Abstract
Human serum albumin (HSA) is used as a plasma expander (PE); however, albumin is readily eliminated from the intravascular space. The objective of this study was to establish the effects of various sized polymerized HSAs (PolyHSAs) during small volume resuscitation from hemorrhagic shock on systemic parameters, microvascular hemodynamics and functional capillary density (FCD) in the hamster window chamber model. PolyHSA size was controlled by varying the cross-link density (i.e. molar ratio of glutaraldehyde to HSA). Hemorrhage was induced by controlled arterial bleeding of 50% of the animal’s blood volume (BV) and hypovolemic shock maintained for 1 h. Resuscitation was implemented in two phases, first by infusion of 3.5% of the BV of hypertonic saline (HTS, 7.5 wt% NaCl), followed by infusion of 10% of BV of each PolyHSAs. Resuscitation provided rapid recovery of blood pressure, blood gas parameters and microvascular perfusion. PolyHSA at a glutaraldehyde to HSA molar ratio of 60:1 (PolyHSA60:1) provided superior recovery of blood pressure, microvascular blood flow and FCD, and acid-base balance, with sustained volume expansion in relation to the volume infused. The high molecular weigh of PolyHSA60:1 increased hydrodynamic radius and solution viscosity. Pharmacokinetic analysis of PolyHSA60:1 indicates reduced clearance and increased circulatory half-life compared to monomeric HSA and other PolyHSA formulations. In conclusion, HSA molecular size and solution viscosity affects central hemodynamics, microvascular blood flow, volume expansion and circulation persistence during small volume resuscitation from hemorrhagic shock. Additionally, PolyHSA can be an alternative to HSA in situations with compromised vascular permeability.
Keywords: Microcirculation, plasma expander, hyperviscous, plasma viscosity, functional capillary density, albumin, polymerized albumin
INTRODUCTION
Colloidal plasma expanders (PEs) are used to increase or maintain intravascular blood volume. PEs increase plasma oncotic pressure (colloid osmotic pressure, COP) and move fluid from the interstitial space into intravascular compartment. However, they further decrease erythrocyte concentration and blood viscosity. Colloids like gelatin, dextran, hydroxyethyl starch (HES) and human serum albumin (HSA) have different advantages and disadvantages [1, 2]. Gelatin exhibits poor volume expansion and is associated with allergic reactions and edema. Dextran and HES are able to effectively restore circulatory volume and microvascular perfusion. Unfortunately, dextran and HES have both been shown to inhibit coagulation, promote aggregation of RBCs and can even induce renal failure [3]. Conversely, HSA is naturally produced protein and accounts for more than 50% of the total plasma proteins. Albumin is the major contributor to plasma COP; it binds toxic species, drugs and consumes free radicals [4, 5]. Despite all of these beneficial properties, clinical trials and meta-analyses of HSA have provided some contradicting results, since the use of HSA has been shown to be generally safe [1, 6]. However, the negative responses to HSA are likely due to extravasation in situation with increased vascular permeability (i.e. shock, sepsis, burns etc.) and associated with edema and tissues exposed to potentially toxic molecules bound to the HSA [7]. Therefore, there is a need to develop a new generation of effective PEs based on HSA with increased vascular retention.
Inspired by this need, glutaraldehyde-based cross-linking represents a simple and cost-effective strategy to synthesize large proteins with high molecular weight (MW). Glutaraldehyde is able to react with lysine, histidine, tyrosine, arginine, and primary amine residues on the surface of HSA, forming both intra- and inter-molecular cross-links [8]. The abundance of these residues on the surface of the HSA facilitates its polymerization with a simple and effective chemistry. Polymeric HSA (PolyHSA) MW is controlled by simply varying the molar ratio of glutaraldehyde to HSA. Payne et al. was the first to increase the size of bovine serum albumin (BSA) by reacting it with various concentrations of glutaraldehyde [9, 10]. However, the resultant Schiff bases in the polymerized BSA molecules were not chemically stabilized, leaving the polymerized BSA susceptible to hydrolysis back into HSA and glutaraldehyde. Recently, PolyHSA was synthesized with chemically stabilized Schiff bases, preserving HSA secondary structure, increasing solution viscosity and decreasing COP compared to unpolymerized HSA [9, 10]. Therefore, glutaraldehyde cross-linking represents an attractive option to increase HSA size and intravascular retention.
The present study was carried on to test the hypothesis that the biophysical properties of PolyHSA (size, viscosity and COP) affect the recovery of systemic and microvascular hemodynamics, volume expansion, and plasma protein retention during small volume resuscitation from hemorrhagic shock model. To achieve this objective, our experimental hamster model was subjected to a hemorrhage of 50% of the animal’s blood volume (BV), followed by one hour of hypovolemic shock. The resuscitation was implemented in two steps. The initial phase consisted of the infusion of hypertonic saline (HTS, 7.5% NaCl) at 3.5% of BV, five minutes after a 10% of the BV infusion of the test solutions (PolyHSA) was administered. The volume resuscitation solutions evaluated in this study were adjusted to a protein concentration of 10 g/dL, namely: HSA (unpolymerized HSA), PolyHSA24:1 (molar ratio of glutaraldehyde to HAS = 24:1), PolyHSA60:1 (molar ratio of glutaraldehyde to HSA = 60:1), and PolyHSA94:1 (molar ratio of glutaraldehyde to HSA = 94:1).
METHODS
PolyHSA synthesis
HSA polymerization has been previously published [10]. Briefly, Albuminar® (ABO Pharmaceuticals, San Diego, CA) was diluted to 25 mg/mL with phosphate buffered saline, and 70% glutaraldehyde (Sigma Aldrich, Atlanta, GA) was then added to HSA solutions at the following molar ratios of glutaraldehyde to HSA: 24:1, 60:1, and 94:1. The polymerization reaction was incubated at 37°C for 3 hours, quenched with 25 mL of 1 M sodium borohydride, and incubated for 30 minutes. PolyHSA solutions were subjected to diafiltration against a modified lactated Ringer’s buffer (115 mM NaCl, 4 mM KCl, 1.4 mM CaCl2, 13 mM NaOH, 27 mM sodium lactate, and 2 g/L N-acetyl-L-cysteine) on a 100 kDa hollow fiber filter (Spectrum Labs, Rancho Dominguez, CA) a total of 4 times. PolyHSA solutions were then filtered through 0.2 µm filters and stored at −80°C until needed.
Light scattering
The absolute MW distribution of HSA/PolyHSA solutions was measured using a SEC column (Ultrahydrogel linear column, 10 µm, 7.8×300 mm, Waters, Milford, MA) driven by a 1200 HPLC pump (Agilent, Santa Clara, CA), controlled by Eclipse 2 software (Wyatt Technology, Santa Barbara, CA) connected in series to a DAWN Heleos (Wyatt Technology) light scattering photometer and an OptiLab Rex (Wyatt Technology) differential refractive index detector. All data were collected and analyzed using Astra 5.3 software (Wyatt Technology).
Viscosity, COP, and protein concentration
The viscosity of HSA/PolyHSA solutions was measured in a cone/plate viscometer DV-II plus with a cone spindle CPE-40 (Brookfield Engineering Laboratories, Middleboro, MA) at a shear rate of 160/sec, while the COP was measured using a Wescor 4420 Colloid Osmometer[11] (Wescor, Logan, UT). Protein concentration was determined using spectrophotometric (Lambda 20 Perkin-Elmer, Norwalk, CT) analysis in the UV domain (280 nm).
Animal preparation
Investigations were performed in 55 – 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies without anesthesia, and the complete surgical technique is described in detail elsewhere [12, 13]. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion criteria
Animals were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic Hct > 45%, and arterial partial pressure of oxygen (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under a 650× magnification did not reveal signs of edema or bleeding.
Acute hemorrhage and volume replacement protocol
Acute hemorrhage was induced by withdrawing 50% of estimated BV via the carotid artery catheter within 5 min. Total BV was estimated as 7% of body weight. One hour after hemorrhage induction, animals received a single bolus infusion of HTS comprising 3.5% of BV, and five minutes after HTS, 10% of the BV comprising of volume resuscitation fluids (see experimental groups) were infused within 10 min via the jugular vein catheter. Parameters were analyzed before hemorrhage (baseline) after 45 min after the hemorrhage (shock), and over 90 min after volume infusion (resuscitation). Animals did not receive any additional fluid during the experiment. At the end of the experiment, blood (1.5 ml) was collected in a heparinized syringe for plasma and blood viscosity, COP and protein concentration.
Resuscitation groups
Animals were randomly divided into the following four experimental groups before the experiment: 1) HSA - animals were resuscitated with 10 wt% HSA; 2) PolyHSA24:1 - animals were resuscitated with 10 wt% PolyHSA24:1; 3) PolyHSA60:1 - animals were resuscitated with 10 wt% PolyHSA60:1; and 4) PolyHSA94:1 - animals were resuscitated with 10 wt% PolyHSA94:1. Solution biophysical properties are presented in Table 1.
Table 1.
Biophysical properties.
Test solution (before infusion) and blood and plasma (after resuscitation)
| Test solutions |
Blood |
Plasma |
|||||
|---|---|---|---|---|---|---|---|
| g/dl | Viscosity* cP |
COP mmHg |
M.W. MDa |
Viscosity* cP |
Viscosity* cP |
COP mmHg |
|
| HSA | 10 | 1.5 | 42.0 | 0.67 | 2.4 ± 0.2 | 1.1 ± 0.2 | 16 ± 2 |
| PolyHSA24:1 | 10 | 1.6 | 22.0 | 0.24 | 2.7 ± 0.3 | 1.2 ± 0.2 | 15 ± 2 |
| PolyHSA60:1 | 10 | 11.2 | 4.0 | 2.00 | 3.2 ± 0.4 | 1.8 ± 0.3 | 14 ± 1 |
| PolyHSA94:1 | 10 | 15.2 | 1.0 | 11.84 | 4.3 ± 0.3 | 2.5 ± 0.4 | 12 ± 2 |
Shear rate of 160 s−1 1 at 37°C; COP, colloid osmotic pressure at 27°C.
Systemic parameters
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hemoglobin (Hb) content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood chemistry and biophysical properties
Arterial blood was collected in heparinized glass capillaries (0.05 ml) and immediately analyzed for PaO2, PaCO2, base excess (BE) and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA).
Microvascular experimental setup
The awake animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, and then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The animals were given 20 min to adjust to the change in the tube environment before measurements. Measurements were carried out using a 40× (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. The same sites of study were followed throughout the experiment so that comparisons could be made directly to baseline levels.
Microhemodynamics
Arteriolar and venular blood flow velocities were measured online via the photodiode cross-correlation method (Photo-Diode/Velocity, Vista Electronics, San Diego, CA) [14]. The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity [15]. A video image-shearing method was used to measure vessel diameter (D) [16]. Blood flow (Q) was calculated from the measured values as Q = p × V (D/2)2. Wall shear stress (WSS) was defined by WSS = WSR × ?, where WSR is the wall shear rate given by 8VD−1, and ? is the microvascular blood viscosity or plasma viscosity.
Functional capillary density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least one RBC in a 60 s period in 10 successive microscopic fields, were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. FCD (cm−1) is the total length of RBC perfused capillaries divided by the area of the microscopic field of view. Measurement includes length of capillaries perfused with RBCs in the field of view. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion [17].
Volume expansion
Changes in intravascular volume were calculated as the difference in Hct before infusion (Hct0) and the Hct at a specific time after infusion (Hctt) as VE = (Hct0−Hctt)/Hctt.
Pharmacokinetic analysis of PolyHSA solutions
PolyHSA pharmacokinetics was studied in hamsters instrumented with the dorsal window chamber (described in animal preparation section) after a 40% of the estimated BV exchange transfusion, to determine the role of PolyHSA molecular size on circulation persistence in a normal vasculature with (uncompromised) permeability. Pharmacokinetic parameters were determined for total PolyHSA in the plasma using a noncompartmental method (NCOMP Pharmacokinetics Analysis Software; Paul B. Laub and James M. Gallo) [18]. At each time point (30 min, and 1, 2, 4, 8, 12, 24, and 48 h) after the exchange transfusio, a 50 µL aliquot of blood was collected, and plasma was obtained by centrifugation (6000 g, 5 min). At 48 h after the exchange, the experiment was terminated.
PolyHSA concentration
PolyHSA presented in the sampled plasma was labeled with a goat anti-HSA polyclonal antibody conjugated to FITC (Bethyl Laboratories, montgomery, TX). This polyclonal antibody does not cross-react with albumin derived from hamsters or other animals. PolyHSAs were fluorescently labeled before infusion in the pharmacokinetic analysis. The antibody was diluted 500:1 in 10 ml of each HSA or PolyHSA solutions and incubated at 4°C for 2 hours. Standard relationships between concentration and fluorescence for all solutions were measured after several dilutions using fresh hamster plasma. The fluorescent signals were determined by an automated fluorescence system FluoroMax-2 (HORIBA Ltd, Japan) [19].
Data analysis
Results are presented as the mean ± standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Apriori, animal sample size based on MAP was calculated. The calculation of the sample size to detect changes in MAP after small volume resuscitation hemorrhagic shock in hamsters was based on our previous results [20–22]. We previously found that during small volume resuscitation hemorrhagic shock MAP changed by 30% (SD 12%). Therefore, the number of animals was based on a power analysis using an alpha of 0.05 and a 1-beta of 0.9, and resulted in an estimated of 7 animals to be required to identify differences in MAP. However, as data was collected, statistical analysis was implemented, and following animal care regulation at our institution, no more animals were included as statistical significance was reached. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline, while lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics for small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P<0.05.
RESULTS
Twenty-four animals were entered into this study; all tolerated the entire protocol without visible signs of discomfort. All animals included in the study passed the Grubbs' test, ensuring that all the measured parameter values at baseline were within a similar population (P<0.05). Animals were randomly assigned to the following experimental hemorrhage resuscitation groups: HSA (n = 6; 62.6 ± 4.2 g); PolyHSA24:1 (n = 6; 64.2 ± 3.6 g); PolyHSA60:1 (n = 6; 60.6 ± 4.1 g); and PolyHSA94:1 (n = 6; 59.8 ± 3.0 g). Systemic data for baseline and shock was obtained by combining all experimental groups. Similarities between groups at baseline and shock were statistically verified among groups (P > 0.30).
Systemic parameters
Basic blood hematology and blood gas parameters are presented in Table 2. Hemorrhage significantly reduced the Hct and Hb concentration compared to baseline. Resuscitation further decreased the Hct and Hb concentration compared to shock. The Hct and Hb concentration decreased as a function of the COP of the PolyHSA solution, which resulted in a larger decrease for the lower MW PolyHSA (PolyHSA24:1) and in a smaller decrease for the higher MW PolyHSA (PolyHSA94:1).
Table 2.
Blood gas parameters
| Hct % |
Hb g/dL |
pH | PO2 mmHg |
PCO2 mmHg |
BE mmol/L |
|
|---|---|---|---|---|---|---|
| Baseline | ||||||
| HSA | 47.5 ± 2.4 | 14.4 ± 0.6 | 7.34 ± 0.02 | 60 ± 9 | 56 ± 8 | 3.7 ± 2.0 |
| PolyHSA24:1 | 48.8 ± 3.0 | 14.8 ± 0.7 | 7.35 ± 0.02 | 62 ± 10 | 57 ± 7 | 4.1 ± 2.3 |
| PolyHSA60:1 | 48.7 ± 3.2 | 14.9 ± 0.9 | 7.36 ± 0.02 | 57 ± 6 | 56 ± 7 | 4.0 ± 2.0 |
| PolyHSA94:1 | 48.4 ± 2.4 | 14.6 ± 0.7 | 7.36 ± 0.02 | 62 ± 5 | 54 ± 2 | 4.8 ± 1.7 |
| Shock | ||||||
| PolyHSA24:1 | 28.2 ± 1.1 | 9.4 ± 0.5 | 7.24 ± 0.12 | 95 ± 11 | 39 ± 7 | −4.8 ± 2.2 |
| PolyHSA24:1 | 28.4 ± 1.0 | 9.5 ± 0.7 | 7.21 ± 0.13 | 97 ± 12 | 38 ± 6 | −5.9 ± 2.4 |
| PolyHSA60:1 | 28.6 ± 0.9 | 9.4 ± 0.8 | 7.22 ± 0.09 | 94 ± 8 | 39 ± 7 | −4.6 ± 2.0 |
| PolyHSA94:1 | 28.0 ± 1.4 | 9.6 ± 0.7 | 7.22 ± 0.08 | 92 ± 10 | 38 ± 7 | −4.9 ± 2.4 |
| Resuscitation 60 min | ||||||
| PolyHSA24:1 | 18.2 ± 1.0 | 5.6 ± 0.5 | 7.38 ± 0.04 | 76 ± 6 | 46 ± 5 | 2.3 ± 1.5 |
| PolyHSA24:1 | 19.3 ± 1.2 | 6.1 ± 0.5 | 7.38 ± 0.06 | 72 ± 9 | 45 ± 6 | 2.1 ± 1.6 |
| PolyHSA60:1 | 21.3 ± 1.4† | 6.7 ± 0.8† | 7.37 ± 0.04 | 76 ± 11 | 47 ± 5 | 3.0 ± 1.3 |
| PolyHSA94:1 | 25.1 ± 1.3†‡§ | 7.9 ± 0.7†‡§ | 7.35 ± 0.04 | 93 ± 6†‡§ | 43 ± 6 | −0.5 ± 1.6†‡§ |
| Resuscitation 60 min | ||||||
| PolyHSA24:1 | 18.5 ± 1.4 | 5.8 ± 0.3 | 7.35 ± 0.03 | 76 ± 6 | 47 ± 6 | 2.2 ± 1.5 |
| PolyHSA24:1 | 18.8 ± 1.2 | 6.0 ± 0.6 | 7.36 ± 0.04 | 80 ± 8 | 46 ± 5 | 1.8 ± 1.4 |
| PolyHSA60:1 | 19.3 ± 1.1† | 6.2 ± 0.5† | 7.39 ± 0.03 | 74 ± 10 | 49 ± 7 | 3.2 ± 1.6 |
| PolyHSA94:1 | 25.0 ± 1.3†‡§ | 7.7 ± 0.7†‡§ | 7.35 ± 0.03 | 81 ± 8†‡§ | 42 ± 6 | −0.7 ± 1.5†‡§ |
Values are means ± SD. Baseline and moderated hemodilution (MH) included all the animals. No significant differences were detected between baseline or during shock between groups. Hct, hematocrit; Hb, hemoglobin; PO2, arterial partial O2 pressure; PCO2, arterial partial pressure of CO2; BE, arterial base excess.
P<0.05 compared to HSA;
P<0.05 compared to polyHSA24:1;
P<0.05 compared to polyHSA60:1.
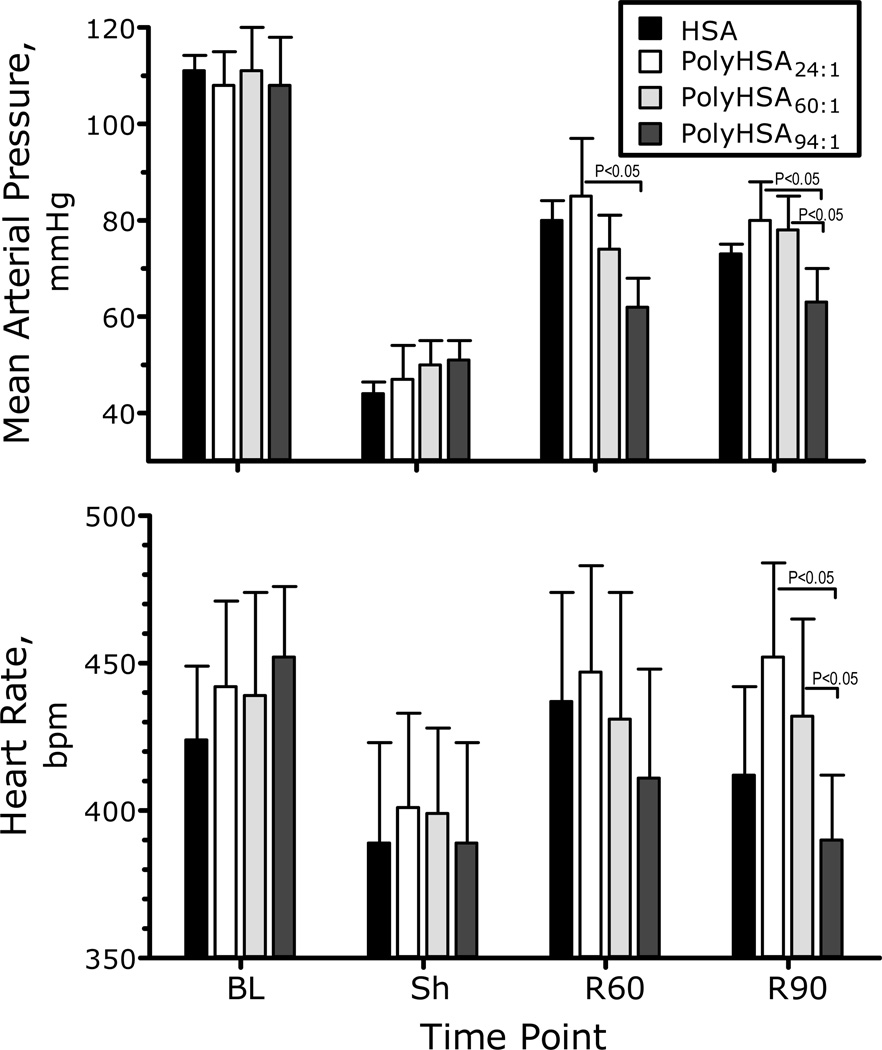
Blood pressure and heart rate at baseline, shock and after resuscitation are shown in Figure 2. Blood pressure was significantly decreased after hemorrhagic shock. Small volume resuscitation with PolyHSA24:1 and PolyHSA60:1 increased MAP compared to the hypovolemic shock, although the MAP was still significantly lower than baseline. The MAP of the group resuscitated with PolyHSA94:1 was not different compared to shock. There were no statistically significant changes in the heart rate (neither from baseline nor after resuscitation) among the study groups at any time during the experiment.
Figure 2.
Mean arterial blood pressure (MAP) during the hemorrhagic shock resuscitation protocol for PolyHSA24:1, PolyHSA60:1 and PolyHSA94:1. Parameters were analyzed before hemorrhage (Baseline, BL), after hemorrhage (Shock, Sh, 60 min after hemorrhage induction), and at 60 and 90 min after fluid resuscitation (Resuscitation 60 min, R60 and Resuscitation 90 min, R90). All MAPs were significantly lower than baseline during shock and after resuscitation.
Blood gas parameters and calculated acid base balance are presented in Table 2. Blood gas parameters (PaO2 and PaCO2) were significantly affected by hemorrhagic shock compared to baseline. Resuscitation with PolyHSA24:1 and PolyHSA60:1 recovered blood gases from shock; however, arterial pO2 remained higher than baseline and pCO2 was decreased compared to baseline. Arterial blood pH in animals resuscitated with PolyHSA24:1 and PolyHSA60:1 were not different compared to baseline. The arterial pO2, pCO2 and pH of the group resuscitated with PolyHSA94:1 was not different compared to shock, and significantly different compared to baseline. There were no significant changes in blood gases between 60 min after resuscitation compared to 90 min after resuscitation.
Blood and plasma viscosities were significantly different among resuscitation groups. Blood and plasma viscosities are presented in Table 1. The group resuscitated with PolyHSA24:1 showed lower blood and plasma viscosities compared to PolyHSA60:1 and PolyHSA94:1. Additionally, the PolyHSA60:1 group presented lower blood and plasma viscosities compared to and PolyHSA94:1. Blood and plasma viscosities for the PolyHSA24:1 and PolyHSA60:1 groups were lower than baseline viscosity. Baseline viscosity data (blood: 4.2 cp, plasma: 1.2 cp) were obtained from hamsters that did not undergo the shock resuscitation protocol. Plasma COP was lower for the group resuscitated with PolyHSA24:1 compared to PolyHSA94:1. Calculated volume expansion using Hct changes at 60 and 90 min after resuscitation was significantly higher for HSA (60 min: 53 ± 7%; 90 min: 51 ± 9%) and PolyHSA24:1 (60 min: 46 ± 8%; 90 min: 55 ± 10%) than PolyHSA60:1 (60 min: 32 ± 7%; 90 min: 46 ± 8%) and PolyHSA94:1 (60 min: 17 ± 7%; 90 min: 12 ± 6%). PolyHSA24:1 and PolyHSA60:1 had significantly higher volume expansion compared to PolyHSA94:1, as expected due to its lower COP. HSA, PolyHSA24:1 and PolyHSA94:1 maintained and increased their volume expansion between 60 and 90 min after infusion, even though PolyHSA94:1 had a low COP. At the end of the experiment, the plasma protein concentration of the groups resuscitated with HSA and PolyHSA24:1 was lower compared to PolyHSA60:1 and PolyHSA94:1, respectively. No difference in plasma protein concentration was measured between the PolyHSA60:1 and PolyHSA94:1.groups.
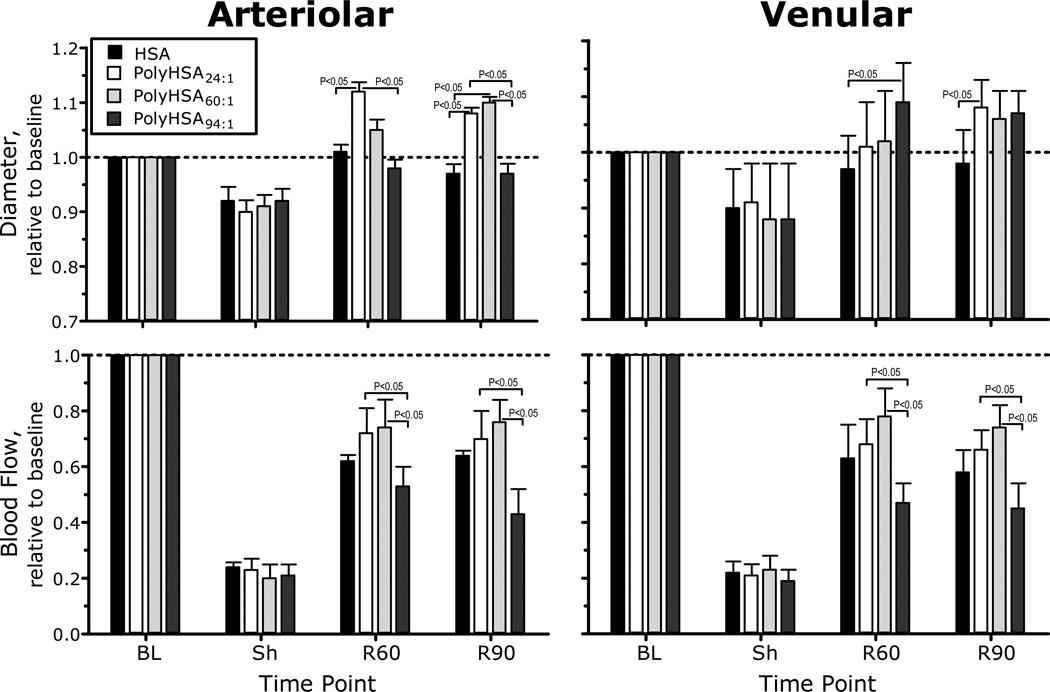
Microvascular hemodynamic measurements are presented in Figure 3. Arteriolar and venular diameters were statistically vasoconstricted during shock for all experimental groups. Arteriolar diameters in animals resuscitated with PolyHSA24:1 and PolyHSA60:1 were significantly different compared to shock, but no different compared to baseline. The diameter of arterioles in the group resuscitated with PolyHSA94:1 was significantly lower compared to resuscitation with PolyHSA24:1 and PolyHSA60:1, and no recovery was observed compared to shock. Arteriolar blood flow during shock was significantly lower compared to baseline in all the experimental groups. In general, resuscitation significantly increased blood flow compared to shock in all groups, although all the groups were significantly lower compared to baseline. 90 min after resuscitation with PolyHSA94:1, arteriolar blood flows were significantly lower compared to PolyHSA24:1 and PolyHSA60:1. Venular diameters were significantly recovered after resuscitation compared to shock in all groups. No differences in venular diameters were measured between groups resuscitated with PolyHSA, however, animals resuscitated with HSA showed were significantly constricted compared to PolyHSA94:1 at 60 min after resuscitation and compared to PolyHSA24:1 at 90 min after resuscitation, respectively. Venular blood flow during shock was statistically lower than baseline, and all resuscitation solutions exhibited statistically increased blood flow compared to shock. Venular blood flow after resuscitation with PolyHSA94:1 was statistically lower compared to PolyHSA24:1 and PolyHSA60:1.
Figure 3.
Relative changes with respect to baseline in arteriolar and venular hemodynamics for PolyHSA24:1, PolyHSA60:1 and PolyHSA94:1. The broken line represents the baseline level. Diameters (µm, mean ± SD) for each animal group at baseline were as follows: PolyHSA24:1 (arterioles (A): 54.6 ± 9.7, n = 38; venules (V): 55.8 ± 8.2, n = 46); PolyHSA60:1 (A: 56.7 ± 9.1, n = 40; V: 57.6 ± 9.2, n = 47) and PolyHSA94:1 (A: 55.7 ± 7.2, n = 36, V: 54.8 ± 8.5, n = 38). n = number of vessels studied. RBC velocities (mm/s, mean ± SD) for each animal group at baseline were as follows: PolyHSA24:1 (A: 4.3 ± 1.2, V: 2.1 ± 0.6); PolyHSA60:1 (A: 4.0 ± 1.1; V: 2.2 ± 0.9) and PolyHSA94:1 (A: 4.2 ± 0.9; V: 2.3 ± 0.8). Calculated flows (nl/s, mean ± SD) for each animal group at baseline were as follows: PolyHSA24:1 (A: 10.8 ± 3.2; V: 6.5 ± 2.1); PolyHSA60:1 (A: 10.4 ± 3.2; V: 6.0 ± 1.9) and PolyHSA94:1 (A: 10.7 ± 2.4; V: 6.3 ± 2.3). Parameters were analyzed before hemorrhage (Baseline, BL), after hemorrhage (Shock, Sh, 60 min after hemorrhage induction), and at 60 and 90 min after fluid resuscitation (Resuscitation 60 min, R60 and Resuscitation 90 min, R90).
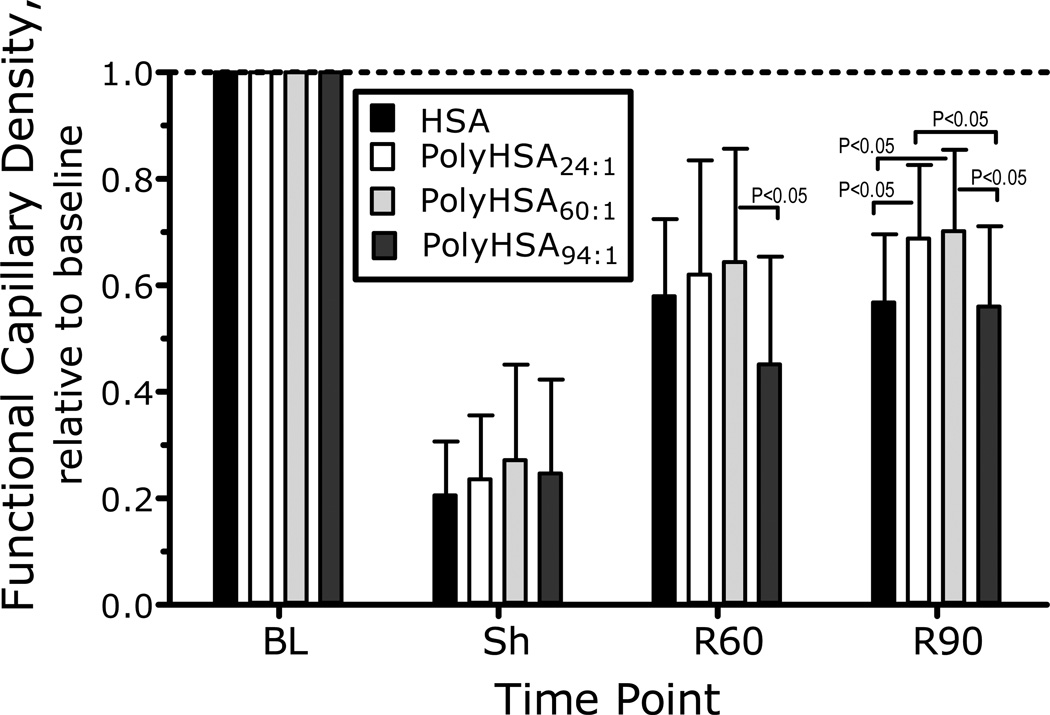
Changes in FCD are presented in Figure 4. FCD was statistically reduced during shock, while resuscitation statistically increased FCD compared to shock in all experimental groups. FCD was no different between groups. Calculated arteriolar and venular wall shear rate was significantly reduced during shock compared to baseline. Resuscitation with PolyHSA60:1 significantly increased the arteriolar and venular wall shear rate compared to the group resuscitated with PolyHSA94:1. Calculated arteriolar and venular wall shear stresses were not different between experimental groups.
Figure 4.
Relative changes with respect to baseline in capillary perfusion during the shock resuscitation protocol. Functional capillary density (FCD) was drastically reduced after hemorrhage. FCD (cm−1) at baseline was as follows: PolyHSA24:1 (112 ± 12); PolyHSA60:1 (116 ± 10); and PolyHSA94:1 (108 ± 9). Parameters were analyzed before hemorrhage (Baseline, BL), after hemorrhage (Shock, Sh, 60 min after hemorrhage induction), and at 60 and 90 min after fluid resuscitation (Resuscitation 60 min, R60 and Resuscitation 90 min, R90).
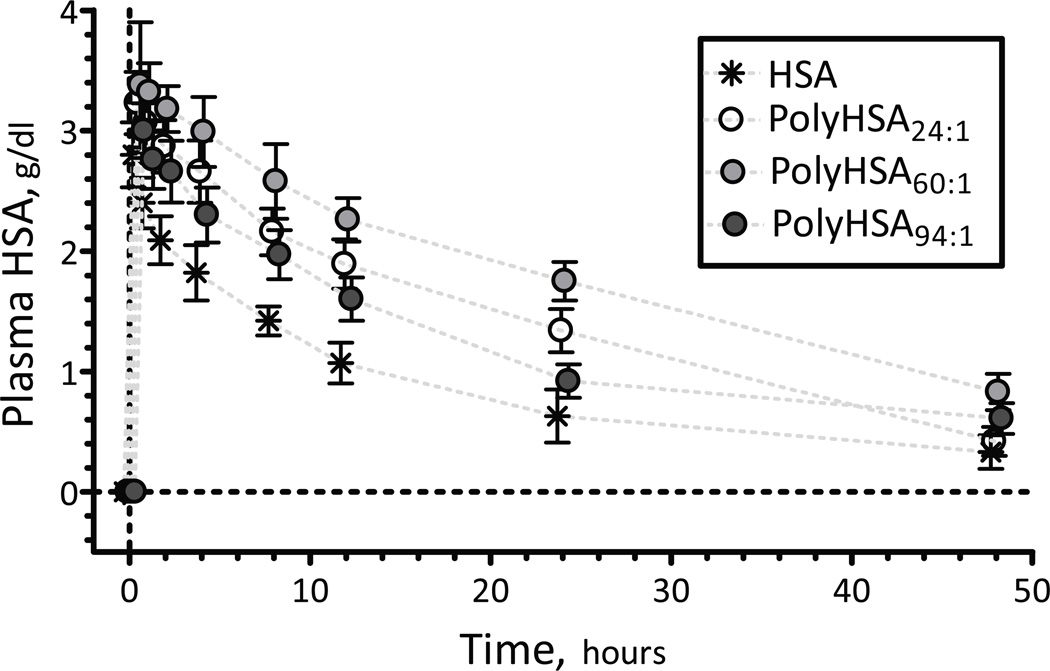
Twelve animals were entered into the pharmacokinetic study. All animals included in the study passed the Grubbs' test, ensuring that all the measured parameter values at baseline were within a similar population (P<0.05). Animals were randomly assigned to the following experimental groups: HSA (n = 3; 63.6 ± 3.6 g) PolyHSA24:1 (n = 3; 60.1 ± 4.2 g); PolyHSA60:1 (n = 3; 62.5 ± 4.5 g); and PolyHSA94:1 (n = 3; 64.1 ± 3.5 g). Plasma HSA/PolyHSA concentration following a 40% exchange transfusion is shown in Figure 5. The pharmacokinetic parameter estimates for HSA/PolyHSA are listed in Table 3. Doses received determined at the completion of exchange transfusion were similar among all groups 158 ± 37 mg (HSA), 145 ± 34 mg (PolyHSA24:1), 156 ± 48 mg (PolyHSA60:1) and 161 ± 42 mg (PolyHSA94:1). Transfusions resulted in total plasma HSA/PolyHSA maximum plasma concentrations (Cmax) of 3.62 ± 0.52 g/dL (HSA), 3.33 ± 0.62 g/dL (PolyHSA24:1), 3.41 ± 0.42 g/dL (PolyHSA60:1) and 3.41 ± 0.42 g/dL (PolyHSA94:1), respectively. The volume of distribution suggest a higher distribution within the central compartment for PolyHSA24:1 and PolyHSA60:1 formulations compared to HSA and PolyHSA94:1.
Figure 5.
Pharmacokinetics of PolyHSA24:1, PolyHSA60:1 and PolyHSA94:1. The pharmacokinetic parameters are listed in Table 3. Doses received determined at the completion of exchange transfusion were similar among groups: 158 ± 37 mg (HSA), 145 ± 34 mg (PolyHSA24:1), 156 ± 48 mg (PolyHSA60:1) and 161 ± 42 mg (PolyHSA94:1).
Table 3.
Pharmacokinetic parameters for PolyHSA solutions
| HSA | PolyHSA24:1 | PolyHSA60:1 | PolyHSA94:1 | |
|---|---|---|---|---|
| CL, mL h−1 | 3.60 | 1.82 | 1.34 | 2.22 |
| MRT, h | 24.8 | 24.9 | 34.9 | 31.3 |
| Vss, mL | 89.2 | 45.3 | 46.6 | 69.4 |
| Terminal K, h−1 | 0.042 | 0.041 | 0.029 | 0.033 |
| Terminal T1/2, h | 16.7 | 16.9 | 24.0 | 21.2 |
| Cmax, mg h−1 | 2.92 | 3.33 | 3.41 | 3.67 |
| AUC to Tlast, mg h mL−1 | 39.6 | 69.1 | 87.8 | 56.5 |
| AUC extrap to time infinity, mg h mL−1 | 46.4 | 79.8 | 116.8 | 72.59 |
| % extrapolated AUC | 14.8 | 13.5 | 24.8 | 22.2 |
| AUMC, mg h mL−1 | 1151 | 1989 | 4075 | 2271 |
| % extrapolated AUMC | 43.1 | 39.2 | 58.9 | 55.52 |
CL, plasma clearance; MRT, mean residence time; Vss volume of distribution at steady-state, terminal k, terminal slope; terminal T1/2, terminal half-life; Cmax, concentration maximal, AUC, area under the curve; Tlast, last measurable concentration; AUMC, area under the first moment curve.
DISCUSSION
The principal finding of this study is that resuscitation with 10 g/dL PolyHSA (PolyHSA24:1 and PolyHSA60:1) provided similar recovery of systemic parameters and microvascular hemodynamics. These hyperviscous PEs provided rapid restoration of blood pressure and blood gas parameters. Remarkably, the restoration trend was maintained with PolyHSA24:1 and PolyHSA60:1 during the entire observation period. These PolyHSA formulations reinstated microvascular perfusion and recovery from metabolic imbalances after infusion. Hemorrhage after penetrating trauma is a major combat hazard, whose therapy relies on aggressive fluid resuscitation strategies [23, 24]. Rapid and large volume fluid resuscitation targets the recovery of intravascular volume to restore blood pressure and metabolic balance, under the assumption that it will prolong survival. This strategy can resolve the hypovolemic syndrome, but may lead to fluid overload disorders that may influence the length of recovery, days requiring mechanical ventilation and patient mortality [25]. Our small volume resuscitation strategy, combining HTS with a colloidal PE based on PolyHSA, can prevent overload morbidity, rebleeding and thrombus dislodging by using permissive hypotensive resuscitation.
This study compares the effects of small volume resuscitation from hemorrhagic shock and correlates the volume expansion attained with the physical properties of the resuscitation solution. The initial similarity of responses observed with all PolyHSA solutions was due to blood volume restoration, and the later results observed at 60 and 90 min after resuscitation reflect the effects of volume expansion, microvascular recovery and the reversal of capillary collapse created during the hypovolemic shock. Volume expansion of PolyHSA was proportional to its COP, and the larger sized PolyHSA molecules increase the natural volume expansion of HSA by increasing its retention in the intravascular compartment. Pharmacokinetic results obtained after exchange transfusion further validate this analysis. The mean residence time in the plasma for PolyHSA60:1 was 1.4 times higher than of the HSA and PolyHSA24:1, respectively. Additionally, the clearance from the plasma for PolyHSA60:1 was 0.37 of the HSA clearance and 0.73 of the PolyHSA24:1 clearance, respectively. This suggests the existence of an optimal molecular size for PolyHSA molecules that increase their circulation time and minimizes their clearance. In fact, the pharmacokinetic results show that increasing molecular size to PolyHSA94:1 did not increase retention or reduce clearance. The pharmacokinetic retention data is in agreement with the basic volume expansion results, calculated based on changes in the Hct and the plasma protein concentration in the hemorrhagic shock experiment.
Volume expansion with HSA requires frequent administration of HSA to maintain elevated albumin concentration, increasing the cost of treatment [25, 26]. Polymerization of HSA with bifunctional cross-linking reagents can reduce the amount HSA needed to maintain intravascular volume, since PolyHSA is larger in size compared to monomeric HSA. PolyHSA large size increases the solution viscosity and decreases the solution COP, limiting intravascular volume expansion to the volume infused without moving fluid from the perivascular space due to the COP of the solutions. The volume expansion attained with PolyHSA24:1 and PolyHSA60:1 were not different, suggesting that structural modification of the HSA by the cross-linking process may also affect volume expansion. Theoretically, PolyHSA should remain in the intravascular compartment long as in the pharmacokinetic study, but hemorrhagic shock induced changes in vascular permeability may affect the volume expansion. The rationale for this analysis is supported by the plasma protein at the end of the experiment. Additionally, the differences in Hct after resuscitation also present a snapshot of volume expansion under pseudo-equilibrium conditions. Unfortunately, it was impossible to perform long-term pharmacokinetic analysis post resuscitation, since animal care regulation does not allow us to keep animals hypovolemic beyond the extent of the shock resuscitation protocol. Differences in volume expansion resulted differences in the Hct and Hb concentration, which also affected the oxygen transport capacity, and whole blood rheological properties.
Our small volume resuscitation strategy from hemorrhagic shock focuses on restoration of microvascular perfusion, which determines oxygen delivery and washout of metabolic waste products, leading to an improved long-term outcome [27]. To insure the recovery of microvascular perfusion and prevent tissue injury, the biophysical properties of the resuscitation fluid needs to reinstate FCD, since FCD reflects the overall function of the microcirculation. Microvascular oriented therapies have shown clinical prevention of multiorgan failure [28, 29]. On the other hand, resuscitation strategies that aim to restore systemic parameters fail to recover microvascular function, such as vasopressin treatment, which only recovers pressure, shunting blood flow away from the microcirculation and decreasing tissue oxygenation [30]. Given that volume restoration and microvascular function are special during resuscitation from hemorrhagic shock, our novel PolyHSAs, when used during small volume resuscitation, produced rapid microvascular flow and FCD and sustained recovery of blood pressure. PolyHSAs are biocompatible and do not induce RBC aggregation [10]. Our findings suggest that an increase in plasma viscosity, without necessarily restoring blood viscosity to normal with PolyHSA, is beneficial during resuscitation. In previous studies increasing plasma viscosity during anemic conditions was associated with increased capillary pressure, a critical determinant of FCD [17]. The importance of increasing plasma viscosity during anemic and hypovolemic conditions is shown by the sustained recovery of microhemodynamic conditions post resuscitation. Pressure redistribution are due to arteriolar vasodilatation, likely due to the restoration of shear stress [17]. Shear stress exerted by the flowing plasma on the vascular endothelial cells influences vessel diameter by the release of vasodilatory autocoids (prostacyclin, nitric oxide, etc) [31].
In our study, PolyHSA60:1 produced the more significant resuscitation, by expanding blood volume and increasing plasma viscosity to 1.8 cP and blood viscosity to 3.2 cP, both values are significantly different than normal blood, where plasma is 1.2 cP and blood is 4.2 cP. The role of plasma viscosity, and consequently blood viscosity in maintaining systemic blood pressure and blood gases, is highlighted by the results obtained here, since increasing plasma viscosity by means of hyperviscous PolyHSA provided consistent restoration of homeostasis, compared to volume resuscitation with conventional PEs. Previous studies evaluated several conventional PEs, including Voluven™ and Hextend™(Hospira, Inc., Lake Forest, IL), in identical small volume resuscitation from hemorrhagic shock protocol as described here, and PolyHSA60:1 shows superior recovery of systemic and microvascular conditions [22]. This study suggests a limit for the benefit attained by increasing plasma viscosity. The upper limit appears to be lower than the plasma viscosity attained with PolyHSA94:1, although the exact value has not yet been defined. Currently in the marketplace, the only colloid with high viscosity is Hextend™(Hospira, Inc., Lake Forest, IL), a high MW HES with a high degree of substitution [32]. However, HES has shown adverse effects on coagulation, mainly on factor VIII, von Willebrand's factor and platelet function [32]. PolyHSA are novel PEs, preserving HSA ligand-binding capacity, and compatibility and potentially serving as a substitute for HSA in patients with high blood vessel and glomerular permeability.
Hemorrhagic shock is a critical situation in which rapid correction of hemodynamics can improve outcome. The recovery observed after infusion of HTS is due to rapid inter-compartmental fluid shifts, and the small volume of hyperviscous PolyHSA stabilized and limited the extravasation of the fluid drawn out of the extravascular space. The gradual and sustained increase of FCD, blood pressure and flow obtained with the PolyHSA indicates a gradual increase in cardiac output combined with a volume effect, which preserves hemostatic mechanisms, reducing the risk for further bleeding. In conclusion, this study shows that, to recover microvascular function after hemorrhagic shock, the biophysical properties of the fluid used during resuscitation must maintain intravascular volume and increase plasma viscosity. PolyHSA solution's potential as new plasma volume expanders is inspired by their tunable intravascular retention and rheological characteristics, controlled by the molar ratio of glutaraldehyde to HSA, and biocompatibility. Although, this study has restricted clinical significance, it presents a comprehensive experimental study, with the objective of defining the mechanistic principles for translational developments and that will need to be evaluated in clinical trials. PolyHSAs used will reduce the multiple administrations of HSA, reducing HSA associate toxicity and cost. Our results with PolyHSA solutions suggest the existence of an optimal size that maintains intravascular volume, extends circulation time and minimizes clearance.
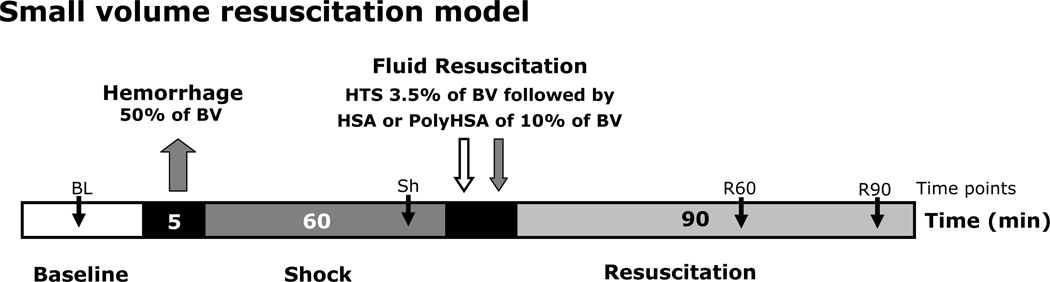
Figure 1.
Hemorrhage was induced by withdrawing 50% of the animal’s BV. Hypovolemia was maintained for 1 hour. Small fluid resuscitation was implemented initially by infusion of 3.5% of the BV with HTS, and five minutes after HTS, followed by infusion of 10% of the BV with PolyHSA resuscitation fluids. The resuscitation fluids comprised of three different formulations of PolyHSA at 10 g/dl, namely: PolyHSA24:1 (synthesized at a 24:1 molar ratio of glutaraldehyde to HSA), PolyHSA60:1 (synthesized at a 60:1 molar ratio of glutaraldehyde to HSA), and PolyHSA94:1 (synthesized at a 94:1 molar ratio of glutaraldehyde to HSA). Parameters were analyzed before hemorrhage (Baseline, BL), after hemorrhage (Shock, Sh, 60 min after hemorrhage induction), and at 60 and 90 min after fluid resuscitation (Resuscitation 60 min, R60 and Resuscitation 90 min, R90).
ACKNOWLEDGMENTS
This work was partially supported by Program project P01-HL071064 and grants R01-HL52684, R01-HL62354, R01-HL078840 and R01-DK070862. The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Groeneveld AB, Navickis RJ, Wilkes MM. Update on the comparative safety of colloids: a systematic review of clinical studies. Ann Surg. 2011;253(3):470–483. doi: 10.1097/SLA.0b013e318202ff00. [DOI] [PubMed] [Google Scholar]
- 2.Kreimeier U, Messmer K. Perioperative hemodilution. Transfus Apher Sci. 2002;27(1):59–72. doi: 10.1016/s1473-0502(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 3.McCahon R, Hardman J. Pharmacology of Plasma Expanders. Anaesthesia & Intensive Care Medicine. 2007;8(2):79–81. [Google Scholar]
- 4.Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002;25(6):695–704. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- 5.Kouoh F, Gressier B, Luyckx M, et al. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco. 1999;54(10):695–699. doi: 10.1016/s0014-827x(99)00082-8. [DOI] [PubMed] [Google Scholar]
- 6.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 7.Haupt MT, Kaufman BS, Carlson RW. Fluid resuscitation in patients with increased vascular permeability. Crit Care Clin. 1992;8(2):341–353. [PubMed] [Google Scholar]
- 8.Sugio S, Kashima A, Mochizuki S, et al. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999;12(6):439–446. doi: 10.1093/protein/12.6.439. [DOI] [PubMed] [Google Scholar]
- 9.Payne JW. Polymerization of proteins with glutaraldehyde. Soluble molecular-weight markers. Biochem J. 1973;135(4):867–873. doi: 10.1042/bj1350867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmer J, Cabrales P, Wang Q, et al. Synthesis and biophysical properties of polymerized human serum albumin. Biotechnol Prog. 2011;27(1):290–296. doi: 10.1002/btpr.531. [DOI] [PubMed] [Google Scholar]
- 11.Webb AR, Barclay SA, Bennett ED. In vitro colloid osmotic pressure of commonly used plasma expanders and substitutes: a study of the diffusibility of colloid molecules. Intensive Care Med. 1989;15(2):116–120. doi: 10.1007/BF00295988. [DOI] [PubMed] [Google Scholar]
- 12.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 13.Endrich B, Asaishi K, Götz A, et al. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 14.Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975;10:165–179. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- 15.Lipowsky HH, Zweifach BW. Application of the "two-slit" photometric technique to the 32 measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 16.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5(3):309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 17.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287(1):H363–H373. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 18.Laub PB, Gallo JM. NCOMP--a windows-based computer program for noncompartmental analysis of pharmacokinetic data. J Pharm Sci. 1996;85(4):393–395. doi: 10.1021/js9503744. [DOI] [PubMed] [Google Scholar]
- 19.Raab S, Thein E, Harris AG, et al. A new sample-processing unit for the fluorescent microsphere method. Am J Physiol. 1999;276(5 Pt 2):H1801–H1806. doi: 10.1152/ajpheart.1999.276.5.H1801. [DOI] [PubMed] [Google Scholar]
- 20.Palmer AF, Zhang N, Zhou Y, et al. Small-Volume Resuscitation From Hemorrhagic Shock Using High-Molecular-Weight Tense-State Polymerized Hemoglobins. J Trauma. 2011 doi: 10.1097/TA.0b013e3182028ab0. [DOI] [PubMed] [Google Scholar]
- 21.Cabrales P, Tsai AG, Intaglietta M. Polymerized bovine hemoglobin can improve small-volume resuscitation from hemorrhagic shock in hamsters. Shock. 2009;31(3):300–307. doi: 10.1097/SHK.0b013e318180ff63. [DOI] [PubMed] [Google Scholar]
- 22.Cabrales P, Tsai AG, Intaglietta M. Increased plasma viscosity prolongs microhemodynamic conditions during small volume resuscitation from hemorrhagic shock. Resuscitation. 2008;77(3):379–386. doi: 10.1016/j.resuscitation.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149(2):55–62. [PubMed] [Google Scholar]
- 24.Advanced Trauma Life Support Course for Physicians. Committee on Trauma. Chicago: American College of Surgeons; 1997. pp. 87–107. [Google Scholar]
- 25.Malhotra K, Axisa B. Low plasma albumin linked to fluid overload in postoperative epidural patients. Ann R Coll Surg Engl. 2009;91(8):703–707. doi: 10.1308/003588409X12486167522072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulimood TB, Park GR. Debate: Albumin administration should be avoided in the critically ill. Critical Care. 2000;4(3):151–155. doi: 10.1186/cc688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27(4):380–389. doi: 10.1097/01.shk.0000239782.71516.ba. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Backer DD. Microcirculatory alterations in the critically ill. Hosp Pract (Minneap) 2009;37(1):107–112. doi: 10.3810/hp.2009.12.262. [DOI] [PubMed] [Google Scholar]
- 29.De Backer D, Creteur J, Dubois MJ, et al. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147(1):91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Cheung ATW, To PL, Chan DM, et al. Comparison of treatment modalities for hemorrhagic shock. Artif Cell Blood Sub. 2007;35(2):173–190. doi: 10.1080/10731190601188257. [DOI] [PubMed] [Google Scholar]
- 31.Ballermann BJ, Dardik A, Eng E, et al. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 32.Westphal M, James MF, Kozek-Langenecker S, et al. Hydroxyethyl starches: different products--different effects. Anesthesiology. 2009;111(1):187–202. doi: 10.1097/ALN.0b013e3181a7ec82. [DOI] [PubMed] [Google Scholar]