15–17 contained within the floxed region of the Dicer conditional allele. Error bars represent SD of values from 5 independent experiments.
Differentiation of primary pre-adipocytes requires Dicer
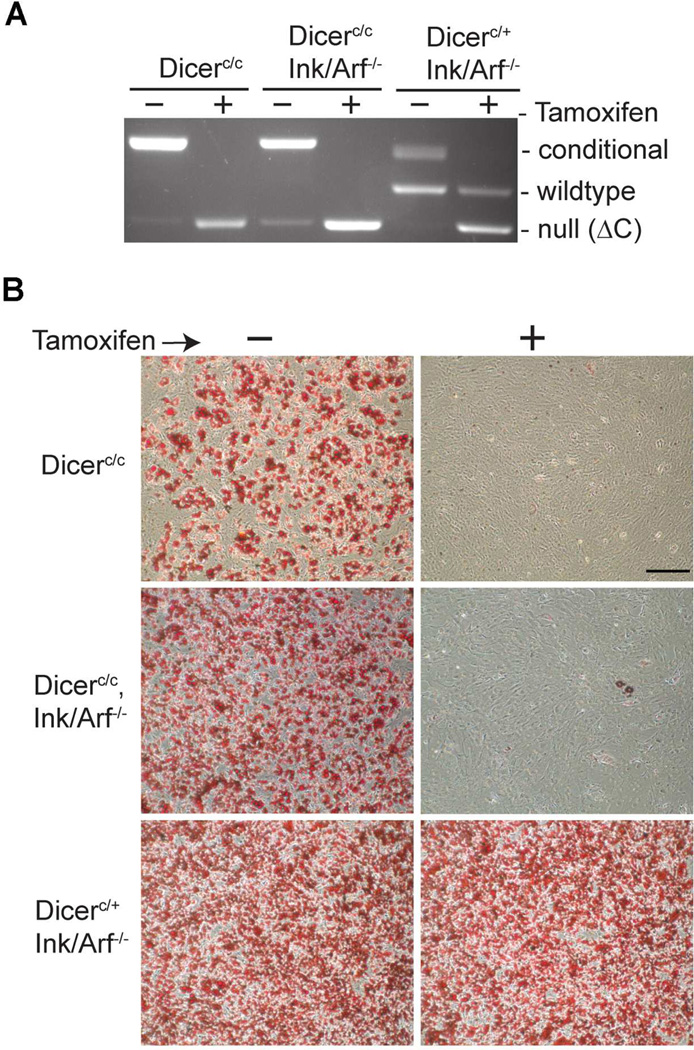
The inability of fibroblasts lacking Dicer to undergo adipogenic differentiation prompted us to examine if Dicer is similarly required for the differentiation of pre-adipocytes. Primary cultures of pre-adipocytes were generated from Dicer-conditional mice bearing the tamoxifen-inducible, CAG-CreER transgene and either wildtype or null for Ink4a. Primary pre-adipocytes were expanded for 6 days in culture, then plated at equal densities and induced to differentiate. Analysis of genomic DNA confirmed the rearrangement of the conditional Dicer allele in primary pre-adipocytes following tamoxifen treatment (Figure 3A). Tamoxifen-induced Cre-ablation of Dicer in the pre-adipocytes blocked differentiation of these cells (Fig. 3B, top panels). As seen in the previous MEF experiments, deletion of the Ink4a alleles slightly increased the percentage of pre-adipocytes undergoing cell differentiation when treated with the hormone cocktail, and co-deletion of functional Ink4a genes did not alter the differentiation block imposed upon Ink/Arf-null pre-adipocytes by Dicer loss (Figure 3B middle panels), These data indicate that the block in adipogenesis seen in Dicer-ablated pre-adipocytes is not due to the induction of premature cell senescence in these primary cells. In contrast, control pre-adipocytes retaining one functional allele of Dicer were able to undergo robust differentiation regardless of tamoxifen treatment (Figure 3B-bottom panels).
Figure 3. Differentiation of mouse pre-adipocytes requires Dicer.
(A) PCR analysis of Dicer alleles in pre-adipocytes after mock-treatment (−) or treatment (+) with tamoxifen to induce Dicer ablation. (B) ORO staining of R26-CreESR transgenic, Dicer-conditional pre-adipocytes in the presence (+) or absence (−) of tamoxifen (top panels), of CAG-CreESR transgenic, Dicer-conditional pre-adipocytes deleted for Ink4a (middle panels), and of control CAG-CreESR transgenic, Dicer-heterozygous preadipocytes deleted for Ink4a (bottom panels). Scale bar represents 250µm.
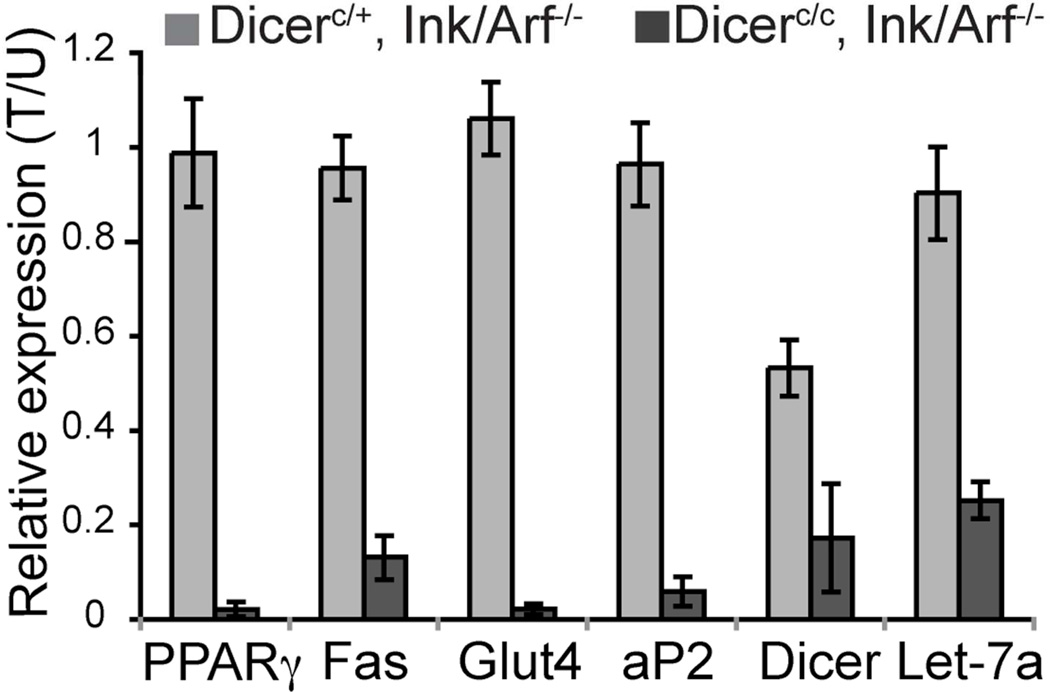
Quantitative PCR analysis of gene expression in the pre-adipocytes (Figure 4) confirmed a 40–50% reduction in Dicer transcript levels in Dicer haploinsufficient cells (Dicerc/+) following Cre induction, and mature Let-7a molecules were reduced to approximately 20% of wild type levels in Dicerc/c pre-adipocytes following Cre induction, indicating robust Dicer ablation in these tamoxifen-treated cells. Similar to what we observed in MEFs, the expression levels of mature adipogenic marker genes (PPARγ2, FAS, Glut4, and aP2) were greatly reduced following Dicer ablation in pre-adipocytes, regardless of Ink4a status, confirming that the block in pre-adipocyte differentiation is not due to induction of senescence in these primary cells.
Figure 4. Expression of adipogenic genes in pre-adipocyte differentiation.
Quantitative PCR analysis of various RNA transcripts and miRNA levels during differentiation. Values are given as relative expression levels in tamoxifen treated (Dicer-ablated) cells compared to untreated (Dicer-wt) cells (T/U). Error bars represent SD of values from 6 independent experiments.
DISCUSSION
The ability of primary fibroblasts and pre-adipocytes to differentiate into adipose-like cells has been very useful for dissecting the molecular intricacies of adipocyte differentiation [Christian et al., 2005]. In this present study, our analysis of embryonic fibroblasts and pre-adipocytes reveal that Dicer is required for adipogenic differentiation. Hormonal induction of cells ablated for Dicer failed to induce the master adipogenic transcriptional regulator PPARγ, and these cells also failed to display other hallmarks of differentiated adipocytes, including lipid accumulation and the expression of downstream adipogenic marker genes such as aP2 and Glut4. These results offer genetic proof for a role for Dicer and miRNA biogenesis in adipocyte differentiation.
We have previously found that deletion of Ink/Arf alleles in the MEFs prevents cell senescence following Dicer ablation [Mudhasani et al., 2008], yet co-deletion of Ink/Arf did not facilitate adipogenic differentiation of Dicer-ablated MEFs. These findings are confirmed by the results of our pre-adipocyte studies, as these cells are similarly unable to differentiate into adipocytes following Dicer ablation, regardless of their Ink/Arf status. Thus, our results also indicate that the essential role of Dicer in adipogenic differentiation of cultured cells is unlinked to the ability of Dicer and miRNA biogenesis to prevent growth senescence of primary cells.
Although our data reveal that Dicer and miRNA biogenesis are required for the differentiation of pre-adipocytes into mature adipocytes, it is possible that the adipogenic functions of fully differentiated cells might also be impacted by loss of miRNA biogenesis. Additional studies focused upon the role of miRNA biogenesis in adipose formation in mice utilizing the Dicer-conditional allele are ongoing. Identification of the roles of Dicer in adipose formation and function and the specific miRNAs involved in regulating adipocyte differentiation and adipose tissue formation in vivo should facilitate the identification of target molecules involved in these processes, and add to our understanding of the molecular mechanisms involved in the regulation of adipogenesis.
ACKNOWLEDGEMENTS
We thank Kathleen Hoover for assistance with the mouse colonies, Zdenka Matijasevic and Nunciada Salma for assistance with cell culturing and differentiation assays, and Charlene Baron for help with manuscript preparation. This work was supported by grants from the National Institutes of Health to SNJ (DK073324 and CA077735) and to ANI (DK079239 and DK084278). RM was supported by an American Heart Award (0625823T) and by NIH-T32CA130807. ANI and SNJ are members of the UMass Diabetes Endocrinology Research Center, and core facilities used in this study were partially supported by DERC grant 5P30DK32520.
REFERENCES
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S, Loughlin AJ, Macqueen HA. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation. 2007;75:360–370. doi: 10.1111/j.1432-0436.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte differentiation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl H, Burkard TR, Sturn A, Rubio R, Schleiffer A, Tian S, Quackenbush J, Eisenhaber F, Trajanoski Z. Molecular processes during fat cell development revealed by gene expression profiling and functional annotation. Genome Biol. 2005;6:R108. doi: 10.1186/gb-2005-6-13-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA. 2006;12:1626–1632. doi: 10.1261/rna.7228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leftorova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, Lawrence JB, Imbalzano AN, Jones SN. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055–1063. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salma N, Xiao H, Mueller E, Imbalzano AN. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor gamma nuclear hormone receptor. Mol Cell Biol. 2004;24:4651–4663. doi: 10.1128/MCB.24.11.4651-4663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Sparks RL, Wharton W. Proadipocyte cell lines: models of cellular proliferation and differentiation. J Cell Sci. 1993;106(Pt 1):1–9. doi: 10.1242/jcs.106.1.1. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Ann Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]