Abstract
Induced biomineralization of materials has been employed as a strategy to increase integration with host tissue, and more recently as method to control cell function in tissue engineering. However, mineralization is typically performed in the absence of cells, since hypertonic solutions that lack the nutrients and culture components required for maintenance of cell viability are often used. In the present study, we exposed fibroblast-seeded 3D collagen-chitosan hydrogels to a defined culture medium modified to have specific concentrations of ions involved in biomineralization. Modified medium caused a significant increase in calcium deposition in collagen-chitosan gels, relative to constructs incubated in standard medium, though serum supplementation attenuated mineral deposition. Collagen-chitosan constructs became opaque over three days of mineralization in modified DMEM, in contrast to translucent control gels incubated in standard DMEM. Histological staining confirmed increased levels of mineral in the treated constructs. Rheological characterization showed that both the storage and loss moduli increased significantly in mineralized materials. Mineralization of fibroblast-seeded constructs resulted in decreased cell viability and proliferation rate over three days of incubation in modified medium, but the cell population remained over 75% viable and regained its proliferative potential after rescue in standard culture medium. The ability to mineralize protein matrices in the presence of cells could be useful in creating mechanically stable tissue constructs, as well as to study the effects of the tissue microenvironment on cell function.
Introduction
There is a clear need for materials and methods to improve bone healing outcomes, particularly in cases of large defects and non-unions. The natural healing response is often not adequate to obtain full repair, and in such cases strategies to augment bone regeneration can be applied. Autografts and allografts are currently used clinically, but are hampered by issues of tissue availability and consistency [1]. The generic tissue engineering approach is to combine cells, biomaterials, and growth factors in a controlled fashion to create living materials that can replace damaged tissue and/or enhance regeneration. In the case of bone tissue, a wide range of strategies have been employed, using a variety of cell types, materials, and biochemical factors[2].
One strategy for potentiating the bone healing response is to use materials that have been exogenously mineralized using defined ionic solutions. Simulated body fluid (SBF) is a solution formulated with ion concentrations similar to blood plasma, which mineralizes the surfaces and pore walls of both natural[3–7] and synthetic[8–10] scaffolds if thermodynamic conditions are appropriate. Kokubo et. al[11] first described that soaking a biomaterial in SBF leads to the ex vivo formation of a bone-like apatite coating, and later studies showed that such coatings can be both osteoconductive[10] and osteoinductive[13], and can facilitate the regeneration of bone [12, 14]. SBF-induced mineralization has been further examined as a method for controlling osteoconductivity [5, 9, 15], as well as for protein [13, 16] and gene delivery [17, 18]. Taken together, this body of work has shown that SBF can be a useful tool to modify biomaterials for bone tissue engineering applications.
Previous studies using SBF to modify material scaffolds have been performed in the absence of cells, since the high ionic concentrations and lack of nutrients in SBF are not conducive to the maintenance of cell growth. However, cell-seeded materials have been proposed for a number of orthopaedic applications. Natural biomaterial hydrogels are of interest in such cases due to their ability to mimic the natural extracellular matrix [19] and provide tissue-specific cues to enhance cell attachment and stem cell differentiation [20]. Direct encapsulation of cells during gel formation can be used to facilitate homogenous cell distribution in hydrogels. Numerous natural polymers including collagen [3], chitosan [21], and composite matrices [22–25] have been employed to engineer tissues and have shown promise in bone regeneration[26]. A drawback of natural hydrogel materials is that they often lack mechanical strength and represent only the protein component of the native bone tissue. Mineralization of such matrices has been pursued as a strategy to improve their mechanical properties and more closely mimic the native matrix[6], however the cellular component is typically not included during the mineralization process.
In the present study, we mineralized 3D hydrogels using a modified culture medium that combined the ionic constituents of SBF with the nutrients, vitamins, and amino acids needed to maintain cell viability. The model tissue constructs consisted of fibroblast cells embedded in collagen-chitosan hydrogel matrices developed previously in our laboratory[24, 26]. Fibroblasts were used as a model cell type to examine the feasibility of mineralization in the presence of cells, since this non-mineralizing cell type allowed us to isolate the effects of mineralization to the medium alone. Both unseeded and fibroblast-seeded hydrogels were exposed to mineralizing solutions that were formulated to induce biomineralization while also supporting cell growth, and the effects of such treatment on mineral content, mechanical properties, and cellular viability were determined. Our primary goal was to demonstrate that mineralization of protein-based hydrogels is possible in the presence of cells. The ability to mineralize cell-seeded protein matrices could be useful in creating mechanically stable and osteogenic tissue constructs, as well as in studying the process of biomineralization.
2. Materials and Methods
2.1 Media formulations
The composition of the mineralization medium was based on previously studied simulated body fluid (SBF) formulations, with modifications to enhance both mineralization and the ability to support cell growth. The base medium was Dulbecco’s modified Eagle medium (DMEM; high glucose, Invitrogen, Carlsbad, CA), which was supplemented with ionic salts. Table 1 shows the ion concentrations of relevant biological fluids and mineralizing media. The main augmentation to the modified medium formulation was a 4-fold increase in calcium (Ca2+) and phosphate (PO43−) in order to promote biomineralization, and an increased carbonate (HCO3−) level to provide buffering capacity. These modifications are further discussed in the Results and Discussion section.
Table 1.
Media Formulation Ion Concentrations (mM)
| Na+ | K+ | Ca2+ | Mg2+ | HCO3− | SO42− | HPO32− | pH | |
|---|---|---|---|---|---|---|---|---|
| Blood Plasma | 142 | 3.6–5.5 | 2.1–2.6 | 1.0 | 27 | 1.0 | 0.65–1.45 | 7.2–7.4 |
| Simulated Body Fluid (SBF) | 141 | 4.0 | 2.5 | 1.0 | 4.2 | 0.5 | 1.0 | 7.4 |
| Dulbecco’s modified Eagle’s Medium (DMEM) | 110 | 5.3 | 1.8 | 0 | 20 | 0.8 | 0.9 | 7.4 |
| Modified DMEM (mDMEM) | 141 | 5.3 | 10 | 1.0 | 8.4 | 0.8 | 4.0 | 7.4 |
The modified medium (mDMEM) formulation was prepared by adding salts directly to DMEM to achieve final concentrations of 141 mM NaCl, 5.3 mM KCl, 10 mM CaCl2·H2O, 1.0 mM MgCl2, 8.4 mM NaHCO3, 0.8 mM MgSO4, and 4.0 mM KH2PO4. The medium was prepared at 25°C and titrated to a pH of 7.4. In experiments examining the effect of serum, mDMEM and control DMEM were supplemented with varying concentrations of fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (P/S; Invitrogen). As per previous protocols, mDMEM was changed every 12 hours to avoid precipitation in the culture solution and DMEM was changed every 3 days. In subsequent mineralization experiments using cells, the mineralization medium (MM) used was mDMEM supplemented with 2% FBS. The control medium (DM) was DMEM supplemented with 10% FBS.
2.2 Collagen-chitosan Gel Fabrication
Collagen-chitosan gel composites were formed through a β-GP induced mechanism as previously described[24]. Briefly, 4.0 mg/ml bovine Type I collagen (MP Biomedicals, Solon, OH) was dissolved in 0.02 N acetic acid (Sigma) and was mixed with 2.0 wt% chitosan (93% DDA; Biosyntech, Quebec, Canada) dissolved in 0.1 N acetic acid at a mass ratio of 50/50 collagen/chitosan. Beta-glycerophosphate (β-GP) and glyoxal were added as physical and chemical cross-linkers, respectively, at concentrations of 7.0wt% β-GP and 0.5 mM glyoxal. A 400 µl aliquot of the pre-gelled mixture was injected into a well of a 24-well plate to create a disk-shaped construct with diameter of 1.5 cm. Gelation was then initiated by incubation of the mixture at 37°C for 30 min. Gels were washed three times in phosphate buffered saline (PBS; Invitrogen) for 10 min to remove excess β-GP prior to use.
2.3 Calcium Quantification
Calcium deposition on acellular gels after 3 days incubation in either modified medium or DMEM containing 0, 2, 5, 10% FBS was quantified using an orthocresolphthalein complex-one (OCPC) method as previously described [27]. Briefly, collagen-chitosan gels were washed three times in PBS for 10 minutes and frozen at days 0, 1, and 3. Samples were then digested in 0.5 ml of 1.0 N acetic acid overnight. Ten microliters of the dissolved solution was then incubated at 10 min at 25°C with 300 µl of a working solution consisting of 0.05 mg/ml of OCPC solution and ethanolamine/boricacid/8-hydroxyquinoline buffer (Sigma). Samples were read spectrophotometrically at 575 nm. Calcium values were quantified via a standard curve prepared from 0.0 to 100 µg/ml.
2.4 Gel Morphology and Von Kossa staining
Acellular gel morphology was examined 3 days after incubation in mineralization medium (MM = mDMEM + 2% FBS) and in control medium (DM = DMEM + 10% FBS). Gels were washed three times in PBS for 10 min each and then transferred to a 12-well plate for imaging using a standard CCD camera in manual mode with a constant exposure setting.
For von Kossa staining, acellular gels were washed three times in PBS for 10 min and then placed in zinc-buffered formalin (Anatech LTD, Battle Creek, MI) for 30 minutes, followed by immersion in 70% ethanol (Fisher Scientific, Pittsburgh, PA). Samples were cryosectioned into the top, middle (300 µm from the top face), and bottom face at the Histology Core Facility at the University of Michigan Dental School. Gels were stained with von Kossa reagent, embedded in paraffin and then mounted on slides. Images were taken at 4X magnification using an Olympus IX15 Microscope system (Olympus America, Center Valley, PA) and stitched together using Metamorph Premier software (Molecular Devices, Sunnyvale, CA). Images were quantified using ImageJ software (National Institute of Health, Bethesda, MD) using thresholding and color discrimination to define positive staining. The ratio of the stained to unstained area was used to determine the fraction of the sample that was positively stained in each sample.
2.5 Gel Rheology
Acellular collagen-chitosanconstructs incubated in MM or DM for 3 days were washed three times in PBS for 10 min each and then evaluated by gel rheometry using an AR-G2 rheometer (TA Instruments, New Castle, DE). Gels were loaded on to a Peltier stage preheated to 37°C. A 8 mm steel parallel plate was used with a gap height of 1500 microns. A strain sweep was performed over the course of 45 minutes with strain rates from 0.1 to 100% and a constant frequency of 1 radian/second. Reported values were taken over the linear range of the samples.
2.6 Cell Culture and Assays
Prior to gel fabrication, human neonatal dermal fibroblasts (hFb; Lonza Inc., Walkersville, MD) were cultured in DMEM containing 10% FBS and 1% PS. For cell assays, hFb were used at passage 7–9 and placed directly into the solution of collagen and chitosan at a concentration of 1.0 × 106 cells/ml prior to gelation, to allow for homogenous encapsulation within formed gels. Previous studies have shown that cells survive the encapsulation process [24, 26], and hFB were used in this study as a model cell type to isolate the effects of mineralization to the modified media solution.
Cell-seeded constructs in the MM condition were cultured for 1 day in DM, 3 days in MM, and then 7 days in DM for a recovery period. Samples were collected at days 0, 1, 2, 4, 5, 7, 11 corresponding to initial conditions, 1 day in DM, 1 day in MM, 3 days in MM, 1 day recovery in DM, 3 days recovery in DM, and 7 days recovery in DM (Figure 1). Gels were washed three times in PBS for 10 min when switching between culture medium. Cell-seeded gels cultured in DM only were used as controls and samples were collected at corresponding timepoints.
Figure 1.
Experimental protocol showing timing of control cultures in standard DMEM (DM) and mineralizing cultures in modified DMEM (MM). All cell-seeded hydrogels were cultured in DM for 1 day. Mineralizing constructs were then incubated in MM for 3 days, followed by a 7 day recovery period in DM. Control constructs were incubated in DM for 11 days. Arrows on time scale indicate points at which constructs were sampled and analyzed.
To evaluate toxicity of the MM media, cell viability was examined using a vital stain kit (Live/Dead®, Molecular Probes, Eugene, OR). Constructs were washed three times in sterile PBS and incubated at 37°C for 45 min in a solution containing 4.0 µm calcein-AM and 4.0 µm ethidium homodimer-1 in PBS. Gels were then washed again in PBS and imaged using a laser scanning confocal microscope (Olympus FluoView 500 Laser Scanning Confocal Microscope, Olympus). Image scans were captured at a horizontal plane 150 µm above the bottom surface of the gel and quantified using ImageJ software.
To quantify cell number during the mineralization period, DNA was extracted in 4.0 M guanidine hydrochloride solution and measured using a commercially available DNA assay (PicoGreen kit, Invitrogen). Calcium deposited on the constructs during the mineralization period was also measured using the OCPC assay described above.
2.7 Statistical Analysis
One-way ANOVA testing with Tukey's post hoc analysis was used to analyze the effect of FBS concentration over time on calcium deposition in acellular hydrogels. Student's T-test was used to assess the significance of the fractional area of von Kossa staining, rheological data, cell viability, DNA content, and calcium deposition in cell-seeded hydrogels treated with mineralizing medium, compared to control medium. One-way ANOVA with Tukey's post hoc analysis was used to analyze the effect of medium type over time on DNA content in cellular hydrogels. Statistical significance was set at p < 0.05. Numerical values are presented as mean +/− standard error of the mean (SEM). N = 4 for each assay, and error bars on graphs represent the standard error of the mean.
3. Results and Discussion
3.1 Rationale for media formulations
This work demonstrated the ability to mineralize protein biomaterials and change their properties both in the presence and absence of embedded cells. Mineralization techniques typically employ simulated body fluid (SBF), however, the lack of nutrients, vitamins, amino acids, and glucose prevent these media from being used for cell culture. DMEM is a commonly used medium for cell culture that can lead to the precipitation of mineral nodules on hydroxyapatite and tricalcium phosphate scaffolds in normal cell culture environments[28]. Therefore we used DMEM as a base medium and supplemented it with the specific salts to enhance the mineralization process. Table 1 shows the ion concentrations of SBF, DMEM, and mDMEM, as well as blood plasma for reference. The mineralizing DMEM(mDMEM) used in this study was formulated to have specific ionic concentrations aimed at maximizing mineral deposition[28]. mDMEM contained concentrations of sodium (Na+), magnesium (Mg2+), and sulfate (SO42−) similar to conventional SBF. Calcium (Ca2+) and phosphate (PO43−) are the primary ions required for biomineralization, and were therefore added at concentrations approximately 4-fold higher than conventional SBF to promote rapid mineralization of substrates. The carbonate (HCO3−) level was maximized to serve as a pH buffer in the media to allow culture in a CO2 incubator, though this anion may be associated with decreased mineralization. Each of these solutions was adjusted to physiological pH to provide an appropriate environment for cell culture.
3.2 Effect of serum on calcium deposition
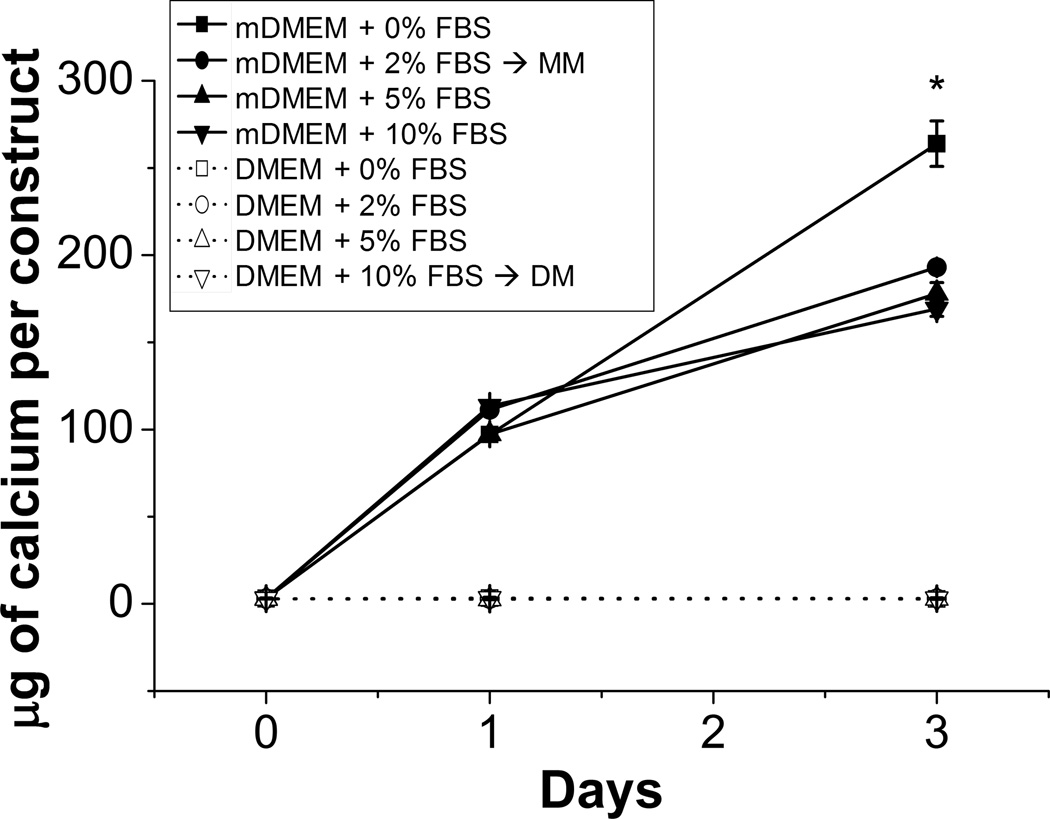
Serum is a necessary supplement in cell culture medium because it contains growth factors that maintain cell viability and growth [28, 29]. However, in some systems serum proteins can detrimentally effect mineralization by delaying or inhibiting deposition [13, 30, 31]. To investigate the effect of serum on calcium deposition in collagen-chitosan materials, acellular hydrogel constructs were exposed to mineralizing DMEM supplemented with 0, 2, 5, and 10% FBS. Gels incubated in control DMEM with 0, 2, 5, 10% FBS served as controls. Figure 2 shows that calcium deposition increased significantly (p<0.01) from day 0 in all samples incubated in mineralizing DMEM by day 1, and that mineralization continued to increase to day 3 in culture. In contrast, samples exposed to control DMEM showed no evidence of calcium deposition regardless of serum content or timepoint. In mDMEM samples, the presence of serum had no effect on the degree of calcium deposition at the day 1 timepoint, but by day 3 serum-supplemented samples showed decreased mineral deposition, relative to the sample with no serum (p<0.01). There was no significant difference between 2, 5 and 10% serum. These data show that FBS had an inhibitory effect on calcium deposition, but that mineralization did occur in the presence of serum proteins. Because of the need for at least a low level of serum for cell maintenance, mineralization studies conducted with cells were performed using mDMEM supplemented with 2% FBS as the mineralization medium (MM). The control medium (DM) was standard DMEM supplemented with 10% FBS, which is widely used for cell culture.
Figure 2.
Calcium deposition in collagen-chitosan gels through incubation in mineralizing DMEM (mDMEM). (*) denotes p < 0.05 from the groups containing FBS. Values are mean +/− standard error, n = 4.
3.3 Mineralization of collagen-chitosan materials
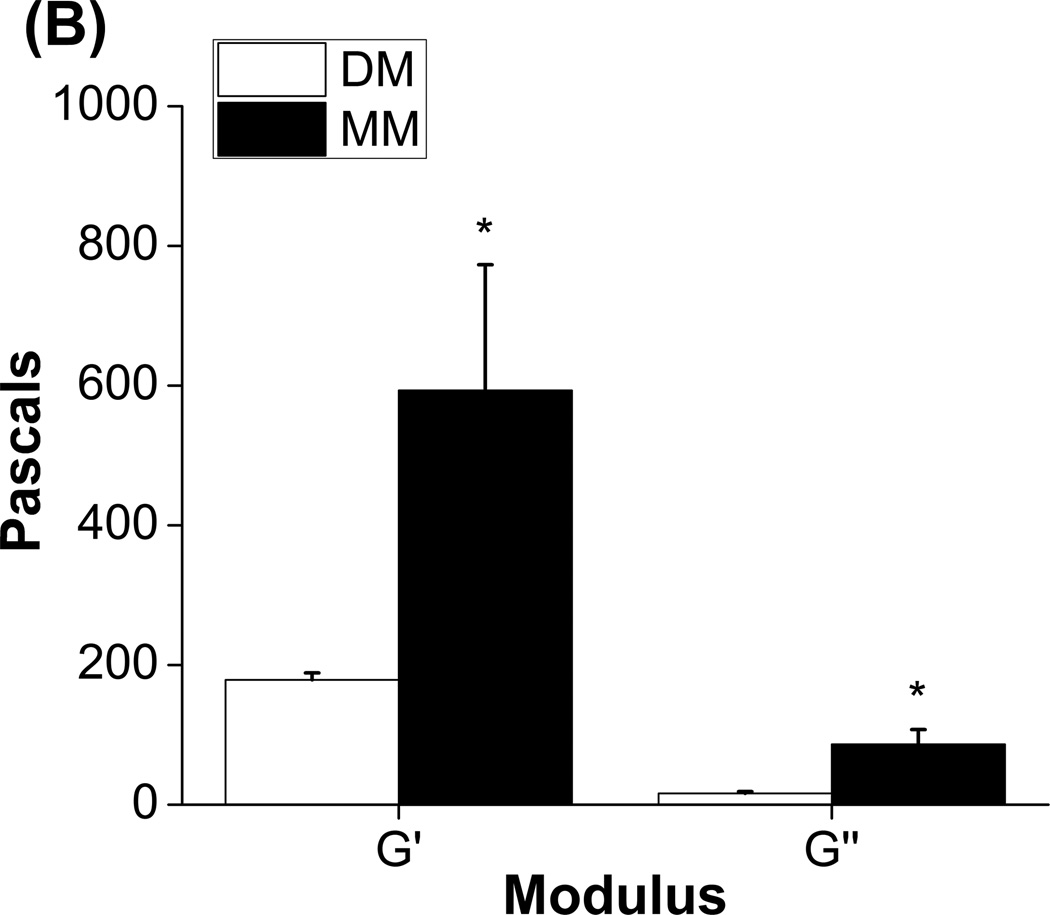
Figure 3 shows data on mineralization and mechanical properties of acellular collagen-chitosan matrices incubated in MM and DM for three days. Histological evaluation of Von Kossa stained sections taken at the top, middle, and bottom of the constructs showed clear differences in degree of phosphate deposition between mineralizing and control conditions (Fig. 3A). After three days in incubation, constructs in DM remained translucent, where as those in MM were opaque, and dark staining for calcium was more prominent in MM samples (Fig. 3A inset). Quantification of relative phosphate staining showed that hydrogels in MM exhibited significantly higher phosphate levels at the top and middle of the constructs, though staining in the bottom section was not statistically different compared to constructs in DM. These results support the finding that MM induces mineralization, but show that mineral deposition was not evenly distributed through the material.
Figure 3.
A) Quantification of calcium deposition in different regions of collagen-chitosan constructs using histological staining for calcium (n=4 at each location). Inset shows control and mineralized collagen-constructs (top row, scale bar = 1 mm) and representative histology sections (bottom row, scale bar = 500 µm). B) Storage (G’) and loss (G”) moduli for control and mineralized collagen-chitosan constructs after three days of culture (n=4, * denotes p < 0.05 from the control DM group).
Figure 3B shows the storage (G’) and loss (G”) modulus from acellular collagen-chitosan gels exposed to DM or MM for three days. Relative to control gels, mineralized gels exhibited a 3-fold increase in stiffness as reflected by the storage modulus (p<0.05). The loss modulus also increased significantly (p<0.001) in the MM condition, as compared to the DM condition. These data show that the mechanical properties of hydrogel constructs can be augmented by mineralization even over relatively short time periods. SBF treatment can affect the properties of scaffolds, though the effects can vary depending on the distribution of the mineral throughout the scaffolding material [8, 18]. Increasing the stiffness and toughness of protein hydrogel materials is desirable in order to allow implantation of constructs, particularly in load-bearing applications. A stiffer matrix may also attenuate the cell-mediated matrix remodeling that occurs in 3D protein hydrogels [32], and may direct the phenotype of progenitor cells toward an osteogenic phenotype [33].
3.4 Effect of mineralization on cell function
Viability data for hFb embedded in 3D collagen-chitosan materials are shown in Figure 4 and Figure 5A. Green staining indicates the cytoplasm of living cells, whereas red-stained nuclei identify dead cells. The culture protocol is shown schematically by the arrows in Figure 4. Control constructs were cultured in DM for 11 days, whereas mineralized constructs were cultured in DM for one day, followed by three days of incubation in MM, and then 7 days of culture recovery in DM. All samples were imaged and assayed for cell function at days 1, 2, 4, 5, 7, and 11. After one day of culture in DM, cells began to spread through the collagen-chitosan matrix (Fig. 4A,4B) and exhibited very high viability (>90%). Constructs subsequently exposed to MM showed significantly decreased viability (p<0.05) over the incubation period in MM (days 2 through 4, Fig. 4D, 4F), but viability was still high (~80%). The morphology of cells incubated in MM was more rounded than the control cells, which were highly stellate and exhibited viability over 90% (Fig. 4C, 4E). Upon reintroduction into standard medium, cells in the mineralized constructs recovered a spread morphology (Fig. 4H, 4J, 4L) and viability returned to the same level as in control constructs by day 11 (>90%).
Figure 4.
Confocal micrographs of fibroblast-seeded collagen-chitosan gels cultured in control medium (A, C, E, G, I, K) or under mineralizing conditions (B, D, F, H, J, L). Cells are stained so that the cytoplasm of living cells is green and the nuclei of dead cells are red. Arrows at sides show culture protocol. Scale bar = 200 µm.
Figure 5.
Quantification of A) cell viability (n=4), B) DNA content (n=4), and C) calcium content (n=4) of collagen-chitosan constructs cultured in control and mineralizing medium. (# denotes statistical significance from day 0 controls; * denotes statistical significance from DM controls; ^ denotes statistical significance from day 7)
The DNA content of hydrogel constructs was used as a measure of cell number and is shown in Figure 5B. Cell number increased steadily and significantly (p<0.001) over time in the DM condition, resulting in an approximately 5-fold increase by day 11. Constructs in the mineralization group showed an initial rise in DNA content, which subsequently dropped significantly upon incubation in MM from days 2 through 4 (p<0.0001). Similar to cell viability, cell number in mineralized constructs recovered after transfer to DM, although the DNA content did not recover to the same levels as controls. The cell viability and cell number data provide different but complementary information on cell “health” in the constructs. A lack of cell proliferation would lead to a decrease in overall cell number over time, though viability observed as a snapshot at any given time can still be high, as observed in MM at the day 1 and 2 time points. Cell death is suggested by the data at the day 4 and 5 time points, since both viability and cell number were decreased. In contrast, when cells are proliferating then cell number will increase and viability can also be high. This corresponds to the trends observed in DM. Taken together these data suggest that incubation in mineralizing medium significantly reduces, and perhaps completely inhibits, the proliferation of the embedded cells while cell viability is also affected but remains high overall.
Figure 5C shows calcium deposition in cell-seeded constructs over the time course of the experiment. Upon transfer to MM at day 2, constructs in the mineralizing group showed a sharp and significant increase in calcium content and reached a level approximately 140-fold higher that controls after three days of incubation in MM (p<0.001). The calcium levels remained similarly high after transfer to recovery medium for the remainder of the experimental period. In contrast, constructs cultured in control medium (DM), showed very little calcium deposition, though a statistically significant increase over day 0 was observed at days 7 and 11 in DM (p<0.001).
Fibroblasts were used as a model cell type in this study in order to isolate the effects of the modified medium. Unlike bone marrow stromal cells and osteoblasts, fibroblasts are a non-mineralizing cell type and therefore increases in mineral content of the matrices can be attributed purely to the effect of the medium, and not cellular action. Preliminary studies showed that hFb could survive three days incubation in MM, though longer culture periods lead to increased cell death (data not shown). In 3D collagen-chitosan hydrogels, cell viability and proliferation rate decreased over three days of incubation in MM, but the cell population remained over 75% viable. These effects can be attributed to the hypertonic nature of MM, which is required to induce mineralization but can also modify cell function. Importantly, both the viability and proliferative capacity of fibroblasts recovered when collagen-chitosan constructs were cultured in standard DMEM after mineralization. These results suggest that the medium and protocol employed were sufficient to mineralize 3D protein constructs in the presence of living fibroblasts, and it is likely that similar results would be observed with other cell types.
A number of further questions remain to be answered in future studies. The specific structure and type of mineral being deposited is of interest because it has relevance to creating biomimetic materials. In addition, it is known that the presence of mineral, either as a substrate or in solution, can affect differentiation of progenitor and stem cells. The influence of material stiffness on cell phenotype is of great interest in the field of tissue engineering, and mineralized matrices may provide a tool to examine such effects. While the biochemical and mechanical effects of matrix mineralization can be difficult to decouple, the ability to mineralize 3D protein-based materials may be another tool to study the effects of the tissue microenvironment on cell function. In addition, creating mineralized matrices in the presence of cells may be used to direct progenitor cell differentiation and/or to create more mechanically stable biomimetic constructs for orthopaedic and other applications.
4. Conclusions
This study represents an initial step in the development of techniques to mineralize 3D protein constructs in the presence of living cells. A modified culture medium and protocol were developed to achieve mineralization of collagen-chitosan matrices at physiological pH and temperature. Typically used SBF formulations do not support cell viability and growth and therefore are limited in their utility in the presence of cells. The modified medium used in this study contained a similar panel of mineralizing ions similar to SBF, though at modified concentrations, and also contained the nutrients and buffers needed to sustain cell function. It was shown that three day incubation of 3D collagen-chitosan materials resulted in mineral deposition and stiffening of the gels. Cell viability was reduced and cell number decreased during incubation in mineralizing medium, however a viable cell population was maintained and it was shown that viability and proliferation recovered after rescue in standard medium. These results suggest that in vitro mineralization in defined media can be used to modulate the composition and properties of engineered tissues in the presence of living cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering Complex Tissues. Tissue Engineering. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnology Progress. 2009;25:1539–1560. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 3.Al-Munajjed AA, et al. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90B:584–591. doi: 10.1002/jbm.b.31320. [DOI] [PubMed] [Google Scholar]
- 4.Suárez-González D, Barnhart K, Saito E, Vanderby R, Hollister SJ, Murphy WL. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. Journal of Biomedical Materials Research Part A. 2010;95A:222–234. doi: 10.1002/jbm.a.32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasuriya AC, Kibbe S. Rapid biomineralization of chitosan microparticles to apply in bone regeneration. Journal of Materials Science: Materials in Medicine. 2009;21:393–398. doi: 10.1007/s10856-009-3874-2. [DOI] [PubMed] [Google Scholar]
- 6.Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA. Mineralization of Hydrogels for Bone Regeneration. Tissue Engineering Part B: Reviews. 2010;16:577–585. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–2258. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis HE, Rao RR, He J, Leach JK. Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. Journal of Biomedical Materials Research Part A. 2009;90A:1021–1031. doi: 10.1002/jbm.a.32169. [DOI] [PubMed] [Google Scholar]
- 9.Kretlow JD, Mikos AG. Review: Mineralization of Synthetic Polymer Scaffolds for Bone Tissue Engineering. Tissue Engineering. 2007;13:927–938. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- 10.Murphy WL, Kohn DH, Mooney DJ. Growth of a continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. Journal of Biomedical Materials Research Part A. 1999;50:50–58. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. Journal of Biomedical Materials Research. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 12.Kohn DH, Shin K, SI H, Jayasuriya AC, Leonova EV, Rosello RA, et al. Self-assembled mineral scaffolds as model systems for biomineralization and tissue engineering. Toronto: University of Toronto Press; 2005. [Google Scholar]
- 13.Luong LN, Hong SI, Patel RJ, Outslay ME, Kohn DH. Spatial control of protein within biomimetically nucleated mineral. Biomaterials. 2006;27:1175–1186. doi: 10.1016/j.biomaterials.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Murphy W, Hsiong S, Richardson T, Simmons C, Mooney D. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303–310. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsuki C, Kamitakahara M, Miyazaki T. Coating bone-like apatite onto organic substrates using solutions mimicking body fluid. Journal of Tissue Engineering and Regenerative Medicine. 2007;1:33–38. doi: 10.1002/term.3. [DOI] [PubMed] [Google Scholar]
- 16.Yang HS, La W-G, Bhang SH, Lee T-J, Lee M, Kim B-S. Apatite-Coated Collagen Scaffold for Bone Morphogenetic Protein-2 Delivery. Tissue Engineering Part A. 2011 doi: 10.1089/ten.TEA.2010.0702. [DOI] [PubMed] [Google Scholar]
- 17.Luong LN, McFalls KM, Kohn DH. Gene delivery via DNA incorporation within a biomimetic apatite coating. Biomaterials. 2009;30:6996–7004. doi: 10.1016/j.biomaterials.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao RR, He J, Leach JK. Biomineralized composite substrates increase gene expression with nonviral delivery. Journal of Biomedical Materials Research Part A. 2010;94:344–354. doi: 10.1002/jbm.a.32690. [DOI] [PubMed] [Google Scholar]
- 19.Ma PX. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund AW, Yener B, Stegemann J, Plopper GE. The Natural and Engineered 3D Microenvironment as a Regulatory Cue During Stem Cell Fate Determination. Tissue Engineering: Part B. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat A, Dreifke MB, Kandimalla Y, Gomez C, Ebraheim NA, Jayasuriya AC. Evaluation of cross-linked chitosan microparticles for bone regeneration. Journal of Tissue Engineering and Regenerative Medicine. 2010;4:532–542. doi: 10.1002/term.270. [DOI] [PubMed] [Google Scholar]
- 22.Batorsky A, Liao J, Lund AW, Plopper GE, Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnology and Bioengineering. 2005;92:492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- 23.Arpornmaeklong P, Pripatnanont P, Suwatwirote N. Properties of chitosan–collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. International Journal of Oral and Maxillofacial Surgery. 2008;37:357–366. doi: 10.1016/j.ijom.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Stegemann JP. Glyoxal cross linking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomaterialia. 2011;7:2410–2417. doi: 10.1016/j.actbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen–fibrin mixtures. Biomaterials. 2004;25:3699–3706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Stegemann JP. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31:3976–3985. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Singh M, Bonewald LF, Detamore MS. Signalling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2009;3:398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 28.Lee JTY, et al. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomaterialia. 2011 doi: 10.1016/j.actbio.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Tan EML, Uitto J, Bauer EA, Eisen AZ. Human Skin Fibroblasts in Culture: Procollagen Synthesis in the Presence of Sera from Normal Human Subjects and from Patients with Dermal Fibroses. The Journal of Investigative Dermatology. 1981;76:462–467. doi: 10.1111/1523-1747.ep12521119. [DOI] [PubMed] [Google Scholar]
- 30.Combes C, Rey C, Freche M. In vitro crystallization of ostacalcium phosphate on type I collagen: influence of serum albumin. Journal of Materials Science: Materials in Medicine. 1999;10:153–160. doi: 10.1023/a:1008933406806. [DOI] [PubMed] [Google Scholar]
- 31.Juhasz JA, Best SM, Auffret AD, Bonfield W. Biological control of apatite growth in simulated body fluid and human blood serum. Journal of Materials Science: Materials in Medicine. 2007;19:1823–1829. doi: 10.1007/s10856-007-3344-7. [DOI] [PubMed] [Google Scholar]
- 32.Hong H, McCullough CM, Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28:3824–3833. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG., Jr Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32:2256–2264. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]