Abstract
Cochlear implant performance in difficult listening situations is limited by channel interactions. It is known that partial tripolar (PTP) stimulation reduces the spread of excitation (SOE). However, the greater the degree of current focusing, the greater the absolute current required to maintain a fixed loudness. As current increases, so does SOE. In experiment 1, the SOE for equally loud stimuli with different degrees of current focusing is measured via a forward-masking procedure. Results suggest that at a fixed loudness, some but not all patients have a reduced SOE with PTP stimulation. Therefore, it seems likely that a PTP speech processing strategy could improve spectral resolution for only those patients with a reduced SOE. In experiment 2, the ability to discriminate different levels of current focusing was measured. In experiment 3, patients subjectively scaled verbal descriptors of stimuli of various levels of current focusing. Both discrimination and scaling of verbal descriptors correlated well with SOE reduction, suggesting that either technique have the potential to be used clinically to quickly predict which patients would receive benefit from a current focusing strategy.
Keywords: Cochlear Implants, Current Focusing, Spread of Excitation, Monopolar, Tripolar, Psychophysics
1. Introduction
Auditory prostheses have restored functional hearing to many deaf individuals. Many cochlear implant (CI) users are capable of good speech understanding under optimal listening conditions. However, even top CI performers have great difficulty in challenging conditions (e.g., speech in noise, competing speech, music, etc). Their difficulty is likely to be due to poor spectral resolution provided by the implant. Different listening conditions require different degrees of spectral resolution. For example, previous CI studies have shown that speech recognition in quiet requires only 4 spectral channels (Shannon et al., 1995), while speech recognition in noise at a 10 dB signal-to-noise ratio (SNR) or less requires 8 channels or more. (Fu et al., 1998; Friesen et al., 2001; Smith et al., 2002). Many more channels are required to perceive music or to segregate competing talkers (Shannon et al., 2004). Commercial CI signal processing strategies currently provide up to 22 physical channels (SPEAK and ACE, Cochlear Corporation) or 120 virtual channels (Fidelity 120, Advanced Bionics Corporation). However, CI users perform as if they only have 8-10 effective channels, far fewer than the number of physical electrodes. Over the last 10 – 15 years, there have been many modifications to CI design and speech processing, e.g., increased numbers of physical electrodes (Filipo et al., 2004), increased stimulation rates (Vandali et al., 2000), virtual channels (Koch et al., 2007), alternate analysis filterbanks (Geurts and Wouters, 2004), etc. These modifications have provided only modest gains in performance (at best). Thus, it seems that even state-of-the-art CI technology is unable to effectively transmit more than ~8 spectral channels.
When compared in isolation (e.g., single-channel electrode discrimination), CI users are often able to discriminate most (if not all) electrodes. However, two adjacent electrodes that are discriminable may not provide independent channels of information when placed in a multi-channel context. For example, McDermott and McKay (1994) showed that two different modulation rates delivered to two adjacent electrodes were perceived as having a pitch between the modulation rates, suggesting that although the electrodes were discriminable, they were not independent. Thus, CI users' functional spectral resolution may be limited by channel interactions. Fu and Nogaki (2005) tested speech recognition in fluctuating noise in CI subjects and normal hearing (NH) subjects listening to acoustic simulations. While NH listeners experienced some release from masking by listening in the dips of the modulated noise, CI users experienced little release from masking. Fu and Nogaki (2005) suggested that channel interaction was the limiting factor in CI performance, which was most comparable to that of NH subjects listening to four broad noise bands (to simulate strong channel interactions). In the CI simulations, both increasing the number of channels or reducing the carrier bandwidth (to simulate reduced channel interactions) produced better performance than obtained in CI users. Similarly, Bingabr et al. (2008) found that with CI simulations, patients with only a few channels (i.e. poor spectral resolution), a reduction in spread of excitation improved performance with HINT sentences. However, with extremely narrow spread of excitation, performance is reduced, suggesting that there is an optimal spread of excitation for CI performance.
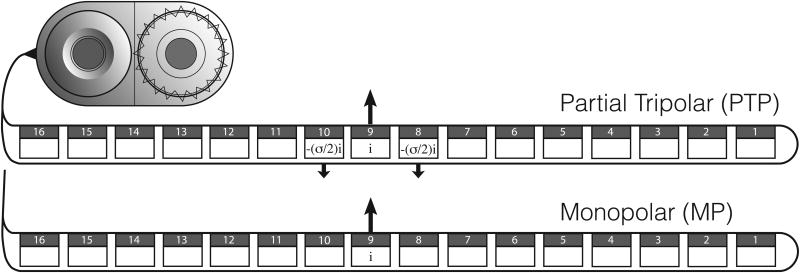
One method to reduce channel interaction would be to reduce the current spread produced by each channel. The default stimulation mode for most contemporary speech processing strategies is monopolar (MP), which has been shown to produce a relatively broad spread of excitation (Bierer and Middlebrooks, 2002; Bierer, 2007). With MP stimulation, current is delivered to an intra-cochlear electrode using an extra-cochlear electrode as a ground. With partial tripolar (PTP) stimulation, current is delivered to one electrode and two adjacent electrodes are used as grounds (Figure 1); the ratio and phase of current delivered to the electrodes determines the degree of “current focusing.” Computational modeling of current flow has shown that PTP stimulation produces a narrower spread of excitation than does bipolar (BP) stimulation, and that both PTP or BP stimulation produce a narrower spread of excitation than does MP stimulation (Spelman et al., 1995; Jolly et al., 1996; Kral et al., 1998; Briaire and Frijns, 2000). PTP stimulation has also been shown to produce a narrower current spread than that with MP or BP stimulation, in physiological (Bierer and Middlebrooks, 2002; Snyder et al., 2004) and psychophysical studies (Bierer, 2007; Bierer et al., 2010). When only the intra-cochlear electrodes are used as grounds (as in BP or PTP stimulation), higher current levels are required to achieve comfortable loudness levels (e.g., Berenstein et al., 2008).
Figure 1.
Illustration of monopolar (MP) and partial tripolar (PTP) stimulation centered on electrode 9. The value i represents the amplitude of the current on electrode 9 and σ is the current focusing coefficient (ranging between 0 and 1). Note that the amplitudes only represent the anodic phase of a biphasic pulse.
To achieve adequate loudness with tripolar stimulation, the extra-cochlear electrode can be used as an additional ground creating a PTP stimulation mode. The ratio between the intra-and extra-cochlear electrode grounds is designated σ (e.g. Litvak et al., 2007). When σ = 1, stimulation is completely intra-cochlear (TP); when σ = 0, stimulation is completely MP. When σ = 0.75, 75% of the current is delivered to the two intra-cochlear ground electrodes and 25% is delivered to the extra-cochlear ground electrode; each intra-cochlear ground receives half of the current remaining in the cochlea (σ/2). Note, in this manuscript, when specifying PTP stimuli with specific values for σ, we will indicate the value of σ as a subscript. (Bonham and Litvak, 2008) and Bierer et al. (2010) reported that PTPσ > 0.5 produced more spatially selective neural activity in the central nucleus of the inferior colliculus (ICC) of the guinea pig, indicating that TP stimulation reduced current spread. For PTPσ < 0.5 (which was only explored in Bonham and Litvak, 2008), the neural activity in the ICC was indistinguishable from MP.
When implementing a speech processing strategy, sounds must be presented within a patient's dynamic range, regardless of the mode of stimulation implemented in the strategy. In order to reach a fixed level of loudness, a focused stimulation mode requires a higher stimulation amplitude than an unfocused stimulation mode (e.g. Litvak et al., 2007; Berenstein et al., 2008; Landsberger and Srinivasan, 2009). However, as the amplitude of a stimulus increases, so does the spread of current from that stimulus (Chatterjee and Shannon, 1998). It is possible that the reduction in current spread from a current focused stimulation mode is counteracted by the increased current spread resulting from the increased amplitude required to maintain a fixed loudness. Kwon and van den Honert (2006) measured the spread of excitation for loudness balanced MP and BP+1 pulse trains and found no consistent reduction in spread of excitation with BP+1 stimulation. This may explain why no significant differences in speech performance have been observed between the relatively broad MP and narrow BP stimulation modes (Pfingst et al., 2001). If a TP (or other current focused stimulation mode) speech processing strategy is going to improve spectral resolution, the spread of excitation from a current focused stimulation must be narrower than a MP spread of excitation at an equal loudness and not at an equal amplitude. Srinivasan et al. (2010) has shown that using quadrupolar virtual channels (QPVCs), which are effectively a broader PTP stimulus created around a virtual channel, provide sharper peaks of stimulation at a fixed loudness than monopolar virtual channels (MPVCs) but did not measure spread of excitation beyond the two electrodes used to create the virtual channel.
The first of three experiments (experiment 1) presented in this manuscript measures the spread of excitation of equally loud MP and PTP stimuli with different values for the focusing coefficient (σ) using a psychophysical forward masking technique. A similar experiment has recently been reported by Bierer and Faulkner (2010) in which psychophysical tuning curves (PTCs) were measured using a fixed masker (PTPσ = 0.5) to detect the presence of either a MPσ = 0.0 or PTPσ ≥ 0.55 probe. While a significantly sharper tuning was detected for PTP PTCs, a large number of the PTCs were very similar for both the MP and PTP condition. However, because this methodology estimates spread of excitation near threshold, it may not be an accurate measure of the relative spread of excitation between MP and PTP stimuli at a comfortably loud level. Nevertheless, results from the present study were consistent with Bierer and Faulkner (2010) in that our results indicated that at a fixed loudness, PTP stimulation reduced the spread of excitation for some but not all subjects. We would therefore predict that in a current-focused speech processing strategy, a user with a reduced spread of excitation might have better spectral resolution, but users for whom there is no reduced spread of excitation would not have any benefit in spectral resolution. If a current focused strategy were to be implemented clinically, it is important to be able to easily predict which patients would benefit from current focusing (and possibly on which electrodes), using measurements that are sufficiently time efficient to be collected in a clinical setting.
In an attempt to find clinically relevant methods to determine which patients had a reduced spread of excitation, two follow up experiments (experiments 2 and 3) were conducted. In experiment 2, we measured the ability of patients to discriminate between equally loud stimuli with varying levels of current focusing. Our hypothesis was that patients for whom current focusing provided a reduced spread of excitation would be better able to discriminate different degrees of current focusing (σ).
Experiment 3 investigated the possibility that a patient's subjective perception of current focused and unfocused stimuli might be useful in predicting which patients would have a reduced spread of excitation with current focusing. Anecdotal reports from our lab and other labs (Berenstein, 2007; Saoji, 2007) suggest that increased σ values may produce higher pitches. Qualitative reports from CI users (Marzalek et al., 2007) suggest that increased σ values may produce better “tonal quality.” These reports are consistent with the findings that cortical activation patterns from MP stimulation have been shown to resemble that of broadband noise, while activation patterns from TP stimulation have been shown to resemble that of an acoustic pure tone (Arenberg et al., 2000; Bierer and Middlebrooks, 2002). We hypothesized that a patient's subjective ratings on a perceptual dimension (such as “clean”, “pure”, or “high”) could be used to predict whether or not a patient actually receives a reduced spread of excitation with current focusing. In experiment 3, we examined the relationship between a patient's perceptual reports of different levels of current focusing (σ) with the relative reduction in spread of excitation between focused and unfocused stimuli.
2. Materials and Methods
2.1 Subjects
Six users of the Advanced Bionics CII or HiRes90K cochlear implants with HiFocus 1J electrodes participated in all three experiments. None of the subjects were implanted using an electrode positioner. All subjects were post-lingually deafened and had at least a year of experience with the implant. All subjects provided informed consent in accordance with local IRB regulations and were compensated for their participation. Specific demographic information about the subjects is presented in Table 1.
Table 1.
CI subject demographics.
| Subject | Age | Gender | Etiology | Prosthesis | Strategy | CI Experience (Years) |
|---|---|---|---|---|---|---|
| C1 | 77 | M | Sudden sensorineural hearing loss | CII | HiRes-P w/Fidelity 120 | 7 |
| C3 | 54 | F | Genetic | HR90K | HiRes-S w/Fidelity 120 | 3 |
| C4 | 62 | F | Cochlear otosclerosis | HR90K | HiRes-S | 4 |
| C7 | 60 | F | Fever + streptomycin | HR90K | HiRes-P w/Fidelity 120 | 3 |
| C8 | 62 | F | Hereditary (Possible otosclerosis) | HR90K | HiRes-S w/Fidelity 120 | 1 |
| C9 | 67 | M | Possible Spinal Meningitis | CII | HiRes-S | 7 |
2.2 Stimuli
Stimuli for all experiments were single channel cathodic-first biphasic PTP pulse trains with a 226 μs phase duration at 1000 pulses per second (pps). Stimuli were presented using the Bionic Ear Data Collection System (BEDCS) software and the standard Advanced Bionics clinical fitting hardware. The current focusing coefficient (σ) ranged from 0 to 0.75. Probe stimuli (used in the masker-probe paradigm in experiment 1) were 20 ms in duration. All other stimuli were 300 ms in duration.
2.3 Experiment 1: Estimating spread of excitation using forward masking
2.3.1 Estimation of Dynamic Range
The dynamic range was quickly estimated for stimuli used in this experiment in order to determine the loudest acceptable stimulation, the approximate value for stimulus threshold, and the loudness growth for each stimulus. Initial stimulation was presented at very low (sub threshold) levels. Stimulation was gradually increased in 5 μa steps. Subjects used an 11 point loudness scale provided by Advanced Bionics to report the loudness of the stimuli. The scale was as follows: 0 – No Sound, 1 – Barely Audible, 2 – Very Soft, 3 - Soft, 4 – Medium Soft, 5 – Medium, 6 – Most Comfortable, 7 – Loud But Comfortable, 8 – Maximal Comfort, 9 – Uncomfortable, 10 – Very Uncomfortable. The amplitudes which corresponded to “Barely Audible” (1), “Soft” (3), “Most Comfortable Level” (6) and “Maximum Comfortable Level” (8) were recorded. When the loudness reached “Maximum Comfortable Level” (8), the procedure stopped. The dynamic range was estimated for PTP stimulation with σ = 0.75 on electrodes 6, 7, 8, 9, 10, 11, and 12. Additionally the dynamic range was estimated on electrode 9 for PTP stimulation with σ = 0, 0.125, 0.0.25, 0.375, 0.5, and 0.625.
2.3.2 Loudness Balancing
The loudness of each of the PTP stimuli with σ between 0 and 0.625 were balanced to the loudness of the σ = 0.75 stimulus at the amplitude corresponding to “Most Comfortable Level”. A 2 interval forced choice (2IFC) double staircase procedure was used. In each trial, one of the two stimuli was the reference stimulus (σ = 0.75 at the amplitude corresponding to the most comfortable level.) The other interval consisted of a stimulus with the value of σ being balanced. Stimuli were both presented for 300 ms with a 300 ms inter-stimulus interval (ISI). The loudness of the target stimulus was adjusted according to either a 1 up 3 down or 3 up 1 down rule resulting in an estimate of when the adaptive stimulus was reported as equally loud as the target stimulus either the 79.4% or 21.6% of the time (Jesteadt, 1980). Ten reversals were recorded: the adaptive step size was 1 dB for the first 2 reversals and 0.5 dB for the remaining 8. The mean of the last 6 reversals for both traces were averaged to estimate the amplitude of the target stimulus which was equally loud as the reference stimulus. The procedure was repeated at least 3 times per patient and all estimates of equal loudness were averaged.
2.3.3 Measuring of Forward Masking Curves
Measuring a forward-masked curve involves measuring the unmasked and masked detection thresholds for the probe stimuli. Unmasked thresholds were measured using a 2-interval forced-choice (2IFC) task. One interval was silent while the other interval contained a 20 ms probe stimulus. A number (1 or 2) was displayed on a computer screen to indicate which interval was being presented. The subject was told to indicate which of the two intervals contained the stimulus by pressing a corresponding button on the computer screen. A 3-down/1-up adaptive procedure was used to converge at the point where subjects could correctly identify which interval contained the stimulus 79.4% of the time (Levitt, 1971). A total of 10 reversals were measured and the mean of the last 6 reversals was used as an estimate of threshold in μa. Each threshold was measured three times and averaged together to improve our threshold estimates. The process was repeated for probe stimuli consisting of PTPσ = 0.75 centering on each of the electrodes between 6 and 12.
Masked thresholds were also measured using a 2IFC task. In each interval, the same masker was presented. In one of the two intervals, a 20 ms probe stimulus was presented after a 300 ms masker and a 5 ms masker-probe interval. The subject's task was to indicate which of the two intervals had an additional sound (i.e. the probe stimulus) after the masker sound ended. A 3-down/1-up adaptive procedure was used to measure the amplitude of the probe stimulus could be detected 79.4% of the time. A total of 10 reversals were measured and the mean of the last 6 reversals were used as an estimate for the masked-threshold. Probed thresholds for all probes centered between electrodes 6 and 12 were measured for four maskers. Maskers consisted of PTP stimulation on electrode 9 with σ = 0, 0.25, 0.5, and 0.75 at equally loud amplitudes as measured by the previous loudness balancing procedure. The probe stimuli were the same probes used to measure the unmasked threshold.
2.4 Experiment 2: Perceptual discrimination of different degrees of current focusing
Stimuli consisted of cathodic-first biphasic MP and PTP pulse trains on electrode 9. Values for σ ranged from 0 to 0.75 in 0.25 steps. Dynamic ranges for all stimuli were measured using the procedure described for experiment 1.
All stimuli were loudness balanced to the PTPσ = 0.75 stimulus at the amplitude described as the most comfortable level. Loudnesses were balanced by alternatively presenting the reference stimulus (PTPσ = 0.75) and the target stimulus at an amplitude adjustable by the subject. Stimuli were each presented for 300 ms with a 300 ms interstimulus interval. The amplitude of the reference stimulus was adjusted by turning a knob (Griffin Powermate) until the two sounds were perceived to be of equal loudness. This procedure was repeated at least three times for each stimulus and averaged to estimate equally loud amplitudes for the different stimuli.
A 3 interval forced-choice task was used to measure discrimination between stimuli with different σ values. Two of the intervals contained a reference stimulus with the same σ value while the third interval contained a stimulus (the target) with a different σ value. The interval containing the target stimulus was randomized for each trial. To mask any residual loudness cues, the amplitudes of the stimuli were roved ± 0.6 dB. Subjects were asked to indicate which of the three intervals were different in any way other than loudness by pressing a corresponding button on a response box (Ergodex DX-1). In a block, all combinations of stimuli were compared using this method once. Fifteen blocks were collected for each patient.
2.5 Experiment 3: Qualitative ratings of current focusing
For experiment 3, three new equally loud stimuli (PTP with σ = 0.125, 0.375, and 0.625) were added to the set of stimuli used for experiment 2. In a trial, a single stimulus was presented, accompanied by a response window on the computer screen. At the top of the screen, a message was displayed asking “How adjective is the sound?” where the word “adjective” was substituted with one of the following adjectives: clean, dirty, high, low, pure, noisy, full, thin, flute-like, or kazoo-like. The adjectives were chosen as they represented qualitative terms that have been anecdotally offered by patients in our lab to describe the difference between focused and unfocused stimuli in previous experiments. The 10 adjectives were picked to be approximate conceptual opposites in the pairs of clean/dirty, high/low, pure/noisy, full/thin, flute-like/kazoolike. It is worth noting that no validation was done to verify that these adjectives were appropriate perceptual opposites. Subjects were asked to rate how well the given adjective described the sound by clicking on a location on a horizontal line corresponding to a continuum from less-adjective to more-adjective. The location of the horizontal line was randomized from trial to trial to force a subject to actively select a new location for a response after each trial. A block of trials consisted of randomly ordered presentations of all stimuli. Within a block, subjects were only asked to rate one adjective. Three blocks of data for each adjective tested were collected, yielding a total of 15 observations for each level of current focusing for each adjective.
3. Results
3.1 Loudness Balancing
Consistent with previous experiments examining current focusing (e.g. Berenstein et al., 2008; Landsberger and Srinivasan, 2009; Srinivasan et al., 2010), an increase in current was required to maintain a fixed level of loudness for an increased value of the current focusing coefficient (σ). Figure 2 shows that a PTPσ = 0.75 stimuli at a “most comfortable” level requires on average 10.13 dB more current than an equally loud MP stimulus on the same electrode. A linear relationship was found between σ and the dB increment required to maintain equal loudness. A regression line was fit through the origin (y = 11.73x) and found to be statistically significant (r2 = 0.947, p < 0.001).
Figure 2.
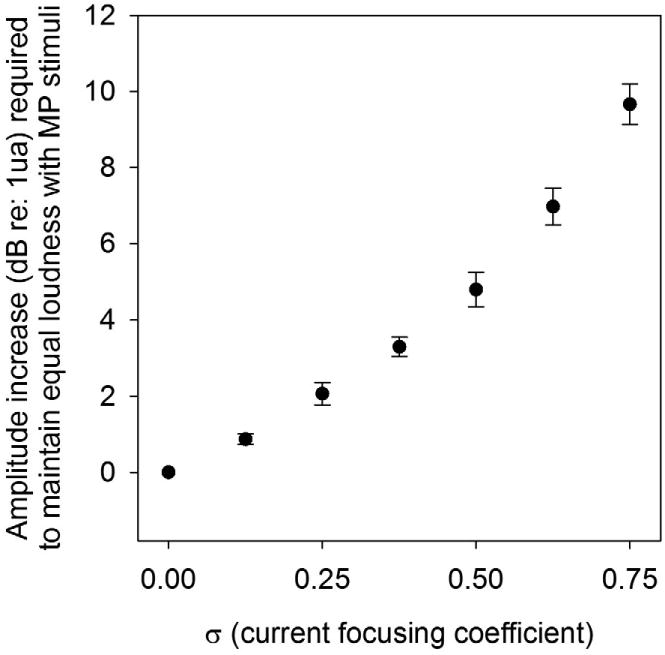
Plot of incremental amount of current (in dB re: 1μa) required for PTP stimuli (of differing σ values) to maintain equal loudness of as a MP (σ = 0.00) stimulus at the “Most Comfortable Level.” Data is averaged across all subjects. Error bars are ± 1 standard error of the mean.
3.2 Experiment 1: Estimating spread of excitation using forward masking
The differences in the spread of excitation for MP and PTPσ = 0.75 were estimated by subtracting unmasked thresholds from the masked thresholds for each of the probe electrodes in μa. The masked threshold curves for each masker (σ = 0, 0.25, 0.5, or 0.75) were normalized to the masked threshold for a probe presented on electrode 9. As expected, the peak of the spread of excitation is typically found within ± 1 electrode from the location of the masker (electrode 9). The area under each forward masking curve was calculated for each subject. The reduction in spread of excitation from current focusing was estimated as the area under the curve for each focused masker (σ = 0.25, 0.5, or 0.75) divided by the area under the curve for the MP masker (σ = 0). For all 6 subjects, the area under the curve was reduced in the σ = 0.75 condition although there was great variability in the reduction of area under the PTPσ = 0.75 curve, ranging from 1% (C3) to 24% (C1). The mean reduction was 12.33%. A repeated-measures one-way ANOVA finds an effect of current focusing coefficient for the masker (F(3,18)=4.56, p<0.015). A post-hoc test using the Holm-Sidak method only detects a significant difference between the σ = 0 and the σ = 0.75 conditions. The raw and normalized forward masked thresholds for each subject for the σ = 0 and σ = 0.75 maskers are presented in Figure 3. Figure 4 shows the reduction in area under the forward masked thresholds curve for all subjects at all 4 current focusing levels. Visual inspection reveals that little to no reduction in current spread is observed for levels of current focusing below σ = 0.75. At σ = 0.75, the reduction is less than 5% for 3 subjects (C3, C8, and C9) and between 19% and 24% for 3 subjects (C1, C4, and C7).
Figure 3.
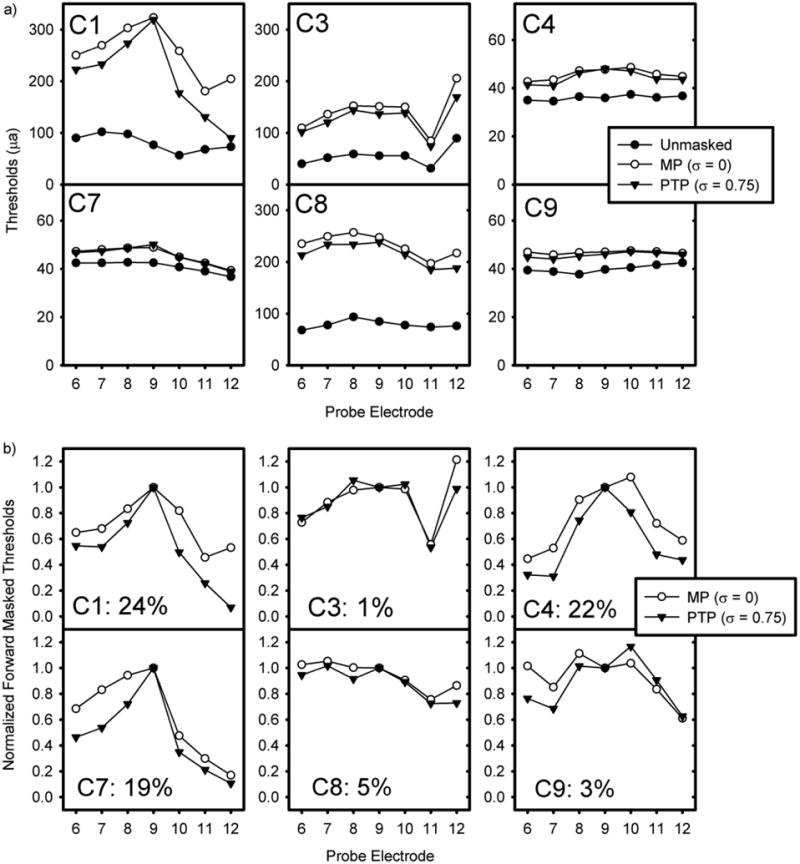
Raw (a) and normalized (b) forward-masking curves for 6 subjects for loudness balanced MP (σ = 0) and PTP (σ = 0.75) maskers in μa. Unmasked thresholds are additionally presented in 3a. For figure 3b, data is normalized to the forward-masked threshold for electrode 9 for both curves. In each box (representing a subject), the percentage reduction of area under the MP (σ = 0) forward masked curve that represents the area under the PTP (σ = 0.75) forward masked curve is presented.
Figure 4.
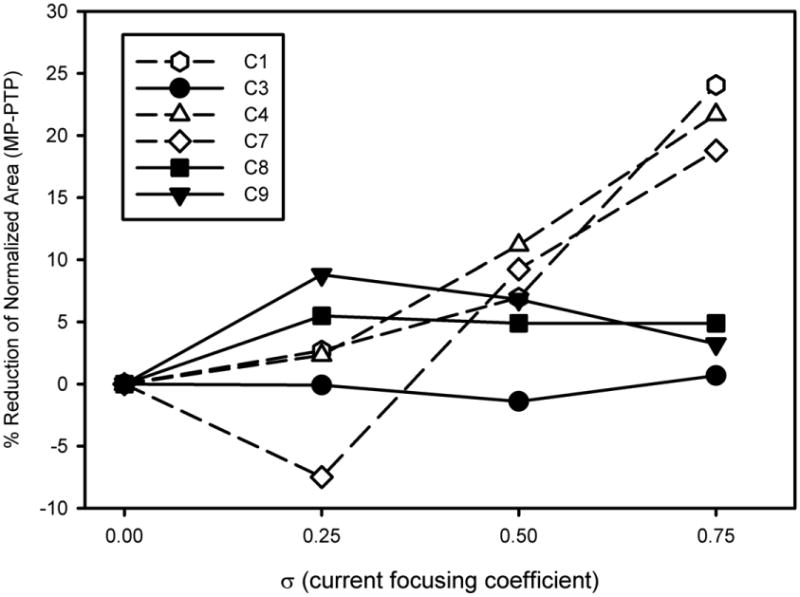
The reduction in area under the MP (σ = 0) forward-masked curves represented by the area under the corresponding PTP curves. Data is plotted as a function of PTP current focusing coefficient for each subject.
3.3 Experiment 2: Discriminating levels of current focusing
Each patient's ability to discriminate adjacent σ values (in σ = 0.25 steps) was calculated. The percentage correct for each adjacent σ value was converted to a d' score based on the tables provided by Hacker and Ratcliff (1979). The d' scores for each interval were summed together to calculate a cumulative d'. The cumulative d' scores are plotted in figure 5 as a function of the reduction in area under the curve between MP and PTPσ = 0.75 stimulation (as calculated for figure 3). A significant correlation between the cumulative d' measurement and the reduction in area under the curves (r2 = 0.84, p = 0.01) suggests that greater reductions in spread of excitation can be predicted by a patient's ability to discriminate between varying degrees of current focusing. Patients for whom there is a cumulative d' below 1.5 had at most a 5% reduction in area between the two curves while patients for whom there was a cumulative d' greater than 2.0 had at least a 19% reduction in area under the forward masked curves.
Figure 5.
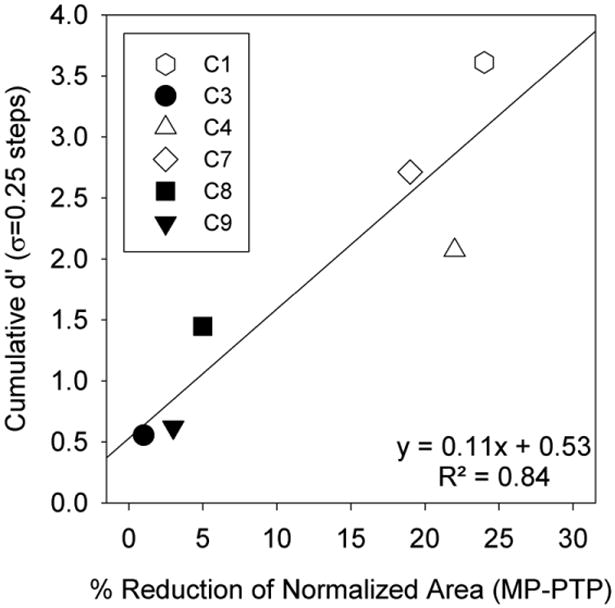
Cumulative d' for discrimination of differing levels of current focusing in σ = 0.25 steps is plotted as a function of the percent reduction in area under the forward masked curves between the MP and PTP (σ = 0.75) maskers.
3.4 Experiment 3: Qualitative ratings of current focusing
All qualitative ratings were scaled from 0 to 1 where 0 corresponded to no agreement with the qualitative description and 1 corresponded to complete agreement with the qualitative description. These scaled ratings were called “agreement scores”. Figure 6 presents the agreement scores for all subjects and adjectives as a function of σ. Typically patients who showed little reduction in spread of excitation in experiment 1 (C3, C8, and C9) provided one pattern of results while the patients who showed at least a 19% reduction in spread of excitation (C1, C4, C7) provided a different pattern of results. Subjects C1, C4, C7 (who were the three subjects with the greatest current reduction) showed a clear pattern of results for the clean/dirty adjective pair. For these subjects, agreement scores suggest that MP (σ = 0) stimuli sound “dirty” and not “clean.” As σ increased, the stimuli sounded more “clean” and less “dirty.” For these subjects, similar patterns were observed for pure/noisy and high/low adjective pairs. Subject C7 finds increasing focusing sound more “thin” and less “full” while C4 reports the opposite. Subjects C1 and C4 consider MP stimuli to be more “kazoo-like” and PTPσ = 0.75 sound more “flute-like.” Subjects C3, C8, and C9, (who were the three subjects with 5% or less reduction in spread of excitation) showed a consistent pattern for all adjectives. Specifically, at a given level of σ, each adjective is rated similarly. Subject C8 rates all 10 adjectives at all focus levels at about 0.5 while subjects C3 and C9 tend to have higher agreement scores for MP stimuli than for focused stimuli, regardless of adjective being scaled.
Figure 6.
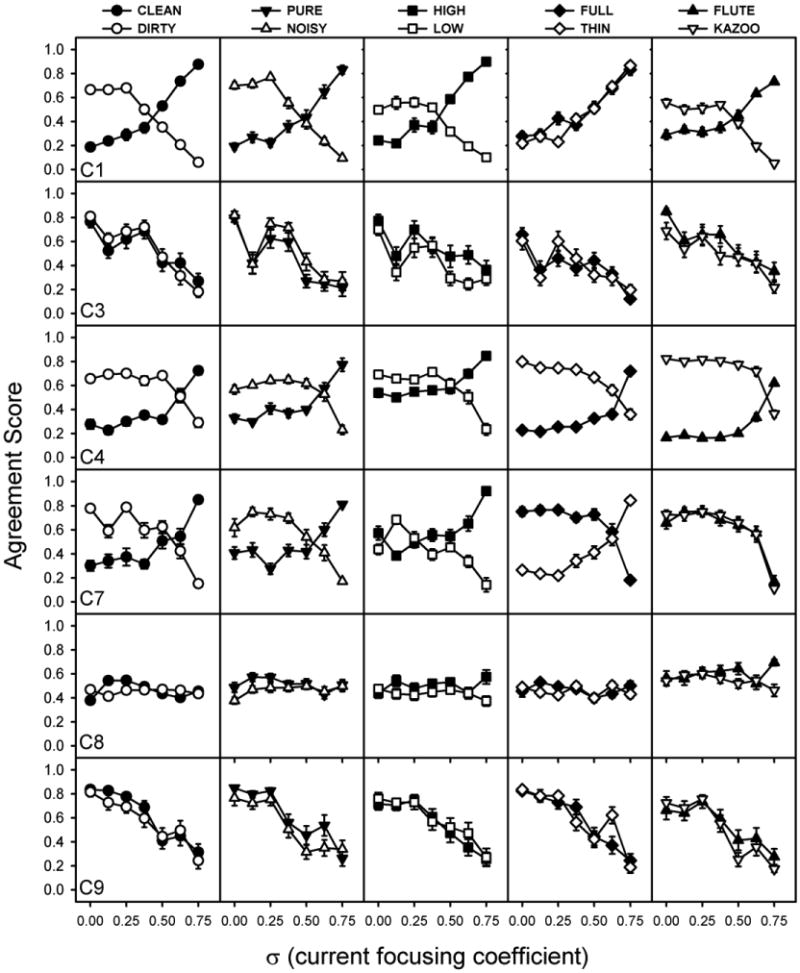
Agreement scores plotted as a function of current focusing coefficient (σ). Each row represents data for an individual subject. Each column represents agreement scores for a set of adjective pairs.
The qualitative scaling task could be simplified to scale only the MP and PTPσ = 0.75 stimuli to make the task more clinically appropriate. The data collected was reanalyzed looking only at scaling data from MP and PTPσ = 0.75. A clean/dirty index score was calculated as the absolute value of the difference in clean and dirty response for σ = 0 and σ = 0.75 (the most and least focused stimuli); see equation 1 for details. A similar index was calculated for Full/Thin, Flute/Kazoo, Pure/Noisy, and High/Low adjective pairs. The various indices were plotted as a function of the reduction in spread of excitation from a MP to a PTPσ = 0.75 masker in figure 7. The figure shows that all subjects for whom there was less than 5% reduction in current had indices of approximately 0 for all 5 adjective pairs. Typically indices were approximately 1 for subjects who had a reduction in spread of excitation greater than 5%. However, subject C7 with a reduction of 19% has a Flute / Kazoo index of 0.129. The Full/Thin index provided no consistent index values for patients with a reduction in current spread more than 5%. Pearson correlations reveal significant relationships between the reduction of current spread and the Clean/Dirty Index (r = -0.944, p < 0.005), the Pure/Noisy Index (r = -0.968, p < 0.002), High/Low Index (r = -0.981, p <0.001), and the Flute/Kazoo Index (r = -0.812, p < 0.05). No significant relationship was detected between the reduction in current spread and the Full/Thin Index (r = -0.005, p > 0.9).
Figure 7.
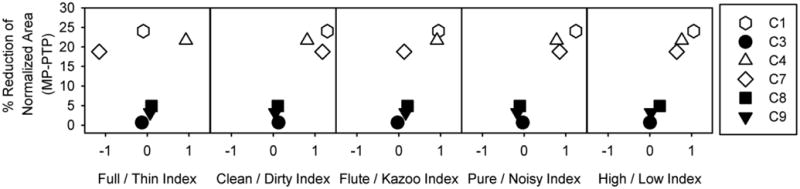
Adjective pair indices plotted as a function reduction in spread of excitation from MP to PTP (σ = 0.75). Adjective pair indices were calculated as the sum of the absolute values of the difference between agreement scores for paired adjectives for σ = 0 and σ = 0.75.
4. Discussion
Results from experiment 1 have shown that at a fixed loudness, PTPσ = 0.75 stimulation reduces the spread of current relative to MP stimulation for some patients but not all. These results are consistent with Bierer and Faulkner (2010). Reducing σ to 0.5 (or 0.25) eliminates any reduction in spread of current. Therefore, if a speech processing strategy is to be implemented using current focusing with the end goal of reducing channel interaction, a current focusing coefficient greater than σ = 0.5 should be used. However, even with PTPσ = 0.75 stimulation, a reduction greater than 5% was observed for roughly only half of the patients. Perhaps a greater current reduction would have been achieved with a greater current focusing coefficient providing either a further reduction in spread of excitation or a reduction in spread of excitation for more patients. In pilot studies, we found that many patients were unable to achieve a full dynamic range with PTPσ = 0.875 before stimulation went beyond device compliance limits and therefore restricted our study to a maximum σ = 0.75.
If current focusing is to be implemented clinically to reduce channel interactions, it is important to know that the patient will actually receive a reduction in spread of excitation with the amount of current focusing used. Measuring spread of excitation forward-masking curves takes too much time to implement clinically. Figure 5 suggests that it may be possible to determine which patients will receive a reduction in current spread simply by asking a patient to discriminate a focused from a non-focused stimulus. An alternative way of predicting which patients actually have a reduced spread of excitation would be through an adjective scaling procedure similar to the one implemented in experiment 3. When examining the relative responses for the MP and PTPσ = 0.75 stimuli (i.e. the pairwise adjective indices plotted in figure 7), the indices were always about 0 for the patients who had at most a 5% reduction in spread of excitation. Typically, the indices were non-zero for patients with greater reductions in spread of excitation, although the consistency of values varied across the different indices. The four patients with the reduced spread of excitation had Clean / Dirty index values clustered around 1. Although a little bit noisier, similar results were also observed for the Pure / Noisy index and High / Low index. It seems likely from this data that scaling the appropriate adjectives could be used to determine quickly (i.e. in a clinical setting) which patients would have a reduced spread of excitation from current focusing and would therefore be strong candidates for a current focused strategy. However, before being able to create a useful clinical test based on a scaling technique, more data needs to be collected both to verify the relationship between a reduced spread of excitation and a patient's scaling index and to determine the optimal adjectives for the test. It is also important to note that unlike the discrimination task previously discussed, this scaling task requires the patient to have an understanding of the sound qualities described by the adjectives used. Bierer and Faulkner (2010) suggested that thresholds might be useful for predicting spread of excitation. However, we found no relationship between PTPσ = 0.75 thresholds and either area under the PTPσ = 0.75 forward masked curve (r2 = 0.09, p = 0.563) or reduction in spread of excitation (r2 = 0.026, p = 0.763).
In the scaling task, subjects C3 and C9 rate focused stimuli as being less in agreement with the adjective than an unfocused stimulus. Because their ratings are the same for each adjective pair (yielding near zero values for their respective indices), the indices correctly suggest that little reduction in spread of excitation is observed for C3 and C9. Nevertheless, these results are surprising because it suggests that patients are able to discriminate levels of current-focusing, even though cumulative d' scores (Figure 5) suggest that they have difficulty with discrimination. In the discrimination task, stimuli were level roved ± 0.6 dB while no level roving was used in the scaling task (as only one stimulus was presented in a trial.) Possibly the difference that is being scaled is mostly masked by the level rove in the discrimination task. Even so, it is surprising that for these patients focusing would simultaneously be considered more clean and more dirty as well as more high and more low.
While we have shown that at a fixed loudness, PTP stimulation can reduce the spread of excitation for some patients, it is still unknown if PTP stimulation can provide better performance in difficult listening situations. Two previous studies (Mens and Berenstein, 2005 and Berenstein et al., 2008) have studied performance with speech processing strategies using PTP stimuli. Mens and Berenstein (2005) compared performance of a MP speech processing strategy with a PTPσ = 0.5 speech processing strategy on monosyllabic word recognition in quiet and in noise. No significant differences were found between the two strategies. However, according to the data collected in experiment 1, as well as physiological data collected by Bonham and Litvak (2008) and Bierer et al. (2010), one would expect the current spread for MP and PTPσ = 0.5 to be very similar and therefore, despite differences in the implementation of the two strategies, one would expect the two strategies to be effectively identical at the neural level. Berenstein et al. (2008) compared performance with a MP and PTP strategy on a spectral ripple task and on monosyllabic phonemes in quiet and noise. Again, no statistical differences were detected between performance with the MP and PTP strategies for the speech test. Of the nine subjects tested, four were tested with a PTP strategy with σ = 0.25 and five were tested with σ = 0.75. Based on the results from experiment 1, we would expect the spread of excitation for the MP and PTP strategies to be effectively identical for the four patients using PTPσ = 0..25. Additionally, we would expect a reduction in current spread for about half of the patients who used the PTPσ = 0.75. Based on these estimates, the MP and PTP strategies would effectively have the same spread of excitation for 6 or 7 of the 9 subjects, and 2 or 3 subjects would have a reduced spread of excitation. Therefore, even if a PTP strategy can increase performance in difficult listening situations, one would not expect to detect any differences in performance between the MP and PTP in the word recognition task. However, despite the combination of PTP stimuli used, a significant difference was detected in spectral ripple discrimination between the MP and PTP strategies. Despite the limited power of only 5 subjects (of which likely a subset had no reduction in spread of excitation), post-hoc tests revealed that patients using PTPσ = 0.75 performed better at the spectral ripple task than when they used the MP strategy. However, the four patients using PTPσ = 0.25 did not perform significantly differently on the spectral ripple task than when they used the MP strategy.
It is worth noting that the reductions in spread of excitation measured on electrode 9 may not be representative of a reduction in spread of excitation across the electrode array. Bierer and Faulkner (2010) demonstrated considerable differences in the widths of psychophysical tuning curves for PTP stimulation across the electrode array. If the reduction in spread of excitation from current focusing is observed only for a subset of electrodes, it is possible that an optimal sound processing strategy would provide current focusing on only a subset of electrodes. Furthermore, this would suggest that our results may only predict which patients would benefit from current focusing on electrode 9 and not which patients would benefit from a strategy implementing current focusing.
Assuming that a reduction in spread of excitation provides better spectral resolution, it is still unknown how much of a reduction in spread is needed to provide a benefit. Similarly, it is unknown if a reduction in current spread is required to be uniform across the entire electrode array, or if certain regions are more important. Nevertheless the results from the experiments presented in this manuscript (as well as previously reported results) are highly encouraging of the possibility that current focusing could produce better spectral resolution for patients for whom current focusing actually provides a narrower spread of excitation. Similarly, the results are promising that a test could be implemented to accurately predict which patients would benefit from a current focusing strategy which could be conducted efficiently enough to be implemented clinically.
| Equation 1 |
Spread of excitation was estimated for equally loud monopoles and tripoles.
Half of the subjects had a reduced spread of excitation with tripolar stimulation.
Half of the subjects had no difference in spread of excitation.
Discrimination between levels of current focusing predict spread of excitation.
Subjective scaling of current focusing predicts reduction in spread of excitation.
Acknowledgments
We thank all of the CI subjects for their time and effort in this research. We also thank John J. Galvin III for editorial assistance.
Abbreviations
- 2IFC
Two Interval Forced Choice
- ACE
Advanced Combination Encoder
- ANOVA
Analysis of Variance
- BEDCS
Bionic Ear Data Collection System
- BP
Bipolar
- CI
Cochlear Implant
- ISI
Inter-Stimulus Interval
- ICC
Inferior Colliculus
- MP
Monopolar
- MPVC
Monopolar Virtual Channel
- NH
Normal Hearing
- PPS
Pulses Per Second
- PTP
Partial Tripolar
- QPVC
Quadrupolar Virtual Channel
- SNR
Signal-to-Noise Ratio
- SOE
Spread of Excitation
- SPEAK
Spectral Peak
- TP
Tripolar
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arenberg JG, Furukawa S, Middlebrooks JC. Auditory cortical images of tones and noise bands. J Assoc Res Otolaryngol. 2000;1:183–94. doi: 10.1007/s101620010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenstein CL. Personal Communication 2007 [Google Scholar]
- Berenstein CK, Mens LHM, Mulder JJS, Vanpouke FJ. Current Steering and Current Focusing in Cochlear Implants: Comparison of Monopolar, Tripolar, and Virtual Channel Electrode Configurations. Ear Hear. 2008;29:250–260. doi: 10.1097/aud.0b013e3181645336. [DOI] [PubMed] [Google Scholar]
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–53. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–92. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–58. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Bierer SM, Middlebrooks JC. Partial tripolar cochlear implant stimulation: Spread of excitation and forward masking in the inferior colliculus. Hear Res. 2010;270:134–42. doi: 10.1016/j.heares.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingabr M, Espinoza-Varas B, Loizou PC. Simulating the effect of spread of excitation in cochlear implants. Hear Res. 2008;241:73–9. doi: 10.1016/j.heares.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham BH, Litvak LM. Current focusing and steering: modeling, physiology, and psychophysics. Hear Res. 2008;242:141–53. doi: 10.1016/j.heares.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briaire JJ, Frijns JH. Field patterns in a 3D tapered spiral model of the electrically stimulated cochlea. Hear Res. 2000;148:18–30. doi: 10.1016/s0378-5955(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Shannon RV. Forward masked excitation patterns in multielectrode electrical stimulation. J Acoust Soc Am. 1998;103:2565–72. doi: 10.1121/1.422777. [DOI] [PubMed] [Google Scholar]
- Filipo R, Mancini P, Ballantyne D, Bosco E, D'Elia C. Short-term study of the effect of speech coding strategy on the auditory performance of pre- and post-lingually deafened adults implanted with the Clarion CII. Acta Otolaryngol. 2004;124:368–70. doi: 10.1080/00016480410016324. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–63. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–96. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Chinchilla S, Nogaki G, Galvin JJ., 3rd Voice gender identification by cochlear implant users: the role of spectral and temporal resolution. J Acoust Soc Am. 2005;118:1711–8. doi: 10.1121/1.1985024. [DOI] [PubMed] [Google Scholar]
- Geurts L, Wouters J. Better place-coding of the fundamental frequency in cochlear implants. J Acoust Soc Am. 2004;115:844–52. doi: 10.1121/1.1642623. [DOI] [PubMed] [Google Scholar]
- Hacker MJ, Ratcliff R. A revised table of d' for M-alternative forced choice. Percept Psychophys. 1979;26:168–170. [Google Scholar]
- Jesteadt W. An adaptive procedure for subjective judgments. Percept Psychophys. 1980;28:85–8. doi: 10.3758/bf03204321. [DOI] [PubMed] [Google Scholar]
- Jolly CN, Spelman FA, Clopton BM. Quadrupolar stimulation for Cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng. 1996;43:857–65. doi: 10.1109/10.508549. [DOI] [PubMed] [Google Scholar]
- Koch DB, Downing M, Osberger MJ, Litvak L. Using current steering to increase spectral resolution in CII and HiRes 90K users. Ear Hear. 2007;28:38S–41S. doi: 10.1097/AUD.0b013e31803150de. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Kwon BJ, van den Honert C. Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners. J Acoust Soc Am. 2006;119:2994–3002. doi: 10.1121/1.2184128. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Srinivasan AG. Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hear Res. 2009;254:34–41. doi: 10.1016/j.heares.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49 2:467+. [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Emadi G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am. 2007;122:967–81. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- Marzalek MS, Dorman MF, Spahr AJ, Litvak LM. Effects of Multi-Electrode Stimulation on Tone Perception: Modeling and Outcomes. CIAP. 20072007 [Google Scholar]
- McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J Acoust Soc Am. 1994;96:155–62. doi: 10.1121/1.410475. [DOI] [PubMed] [Google Scholar]
- Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: a crossover study. Otol Neurotol. 2005;26:957–64. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Franck KH, Xu L, Bauer EM, Zwolan TA. Effects of electrode configuration and place of stimulation on speech perception with cochlear prostheses. J Assoc Res Otolaryngol. 2001;2:87–103. doi: 10.1007/s101620010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji AA. Personal Communication 2007 [Google Scholar]
- Shannon RV, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol Suppl. 2004:50–4. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Clopton BM, Jolly CN, Rodenhiser KL. Effects of electrical current configuration on potential fields in the electrically stimulated cochlea: field models and measurements. Ann Otol Rhinol Laryngol Suppl. 1995;166:131–6. [PubMed] [Google Scholar]
- Srinivasan AG, Landsberger DM, Shannon RV. Current focusing sharpens local peaks of excitation in cochlear implant stimulation. Hear Res. 2010;270:89–100. doi: 10.1016/j.heares.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–24. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]