Abstract
Delivery of neurotrophic factors to treat neurodegenerative diseases has not been efficacious in clinical trials despite their known potency for promoting neuronal growth and survival. Direct gene delivery to the brain offers an approach for establishing sustained expression of neurotrophic factors but is dependent on accurate surgical procedures to target specific anatomical regions of the brain. Serotype-2 adeno-associated viral (AAV2) vectors have been investigated in multiple clinical studies for neurological diseases without adverse effects, however the absence of significant clinical efficacy after neurotrophic factor gene transfer has been largely attributed to insufficient coverage of the target region. Our pre-clinical development of AAV2-glial-derived neurotrophic factor (GDNF) for Parkinson’s disease involved real-time image guided delivery and optimization of delivery techniques to maximize gene transfer in the putamen. We have demonstrated that AAV2 vectors are anterogradely transported in the primate brain with GDNF expression observed in the substantia nigra after putaminal delivery in both intact and nigrostriatal lesioned primates. Direct midbrain delivery of AAV2-GDNF resulted in extensive anterograde transport to multiple brain regions and significant weight loss.
Keywords: Glial-derived neurotrophic factor, Adeno-associated viral vectors, Anterograde transport, Parkinson’s disease, Gene therapy, Drug delivery
1. Introduction
An important therapeutic innovation with the potential to dramatically inhibit progression of the motor deficit in Parkinson’s disease is the delivery of potent neurotrophic factors directly into the degenerated region of the brain. Delivery of a gene therapy vector or therapeutic agent into the brain via direct injection offers an effective method for bypassing the blood brain barrier and targeting a specific anatomical site. However, the large size and complex architecture of the human brain often prevents scalable translation of small animal experimental methods involving direct brain delivery into effective medical procedures. Previous clinical studies investigating the direct delivery of recombinant glial-derived neurotrophic factor (GDNF) protein into the putamen did not yield significant efficacy (Patel and Gill, 2007). The reason for this disappointing outcome is still unclear, but sub-optimal distribution of infused GDNF protein is suspected. The generation of anti-GDNF antibodies in some subjects, along with reported cerebellar toxicity in Rhesus macaques after cessation of prolonged putamen infusion of GDNF, diminished enthusiasm for this approach (Hovland et al., 2007; Lang et al., 2006). In contrast, gene therapy has become a more favored approach primarily because targeting of much lower levels of growth factor only to the nigrostriatal neurons seems to provide a way of harnessing the potency of neurotrophic factors while limiting the potential side effects that might be triggered through inadvertent escape of the neurotrophic factor into other parts of the brain or into peripheral circulation. Adeno-associated viral (AAV) vectors have emerged as the current vehicle of choice for neurological gene transfer with multiple clinical trials completed or initiated. Over 100 patients have received direct cranial delivery of AAV serotype 2 (AAV2) vectors in clinical trials for Parkinson’s disease without any serious adverse events attributable to the vector (Christine et al., 2009; Kaplitt et al., 2007; LeWitt et al., 2011; Marks et al., 2010; Marks et al., 2008; Muramatsu et al., 2010; Muramatsu et al., 2009). The recently completed AAV2-GAD (glutamic acid decarboxylase) gene therapy trial for advanced Parkinson’s disease is the first phase 2 double-blinded clinical study to demonstrate the efficacy of gene therapy in a neurological disorder (LeWitt et al., 2011).
The exploration of this technology, however, has revealed complex parameters that must be controlled in order to achieve efficacy without unacceptable side effects. The difficulty of achieving the requisite level of transgene expression over sufficient areas of the striatum to exert a positive effect in Parkinson’s disease patients was encountered in a 58-patient controlled (sham surgery) phase 2 trial of AAV2-Neurturin (CERE-120). This trial was sufficiently powered to overcome the anticipated placebo effect prevalent in Parkinson’s disease studies. In this study, a total of 16 vector injections were made via 8 needle passes that resulted in only about 15% coverage of the putamen, based on postmortem analysis of 2 participants in the study who had died from unrelated causes (Bartus et al., 2011). This result suggested that perhaps inadequate distribution of vector within the putamen was responsible for the lack of clinical effect. In contrast, AAV2-mediated putaminal expression of aromatic L-amino acid decarboxylase (AADC), the rate-limiting enzyme required for conversion of L-dopa into dopamine, was substantial as shown by PET with the AADC-specific substrate, 6-[18F]-fluoro-metatyrosine (FMT) (Christine et al., 2009; Eberling et al., 2007; Muramatsu et al., 2010). The use of only two reflux-resistant infusion cannulae per hemisphere in the AAV2-hAADC study enabled pressurized convection-enhanced delivery (CED) that increases parenchymal distribution surrounding the cannula tip. Distribution of the vector infusate in these patients was visualized by postoperative MR imaging within 7 hours of vector delivery and was calculated to cover 22% of the putamen volume (Valles et al., 2010). Although the coverage of the putamen does not appear to be substantially different between the two delivery procedures adopted for these clinical trials, the CED approach required a smaller number of infusions and, therefore, lowered the risk of adverse events associated with intracranial cannula placement. It is, however, evident from these early studies that, without a standardized delivery system that can be implemented in multicenter studies and reliably provide a larger distribution of vector within the target brain structure, it will not be possible to fully evaluate the efficacy of therapeutic gene therapy vectors. Even in the successful AAV2-GAD study where gene transfer was targeted to a relatively small deep brain nucleus, the subthalamic nucleus, it was found that precise targeting was critical to clinical benefits, and no motor improvements were observed when the cannula was not correctly positioned (LeWitt et al., 2011).
Therefore, one of the greatest challenges in the development of therapeutic gene therapy for the treatment neurological disorders is to gain control over the distribution of gene transfer within the central nervous system (CNS). Neurotrophic factors play a critical role in maintaining the CNS and have long been proposed as therapeutic agents for neurodegenerative diseases. However, despite the promise that neurotrophic factors offer for neurodegenerative diseases, including nerve growth factor for Alzheimer’s disease (Cattaneo et al., 2008), no neurotrophic factor has established clinical efficacy in a placebo-controlled trial. The challenges in delivering proteins, such as neurotrophic factors, to the brain are evident in the large number of animal and human studies that have focused on developing GDNF, a potent growth factor for dopaminergic neurons, into a treatment for Parkinson’s disease (Kirik et al., 2004). Sub-optimal distribution has been blamed for the lack of clinical efficacy in investigations of GDNF or its homolog, Neurturin, to inhibit the progression of motor deficits in Parkinson’s disease (Lang et al., 2006; Marks et al., 2010; Nutt et al., 2003). Therefore, a crucial component of our ongoing AAV2-GDNF gene therapy program for Parkinson’s disease has been the parallel development of a real-time image-guided neurosurgical drug delivery system. This platform technology significantly enhances the accuracy of targeting deep brain structures and maximizes distribution of vector within the parenchyma surrounding the delivery catheter to ensure optimal coverage of the target structure. Repetitive magnetic resonance (MR) imaging during infusions into the non-human primate (NHP) brain provided real-time feedback of vector distribution that enabled us to optimize the cannula design and prescribe infusion protocols (i.e. infusion rates, volumes and cannula positioning) that minimize the risk of off-target distribution or backflow while maximizing target coverage (Yin et al., 2010; Yin et al., 2009). In collaboration with MRI Interventions (Irvine, CA, USA; formally SurgiVision Inc.), we have developed a reflux-resistant cannula with a 3-mm fused silica distal tip that is compatible with the FDA-approved Clearpoint® intra-operative MR trajectory guidance frame (Richardson et al., 2011a).
An unanticipated consequence of enhanced target coverage has been the repeated observation of AAV2-mediated transduction of neurons in anatomical areas of the primate brain not directly targeted but known to receive axonal projections originating from the site of vector infusion (Kells et al., 2009). A retrospective analysis of our recent studies shows significant evidence for anterograde, but not retrograde, transport of AAV2 vectors in the NHP brain. This anterograde trafficking of AAV2 may be advantageous when the secondary region is also a valid target for gene delivery. However, transgene expression within non-targeted areas of the brain may equally lead to serious adverse events such as the weight loss that occurred after intracerebroventricular delivery of GDNF protein to Parkinson’s disease patients (Nutt et al., 2003).
2. Anterograde distribution of AAV2 in the non-human primate brain
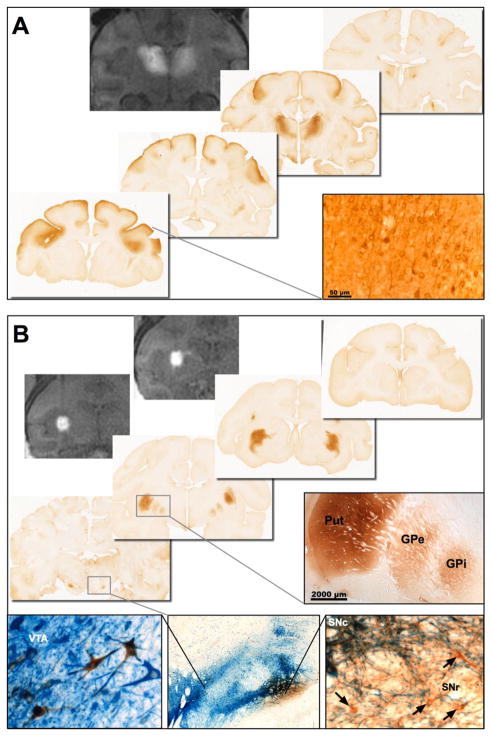
Anterograde distribution of AAV2 vectors in the NHP brain was initially observed after delivery of AAV2-thymidine kinase into the putamen (Hadaczek et al., 2006), and after infusion of AAV2-GFP (green fluorescent protein) into the thalamus (Kells et al., 2009). Neither of the proteins produced by these vectors are secreted, and therefore only directly transduced cells contain TK or GFP. The thalamic infusions of AAV2-GFP resulted in extensive GFP expression within layer V and VI pyramidal neurons throughout various regions of the cortex known to receive axonal projections from the same region of the thalamus transduced by AAV2-GFP. More recently, we have performed real-time MRI-guided infusion of AAV2-GDNF into both the thalamus and putamen of naïve rhesus macaques (n=9) via a novel FDA-approved neuro-navigational guidance system (Clearpoint®, MRI Interventions, Irvine, CA) and reflux-resistant cannula specifically designed for clinical application (Richardson et al., 2011a; Richardson et al., 2011b). Repeated MR scanning of the subject’s brain while the vector is being co-infused with an MR-visible contrast agent (e.g. Gadoteridol) provides rapid confirmation of the cannula placement, actual vector distribution, the extent of any reflex along the cannula tract, and whether there is any leakage into the cerebrospinal fluid. No leakage was observed during the infusions, although some gadoteridol was observed along some of the cannula tracts after removal of the cannula. High levels of GDNF protein expression were evident in the primary targets of transduction, i.e. putamen or thalamus, directly surrounding the site of infusion (Fig. 1).
Fig. 1. Prediction of vector distribution by real-time MR imaging.
Co-infusion of gadoteridol and AAV2-GDNF allows real-time monitoring of distribution at the site of infusion that is highly predictive of the area of GDNF expression. Representative MR images and immunohistochemical staining for GDNF after bilateral infusion into the NHP (A) thalamus or (B) putamen (2 sites per hemisphere). MR imaging immediately after completion of the infusions showed that the gadoteridol tracer was confined to the target structures with no leakage or backflow along the cannula tracts. The distribution of GDNF in the thalamus and putamen correlated exactly with the MR signal. In addition, anterograde transport of AAV2-GDNF to secondary brain regions was observed with GDNF present in multiple regions of the cortex after infusion into the thalamus, and basal ganglia nuclei after infusion into the putamen. Dual staining for GDNF (brown) and tyrosine hydroxylase (blue) shows some overlap between GDNF expression in the substantia nigra and TH-positive neurons in the pars compacta. However, many GDNF-positive neurons (arrows) were found in the TH-negative SNr. Abbreviations: GPe: Globus Pallidus external; GPi: Globus Pallidus internal; Put: Putamen; SNc: Substantia nigra pars compacta; SNr: Substantia nigra pars reticulata; VTA: ventral tegmental area.
Comparison of the expression patterns in the putamen and thalamus showed identical distribution to the MR images obtained during vector delivery as previously reported for AAV2-GDNF and AAV2-hAADC (Su et al., 2010). When we directly compared the distribution of GDNF and AADC after co-infusion of the two vectors into the same structure, we observed identical distribution patterns indicating that the secreted GDNF protein was not diffusing into adjacent non-infused tissue but remained localized to the site of gene transfer. This absence of detectable GDNF protein diffusion to un-transduced regions of the brain further highlights the importance of ensuring optimal distribution of the vector during initial delivery. However, in contrast to the restricted spread of GDNF within the primary infusion site, GDNF protein was present in secondary brain structures that were not directly targeted during the infusion procedure. Specifically, after delivery to the thalamus, GDNF-positive neurons and fibers were identified in deep layers of the frontal cortex and cingulate gyrus (Fig. 1A); both cortical regions are known to receive afferent connections from ventral thalamic nuclei (Kells et al., 2009; Su et al., 2010). Similarly, infusion into the putamen resulted in the presence of GDNF protein in multiple basal ganglia nuclei that receive direct or indirect afferent connections from the putamen including medial and lateral segments of the globus pallidus, subthalamic nucleus, substantia nigra pars compacta and substantia nigra pars reticulata (Fig. 1B). No cortical GDNF expression was observed in animals that received putaminal AAV2-GDNF, except for a few GDNF-positive cells adjacent to the cannula tract. The absence of cortical GDNF expression is indicative of absence of retrograde transport of AAV2 by corticostriatal neurons. Co-staining of sections with GDNF and TH antibodies to identify dopaminergic neurons in the midbrain or animals that received putaminal delivery revealed considerable GDNF in TH-negative neurons and fibers in the SNr. GDNF also co-localized with TH-positive cells in the SNc and ventral tegmental area.
3. AAV2-GDNF distribution in the dopamine-depleted brain
Axonal trafficking has direct implications for treating basal ganglia disorders such as Parkinson’s disease where neuro-protective and neuro-restorative treatments have frequently focused on the maintenance or restoration of dopaminergic activity in the caudate-putamen rather than on the vulnerable dopaminergic neuronal cell bodies residing in the ventral midbrain. The prevailing hypothesis has been that delivery of GDNF, a potent neurotrophic factor for dopaminergic neurons (Lin et al., 1993), or alternative GDNF family ligands, to the caudate-putamen would provide trophic support to dopaminergic neurons through retrograde trafficking mechanisms that are known to have a critical role in directing the initial development and maintenance of these nigrostriatal connections (Airaksinen and Saarma, 2002; Tomac et al., 1995b). During normal brain development GDNF acts as a target derived trophic factor for nigrostriatal dopaminergic neurons with very high expression levels in the developing caudate-putamen that rapidly decline soon after birth (Stromberg et al., 1993). Despite the low level of GDNF in the adult brain, GDNF expression within the caudate-putamen remains critical for the maintenance of dopaminergic fibers and synapses in the adult (Granholm et al., 2000; Pascual et al., 2008). Although retrograde trafficking plays a crucial role in facilitating neurotrophic support for the survival and function of nigrostriatal connections, by the time Parkinson’s disease can be clinically diagnosed the integrity of nigrostriatal connections is severely compromised with the majority of dopaminergic neurons already lost. This degeneration obviously would ablate retrograde trafficking to the midbrain dopaminergic neurons after striatal delivery in Parkinson’s disease patients. However, anterograde connections from the striatum to the midbrain do not degenerate in idiopathic Parkinson’s disease and can be expected to facilitate transport of AAV2 vector particles, thereby resulting in transduction of neurons in the substantia nigra pars reticulata as observed in NHP.
Many non-clinical studies have been conducted that demonstrate the potent neuroprotective properties of GDNF against nigrostriatal insults including 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in rodents (Choi-Lundberg et al., 1997; Choi-Lundberg et al., 1998; Connor et al., 1999; Kearns and Gash, 1995; Kirik et al., 2001; Kirik et al., 2000a; Kirik et al., 2000b; Tomac et al., 1995a) or non-human primates (Eslamboli et al., 2003; Gash et al., 1996; Grondin et al., 2002; Kordower et al., 2000). However, protection alone is challenging to measure clinically and arguably of limited therapeutic value in the absence of pre-symptomatic diagnostic tests for Parkinson’s disease. A more clinically relevant goal, particularly for advanced Parkinson’s disease patients included in clinical studies, is to determine whether GDNF also stimulates restorative processes that would lead to a meaningful and measurable reversal of Parkinsonian symptoms. To demonstrate GDNF-induced restoration and maintenance of the dopaminergic system as a potential disease-modifying treatment of Parkinson’s disease, we performed convective delivery of AAV2-GDNF (n=8) or vehicle (n=7) to the putamen of MPTP-lesioned adult macaques (Kells et al., 2010). Severe lesioning of the dopaminergic system in the right neostriatum was achieved by right-side intracarotid artery (ICA) infusion of MPTP followed by repeated intravenous MPTP dosing until the animals displayed substantial motor deficits indicative of dopamine lesioning. This model of PD is characterized by an almost complete lesion in the neostriatum ipsilateral to the ICA injection, and mild lesioning on the contralateral side that can be assessed by tyrosine hydroxylase staining (Fig. 2B). In this way the effect of GDNF expression can be determined under conditions that model both early and late disease. At least 3 months after the last administration of MPTP, animals received bilateral infusions of AAV2-GDNF or phosphate buffered saline (PBS) and were closely monitored for up to 24 months with a standardized clinical rating scale (CRS) to assess changes in motor function. Although some of the control animals displayed some early improvements in motor control, all of the AAV2-GDNF treated animals showed progressive improvement over an extended 9-month period, leading to a mean 56% reduction in CRS. The magnitude of motor recovery was directly correlated with enhanced FMT PET signal in the putamen that is indicative of increased AADC activity. Direct quantification of dopamine and dopamine metabolite levels in putamen tissue punches provided confirmation of enhanced dopaminergic activity in AAV2-GDNF-treated animals in contrast to the significant loss of dopamine in the control animals.
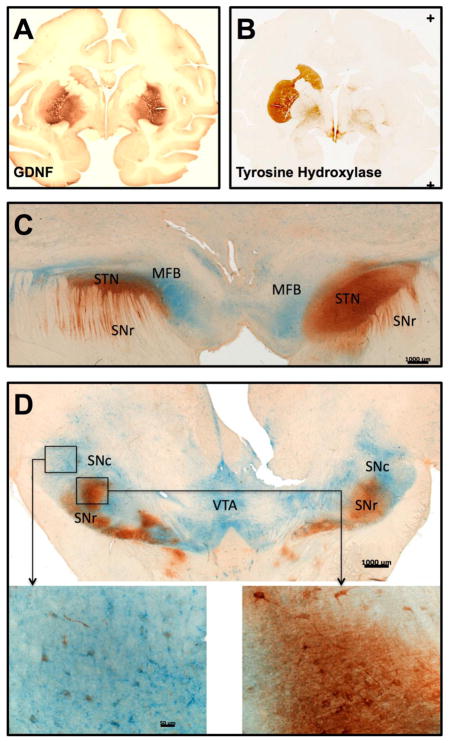
Fig. 2. Anterograde transport by striatonigral neurons is not impaired by MPTP lesioning of the nigrostriatal neurons.
Delivery of AAV2-GDNF into the putamen of severely MPTP lesioned NHP resulted in progressive recovery of motor function and partial restoration of dopaminergic activity. (A) High levels of GDNF were expressed in the putamen and globus pallidus after AAV2-GDNF delivery (150 μL per hemisphere). (B) Complete loss of TH staining in the right side caudate and putamen demonstrated the extensive degeneration of the nigrostriatal neurons caused by right side intracarotid artery administration of MPTP, whereas the left side is only mildly lesioned. (C, D) Dual staining for GDNF (brown) and tyrosine hydroxylase (blue) show identical distribution of GDNF in both hemispheres with the majority of GDNF localized in TH-negative areas including the substantia nigra pars reticulata (SNr) and subthalamic nucleus (STN), both of which receive direct and indirect axonal projections from the putamen.
MPTP-induced degeneration of the right-side dopaminergic nigrostriatal neurons was confirmed by tyrosine hydroxylase (TH) staining that showed almost complete loss of TH-positive neurons in the substantia nigra pars compacta and their axonal projections to the caudate-putamen of control treated animals. TH expression within the left neostriatum was only mildly affected by the right-side intracarotid artery infusion of MPTP (Fig. 2B). Despite this substantial imbalance in the integrity of the dopaminergic nigrostriatal pathway, we did not find any significant left/right side difference in the levels of GDNF present in the putamen or substantia nigra. Therefore, transport of AAV2-GDNF vector particles and/or GDNF protein to the substantia nigra is not dependent on the integrity of nigrostriatal dopaminergic neurons. Dual immunohistochemical staining for GDNF and TH showed extensive GDNF expression within neuronal fibers and cell bodies in other regions of the brain devoid of TH, including the internal and external globus pallidus, subthalamic nucleus and substantia nigra reticulata (Fig. 2C–D). These basal ganglia nuclei are all connected to the striatum via direct and indirect pathways of the basal ganglia circuitry, and receive afferent projections from the putamen. The presence of GDNF-positive cell bodies is indicative of anterograde transport of AAV2 vector particles away from the striatum by striatal projection neurons leading to the trans-synaptic transduction of recipient neurons in other basal ganglia nuclei. Neurons containing both GDNF and TH were observed in substantia nigra pars compacta and ventral tegmental area, however, the intensity of GDNF immunostaining was considerably lower than in the adjacent TH-negative substantia nigra reticulata neurons, and is conceivably due to direct retrograde transport of GDNF protein from the putamen. No GDNF staining was found in the cerebral cortex, brainstem, cerebellum or other regions of the brain that are not part of the basal ganglia. Taken together these observations provide strong evidence for the controlled distribution of AAV2 vector along known axonal projections in an anterograde direction.
In a separate investigation of anterograde vector transport within the striato-nigral-striatal loop of the basal ganglia network, we bilaterally infused AAV2-GDNF into the striatum or substantia nigra of adult male rats (n=16) with severe 6-OHDA-induced degeneration of the nigrostriatal pathway on one side of the brain (Ciesielska et al., 2011). Consistent with our observations in the MPTP-lesioned non-human primate brain we found that the presence of GDNF in the substantia nigra was not mediated by the nigrostriatal pathway but due to anterograde transport along striatonigral projections. GDNF-positive neurons were present within the non-lesioned substantia nigra pars compacta. However, due to the complexity of the nigrostriatal connections, there are multiple ways in which this may have occurred. These include direct transduction via axon terminals in the striatum, anterograde transport of AAV2-GDNF to the pars compacta via indirect neuronal connections, or retrograde transport of GDNF protein from the striatum where GDNF is secreted by transduced neurons. More salient, however, was the complete absence of GDNF in the striatum on the lesioned side of animals that received a nigral infusion of AAV2-GDNF. Nigral infusion of AAV2-GDNF in the intact side of the brain resulted in broad distribution of GDNF throughout the rat striatum, thus demonstrating the extensive innervation of the striatum by nigrostriatal neurons. The inability of GDNF to reach the striatum after lesioning of the dopaminergic nigrostriatal neurons indicates that this distribution is facilitated by anterograde transport and that neither AAV2-GDNF vector or GDNF protein are retrogradely transported by striatonigral neurons.
4. Anterograde distribution of AAV2-GDNF in the aged primate brain
Early in our pre-clinical development program, we performed a safety and tolerability study of AAV2-GDNF in aged Rhesus macaques (over 20 years of age, n=17) that investigated direct delivery to the putamen or midbrain (Johnston et al., 2009; Su et al., 2009). Aged primates typically have reduced dopaminergic function compared to younger animals and, in addition to being a relevant model for assessing safety of targeted gene transfer in a setting relevant to Parkinson’s disease, they can afford efficacy data related to changes in dopamine activity. Delivery of AAV2-GDNF to the substantia nigra resulted in much broader GDNF distribution than putamen delivery. However, only direct infusion of AAV2-GDNF into the putamen resulted in a significant increase in the density of dopaminergic fibers innervating the putamen. These findings were consistent with earlier studies by Kirik and colleagues that demonstrated a requirement for the localized striatal presence of GDNF to promote functional regeneration of the nigrostriatal fibers. Direct midbrain delivery of GDNF to the rat substantia nigra was sufficient for protection of the dopaminergic cell bodies, but not their axonal projections to the striatum. Therefore, GDNF in the midbrain alone did not prevent the functional motor deficits induced by subsequent 6-OHDA lesioning (Kirik et al., 2000a; Kirik et al., 2000b).
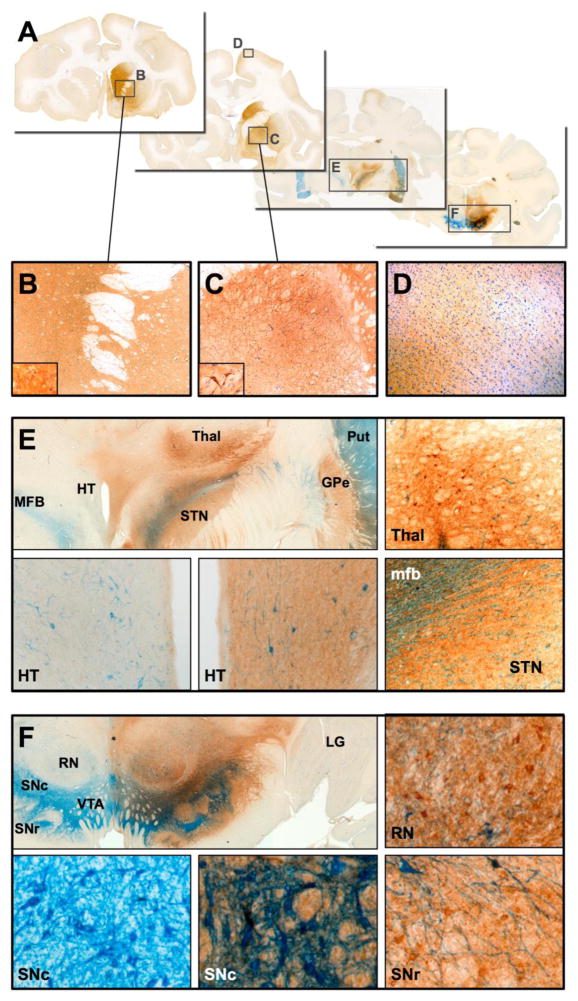
Putaminal infusions of AAV2-GDNF in the aged NHP brain resulted in an identical distribution of GDNF expression within the putamen and basal ganglia nuclei, including the substantia nigra pars reticulata, similar to that seen in younger MPTP-lesioned NHP. In contrast to the contained distribution pattern of GDNF that was observed after delivery of AAV2-GDNF to the putamen, direct infusion into the substantia nigra resulted in very broad transduction of midbrain neurons including pars compacta, pars reticulata, ventral tegmental area, subthalamic nucleus and red nucleus (Fig. 3A,F). GDNF was also observed in distal regions of the brain including the caudate nucleus, medial putamen, globus pallidus, frontal cortex, cingulate cortex, septum, ventral lateral thalamus and hypothalamus (Fig. 3A–E). This extensive distribution of GDNF throughout the ipsilateral hemisphere only is further evidence of anterograde transport of AAV2-GDNF. Surprisingly there was a noticeable lack of GDNF in the dorsal-medial putamen, the region most severely depleted of dopaminergic activity in Parkinson’s disease and the primary site for coordinating movement. Expression of GDNF was not observed in the contralateral midbrain suggesting a lack of GDNF diffusion despite the detection of high GDNF concentrations in the regions adjacent to the mid-line (e.g. ventral tegmental area and hypothalamus). The unintended dissemination of GDNF to non-targeted regions of the primate brain that are not primary areas of pathology in Parkinson’s disease is of some concern for midbrain delivery of AAV2-GDNF. Although we did not specifically identify any adverse pathological changes in the aged NHP that received either striatal or nigral delivery of AAV2-GDNF, all of the NHP that received nigral infusions experienced reduced body weight 6 months after treatment (mean body weight loss of 19% compared with 4% weight gain in putaminally treated NHP and 12% gain in sham controls). GDNF was found to induce loss of weight in humans when delivered via intraventricular infusion (Kordower et al., 1999; Nutt et al., 2003), and weight loss can also be induced in rats after direct delivery of AAV5-GDNF to the substantia nigra (Manfredsson et al., 2009a; Manfredsson et al., 2009b). Manfredsson and colleagues attributed this weight loss to high GDNF protein levels in the substantia nigra with subsequent transport and secretion of GDNF in the hypothalamus, activating of a population of corticotrophin-releasing factor neurons. Delivery of the same AAV5-GDNF vector to the rat striatum did not affect metabolic rate or cause weight loss despite GDNF still being expressed in the substantia nigra. Loss of body weight presents a serious safety concern for the delivery of an unregulated gene that will be expressed by transduced cells for the life of the recipient. Direct infusions into the midbrain carries a higher surgical risk than delivery to the putamen only and, therefore, we do not consider direct AAV2-GDNF gene delivery to the substantia nigra of Parkinson’s disease patients to be an optimal approach for clinical development.
Fig. 3. Distribution of GDNF after delivery of AAV2-GDNF to the midbrain.
(A) Direct infusion of AAV2-GDNF into the substantia nigra of aged NHP resulted in extensive GDNF expression in many distal areas of the primate brain, including (B) caudate nucleus, medial putamen, (C) globus pallidus and (D) cerebral cortex. GDNF-positive neurons and fibers (E) were also found in the ventral thalamus, hypothalamus and subthalamic nucleus, none of which were directly targeted during AAV2-GDNF infusion. Within the targeted midbrain, extensive GDNF expression was present (F) in the substantia nigra, ventral tegmental area and red nucleus. No GDNF was observed in the contralateral hemisphere. IHC staining: tyrosine hydroxylase (blue), GDNF (brown).
5. Conclusion
Multiple non-clinical primate and rodent studies have been conducted that demonstrate that direct delivery of AAV2-GDNF to the putamen induces sustained GDNF expression in the basal ganglia at levels that can both protect and restore nigrostriatal function. However, until recently, a safe and reliable clinical procedure has not been advanced that would result in the broad distribution of GDNF throughout the putamen and substantia nigra that we believe is necessary for clinical efficacy. We have shown that anterograde transport of AAV2 from the putamen to the substantia nigra is completely independent of dopaminergic neurons and displays a close correlation with the extent of putaminal transduction. The most intense GDNF staining in the substantia nigra was observed when there was broad transduction of the ipsilateral putamen. This positive correlation between the extent of putaminal transduction and GDNF expression in the substantia nigra may explain the very small amount of Neurturin that was detected in the substantia nigra of the Parkinson’s disease patients that received AAV2-Neurturin in the Phase 2 CERE-120 study. Neurturin expression in these patients covered less than 15% of the putamen (Bartus et al., 2011). Despite the relatively large number of patients that have now received direct central nervous system delivery of AAV vectors in clinical studies, very little post-mortem data has become available to directly assess vector distribution and long-term transgene expression in humans. Over time, we expect more such data to become available that would enable retrospective analysis of the relative performance of various delivery paradigms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Bartus RT, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, et al. Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J Alzheimers Dis. 2008;15:255–83. doi: 10.3233/jad-2008-15210. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–41. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–75. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Christine CW, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009 doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A, et al. Anterograde Axonal Transport of AAV2-GDNF in Rat Basal Ganglia. Mol Ther. 2011;19:922–7. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor B, et al. Differential effects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged Parkinsonian rat. Gene Ther. 1999;6:1936–51. doi: 10.1038/sj.gt.3301033. [DOI] [PubMed] [Google Scholar]
- Eberling JL, et al. PET 6-[F]fluoro-L-m-tyrosine Studies of Dopaminergic Function in Human and Nonhuman Primates. Front Hum Neurosci. 2007;1:9. doi: 10.3389/neuro.09.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, et al. Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus) Exp Neurol. 2003;184:536–48. doi: 10.1016/j.expneurol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Gash DM, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–5. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Granholm AC, et al. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20:3182–90. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- Hovland DN, Jr, et al. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicol Pathol. 2007;35:676–92. doi: 10.1080/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Johnston LC, et al. Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum Gene Ther. 2009;20:497–510. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–11. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Kells AP, et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci. 2010;30:9567–77. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proceedings of the National Academy of Sciences, USA. 2009;106:2407–11. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, et al. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–10. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kirik D, et al. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson’s disease. Eur J Neurosci. 2001;13:1589–99. doi: 10.1046/j.0953-816x.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, et al. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci. 2000a;12:3871–82. doi: 10.1046/j.1460-9568.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, et al. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000b;20:4686–700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–73. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol. 1999;46:419–24. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lang AE, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–19. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, et al. Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector. Mol Ther. 2009a;17:1857–67. doi: 10.1038/mt.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, et al. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther. 2009b;17:980–91. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WJ, Jr, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010 doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–8. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther. 2010;18:1731–5. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S-i, et al. Aromatic L-Amino Acid Decarboxylase Gene Therapy for Parkinson’s Disease: Results from an Open-Label, Phase I Trial. American Society for Gene Therapy; San Diego, CA, USA: 2009. [Google Scholar]
- Nutt JG, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Pascual A, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–61. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Patel NK, Gill SS. GDNF delivery for Parkinson’s disease. Acta Neurochir Suppl. 2007;97:135–54. doi: 10.1007/978-3-211-33081-4_16. [DOI] [PubMed] [Google Scholar]
- Richardson RM, et al. Novel Platform for MRI-Guided Convection-Enhanced Delivery of Therapeutics: Preclinical Validation in Nonhuman Primate Brain. Stereotact Funct Neurosurg. 2011a;89:141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, et al. Interventional MRI-guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson’s Disease. Mol Ther. 2011b;19:1048–57. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg I, et al. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–12. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Su X, et al. Real-time MR imaging with Gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther. 2010;18:1490–5. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, et al. Safety Evaluation of AAV2-GDNF Gene Transfer into the Dopaminergic Nigrostriatal Pathway in Aged and Parkinsonian Rhesus Monkeys. Human Gene Therapy. 2009 doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995a;373:335–9. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tomac A, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995b;92:8274–8. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, et al. Cannula placement for effective convection-enhanced delivery in the non-human primate thalamus and brainstem: Implications for clinical delivery of therapeutics. J Neurosurg. 2010 doi: 10.3171/2010.2.JNS091744. In Press. [DOI] [PubMed] [Google Scholar]
- Yin D, et al. Optimal region of the putamen for image-guided convection-enhanced delivery of therapeutics in human and non-human primates. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]