Abstract
The click-evoked auditory brainstem response (ABR) is widely used in clinical settings, partly due to its predictability and high test-retest consistency. More recently, the speech-evoked ABR has been used to evaluate subcortical processing of complex signals, allowing for the objective assessment of biological processes underlying auditory function and auditory processing deficits not revealed by responses to clicks. Test-retest reliability of some components of speech-evoked ABRs has been shown for adults and children over the course of months. However, a systematic study of the consistency of the speech-evoked brainstem response in school-age children has not been conducted. In the present study, speech-evoked ABRs were collected from 26 typically-developing children (ages 8-13) at two time points separated by one year. ABRs were collected for /da/ presented in quiet and in a 6-talker babble background noise. Test-retest consistency of response timing, spectral encoding, and signal-to-noise ratio was assessed. Response timing and spectral encoding were highly replicable over the course of one year. The consistency of response timing and spectral encoding found for the speech-evoked ABRs of typically-developing children suggests that the speech-evoked ABR may be a unique tool for research and clinical assessment of auditory function, particularly with respect to auditory-based communication skills.
Keywords: electrophysiology, auditory brainstem, speech, children, test-retest reliability
1. Introduction
The auditory brainstem response (ABR) is a far-field measure that reflects the synchronous neural activity generated by nuclei along the brainstem in response to an acoustic signal. For many reasons, including the high replicability of the response, the click-evoked ABR has been widely used as a clinical measure of auditory thresholds and neural integrity (Hall, 2006; Hood, 1998; Sininger, 2007). Within an individual, the variability in response timing (peak latencies) from one test to another is small, even when the tests are conducted months apart (Edwards et al., 1982; Lauter et al., 1992; Tusa et al., 1994). Response variability for both adults and children is higher across individuals than within individuals (Edwards et al., 1982; Lauter et al., 1992). The replicability and precise temporal resolution of the click-evoked ABR is such that changes in the absolute latencies from test to retest on the order of fractions of a millisecond are considered clinically significant (Hall, 2006). In children, the click-evoked response is mature and adult-like by the age of two years (Hood, 1998; Ponton et al., 2000; Salamy, 1984; Sininger, 2007), although there are additional maturational changes that occur throughout childhood such as decreases in response amplitude and response variability (Issa et al., 1995; Lauter et al., 1992; Ponton et al., 2000; Salamy, 1984).
ABRs can also be elicited by more complex stimuli such as speech or music. Similar to the click-evoked ABR, the speech-evoked ABR is highly replicable in young adults (Song et al., 2011). Because the speech-evoked ABR appears to be mature by the age of five (Johnson et al., 2008), it is also likely to be replicable in school-age children. ABRs to more complex stimuli, such as speech or music, retain both the temporal and spectral characteristics of the evoking sound with remarkable fidelity (Russo et al., 2004; Skoe et al., 2010), requiring precise neural phase-locking and synchronous responses to transient stimuli.
While the test-retest reliability of speech-ABR measures has been established in adults (Song et al., 2011), a systematic study of the test-retest reliability of speech-evoked brainstem responses in typically-developing children has not been conducted. Some response measures appear to be stable in children with a range of neurodevelopmental disorders (Russo et al., 2005; Russo et al., 2010), however the test-retest interval was short, on average five months, and response components assessed did not include measures of response timing and spectral encoding recently shown to relate to auditory-based communication skills (Anderson et al., 2010a; Anderson et al., 2010b; Banai et al., 2009; Hornickel et al., 2011; Hornickel et al., 2009; Wible et al., 2004). If the speech-evoked ABR is to be used to investigate and clinically evaluate auditory function and possibly serve as an objective metric of experience-dependent change, the reliability of speech-evoked ABR measures needs to be systematically assessed in school-age children.
The present study investigates the test-retest reliability of speech-evoked auditory brainstem response measures of timing, spectral encoding, and within-session consistency in typically-developing children. Importantly, these measures, particularly response timing, have been linked to auditory communication skills such as reading and listening to speech in noisy environments (Anderson et al., 2010a; Anderson et al., 2010b; Banai et al., 2009; Hornickel et al., 2011; Hornickel et al., 2009; Wible et al., 2004). Given that both the click- and speech-evoked ABRs are thought to be mature by five years of age (Johnson et al., 2008; Salamy, 1984), we do not expect to see a change in brainstem response properties over a 12 month time interval in typically-developing children ages eight to thirteen years old. By demonstrating the consistency of these measures, we can establish the stability of the speech-evoked brainstem response and provide norms for test-retest change in typically-developing children which can be used to assess auditory function and evaluate change in groups undergoing training or receiving intervention.
2. Materials and Methods
2.1 Participants
Participants were 26 typically-developing children, ages 8-13 years (mean = 10.5, 12 girls). All children were recruited from the Chicago area. All participants had normal hearing defined as air conduction thresholds < 20 dB HL for octaves from 250-8000 Hz with air-bone threshold gaps < 10 dB for octaves 500-4000 Hz, normal click-evoked brainstem responses (100 s stimulus presented at 31.3 Hz and 80 dB SPL), full-scale IQ scores ≥ 85 on the Wechsler Abbreviated Scale of Intelligence (Woerner et al., 1999), no current or prior neurological disorders, no family history of learning impairments, scores ≥ 95 on the Test of Word Reading Efficiency (Torgensen et al., 1999), and were not receiving special services in school. The participants were tested during two sessions (Year 1 and Year 2) that occurred an average of 11.9 months apart (SD = 1.85). All procedures were approved by the Northwestern University Institutional Review Board and complied with the Declaration of Helsinki, and the children and their parents gave informed assent and consent, respectively. Due to the corruption of data files, one participant’s response in background noise from Year 1 was lost. This participant was excluded from the test-retest analyses of the response measures for /da/ presented in noise.
2.2 Electrophysiological Stimuli and Recording Parameters
The six-formant speech stimulus /da/ was 170 ms in length (50 ms formant transition and 120 ms steady-state vowel) with a stable fundamental frequency (100 Hz) and fourth (3300 Hz), fifth (3750 Hz), and sixth (4900 Hz) formants. During the formant transition period, the first, second, and third formants were dynamic, rising from 400 to 720 Hz, falling from 1700 to 1240 Hz, and falling from 2580 to 2500 Hz, respectively. The /da/ stimulus was presented in quiet and in the presence of six-talker babble background noise. The six-talker babble was made up of four female and two male voices speaking grammatically correct but nonsensical sentences, created in Cool Edit Pro, Version 2.1 (Syntrillium Software, 2003). The babble track was 4.7 s long with the signal-to-noise ratio set at +10 dB based on the root mean square amplitude of the entire track. The /da/ stimulus was synthesized using KLATT (Klatt, 1980).
Stimuli of alternating polarity were presented with a 60 ms interstimulus interval at 80 dB SPL through an insert earphone (ER-3, Etymotic Research) to the right ear using the stimulus presentation software Neuroscan Stim 2 (Compumedics). Responses were recorded with a vertical electrode montage (active Cz, forehead ground, and ipsilateral earlobe reference) using Neuroscan Acquire 4.3 (Neuroscan Scan, Compumedics). Responses were collected at a sampling rate of 20 kHz. During electrophysiological recording, participants watched a movie of their choice while seated in a comfortable chair. Participants were able to hear the soundtrack of the movie, played at <40 dB SPL in the testing booth, through their unoccluded left ear. Allowing participants to view a movie during testing encouraged participant cooperation in sitting quietly and relaxed for the testing session.
2.3 Data processing and analyses
All data processing and analysis techniques replicated previously published studies (Anderson et al., 2010a; Song et al., 2011). Responses were bandpass filtered from 70-2000 Hz (12 dB/octave roll-off) and broken into 230 ms analysis windows (40 ms of pre-stimulus activity). Trials with amplitude greater than ± 35 μV were excluded. Responses to individual polarities were averaged across the first and second halves of the recording independently and also across the entire recording and then added to form two replications of 3000 sweeps (1500 of each polarity) and one final average of 6000 sweeps (3000 of each polarity). The addition of responses to alternating polarities eliminates the cochlear microphonic and reduces the impact of stimulus artifact (which we did not observe as the use of tube-insert earphones, alternating polarities, and common mode referencing effectively minimizes stimulus artifact; (Aiken et al., 2008; Campbell et al., in press; Gorga et al., 1985).
2.3.1 Response Timing
2.3.1.1 Response Latencies
Response peaks within the region corresponding to the onset and formant transition of the stimulus (0-60 ms) were manually identified for responses to /da/ presented in quiet and in noise. These peaks included the onset response (p9 and p10) and the peaks and troughs mimicking the glottal pulse of the stimulus occurring approximately every 10 ms (p42, p43, p52, p53). Peak and trough names were determined by their approximate latencies in milliseconds. Response peaks and troughs occurring at approximately 22, 23, 32, and 33 ms were excluded from the analysis due to their limited reliability in the noise responses. Manual identification of the peaks and troughs was conducted by the first author for both Year 1 and Year 2.
2.3.1.2 Phase Shift
Replicability of response timing was further evaluated through comparisons of response phase. Using the cross-phaseogram methods of Skoe and colleagues, we assessed the difference in phase between responses in quiet and those in noise (Skoe et al., 2011). Because the response in quiet was expected to occur earlier than the response in noise (Anderson et al., 2010a; Song et al., 2011), the response in noise was predicted to phase lag the response in quiet. Inspection of the difference in phase between the responses over frequency and across time and an a priori hypothesis that differences might be largest for lower harmonics (Anderson et al., 2010b; Song et al., 2010) revealed that the largest phase differences between quiet and noise in Year 1 were found in the formant transition region of the response (20-60 ms) in the low harmonics (0-200 Hz). The average phase difference between responses in quiet and those in noise was calculated over this region for Year 1 and Year 2.
2.3.2 Within-Session Consistency
The consistency of the response within a recording session was determined by calculating the straight correlation between the two 3000 sweep replications generated for each recording (/da/ in quiet and /da/ in noise) over the entire response (0-180ms). The resulting correlation coefficients were Fisher transformed prior to statistical analyses. Correlations reported were converted to r-values after statistical analyses.
2.3.3 Across-Year Consistency
Similar to the within-session consistency measure, the across-year consistency measure was calculated as the straight correlation of an individual’s response from Year 1 with their response from Year 2 for both /da/ in quiet and /da/ in noise over the entire response (0-180ms). The resulting correlation coefficients were Fisher transformed prior to averaging across the group. Correlations reported were converted to r-values after statistical analyses.
2.3.4 Response Amplitude
2.3.4.1 Signal-to-Noise Ratio
Root mean square amplitude over the entire response (0-180 ms) served as a measure of overall response amplitude for both /da/ in quiet and /da/ in noise. Signal-to-noise ratios were calculated by dividing these overall response amplitudes by the amplitudes calculated over the pre-stimulus period (−40-0 ms) for each response. We elected not to include an analysis of raw response amplitude because it is known to be unreliable in clinical evaluations of subcortical function and may be influenced by a number of subject factors that are not related to maturation of the nervous system, such as head size (Ponton et al., 2000; Salamy, 1984).
2.3.4.2 Spectral Amplitudes of Responses
Spectral amplitudes of responses to /da/ in quiet and in noise were calculated using Fast Fourier Transforms conducted over the formant transition region of the response (20-60ms) and averaging over 40 Hz wide bins centered at the fundamental frequency (F0) and its integer harmonics through 1000 Hz (i.e., harmonics 2-10).
2.4 Statistics
Multivariate Repeated Measures ANOVAs were used for the analysis of response latencies and spectral amplitudes, while Repeated Measures ANOVAs were used for the analysis of signal-to-noise ratio and within-session consistency. In all cases, time (Year 1, Year 2) and condition (quiet, noise) served as within-subjects factors and main effects and interactions are reported. Follow-up paired t-tests were used when appropriate and for the comparison of the phase shift between responses. Because we hypothesized little change in response measures from Year 1 to Year 2, a conservative approach was to let α equal 0.05, allowing the greatest possibility of finding a significant result (contrary to our hypothesis). Spearman’s correlations were also conducted between Year 1 and Year 2 to assess the reliability of the response measures.
3. Results
Speech-evoked brainstem response measures were highly replicable over one year. No change was found for any measure from Year 1 to Year 2 (see Table 1). As would be predicted, a main effect of condition was found for all analyses, with responses to /da/ in quiet occurring earlier and being larger than responses to /da/ in noise. Because test-retest effects were the focus of the manuscript, these main effects of condition will not be discussed.
Table 1.
Means and standard deviations for response measures for Year 1, Year 2, and the test-retest change (computed as Year 2 minus Year 1) and reliability of measures (Year 1 correlated with Year 2). Measures that showed a significant change from Year 1 to Year 2 or a significant reliability correlation coefficient are bolded (p < 0.05).
| Year 1 | Year 2 | Change | Reliability | |
|---|---|---|---|---|
| Timing | Mean (Standard Deviation) |
Spearman’s rho | ||
| Response Latencies in Quiet (ms) | ||||
| Peak 9 | 9.165 (0.48) | 9.196 (0.37) | 0.031 (0.62) | 0.123 |
| Trough 10 | 10.235 (0.60) | 10.279 (0.48) | 0.044 (0.79) | 0.139 |
| Peak 42 | 42.844 (0.25) | 43.054 (0.47) | 0.210 (0.46) | 0.565 |
| Trough 43 | 44.402 (0.75) | 44.285 (0.71) | −0.117 (0.72) | 0.456 |
| Peak 52 | 52.889 (0.22) | 52.985 (0.29) | 0.096 (0.30) | 0.473 |
| Trough 53 | 54.004 (0.30) | 54.112 (0.32) | 0.108 (0.29) | 0.484 |
| Response Latencies in Noise (ms) | ||||
| Peak 9 | 9.914 (0.65) | 9.906 (0.64) | −0.002 (0.99) | −0.185 |
| Trough 10 | 10.748 (0.61) | 10.712 (0.73) | −0.024 (1.01) | −0.154 |
| Peak 42 | 43.682 (0.76) | 43.810 (0.83) | 0.096 (0.73) | 0.566 |
| Trough 43 | 45.050 (0.86) | 45.298 (0.84) | 0.202 (0.86) | 0.401 |
| Peak 52 | 53.142 (0.47) | 53.265 (0.58) | 0.054 (0.54) | 0.590 |
| Trough 53 | 54.284 (0.66) | 54.415 (0.82) | 0.094 (0.92) | 0.305 |
| Quiet-to-Noise Phase Shift (π radians) | ||||
| Low Harmonics | 0.531 (0.39) | 0.481 (0.43) | −0.041 (0.43) | 0.355 |
|
| ||||
| Within-Session Replicability ( r ) | ||||
|
| ||||
| Quiet | 0.724 (0.30) | 0.707 (0.29) | −0.035 (0.23) | 0.664 |
| Noise | 0.534 (0.30) | 0.591 (0.29) | 0.086 (0.24) | 0.667 |
|
| ||||
| Amplitude (signal-to-noise ratio) | ||||
| Quiet SNR | 2.54 (0.63) | 2.37 (0.66) | −0.171 (0.65) | 0.752 |
| Noise SNR | 1.92 (0.54) | 1.96 (0.56) | 0.058 (0.49) | 0.601 |
|
| ||||
| Spectral Encoding (μV) | ||||
|
| ||||
| Quiet | ||||
| F0 | 0.1126 (0.036) | 0.1030 (0.041) | −0.0112 (0.024) | 0.815 |
| H2 | 0.0614 (0.023) | 0.0565 (0.022) | −0.0048 (0.017) | 0.662 |
| H3 | 0.0275 (0.113) | 0.0252 (0.012) | −0.0024 (0.014) | 0.319 |
| H4 | 0.0192 (0.010) | 0.0154 (0.007) | −0.0036 (0.011) | 0.339 |
| H5 | 0.0213 (0.010) | 0.0193 (0.009) | −0.0024 (0.009) | 0.586 |
| H6 | 0.0126 (0.007) | 0.0116 (0.006) | −0.0012 (0.007) | 0.510 |
| H7 | 0.0089 (0.006) | 0.0083 (0.005) | −0.0012 (0.007) | 0.740 |
| H8 | 0.0056 (0.003) | 0.0050 (0.002) | 0.0004 (0.008) | 0.202 |
| H9 | 0.0058 (0.003) | 0.0060 (0.004) | 0.0004 (0.005) | 0.540 |
| H10 | 0.005 (0.005) | 0.0052 (0.004) | 1.39e-19 (0.006) | 0.358 |
| Noise | ||||
| F0 | 0.0617 (0.024) | 0.0689 (0.027) | 0.0029 (0.022) | 0.656 |
| H2 | 0.0374 (0.022) | 0.0392 (0.020) | 0.0013 (0.028) | 0.231 |
| H3 | 0.0189 (0.010) | 0.0165 (0.008) | −0.0025 (0.015) | −0.195 |
| H4 | 0.0163 (0.009) | 0.0142 (0.006) | −0.0013 (0.014) | −0.117 |
| H5 | 0.0129 (0.008) | 0.0107 (0.006) | −0.0013 (0.007) | 0.328 |
| H6 | 0.0081 (0.005) | 0.0076 (0.003) | 0.0004 (0.005) | 0.429 |
| H7 | 0.0052 (0.003) | 0.0052 (0.003) | −0.0008 (0.006) | 0.336 |
| H8 | 0.0038 (0.002) | 0.0041 (0.002) | 0.0013 (0.005) | 0.142 |
| H9 | 0.0053 (0.003) | 0.0043 (0.002) | −0.0008 (0.005) | 0.511 |
| H10 | 0.0044 (0.004) | 0.0039 (0.002) | −0.0008 (0.005) | 0.598 |
3.1 Consistency of response timing
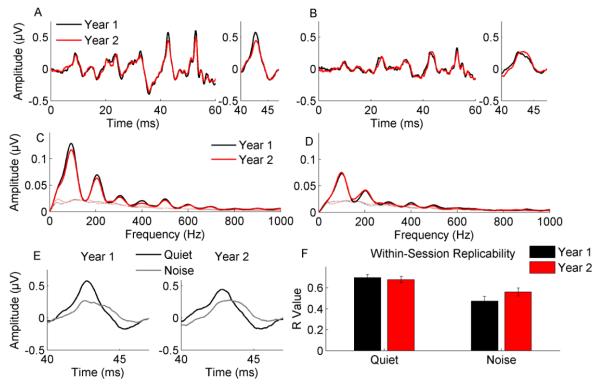
From Year 1 to Year 2, there was no difference in response latencies in quiet and noise (F6,19 = 0.875, p > 0.50) and no interaction between condition and time (F6,19 = 0.705, p > 0.65), indicating that there is no change in the quiet-to-noise timing shift of response peaks over the course of one year of growth (see Figure 1A, B, and E and Table 1).
Figure 1. Speech-evoked brainstem response timing and spectral encoding is stable over the course of one year in school-age children.
Response peak latencies during the formant transition region (0-60 ms) do not change over the course of one year for responses to /da/ presented in quiet (A) or /da/ presented in noise (B). See insets for greater detail. The magnitude of the timing shift between responses in quiet and responses in noise, with responses in noise occurring later, is also stable (E). Spectral amplitudes of responses to /da/ in quiet (C) and /da/ in noise (D) also do not change over the course of one year. Additionally the consistency of the responses within the recording session is stable over one year of growth (F). The predicted main effect of condition was replicated for all measures, with responses in quiet being larger, earlier, and more consistent than responses in noise (A-F). Note: Year 1 is marked by black and Year 2 by red for all plots except (E) for which responses in quiet are marked by black and responses in noise by gray. Noise floors for plots C and D are marked by hashed lines.
The lack of an interaction between time and condition was further supported by a lack of change from Year 1 to Year 2 in average response phase lag between responses to /da/ in quiet and /da/ in noise during the formant transition across the low harmonics (t24 = 0.475, p > 0.60; see Table 1).
3.2 Consistency of responses within the recording session and across years
The within-session consistency of the responses did not change from Year 1 to Year 2. Correlation strength between responses from the first half of the recording and those from the second half in Year 2 was not different from the split-half correlation of responses in Year 1 for the responses to /da/ in quiet and noise (F1,24 = 0.769, p > 0.389; see Figure 1F and Table 1). Additionally there was no interaction between time and condition (F1,24 = 3.055, p > 0.05).
The across-year consistency correlations were 0.799 (SD = 0.29) for responses to /da/ in quiet and 0.689 (SD = 0.28) for responses to /da/ in noise, suggesting that overall the responses from the two years were very similar.
3.3 Signal-to-noise ratio
There was no difference from Year 1 to Year 2 in the signal-to-noise ratio of the response for responses in quiet or in noise (F1,24 = 0.426, p > 0.50) or interaction between time and condition (F1,24 = 2.417, p > 0.13). Although electrophysiological amplitudes are known to decrease with age (Ponton et al., 2000; Salamy, 1984), there is no indication of any change in response robustness as assessed by the signal-to-noise ratio.
3.4 Stability of frequency representation
For responses to /da/ in quiet and in noise, there was no change in spectral magnitudes from Year 1 to Year 2 (F10,15 = 1.175, p > 0.35) and no significant interaction of condition and time (F10,15 = 1.199, p > 0.35; see Figure 1C and D and Table 1).
3.5 Reliability of response metrics
In order to assess reliability of the measures discussed here, Spearman’s correlations were conducted between Year 1 and Year 2 values (see Table 1). Reliability estimates were generally good, although not for every measure, and appear to be larger for metrics that were automated (signal-to-noise ratio, spectral amplitudes, and within-session response replicability).
4. Discussion
The timing and spectral amplitudes of speech-evoked auditory brainstem response measures are remarkably consistent over one year of growth in school-age children. The stability and consistency of speech-evoked auditory brainstem responses is clinically and theoretically important because response measures such as these have been linked to learning and communication skills in children and been shown to be malleable with short-term and life-long experience with sound (Anderson et al., 2010a; Banai et al., 2009; Hornickel et al., 2011; Krishnan et al., 2005; Russo et al., 2010; Song et al., in press; Wong et al., 2007). The results here suggest that speech-evoked ABRs are a possible tool for clinical and research assessment of auditory-based communication skills and experience-dependent neuroplasticity.
The clinically-viable reliability of the click-evoked ABR is well established (Edwards et al., 1982; Hood, 1998; Lauter et al., 1992; Musiek et al., 2007; Sininger, 2007; Tusa et al., 1994). The highly replicable, consistent response pattern evoked by click stimuli and characteristic response changes with alterations of intensity or presentation rate underlies the use of the click-evoked brainstem response in infant hearing screening and assessments of central auditory function (Hall, 2006; Hood, 1998; Musiek et al., 2007; Sininger, 2007). The reliability and replicability of ABRs to speech has been shown in adults (Song et al., 2011) and here we confirm a similar consistency of responses in children over one year of growth.
Song and colleagues (Song et al., 2011) assessed retest reliability over the course of three months in adults and reported that response measures such as overall amplitude, signal-to-noise ratio, response peak latencies, and spectral amplitudes were stable. The reliability coefficients of these measures were robust (Song et al., in press). Other work investigating training-related improvements in children suggested that responses from children are replicable and consistent, due to the lack of significant change in control groups from pre-test to post-test (Russo et al., 2005), similar to reliability shown in adults (Carcagno et al., 2011; Song et al., 2011). The present study expanded those results to a larger set of response measures and found similar replicability in children as in adults. Importantly, unlike the previous training studies, the test-retest period in the current study was an entire calendar year on average, which increased the chances of detecting maturational or experiential change if it occurred. In the current study no changes in response timing measures or in spectral encoding were found over the course of one year. The consistency of response timing and spectral measures over one year of growth in the school-age years suggests that speech-evoked brainstem responses are at a maturational plateau in this age group, a theory that is supported by developmental studies.
The click-evoked ABR matures quickly, reaching adult-like response morphology at approximately two years of age (Hood, 1998; Salamy, 1984; Sininger, 2007). That the click-evoked response is measurable in neonates suggests that the basic neural structure of the auditory pathway is established by birth, congruent with the onset of hearing at approximately 20 weeks in utero (Graven et al., 2008), and supports the use of click-evoked responses to detect peripheral auditory function and nervous system pathologies (Hall, 2006; Hood, 1998; Sininger, 2007). The speech-evoked ABR, on the other hand, does not appear to mature until at least age five. Speech-evoked ABRs of five-year-old children are not significantly different than responses from school-age children (8-12 years old) while responses of children who are three and four years old differ in response morphology and timing (Johnson et al., 2008). The slower maturation of speech-evoked responses may be due to the acoustic complexity of speech relative to click stimuli, requiring more continuous and synchronous phasic activity from a variety of neural nuclei. Within-subject variability in click-evoked responses is greatest for young children (5-7 years old), reduced for older children (10-12 years old), and least for adults (Lauter et al., 1992), suggesting that neural synchrony and consistency is increasing during the same period that speech-evoked responses are maturing.
In addition to demonstrating that speech-evoked ABR measures do not significantly differ over the course of one year we estimated the reliability of these measures. In general reliability estimates were adequate, although reliability was sometimes low for individual response peaks or spectral amplitudes. Reliability coefficients above 0.7-0.8 are typically preferred (Nunnally, 1959) and were found for speech-evoked auditory brainstem response measures collected in adults (Song et al., in press). As stated above, there is evidence that the variability of auditory brainstem responses decreases with age (Lauter et al., 1992) and the weaker reliability estimates observed here relative to previous estimates in adults may be due to slightly greater response variability in this group of school-age subjects. Additionally, the test-retest period is one full year in the present study which is substantially longer than test-retest periods of months or days in previous studies showing speech-evoked response reliability (Russo et al., 2004; Song et al., 2011; Song et al., in press), click-evoked response reliability (Edwards et al., 1982; Lauter et al., 1992), and test-retest reliability for well-utilized behavioral assessments of learning and achievement (McGrew et al., 2001; Torgesen et al., 1999; Wagner et al., 1999). Click-evoked response reliability is weaker for responses collected two years apart than responses collected on the same day (Tusa et al., 1994) and it is very likely that the reliability estimates in the present study would be higher if the test-retest interval were shorter.
Altering stimulus and recording parameters, such as stimulus presentation rate and response sampling rate, may also lead to enhancements of reliability. While the fast sampling rate used here allows for finer temporal resolution, it also reveals the effects of very subtle differences in external factors, such as slight differences in electrode impedance. Additionally, the extremely high temporal resolution may impact the subjective peak picking analyses by reflecting these subtle differences in external factors that can impact response morphology. If speech-evoked ABRs are to be used for clinical diagnoses, manipulations of the stimulus and recording paradigms should be made to maximize reliability and objective response metrics should be relied upon as the key measures.
Despite the long test-retest interval and potentially greater variability in responses due to age and external factors, no changes were seen in response timing and spectral encoding over the course of one year of growth, highlighting that within-subject variability is minimal and responses are consistent from test to retest. Additionally, the straight correlations between responses from Year 1 and Year 2 were on average 0.7 and higher for both /da/ collected in quiet and /da/ collected in noise, indicating that the responses themselves were highly consistent across years and lower reliabilities seen for some of the reported measures, such as peak latencies, may be due to the more subjective nature of the analyses. It is important to note that automated response measures, particularly spectral magnitudes and within-session consistency, appear to be more reliable than measures requiring manual identification of response peaks, suggesting they may be preferred in a clinical setting.
The lack of test-retest changes found here strengthens the notion that changes in neural function following auditory training in school-age children reflect fundamental changes in sensory processing associated with meaningful engagement with sound, and not test-retest factors. Although auditory brainstem responses are known to be malleable with meaningful or impaired interaction with sound (Anderson et al., 2010a; Anderson et al., 2010b; Banai et al., 2009; Hornickel et al., 2011; Hornickel et al., 2009; Kraus et al., 2010; Krishnan et al., 2005; Musacchia et al., 2007; Wible et al., 2004; Wong et al., 2007; Xu et al., 2006), in the absence of such active engagement they are expected to be stable. If positive changes are found in the speech-evoked response measures included here, which have been linked to auditory-based communication skills (Anderson et al., 2010a; Banai et al., 2009; Hornickel et al., 2011), it is possible that behavioral improvements may follow. Additionally, these results suggest that differences in speech-evoked response measures between groups of school-age children, for example good and poor readers, are not due to a difference in maturational differences or response inconsistencies. The observed consistency of the speech-evoked ABR during the school-age years and repeat evidence of relationships between response measures and behavioral measures of communication skills suggests that speech-evoked brainstem responses could possibly serve as a unique metric of biological correlates of auditory-based communication skills and experience-dependent modulation of auditory function.
4.1 Conclusion
The speech-evoked brainstem response is replicable and consistent over one year of growth in typically-developing children. These results mirror recent analyses of test-retest reliability of speech-evoked brainstem response measures in adults showing that measures of response timing and frequency representation are reliable across months. Our expansion of these results to typically-developing children assessed over one year supports a theory that speech-evoked brainstem responses are at a maturational plateau in this age group and may contribute unique information for research and clinical assessment of auditory processing within groups of school-age children. Future studies should investigate the consistency of these response measures in children with auditory-based communication and/or learning deficits to determine if children with impairments show a similar response consistency as seen in typically-developing children or whether response variability is a biological hallmark of clinical impairments.
Speech-evoked auditory brainstem responses (ABR) reflect complex acoustics.
Speech-ABR timing and spectra are consistent over one year of growth in children.
Speech-ABR may be unique index of auditory and communication skills in children.
Acknowledgements
This work was supported by the National Institutes of Health (R01DC01510) and the Hugh Knowles Center of Northwestern University. The authors would like to thank Steven Zecker for his advisement, Dana Strait, Samira Anderson, and Trent Nicol for their review of the manuscript and the children and their families for participating.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: JH developed the study design, collected and processed the data, analyzed and interpreted the data, and prepared the manuscript. EK analyzed and interpreted the data and prepared the manuscript. NK developed the study design and prepared the manuscript. None of the authors have any conflicts of interest, financial or otherwise.
References
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear. Res. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. J. Neurosci. 2010a;30:4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Zecker SG, Kraus N. Brainstem correlates of speech-in-noise perception in children. Hear. Res. 2010b;270:151–157. doi: 10.1016/j.heares.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai K, Hornickel J, Skoe E, Nicol T, Zecker SG, Kraus N. Reading and subcortical auditory function. Cerebral Cortex. 2009;19:2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T, Kerlin JR, Bishop CW, Miller LM. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear and Hearing. doi: 10.1097/AUD.0b013e3182280353. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcagno S, Plack CJ. Subcortical plasticity following perceptual learning in a pitch discrimination task. J. Assoc. Res. Otolaryngol. 2011;12:89–100. doi: 10.1007/s10162-010-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RM, Buchwald JS, Tanguay PE, Schwafel JA. Sources of variability in auditory brain stem evoked potential measures over time. Electroencephalogr. Clin. Neurophysiol. 1982;53:125–132. doi: 10.1016/0013-4694(82)90018-9. [DOI] [PubMed] [Google Scholar]
- Gorga M, Abbas P, Worthington D, Jacobsen J. The Auditory Brainstem Response. College-Hill Press; San Diego: 1985. Stimulus calibration in ABR measurements; pp. 49–62. [Google Scholar]
- Graven SN, Browne JV. Auditory development in the fetus and infant. Newborn and Infant Nursing Reviews. 2008;8:187–193. [Google Scholar]
- Hall JW. New Handbook of Auditory Evoked Responses. Allyn & Bacon; Boston, MA: 2006. [Google Scholar]
- Hood LJ. Clinical applications of the auditory brainstem response Delmar Learning. Clifton Park, NY: 1998. [Google Scholar]
- Hornickel J, Chandrasekaran B, Zecker SG, Kraus N. Auditory brainstem measures predict reading and speech-in-noise perception in school-aged children. Behav. Brain Res. 2011;216:597–605. doi: 10.1016/j.bbr.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J, Skoe E, Nicol T, Zecker SG, Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proceedings of the National Academy of Sciences. 2009;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A, Ross HF. An improved procedure for assessing ABR latency in young subjects based on a new normative data set. Int. J. Pediatr. Otorhinolaryngol. 1995;32:35–47. doi: 10.1016/0165-5876(94)01110-j. [DOI] [PubMed] [Google Scholar]
- Johnson K, Nicol T, Kraus N. Developmental plasticity in the human auditory brainstem. J. Neurosci. 2008;28:4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthesizer. J. Acoust. Soc. Am. 1980;67:971–995. [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nature Reviews Neuroscience. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Xu Y, Gandour J, Cariani P. Encoding of pitch in the human brainstem is sensitive to language experience. Cognitive Brain Research. 2005;25:161–168. doi: 10.1016/j.cogbrainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lauter JL, Oyler RF. Latency stability of auditory brainstem responses in children aged 10-12 compared with younger children and adults. Br. J. Audiol. 1992;26:245–253. doi: 10.3109/03005369209076643. [DOI] [PubMed] [Google Scholar]
- McGrew KS, Woodcock RW. Technical Manual: Woodcock-Johnson III. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Musacchia G, Sams M, Skoe E, Kraus N. Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proceedings of the National Academy of Sciences. 2007;104:15894–15898. doi: 10.1073/pnas.0701498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek F, Shinn JB, Jirsa RE. The auditory brainstem response in auditory nerve and brainstem dysfunction. In: Burkard RF, Eggermont JJ, Don M, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Lippincott Williams & Wilkins; Baltimore, MD: 2007. pp. 291–312. [Google Scholar]
- Nunnally JC. Tests and measurements: Assessment and prediction. McGraw Hill, New York: 1959. [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Russo N, Nicol T, Musacchia G, Kraus N. Brainstem responses to speech syllables. Clin. Neurophysiol. 2004;115:2021–2030. doi: 10.1016/j.clinph.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Nicol T, Zecker SG, Hayes E, Kraus N. Auditory training improves neural timing in the human brainstem. Behav. Brain Res. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Russo N, Hornickel J, Nicol T, Zecker SG, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav. Brain Funct. 2010:6. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamy A. Maturation of the auditory brainstem response from birth through early childhood. J. Clin. Neurophysiol. 1984;1:293–329. doi: 10.1097/00004691-198407000-00003. [DOI] [PubMed] [Google Scholar]
- Sininger YS. The use of auditory brainstem response in screening for hearing loss and audiometric threshold prediction. In: Burkard RF, Eggermont JJ, Don M, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Lippincott Williams & Wilkins; Baltimore, MD: 2007. pp. 254–274. [Google Scholar]
- Skoe E, Kraus N. Auditory brainstem response to complex sounds: A tutorial. Ear and Hearing. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Nicol T, Kraus N. Cross-phaseogram: Objective neural index of speech sound differentiation. J. Neurosci. Methods. 2011;196:308–317. doi: 10.1016/j.jneumeth.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nicol T, Kraus N. Test-retest reliability of the speech-evoked auditory brainstem response. Clin. Neurophysiol. 2011;122:346–355. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nicol T, Kraus N. A reply to Drs. McFarland and Cacace. Clin. Neurophysiol. in press. [Google Scholar]
- Song JH, Skoe E, Banai K, Kraus N. Perception of speech in noise: Neural correlates. J. Cogn. Neurosci. 2010 doi: 10.1162/jocn.2010.21556. doi:10.1162/jocn.2010.21556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgensen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Pro-Ed; Austin, TX: [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Examiner’s manual: Test of word reading efficiency. Pro-Ed; Austin, TX: 1999. [Google Scholar]
- Tusa RJ, Stewart WF, Shechter AL, Simon D, Liberman JN. Longitudinal stud of brainstem auditory evoked responses in 87 normal human subjects. Neurology. 1994;44:528–532. doi: 10.1212/wnl.44.3_part_1.528. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Examiner’s manual: The comprehensive test of phonological processing. Pro-Ed; Austin, TX: 1999. [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol. Psychol. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Woerner C, Overstreet K. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wong PCM, Skoe E, Russo N, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat. Neurosci. 2007;10:420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Krishnan A, Gandour J. Specificity of experience-dependent pitch represenation in the brainstem. Neuroreport. 2006;17:1601–1605. doi: 10.1097/01.wnr.0000236865.31705.3a. [DOI] [PubMed] [Google Scholar]