Abstract
An N-halamine precursor, 5, 5-dimethyl hydantoin (DMH), was covalently linked to the surface of polyurethane (PU) with 1,6-hexamethylene diisocyanate (HDI) as a coupling agent. The reaction pathways were investigated using propyl isocyanate (PI) as a model compound, and the results suggested that the imide and amide groups of DMH had very similar reactivity toward the isocyanate groups on PU surfaces activated with HDI. After bleach treatment, the covalently bound DMH moieties were transformed into N-halamines. The new N-halmaine-based PU provided potent antimicrobial effects against Staphylococcus aureus (S. aureus, Gram-positive), Escherichia coli (E. coli, Gram-negative), methicillin-resistant staphylococcus aureus (MRSA, drug resistant Gram-positive bacteria), vancomycin-resistant enterococcus (VRE, drug resistant Gram-positive bacteria), and Candida albicans (C. ablicans, fungi), and successfully prevented bacterial and fungal biofilm formation. The antimicrobial and biofilm-controlling effects were stable for longer than 6 months under normal storage in open air. Furthermore, if the functions were lost due to prolonged use, they could be recharged by another chlorination treatment. The recharging could be repeated as needed to achieve long-term protection against microbial contamination and biofilm-formation.
Keywords: Antimicrobial, Biofilm-controlling, N-halamine, Chlorination, Rechargeable, Polyurethane
1. Introduction
Thanks to its wide availability, low cost, ease of fabrication, and excellent physical and biological properties, polyurethane (PU) has become one of the most versatile polymers in medical, dental, industrial, institutional, and environmental applications.[1–3] Unfortunately, like most conventional polymers, PU surface is susceptible to contamination of microorganisms, which can act as sources of cross-contamination and cross-infection.[4–6] Moreover, adherent microbial species can secrete “slimes” (extracellular polymeric substances, or EPS) that attach them firmly to the material surfaces, resulting in irreversible adhesion and biofilm formation.[7–9] Once formed, biofilms are very difficult to destroy. Protected by the EPS, microbes living in a biofilm are up to 1,000 times more resistant against disinfection, causing serious problems including device-related infections, healthcare-acquired infections, waterborne/foodborne illnesses, corrosions, blockage of filters, etc.
Consequently, there has been a growing interest in the development of antimicrobial polymers, including antimicrobial PUs, to solve the problems. The majority of reported antimicrobial PU was prepared by either physically mixing antimicrobial agents (e.g., antibiotics, metal ions, quaternary ammonium salts, iodine, etc.) into or covalently binding the antimicrobial agents onto the target polymers.[11–14] Nonetheless, since microbial contamination and biofilm-formation mainly occur on polymer surfaces, it could be advantageous to utilize the surface-modification technique to impart the desired antimicrobial effects without causing bulk structure/property changes of the polymers.[10,15] The research interest of this lab focuses on N-halamine-based antimicrobial and biofilm-controlling polymers. An N-halamine is a compound containing one or more nitrogen-halogen covalent bonds. N-halamines have antimicrobial efficacy similar to that of hypochlorite bleach, one of the most widely used disinfectants, but they are more stable, less corrosive, and have much less tendency to generate halogenated hydrocarbons. Therefore, N-halamines have found wide applications as food and water disinfectants.[16, 17] Moreover, once N-halamine structures are covalently linked to the backbones of ordinary polymers, the antimicrobial effects can be retained and the resulting polymers are transformed into antimicrobial polymers.[18–23]
Building on these results, in the current study, we report a strategy to covalently bind an N-halamine precursor, 5, 5-dimethyl hydantoin (DMH), onto PU surface using hexamethylenediisocyanate (HDI) as a coupling agent. The bound DMH moieties on PU surface are transformed into N-halamines through a simple bleach treatment, leading to potent antimicrobial and biofilm-controlling functions against Staphylococcus aureus (S. aureus, Gram-positive bacteria), Escherichia coli (E. coli, Gram-negative bacteria), methicillin-resistant staphylococcus aureus (MRSA, drug resistant Gram-positive bacteria), vancomycin-resistant enterococcus (VRE, drug resistant Gram-positive bacteria), and Candida albicans (C. ablicans, fungi), representative microorganisms that are responsible for various infections.[24–27] The antimicrobial and biofilm-controlling effects of the new polymers are both durable and rechargeable, pointing to even greater potentials of the new systems for a broad range of related applications.
2. Experimental
2.1. Materials
5, 5-dimethyl hydantoin (DMH), 1,6-hexamethylene diisocyanate (HDI), and propyl isocyanate (PI) were purchased from Sigma-Aldrich and used as received. Polyurethane (PU, Estane® 5707), a polyester-based thermoplastic PU, was kindly supplied by Lubrizol Advanced Materials Inc (Ohio, USA). A household bleach (Clorox® Regular-Bleach) containing 6.0 wt% of sodium hypochlorite was used throughout this study. Other chemicals were analytical grade and used without further purification.
The microorganisms, Staphylococcus aureus (S. aureus, ATCC 6538), Escherichia coli (E. coli, ATCC 15597), methicillin-resistant S. aureus (MRSA, ATCC BAA-811), vancomycin-resistant E. faecium (VRE, ATCC 700221), and Candida albicans (C. ablicans, ATCC10231) were obtained from the American Type Culture Collection (ATCC).
2.2. Instruments
Attenuated total reflectance (ATR) spectra of the samples were recorded on a Thermo Nicolet 6700 infrared spectroscopy (Woburn, MA). 1H-NMR studies were carried out using a Varian Unity-300 spectrometer (Palo Alto, CA) at ambient temperature in DMSO-d6. A FEI Quanta 450 scanning electron microscope (SEM) was used to study the surface morphology of the samples. UV/VIS spectra were recorded on a Beckman DU® 520 UV/VIS spectrophotometer.
2.3. Surface modification of PU
PU pellets were extracted with methanol and distilled water for 24 h to remove low molecular weight components and other potential contaminants. After extraction, the pellets were dried in a vacuum drier at 25 °C for 48 h. PU films were obtained by solution casting. To prepare PU films, 2 g PU was dissolved in 20 mL tetrahydrofuran (THF) at room temperature. After filtration, the PU solution was poured into a glass dish (100×15 mm) and allowed to dry in a fume hood overnight at room temperature. The resulting film was uniform and optically transparent with a thickness around 0.1 mm and was cut into square-shaped test films.
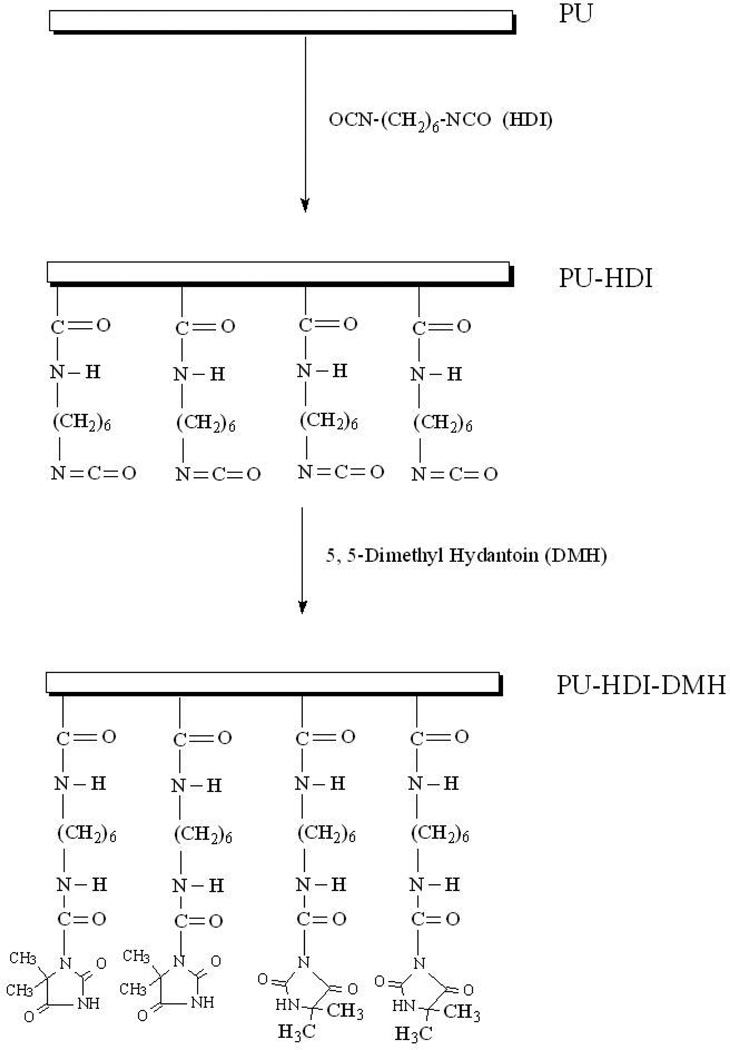
DMH was covalently linked onto PU surface through a two-step procedure, as illustrated in Scheme 1. In the first step, 1,6-hexamethylene diisocyanate (HDI) was dissolved in anhydrous toluene at a volume ratio of 1:10. PU films were immersed into 150 mL of the HDI-toluene in a 250 mL 3-neck round flask with the presence of 0.5% (v/v) dibutyltindilaurate (DBTDL) as catalyst. The mixture was stirred under N2atmosphere at 60 °C for 2 h. The isocyanate-containing PU (PU-HDI) films were taken out and washed repeatedly with anhydrous toluene to remove un reacted HDI. In the second step, DMH was immobilized onto the PU-HDI surface. PU-HDI films were dispersed in 100 mL anhydrous toluene containing 10 g DMH, and the mixtures were stirred under N2atmosphere at 70 °C for 3 h. All films after surface modifications (PU-HDI-DMH) were extracted with anhydrous toluene and ethanol three times, respectively, and driedin vacuum.
Scheme 1.
Covalently binding DMH onto PU surfaces
2.4. Synthesis of the model compound
To study the reaction mechanism between PU-HDI and DMH, propyl isocyanate (PI) was used as a model compound of PU-HDI. In the reaction, equal molar of DMH and PI were dissolved in anhydrous toluene containing 0.5% (v/v) DBTDL. The reaction continued in a 250 mL 3-neck round flask under N2atmosphere at 70 °C for 3 h. After reaction, the solvent was removed under reduced pressure, and the residuals were dried in a vacuum oven. The final product was recrystallized twice from deionized water, and dried over CaCl2 in a vacuum oven.
2.5. Determination of isocyanate content
The −NCO content on the surface of PU-HDI was determined by reacting the isocyanate groups on PU-HDI with an excess amount of dibutylamine (Bu2NH) in anhydrous toluene, followed by back-titration of the remaining Bu2NH with hydrochloric acid in anhydrous ethanol using bromphenol blue as an indicator, as reported previously.[28, 29] The mechanism is shown in Equations 1 and 2:
| (1) |
| (2) |
The un-modified original PU was titrated under the same conditions to serve as controls. Each test was repeated three times.
2.6. Chlorination of PU-HDI-DMH
To transform the grafted DMH moieties into N-halamines, PU-HDI-DMH films were immersed in 10% bleach solution sat room temperature for 45 min under constant stirring. Afterwards, the samples were washed copiously with deionized water (the washing water was tested with potassium iodide/starch to ensure that the free chlorine was removed), air-dried and stored in a desiccator for 48 h to reach constant weight. The content of active chlorineon the surface of the chlorinated PU-HDI-DMH film was determined by iodometric titration following our published procedures.[18–20, 22, 23]
2.7. Antibacterial and antifungal functions
In the antibacterial study, Staphylococcus aureus (S. aureus, ATCC 6538) and Escherichia coli (E. coli, ATCC 15597) were used as examples of non-resistant Gram-positive and Gram-negative bacteria, respectively. Methicillin-resistant S. aureus (MRSA, ATCC BAA-811) and vancomycin-resistant E. faecium (VRE, ATCC 700221) were selected to represent drug-resistant strains because these species have caused serious healthcare-associated infections (HAIs) and community-acquired infections.[30] Candida albicans (C. ablicans, ATCC 10231) was employed to challenge the antifungal activities of the samples.
To prepare the bacteria or fungi suspensions, an inoculating loop (10µL) of S. aureus 6538, E. coli 15597, MRSA BAA-811, and VRE 700221 were grown in the corresponding 5 mL broth solutions (see Table 1) at 37 °C for 24 h, and C. ablicans 10231 was grown in 5 mL YM broth at 26 °C for 36 h. Cells were harvested by centrifuge, washed twice with sterile phosphate buffered saline (PBS), and then re-suspended in sterile PBS.
Table 1.
Microorganisms tested in this study and the media used for their growth
| Bacteria | Drug-resistant bacteria | Fungi | |||
|---|---|---|---|---|---|
|
S. aureus 6538a |
E. coli 15597b |
MRSR BAA-811a |
VRE 700221a |
C. albicans 10231 |
|
| Broth | Tryptic soy broth |
LB broth | Tryptic soy broth | Tryptic soy broth |
YM broth |
| Agar | Tryptic soy agar |
LB agar | Tryptic soy agar | Tryptic soy agar |
YPD agars |
Gram-positive bacteria
Gram-negative bacteria
In the current study, waterborne microbial challenging conditions were used to evaluate the antimicrobial effects of the film samples. All microbial tests were performed in a Bio safety Level-2 hood to ensure safety, and the films were sterilized 15 min each side under UV light before microbial studies. In each test, 10 µL of an aqueous suspension containing 108–109 CFU/mL of each bacteria (S. aureus 6538, E. coli 15597, MRSA BAA-811, and VRE 700221) or fungi (C. ablicans 10231) was placed onto the surface of a chlorinated PU-HDI-DMH film (3×3 cm). Another identical film was used to coverit, so the bacterial/fungal aqueous suspension was entrapped inside the films like “fillings”in a “sandwich” to improve contact between the bacteria and the film surfaces. After a certain period of contact time, the entire “sandwich” was transferred into 10 mL 0.03 wt% sodium thiosulfate aqueous solution to quench the active chlorine and stop the microbial tests.[20] The mixture was vortexed for 1 min and then sonicated for 5 min. An aliquot of the solution was serially diluted, and 100 µL of each dilution was plated onto the corresponding agar plates. The same procedure was also applied to the original PU films to serve as controls. Bacterial colonies were counted after incubation at 37 °C for 24 h, and fungi colonies were counted after incubation at 26 °C for 36 h. Each test was repeated three times.
2.8. Kirby-Bauer test
In this test, the surfaces of a tryptic soy agar plate and Luria-Bertant (LB) agar plate were overlaid with 1 mL 108–109 CFU/mL of S. aureus 6538 and E. coli 15597, respectively. The plates were then allowed to stand at 37 °C for 2 h. Each chlorinated PU-HDI-DMH film (2×2 cm) was placed onto the surface of each of the bacteria-containing agar plates. The film was gently pressed with a sterile forceps to ensure full contact between the film and the agar. After incubation at 37 °C for 24 h, the inhibition zone around the films (if any) was measured. Afterwards, the films were removed sterilely from the agar plates, and washed gently with non-flowing sterile PBS (3×10 mL) to remove loosely attached bacteria and/or possible agar components. The resulting films were vortexed for 1 min and sonicated for 5 min in 10 mL PBS to detach adherent bacteria. The sonicated solution was serially diluted, and 100 µL of each dilution was placed onto the corresponding agar plates (see Table 1). Recoverable microbial colonies were counted after incubation at 37 °C for 24 h. The same procedure was also applied to the original PU films to serve as controls.
2.9. Biofilm-controlling function
The ability of the chlorinated PU-HDI-DMH films to prevent biofilm formation was evaluated using scanning electron microscope (SEM) analysis. In this study, S. aureus, E. coli and C. albicans were grown and harvested as described above. A chlorinated PU-HDI-DMH film (2×2 cm) was immersed in 10 mL sterile PBS containing 108–109 CFU/mL of S. aureus, E. coli or C. albicans, respectively. The mixture was gently shaken at 37 °C for 30 min to allow microbial adhesion. The films were taken out of the bacteria or fungi solution, gently washed 3 times with 10 mL sterile PBS to remove loosely attached cells. The films were then immersed in broth solutions (tryptic soy broth for S. aureus, LB broth for E. coli, and YM broth for C. albicans), and incubated at 37 °C for 72 h to facilitate biofilm formation. The films were taken out, gently washed 3 times with 10 mL sterile PBS, and fixed with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (SCB) at 4 °C for 24 h. After being gently washed with SCB, the samples were dehydrated through an alcohol gradient, dried in a critical point drier, mounted onto sample holders, sputter coated with gold-palladium, and observed under a FEI Quanta 450 SEM to check for the presence of biofilms. The same procedure was also applied to the un-chlorinated PU-HDI-DMH films and the original PU films to serve as controls.
2.10. Stability and recharge ability of the N-halamines
To evaluate the stability of the covalently bound chorines in the N-halamines under wet conditions, a series of chlorinated PU-HDI-DMH films (3×3 cm) were immersed in 10 mL PBS under constant shaking (50 rpm) at room temperature. After a certain period of time, 1 mL solution was taken out of the immersing PBS and tested with a Beckman DU® 520 UV/VIS spectrophotometer in the range of 190–400 nm to determine whether DMH or Cl-DMH-containing compounds were released from the PU film into the solution (characteristic absorption peaks of pure DMH: 218 nm, and Cl-DMH: 348 nm). The chlorine contents and the antibacterial and antifungal functions of the film samples were tested periodically over a one-month period.
The stability of the N-halamine-based PUs was also investigated under dry-storage conditions. A series of chlorinated PU-HDI-DMH films with known chlorine contents were stored under normal lab conditions (25 °C, 40–60 % RH) in open air. The chlorine contents and the antibacterial and antifungal functions were tested periodically over a 6-month storage period.
To test recharge ability, the N-halamine-based PU films were first treated with 0.1 M sodium thiosulfate aqueous solution at room temperature for 24 h to quench the bound chlorine, and then recharged using a 10% Clorox® regular-bleach aqueous solution for 45 min. The chlorinated PU films were washed with distilled water, air dried, and then quenched and recharged again. After different cycles of this “quenching-recharging” treatment, the chlorine contents and antibacterial and antifungal functions of the resulting films were retested to fully evaluate recharge ability. Each test was repeated three times.
2.11 Statistical analysis
Data management and analysis was conducted using SPSS version 11.0 (SPSS Inc., Chicago, IL). One-way analysis of variance was used to assess statistical significance with 95% of confidence level.
3. Results and discussion
3.1. Surface modification of PU
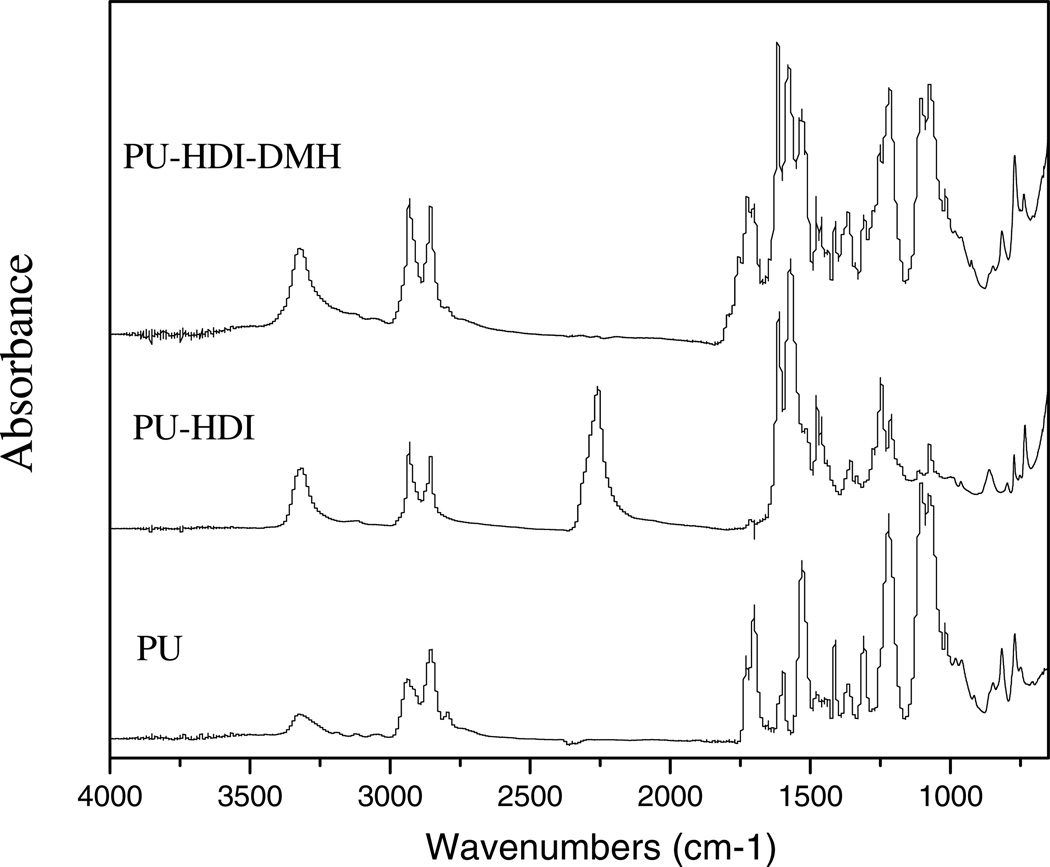
DMH was covalently linked to the surface of PU with a 2-step chemical reaction using HDI as a coupling agent, as shown in Scheme 1. The reaction was confirmed by ATR-FTIR studies (see Figure 1). In the spectrum of PU, the bands around 3420-3200 cm−1 were attributable to N-H stretching vibrations, the peaks at 3000-2800 cm−1 were assigned to CH2 and CH3 stretching, the peak at 1725 cm−1 was caused by C=O (amide I) stretching, and the signal at 1530 cm−1 was attributable to N-H (amide II) deforming, in good agreement with the literature data.[31] Each HDI has two isocyanate (−NCO) groups, both of which could react with the urethane groups of PU, causing crosslinks of the polymer (see below). On the other hand, some HDI molecules could also contribute only one −NCO group to react with PU, leaving a free −NCO group on PU surface for further functionalization with DMH.
Figure 1.
FT-IR spectra of PU, PU-HDI, and PU-HDI-DMH
The presence of free −NCO groups on PU-HDI was confirmed by both ATR study and Bu2NH/HCL titration. In the ATR spectrum of PU-HDI (Figure 1), in addition to the characteristic bands of PU, a new band at 2260 cm−1 could be clearly detected, which was attributable to the stretching oscillation of free −NCO groups. In Bu2NH/HCL titration, although the original PU did not have any detectable −NCO groups, after reacting with HDI under our reported conditions, the resulting PU-HDI surface had (1.81 ± 0.15) × 1016 groups/cm2 of free −NCO (see Table 2).
Table 2.
Determination of NCO content and active chlorine content
| Sample | NCO (groups / cm2) |
Active Chlorine (atoms / cm2) |
|---|---|---|
| Original PU | 0 | (1.18 ± 0.13) × 1014 |
| PU-HDI | (1.81 ± 0.15) × 1016 | N/A |
| PU-HDI-DMH | 0 | (1.76 ± 0.11) × 1016 |
The free −NCO groups on PU-HDI enabled the polymer to covalently bind DMH onto the film surfaces. As shown in Figure 1, after reacting with DMH, the 2260 cm−1 isocyanate signal disappeared. On the other hand, a new band at 1760 cm−1 could be observed in the ATR spectrum of PU-HDI-DMH as a weak shoulder, which must be caused by the carbonyl groups of the covalently bound DMH.[32]
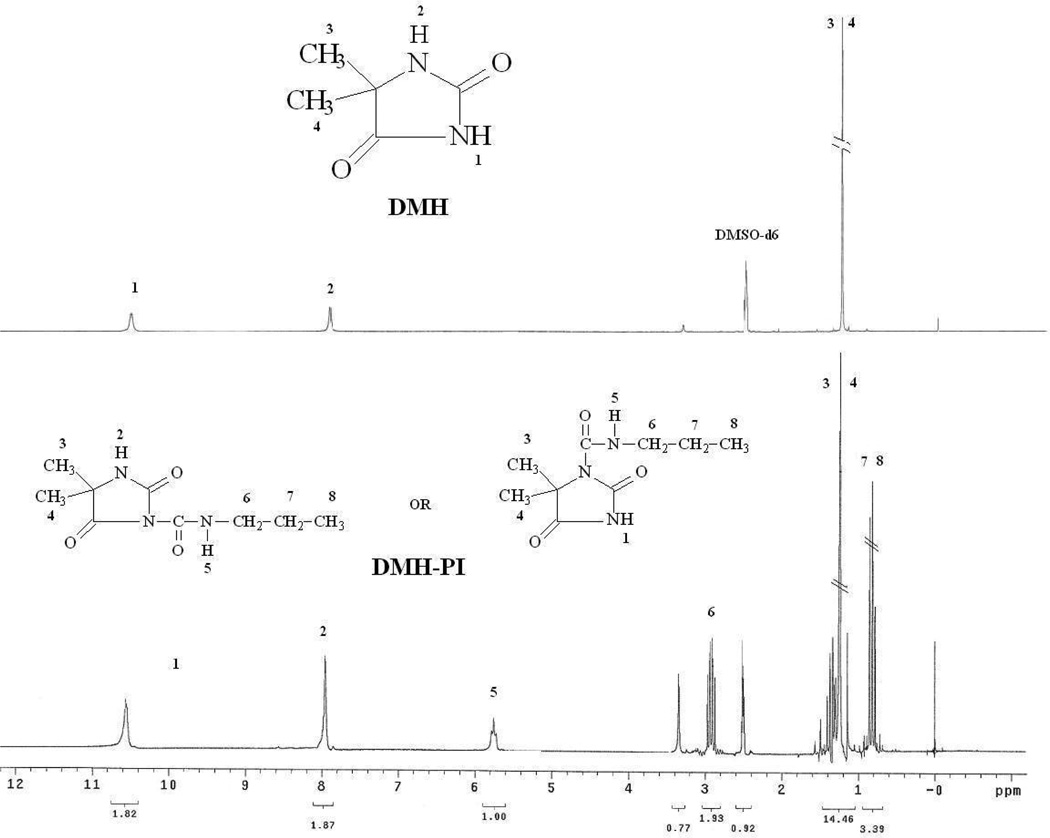
Each DMH molecule has two potential sites, the amide −NH and imide −NH, to react with the free isocyanate groups on PU-HDI. It was of great interest to determine which site(s) reacted with −NCO. Normally, such information could be readily obtained through 1H-NMR studies. However, under our reaction conditions (see the Experimental section), the resulting PU-HDI and PU-HDI-DMH were only partially soluble in DMSO, DMF, THF, and other good solvents of PU. These results suggested that part of the PU polymer chains were crosslinked by HDI, making 1H-NMR studies difficult to perform on these polymers. On the other hand, since the reaction of PU-HDI with DMH occurred as one free −NCO on PU-HDI choosing a reaction site from DMH (amide −NH or imide −NH), we believed that the reaction pathway could be reasonably deduced using a mono isocyanate, such as propyl isocyanate (PI), as a model compound of PU-HDI to react with DMH.
Figure 2 presented the 1H-NMR spectra of DMH and the product of DMH reacting with PI (DMH-PI). In the spectrum of DMH, the signals of the imide protons and amide protons appeared at 10.56 ppm and 7.92 ppm, respectively. The signals at 1.23 ppm were correspondent to the protons of methyl groups. Upon reacting with PI, if the binding site was primarily the imide −NH group of DMH, the signal at 10.56 ppm in the spectrum of the resulting DMH-PI would significantly decrease. On the other hand, if the reaction mainly occurred at the amide −NH group of DMH, the signal around 7.92 ppm of the product would become much weaker.[32, 33] However, in the 1H-NMR spectrum of DMH-PI, both signals of the imide and amide protons were clearly detectable, with very similar proton integration values (1.82 vs. 1.87). These results might suggest that due to the high reactivity of isocyanate groups, the imide −NH group and amide −NH group of DMH had very similar probability in reacting with PU-HDI. Thus, the resulting PU-HDI-DMH could have both amide and imide −NH groups for chlorination to produce N-halamines.
Figure 2.
1H-NMR spectra of DMH and DMH-propyl isocyanate (DMH-PI)
After treatment with 10% bleach solutions, the amide or the imide groups on PU-HDI-DMH were readily transformed into N-halamine structures. Iodometrictitration revealed that the chlorinated PU-HDI-DMH had a total chlorine atom area density of (1.76 ± 0.11) × 1016 atoms / cm2, which was relatively unchanged after storage of up to 6 months in open air. As a comparison, after the same chlorine bleach treatment, the original PU film had (1.18 ± 0.13) × 1014 atoms / cm2 of chlorine; after only 1 week of storage in open air, almost no chlorines could be detected in titration. These findings strongly suggested the presence of stable N-halamines on the surface of chlorinated PU-HDI-DMH films.
3.2. Antibacterial and antifungal activity
In this study, the antibacterial and antifungal efficacies of the chlorinated PU-HDI-DMH films were evaluated under waterborne contact mode.[18, 20, 34] The antimicrobial results were shown in Table 3 using the untreated original PU films as negative controls. The chlorinated PU-HDI-DMH films provided a 4-log reduction (the highest antimicrobial potency that could be observed under the current testing conditions)of S. aureus (Gram-positive bacteria) in 60 min, and a 4-log reduction of E. coli (Gram-negative bacteria) in 30 min, respectively. On the other hand, the freshly prepared chlorinated original PU films only provided a 90% reduction of the same bacteria after as long as 8 h of contact (data not shown in Table 3), suggesting that the antimicrobial effects of the chlorinated PU-HDI-DMH mainly originated from the N-halamines on the covalently bound DMH moieties.
Table 3.
Log reduction of microbial cells in the antimicrobial tests*
| Contact time (min) |
S. aureus | E. coli | MRSA | VRE | C. albicans |
|---|---|---|---|---|---|
| 5 | 1-log | 2-log | 1-log | 1-log | 1-log |
| 10 | 2-log | 3-log | 3-log | 3-log | 2-log |
| 30 | 3-log | 4-log** | 4-log | 4-log | 3-log |
| 60 | 4-log | 4-log |
S. aureus, E. coli, MRSA, VRE, and C. albicans concentrations were 108–109 CFU/mL, and 10 µL of such microbial suspension was used in each test (see text for details); the chlorinated PU-HDI-DMH surface contained (1.76 ± 0.11) × 1016 atoms / cm2 of active chlorine.
Highest antimicrobial potency that could be observed under the current testing conditions.
Drug-resistant bacteria including MRSA and VRE are of major concerns in healthcare settings and a wide range of related community facilities, causing serious healthcare-related infections and community acquired infections. It was encouraging to find that the chlorinated PU-HDI-DMH films provided potent antimicrobial effects against MRSR and VRE, achieving a 4-log reduction of both species within 30 min (see Table 3). This phenomenon could be explained by the antimicrobial mechanisms. MRSA is resistant to certain antibiotics via the mecA gene, and VRE acquires resistance to vancomycin through the introduction of new DNAs to prevent vancomycin-binding.[6] On the other hand, N-halamines provide antimicrobial effects by the oxidative reactions of Cl+ from N-halamines to microbial cells, which was apparently not affected by the drug-resistant actions of MRSR or VRE.[35]
Candida species are emerging as important nosocomial pathogens, and implanted devices with detectable biofilms are frequently associated with these species.[36] The antifungal function of the chlorinated PU-HDI-DMH samples was evaluated against C. albicans, which showed that the new N-halamine-based PU samples provided a 4-log reduction of the yeast within 60 min, pointing to even greater potentials of the new samples.
3.3. Kirby-Bauer test
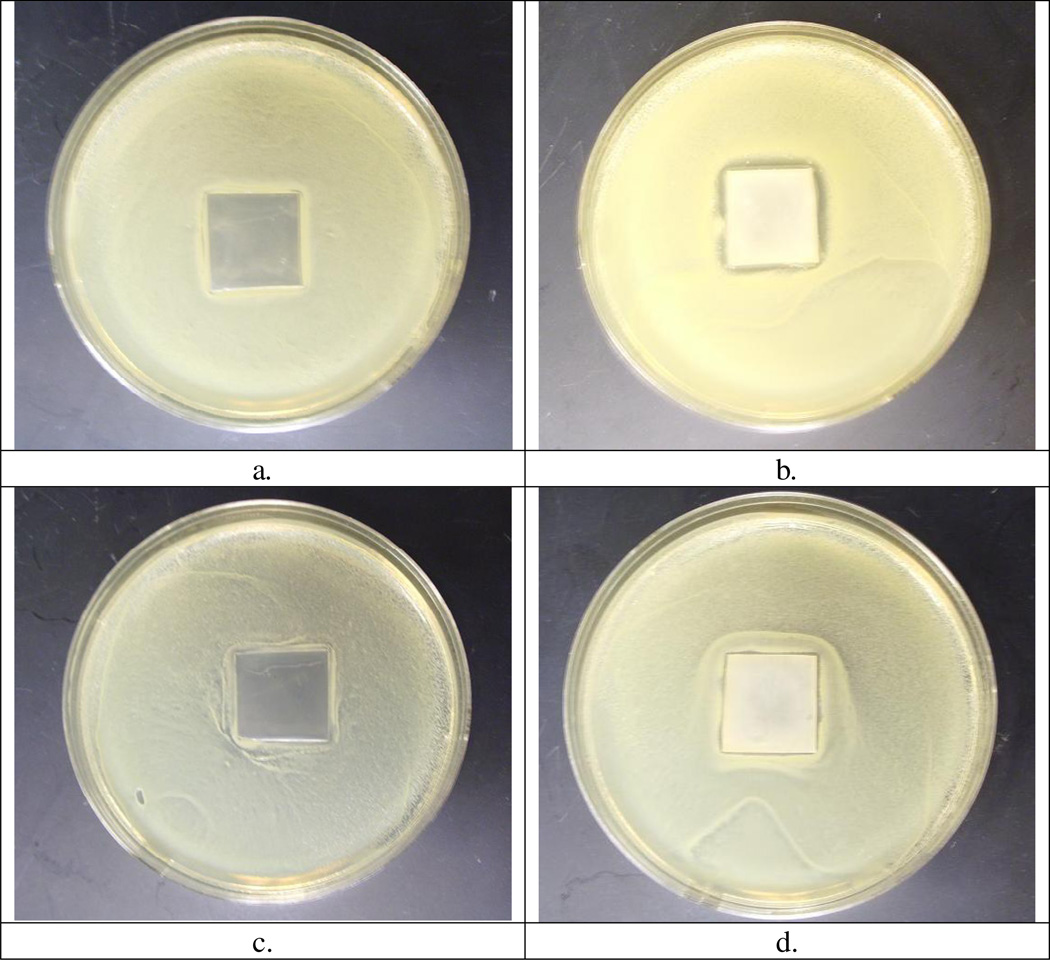
The antibacterial activity of the chlorinated PU-HDI-DMH samples was further assessed with Kirby-Bauer test. As shown in Figure 3, the original PU film did not show any inhibition zones. In the case of the chlorinated PU-HDI-DMH samples containing (1.76 ± 0.11) × 1016 atoms / cm2 of active chlorine, a clear inhibition zone could be detected, with a zone size around 1.8 mm against S. aureus, and about 0.9 mm against E. coli, respectively. These findings suggested that during the antimicrobial tests, active chlorines diffused away from the chlorinated PU-HDI-DMH to kill the bacteria. To confirm this, chlorinated PU-HDI-DMH films were immersed in deionized water under constant shaking at room temperature, and UV/VIS spectrophotometer was used to test the immersing solutions. Within the test period of 72h, the soaking solution was very clear, with no suspensions/precipitation could be observed. In the wavelength range of 190–400 nm, no UV absorption could be detected, suggesting that no detectable DMH or Cl-DMH-containing compounds released into the water system. On the other hand, iodometric titration found that the solution contained about 0.2 ppm of active chlorine, indicating that the inhibition zones were most likely caused by Cl+ originated from the disassociation of N-halamines. Such a low level of chlorine release pointed to good stability and safety of the new samples (As a comparison, EPA allows drinking water contain up to 4 ppm of active chlorine as disinfection residues).
Figure 3.
Inhibition zone of (a) the original PU film against S. aureus (b) the chlorinated PU-HDI-DMH film against S. aureus (c) the original PU film against E. coli, and (d) the chlorinated PU-HDI-DMH film against E. coli.
To provide more information about the antimicrobial effect, immediately after the Kirby-Bauer test, the films were washed and sonicated to remove surface adherent bacteria. As shown in Table 4, from the original PU film, as high as (5.4±0.11) × 106 CFU/cm2 of S. aureus or (4.9 ± 0.18) × 106 CFU/cm2 of E. coli could be recovered (n=3). From the chlorinated PU-HDI-DMH films, however, the recoverable levels of S. aureus and E. coli were only in the range of 102 CFU/cm2, further confirming the antimicrobial effects of the new samples.
Table 4.
Inhibition zones of the films and levels of recoverable bacteria
| Inhibition zone (mm) |
Bacteria recovered (CFU/cm2) |
|||
|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | |
| Control* | 0 | 0 | (5.4 ± 0.11)×106 | (4.9 ± 0.18)×106 |
| Sample** | 1.8 ± 0.1 | 0.9 ± 0.1 | (4.9 ± 0.36)×102 | 3.1 ± 0.21)×102 |
The original PU.
Chlorinated PU-HDI-DMH with (1.76 ± 0.11) × 1016 atoms / cm2 of active chlorine.
3.4. Biofilm-controlling effects
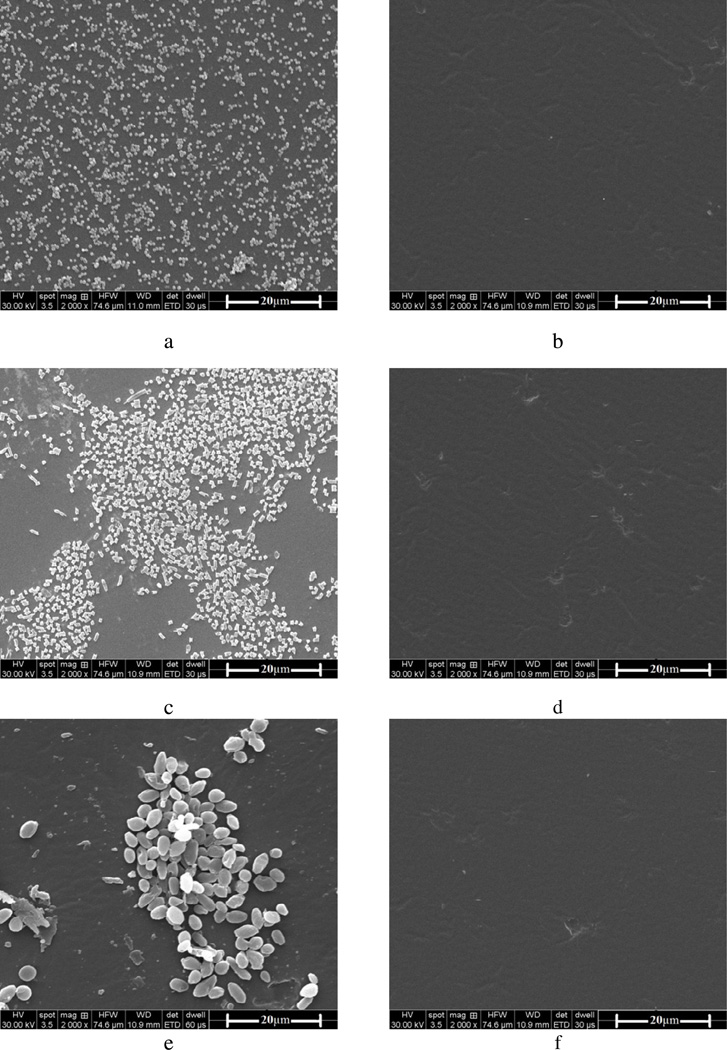
The potent antimicrobial activity of the new chlorinated PU-HDI-DMH samples pointed to effective biofilm-controlling functions. To evaluate this effect, Figure 4 presented the SEM results of the samples in biofilm-controlling studies. After 72 h of growth in the corresponding broth solutions, a large quantity of layered S. aureus (Figure 4 a), E. coli (Figure 4 c) or C. albicans (Figure 4 e) attached and aggregated on the original PU surfaces, indicating formation and development of biofilms. Similar results were observed on the un-chlorinated PU-HDI-DMH films (images not shown). On the surfaces of the chlorinated PU-HDI-DMH films, however, no adherent microbial cells could be observed, suggesting powerful biofilm-controlling functions of the new N-halamine-based PU against Gram-positive bacteria, Gram-negative bacteria, and fungi. The biofilm-controlling function of the chlorinated PU-HDI-DMH films could be attributed to the antimicrobial activities of the N-halamines, i.e., when microbial cells came into contact with the films, most of them were inactivated during and/or after adherence/colonization. The inactivated cells could be easily removed duration the SEM preparation process (e.g., rinsing, fixation, alcohol dehydration), resulting in cleaner surfaces.[20]
Figure 4.
Biofilm-controlling function of (a) the original PU film against S. aureus, (b) the chlorinated PU-HDI-DMH film against S. aureus, (c) the original PU film against E. coli, (d) the chlorinated PU-HDI-DMH film against E. coli, (e) the original PU film against C. albicans, and (f) the chlorinated PU-HDI-DMH film against C. albicans.
3.5. Stability and recharge ability of the N-halamines
In the soaking studies, during the test period of 1 month under constant stirring, the soaking solution was very clear, and no suspensions/precipitation could be observed. In the wavelength range of 190–400 nm, no UV absorption could be found, suggesting that no detectable DMH or Cl-DMH-containing compounds were released out from the chlorinated PU-HDI-DMH films. Moreover, after 1 month in the solution, the antimicrobial efficacies of the samples against the bacterial and fungi species were unchanged.
In the dry-storage studies, under normal lab conditions, after six months of storage in open air, 91% the original active chlorines were retained on the chlorinated PU-HDI-DMH films (as shown in Table 5). The antimicrobial activity against the bacterial and fungal species were not changed at the end of the six-month storage tests. Furthermore, after 10 cycles of the “quenching–recharging” treatments, the chlorine contents and antimicrobial activities of the new N-halamine-based PU sample were essentially unchanged, indicating that the antimicrobial function was fully rechargeable.
Table 5.
Durability and Recharge ability of the N-halamines in the chlorinated PU-HDI-DMH
| Sample | chlorine atom area density (atoms / cm2) |
Percentage of chlorine left (%) |
|---|---|---|
| Fresh samples | (1.76 ± 0.11) × 1016 | 100 |
| After six-month of storage | (1.61 ± 0.23) × 1016 | 91 |
| After 10 cycles of “quenching–recharging” |
(1.74 ± 0.21) × 1016 | 98.6 |
4. Conclusions
DMH, an N-halamine precursor, was covalently bound onto PU surfaces using HDI as a coupling agent. The reactions were confirmed by ATR, 1H-NMR, −NCO content determination, and iodometric titration. Model compound studies suggested that the imide and amide groups of DMH had very similar reactivity toward −NCO groups on PU-HDI surfaces. Upon bleach treatment, the DMH moieties on the PU-HDI-DMH surfaces were transformed into N-halamine, providing powerful, durable, and rechargeable antimicrobial activities against Gram-positive bacteria, Gram-negative bacteria, drug-resistant bacteria, and fungi. Kirby-Bauer test showed that the antimicrobial function was at least partly provided by the positive chlorines generated from the disassociation of the newly formed N-halamine structures. SEM studies demonstrated that the N-halamine-based PU could effectively prevent the formation of bacterial and fungal biofilms. These encouraging results point to great potentials of the new N-halamine-based PU for a wide range of long-term antimicrobial and biofilm-controlling applications.
Acknowledgements
This study was sponsored by NIH, NIDCR (Grant number R01DE018707).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Oertel G, Abele L. Polyurethane handbook: chemistry, raw materials, processing, application, properties. Hanser Gardner Publications; 1994. [Google Scholar]
- 2.Oertel G, Abele L. Polyurethane. Hanser Gardner Publications; 1993. [Google Scholar]
- 3.Szycher M. Szycher's handbook of polyurethanes. CRC Press; 1999. [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Haley RW, Hooton TM, Culver DH, Stanley RC, Emori TG, Hardison CD, et al. Nosocomial infections in U.S. hospitals 1975–1976: estimated frequency by selected characteristics of patients. Am J Med. 1981;70:947–959. doi: 10.1016/0002-9343(81)90561-1. [DOI] [PubMed] [Google Scholar]
- 6.Linde H, Wagenlehner F, Strommenger B, Drubel I, Tanzer J, Reischl U, et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur J Clin Microbiol Infect Dis. 2005;24:419–422. doi: 10.1007/s10096-005-1341-7. [DOI] [PubMed] [Google Scholar]
- 7.von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs. 2005;65:179–214. doi: 10.2165/00003495-200565020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev. 2005;57:1539–1550. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Pavithra D, Doble M. Biofilm formation, bacterial adhesion and host response on polymeric implants--issues and prevention. Biomed. Mater. 2008;3:1–13. doi: 10.1088/1748-6041/3/3/034003. [DOI] [PubMed] [Google Scholar]
- 10.Goddard JM, Hotchkiss JH. Rechargeable antimicrobial surface modification of polyethylene. J Food Protect. 2008;71:2042–2047. doi: 10.4315/0362-028x-71.10.2042. [DOI] [PubMed] [Google Scholar]
- 11.Tan KT, Obendorf SK. Development of an antimicrobial microporous polyurethane membrane. J Membrane Sci. 2007;289:199–209. [Google Scholar]
- 12.Makal U, Wood L, Ohman DE, Wynne KJ. Polyurethane biocidal polymeric surface modifiers. Biomaterials. 2006;27:1316–1326. doi: 10.1016/j.biomaterials.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Grapski JA, Cooper SL. Synthesis and characterization of non-leaching biocidal polyurethanes. Biomaterials. 2001;22:2239–2246. doi: 10.1016/s0142-9612(00)00412-9. [DOI] [PubMed] [Google Scholar]
- 14.Sun YY, Luo J, Chen ZB. Controlling biofilm formation with an N-halamine-based polymeric additive. J Biomed Mater Res A. 2006;77A:823–831. doi: 10.1002/jbm.a.30689. [DOI] [PubMed] [Google Scholar]
- 15.Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS. Polymeric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces. 2011;3:2845–2850. doi: 10.1021/am200351w. [DOI] [PubMed] [Google Scholar]
- 16.Worley SD, Williams DE. Halamine Water Disinfectants. Crit Rev Env Contr. 1988;18:133–175. [Google Scholar]
- 17.Lauten SD, Sarvis H, Wheatley WB, Williams DE, Mora EC, Worley SD. Efficacies of Novel N-Halamine Disinfectants against Salmonella and Pseudomonas Species. Appl Environ Microb. 1992;58:1240–1243. doi: 10.1128/aem.58.4.1240-1243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Sun Y. Polymeric N-halamine latex emulsions for use in antimicrobial paints. ACS Appl. Mater. Interfaces. 2009;1:494–504. doi: 10.1021/am800157a. [DOI] [PubMed] [Google Scholar]
- 19.Yao JR, Sun YY. Preparation and characterization of polymerizable hindered amine-based antimicrobial fibrous materials. Ind Eng Chem Res. 2008;47:5819–5824. [Google Scholar]
- 20.Cao Z, Sun Y. N-halamine-based chitosan: preparation, characterization, and antimicrobial function. J Biomed Mater Res A. 2008;85:99–107. doi: 10.1002/jbm.a.31463. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Sun YY. Acyclic N-halamine-based biocidal tubing: Preparation, characterization, and rechargeable biofilm-controlling functions. J Biomed Mater Res A. 2008;84A:631–642. doi: 10.1002/jbm.a.31301. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZB, Luo J, Sun YY. Biocidal efficacy, biofilm-controlling function, and controlled release effect of chloromelamine-based bioresponsive fibrous materials. Biomaterials. 2007;28:1597–1609. doi: 10.1016/j.biomaterials.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Sun YY. Acyclic N-halamine-based fibrous materials: Preparation, characterization, and biocidal functions. J Polym Sci Pol Chem. 2006;44:3588–3600. [Google Scholar]
- 24.Hebert C, Robicsek A. Decolonization therapy in infection control. Curr Opin Infect Dis. 2010;23:340–345. doi: 10.1097/QCO.0b013e32833ae214. [DOI] [PubMed] [Google Scholar]
- 25.Ouwendyk M, Helferty M. Central venous catheter management: how to prevent complications. ANNA J. 1996;23:572–577. [PubMed] [Google Scholar]
- 26.Pournaras S, Iosifidis E, Roilides E. Advances in antibacterial therapy against emerging bacterial pathogens. Semin Hematol. 2009;46:198–211. doi: 10.1053/j.seminhematol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Brun-Buisson C. New technologies and infection control practices to prevent intravascular catheter-related infections. Am J Resp Crit Care. 2001;164:1557–1558. doi: 10.1164/ajrccm.164.9.2109051. [DOI] [PubMed] [Google Scholar]
- 28.Wissman HG, Rand L, Frisch KC. Kinetics of polyether polyols–diisocyanate reactions. J Appl Polym Sci. 1964;8:2971–2978. [Google Scholar]
- 29.Xu J, Dimonie VL, Sudol ED, El-Aasser MS. Crosslinking of isocyanate functional acrylic latex with telechelicpolybutadiene. I. Synthesis and characterization. J Appl Polym Sci. 1998;69:965–975. [Google Scholar]
- 30.Gastmeier P, Stamm-Balderjahn S, Hansen S, Zuschneid I, Sohr D, Behnke M, et al. Where should one search when confronted with outbreaks of nosocomial infection? Am J Infect Control. 2006;34:603–605. doi: 10.1016/j.ajic.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Guan JJ, Gao GY, Feng LX, Sheng JC. Surface photo-grafting of polyurethane with 2-hydroxyethyl acrylate for promotion of human endothelial cell adhesion and growth. J Biomat Sci-Polym E. 2000;11:523–536. doi: 10.1163/156856200743841. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Sun G. Novel regenerable N-halamine polymeric biocides. I. Synthesis, characterization, and antibacterial activity of hydantoin-containing polymers. J Appl Polym Sci. 2001;80:2460–2467. [Google Scholar]
- 33.Sun Y, Sun G. Novel regenerable N-halamine polymeric biocides. II. Grafting hydantoin-containing monomers onto cotton cellulose. J Appl Polym Sci. 2001;81:617–624. [Google Scholar]
- 34.Sun XB, Cao ZB, Porteous N, Sun YY. Amine, Melamine, and Amide N-Halamines as Antimicrobial Additives for Polymers. Ind Eng Chem Res. 2010;49:11206–11213. doi: 10.1021/ie101519u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang J, Chen Y, Barnes K, Wu R, Worley SD, Huang TS. N-halamine/quatsiloxane copolymers for use in biocidal coatings. Biomaterials. 2006;27:2495–2501. doi: 10.1016/j.biomaterials.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Cao Z, Sun X, Yeh CK, Sun Y. Rechargeable infection-responsive antifungal denture materials. J Dent Res. 2010;89:1517–1521. doi: 10.1177/0022034510379604. [DOI] [PMC free article] [PubMed] [Google Scholar]