Abstract
The use of functional nanogels based on poly(N-isopropylacrylamide) for effectively scavenging compounds (here, the model drug bupivacaine) is demonstrated using an in vitro cell-based assay. Nanogels containing higher loadings of acidic functional groups or more core-localized functional group distributions bound more bupivacaine, while nanogel size had no significant effect on drug binding. Increasing the dose of nanogel applied also facilitated more bupivacaine binding for all nanogel compositions tested. Binding was driven predominantly by acid-base interactions between the nanogels (anionic) and bupivacaine (cationic) at physiological pH, although both non-specific absorption and hydrophobic partitioning also contributed to drug scavenging. Nanogels exhibited minimal cytotoxicity to multiple cell types and were well-tolerated in vivo via peritoneal injections, although larger nanogels caused limited splenic toxicity at higher concentrations. The cell-based assay described herein is found to facilitate more robust drug uptake measurements for nanogels than conventional centrifugation-based assays, in which nanogels can be compressed (and thus drug released) during the measurement.
Keywords: nanogels, drugs, molecular scavenging, poly(N-isopropylacrylamide), structure-property correlations
1. INTRODUCTION
Drug overdose remains a common clinical problem across the globe, occurring both inadvertently and intentionally. Ideally, an antidote is provided, but specific antidotes are frequently not available. Consequently, a broad range of non-specific therapies have been developed to approach this problem, ranging from cathartics to more invasive and sophisticated approaches such as plasmapheresis[1], hemoperfusion[2], hemodialysis[3], or a combination thereof[4] [5].
Recently, several nanoparticle-based strategies have been investigated to achieve effective drug scavenging in vivo. Nanoparticles are attractive vehicles for this purpose because of easy injectability, large surface area-to-volume ratios, and low probability of embolic phenomena. Lipid microemulsions or nanoemulsions[6–8], liposomes[9–11], vesicles[12], nanocapsules[13], and polymer conjugates[14, 15] have been developed for scavenging various drugs; indeed, lipid microemulsions have been demonstrated to provide superior performance compared to drug-based therapies for treating anesthetic overdoses[7, 16].
Nanogels, sub-micron hydrogel nanoparticles, have highly desirable properties that may make them particularly suitable for such applications. Like hydrogels, nanogels have a three-dimensional, internally-crosslinked microstructure, swell in aqueous solvents to provide free volume for non-specific sorption, and can shrink and swell according to changes in the gel environment. Like nanoparticles, nanogels are injectable, have extremely high specific surface areas available for interaction with chemicals in the gel environment, and respond much faster to environmental stimuli. Based on these properties, nanogels have already attracted significant interest as sensors, rheological modifiers, optical devices, mechanical actuators, diagnostics supports, and drug delivery vehicles, among other applications[17]. Included among these applications is the use of nanogels as a peptide scavenger synthesized by molecular imprintation[18], although few other examples of nanogel drug scavengers have been reported in the literature.
To investigate the capacity of nanogels to scavenge drugs, we have used nanogels based on poly(N-isopropylacrylamide) (PNIPAM). Aside from their useful thermosensitive properties, the size, morphology and chemical composition of PNIPAM particles can easily be tuned, making them ideal model systems for investigating the design of nanoparticle-based drug scavengers. Here, we study the effect of altering nanogel size, charge, and chemistry on the scavenging of a model drug (bupivacaine, an amphiphilic local anesthetic that is cationic at physiological pH). The capacity of nanogels to absorb and significantly reduce the toxicity of bupivacaine is analyzed, as is their cytotoxicity in vitro and biocompatibility in vivo. Cell-based assay results are compared to the conventional centrifugation assay results for measuring drug binding to nanogels.
2. MATERIALS AND METHODS
2.1 Materials
N-isopropylacrylamide (NIPAM, 99%), acrylic acid (AA, 99%), methacrylic acid (MAA, 99%), vinylacetic acid (VAA, 97%), fumaric acid (FA, 99%), dimethylaminoethyl methacrylate (DMAEA, 99%), N,N-methylenebisacrylamide (BIS, 99%), sodium dodecyl sulfate (SDS, 99.5%), and bupivacaine hydrochloride (BPV, 99%) were purchased from Sigma-Aldrich. Ammonium persulfate (APS, 99%) was purchased from Fluka. Sterile saline for injections was purchased from Baxter Pharmaceuticals. Phosphate buffered saline (PBS) was purchased from Invitrogen. All water used in the synthesis and purification was of Milli-Q grade.
2.2 Nanogel Synthesis
Recipes used to prepare the nanogels are shown in Table 1. The nanogel codes are assigned to indicate the type of functional monomer used to prepare the nanogel (AA, MAA, VAA, FA or DMAEA), the mole percentage of functional monomer used to prepare the nanogel (6 mol% or 20 mol% - the remainder being NIPAM), and the relative size of the nanogel (S = small, 100–200 nm diameter, M = medium, 250–400 nm diameter, L = large, 800–1000 nm diameter). Thus AA-20S indicates a small particle containing 20 mol% acrylic acid.
Table 1.
Recipes and % functional monomer content of copolymer nanogels prepared
| Nanogel | NIPAM (g) | AA (g) | MAA (g) | VAA (g) | FA (g) | DMAEA (g) | BIS (g) | SDS (g) | APS (g) | Water (mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| AA-6L | 1.4 | 0.058 | 0 | 0 | 0 | 0 | 0.10 | 0 | 0.10 | 155 |
| AA-6M | 1.4 | 0.058 | 0 | 0 | 0 | 0 | 0.10 | 0.05 | 0.10 | 155 |
| AA-6S | 1.4 | 0.058 | 0 | 0 | 0 | 0 | 0.10 | 0.20 | 0.10 | 155 |
| AA-20L | 1.4 | 0.232 | 0 | 0 | 0 | 0 | 0.10 | 0 | 0.10 | 155 |
| AA-20M | 1.4 | 0.232 | 0 | 0 | 0 | 0 | 0.10 | 0.05 | 0.10 | 155 |
| AA-20S | 1.4 | 0.232 | 0 | 0 | 0 | 0 | 0.10 | 0.20 | 0.10 | 155 |
| MAA-6M | 1.4 | 0 | 0.068 | 0 | 0 | 0 | 0.10 | 0.05 | 0.10 | 155 |
| VAA-6M | 1.4 | 0 | 0 | 0.30 | 0 | 0 | 0.10 | 0.05 | 0.10 | 155 |
| FA-6M | 1.4 | 0 | 0 | 0 | 0.10 | 0 | 0.10 | 0.05 | 0.10 | 155 |
| DMAEA-6S | 1.4 | 0 | 0 | 0 | 0 | 0.126 | 0.10 | 0 | 0.10 | 155 |
| DMAEA-20S | 1.4 | 0 | 0 | 0 | 0 | 0.504 | 0.10 | 0 | 0.10 | 155 |
All monomers and surfactants were dissolved in 150 mL water inside a 500 mL round-bottom flask and heated to 70°C under a N2 purge and 200 RPM magnetic mixing. After 30 minutes, 0.10 g of APS dissolved in 5 mL water was injected into the flask to initiate polymerization. The polymerization reaction was continued for four hours, after which the nanogels were cooled and decanted into a 500 kDa MWCO poly(vinylidene fluoride) dialysis membrane. Nanogels were exhaustively dialyzed over 8 cycles against 4L of Millipore water to remove residual surfactant and linear oligomers which are by-products of nanogel synthesis. The nanogels were then lyophilized to dryness for storage.
2.3 Nanogel Characterization
The degree of acid monomer incorporation was measured using conductometric titration using a Burivar-I2 automatic buret (ManTech associates) with PC-Titrate software (Version 2.0.0.79). Samples of 50 mg of nanogel were suspended in 50 mL of 10−3M NaCl. Titration was conducted using 0.1 M HCl and 0.1 M NaOH Acculute standards, with injections performed at a rate of 10 min/unit pH over the course of the titration. The particle size of the nanogels was evaluated using a Zeta Plus dynamic light scattering instrument operating at a 90° detection angle (Brookhaven Instruments Inc.). Results are reported as means of ten replicate particle size measurements; errors represent the standard deviation of those measurements. All particle sizes were conducted in PBS, both in the absence and presence and bupivacaine; bupivacaine concentrations between 0 – 12wt% relative to the dry mass of nanogel in the suspension were tested, with drug incubated with the nanogel suspensions for 24 hours at room temperature prior to measurement. The electrophoretic mobility of the nanogels was measured using the Zeta Plus instrument (Brookhaven Instruments Inc.) operating in phase analysis light scattering (PALS) mode. Results are reported as means of ten independent measurements, each consisting of 15 repeat measurements; errors represent the standard errors of those measurements. It should be noted that measurements were not possible in cell media, which absorbed the incident (red) laser light used in the size and mobility instrument.
2.4 Centrifugation-Based Drug Loading Assay
The drug loading capacity of functionalized nanogels for bupivacaine was assayed using a conventional centrifugation technique. A 1 mg fraction of nanogel was suspended in a 1 mg/mL bupivacaine solution in 0.15 M saline and mixed for 24 hours in an incubating shaker maintained at 37°C. The samples were then centrifuged for one hour at 73000 RPM; UV/VIS spectrophotometry indicated complete removal of the highly scattering nanogel particles from the suspension. The supernatant was then removed and assayed for bupivacaine concentration using UV/VIS spectrophotometry (wavelength = 262 nm), with absorbances converted to concentrations via the use of a calibration curve prepared in the same 0.15 M saline solution (R2 = 0.99).
2.5 Cytotoxicity and Drug Scavenging Assay
A MTT assay was used to evaluate the biocompatibility of the nanogels with mouse-derived C2C12 myoblasts, mouse-derived 3T3 fibroblasts, mouse-derived J.1774 macrophage-like cells, and MeT-5A human mesothelial cells, each in ATTC-recommended media. Each cell line was plated in 1mL aliquots in a 24-well plate at a density of 30000 cells/well and permitted to adhere and stabilize over 24 hours. In the case of the C2C12 myoblasts, the FBS growth medium was replaced with 2% horse serum and 1% penicillin streptomycin-supplemented DMEM media to differentiate the myoblasts into myotubes over the course of 8 days, with regular media changes every 3 days. Passages 3–35 were used for cytotoxicity studies. Materials were sterilized dry under a UV lamp over a period of three hours, after which 0.9% saline solution was added aseptically and the nanogels were resuspended under gentle mixing at the targeted concentration. 0.1mL aliquots of nanogels were then added to each of the wells of the multiwell plates, with 0.1 mL of sterile saline added to the cell-only and media-only controls to conserve volume. Subsequently, a 0.1 mL aliquot of bupivacaine hydrochloride dissolved in sterile saline at the desired concentration was added to drug-containing cell wells. Four replicate wells were tested for each material, with media-only and cell-only controls (also performed in quadruplicate) included on each 24-well plate tested. At time points of 24 hours and 4 days after material addition, both the media and the test material were removed and replaced with 1 mL of fresh media and 100 μL of MTT reagent. Solubilization solution (Promega) was added after four hours of incubation and the plates were mixed on an orbital stirrer for 24 hours. The absorbances of each of the wells were then measured in duplicate in a 96-well plate using a multi-well plate reader (Molecular Devices) operating at 570 nm. Results are baseline-corrected to eliminate the impact of media absorbance and are normalized relative to the cell-only results.
2.6 Live-Dead Assay of Cytotoxicity
A LIVE/DEAD Reduced Biohazard Viability/Cytotoxicity Kit (Invitrogen) was used to assess the viability of cells in the presence of nanogels and bupivacaine. Cells were cultured and tested for nanogel and bupivacaine toxicity as outlined previously for the MTT assay. After the materials are removed from the cells, solutions of SYTO 10 green fluorescent nucleic stain (live cell staining) and DEAD red (ethidium homodimer-2) nucleic acid stain (dead cell staining) were prepared in Hank's balanced salt solution (HBSS) according to the recommended protocol for the assay. Aliquots of 300 μL/well of the dye mixture were added to each of the test wells and incubated in darkness for 15 minutes at room temperature. Cells were then washed twice with fresh HBSS buffer, after which a 4% glutaraldehyde solution prepared in HBSS was added. After one hour of incubation, HBSS buffer was added to the cells and the wells were imaged by fluorescence microscopy.
2.7 Peritoneal Injections
To assay the biocompatibility of nanogels in vivo, 1 mL aliquots of AA-20S and AA-20L nanogels were injected into the peritoneal cavities of mice (using a 25G needle) at concentrations of 10 mg/mL and 20mg/mL (n=4 at each concentration). SV129 mice (20–25 g in mass) were purchased from Charles River Laboratories (Wilmington, MA) and housed in groups of four using a 7AM–7PM light-dark cycle. Animals were cared for in compliance with protocols approved by the Animal Care and Use Committee at the Massachusetts Institute of Technology. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed. Mice were briefly (< 2 minutes) anesthetized with isofluorane in 100% oxygen prior to injection. Animals were visually observed over three days to identify any acute toxic response and sacrificed on day 3 for histological analysis. Tissues were sectioned and stained with hematoxylin-eosin using standard techniques. Slides were analyzed by an observer (MWL) blinded to the nature of the material injected into the animal being observed.
3. RESULTS
3.1 Nanogel Characterization
The library of nanogels synthesized in this study, based on the thermoresponsive polymer poly(N-isopropylacrylamide) (PNIPAM), was designed to allow for the evaluation of the effects of nanogel concentration, functional group content, the type of functional group incorporated, particle size and charge, and the distribution of functional groups within the nanogel matrix on the capacity of nanogels to bind cationic drugs and scavenge excess local anesthetic. The particle sizes and electrophoretic mobilities of nanogels evaluated in this study are shown in Table 2. Syntheses were performed to generate a library of nanogels encompassing: a) a large range of sizes: 100–1000 nm, separated in groups denoted as “small” (100–250 nm), “medium” (250–400 nm) and “large” (800–1000 nm); b) various degrees of functionalization: 0–20 mol%; and c) various types of functionalization: -COOH groups in acrylic acid (AA), methacrylic acid (MAA), vinylacetic acid (VAA), and fumaric acid (FA) or tertiary amine groups in dimethylaminoethyl methacrylate (DMAEA). In addition, the use of the four different carboxylic acid-containing comonomers (AA, MAA, VAA, and FA) facilitated the synthesis of nanogels with different distributions of functional groups. Previous work has shown that methacrylic acid is localized in the core of the nanogel, acrylic acid is uniformly dispersed throughout the nanogel, vinylacetic acid is isolated at chain ends near the nanogel surface, and fumaric acid presents paired functional groups localized near the nanogel surface[19].
Table 2.
Particle size and electrophoretic mobilities of nanogels at 25°C and 37°C (measured in PBS).
| Nanogel | Functional group loading (mol%) (theory)a | Functional groups in microgel (mol%) (measured)b | 25°C | 37°C | ||
|---|---|---|---|---|---|---|
| Particle size (nm) | Electrophoretic mobility (x 10−8m2/Vs) | Particle size (nm) | Electrophoretic mobility (x 10−8m2/Vs) | |||
| AA-6L | 5.8 | 5.6 ± 0.4 | 840 ± 28 | −0.92 ± 0.05 | 572 ± 8 | −1.21 ± 0.06 |
| AA-6M | 5.8 | 5.5 ± 0.4 | 265 ± 1 | −0.78 ± 0.06 | 222 ± 4 | −1.05 ± 0.10 |
| AA-6S | 5.8 | 5.4 ± 0.4 | 110 ± 1 | −0.77 ±0.10 | 97 ± 1 | −0.91 ± 0.13 |
| AA-20L | 19.8 | 19.0 ± 0.6 | 974 ± 16 | −1.98 ± 0.08 | 815 ± 12 | −2.28 ± 0.11 |
| AA-20M | 19.8 | 18.7 ± 0.9 | 458 ± 3 | −1.12 ± 0.12 | ^346 ± 3 | −1.68 ± 0.29 |
| AA-20S | 19.8 | 19.2 ± 0.7 | 198 ± 7 | −0.73 ± 0.07 | 168 ± 2 | −1.78 ± 0.09 |
| MAA-6M | 5.8 | 5.9 ± 0.3 | 234 ± 6 | −0.52 ± 0.05 | 194 ± 5 | −0.61 ± 0.04 |
| VAA-6M | 21.1 | 5.7 ± 0.3 | 245 ± 6 | −0.97 ± 0.05 | 207 ± 4 | −1.16 ± 0.08 |
| FA-6M | 12.1 | 5.9 ± 0.3 | 274 ± 8 | −1.15 ± 0.06 | 223 ± 5 | −1.37 ± 0.06 |
| DMAEA-6S | 5.8 | N/A | 122 ± 4 | −0.49 ± 0.04 | 103 ± 4 | −1.02 ± 0.04 |
| DMAEA-20S | 19.8 | N/A | 216 ± 2 | −0.46 ± 0.06 | 144 ± 1 | −1.16 ± 0.06 |
The number in the nanogel label corresponds to the mole % of functional monomer contained in the nanogel, while the letter corresponds to the nanogel size (L = large, M = medium, and S = small, as defined in text).
Molar percentage of monomers containing a -COOH group (AA, MAA, VAA, FA) or a -N(CH3)2 group (DMAEA) added to the nanogel recipe
Molar percentage of monomer residues containing a -COOH group in final nanogel, as measured via conductometric titration
Nanogel size increased as the amount of functional monomer used in the preparation increased and the amount of surfactant decreased. This result was consistent with previous studies[20]. At each degree of functionalization tested (“functional group loading” in Table 2; 5.8% or 19.8 mol% acrylic acid), nanogel size was inversely related to the presence of surfactant during nanogel synthesis; nanogels prepared with 0 g, 0.05 g, and 0.2 g of surfactant (SDS) had particle sizes on the order of 800–1000 nm (large, L), 250–450 nm (medium, M), and 100–280 nm (small, S) respectively. Thus, comparisons can be made between nanogels with different functional monomer contents but similar sizes. Note that polydispersities of <5% were noted for each nanogel, suggesting the particles were highly monodisperse and isotropic. Furthermore, both crosslinker contents (< 5 mol%) and functional monomer contents (< 20mol%) were kept within ranges in which copolymerization kinetics have been found to directly regulate the distribution of crosslinker and functional monomer within the nanogels[19, 21]. Therefore, we do not expect differences in nanogel morphology between acrylic acid-functionalized nanogels to significantly confound the analysis of the effects of other parameters (e.g. particle size and degree of functionalization) on drug binding.
Functional monomer incorporation into the nanogels was statistically quantitative in AA- and MAA-functionalized nanogels (i.e. the conductometric titration result matched the nanogel recipe prediction, Table 1) but was significantly lower than the theoretical prediction in FA and VAA-functionalized nanogels. This result was consistent with previous studies[19, 22]. The recipes for these latter two nanogels were adjusted such that the final concentration of –COOH groups in each FA- and VAA-functionalized nanogels was matched with the AA- and MAA-functionalized nanogels; as a result, direct comparisons between nanogels with different functional group distributions may be achieved.
The electrophoretic mobility relates to the zeta potential of the nanogel; the higher the absolute value of the electrophoretic mobility, the higher the surface charge on the nanogel. The electrophoretic mobility (μe) of the nanogels at 37°C scaled with the degree of acid functionalization. AA-6 nanogels had mobilities in the range −0.9 > μe > −1.2 while AA-20 nanogels had mobilities in the range −1.7 > μe > 2.3. Electrophoretic mobility is a weak function of particle size at a given degree of functionalization (R2 = 0.77 for AA-20 nanogels and R2 = 0.92 for AA-6 nanogels), although pair-wise t-test comparisons indicate that among all the AA-6 and AA-20 nanogels tested, only AA-20L and AA-20S nanogels exhibit mobility results that are statistically different (t = 3.52 > tcrit = 2.228). Thus, overall, the library of nanogels synthesized in this study allowed for the independent evaluation of the biological responses and drug delivery capacities of nanogels as a function of both the degree of functionalization and the particle size.
The presence of bupivacaine in the ambient solution induced deswelling in the nanogel particles, with larger nanogels deswelling significantly more than smaller nanogels upon drug binding (Supplementary Figure S1). This phenomenon has previously been reported in other work where cationic drugs interact with anionic nanogels[17, 23, 24]. Note that all drug binding experiments were conducted in high ionic strength PBS suspensions, such that nanogel deswelling could not be attributed solely to an increase in the ionic strength due to the addition of cationic bupivacaine. No nanogel aggregation was observed in PBS at any tested drug concentration. Furthermore, despite the relatively large size changes observed in some nanogels upon bupivacaine exposure, all nanogels remained in their general size categories (“small”, “medium”, or “large”) at all tested bupivacaine concentrations, enabling comparisons between nanogels of different sizes at any bupivacaine concentration.
Nanogels prepared with MAA had low absolute electrophoretic mobilities (implying MAA localization primarily in the core of the nanogel) and both FA and VAA nanogels had high absolute electrophoretic mobilities (implying functional group localization toward the shell of the nanogel). These findings were consistent with previously reported trends[23].
DMAEA-20S nanogels were similar in size to AA-20S nanogels, making them useful controls to independently assess the impact of functional group type on anesthetic binding. Although DMAEA nanogels had a net cationic charge in water or dilute salt solutions (for example, mobility of (+0.37 ± 0.06) × 10−8 m2/Vs in 1mM NaCl adjusted to pH 7.4), DMAEA nanogels in PBS exhibited a net anionic charge due to divalent phosphate ion adsorption to cationic functional groups on the nanogel surface.
All nanogels reported in Table 2 maintained colloidal dispersion when suspended in cell media at physiological temperature, although size and mobility testing were not possible due to absorption of the instrument laser wavelength (632 nm) by the red-colored media. Nanogels containing no functional groups (i.e. a poly(NIPAM) nanogel) aggregated in cell media at 37°C and were therefore excluded from the subsequent analysis.
The ability of nanogels to bind with the local anesthetic bupivacaine was assayed by testing the capacity of nanogels with different characteristic properties to prevent cytotoxicity to differentiated C2C12 myotubes from bupivacaine exposure[25].
3.2 Effect of Nanogel Concentration
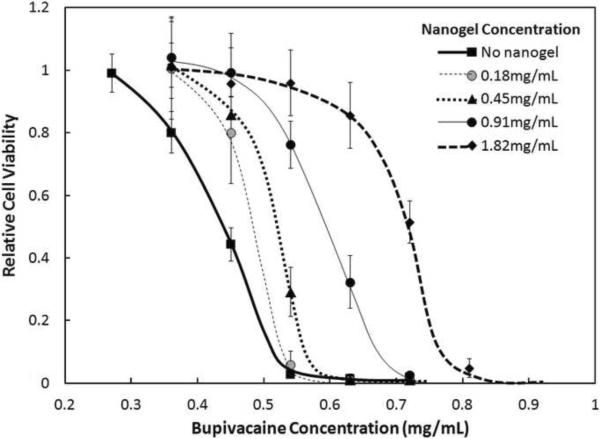
The addition of acrylic acid-functionalized nanogels to cell media reduced myotube cytotoxicity to bupivacaine (Figure 1; the same pattern was seen for all acid-functionalized nanogels tested, Supplementary Figures S2–S5). In the absence of nanogels, myotube cultures became completely non-viable when ~ 0.5 mg/mL bupivacaine was added to the cell media. However, the addition of as little as 0.18 mg/mL nanogel resulted in approximately 70% cell viability at the same concentration of bupivacaine, while close to 100% cell viability was maintained at the same bupivacaine concentration when the nanogel concentration was increased to 0.91 mg/mL. This protective effect of nanogels against bupivacaine-induced cytotoxicity was confirmed by live/dead fluorescence staining (Supplementary Figure S6). In the absence of nanogel, loss of confluence and cell viability was first significantly observed at 0.36 mg/mL, with complete loss of viability observed at 0.73 mg/mL. However, in the presence of the nanogel, no significant loss in viability was observed at 0.36 mg/mL bupivacaine and significant viability was maintained even in the presence of 0.73 mg/mL bupivacaine, although the cell layer was less confluent and “live” cells appeared more rounded.
Figure 1.
Viability (MTT assay) of C2C12 myotubes after 1 day exposure to various concentrations of AA-6S nanogels and bupivacaine. Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point).
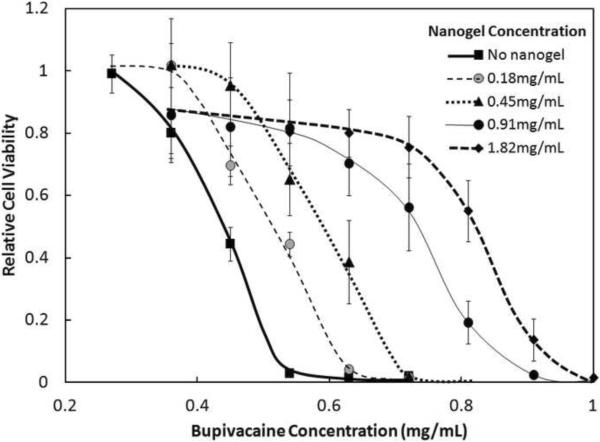
3.3 Effect of Functional Group Content
The protective effect of nanogels against bupivacaine cytotoxicity was enhanced by increasing the concentration of -COOH functional groups contained within the nanogel phase, as demonstrated by the significantly higher cell viabilities achieved with AA-20S (Figure 2) relative to AA-6S (Figure 1). For example, 0.91mg/mL AA-6S failed to preserve any cell viability after one day of exposure to 0.72 mg/mL bupivacaine, while 0.91 mg/mL AA-20S preserved approximately 60% cell viability over the same time period. This increase in viability was likely related to the increased capacity of the more highly acid-functionalized nanogel for binding bupivacaine, which is cationic at physiological pH. Increased nanogel functionalization permits increased electrostatic binding between the anionic acid groups and the cationic bupivacaine, inducing electrostatically-driven partitioning of bupivacaine into the nanogel phase and reducing the concentration of free bupivacaine in the cell media available to induce a cytotoxic response. Similar trends were observed for both the medium (Supplementary Figures S2 and S4) and large (Supplementary Figures S3 and S5) nanogels tested, with higher cell viability maintained in the presence of nanogels with higher degrees of acrylic acid functionalization.
Figure 2.
Viability (MTT assay) of C2C12 myotubes after 1 day exposure to various concentrations of AA-20S nanogels and bupivacaine. Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point)
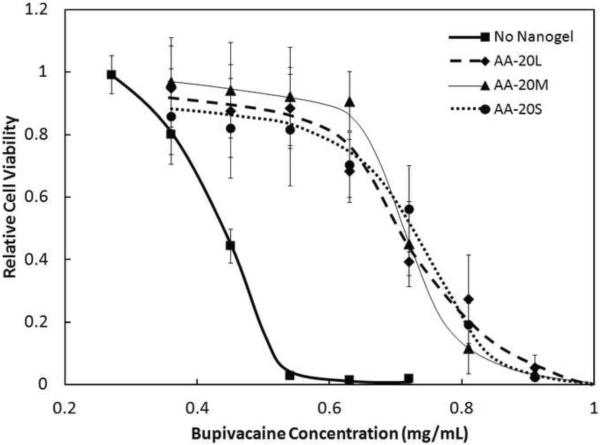
3.4 Effect of Nanogel Size
There was no significant difference in the reduction in bupivacaine cytotoxicity to C2C12 myoblasts from 20 mol% functionalized nanogels of large (~815nm), medium (~345nm), and small (~170nm) sizes (Figure 3). Thus, the effective surface area of the nanogels has no significant impact on the overall capacity of the nanogels for drug binding, at least over the one day interval used for testing in this study. The same result was observed for 6 mol% functionalized nanogels (Supplementary Figure S7).
Figure 3.
Viability (MTT assay) of C2C12 myotubes after 1 day exposure to various concentrations of bupivacaine, in the presence of 0.91 mg/mL of 20 mol% acrylic acid-functionalized nanogels of various sizes. Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point)
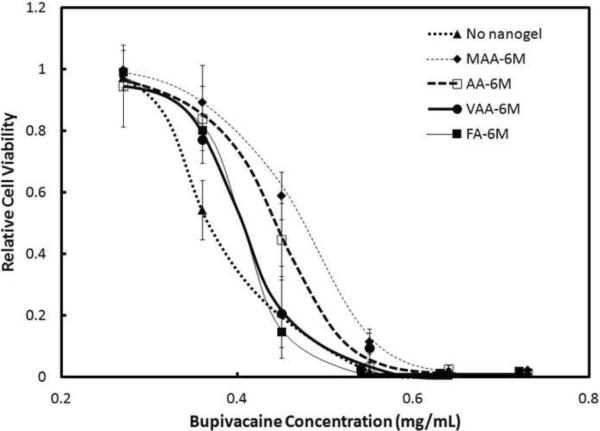
3.5 Effect of Functional Group Distributions
It has previously been shown that the type of functional comonomer used to prepare the nanogel affects the distribution of functional groups within the nanogel[23]. For example, with methacrylic acid (MAA), groups are incorporated primarily in the core, with acrylic acid (AA) -COO− groups are distributed throughout the nanogel, with vinylacetic acid (VAA) they are on the surface only, and with fumaric acid (FA) they are paired on the surface. Figure 4 indicates that these different distributions of functional groups within nanogels with the same total acid content affect the bupivacaine binding capacity of the nanogels. Nanogels prepared using MAA bound the greatest amount of drug (as evidenced by the greatest preservation of cell survival; Fig. 4), followed by AA, VAA, and FA. These results were consistent with previous findings from in vitro uptake studies in the absence of cells that nanogels with functional groups in the core of the nanogel have improved drug binding properties over nanogels with functional groups on the surface [23]. However, this difference in anesthetic binding capacity related to changing the functional group distribution is small compared to the effect of changing the number of functional groups in the nanogel (e.g. Figs. 1 and 2), and is limited to a relatively narrow range of concentrations.
Figure 4.
Viability (MTT assay) of C2C12 myotubes after 1 day exposure to various concentrations of bupivacaine, in the presence of 0.45 mg/mL of 6 mol% nanogels of various sizes with different functional group distributions. Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point)
3.6 Effect of Functional Group Type
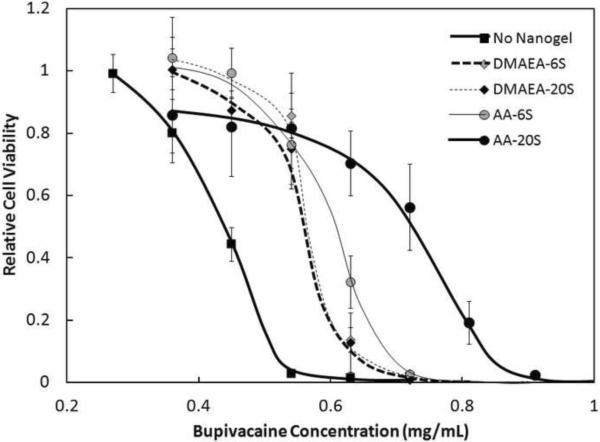
Acrylic acid-functionalized nanogels are predominantly ionized at physiological pH and thus have an electrostatic affinity for bupivacaine uptake (since bupivacaine is a cationic weak base at pH 7.4). However, non-specific absorption or partitioning of bupivacaine into the nanogel phase may also contribute to the protective effect of nanogels toward bupivacaine-sensitive cells. This was evidenced in the fact that nanogels functionalized with N,N-dimethylaminoethylacrylate (DMAEA, a cationic weak base at pH 7.4) could adsorb and/or absorb cationic bupivacaine into the nanogel matrix (Figure 5). Drug binding did not increase as the DMAEA concentration increased from 6 mol% to 20 mol% (Figure 5), demonstrating the lack of functional group-specific binding in cationic nanogels. In comparison, AA-functionalized nanogels showed higher bupivacaine binding than DMAEA-functionalized nanogels at both degrees of functionalization and bupivacaine binding increased with the degree of acrylic acid functionalization, according to the increase in electrostatically-driven drug binding at higher acrylic acid loadings (Figure 5). The same result was observed for the large nanogels tested (Supplementary Figure S8). Therefore, both non-specific partitioning and electrostatic interactions play significant roles in facilitating drug loading into nanogels and protecting cells from bupivacaine-induced toxicity.
Figure 5.
Viability (MTT assay) of C2C12 myotubes after 1 day exposure to 0.91 mg/mL of nanogels functionalized with different mole percentages of DMAEA (base) and AA (acid). Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point)
3.7 Cytotoxicity of Nanogels
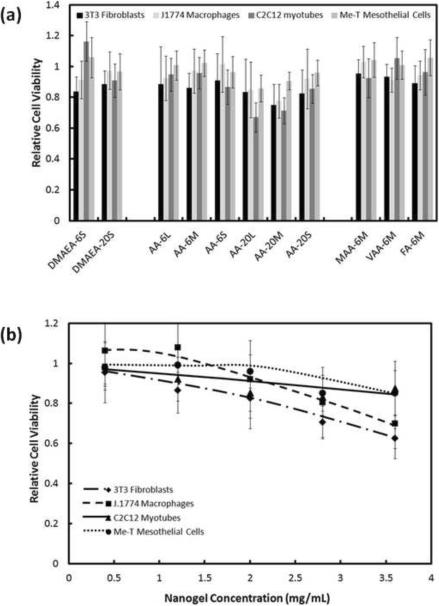
The cytotoxicity of a high concentration (2 mg/mL) of nanogels to four cell lines was examined (Figure 6). The 6 mol% functionalized nanogels exhibited no significant cytotoxicity regardless of the type or distribution of functional group (Fig. 6a). With 20% functionalized nanogels, smaller particles induced no significant cytotoxicity but the larger nanogels did exhibit mild-to-moderate toxicity (Fig. 6a). This effect may be attributable to the local acidity of the unbuffered AA-20 nanogel suspensions in saline used for the assay; the red buffer media turned orange for higher concentrations of AA-20 nanogels, indicating acidification inside the cell wells that would not be fully representative of the infinite sink in vivo environment.
Figure 6.
Viability (MTT assay) of various cell types after 1 day exposure to nanogels (a) cell viability in response to 2 mg/mL of all nanogels tested in this study; (b) cell viability in the presence of various concentrations of AA-20S nanogels. Cell viability is relative to untreated cells. Data represent means ± standard deviations (n = 4 at each data point)
No significant cytotoxic effects were observed as a result of changing the distribution or type of functional groups in nanogels with the same overall functional group content (Fig. 6a). All cell types also exhibited minimal or no cytotoxic effects over a wide range of nanogel concentrations (Fig. 6b for AA-20S is representative of data obtained for other nanogels). At nanogel concentrations up to 0.5 mg/mL, none of the cell types screened exhibited a statistically significant reduction in viability in the presence of nanogels, while only fibroblasts (p = 0.002) exhibited a significant reduction in cell viability at concentrations up to 2 mg/mL. At nanogel concentrations greater than 2.5 mg/mL, macrophages and fibroblasts exhibited mild but statistically significant cytotoxic responses, while myotubes and mesothelial cells maintained high viability even at higher nanogel concentrations.
3.8 Biocompatibility of Nanogels
To assay the in vivo biocompatibility of nanogels, 1 mL aliquots of AA-6S, AA-6L, AA-20S, and AA-20L were injected into the peritoneal cavity of SV129 mice at concentrations of 10 mg/mL and 20 mg/mL. These concentrations are between 5 and 10-fold higher than any concentration tested in the in vitro cell experiments and represent extremely high doses of up to 600 mg/kg in the mouse. Peritoneal injections were chosen due to the sensitivity of the peritoneum to injected biomaterials[26]. In all cases, mice did not show systemic signs of illness after injection.
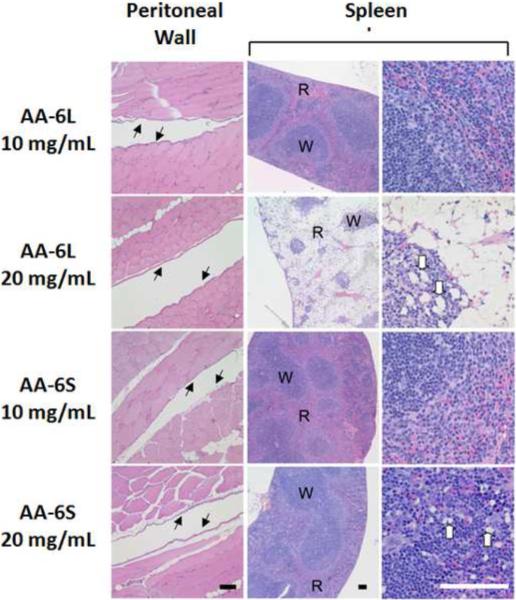
Histological analysis was performed on paraffin-embedded sections of the abdominal wall, liver, spleen, and pancreas to determine the effect of intraperitoneal injections of nanogel to the organs and peritoneal surface of the abdominal cavity. Evaluation of two animals from each treatment group revealed no significant inflammation in the parietal peritoneum in any of the animals tested, suggesting that none of the nanogel doses or infusions caused peritonitis (Figure 7 and Supplementary Figure S9). Similarly, no significant changes were noted in the liver or pancreas with respect to treatment group, with the exception of a focus of infarcted, necrotic tissue seen in one animal that had been treated with 10 mg/mL of AA-20L. The pattern of injury was most consistent with a local interruption of vascular supply to a portion of an abdominal organ, and was attributable to injection trauma. However, nanogel doses and formulations had a marked impact on the histology of the spleen. Animals injected with 10 mg/ml of AA-6L or AA-6S displayed normal splenic histology, consisting of well-delineated zones of red pulp and white pulp (Fig. 7). In contrast, increasing the dose of AA-6L to 20 mg/mL produced a marked loss of cells within the red pulp, consistent with a toxic injury to these cell populations, with a preservation of the white pulp containing lymphoid follicles and foamy macrophages (Fig. 7). No such cell loss was observed upon injection of 20mg/mL of AA-6S, while numerous foamy macrophages were noted in the red pulp and lymphoid follicles. The presence of foamy macrophages is consistent with the phagocytosis of nanogel material. Injection of AA-20 nanogels showed a similar trend with respect to nanogel size (Supplementary Figure S9). AA-20L injections at concentrations of 10 mg/mL and 20 mg/mL both showed cell loss from the red pulp (with more severe cell loss seen at the higher dose), while injection of AA-20S showed no significant cell loss in either pulp. The lymphoid follicles of the white pulp contained numerous foamy macrophages with both nanogels, while foamy macrophages were also present in the red pulp with AA-20S injections.
Figure 7.
Representative histological findings following injection of AA-6 nanogels. Hematoxylin and eosin-stained sections of body wall musculature revealed an absence of inflammation on the peritoneal surface (black arrows) independent of the dose and size of AA-6 nanogels injected. Examination of the spleen revealed marked cellular depletion within the red pulp (R) with comparative sparing of the white pulp (W) in animals injected with 20 mg/mL of AA-6L. Foamy macrophages (white arrows) were also observed at higher doses of all AA-6 nanogels. (Scale bar = 100 μm).
4. DISCUSSION
Acid-functionalized nanoparticles are able to bind bupivacaine with high affinity, significantly reducing bupivacaine-induced cytotoxicity of C2C12 mouse myotubes. In general, increasing the amount of nanogel and/or the degree of (acid) functionalization of each nanogel increased the amount of bupivacaine that could be scavenged. These findings provide proof-of-principle support for the view that particles of this type could be used as drug scavenging systems. Nanogels have several attractive properties, such as their ability to absorb compounds (and, in fact, elements) with a wide range of properties[17, 27] and their facile tuning of specificity and effectiveness modifying the particle size and surface functionalization[23]. These advantages are not shared by the extant approaches mentioned in the Introduction.
The nanogels displayed little cytotoxicity and had acceptable biocompatibility in the peritoneum, with smaller nanogels exhibiting no significant toxic effects on any tissue evaluated even at high concentrations up to 600 mg/kg. (This is equivalent to a dose of approximately 56 g in a 70 kg human.) There was, however, a loss of red pulp observed in the spleen only when larger particles were administered; the significance of this finding is unknown. Nonetheless, the specific particles employed here for proof-of-principle may not be ideal for in vivo use. There is no obvious mechanism by which they would be biodegradable, although that might not be an absolute contraindication to their use if they were to be used as a one-time administration for life-threatening drug overdoses. Such particles could be modified to be degradable, or biodegradable analogues constructed. Nevertheless, the well-controlled size, functionalization, and morphology of the systems described herein provided excellent models for identifying rules for designing nanogel-based drug scavengers with optimal performance.
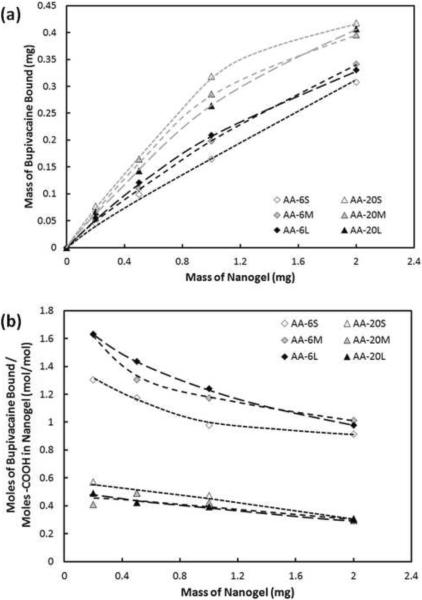
To quantify the cell results for comparisons between different samples, the bupivacaine concentration at which cell viability was 50% in the absence of nanogel was measured and subtracted from the bupivacaine concentration at which cell viability reached 50% in the presence of a particular nanogel (from Figures 1, 2 and 3 as well as Supplementary Figures S2–S5) (Figure 8). This analysis provides quantitative support for the qualitative trends described in the Results section. Increasing the nanogel concentration increases the capacity for bupivacaine binding for all nanogels tested. The scavenging capacity of the nanogel was adjustable over a large range of concentrations, without inducing significant cytotoxicity from the particles (Fig. 8a). Nanogels containing 20 mol% -COOH bind more bupivacaine than nanogels containing 6 mol% -COOH (consistent with Figures 1 and 2), although no significant trend was observed with regards to nanogel size (consistent with Figure 3).
Figure 8.
Bupivacaine binding of acrylic acid-functionalized nanogels: (a) mass of bupivacaine bound as a function of nanogel mass; (b) moles of bupivacaine bound per mole of nanogel-bound carboxylic acid group as a function of nanogel mass added. Data are calculated from Figures 1–3 and S2–S5 by measuring the bupivacaine concentration at which half the C2C12 myoblasts are viable in the presence of nanogel minus the bupivacaine concentration for half cell viability in the absence of nanogel.
Insight into the mechanism of drug uptake can also be achieved when the binding results are expressed per unit charge in the nanogel phase (Fig. 8b). Nanogels with lower –COOH contents (the AA-6 series) bind more bupivacaine per -COO− residue than nanogels with higher –COO− contents (the AA-20 series). This observation may be attributable to steric hindrance preventing binding of additional bupivacaine to clustered ionic functional groups inside the nanogel matrix. Nanogel condensation (i.e. local deswelling) induced by the neutralization of nanogel-bound charges upon bupivacaine binding is also observed to occur to a greater extent in more highly functionalized nanogels (Supplementary Figure S1). This condensation phenomenon has previously been demonstrated to limit drug uptake into thermosensitive nanogels depending on the hydrophobicity of the cationic drug tested [23]. Bupivacaine:COO− binding ratios greater than one are observed with 6 mol% acrylic acid-functionalized nanogels (Fig. 8b); that is, more bupivacaine was trapped inside the nanogel than would be predicted based on electrostatic interactions alone. This provides additional evidence that bupivacaine loading into the nanogels occurs due to a combination of electrostatic binding, affinity partitioning, and non-specific absorption into the high free volume nanogel interior. Affinity binding is likely to occur in this system since cationic bupivacaine has a logP value of 0.18 [28] while a polymerized NIPAM residue has a logP value of 0.06 [28]; these similar logP values suggest that bupivacaine would partition into a NIPAM-rich phase in relative to an aqueous phase even in the absence of an ionic driving force. Based on these observations, we hypothesize that the hydrophobicity of nanogels could analogously be tuned via copolymerization to match the hydrophobicity of a targeted drug (or toxin) to maximize its binding to the nanogel phase. Analysis of the percentage of bupivacaine bound to nanogels indicates that up to ~45% of all free bupivacaine in the system (total concentration 5 mg/mL) was bound to AA-20 nanogels while ~40% of free bupivacaine was bound to AA-6 nanogels at nanogel concentrations of 1.8mg/mL (Supplementary Figure S10). This result is on par with reported liposomal results at corresponding drug and total particle concentrations in the presence of serum proteins[11]. Comprehensive engineering of the charge, hydrophobicity, and mesh size of the nanogel may be used to optimize nanogel-based scavengers for a variety of molecular targets.
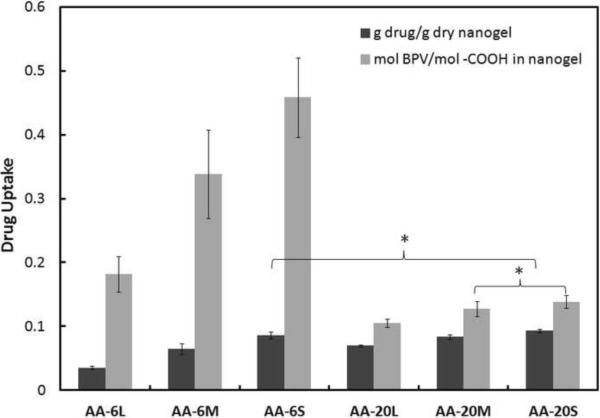
We elected to measure drug-nanogel interactions with a cell-based assay. This differs from the more conventional of measuring drug uptake into microparticles or nanoparticles, which involves centrifuging the particles out of suspension and assaying the supernatant for residual drug concentration. The subtraction of the residual drug concentration from the total amount of drug added to the system yields the amount of drug taken up by the particle[29]. Our principal reason for designing an alternative cell-based approach was concern over the potential for distortion of the nanogels by centrifugation. The results of a centrifugation-based drug uptake assay, collected under the same conditions as used for the cell-based assay (1 mg/mL nanogel suspended in a 1 mg/mL bupivacaine solution in PBS), are shown in Figure 9. The centrifugation results show the same general trend as the cell results in terms of the acrylic acid content of the nanogels, with increased drug binding observed in nanogels with higher acrylic acid contents (p < 0.05 in all pair-wise comparisons except for AA-6S versus AA-20S). However, the centrifugation assay indicates significantly higher drug uptake in smaller nanogels compared to larger nanogels with the same composition, in contrast to the size-independent uptake observed in the cell-based uptake study (Fig. 3). Furthermore, the total amount of drug bound (expressed both on a g drug bound/g dry nanogel basis and in terms of the moles of drug bound per mole of anionic –COO− group present in the nanogels) was significantly lower when drug uptake was measured via centrifugation (Fig. 9) relative to using the cell-based method (Fig. 8). Together, these results suggest that centrifugation (and the resulting compression of nanogels into a dense pellet) induces active transport of drug absorbed or partitioned into the free volume of the nanogel out of the nanogel phase. Larger nanogels are easier to isolate via centrifugation and therefore become compressed into a denser pellet over a defined centrifugation cycle; consequently, larger nanogels show a lower observed capacity for drug uptake relative to smaller nanogels. Therefore, centrifugation-based assays can yield drug binding results that are primarily related to the degree to which centrifugation compacted the nanogel rather than the inherent binding capacity of the nanogel for the drug, particularly for weakly-associated drug systems. The cell-based assay also includes the presence of proteins (in this case, 10% fetal bovine serum used to culture the cells) and therefore directly reflects the impact of protein-drug complexation on effective drug uptake. The use of a cell binding assay to measure drug uptake into nanogels, as described herein, therefore represents a novel and highly relevant method for performing drug uptake measurements without applying any mechanical forces on the particles or altering the pH, ionic strength, or temperature away from physiological conditions.
Figure 9.
Drug uptake expressed in terms of g drug/g dry nanogel (black bar) and mole drug/mole of nanogel-bound –COOH groups (grey bar) for acrylic acid-functionalized nanogels. Stars indicate samples in which pair-wise t –test comparisons of nanogels with the same mole% of acrylic acid but different sizes or nanogels with the same size but different mole% of acrylic acid yielded drug uptake results that were not significantly different (p > 0.05). Data represent means ± standard deviations (n = 4 at each data point).
5. CONCLUSIONS
Acid-functionalized nanogels based on poly(N-isopropylacrylamide) can effectively bind cationic drugs (in this case, the local anesthetic bupivacaine) at high concentrations in physiological fluids. Increasing the concentration or degree of acrylic acid functionalization of the nanogel increased the binding capacity of the nanogel for bupivacaine. Bupivacaine scavenging was driven by both ionic interactions between the cationic anesthetic and anionic –COO− groups in the nanogel as well as non-specific affinity partitioning into the nanogel phase. Biocompatibility studies indicate that the nanogels are well-tolerated in vivo even at high concentrations, particularly when the nanogel size is small (< 200 nm). On this basis, nanogels have potential as highly effective drug scavengers for the treatment local anesthetic overdoses. By generalizing these results, we anticipate that nanogels engineered to contain affinity groups or phases for other target compounds would exhibit similar scavenging capabilities for other drugs or toxins of interest.
Supplementary Material
ACKNOWLEDGEMENTS
DSK acknowledges NIH GM073626 for research funding. TH acknowledges the Natural Sciences and Engineering Research Council of Canada for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- [1].Bachmann B, Biscoping J, Violka T, Schurg R, Hempelmann G. Acute Preoperative Hemodilution and Lumbar Epidural-Anesthesia - Effects on Bupivacaine Pharmacokinetics. Anaesthesist. 1991;40:32–7. [Google Scholar]
- [2].Smith JC, Bolon B. Comparison of three commercially available activated charcoal canisters for passive scavenging of waste isoflurane during conventional rodent anesthesia. Contemp Top Lab Anim. 2003;42:10–5. [PubMed] [Google Scholar]
- [3].Hansen SK. Advances in sorbent dialysis. Dialysis Transplant. 2005;34:652–+. [Google Scholar]
- [4].Khan E, Huggan P, Celi L, MacGinley R, Schollum J, Walker R. Sustained low-efficiency dialysis with filtration (SLEDD-f) in the management of acute sodium valproate intoxication. Hemodial Int. 2008;12:211–4. doi: 10.1111/j.1542-4758.2008.00254.x. [DOI] [PubMed] [Google Scholar]
- [5].Sen S, Ratnaraj N, Davies NA, Mookerjee RP, Cooper CE, Patsalos PN, et al. Treatment of phenytoin toxicity by the Molecular Adsorbents Recirculating System (MARS) Epilepsia. 2003;44:265–7. doi: 10.1046/j.1528-1157.2003.31402.x. [DOI] [PubMed] [Google Scholar]
- [6].Morey TE, Varshney M, Flint JA, Rajasekaran S, Shah DO, Dennis DM. Treatment of local anesthetic-induced cardiotoxicity using drug scavenging nanoparticles. Nano Lett. 2004;4:757–9. doi: 10.1021/nl049880w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071–5. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- [8].Varshney M, Morey TE, Shah DO, Flint JA, Moudgil BM, Seubert CN, et al. Pluronic microemulsions as nanoreservoirs for extraction of bupivacaine from normal saline. J Am Chem Soc. 2004;126:5108–12. doi: 10.1021/ja0394479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Petrikovics I, Hong K, Omburo G, Hu QZ, Pei L, McGuinn WD, et al. Antagonism of paraoxon intoxication by recombinant phosphotriesterase encapsulated within sterically stabilized liposomes. Toxicol Appl Pharm. 1999;156:56–63. doi: 10.1006/taap.1998.8620. [DOI] [PubMed] [Google Scholar]
- [10].Fallon MS, Chauhan A. Sequestration of amitriptyline by liposomes. J Colloid Interf Sci. 2006;300:7–19. doi: 10.1016/j.jcis.2006.03.065. [DOI] [PubMed] [Google Scholar]
- [11].Howell BA, Chauhan A. Bupivacaine Binding to Pegylated Liposomes. Anesthesia and Analgesia. 2009;109:678–82. doi: 10.1213/ane.0b013e3181a8da61. [DOI] [PubMed] [Google Scholar]
- [12].Dhanikula AB, Lamontagne D, Leroux JC. Rescue of amitriptyline-intoxicated hearts with nanosized vesicles. Cardiovasc Res. 2007;74:480–6. doi: 10.1016/j.cardiores.2007.02.017. [DOI] [PubMed] [Google Scholar]
- [13].Underhill RS, Jovanovic AV, Carino SR, Varshney M, Shah DO, Dennis DM, et al. Oil-filled silica nanocapsules for lipophilic drug uptake: Implications for drug detoxification therapy. Chem Mater. 2002;14:4919–25. [Google Scholar]
- [14].Lee DW, Baney RH. Oligochitosan derivatives bearing electron-deficient aromatic rings for adsorption of amitriptyline: Implications for drug detoxification. Biomacromolecules. 2004;5:1310–5. doi: 10.1021/bm049935o. [DOI] [PubMed] [Google Scholar]
- [15].Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex - A dose-finding and safety study. Anesthesiology. 2006;104:667–74. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- [16].Weinberg GL, Di Gregorio G, Ripper R, Kelly K, Massad M, Edelman L, et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–13. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- [17].Eichenbaum GM, Kiser PF, Dobrynin AV, Simon SA, Needham D. Investigation of the swelling response and loading of ionic microgels with drugs and proteins: The dependence on cross-link density. Macromolecules. 1999;32:4867–78. [Google Scholar]
- [18].Hoshino Y, Kodama T, Okahata Y, Shea KJ. Peptide Imprinted Polymer Nanoparticles: A Plastic Antibody. J Am Chem Soc. 2008;130:15242–3. doi: 10.1021/ja8062875. [DOI] [PubMed] [Google Scholar]
- [19].Hoare T, McLean D. Kinetic prediction of functional group distributions in thermosensitive microgels. J Phys Chem B. 2006;110:20327–36. doi: 10.1021/jp0643451. [DOI] [PubMed] [Google Scholar]
- [20].McPhee W, Tam KC, Pelton R. Poly(N-Isopropylacrylamide) Latices Prepared with Sodium Dodecyl-Sulfate. J Colloid Interface Sci. 1993;156:24–30. [Google Scholar]
- [21].Zhou SQ, Chu B. Synthesis and volume phase transition of poly(methacrylic acid-co-N-isopropylacrylamide) microgel particles in water. J Phys Chem B. 1998;102:1364–71. [Google Scholar]
- [22].Hoare T, Pelton R. Titrametric characterization of pH-induced phase transitions in functionalized microgels. Langmuir. 2006;22:7342–50. doi: 10.1021/la0608718. [DOI] [PubMed] [Google Scholar]
- [23].Hoare T, Pelton R. Impact of Microgel Morphology on Functionalized Microgel-Drug Interactions. Langmuir. 2008;24:1005–12. doi: 10.1021/la7024507. [DOI] [PubMed] [Google Scholar]
- [24].Tam KC, Tan JPK, Zeng AQF, Chang CC. Release kinetics of procaine hydrochloride (PrHy) from pH-responsive nanogels: Theory and experiments. Int J Pharm. 2008;357:305–13. doi: 10.1016/j.ijpharm.2008.01.058. [DOI] [PubMed] [Google Scholar]
- [25].Padera R, Bellas E, Tse JY, Hao D, Kohane DS. Local myotoxicity from sustained release of bupivacaine from microparticles. Anesthesiology. 2008;108:921–8. doi: 10.1097/ALN.0b013e31816c8a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yeo Y, Burdick JA, Highley CB, Marini R, Langer R, Kohane DS. Peritoneal application of chitosan and UV-cross-linkable chitosan. J Biomed Mat Res A. 2006;78A:668–75. doi: 10.1002/jbm.a.30740. [DOI] [PubMed] [Google Scholar]
- [27].Eichenbaum GM, Kiser PF, Shah D, Meuer WP, Needham D, Simon SA. Alkali earth metal binding properties of ionic microgels. Macromolecules. 2000;33:4087–93. [Google Scholar]
- [28].Stefanis E, Constantinou L, Panayiotou C. A group-contribution method for predicting pure component properties of biochemical and safety interest. Ind Eng Chem Res. 2004;43:6253–61. [Google Scholar]
- [29].Xu QB, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, et al. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow-Focusing Device for Controlled Drug Delivery. Small. 2009;5:1575–81. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.