Abstract
There is an urgent global need for effective and affordable approaches to cervical cancer screening and diagnosis. For developing nations, cervical malignancies remain the leading cause of cancer death in women. This reality is difficult to accept given that these deaths are largely preventable; where cervical screening programs are implemented, cervical cancer deaths decrease dramatically. In the developed world, the challenges with respect to cervical disease stem from high costs and over-treatment. We are presently eleven years into a National Cancer Institute-funded Program Project (P01 CA82710) that is evaluating optical technologies for their applicability to the cervical cancer problem. Our mandate is to create new tools for disease detection and diagnosis that are inexpensive, require minimal expertise to use, are more accurate than existing modalities, and will be feasibly implemented in a variety of clinical settings. Herein, we update the status of this work and explain the long-term goals of this project.
Introduction
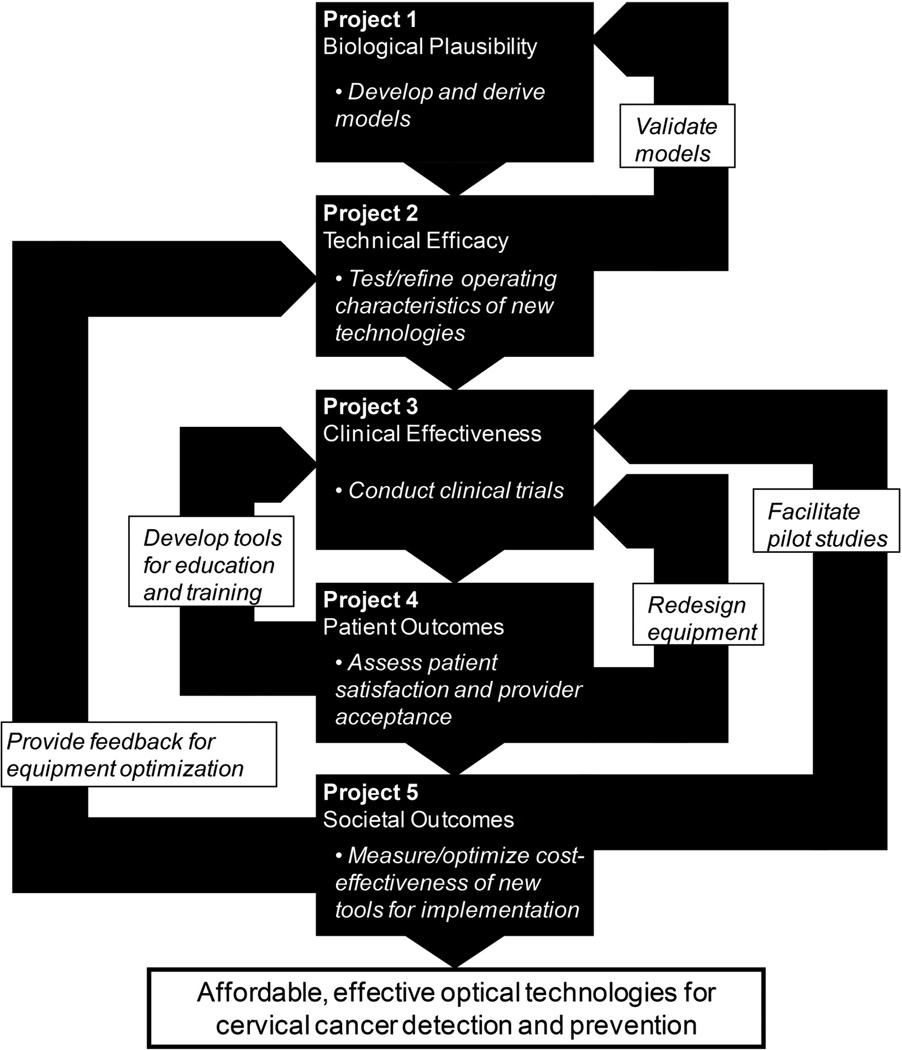
Eleven years ago, we assembled a multi-disciplinary research group to evaluate optical technologies for cervical cancer screening and diagnosis in both developed and developing nations. This team was comprised of optical engineers, gynecologic oncologists, clinician-pathologists, statisticians, computer scientists, epidemiologists, behavioral scientists, instrumentation engineers, and decision scientists. This National Cancer Institute (NCI)-funded study (P01 CA82710) has investigated the biologic plausibility, technical efficacy, clinical effectiveness, patient satisfaction, provider satisfaction, and cost-effectiveness of implementing optical technologies in a cervical cancer detection setting, as per the Littenberg technology assessment model (1) (the framework used to address each of these points is presented in Figure 1). Since we last discussed this project (2), our work has progressed significantly. Additionally, the field of cervical screening research has evolved. These changes – and the fact that cervical malignancies remain a leading cause of cancer death for women around the world – make timely this updated summary of our translational work.
Figure 1.
Framework of interaction between the five subprojects of the larger program project undertaking. Regular, effective interactions between project personnel have ensured that these connections remain robust.
Background
Cervical cancer remains the highest cause of cancer death for women in the developing world. For 2007, it is estimated that the number of new cervical cancer cases worldwide was 555,000 and the number of cervical cancer deaths was 310,000 (3). These advanced disease diagnoses and high death rates are due in large part to the absence of effective prevention and screening programs; where such programs exist, invasive disease and cancer deaths are reduced dramatically. For example, in British Columbia (Canada), cervical cancer incidence has declined by more than two thirds over the last 50 years (4). Eighty percent of cervical cancer deaths occur in the developing world, where access to effective screening and treatments is limited (3).
Approaches to disease screening and prevention
There are several methods to prevent and detect cervical neoplasias. The feasibility of adopting these around the globe can vary. It is known that the advent of vaccines against human papillomavirus (HPV) has the potential to greatly reduce cervical cancer incidence, since this virus is a key etiological factor in cervical neoplasia. Currently, HPV vaccines from Merck & Co. (Whitehouse Station, NJ; covering types 6, 11, 16, and 18) and GlaxoSmithKline (Brentford, UK; covering HPV 16 and 18) are licensed for use in 60 countries (5). At this time, these vaccines will only realistically be administered in developed countries, an unfortunate reality given that cervical cancer deaths occur at the low rate of 1/50,000 in these nations (6–11). There are multiple challenges associated with administering HPV vaccines in the developing world. First, the cost is prohibitive: both the bivalent and quadrivalent commercial HPV vaccines cost more than USD$120/dose – and three doses are needed for the vaccines to be effective (5). Second, it is challenging to achieve these repeated clinical visits in developing world populations, therefore not all required doses will be given. Third, the need for a continuous cold chain (i.e. refrigeration) for vaccine materials is often not feasible in developing countries. Fourth, highly prevalent HPV types in developing countries are not necessarily covered by current vaccines.
Testing for the presence of HPV is being used in several clinical contexts, with polymerase chain reaction (PCR) analysis and the HPV Hybrid Capture II test (HC2) used to detect HPV DNA. These assays can be used in several ways, including 1) to triage cytologically-defined low-grade squamous intraepithelial lesions (LSIL) and atypical squamous cells of uncertain significance (ASCUS), 2) to follow-up colposcopically-negative but cytologically-positive cases, 3) to predict therapeutic outcomes, and 4) to serve as a primary screening tool in the place of the Papanicolaou smear test. A recent meta-analysis addressed the use of HC2 and PCR in the above contexts, concluding that no study adequately addressed the utility of these approaches in colposcopically-negative and cytologically-positive patient cases (12). In this same meta-analysis, great variability was observed in the specificity and sensitivity values calculated in each of the above contexts (triage for LSIL, etc.). For example, the observed specificities of HC2/PCR in the context of predicting treatment outcome were found to vary from 44% to 100% depending on the study. Although the HC2 test is being studied in the developed and developing world, its USD$80 cost is prohibitive where resources are limited (12–16). The careHPV test – developed by the Gates Foundation and Qiagen – is designed to be a cost-effective HPV DNA assay for the developing world. A recent clinical trial in China measured careHPV sensitivity at 90% and specificity at 85% in predicting high-grade lesions (for patients only evaluated if one screening test was positive) (17). The potential utility of careHPV cannot be evaluated until its per-patient cost is known and a trial involving a large number of patients with histopathological results (for gold standard comparison) has been completed.
Visual inspection with acetic acid (VIA) is a simpler screening methodology. It involves naked-eye examination of the cervix under white-light illumination after application of acetic acid, with caregivers looking for aceto-whitening of tissue (18–19). VIA is used in the developing world due the low costs of the process, which result from the use of inexpensive reagents, a minimal need for equipment, and the ability to see-and-treat patients (precluding the need for downstream sample handling expenses) (20). While the sensitivity of VIA has been reported to be comparable to the Papanicolaou Smear, its specificity been reported as lower (21). Further, approximately 20% of lesions detected by more robust methodologies may fail to exhibit aceto-whitening. Therefore, more effective approaches are needed to address preventable cervical cancer deaths in resource-poor settings.
An ideal approach to screening and diagnosis of cervical disease would have many specific traits. It would provide real-time results, removing the need for cumbersome follow-up appointments and procedures. Ideally, the same approach could be used in both general population screening for cervical abnormalities and in a diagnostic setting (to properly characterize disease stage). This ideal approach would also be inexpensive, thus facilitating its adoption in the developing nations where the majority of cervical cancer deaths occur. Its minimal training requirements would make wide and rapid dissemination of this approach possible. And sensitivity and specificity metrics for this approach would outperform those for established technologies (20).
We have spent the past decade evaluating the capacity of optical technologies to address these criteria in a cervical cancer context.
Biologic Plausibility
Dysplastic cervical tissues harbor many molecular alterations that govern cellular metabolic processes and change cell structures. Since various molecules found in tissue are natural fluorophores, the fluorescence of precancerous tissues differs from the fluorescence of normal tissues. Fluorescence spectroscopy represents a non-invasive approach for detecting tissues with such changes. For example, if collagen breaks down during the development of dysplasia and collagen fluoresces, then decreased fluorescence intensity in cervical tissues may be associated with the presence of diseased tissue. Testing has indeed demonstrated that this can occur in cervical tissues (22). Multiple groups have shown that techniques based on quantitative optical spectroscopy can improve early detection of cervical neoplasia, providing accurate, objective, and real-time diagnostic and screening tools (23–24). Interestingly, the connection between these optical signatures and the underlying morphology and biology of diseased tissues is not completely understood.
Part of the reason for this incomplete understanding is that cervical tissues are complex and comprised of many components. To diagnose disease, discrete measurements of individual tissue components (nicotinamide adenine dinucleotide and its reduced form [NAD/NADH], flavin adenine dinucleotide and its reduced form [FAD/FADH2], aromatic amino acid fluorescence at ultraviolet [UV] wavelengths, etc.) contribute to our understanding of the disease state. Excitation-emission matrices (EEMs) plot fluorescence intensities as a function of excitation and emission wavelengths, providing information about the behavior of many molecular components in tissue. Previous studies have shown that fluorescence and reflectance spectroscopy can provide a highly informative readout for delineating healthy and premalignant tissues (25–26, 27I, 28–29).
Our larger research project has generated methods for quantifying cell morphology changes and also yielded insights into changes to the molecular composition of cells during premalignant progression. For example, we developed the first electromagnetic computational tools to account for changes in nuclear structure while calculating the scattering of normal and dysplastic epithelial cells (30). We also developed tools to measure scattering in normal and dysplastic epithelial cells from intact cervical tissues (31). To verify computer model predictions, we used these tools to assess changes in scattering as a response to acetic acid and to help understand the effect of these changes on tissue spectroscopy (32). The methods we are developing to measure the 3D distribution of nuclei will directly impact the emerging quantitative pathology tools we are using elsewhere in this project.
To better calculate the scattering coefficient of structural protein networks, we also enhanced existing electromagnetic computational tools. This work provided the first means of evaluating how dysplastic changes lead to altered stromal scattering (33). Going forward, these tools will facilitate quantitative studies of epithelial/stromal interactions. Non-invasive approaches based on these tools are also being derived.
During this program project, we have also developed tools measuring fluorescence and reflectance to determine the optimal excitation wavelengths and source detector separations for differentiating between neoplastic and non-neoplastic cervical tissue. Mathematical models of cervical tissue fluorescence and reflectance were then derived to understand these detected biophysical differences in vivo. Monte Carlo simulations were undertaken to validate these models, which were then used to analyze in vivo spectra accumulated during large screening and diagnostic trials associated with this project (34–35). Based on these reflectance and fluorescence data and the use of these model parameters, diagnostic algorithms were derived that performed equivalently to empirical data decomposition methods (e.g. principal component analysis). An additional upside with the model is that it could also be analyzed to extract information regarding the biological basis for disease (as reflected in the results from fluorescence and reflectance spectroscopy) (23, 25, 27, 36–40).
Additional tools and models that we have developed have also contributed to our ability to detect and define the biological changes that underpin cervical dysplasia. To evaluate the fluorescence and reflectance spectra of tissue from different depths within the epithelium and stroma, we developed a fiber optic probe capable of selectively recording optical signals from the epithelium versus stroma. This device may yield enhance diagnostic capability, particularly as it may be able to discriminate columnar tissues from dysplastic ones (41). We also developed automated multi-spectral imaging approaches for the detection of cervical dysplasia. Models of fluorescence were used to select optimal wavelengths and collection geometries for imaging systems (42–46). Upcoming pilot trials will test these metrics. An inverse model capable of distinguishing high grade lesions and cancers from other cervical tissues also contributes to our current best-performing algorithm for delineating cervical lesions (47). We are also working to model cervical carcinogenesis and nuclear architecture using confocal microscopy to gain further disease insights (30, 48–49). This work with both forward and inverse models has conclusively shown that we can understand the biological basis for fluorophore changes observed in the histopathological sections taken in the clinical trial setting.
Technical Feasibility
When cervical abnormalities are detected by Papanicolaou smear, current standard of care involves referral of these patients for colposcopic examination. Diagnoses are then made based on histopathological examination of colposcopically-directed biopsies. This process can take days or weeks to complete and can require additional clinic visits for patients. It can add financial costs and anxiety to health care providers and patients. During our program project, we have worked to facilitate real-time diagnosis of cervical abnormalities with novel imaging devices and quantitative fluorescence and reflectance spectroscopy techniques. Demonstrated efficacy with these techniques would help to improve standard approaches to the identification and treatment of cervical disease. More specifically, our ongoing efforts involve the evaluation of several existing and emerging approaches to detection and diagnosis of cervical disease: colposcopy, repeat Papanicolaou smear, endocervical curettage, point probe optical spectroscopy, multispectral digital colposcopy (MDC), and VIA, and HPV testing. We are also evaluating various combinations of these approaches and devising analytical techniques to improve the diagnostic performance of generated data. This work has been undertaken in the United States, Canada, and Nigeria, generating meaningful data from varied populations. To our knowledge, these represent the largest trials of optical spectroscopy with statistically justified sample sizes and consensus-read biopsies taken at each cervical site for use in gold standard comparisons.
To test efficacy of different devices, we have elicited thousands of measurements from patients. A key lesson after evaluating 1850 patients – and 4767 biopsies – is that instrument noise can be a significant barrier to disease detection. One challenge for this project has been identifying sources of variability in the measurements that produce random or systematic noise; wherever possible we have sought to eliminate this noise through laboratory experiments and pilot patient trials. By having biostatisticians and engineers working in concert to address this challenge, we have facilitated better technology performance and stronger study design. Given the challenges associated with evaluating device algorithms – while our trials finished early, selection of a best-performing algorithm for analysis took more than three years – it has been extremely beneficial to have these diverse researchers in regular contact.
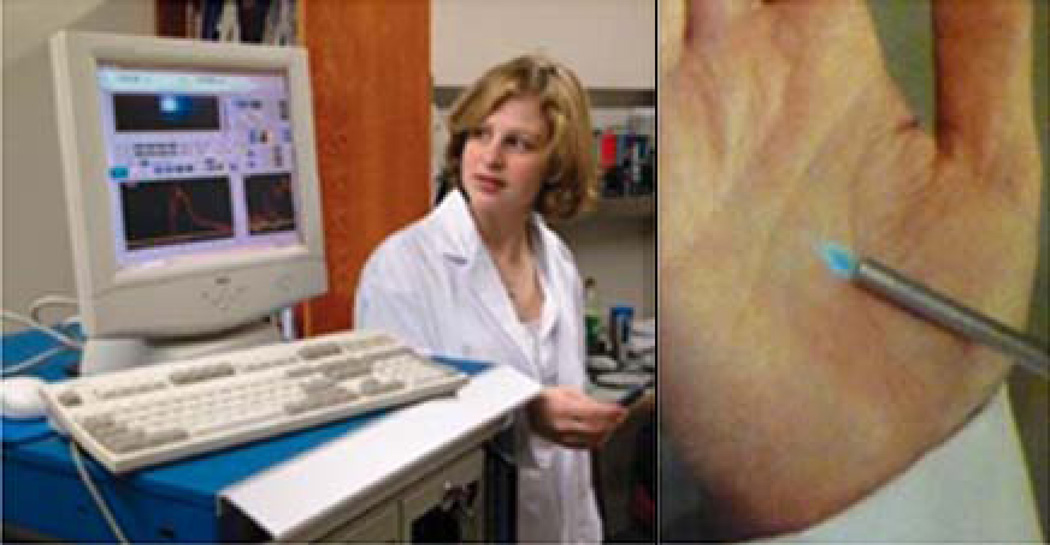
We have done extensive work developing and optimizing a point probe for fluorescence and reflectance spectroscopy that measures a 2 mm by 2 mm area of the cervix (Figure 2). Some of our initial assessments of performance for this device were undertaken in vitro or on ex vivo tissues (31, 50–51). In early in vivo studies, baseline measurements for this device were made from normal epithelial, dysplastic, and tumor tissues (25–26, 31). We also evaluated the influence of several variables on device measurements, including the influence of point probe pressure on the cervix and the impact of having different devices and device users (40, 52–56). The impact of the menstrual cycle on fluorescence spectroscopy measurements was also examined (57– 59). Daily replicate measurements of women with no history of abnormal Papanicolaou smear suggested that intra-individual variation over the course of the menstrual cycle did not significantly impact measurements (though analysis during times of menstrual bleeding should be avoided, as this was the likely cause for observed variation).
Figure 2.
The point probe device. Computer equipment and readouts are shown (left), as is the point probe itself (right).
We also sought to determine which device readouts provided the best diagnostic performance, deriving algorithms for analyzing fluorescence and reflectance spectra that were capable of delineating normal tissues, low grade lesions, high grade lesions, and tumor tissues (23, 60). More recent analytical approaches further evaluated spatial changes in spectra and biological insights into disease (e.g. Monte Carlo modeling; see above) (34–35, 39). We found that the point probe device was capable of accurately delineating high grade squamous intraepithelial lesions (HSILs) from all other types of epithelium – normal and abnormal, squamous and columnar – with a sensitivity of 80% and a specificity of 60–70%. Recent work on instrumentation and data algorithms has improved these values, with sensitivity now measured at 100% and specificity at 71% (61). These values show that the point probe device has equivalent or better performance than colposcopy in the diagnostic setting. This in turn suggests that the point probe could be used as the first effective adjunct to colposcopy. We are currently working to further improve this device’s detection capabilities, allowing it to function independently from colposcopy.
Another major product from this project has been the development and optimization of a multispectral digital colposcope (MDC) (shown in Figure 3). This device visualizes the entire cervix and provides excellent diagnostic performance, even where only two excitation wavelengths are used (330 nm and 440 nm) (44). A pilot study showed that changes in MDC images lined up well with histologically-defined CIN (cervical intraepithelial neoplasia) (62). A further study of 29 patients measured MDC specificity at 88% and sensitivity at 79% for differentiating cancerous lesions and HSILs from normal or LSILs (46). Work to further optimize MDC performance is ongoing and efforts to bring this cost-effective tool to the developing world have begun (63–64).
Figure 3.
The Multispectral Digital Colposcope (MDC) device. The left panel shows the imaging component of the MDC, while the right panel shows the associated computer equipment required to operate the device and store imaging data.
The bedrock for properly evaluating device results is a meaningful ‘gold standard’ diagnosis. We established a robust qualitative histopathologic standard through careful assessment within a team of pathologists evaluating thousands of cervical biopsy specimens (65). A high level of agreement was achieved between evaluators, ensuring that reproducible comparisons could be made over the course of the study. Quantitative histopathologic measurements – nuclear morphology, chromatin texture, and DNA content – were also evaluated and will help to hone disease stratification (30, 66). We are proceeding to calculate means, medians, and ranges for feature data that have been collected from the thousands of consensus-read biopsies we have accrued.
We also worked extensively to optimize quantitative histology methodologies. A three-tiered quality assurance (QA) system for quantitative histology was developed based on a multi-center analysis of imaging results, with QA data derived from both short term (daily) and long term (semi-annual) measurements (67). Methodological challenges – including variation in tissue section staining intensity, inter- and intra-observer variability of results, and the use of intermediate layer cells only – were also evaluated and addressed for specimens collected from a large patient cohort (n = 1800) (66). The diagnostic performance of DNA ploidy measurements of Feulgen-stained thin-prep monolayer specimens were also evaluated against conventional cytological methods and the Hybrid Capture II test (68). Ploidy analysis was shown to have comparable sensitivity, specificity, and negative and positive predictive values compared to the other approaches, with this test having the added benefit of being a semi-automated procedure requiring limited expertise with a quick turnaround for the results (within two days). To further decrease the turnaround time for such analyses, we have been working on a protocol using Azure A stain on cytological specimens. This protocol could potentially be completed in two hours, providing same-day results that would likely decrease the loss of patients to follow-up in developing countries – and developed nations as well (69).
Intermediate Outcomes (Clinical Effectiveness)
Rigorous technology assessment requires well-designed trials. Over the course of this project, we have undertaken multiple pilot and Phase I/II studies. Through Phase II trials of quantitative cytology, we saw 1850 patients at five clinical sites, with this work confirming the feasibility of obtaining quick results by this technique (68, 70). Data from a Phase II trial that evaluated >3500 cervical biopsies using quantitative pathology methodologies helped hone automated components of this technique and identify cell-level changes associated with different neoplastic stages (66–67, 71–73). Our point probe for fluorescence and reflectance spectroscopy was also evaluated in a Phase II study on a similar number of tissue specimens – and these results were comparable to those obtained by other groups (54–55, 74–75). This investigation helped to identify causes of measurement variability in the clinical setting (e.g. differences in menopausal status), giving information essential for developing effective downstream normalization and analysis approaches. A recent pilot study based of the MDC demonstrated that this device could be used effectively in a clinical setting and helped establish operational parameters for larger studies that will follow (unpublished results).
Upcoming trials for this project involve 1) evaluation of the above technologies in expanded patient populations, 2) parallel analysis of these same technologies to determine whether combined application can improve diagnostic performance, and 3) evaluation of newer tools and device algorithms we have not yet assessed in patient populations. One trial we are initiating will evaluate the performance of the MDC in a clinical trial involving over 600 patients. A primary goal of this work is the optimization of an algorithm for MDC data that can effectively delineate the presence/absence of disease compared to gold standard histopathological results. Another trial will evaluate 180 patients to determine whether combined application of the MDC and point probe devices leads to greater diagnostic accuracy.
Pilot trials are planned or have been initiated to assess the utility of other approaches for delineating cervical disease states. Based largely on feedback and ideas from program project members at the University of Ibadan in Nigeria, we have produced a device called the Diagnostic Imaging Aid (DIA). It is essentially a battery-powered, portable version of the MDC. This device will be assessed against standard approaches and other devices we have developed during this project to determine whether it will have utility in the developing world (the DIA is cheaper to build, service, and use – and its portability ensures greater utility in resource-poor clinical settings). In vivo confocal microscopy, which has the capacity to produce real-time cell level images of the cervical epithelium, will also be assessed in a pilot trial. Finally, a variety of contrast agents will be applied to the cervix to determine whether they can improve detection of spectral changes associated with neoplastic processes.
Taken together, these clinical studies will provide a wealth of knowledge regarding the utility and practicality of a wide array of tools for detecting cervical disease. Based on this work we will know which devices perform best in different clinical settings (screening vs. diagnostic populations, developed vs. developing world clinics, etc.). Our work is making real-time diagnosis and treatment of cervical lesions a viable possibility. Furthermore, the improved specificities and sensitivities we are obtaining (by improved instrumentation, diagnostic algorithms, etc.) are reducing the likelihood that disease will go undetected, limiting the possibility of over-treatment, and decreasing the clinical costs associated with managing this disease.
Patient and Provider Outcomes
Technology assessment literature places a strong emphasis on evaluating the impact of new technologies on patient outcomes. These include assessments of physical, functional, or emotional well-being in patients after exposure to new technologies. Evaluating these patient outcomes during development phases can help identify and fix problems before new technologies are disseminated. To ensure that analysis in this area is robust, patients must be of varied ages, drawn from ethnically diverse populations, and exhibit differences along additional socioeconomic measures (e.g. marital status, education, etc.). Significantly, few studies have examined the impact of screening and diagnostic technologies on these outcomes.
We have attempted to integrate meaningful patient outcome evaluations into the clinical trials and pilot tests of our screening and diagnostic technologies. Based on patient feedback, we created and validated tools and approaches for measuring patient distress (26, 76–80). These results taught us that both screening and diagnostic patients perceive optical spectroscopy to be less painful than Papanicolaou smear, colposcopy, or biopsy (81). We also learned that patients were less anxious during spectroscopy than during other tests (77, 82). When queried about their satisfaction after the examination, patients reported biopsy testing to be more frightening than spectroscopy. They also stated a preference for decreased lighting during spectroscopy (i.e. lights out). The only negative aspect of spectroscopy, as defined by the patients, was the extended amount of time needed to collect device measurements. Based on this feedback, study investigators involved in instrumentation have taken (and continue to take) steps to reduce the “time footprint” associated with spectroscopic measurements. Significantly, no long-term or short-term adverse effects have been reported in these patients, providing strong evidence of the safety of study technologies.
We also assessed individual perceptions in addition to those associated with clinical screening (83–84). For example, we queried patient knowledge regarding HPV and cervical dysplasia (84). The limited knowledge evidenced by our results spurred us to produce new educational tools to better explain cervical cancer screening, diagnosis, and treatment. We also evaluated patient attitudes, behaviors, and barriers to participation in trials, screening, and treatments (76, 85–86). One finding from this work was that surveyed diagnostic and screening population patients rated test sensitivity as the most important test characteristic (76). This same analysis showed that some patients preferred not to receive same-day treatment following diagnosis, a finding that has prompted us to being to develop tools predicting whether patients will want realtime disease management. Given its demonstrated role in adoption of new tools in clinical practice, we included knowledge dissemination as a direct goal in our program project design (87–88). We have also conducted a study of health care provider satisfaction with the device (89). In this work, presented elsewhere in this issue, we found that the primary obstacle to implementation in practice was the fear that a device capable of real-time diagnosis would lead to challenges around the length and character of patient visits.
Societal Outcomes (and Economic Evaluation)
We measured the ‘societal’ impact of our technology development by evaluating economic factors, since health care costs significantly impact on all societies. This is particularly true of nations with limited resources, where the relative abundance of cervical cancer cases is directly related to the prohibitive expense of regular screening. It has been established that the annual cost for the diagnosis and evaluation of atypias and LSILs in the United States is ~USD$6 billion (90). With more effective screening and diagnostic tools, this substantial sum could be more effectively applied. For example, the billions of dollars allocated for the cervical cancer problem could be used to reach underserved populations and fund better management of patients with disease that is more likely to progress.
To date, there have been few studies into the cost-effectiveness of emerging imaging technologies. This represents a lost opportunity, as economic considerations can have a positive impact on technology design. In the preliminary work for these optical spectroscopy devices, we found that only a minimal number of light wavelengths were biologically useful in the algorithm (25, 91). Identifying the ideal wavelengths of light allowed the choice of less expensive light source than a laser for a potential commercial device. (It also substantially reduced patient exposure to UV light, though levels were already orders of magnitude below defined thresholds.) During this project, we devised an approach to estimate the diagnostic performance of Bayesian classifiers derived from optical spectra – and then used these results to evaluate the impact of variations in tissue type, sample size, patient population and financial cost of the point probe device (92). The net result of this work was a method for reducing experimental costs associated with cervical cancer diagnostic tools that are based on optical spectra.
We also undertook several comparative analyses of different screening and diagnostic approaches. Specifically, we have evaluated (or are evaluating) the performance and cost-effectiveness of colposcopy, repeat Papanicolaou smear, endocervical curettage, point probe optical spectroscopy, MDC, a combination point probe and MDC, an MDC algorithm, an MDC-point probe algorithm, a DIA device, VIA, and HPV testing (55, 74–75, 90, 93–100). These analyses are being undertaken in the US and Canada, with similar studies planned for Nigeria. (Much of this work has been alluded to above.)
This project has also provided new methodologies with applicability in other clinical trials. For example, we demonstrated that it was feasible to collect and analyze non-health care direct cost data (e.g. costs associated with child/elder care or transportation/ parking) and time cost data (101). This analysis showed that clinic type (community vs. specialty hospital) and patient population (screening vs. diagnostic) impacted such costs. This work also demonstrated that non-health care direct costs could be analyzed for a single large-scale trial, indicating that our approaches are widely applicable. We also developed a quantitative pathology approach that uses measurements of 120 different features from cells on a tumor tissue section to diagnose disease and guide patient management decisions (102). This approach makes use of a cumulative log-odds model score, followed by receiver operating characteristic (ROC) curve analysis and is currently being evaluated in a larger patient cohort. In a third study, we evaluated previous cost-benefit ratio analyses for a variety of diseases, identifying ratio values associated with disease severity (103). For example, directly life threatening but curable clinical scenarios were found to have a cost-benefit ratio of <0.05. Our approaches may have broad utility for guiding the selection of optimal test cutpoints on receiver operating characteristic (ROC) curves during the development of diagnostic tests. Finally, we reviewed in detail the mathematical models being applied to the cervical cancer problem and discussed how they will impact research going forward (100).
The natural history of cervical cancer was also evaluated during this project. We have previously evaluated the utility of intermediate markers as means for guiding management of cervical lesions (104). In this project, a meta-analysis determined the probability of progression from HSIL to invasive disease and from LSIL to HSIL – and the probability of regression from HSIL to LSIL and LSIL to normal (105). This analysis showed that, while the probability of transition between cervical cancer stages may be small at half year intervals, the cumulative risk of cervical cancer is significant. We also compared performance of the HC2 test and colposcopy versus the Papanicolaou smear in a large population, assessing the capacity of these approaches to identify disease in screening and diagnostic settings (97, 99). We found that for women over the age of 30, the HC2 test was more effective for detecting disease in screening populations than the Papanicolaou smear (97). Colposcopy, on the other hand, was found to be more effective in diagnostic populations than screening ones (99).
Any large-scale clinical study targeted towards women also has the inherent benefit of highlighting the value of women. This is particularly true for the developing world, where women can be marginalized. Women’s clinics not only provide opportunities for education about disease – their very existence reinforces the idea that women and women’s health issues are worthy of attention and resources.
Foundations (Project Cores)
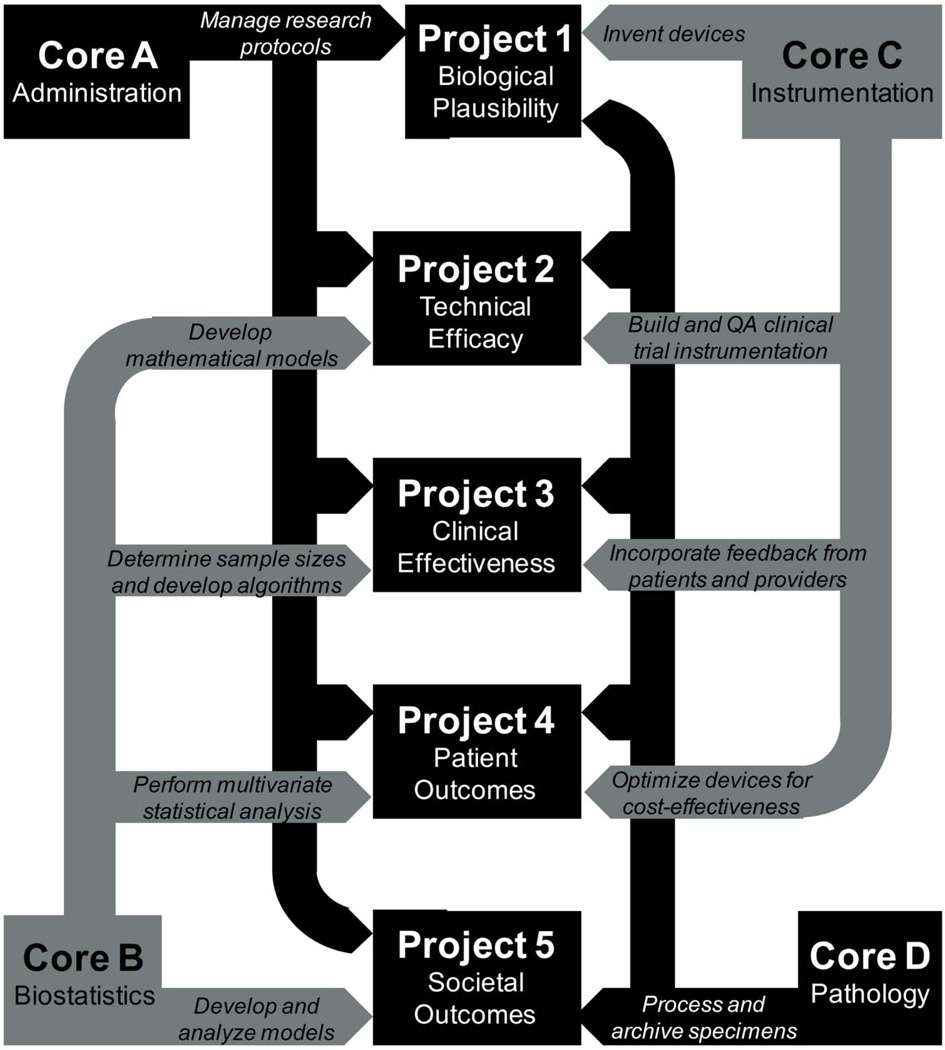
P01 program projects include funding for resources and infrastructure that can backstop multiple research subprojects. Four project cores were defined for our work: an Administrative, Research Compliance, and Epidemiology Core; a Biostatistics and Data Management Core; an Instrumentation Core; and a Pathology and HPV Biomarkers Core. The many facets of technology development described above have only been made possible by the existence of these very effective support elements. The relationship between cores and subprojects is depicted in Figure 4.
Figure 4.
Relationship of project cores to the five subprojects of the wider program project endeavor.
Without our Administrative, Research Compliance, and Epidemiology Core, we would have been unable to secure Nigeria as a major collaborator on this work and we would not have been able to efficiently track patient diversity in clinical trials (64, 87, 106–109). We also would not have derived novel disease-associated biomarkers or glean new insights into disease biology (72, 110–112). Weekly teleconference meetings by this group have helped sustain productivity on this project, as have insights from the internal and external advisory boards associated with this work. The regular communication and interactions mandated by this project core have helped drive the successes detailed in this paper by corralling individuals with disparate research interests and pointing them towards common goals.
The broad purview of the Biostatistics and Data Management Core has also enhanced this project over the past decade. Individuals associated with this core have done extensive work to address each of the technology development categories outlined above, including statistical analyses and algorithm development for several research components (23, 25–27, 30, 36, 38–40, 50, 52–53, 55–59, 62, 65, 67, 69, 71, 74–75, 81, 85–87, 92–93, 95–96, 98–99, 103, 105, 113–118). They have also worked closely with the other research cores associated with this project (30, 64, 72, 106–108, 110–111, 118–121). This group has also been responsible for the creation and proper maintenance of our secure patient database – and the development of software and statistical approaches for analyzing this collected information (102, 122). Members of the Biostatistics and Data Management Core are currently developing new approaches to handle our multi-dimensional data sets and to facilitate the review and integrated analysis of spectral, quantitative cytologic, and quantitative pathologic data.
Our Instrumentation Core has designed, built, calibrated, and maintained all of the devices used during this program project. Bioengineers at each project site have worked directly with health providers to track and improve device performance in real- time. Dialogue with other project partners has helped to make tools more cost effective, user-friendly, and patient-friendly. Specifically, the instrumentation core has built three point probe devices and developed and implemented quality assurance software for these tools (55, 92); developed three MDC devices and meaningful MDC quality assurance metrics (63); developed fiber optic probes for biomedical spectroscopic sensing (123–124); and devised and executed trials to test the impact of intra- and inter-device variation on study measurements (40, 54–56, 114, 125)
Finally, inputs from the Pathology and HPV Biomarkers Core have also been critical to the success of the different parts of this project. Having collected, processed, stained, and reviewed thousands of cervical biopsy slides, this group has provided the ‘gold standard’, consensus-reviewed diagnoses that have been critical to all components of this study (65). All of the cytologic and histopathologic specimens needed for clinical and quantitative assessment have been collected and managed by this core. This core has also developed imaging systems for evaluating morphometric and architectural features on tissue cross-sections, with phenotypic scores calculated by this method correlating well with pathology classifications and HPV status (48, 66–68, 70, 73, 120–121). Algorithms based on this approach have had particular utility in characterizing the underlying biology of cervical disease. Associations between HPV mRNA levels and specific dysplastic stages were also evaluated through the efforts of this core (126–127).
Conclusion
This research initiative has spanned more than ten years and two continents. It has resulted in nearly 200 publications, two dozen inter-institutional patents, and over 50 graduate degrees and post-doctoral fellowships. More significantly, thousands of patients have been reviewed over its course, with hundreds of treatable cervical lesions identified in patient populations that may not have otherwise had access to effective screening. The imaging tools and approaches that we have developed for detecting cervical disease have steadily improved with respect to their accuracy, cost- effectiveness, and ease of use. Analyses that integrate the multiple levels of data we have generated are continuing to improve these parameters. We feel strongly that our work will have a positive impact on the clinical and economic outcomes associated with cervical cancer. We also feel that many of the organizational approaches and methodologies we have taken to this work could be used to augment other translational research projects, making collaborations more effective and sowing the seeds for greater innovation.
Acknowledgement
We would like to thank all the investigators and supporting staff affiliated with the PO1 Program Project, 2PO1-82710-09.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Littenberg B. Technology assessment in medicine. Acad Med. 1992;67(7):424–428. doi: 10.1097/00001888-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Follen M, Crain S, MacAulay C, Basen-Engquist K, Cantor SB, Cox D, et al. Optical technologies for cervical neoplasia: update of an NCI program project grant. Clin Adv Hematol Oncol. 2005;3(1):41–53. [PubMed] [Google Scholar]
- 3.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agency BC. Annual Report. Vancouver, BC: 2004. Cervical Cancer Screening Program. 2004. [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6.Cheaper HPV vaccines needed. Lancet. 2008;371(9625):1638. doi: 10.1016/S0140-6736(08)60701-1. [DOI] [PubMed] [Google Scholar]
- 7.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries--key challenges and issues. N Engl J Med. 2007;356(19):1908–1910. doi: 10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 8.Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine--opportunity and challenge. N Engl J Med. 2007;356(19):1990–1991. doi: 10.1056/NEJMe078088. [DOI] [PubMed] [Google Scholar]
- 9.Dailard C. HPV in the United States and Developing Nations: A Problem of Public Health and Politics. The Guttmacher Report on Public Policy. 2003;6(3):4–15. [Google Scholar]
- 10.Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst. 2004;96(8):604–615. doi: 10.1093/jnci/djh104. [DOI] [PubMed] [Google Scholar]
- 11.Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003;9(1):37–48. doi: 10.3201/eid0901.020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26 Suppl 10:K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 14.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 15.Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 2001;285(24):3107–3115. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- 16.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ. 2001;79(10):954–962. [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9(10):929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370(9585):398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, et al. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer. 1998;83(10):2150–2156. [PubMed] [Google Scholar]
- 20.Thekkek N, Richards-Kortum R. Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat Rev Cancer. 2008;8(9):725–731. doi: 10.1038/nrc2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89 Suppl 2:S4–S12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Drezek R, Sokolov K, Utzinger U, Boiko I, Malpica A, Follen M, et al. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements, and implications. J Biomed Opt. 2001;6(4):385–396. doi: 10.1117/1.1413209. [DOI] [PubMed] [Google Scholar]
- 23.Chang SK, Mirabal YN, Atkinson EN, Cox D, Malpica A, Follen M, et al. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt. 2005;10(2):024031. doi: 10.1117/1.1899686. [DOI] [PubMed] [Google Scholar]
- 24.Georgakoudi I, Sheets EE, Muller MG, Backman V, Crum CP, Badizadegan K, et al. Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo. Am J Obstet Gynecol. 2002;186(3):374–382. doi: 10.1067/mob.2002.121075. [DOI] [PubMed] [Google Scholar]
- 25.Chang SK, Follen M, Malpica A, Utzinger U, Staerkel G, Cox D, et al. Optimal excitation wavelengths for discrimination of cervical neoplasia. IEEE Trans Biomed Eng. 2002;49(10):1102–1111. doi: 10.1109/TBME.2002.803597. [DOI] [PubMed] [Google Scholar]
- 26.Nath A, Rivoire K, Chang S, West L, Cantor SB, Basen-Engquist K, et al. A pilot study for a screening trial of cervical fluorescence spectroscopy. Int J Gynecol Cancer. 2004;14(6):1097–1107. doi: 10.1111/j.1048-891X.2004.14607.x. [DOI] [PubMed] [Google Scholar]
- 27.Mirabal YN, Chang SK, Atkinson EN, Malpica A, Follen M, Richards-Kortum R. Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt. 2002;7(4):587–594. doi: 10.1117/1.1502675. [DOI] [PubMed] [Google Scholar]
- 28.Ramanujam N, Mitchell MF, Mahadevan A, Thomsen S, Malpica A, Wright T, et al. Development of a multivariate statistical algorithm to analyze human cervical tissue fluorescence spectra acquired in vivo. Lasers Surg Med. 1996;19(1):46–62. doi: 10.1002/(SICI)1096-9101(1996)19:1<46::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Ramanujam N, Mitchell MF, Mahadevan A, Thomsen S, Silva E, Richards-Kortum R. Fluorescence spectroscopy: a diagnostic tool for cervical intraepithelial neoplasia (CIN) Gynecol Oncol. 1994;52(1):31–38. doi: 10.1006/gyno.1994.1007. [DOI] [PubMed] [Google Scholar]
- 30.Drezek R, Guillaud M, Collier T, Boiko I, Malpica A, Macaulay C, et al. Light scattering from cervical cells throughout neoplastic progression: influence of nuclear morphology, DNA content, and chromatin texture. J Biomed Opt. 2003;8(1):7–16. doi: 10.1117/1.1528950. [DOI] [PubMed] [Google Scholar]
- 31.Arifler D, Guillaud M, Carraro A, Malpica A, Follen M, Richards-Kortum R. Light scattering from normal and dysplastic cervical cells at different epithelial depths: finite-difference time-domain modeling with a perfectly matched layer boundary condition. J Biomed Opt. 2003;8(3):484–494. doi: 10.1117/1.1578640. [DOI] [PubMed] [Google Scholar]
- 32.Zuluaga AF, Drezek R, Collier T, Lotan R, Follen M, Richards-Kortum R. Contrast agents for confocal microscopy: how simple chemicals affect confocal images of normal and cancer cells in suspension. J Biomed Opt. 2002;7(3):398–403. doi: 10.1117/1.1481047. [DOI] [PubMed] [Google Scholar]
- 33.Arifler D, Pavlova I, Gillenwater A, Richards-Kortum R. Light scattering from collagen fiber networks: micro-optical properties of normal and neoplastic stroma. Biophys J. 2007;92(9):3260–3274. doi: 10.1529/biophysj.106.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arifler D, Schwarz RA, Chang SK, Richards-Kortum R. Reflectance spectroscopy for diagnosis of epithelial precancer: model-based analysis of fiber-optic probe designs to resolve spectral information from epithelium and stroma. Appl Opt. 2005;44(20):4291–4305. doi: 10.1364/ao.44.004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arifler D, MacAulay C, Follen M, Richards-Kortum R. Spatially resolved reflectance spectroscopy for diagnosis of cervical precancer: Monte Carlo modeling and comparison to clinical measurements. J Biomed Opt. 2006;11(6):064027. doi: 10.1117/1.2398932. [DOI] [PubMed] [Google Scholar]
- 36.Brookner C, Utzinger U, Follen M, Richards-Kortum R, Cox D, Atkinson EN. Effects of biographical variables on cervical fluorescence emission spectra. J Biomed Opt. 2003;8(3):479–483. doi: 10.1117/1.1578642. [DOI] [PubMed] [Google Scholar]
- 37.Pavlova I, Sokolov K, Drezek R, Malpica A, Follen M, Richards-Kortum R. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem Photobiol. 2003;77(5):550–555. doi: 10.1562/0031-8655(2003)077<0550:maboon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Chang SK, Arifler D, Drezek R, Follen M, Richards-Kortum R. Analytical model to describe fluorescence spectra of normal and preneoplastic epithelial tissue: comparison with Monte Carlo simulations and clinical measurements. J Biomed Opt. 2004;9(3):511–522. doi: 10.1117/1.1695559. [DOI] [PubMed] [Google Scholar]
- 39.Chang SK, Marin N, Follen M, Richards-Kortum R. Model-based analysis of clinical fluorescence spectroscopy for in vivo detection of cervical intraepithelial dysplasia. J Biomed Opt. 2006;11(2):024008. doi: 10.1117/1.2187979. [DOI] [PubMed] [Google Scholar]
- 40.Marin NM, MacKinnon N, MacAulay C, Chang SK, Atkinson EN, Cox D, et al. Calibration standards for multicenter clinical trials of fluorescence spectroscopy for in vivo diagnosis. J Biomed Opt. 2006;11(1):014010. doi: 10.1117/1.2166389. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz RA, Arifler D, Chang SK, Pavlova I, Hussain IA, Mack V, et al. Ball lens coupled fiber-optic probe for depth-resolved spectroscopy of epithelial tissue. Opt Lett. 2005;30(10):1159–1161. doi: 10.1364/ol.30.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan L, Ramanujam N. Relationship between depth of a target in a turbid medium and fluorescence measured by a variable-aperture method. Opt Lett. 2002;27(2):104–106. doi: 10.1364/ol.27.000104. [DOI] [PubMed] [Google Scholar]
- 43.Ramanujam N, Chen J, Gossage K, Richards-Kortum R, Chance B. Fast and noninvasive fluorescence imaging of biological tissues in vivo using a flying-spot scanner. IEEE Trans Biomed Eng. 2001;48(9):1034–1041. doi: 10.1109/10.942594. [DOI] [PubMed] [Google Scholar]
- 44.Benavides J, Chang S, Park S, Richards-Kortum R, Mackinnon N, Macaulay C, et al. Multispectral digital colposcopy for in vivo detection of cervical cancer. Opt Express. 2003;11(10):1223–1236. doi: 10.1364/oe.11.001223. [DOI] [PubMed] [Google Scholar]
- 45.Park SY, Collier T, Aaron J, Markey M, Richards-Kortum R, Sokolov K, et al. Multispectral digital microscopy for in vivo monitoring of oral neoplasia in the hamster cheek pouch model of carcinogenesis. Opt Express. 2005;13(3):749–762. doi: 10.1364/opex.13.000749. [DOI] [PubMed] [Google Scholar]
- 46.Park SY, Follen M, Milbourne A, Rhodes H, Malpica A, MacKinnon N, et al. Automated image analysis of digital colposcopy for the detection of cervical neoplasia. J Biomed Opt. 2008;13(1):014029. doi: 10.1117/1.2830654. [DOI] [PubMed] [Google Scholar]
- 47.Redden Weber C, Schwarz RA, Atkinson EN, Cox DD, Macaulay C, Follen M, et al. Model-based analysis of reflectance and fluorescence spectra for in vivo detection of cervical dysplasia and cancer. J Biomed Opt. 2008;13(6):064016. doi: 10.1117/1.3013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillaud M, Adler-Storthz K, Malpica A, Staerkel G, Matisic J, Van Niekirk D, et al. Subvisual chromatin changes in cervical epithelium measured by texture image analysis and correlated with HPV. Gynecol Oncol. 2005;99(3 Suppl 1):S16–S23. doi: 10.1016/j.ygyno.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 49.Collier T, Guillaud M, Follen M, Malpica A, Richards-Kortum R. Real-time reflectance confocal microscopy: comparison of two-dimensional images and three-dimensional image stacks for detection of cervical precancer. J Biomed Opt. 2007;12(2):024021. doi: 10.1117/1.2717899. [DOI] [PubMed] [Google Scholar]
- 50.Brookner CK, Follen M, Boiko I, Galvan J, Thomsen S, Malpica A, et al. Autofluorescence patterns in short-term cultures of normal cervical tissue. Photochem Photobiol. 2000;71(6):730–736. doi: 10.1562/0031-8655(2000)071<0730:apistc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Collier T, Lacy A, Richards-Kortum R, Malpica A, Follen M. Near real-time confocal microscopy of amelanotic tissue: detection of dysplasia in ex vivo cervical tissue. Acad Radiol. 2002;9(5):504–512. doi: 10.1016/s1076-6332(03)80326-4. [DOI] [PubMed] [Google Scholar]
- 52.Nath A, Rivoire K, Chang S, Cox D, Atkinson EN, Follen M, et al. Effect of probe pressure on cervical fluorescence spectroscopy measurements. J Biomed Opt. 2004;9(3):523–533. doi: 10.1117/1.1695562. [DOI] [PubMed] [Google Scholar]
- 53.Rivoire K, Nath A, Cox D, Atkinson EN, Richards-Kortum R, Follen M. The effects of repeated spectroscopic pressure measurements on fluorescence intensity in the cervix. Am J Obstet Gynecol. 2004;191(5):1606–1617. doi: 10.1016/j.ajog.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Follen M, MacAulay C, Pikkula B, Serachitopol D, Price R, et al. Sources of variability in fluorescence spectroscopic measurements in a Phase II clinical trial of 850 patients. Gynecol Oncol. 2007;107(1 Suppl 1):S260–S269. doi: 10.1016/j.ygyno.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Freeberg JA, Serachitopol DM, McKinnon N, Price R, Atkinson EN, Cox DD, et al. Fluorescence and reflectance device variability throughout the progression of a phase II clinical trial to detect and screen for cervical neoplasia using a fiber optic probe. J Biomed Opt. 2007;12(3):034015. doi: 10.1117/1.2750332. [DOI] [PubMed] [Google Scholar]
- 56.Pikkula BM, Shuhatovich O, Price RL, Serachitopol DM, Follen M, McKinnon N, et al. Instrumentation as a source of variability in the application of fluorescence spectroscopic devices for detecting cervical neoplasia. J Biomed Opt. 2007;12(3):034014. doi: 10.1117/1.2745285. [DOI] [PubMed] [Google Scholar]
- 57.Chang SK, Dawood MY, Staerkel G, Utzinger U, Atkinson EN, Richards-Kortum RR, et al. Fluorescence spectroscopy for cervical precancer detection: Is there variance across the menstrual cycle? J Biomed Opt. 2002;7(4):595–602. doi: 10.1117/1.1509753. [DOI] [PubMed] [Google Scholar]
- 58.Cox DD, Chang SK, Dawood MY, Staerkel G, Utzinger U, Richards-Kortum RR, et al. Detecting the signal of the menstrual cycle in fluorescence spectroscopy of the cervix. Appl Spectrosc. 2003;57(1):67–72. doi: 10.1366/000370203321165223. [DOI] [PubMed] [Google Scholar]
- 59.Macaulay C, Richards-Kortum R, Utzinger U, Fedyk A, Atkinson E, Cox D, et al. Variation of fluorescence spectroscopy during the menstrual cycle. Opt Express. 2002;10(12):493–504. doi: 10.1364/oe.10.000493. [DOI] [PubMed] [Google Scholar]
- 60.Marin NM, Milbourne A, Rhodes H, Ehlen T, Miller D, Benedet L, et al. Diffuse reflectance patterns in cervical spectroscopy. Gynecol Oncol. 2005;99(3 Suppl 1):S116–S120. doi: 10.1016/j.ygyno.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 61.Cantor SB, M YJ, D CD, Guillaud M, Atkinson EN, Benedet JL, et al. Accuracy of optical spectroscopy for the detection of cervical intraepithelial neoplasia: testing a device as an adjunct to colposcopy. Int J Cancer. 2010 doi: 10.1002/ijc.25667. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milbourne A, Park SY, Benedet JL, Miller D, Ehlen T, Rhodes H, et al. Results of a pilot study of multispectral digital colposcopy for the in vivo detection of cervical intraepithelial neoplasia. Gynecol Oncol. 2005;99(3 Suppl 1):S67–S75. doi: 10.1016/j.ygyno.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 63.Nakappan S, Park SY, Serachitopol D, Price R, Cardeno M, Au S, et al. Methodology of a real-time quality control for the multispectral digital colposcope (MDC) Gynecol Oncol. 2007;107(1 Suppl 1):S215–S222. doi: 10.1016/j.ygyno.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Roblyer D, Richards-Kortum R, Park SY, Adewole I, Follen M. Objective screening for cervical cancer in developing nations: lessons from Nigeria. Gynecol Oncol. 2007;107(1 Suppl 1):S94–S97. doi: 10.1016/j.ygyno.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 65.Malpica A, Matisic JP, Niekirk DV, Crum CP, Staerkel GA, Yamal JM, et al. Kappa statistics to measure interrater and intrarater agreement for 1790 cervical biopsy specimens among twelve pathologists: qualitative histopathologic analysis and methodologic issues. Gynecol Oncol. 2005;99(3 Suppl 1):S38–S52. doi: 10.1016/j.ygyno.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 66.Guillaud M, Cox D, Malpica A, Staerkel G, Matisic J, Van Niekirk D, et al. Quantitative histopathological analysis of cervical intra-epithelial neoplasia sections: methodological issues. Cell Oncol. 2004;26():31–43. doi: 10.1155/2004/238769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiu D, Guillaud M, Cox D, Follen M, MacAulay C. Quality assurance system using statistical process control: an implementation for image cytometry. Cell Oncol. 2004;26(3):101–117. doi: 10.1155/2004/794021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guillaud M, Benedet JL, Cantor SB, Staerkel G, Follen M, MacAulay C. DNA ploidy compared with human papilloma virus testing (Hybrid Capture II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmation. Cancer. 2006;107(2):309–318. doi: 10.1002/cncr.21993. [DOI] [PubMed] [Google Scholar]
- 69.Guillaud M, Benedet JL, Follen M, Crain BT, MacAulay C. Scan-and-treat methodology using Azure A fast stain as a cost-effective cervical cancer screening alternative to visual inspection with acetic acid. Gynecol Oncol. 2007;107(1 Suppl 1):S256–S259. doi: 10.1016/j.ygyno.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Scheurer ME, Guillaud M, Tortolero-Luna G, McAulay C, Follen M, Adler-Storthz K. Human papillomavirus-related cellular changes measured by cytometric analysis of DNA ploidy and chromatin texture. Cytometry B Clin Cytom. 2007;72(5):324–331. doi: 10.1002/cyto.b.20173. [DOI] [PubMed] [Google Scholar]
- 71.Guillaud M, Cox D, Adler-Storthz K, Malpica A, Staerkel G, Matisic J, et al. Exploratory analysis of quantitative histopathology of cervical intraepithelial neoplasia: objectivity, reproducibility, malignancy-associated changes, and human papillomavirus. Cytometry A. 2004;60(1):81–89. doi: 10.1002/cyto.a.20034. [DOI] [PubMed] [Google Scholar]
- 72.Guillaud M, Richards-Kortum R, Follen M. Paradigm shift: a new breed of pathologist. Gynecol Oncol. 2007;107(1 Suppl 1):S46–S49. doi: 10.1016/j.ygyno.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 73.Kamalov R, Guillaud M, Haskins D, Harrison A, Kemp R, Chiu D, et al. A Java application for tissue section image analysis. Comput Methods Programs Biomed. 2005;77(2):99–113. doi: 10.1016/j.cmpb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Freeberg JA, Benedet JL, MacAulay C, West LA, Follen M. The performance of fluorescence and reflectance spectroscopy for the in vivo diagnosis of cervical neoplasia; point probe versus multispectral approaches. Gynecol Oncol. 2007;107(1 Suppl 1):S248–S255. doi: 10.1016/j.ygyno.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Freeberg JA, Benedet JL, West LA, Atkinson EN, MacAulay C, Follen M. The clinical effectiveness of fluorescence and reflectance spectroscopy for the in vivo diagnosis of cervical neoplasia: an analysis by phase of trial design. Gynecol Oncol. 2007;107(1 Suppl 1):S270–S280. doi: 10.1016/j.ygyno.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Basen-Engquist K, Fouladi RT, Cantor SB, Shinn E, Sui D, Sharman M, et al. Patient assessment of tests to detect cervical cancer. Int J Technol Assess Health Care. 2007;23(2):240–247. doi: 10.1017/S0266462307070171. [DOI] [PubMed] [Google Scholar]
- 77.Shinn E, Basen-Engquist K, Le T, Hansis-Diarte A, Bostic D, Martinez-Cross J, et al. Distress after an abnormal Pap smear result: scale development and psychometric validation. Prev Med. 2004;39(2):404–412. doi: 10.1016/j.ypmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Basen-Engquist K, Paskett ED, Buzaglo J, Miller SM, Schover L, Wenzel LB, et al. Cervical cancer. Cancer. 2003;98(9 Suppl):2009–2014. doi: 10.1002/cncr.11681. [DOI] [PubMed] [Google Scholar]
- 79.Boling W, Fouladi RT, Basen-Engquist K. Health-related quality of life in gynecological oncology: instruments and psychometric properties. Int J Gynecol Cancer. 2003;13(1):5–14. doi: 10.1046/j.1525-1438.2003.13051.x. [DOI] [PubMed] [Google Scholar]
- 80.Swartz RJ, de Moor C, Cook KF, Fouladi RT, Basen-Engquist K, Eng C, et al. Mode effects in the center for epidemiologic studies depression(CES-D)scale: personal digital assistant vs. paper and pencil administration. Qual Life Res. 2007;16(5):803–813. doi: 10.1007/s11136-006-9158-0. [DOI] [PubMed] [Google Scholar]
- 81.Basen-Engquist K, Shinn EH, Warneke C, de Moor C, Le T, Richards-Kortum R, et al. Patient distress and satisfaction with optical spectroscopy in cervical dysplasia detection. Am J Obstet Gynecol. 2003;189(4):1136–1142. doi: 10.1067/s0002-9378(03)00540-4. [DOI] [PubMed] [Google Scholar]
- 82.Shinn E, Le T, Gallegos J, Basen-Engquist K. A pilot analysis of multispectral digital colposcopy for women with high-grade squamous intraepithelial lesion (HGSIL) Pap smear results. Gynecol Oncol. 2007;107(1 Suppl 1):S83–S85. doi: 10.1016/j.ygyno.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 83.Pruitt SL, Parker PA, Follen M, Basen-Engquist K. Communicating colposcopy results: what do patients and providers discuss? J Low Genit Tract Dis. 2008;12(2):95–102. doi: 10.1097/LGT.0b013e31815a5142. [DOI] [PubMed] [Google Scholar]
- 84.Pruitt SL, Parker PA, Peterson SK, Le T, Follen M, Basen-Engquist K. Knowledge of cervical dysplasia and human papillomavirus among women seen in a colposcopy clinic. Gynecol Oncol. 2005;99(3 Suppl 1):S236–S244. doi: 10.1016/j.ygyno.2005.07.095. [DOI] [PubMed] [Google Scholar]
- 85.Ho V, Yamal JM, Atkinson EN, Basen-Engquist K, Tortolero-Luna G, Follen M. Predictors of breast and cervical screening in Vietnamese women in Harris County, Houston, Texas. Cancer Nurs. 2005;28(2):119–129. doi: 10.1097/00002820-200503000-00005. quiz 30-1. [DOI] [PubMed] [Google Scholar]
- 86.Shuhatovich OM, Sharman MP, Mirabal YN, Earle NR, Follen M, Basen-Engquist K. Participant recruitment and motivation for participation in optical technology for cervical cancer screening research trials. Gynecol Oncol. 2005;99(3 Suppl 1):S226–S231. doi: 10.1016/j.ygyno.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 87.Miller D, Okolo CA, Mirabal Y, Guillaud M, Arulogun OS, Oladepo O, et al. Knowledge dissemination and evaluation in a cervical cancer screening implementation program in Nigeria. Gynecol Oncol. 2007;107(1 Suppl 1):S196–S207. doi: 10.1016/j.ygyno.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 88.Shinn E, Basen-Engquist K, Crain B, Follen M. A theory-aided dissemination strategy for emerging technologies in cervical cancer screening. Gynecol Oncol. 2007;107(1 Suppl 1):S35–S39. doi: 10.1016/j.ygyno.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 89.Hernandez CL, Shinn E, Qazi U, Gera S, Brodovsky MS, Simpson J, et al. Physician attitudes toward dissemination of optical spectroscopy devices for cervical cancer control: an Industrial-Academic collaborative study. DrexelMed Journal. 2010 doi: 10.1016/j.genm.2011.11.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cantor SB, Mitchell MF, Tortolero-Luna G, Bratka CS, Bodurka DC, Richards-Kortum R. Cost-effectiveness analysis of diagnosis and management of cervical squamous intraepithelial lesions. Obstet Gynecol. 1998;91(2):270–277. doi: 10.1016/s0029-7844(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 91.Ramanujam N, Mitchell MF, Mahadevan-Jansen A, Thomsen SL, Staerkel G, Malpica A, et al. Cervical precancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem Photobiol. 1996;64(4):720–735. doi: 10.1111/j.1751-1097.1996.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 92.Utzinger U, Trujillo EV, Atkinson EN, Mitchell MF, Cantor SB, Richards-Kortum R. Performance estimation of diagnostic tests for cervical precancer based on fluorescence spectroscopy: effects of tissue type, sample size, population, and signal-to-noise ratio. IEEE Trans Biomed Eng. 1999;46(11):1293–1303. doi: 10.1109/10.797989. [DOI] [PubMed] [Google Scholar]
- 93.Cong X, Cox DD, Cantor SB. Bayesian meta-analysis of Papanicolaou smear accuracy. Gynecol Oncol. 2007;107(1 Suppl 1):S133–S137. doi: 10.1016/j.ygyno.2007.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardenas-Turanzas M, Follen M, Benedet JL, Cantor SB. See-and-treat strategy for diagnosis and management of cervical squamous intraepithelial lesions. Lancet Oncol. 2005;6(1):43–50. doi: 10.1016/S1470-2045(04)01712-7. [DOI] [PubMed] [Google Scholar]
- 95.Cardenas-Turanzas M, Follen M, Nogueras-Gonzalez GM, Benedet JL, Beck JR, Cantor SB. The accuracy of the Papanicolaou smear in the screening and diagnostic settings. J Low Genit Tract Dis. 2008;12(4):269–275. doi: 10.1097/LGT.0b013e31816b44bc. [DOI] [PubMed] [Google Scholar]
- 96.Cardenas-Turanzas M, Freeberg JA, Benedet JL, Atkinson EN, Cox DD, Richards-Kortum R, et al. The clinical effectiveness of optical spectroscopy for the in vivo diagnosis of cervical intraepithelial neoplasia: where are we? Gynecol Oncol. 2007;107(1 Suppl 1):S138–S146. doi: 10.1016/j.ygyno.2007.08.082. [DOI] [PubMed] [Google Scholar]
- 97.Cardenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, Adler-Storthz K, Benedet JL, Beck JR, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2865–2871. doi: 10.1158/1055-9965.EPI-08-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davies KR, Cox DD, Swartz RJ, Cantor SB, Follen M. Inverse decision theory with applications to screening and diagnosis of cervical intraepithelial neoplasia. Gynecol Oncol. 2007;107(1 Suppl 1):S187–195. doi: 10.1016/j.ygyno.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 99.Cantor SB, Cardenas-Turanzas M, Cox DD, Atkinson EN, Nogueras-Gonzalez GM, Beck JR, et al. Accuracy of colposcopy in the diagnostic setting compared with the screening setting. Obstet Gynecol. 2008;111(1):7–14. doi: 10.1097/01.AOG.0000295870.67752.b4. [DOI] [PubMed] [Google Scholar]
- 100.Cantor SB, Fahs MC, Mandelblatt JS, Myers ER, Sanders GD. Decision science and cervical cancer. Cancer. 2003;98(9 Suppl):2003–2008. doi: 10.1002/cncr.11680. [DOI] [PubMed] [Google Scholar]
- 101.Cantor SB, Levy LB, Cardenas-Turanzas M, Basen-Engquist K, Le T, Beck JR, et al. Collecting direct non-health care and time cost data: application to screening and diagnosis of cervical cancer. Med Decis Making. 2006;26(3):265–272. doi: 10.1177/027298906288679. [DOI] [PubMed] [Google Scholar]
- 102.Swartz RJ, Cox DD, Cantor SB, Davies KR, Follen M. Inverse decision theory: characterizing losses for a decision rule with applications in cervical cancer screening. Journal of the American Statistical Association. 2006;101(473):1–8. [Google Scholar]
- 103.Cantor SB, Sun CC, Tortolero-Luna G, Richards-Kortum R, Follen M. A comparison of C/B ratios from studies using receiver operating characteristic curve analysis. J Clin Epidemiol. 1999;52(9):885–892. doi: 10.1016/s0895-4356(99)00075-x. [DOI] [PubMed] [Google Scholar]
- 104.Mitchell MF, Hittelman WN, Hong WK, Lotan R, Schottenfeld D. The natural history of cervical intraepithelial neoplasia: an argument for intermediate endpoint biomarkers. Cancer Epidemiol Biomarkers Prev. 1994;3(7):619–626. [PubMed] [Google Scholar]
- 105.Cantor SB, Atkinson EN, Cardenas-Turanzas M, Benedet JL, Follen M, MacAulay C. Natural history of cervical intraepithelial neoplasia: a meta-analysis. Acta Cytol. 2005;49(4):405–415. doi: 10.1159/000326174. [DOI] [PubMed] [Google Scholar]
- 106.Okafor C, Follen M, Adewole I. Opportunities to improve health systems in Africa. A comparative overview of healthcare challenges for stakeholders and strategic planners. Gynecol Oncol. 2007;107(1 Suppl 1):S86–S93. doi: 10.1016/j.ygyno.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 107.Follen M, editor. Participants included teams from Ibadan, Lagos, Port Harcourt, Enugu, Zaria, and Jos. 2006. Monograph: Operation Stop Cervical Cancer; generous funding by T. Boone Pickens, ExxonMobil Foundation, and FedEx, January 2006: This monograph discusses the results of meetings with stakeholders from six diverse sites to understand equipment and funding needs to begin a cervical cancer screning, detection, and treatment program in Ibadan, Nigeria. [Google Scholar]
- 108.Follen M, editor. Results of meeting with stakeholders from six diverse sites to whom the requested equipment was delivered and who participated in a ten day training program for administrators, data managers, physicians, nurses, and scientists in Ibadan, Nigeria. 2006. Monograph: Operation Stop Cervical Cancer; generous funding by T. Boone Pickens, ExxonMobil Foundation, and FedEx, July 2006. [Google Scholar]
- 109.Pham B, Earle N, Rabel K, Follen M, Scheurer ME. Maximizing the diversity of participants in a phase II clinical trial of optical technologies to detect cervical neoplasia. Gynecol Oncol. 2007;107(1 Suppl 1):S208–S214. doi: 10.1016/j.ygyno.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shadeo A, Chari R, Vatcher G, Campbell J, Lonergan KM, Matisic J, et al. Comprehensive serial analysis of gene expression of the cervical transcriptome. BMC Genomics. 2007;8:142. doi: 10.1186/1471-2164-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thiberville C, Guillaud M, Lockwood W, Lam W, Follen M, MacAulay C. Multi-scale system biology applied to cervical inter-epithelial neoplasia. Gynecol Oncol. 2007;107(1 Suppl 1):S72–S82. doi: 10.1016/j.ygyno.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 112.Scheurer ME, Guillaud M, Tortolero-Luna G, Follen M, Adler-Storthz K. Epidemiologic modeling of cervical dysplasia with molecular and cytopathological markers. Gynecol Oncol. 2007;107(1 Suppl 1):S163–S169. doi: 10.1016/j.ygyno.2007.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang SK, Pavlova I, Marin NM, Follen M, Richards-Kortum R. Fluorescence spectroscopy as a diagnostic tool for detecting cervical pre-cancer. Gynecol Oncol. 2005;99(3 Suppl 1):S61–S63. doi: 10.1016/j.ygyno.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 114.Lee JS, Shuhatovich O, Price R, Pikkula B, Follen M, McKinnon N, et al. Design and preliminary analysis of a study to assess intra-device and inter-device variability of fluorescence spectroscopy instruments for detecting cervical neoplasia. Gynecol Oncol. 2005;99(3 Suppl 1):S98–S111. doi: 10.1016/j.ygyno.2005.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mitchell MF, Cantor SB, Brookner C, Utzinger U, Schottenfeld D, Richards-Kortum R. Screening for squamous intraepithelial lesions with fluorescence spectroscopy. Obstet Gynecol. 1999;94(5 Pt 2):889–896. doi: 10.1016/s0029-7844(99)00408-1. [DOI] [PubMed] [Google Scholar]
- 116.Mitchell MF, Cantor SB, Ramanujam N, Tortolero-Luna G, Richards-Kortum R. Fluorescence spectroscopy for diagnosis of squamous intraepithelial lesions of the cervix. Obstet Gynecol. 1999;93(3):462–470. doi: 10.1016/s0029-7844(98)00385-8. [DOI] [PubMed] [Google Scholar]
- 117.Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91(4):626–631. doi: 10.1016/s0029-7844(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 118.Swartz RJ, West LA, Boiko I, Malpica A, Guillaud M, Macaulay C, et al. Classification using the cumulative log-odds in the quantitative pathologic diagnosis of adenocarcinoma of the cervix. Gynecol Oncol. 2005;99(3 Suppl 1):S24–S31. doi: 10.1016/j.ygyno.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 119.Shadeo A, Chari R, Lonergan KM, Pusic A, Miller D, Ehlen T, et al. Up regulation in gene expression of chromatin remodelling factors in cervical intraepithelial neoplasia. BMC Genomics. 2008;9:64. doi: 10.1186/1471-2164-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swartz R, West L, Boiko I, Malpica A, MacAulay C, Carraro A, et al. Use of nuclear morphometry characteristics to distinguish between normal and abnormal cervical glandular histologies. Anal Cell Pathol. 2003;25(4):193–200. doi: 10.1155/2003/207481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.West LA, Swartz R, Cox D, Boiko IV, Malpica A, Macaulay C, et al. Cytometric features of cell nuclei of adenocarcinoma in situ and invasive adenocarcinoma of the cervix. Am J Obstet Gynecol. 2002;187(6):1566–1573. doi: 10.1067/mob.2002.127906. [DOI] [PubMed] [Google Scholar]
- 122.Atkinson EN, Follen M. Interactive dynamic graphical techniques for the exploration of functional data. Gynecol Oncol. 2005;99(3 Suppl 1):S76–S83. doi: 10.1016/j.ygyno.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 123.Myakov A, Nieman L, Wicky L, Utzinger U, Richards-Kortum R, Sokolov K. Fiber optic probe for polarized reflectance spectroscopy in vivo: design and performance. J Biomed Opt. 2002;7(3):388–397. doi: 10.1117/1.1483314. [DOI] [PubMed] [Google Scholar]
- 124.Utzinger U, Richards-Kortum RR. Fiber optic probes for biomedical optical spectroscopy. J Biomed Opt. 2003;8(1):121–147. doi: 10.1117/1.1528207. [DOI] [PubMed] [Google Scholar]
- 125.Pikkula B, Serachitopol D, MacAulay C, MacKinnon N, Lee JS, Cox D, et al. Multicenter clinical trials of in vivo fluorescence: Are the measurements equivalent? Progress in Biomedical Optics and Imaging. 2007;8(7) [Google Scholar]
- 126.Scheurer ME, Tortolero-Luna G, Guillaud M, Follen M, Chen Z, Dillon LM, et al. Correlation of human papillomavirus type 16 and human papillomavirus type 18 e7 messenger RNA levels with degree of cervical dysplasia. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1948–1952. doi: 10.1158/1055-9965.EPI-05-0073. [DOI] [PubMed] [Google Scholar]
- 127.Scheurer ME, Dillon LM, Chen Z, Follen M, Adler-Storthz K. Absolute quantitative real-time polymerase chain reaction for the measurement of human papillomavirus E7 mRNA in cervical cytobrush specimens. Infect Agent Cancer. 2007;2:8. doi: 10.1186/1750-9378-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]