Abstract
Aminoglycoside antibiotics and cisplatin (CDDP) are the major ototoxins of clinical medicine due to their capacity to cause significant, as well as permanent hearing loss by targeting the mammalian sensory cells. Understanding the pathogenesis of damage is the first step in designing effective prevention of drug-induced hearing loss. In-vitro systems greatly enhance the efficiency of biochemical and molecular investigations through ease of access and manipulation. HEI-OC1, an inner ear cell line derived from the immortomouse, expresses markers for auditory sensory cells and, therefore, is a potential tool to study the ototoxic mechanisms of drugs like aminoglycoside antibiotics and CDDP. HEI-OC1 cells (and also HeLa cells) efficiently take up fluorescently tagged gentamicin and respond to drug treatment with changes in cell death and survival signaling pathways. Within hours, the C-jun N-terminal kinase pathway and the transcription factor AP-1 were activated and at later times, the “executioner caspase”, caspase-3. These responses were robust and elicited by both gentamicin and kanamycin. However, despite the initiation of apoptotic pathways and transient changes in nuclear morphology, cell death was not observed following aminoglycoside treatment, while administration of CDDP lead to significant cell death as determined by flow cytometric measurements; β-galactosidase analysis ruled out senescence in gentamicin-treated cells. The ability to withstand treatment with aminoglycosides but not with CDDP suggests that this cell line might be helpful in providing some insight into the differential actions of the two ototoxic drugs.
Keywords: HEI-OC1 cell line, aminoglycoside, cisplatin, cell death, caspase-3, jun-kinase, AP-1, NF-κB, endonuclease G
Introduction
While clinical trials are the ultimate tests to establish rational treatments and preventive measures for inner ear pathogenesis, model systems are often used to explore such therapeutic strategies and to elucidate fundamental pathways. In-vivo animal experiments are a standard approach, but they also present significant complexity and potentially confounding factors, so that simplified systems have frequently been sought. Organ or cell cultures provide more accessible and convenient ways to investigate cellular pathways and have been exploited in neurobiological research (Lendahl and McKay 1990) including visual and olfactory studies (Seigel 1999; Barber and Ronnet 2000).
Likewise, research into auditory and vestibular systems has benefited from cell lines that were developed from different tissues and developmental stages of the inner ear. More than 300 cell lines have been established, some of which express molecular markers of inner ear sensory cells (Helyer et al. 2007; Germiller et al. 2004). For example, HEI-OC1, a conditionally immortalized mouse cell line derived from the postnatal organ of Corti, displays a variety of phenotypes and expresses math1, myosin 7a, and prestin in addition to markers for non-sensory cells. Because of its simplicity and ease of manipulation, it is a potential model system to screen for ototoxic chemicals and to investigate mechanisms of action. In support of this notion, HEI-OC1 cells respond to aminoglycoside antibiotics by activating caspase-3, a reaction that should indicate ensuing cell death (Kalinec et al. 2003).
On the other hand, responses in vitro might vary from responses in vivo. We have recently documented the up-regulation of caspase-independent cell death pathways in the inner ear following chronic administration of aminoglycosides in-vivo (Jiang et al. 2006). In contrast, acute application of the drugs to cultured cells and organ culture may predominantly activate caspase-dependent cell death pathways (Forge and Li et al., 2000; Cunningham et al., 2002). The current study investigates HEI-OC1 cell cultures, both under permissive and non-permissive conditions, in order to explore the potential contribution of cell lines to the analysis of ototoxic mechanisms and, in particular, of aminoglycoside-induced signaling pathways.
Materials and Methods
Materials
Gentamicin sulfate was purchased from Spectrum Quality Products Incorporation (Gardena, CA; Cat #G1005); kanamycin sulfate from USB Corporation (Cleveland, OH; Cat #17924; Lot #110755); poly(dI-dC) double strand (Lot #3127880021) and MicroSpin™ G-50 columns (Cat #27-5330-01; Lot #19544) from Amersham Pharmacia Biotech (Piscataway, NJ); anti-phospho-JNK1/2 from Cell Signaling Technology Inc. (Beverly, MA); anti-cleaved caspase-3 antibody, T4 Polynucleotide Kinase (Cat #M410A; Lot #15453917), and AP-1 oligonucleotide (Cat #E320A; Lot #13326607) from Promega Corporation (Madison, WI, USA). Secondary fluorescent antibodies (Alexa 488), propidium iodide and Hoechst 33342 came from Molecular Probes Inc. (Eugene, OR, USA). Complete™ mini EDTA free protease inhibitor cocktail tablets were purchased from Roche Diagnostic GmbH (Mannheim, Germany), and [γ-32P]-ATP from PerkinElmer Life And Analytical Sciences, Inc (Boston, MA). Galacto-Light Plus™ Chemiluminescent Reporter Gene Assays for β-galactosidase was obtained from AB Applied Biosystems (Foster City, CA). Texas Red and gentamicin linked to Texas Red (GTTR) were the kind gifts of Dr. Peter Steyger (Oregon Health & Science University, Portland, OR). All other reagents came from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Cell line and culture conditions
HEI-OC1, an inner ear cell line, was provided by Dr. Kalinec, House Ear Institute, Los Angeles, CA. The cell line was cultured on plastic culture dishes under permissive conditions (33°C, 10% CO2) in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS; Gibco BRL) and 50 U/ml gamma-interferon (Genzyme, Cambridge, MA) to proliferate. The proliferating cell culture was moved to non-permissive conditions (37°C, 5% CO2) in DMEM containing 10% FBS to differentiate. Cells were grown to ~80% confluency and regular medium was exchanged for medium with or without drugs. Since both the gentamicin and kanamycin preparations contain sulfate as the counter-ion, control incubations were performed with 2.6 molar equivalents of Na2SO4 to test for any potential effects of the sulfate contents.
HeLa cells from an immortalized human cervical cancer cell line were provided by Dr. Omar Moussa (Department of Pathology and Laboratory Medicine, Medical University of South Carolina, Charleston, SC). The cell line was cultured on plastic culture dishes at 37°C, 5% CO2 in Roswell Park Memorial Institute 1640 (RPMI 1640; Thermo Scientific Hyclone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS; Gibco BRL), and 1% penicillin streptomycin (Pen/Strep; Sigma-Aldrich, St. Louis, MO, USA). After cells were grown to ~80% confluency, regular medium was exchanged for medium with or without drugs.
Gentamicin uptake
HEI-OC1 or HeLa cells were transferred to chamber slides (Nalge Nunc International, Rochester, NY) and maintained in culture medium under permissive conditions for 1 d when the medium was exchanged to include 1.56 µg/mL gentamicin coupled to Texas Red (GTTR; Myrdal et al., 2005). Following incubation with GTTR for the desired time points, the cells were washed twice with PBS and fixed with 4% paraformaldehyde at 4°C for 20 min. The cells were then counter-stained with Hoechst 33342 for 30 min at room temperature. After three washes with phosphate-buffered saline, pH 7.4 (PBS), the slides were mounted with GelMount (Biomedia Corp. , Foster City, CA) and observed with an Olympus confocal microscope.
Cell death quantification by staining with trypan blue
Control- and aminoglycoside-treated cell cultures grown on 100 mm Falcon culture plates were washed twice with 10 mM PBS and incubated at 37°C for 10 min in 1 mL 0.05% trypsin and 0.53 mM EDTA in PBS. The trypsin was neutralized with 4 mL DMEM and the cells were mixed into a homogenous suspension by vigorous pipetting. They then were transferred to 15 mL conical tubes. A 500-µL sample was taken from each tube for analysis. The samples were diluted in DMEM and mixed with 0.4% trypan blue (Invitrogen, Carlsbad, CA) solution at a 1:1 ratio. The samples were quickly counted under a light microscope after addition of the trypan blue stain using a hemocytometer loaded with 15 µL per chamber. Counts are presented as dead cells (taking up the stain) per total cells.
Cell death quantification by staining with calcein AM and propidium iodide
Cells were grown and treated on glass cover slips in six-well tissue culture plates. After treatment, cells were incubated with 2 µM calcein AM (Molecular Probes, Eugene, OR) in DMEM for 35 min at room temperature in the dark and washed once with DMEM. The cells were then incubated with 5 µM propidium iodide (PI) in DMEM for 30 min at room temperature in the dark. The cells were washed for 35 min in DMEM prior to counting to allow the calcein AM to diffuse out of cells with disrupted membranes (dead cells). Cells were counted on the cover slips mounted with media. They were scored as percent dead cells per total cells under a fluorescent microscope by separately counting cells with nuclear PI staining at 515–560 nm and calcein AM-stained cells at 450–490 nm excitation wavelengths.
Cell death quantification by MTT Assay
HEI-OC1 cells grown on 100 mm Falcon culture plates were incubated at 37°C for 5 min in 2 mL 0.25% trypsin and 0.53 mM EDTA in PBS. The trypsin was neutralized with 10 mL DMEM and transferred to 15 mL conical tubes. The cells were collected, resuspended in 1 mL of DMEM and mixed into a homogenous suspension by vigorous pipetting. A 10-µL sample was taken from the tube and diluted in 0.4% trypan blue (Invitrogen, Carlsbad, CA) solution at a 9:1 ratio. The samples were quickly counted under a light microscope after addition of trypan blue stain using a hemocytometer loaded with 15 µL per chamber to ensure that viability was not less than 95%. Cells were diluted to 1.1×105 cells in DMEM with 10% FBS and 50U/mL gamma-interferon. Ninety µL of cells were transferred into appropriate wells of a 96-well flat bottom microtiter plate (Corning Incorporated, Corning, NY) and incubated overnight at 33°C and 10% CO2. Once cells have reached the 70 to 80% confluency, 10 µL of medium with or without drugs were added to the appropriate wells and incubated (33°C, 10% CO2) for the desired time points. Following overnight incubation, media was removed from the 96-well microtiter plate and cells were incubated (33°C, 10% CO2) for 4 h with 115 µL of MTT dye solution containing Phenol Red Free DMEM (Invitrogen Gibco, Carlsbad, CA), 10% FBS, 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 50 U/mL gamma interferon, and 15 µL of MTT dye (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Promega, Madison, WI) per well. One hundred µL of Solubilization/Stop solution (Promega, Madison, WI) were added to each well and incubated (33°C, 10% CO2) for an additional 4 h. The microtiter plate was read within 24 h with a µQuant Microplate reader and analysed by Alpha Innotech software. Absorption was read at a primary wavelength of 570 nm and a secondary wavelength of 630 nm.
Cell death quantification by flow cytometry
Drug-treated (gentamicin or CDDP) and control cell cultures were incubated with BD ApoAlert™ Annexin V-FITC Apoptosis Kit (BD Biosciences Clontech, Palo Alto, CA) per the manufacturer’s instruction. First, 5×105 cells were collected and rinsed with binding buffer, then resuspended in 200 µL of binding buffer. Next, 5 µL of Annexin V and 10 µL of propidium iodide were added to the cells and were incubated at room temperature for 10 min in the dark. Another 300 µL of binding buffer was added to bring the reaction volume up to 500 µL before the cells were counted using a single laser emitting excitation light at 488 nm. Cell death was determined by double propidium iodide and annexin-V staining. Viable cells were stained double negative, and early apoptotic cells were stained with annexin-V only.
Extraction of nuclear protein
Cell cultures were rapidly rinsed with ice-cold 10 mM PBS and then lifted in Buffer A consisting of 10 mM sodium HEPES (pH 7.9) with 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 5 mM dithiothreitol, 10 mM NaF, 10 mM sodium β-glycerophosphate, and protease inhibitor (one tablet of inhibitor cocktail per 10 ml of Buffer A). After 15 min on ice, 10% Nonidet P-40 was added as a final concentration of 0.6% to lyse the cells, which remained on ice for another 30 min. A nuclear fraction was pelleted by centrifugation at 750 × g at 4°C for 10 min. The pellet was rinsed with Buffer A, suspended in Buffer B consisting of 50 mM Tris-HCl (pH 7.5) with 5 mM MgCl2, 20% glycerol, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol, 0.25 mg/ml poly(dl-dC)-poly(dl-dC) and kept on ice for 30 min. The nuclear extracts were collected in the supernatant following centrifugation at 15,000 × g for 10 min at 4°C. Protein concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Extraction of total protein
Cell cultures were rapidly rinsed twice with ice-cold 10 mM PBS and then ice-cold RIPA lysis buffer containing RIPA lysis buffer base (50 mM Tris-HCl, 1% IGEPAL, 0.25% Na-deoxycholate, 150 mM NaCl, 1mM EDTA, 1 mM PMSF, 1 mM NaF) plus Phosphatase Inhibitor Cocktails II and III, and Roche Protease Inhibitor were added to the plates. Cells were scraped from the bottom of the dishes moved to conical tubes. After 30 min on ice, tissue debris was removed by centrifugation at 10,000 × g at 4°C for 10 min and the supernatants were retained as the total protein fractions. Protein concentrations were determined using the Bio-Rad Protein Assay dye reagent (Bio-Rad, Hercules, CA) with bovine serum albumin as a protein standard.
Immunocytochemistry
Cell cultures were rinsed with ice-cold PBS three times, then fixed immediately with 4% paraformaldehyde for 10 min and incubated in 0.5% Triton X-100 for 15 min at room temperature. After washing three times with PBS, a blocking solution of 3% goat serum was added to the cells for 30 min at room temperature, followed by the primary antibody of either p-JNK at dilution of 1:100 or of cleaved caspase-3 at a dilution of 1:200 in PBS for 2 h. The cultures were then washed three times with PBS and incubated with secondary antibody conjugated with Alexa 488 in a dilution of 1:500 in PBS for 1 h at room temperature in darkness. The cultures were then stained with propidium iodide (2 µg/mL in PBS) for 40 min in darkness. After washing with PBS, the fixed cultures were mounted and photographed using a laser confocal microscope (Olympus American, Melville, NY).
Electromobility shift assay
Ten ng of double-stranded AP-1 or NF-κB oligonucleotides were end-labeled with [32P]ATP and T4 polynucleotide kinase. Ten µg of nuclear extract and 50,000 cpm of labeled oligonucleotides were added to binding buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 20 mM glycerol, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol (DTT), and 0.25 mg/mL poly (dI-dC). The reactions were incubated at 25°C for 30 min. The protein-DNA complexes were separated on 4.5% acrylamide gel and visualized by autoradiography.
β-Galactosidase assay
At the end of the desired incubation time, HEI-OC1 cells were scraped from the bottom of the dishes into the medium, moved to conical tubes, and centrifuged at 682 × g for 5 min. The medium was decanted from the cell pellet and sterile PBS was added to rinse. While the PBS rinse was repeated, DTT was added to the lysis solution provided in the Galacto-Light Plus System kit from AB Applied Biosystems to a concentration of 0.5 mM. Lysis solution was added to the cell pellet after removing the PBS. The final cells were then mixed thoroughly into the lysis solution, transferred to a micro-centrifuge tube, and centrifuged at 13,000 × g for 2 min to pellet cell debris. The supernatant was retained and stored at −80°C (Dimri et al., 1995). The concentration of β-galactosidase was determined using Galacto-Light Plus™ Chemiluminescent Reporter Gene Assays kit according to the manufacturer’s protocol. Assays were conducted in duplicates and incubations with sodium sulfate served as controls.
Statistical analysis
All data were evaluated statistically by Student’s t-test and by analyses of variance with Student-Newman-Keuls test for significance (p < 0.05) using Primer of Biostatistics software (McGraw-Hill Software, New York, NY). The western blots and EMSA were evaluated by one-sided single-sample t-tests using SPSS software by the University of Michigan’s Center for Statistical Consultation and Research.
Results
Both HEI-OC1 cells and HeLa cells take up gentamicin
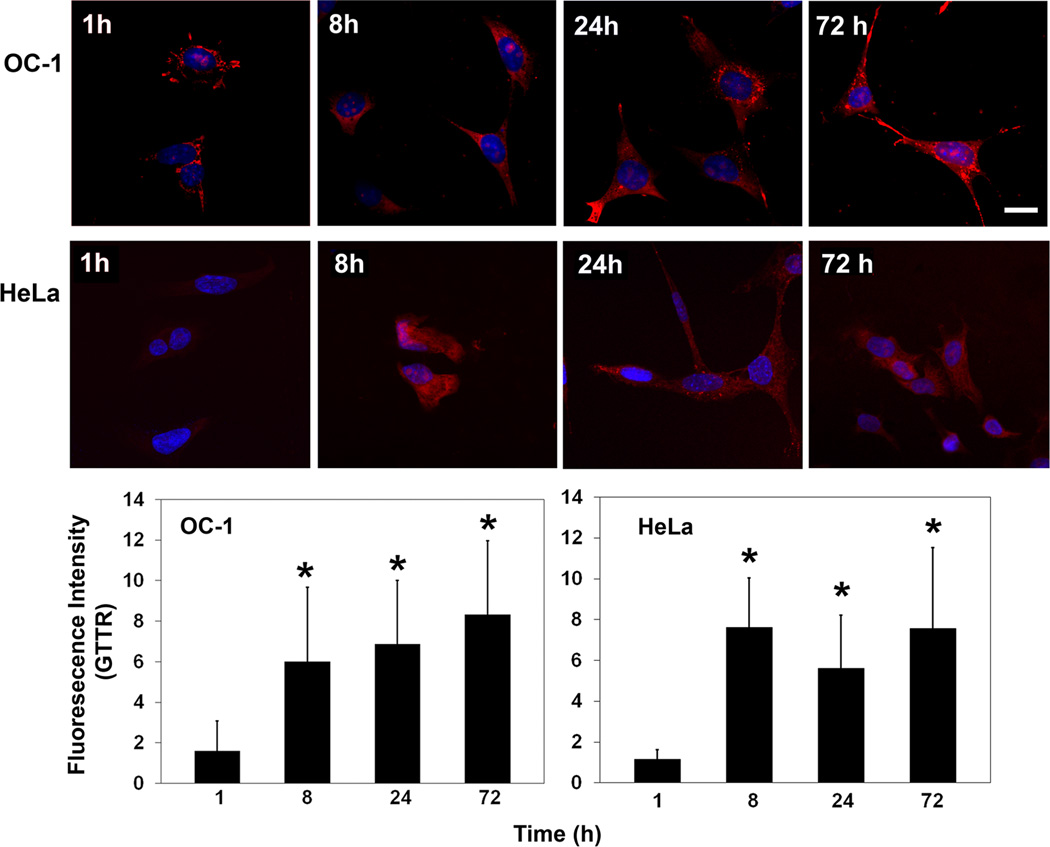
Uptake of gentamicin into HEI-OC1 cells was monitored via the drug tagged to fluorescent Texas Red (GTTR; Myrdal et al., 2005). Uptake was evident at 1 h and intensity of GTTR significantly increased at 8 h and remained stable at later time points as determined at 24 and 72 h. At 24 and 72 h, however, staining became more punctate and heavier in the nucleus (fig. 1). HeLa cells, a cell line that was derived from human cervical cancer tissue, showed similar uptake of gentamicin at 8, 24 and 72 h time points (fig. 1).
Figure 1.
Uptake of gentamicin. Following incubation of HeLa or HEI-OC1 cultures under permissive conditions with GTTR (red) for the times indicated, cells were processed for confocal microscopy. Uptake of GTTR was evident in both types of cells. Representative images of each time point are shown and experiments were repeated three times. Fluorescence intensity was measured by Image J software. Blue is Hoechst 33342 counter stain of nuclei. Scale bar = 20 µm. * indicates p < 0.05 compared to control.
Potentially apoptotic cell death pathways are activated by aminoglycoside treatment
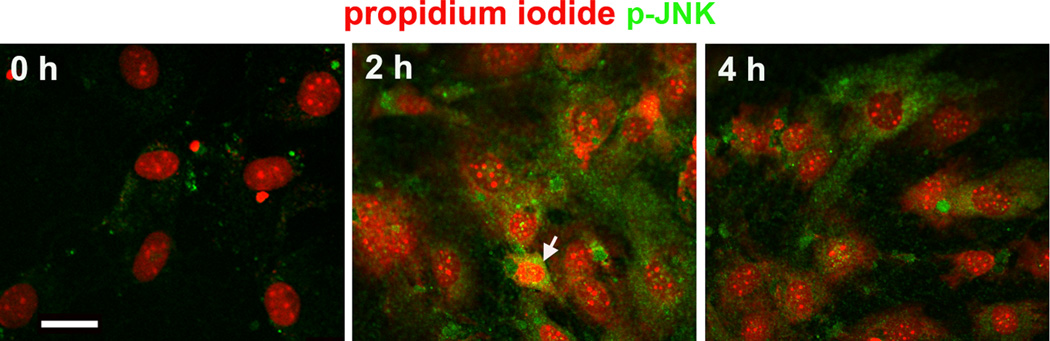
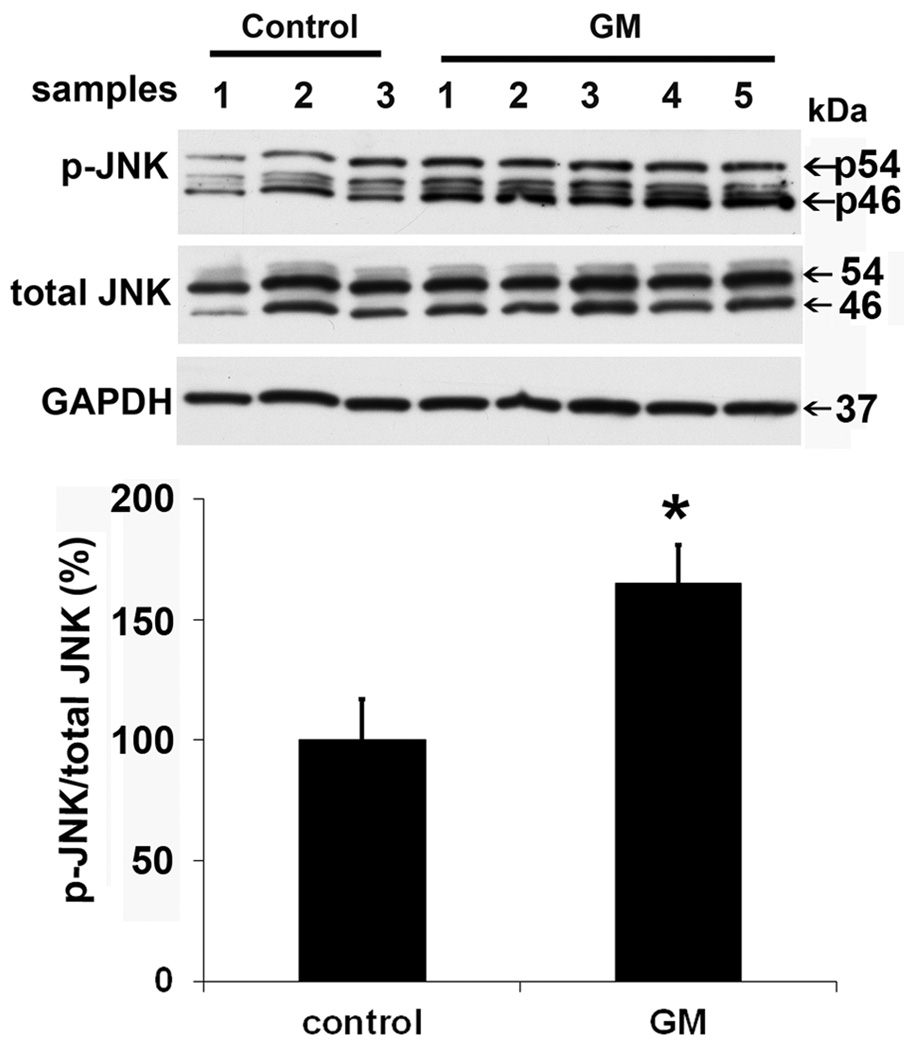
Aminoglycosides trigger the activation of C-jun N-terminal kinase (JNK) by phosphorylation as part of an apoptotic sequence in organ culture and, to some extent, in vivo (Yikoski et al., 2002; Jiang et al., 2006). Immunocytochemical analysis showed that the JNK pathway responds early to aminoglycoside exposure in both permissive and non-permissive conditions of HEI-OC1 cell cultures. Green staining for p-JNK in HEI-OC1 cells increased from 2 to 4 h after gentamicin treatment (fig. 2A). Based on the immunocytochemical staining for p-JNK, we chose the 2 h post-gentamicin time point for further study. The expression of p-JNK by Western blot analysis showed that the ratio of p-JNK to total-JNK significantly increased by 60% (fig. 2B). A similar response was observed with 2 mM kanamycin treatment (data not shown).
Figure 2.
JNK is phosphorylated. (A) This representative image is from an incubation under non-permissive conditions. The arrow indicates a cell with a condensed nucleus. No phosphorylated JNK was detected in control cells treated with 2.6 mM Na2SO4 for 2 h. Each panel is representative of three individual experiments. Green indicates p-JNK; Red represents propidium iodide to counter-stain the nuclei. Scale bar = 20 µm. (B) Western blot for the quantification of p-JNK. OC-1 cells were treated with 1 mM gentamicin for 2 h. GAPDH services as loading control. Data are means ± s. d.; * indicates p < 0.05.
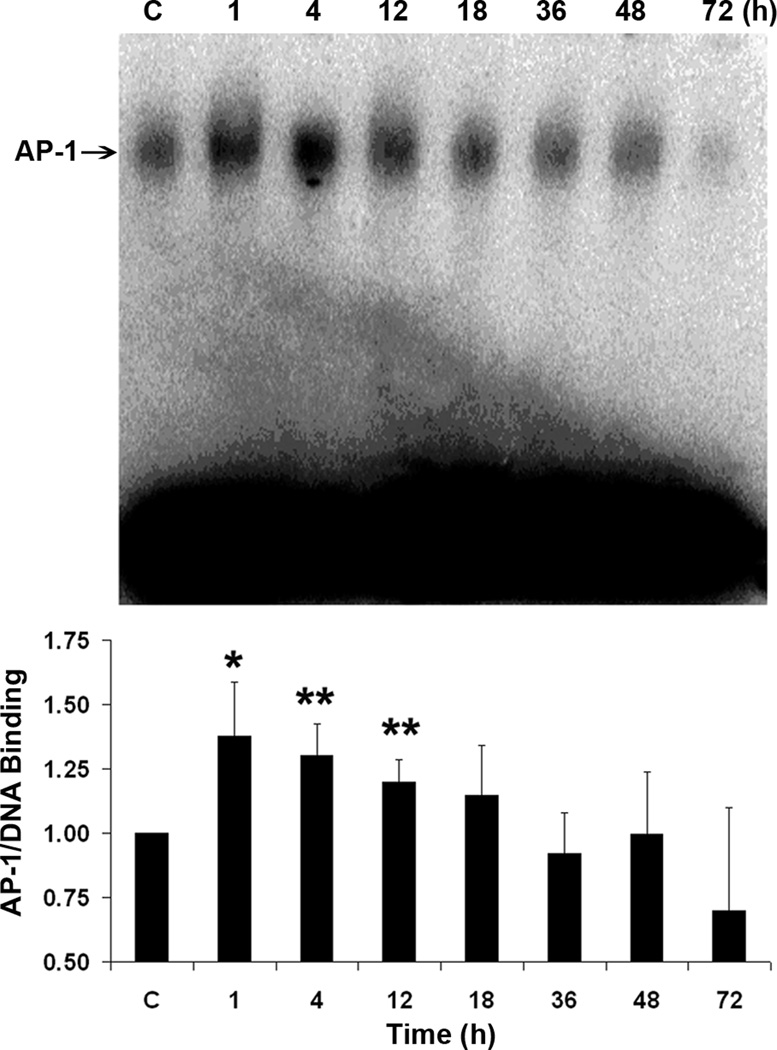
The activator protein-1 (AP-1) transcription factor is a downstream target of JNK. It is a heterodimeric or homodimeric complex of primarily the jun and fos family of proteins. Nuclear extracts of HEI-OC1 cell cultures grown under both permissive and non-permissive conditions were analyzed using an electromobility shift assay for AP-1 binding activity to DNA. Semi-quantitative analysis of the bands showed that treatment with 1 mM gentamicin increased DNA binding activity of AP-1 significantly from 1 to 12 h and decreased later (fig. 3). A similar pattern was seen with 2 mM kanamycin treatment with a significant increase around 2 to 4 h (data not shown).
Figure 3.
AP-1/DNA binding activity increases. Analysis of the AP-1 binding of HEI-OC1 cells under non-permissive conditions showed a significant increase of DNA binding activity of AP-1 after 1, 4, and 12 h of treatment with 1 mM gentamicin (*p < 0.05 at 1 h; **p < 0.01 at 4 and 12 h). Data are presented as mean ± s. d., n = 4. A representative image is presented above the bar graph.
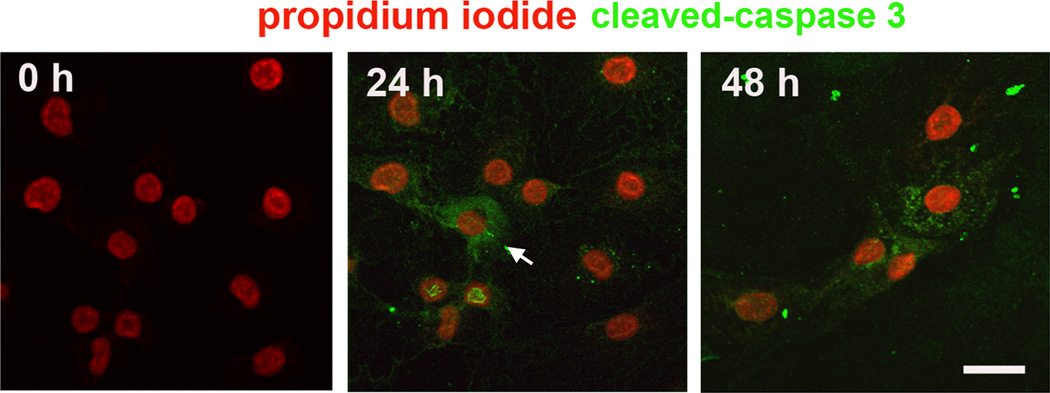
Caspases are a family of cysteine proteases participating in the regulation and execution of apoptosis. They are activated by aminoglycosides in the HEI-OC1 cell line (Kalinec et al. 2003) as well as in vestibular utricle culture (Cunningham et al. 2002). Consistent with these reports, treatment with either gentamicin or kanamycin dramatically increased active caspase-3 in our cultures at 24 and 48 h of treatment (fig. 4).
Figure 4.
Caspase-3 is activated. Active caspase-3 was detected with anti-cleaved caspase-3 antibody shown in green after fixation of HEI-OC1 cells under non-permissive condition. After 24 and 48 h of treatment with 1 mM gentamicin, some cells showed positive staining of active caspase-3 (arrow). Each panel is representative of three individual experiments. Propidium iodide (red) acts as counter-stain for nuclei. Scale bar = 20 µm.
Endonuclease G (endo G) is a highly conserved non-specific mitochondrial nuclease encoded by a nuclear gene. Following potentially apoptotic insults, endo G can be released from mitochondria and translocated to the nucleus to digest nuclear DNA. We analyzed for endo G because it has been shown that activation and translocation occurs after kanamycin treatment in vivo (Jiang et al. 2006). However, there was no evidence that endo G was involved in the response of the HEI-OC1 cell culture to aminoglycosides. Immunofluorescent staining localized endo G to the cytosol in both proliferated and differentiated HEI-OC1 cell cultures and remained cytoplasmic after 1, 2, 4, 12, 24, 48, 72, and 96 h of treatment with either 1 mM gentamicin or 2 mM kanamycin.
Appropriate controls were included in all experiments to account for the presence of sulfate as the counter ion to aminoglycosides. The presence of sodium sulfate (Na2SO4) did not influence the pathways that we tested, nor did it affect any of the parameters in subsequent experiments.
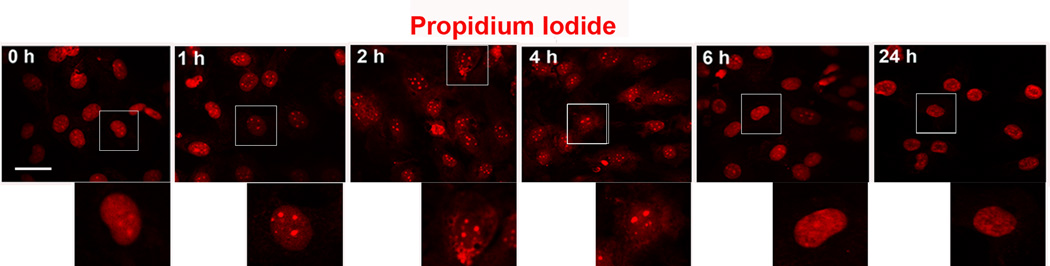
Transient changes in nuclear morphology
Nuclear morphology began to change after exposure to gentamicin of only 2 to 4 h (fig. 5). The sharp outline of the nuclei was lost suggesting a compromised integrity of the nuclear membrane, and punctate formations reminiscent of small chromatin aggregates appeared. However, the appearance of the nuclei reverted to normal after approximately 6 h of exposure to the drug and became indistinguishable from the nuclei in the control cells.
Figure 5.
Nuclear morphology changes transiently. HEI-OC1 cell cultures treated with 1 mM gentamicin were fixed at different time points and stained with propidium iodide (red). At 2 to 4 h following gentamicin treatment, the nuclei lost their sharp outline, but appeared to revert to a normal appearance at longer incubation times in the presence of drug. The panels are representative of three incubations at each time point. Inserted images show higher magnification of nuclear morphology. Scale bar = 20 µm.
Aminoglycosides do not promote cell death in HEI-OC1 cells
The drug concentrations for this study were chosen based on the fact that they will completely eliminate hair cells in short-term culture of the mouse organ of Corti (Chen et al., 2009). Cell death and survival following treatment with gentamicin or kanamycin was assessed in HEI-OC1 cells under both permissive and non-permissive conditions by staining with trypan blue. Since the nuclear morphology changed 2 to 4 h after gentamicin treatment and caspase-3 activation was observed during the first day of exposure to the drug, we selected treatment times of 4 h and 1, 3, and 5 d for the evaluation of cell counts. First, cells were stained with live/dead dyes and counted under the microscope. For each treatment condition approximately 600 – 800 cells were counted and counts were repeated three times. No significant cell death after gentamicin or kanamycin treatment was observed. Even after 5 d of exposure to gentamicin, only 1.0 ± 0.3% (at 1 mM gentamicin) or 0.9 ± 0.2% (at 2 mM kanamycin) of HEI-OC1 cells took up Trypan Blue. This percentage was indistinguishable from the control incubations (0.9 ± 0.3%) indicating that the drugs did not induce cell death.
Interestingly, double-staining of a subset of the cells suggested that the plasma membrane was intact enough to retain calcein AM, but that the nuclear membrane was compromised allowing for the PI to bind nuclear DNA. This subset consisted of 5.2 ± 0.6% of control cells and 12.7 ± 3.5% of cells exposed to 1 mM gentamicin for 24 h (p < 0.05).
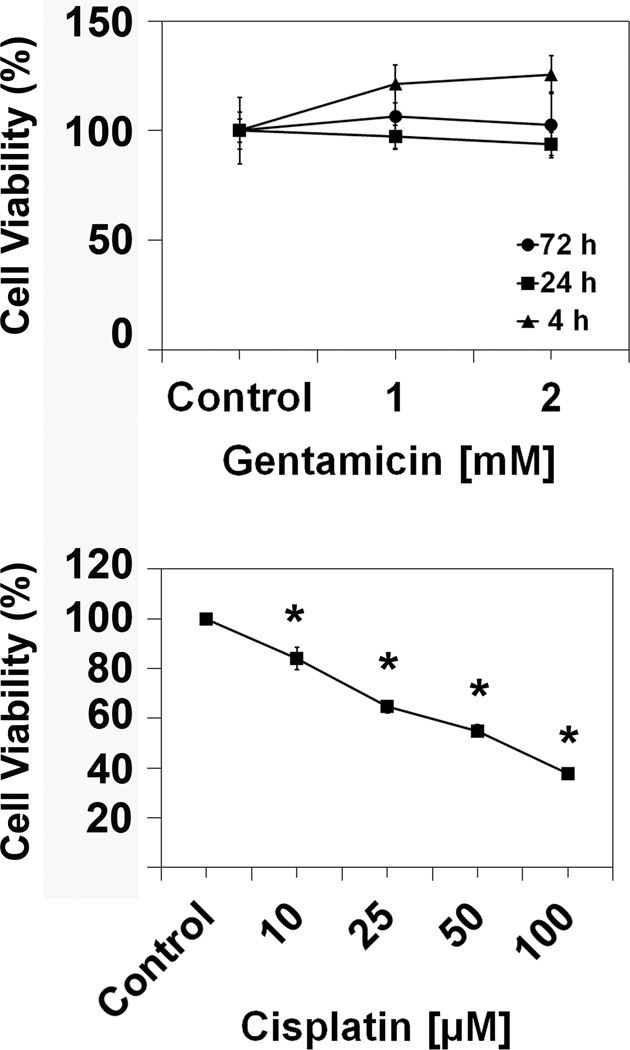
In addition, we determined cell viability by assessing mitochondrial enzyme activity in an MTT assay (fig. 6). At the 4, 24, and 72 h time points, there was no difference in cell viability between control, 1 mM and 2 mM gentamicin treatments, confirming the observations that aminoglycosides did not increase cell death. In contrast, treatment with cisplatin at concentrations as low as 10 µM produced a significant decrease in cell viability and cell death increased with increasing doses of cisplatin reaching 50% at a concentration of 35 µM (fig. 6).
Figure 6.
Gentamicin does not reduce cell viability. Following incubation with 1 and 2 mM gentamicin for 4, 24, and 72 h, the viability of HEI-OC1 cells under permissive conditions was measured via MTT assay as described in the ‘Methods’ section. There was no difference in enzymatic activity between control cells and gentamicin treated cells, but there was a significant difference between controls and cisplatin treatment. Data are presented as mean ± s. d., n = 5 for each drug concentration shown. * represents p < 0.05 compared to control.
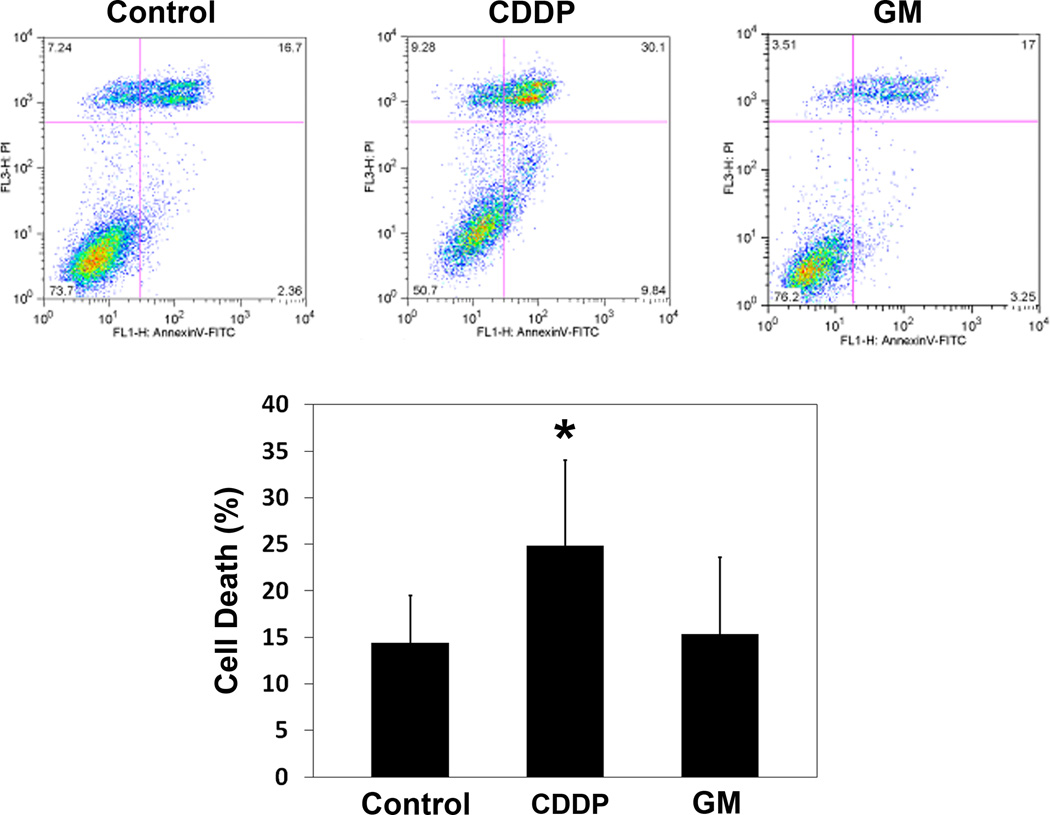
Flow cytometry analysis corroborated that aminoglycosides did not increase cell death while CDDP induced significant cell death (fig. 7). There was no difference between the control and gentamicin-treated cultures. In contrast, a significant reduction in the number of viable cells was achieved by treatment with 35 µM cisplatin.
Figure 7.
Gentamicin does not induce cell death. Following incubation with 1 mM gentamicin for 24 h, HEI-OC1 cells under permissive conditions were stained for apoptotic features and analyzed by a flow cytometry assay. There was no difference in cell death between control and 1 mM gentamicin treated cells. In contrast, incubation with 35 µM cisplatin significantly increased the number of cells with features of cell death. Data are presented as mean + s. d., n = 5 for each treatment. * represents p < 0.05 compared to control.
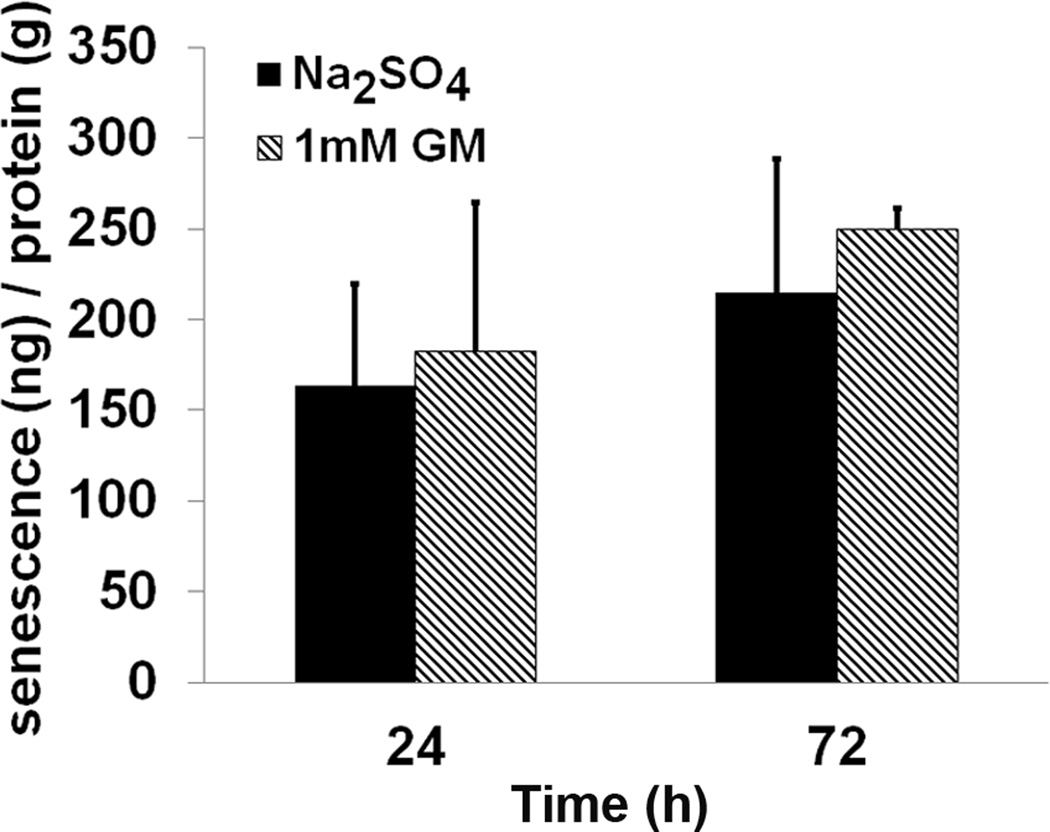
Finally, in order to investigate whether the absence of cell death by gentamicin was due to the cells entering into senescence, the levels of β-galactosidase were measured. Proliferating cells were treated with 1 mM gentamicin for 3, 24 and 72 h. No change in the levels of β-galactosidase was observed (fig 8).
Figure 8.
Gentamicin does not induce cellular senescence. There was no difference in β-galactosidase activity between 1 mM gentamicin treatment or control HEI-OC1 cells under permissive condition at 24 and 72 h. Control cells were treated with 2.6 mM Na2SO4. Data are presented as mean + s. d., n = 3 for each experimental condition.
Discussion
The responses to an aminoglycoside challenge differ significantly between the HEI-OC1 cells employed in this study and in-vivo or organ culture models of ototoxicity. The most significant discrepancy is the absence of cell death in the HEI-OC1 cells despite the transient activation of apoptotic signals and activation of the transcription factor AP-1 upon either gentamicin or kanamycin exposure. We should note in this context that the gentamicin concentrations employed here are ten times higher than the concentrations that will cause complete hair cell death in short-term cultures of organ of Corti explants (Chen et al., 2009). The survival of the HEI-OC1 cells despite the appearance of cleaved caspase 3 and p-JNK was robust regardless of permissive or non-permissive conditions or which aminoglycoside was used. Also of interest is the fact that the apoptotic marker endo G remained unaffected by drug treatment in the HEI-OC1 cells while it translocated to the nuclei of hair cells in vivo after aminoglycoside treatment (Jiang et al., 2005; Jiang et al., 2006). The sum of these observations strongly supports the notion that responses to aminoglycoside challenge are model-dependent.
The resistance of the HEI-OC1 cells to aminoglycoside-induced cell death is remarkable. It is not a simple consequence of a lack of internalization of the drugs since gentamicin is quickly and efficiently taken up. Incidentally, the efficient entry of GTTR into HEI-OC1 and HeLa cells suggests that functional hair cell mechanotransducer channels in stereocilia may not be required for cellular uptake and that other transport mechanisms must exist (Alharazneh et al., 2011; Xie et al., 2011). It is most intriguing that some of the early molecular responses, like jun-kinase and caspase-3, are similar to those documented for ototoxic treatment with aminoglycosides in other systems (Rybak et al., 2008) and should be expected to lead to cell death. For example, studies of vestibular organ explant cultures of guinea pigs and gerbils show that caspase-9 and caspase-3 are activated by aminoglycosides, leading to apoptosis as the primary type of cell death (Cunningham, et al., 2002,Cunningham, et al., 2004,Lee, et al., 2004). Consequently, the presence of caspase inhibitors rescues the majority of sensory cells from ototoxic insults (Forge and Li, 2000; Lee, et al., 2004).
This enigmatic behavior of transient caspase activation is reflected in the morphological changes occurring in the nuclei. The nuclear membrane appears to be compromised during early exposure to gentamicin and there are changes in nuclear morphology that resemble chromatin condensation. Similar morphological alterations can be observed in other cell lines exposed to aminoglycoside antibiotics, such as aminoglycoside-sensitive renal LLC-PK1 or MDCK cell cultures (El Mouedden, et al., 2000) and OC-k3, another immortalized mouse cell line from the organ of Corti (Bertolaso et al., 2003). In contrast to HEI-OC1 cells, however, these other cell lines lose their viability. The nuclei of HEI-OC1 cells regain an essentially normal appearance in the continued presence of the drugs and do not undergo apoptosis. Both MTT assays and flow cytometry of cells labeled with live/dead markers and quantification of cell numbers extend this observation and confirm the lack of significant detrimental effect of aminoglycosides on HEI-OC1 cells.
The reasons for the resistance to apoptotic signals are not entirely clear. Resistance can arise in some cell types when they undergo senescence, for example, in reaction to oxidative stress (Chen et al., 2000). Aminoglycosides trigger oxidative stress in vivo (Jiang et al., 2005) as well as in organ of Corti explants (Choung et al., 2009) and could create a stable senescent cell culture. However, this explanation is ruled out by the fact that β-galactosidase activity, a biomarker of senescence (Dimri et al., 1995), does not increase. One might also consider the fact that the transgenic immortomouse harbors the simian virus 40 (SV40) large tumor antigen (TAg) which is associated with tumorigenesis. The introduction of this gene is expected to change cellular signaling, especially the apoptotic pathways. The T antigen blocks apoptosis by binding to its pro-apoptotic protein, p193 or by abrogating p53 function; blocking p53 induces the expression of pro-apoptotic genes such as Box (Saenz-Robles et al. 2001). The HEI-OC1 cell line developed from the immortomouse utilizes a thermolabile TAg to reduce the levels of functional T antigen (Jat et al. 1991). Thus, while cell death pathways may be initiated, they do not follow the same signaling sequence as in in-vivo models and do not lead to the demise of the cell. Such an explanation is conceivable but remains incomplete since cisplatin will cause cell death in the HEI-OC1 cells.
However, while the activation of caspase-3 has long been considered an irreversible step towards apoptosis (Stennicke and Salvesen, 1998), recent evidence points towards a more complex role of caspases in cell fate determination including context-dependent non-apoptotic functions (Kuranga and Miura, 2007; Feinstein-Rotkopf and Arama, 2009; D’Amelio et al., 2010). Therefore, it is possible that aminoglycosides are only a limited toxic stimulus to HEI-OC1 cells and are able to induce only some early features of apoptosis. The cells would retain their capability for homeostatic responses and allow caspase-3 to execute non-apoptotic processes. In this case, the HEI-OC1 cells have the potential to be useful in providing insights into different mechanisms of cisplatin and aminoglycoside ototoxicity or into specific resistance capabilities against aminoglycosides.
Highlights.
The inner ear immortomouse cell line (HEI-OC1) efficiently takes up gentamicin conjugated with Texas Red (GTTR).
Aminoglycoside antibiotics activate caspase-dependent cell death pathways but do not cause cell death in the inner ear immortomouse cells (HEI-OC1).
Cellular senescence is not observed in the inner ear immortomouse cells (HEI-OC1) following gentamicin treatment.
Cisplatin treatment leads to inner ear immortomouse cell (HEI-OC1) death.
Acknowledgement
This work was partially conducted in Walton Research Building renovated space supported by grant C06 RR014516 from the National Center for Research Resources. Imaging facilities for partial work in this research were supported, in part, by Cancer Center Support Grant P30 CA138313 to the Hollings Cancer Center, Medical University of South Carolina. The research project described was supported by grant R01 DC-03685 and a core center grant P30 DC-05188 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Non-standard abbreviations
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
fetal bovine serum
- GTTR
gentamicin coupled to Texas Red
- MTT
Thiazolyl Blue Tetrazolium Blue
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. Epub 2011 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RD, Ronnett GV. Reconstructing smell. Mol Neurobiol. 2000;21:161–173. doi: 10.1385/MN:21:3:161. [DOI] [PubMed] [Google Scholar]
- Bertolaso L, Bindini D, Previati M, Falgione D, Lanzoni I, Parmeggiani A, et al. Gentamicin-induced cytotoxicity involves protein kinase C activation, glutathione extrusion and malondialdehyde production in an immortalized cell line from the organ of corti. Audiol Neurootol. 2003;8:38–48. doi: 10.1159/000067890. [DOI] [PubMed] [Google Scholar]
- Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem. 2009;108:1226–1236. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung YH, Taura A, Pak K, Choi SJ, Masuda M, Ryan AF. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience. 2009;161:214–226. doi: 10.1016/j.neuroscience.2009.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- D'Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouedden M, Laurent G, Mingeot-Leclercq MP, Tulkens PM. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol Sci. 2000;56:229–239. doi: 10.1093/toxsci/56.1.229. [DOI] [PubMed] [Google Scholar]
- Feinstein-Rotkopf Y, Arama E. Can't live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis. 2009;14:980–995. doi: 10.1007/s10495-009-0346-6. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Germiller JA, Smiley EC, Ellis AD, Hoff JS, Deshmukh I, Allen SJ, et al. Molecular characterization of conditionally immortalized cell lines derived from mouse early embryonic inner ear. Dev Dyn. 2004;231:815–827. doi: 10.1002/dvdy.20186. [DOI] [PubMed] [Google Scholar]
- Helyer R, Cacciabue-Rivolta D, Davies D, Rivolta MN, Kros CJ, et al. A model for mammalian cochlear hair cell differentiation in vitro: effects of retinoic acid on cytoskeletal proteins and potassium conductances. Eur J Neurosci. 2007;25:957–973. doi: 10.1111/j.1460-9568.2007.05338.x. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005;79:644–651. doi: 10.1002/jnr.20392. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha S-H, Forge A, Schacht J. Caspase-independent pathways on hair cell death induced by kanamycin in vivo. Cell Death Diff. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8:177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Kita T, et al. Signaling pathway for apoptosis of vestibular hair cells of mice due to aminoglycosides. Acta Otolaryngol Suppl. 2004:69–74. doi: 10.1080/03655230310016799. [DOI] [PubMed] [Google Scholar]
- Lendahl U, McKay RD. The use of cell lines in neurobiology. Trends Neurosci. 1990;13:132–137. doi: 10.1016/0166-2236(90)90004-t. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Talaska AE, Schacht J. Drug-induced hearing loss. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection and Repair. 2008. pp. 219–256. [Google Scholar]
- Saenz-Robles MT, Sullivan CS, Pipas JM. Transforming functions of Simian Virus 40. Oncogene. 2001;20:7899–7907. doi: 10.1038/sj.onc.1204936. [DOI] [PubMed] [Google Scholar]
- Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999;5:4. [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear. Res. 2011;281 doi: 10.1016/j.heares.2011.05.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yikoski J, Liang X-Q, Jussi V, Ulla P. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear. Res. 2002;166:33–43. doi: 10.1016/s0378-5955(01)00388-4. [DOI] [PubMed] [Google Scholar]