Abstract
Background
In Barrett’s esophagus (BE), the normal esophageal squamous epithelium is replaced by specialized metaplastic columnar epithelium. BE is a premalignant lesion which can progress to esophageal adenocarcinoma (EAC). Currently there are no early molecular indicators that would predict progression from BE to EAC. As the only permanent residents of the epithelium, stem cells have been implicated in this metaplastic progression.
Aim
To determine the expression of DCAMKL-1 and other putative gastrointestinal stem cell markers in normal esophageal mucosa (NEM), BE and EAC.
Methods
Human NEM, BE, EAC and multi-tissue microarrays were analyzed for DCAMKL-1 and immunohistochemically scored based on staining intensity and tissue involvement, with epithelia and stroma scored separately. Total RNA isolated from BE and paired NEM was subjected to real-time RT-PCR analysis for DCAMKL-1, LGR5 and Musashi-1 mRNA expression.
Results
DCAMKL-1 is minimally expressed in squamous NEM, but increased in BE (with and without dysplasia) and EAC tissues. In EAC, we found increased stromal DCAMKL-1 staining compared to adjacent epithelia. Within the sub-mucosa of dysplastic BE tissues, an increase in endothelial cell expression of DCAMKL-1 was observed. Finally, an upregulation of DCAMKL-1, LGR5 and Musashi-1 mRNA was seen in BE compared to squamous NEM.
Conclusions
Here we report progressive increase of DCAMKL-1 expression in BE from dysplasia to EAC. Furthermore, there was an increase in putative stem cell markers DCAMKL-1, LGR5 and Msi-1 mRNA. These data taken together suggest that regulation of resident stem cells may play an important role in progression of BE to EAC.
Keywords: Stem Cell Marker, DCAMKL-1, Dclk-1, Cancer Stem Cell Marker, Esophagus, Barrett’s Esophagus, LGR5, Musashi-1
INTRODUCTION
Barrett’s esophagus (BE) is a widely recognized premalignant lesion detected in the majority of patients with esophageal and gastro-esophageal adenocarcinoma. It has a low 5-year survival rate of approximately 15–20% 1. The incidence of esophageal adenocarcinoma (EAC) has been increasing in the United States for more than 30 years 2. In 2009, nearly 60% of the estimated 16,400 new esophageal cancer cases were adenocarcinomas 3. The risk of EAC is 30–40 fold greater in patients with BE compared to those without this condition. Progression of BE is thought to involve the development of low-grade intraepithelial dysplasia (LGID) and high-grade intraepithelial dysplasia (HGID) prior to the development of cancer. These data support the hypothesis that BE is a pre-malignant condition 4.
BE is diagnosed in 10–15% of reflux patients undergoing upper endoscopy. Among those with chronic reflux symptoms undergoing endoscopic screening, a prevalence of 5.6% was reported 5. Although prevalence in the United States population is not known, a population-based study from Sweden diagnosed BE in 1.6% of all participants 6. Applying this percentage to the US population, it is estimated that 1.5 to 2 million adults have BE. Risk factors for BE include increased age, male gender, non-Hispanic white ethnicity, presence of reflux symptoms, and obesity. Inverse associations for BE include red wine consumption, H. pylori infection, and African-American ethnicity 7, 8.
Despite the clear association between BE and EAC, the low frequency of neoplastic progression of BE to EAC has created a major obstacle in predicting absolute risk of cancer when BE is diagnosed. This is compounded by the requirement of expert pathologic diagnosis of the degree of dysplasia in biopsy samples, and the potential for sampling error during endoscopy. The potential for field effects adjacent to observable lesions complicates the diagnosis. The identification of rational molecular biomarkers based on the cell of origin to categorize patients at increased risk for progression to EAC would be a major advance. Proposed markers have included tumor suppressor genes (CDKN2A and TP53) along with the presence of epithelial aneuploidy or tetraploidy 9. The cumulative 5-year incidence of EAC was 43% and 56% in BE patients with aneuploidy and tetraploidy respectively, compared to 5% without either finding 10. However, the predictive value of these markers has not been confirmed by further studies.
EAC is postulated to arise from a clonal stem-like population of cells, which is potentially responsible for its poor prognosis. Molecular signatures identifying the transition from normal esophageal stem cells to cancer stem/progenitor cells are of paramount importance in the development of novel therapeutics 11. Moreover, recent studies have indicated that tumor-initiating cells are present in EAC, suggesting a hierarchical organization 12. Furthermore, it has been speculated that cells expressing the putative intestinal stem cell marker Musashi-1 may play a significant role in the etiology of BE and EAC, and perhaps even a cell of origin for this disease. Recent studies have demonstrated that expression of Musashi-1 is absent in normal squamous epithelium, with minimal expression in BE, and is markedly increased in EAC 13. These studies suggest that stem cells may play an important role in the pathogenesis and progression of BE and EAC.
Recently, we have reported that the putative gastrointestinal and adenoma stem cell marker DCAMKL-1 is upregulated in human colorectal and pancreatic cancer and may play a functional role in cancer progression via the regulation of endogenous microRNAs 14–17. However, the expression of DCAMKL-1 in human BE or EAC has not yet been reported. In this study, we have employed immunohistochemical analysis to determine the cell specific protein expression patterns of DCAMKL-1 in normal esophageal mucosa, BE and EAC in human esophageal biopsy tissues. This study explores a potential mechanistic link between this key putative intestinal and pancreatic stem cell marker, which is expressed in several distinct regions of the gastrointestinal tract, and the eventual development of EAC in patients with BE.
METHODS
Tissue procurement
Human Barrett’s esophagus tissues were provided by Dr. Rhonda Souza at the University of Texas Southwestern Medical Center, Dallas, Texas; Oklahoma Veteran’s Affairs Medical Center (VAMC) according to the policies and practices of the institution’s IRB. Human multi-tissue microarrays (Tissue Array Network, Rockville, MD) were purchased commercially.
Immunohistochemistry
Heat-induced epitope retrieval was performed on formalin-fixed paraffin-embedded sections by utilizing a pressurized decloaking chamber (Biocare Medical, Concord, CA) in citrate buffer (pH 6.0) at 99°C for 18 min. For brightfield microscopy, slides were incubated in 3% hydrogen peroxide at room temperature for 20 min. After incubation with primary antibody [DCAMKL-1 (Ab31704) and LGR5 (Ab12827) (Abcam, Cambridge, MA)], the slides were incubated in rabbit polymer-horseradish peroxidase secondary detection kit (Biocare Medical). Slides were developed with diaminobenzidine (Sigma, St. Louis, MO).
Microscopic examination
Slides were examined with a Nikon 80i microscope and DXM1200C camera, and processed using NIS-Elements software (Nikon Instruments, Melville, NY).
Real-time RT-PCR Analysis
Real-time RT-PCR analyses were carried out as previously described 14. Detailed descriptions are provided in the supplementary materials and methods.
Scoring
Composite scoring for the immunostaining was performed by senior pathologist Dr. Stan Lightfoot. Detailed descriptions are provided in the supplementary materials and methods.
Statistical analysis
The Student’s t-test was used for comparison of mean values between groups. A p-value < 0.05 was considered statistically significant. SAS version 9.1 software (SAS Institute, Cary, NC) was used to perform all analyses.
RESULTS
DCAMKL-1 is expressed in BE and EAC
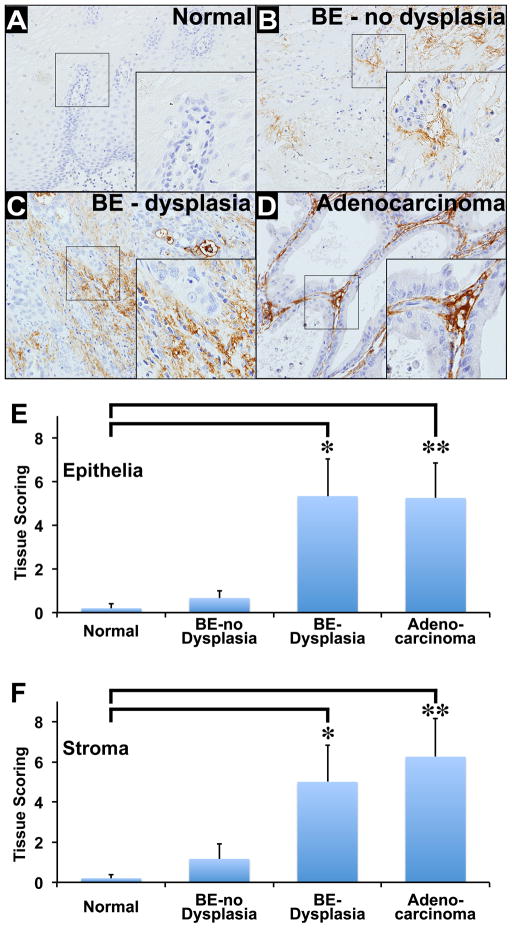
We utilized tissues obtained from biopsy specimens at the Oklahoma VAMC and human multi-tissue microarrays (Tissue Array Network, Rockville, MD) that were immunostained for DCAMKL-1. Normal squamous epithelial tissues (n = 5), BE with no dysplasia (n = 6), BE with dysplasia (n = 6), and Adenocarcinoma (n = 8) were utilized for this study. Minimal DCAMKL-1 epithelial staining was observed in normal squamous epithelial cells (Figure 1A). The staining intensity of DCAMKL-1 progressively increased in BE with no dysplasia, BE with dysplasia, and Adenocarcinoma/EAC (Figure 1 B–D). Distinct DCAMKL-1 stromal staining was observed in BE patients’ tissues (Figure 1 B–D). Quantitative analysis revealed an increase in stromal staining intensity that correlated with increased epithelial staining as the pathologic diagnosis progressed from BE patients with no dysplasia to patients with dysplasia. We observed a significant difference in the epithelial DCAMKL-1 immunostaining: (A) between the normal (95% CL of −0.36 to 0.76) and BE with dysplasia (95% CL 1 to 9.67) (p = 0.03); (B) between the normal and Adenocarcinoma (95% CL of 1.44 to 9.06) (p = 0.02); and (C) between BE with dysplasia and BE without dysplasia (95% CL of −0.19 to 1.52) (p = 0.04). We did not observe a significant difference between: (A) normal and BE without dysplasia (p = 0.28); (B) BE without dysplasia and Adenocarcinoma (p = 0.14); and (C) BE with dysplasia and Adenocarcinoma (p = 0.93) (Table 1 and Supplementary Table 1A and C). Furthermore, we observed a significant difference in the stromal DCAMKL-1 immunostaining: (A) normal (95% CL of −0.36 to 0.76) and BE with dysplasia (95% CL of 0.26 to 9.74) (p = 0.05); and (B) normal and Adenocarcinoma (95% CL of 1.76 to 10.74) (p = 0.04). We did not observe significant difference between: (A) normal and BE without dysplasia (95% CL of −0.76 to 3.09) (p = 0.26); (B) BE with dysplasia and BE without dysplasia (p = 0.08); (C) BE without dysplasia and Adenocarcinoma (p = 0.23); and (D) BE with dysplasia and Adenocarcinoma (p = 0.95) (Table 1 and Supplementary Table 1B and D). In adenocarcinoma, a greater intensity of stromal DCAMKL-1 staining was observed in comparison to patients with BE with or without dysplasia (Figure 1E, 1F; Table 1 and Supplementary Table 1). These data taken together demonstrate increased stromal expression of DCAMKL-1 that correlates with the progression from BE without dysplasia to EAC.
Figure 1.
Immunohistochemical expression of DCAMKL-1 in Normal, BE without dysplasia, BE with dysplasia and Adenocarcinoma/EAC. (A) Minimal DCAMKL-1 epithelial staining in normal squamous epithelium. (B–D) Increased expression of DCAMKL-1 in stroma of biopsies of BE with no dysplasia (B) and BE with dysplasia (C) as well as Adenocarcinoma/EAC in situ (D). Brown indicates cells positive for DCAMKL-1. (E–F) Immunohistochemical scoring of DCAMKL-1 in epithelium (E) and stroma (F) of various tissues as indicated. Values in the bar graphs are given as average ± SEM, and asterisks denote statistically significant differences (* p<0.05 = BE with dysplasia compared to Normal; ** p<0.05 = Adenocarcinoma compared to Normal).
Table 1.
Epithelial and stromal scoring of endoscopically obtained, histologically confirmed squamous esophageal mucosa, BE without dysplasia, BE with dysplasia and Adenocarcinoma/EAC.
| Subject identifier | Epithelial scoring | Stromal scoring | Histologic diagnosis |
|---|---|---|---|
| 8-226-A | 0 | 1×=3 | BE with no Dysplasia |
| 8-1510 | 0 | 0 | BE with no Dysplasia |
| 8-1017-3 | 2×1=2 | 0 | BE with no Dysplasia |
| 8-1017-2 | 1×=1 | 0 | BE with no Dysplasia |
| 8-140 | 0 | 2×2=4 | BE with no Dysplasia |
| 9-414-1A | 1×=1 | 0 | BE with no Dysplasia |
| 8-1497-2 | 3×=6 | 2×=6 | BE with Dysplasia |
| 8-149 | 0 | 4×3=12 | BE with Dysplasia |
| 8-332 | 1×2=2 | 2×3=6 | BE with Dysplasia |
| 8-393 | 3×2=6 | 2×3=6 | BE with Dysplasia |
| 8-1497-4 | 4×3=12 | 0 | BE with Dysplasia |
| 8-1497-3 | 3×2=6 | 0 | BE with Dysplasia |
| 8-1130 | 3×=12 | 3×4=12 | Adenocarcinoma |
| 8-86 | 1×3=3 | 0 | Adenocarcinoma |
| 8-56 | 3×4=12 | 4×3=12 | Adenocarcinoma |
| 9-414-1G | 1×1=1 | 1×1=1 | Adenocarcinoma |
| 9-414-1C | 0 | 1×1=1 | Adenocarcinoma |
| 8-4456 | 2×3=12 | 4×3=12 | Adenocarcinoma |
| 8-2686 | 1×4=4 | 2×2=4 | Adenocarcinoma |
| 8-2856-A | 4×1=4 | 2×4=8 | Adenocarcinoma |
DCAMKL-1 expression progressively increases in the squamous esophageal mucosa of patients with BE
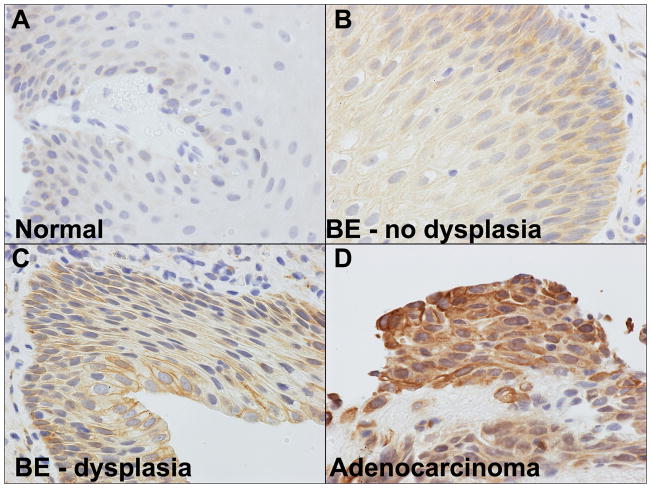
Although minimal DCAMKL-1 protein was observed in the squamous epithelium of patients without BE (Figure 2A), we observed increased DCAMKL-1 expression in the squamous cells of BE without dysplasia (Figure 2B). Furthermore, we observed a progressive increase of perinuclear DCAMKL-1 expression in reactive, irregular cells of BE with dysplasia (Figure 2C) and in the dysplastic cells of EAC (Figure 2D). When examined quantitatively, this pattern of DCAMKL-1 staining again correlated with the progression from BE with no dysplasia to BE with dysplasia and EAC. These data taken together suggests a potential role for DCAMKL-1 in the progression of dysplasia in BE.
Figure 2.
Immunohistochemical expression of DCAMKL-1 in endoscopically obtained, histologically confirmed squamous esophageal mucosa. (A) Minimal DCAMKL-1 expression in normal squamous epithelium. (B) Increased DCAMKL-1 expression in the squamous cells of BE without dysplasia; Progressive increase of perinuclear DCAMKL-1 expression in reactive, irregular cells of BE with dysplasia (C) and in dysplastic cells of Adenocarcinoma/EAC (D). Brown indicates cells positive for DCAMKL-1.
DCAMKL-1 expression in glandular and stromal cells of patients with BE
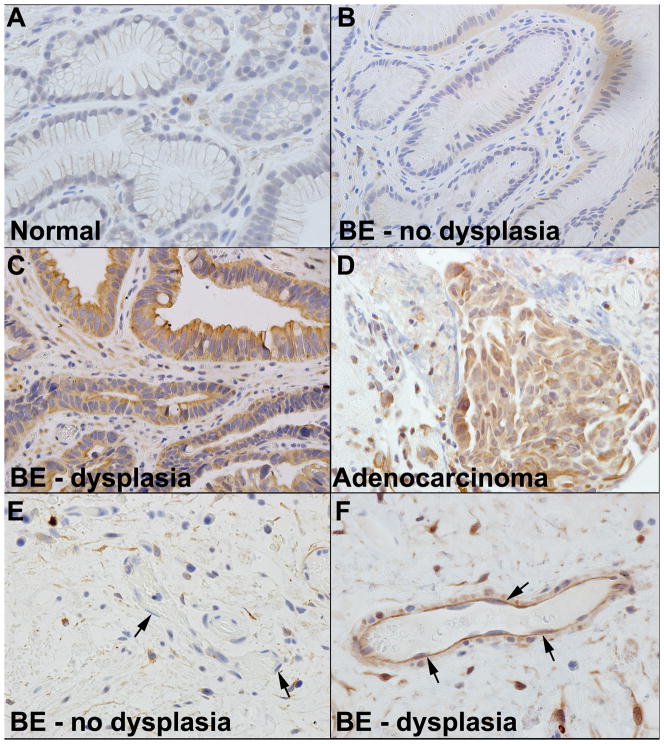
We next sought to determine cell specific expression patterns of DCAMKL-1 in the columnar epithelium of patients with BE. In normal patients, we observed minimal DCAMKL-1 expression in scattered individual epithelial cells (Figure 3A). However, in patients with BE without dysplasia we observed increased DCAMKL-1 expression in columnar epithelial cells (Figure 3B). In BE patients with dysplasia, we observed the appearance of stromal cells that demonstrated intense staining for DCAMKL-1 as well as increased epithelial expression (Figure 3C). In patients with EAC, we observed an overall increase in the number of cells and intensity of DCAMKL-1 expression in both epithelial and stromal compartments (Figure 3D). In sub-mucosa of BE patients without dysplasia, we observed a minority of endothelial cells that expressed DCAMKL-1 (Figure 3E). Whereas in BE patients with dysplasia, a majority of endothelial cells lining blood vessels expressed DCAMKL-1 (Figure 3F). In the biopsy specimens of the patients diagnosed with EAC, there were several cell types that may represent macrophages or mast cells that display immunoreactive DCAMKL-1. The scoring system for DCAMKL-1 staining in the epithelial and stromal cells is as described earlier for individual patients demonstrated in table 1.
Figure 3.
Immunohistochemical expression of DCAMKL-1 within endoscopically obtained, histologically confirmed glandular epithelium, BE without dysplasia, BE with dysplasia and Adenocarcinoma/EAC. (A) Scattered individual epithelial cells were positive for DCAMKL-1 in normal patients without BE. (B–D) The appearance of DCAMKL-1 expression in columnar epithelial cells in BE without dysplasia (B), increased expression of DCAMKL-1 in the columnar epithelia and the appearance of stromal cells positive for DCAMKL-1 in BE with dysplasia (C) overall increase in the number and intensity of DCAMKL-1 expression in both columnar and stromal compartments in Adenocarcinoma/EAC. (D). Minimal DCAMKL-1 immunostaining is observed in endothelial cells in patients with BE without dysplasia (arrows indicates endothelial cells) (E). Increased DCAMKL-1 staining is observed endothelial cells in BE with dysplasia (F). Brown indicates cells positive for DCAMKL-1 (arrows).
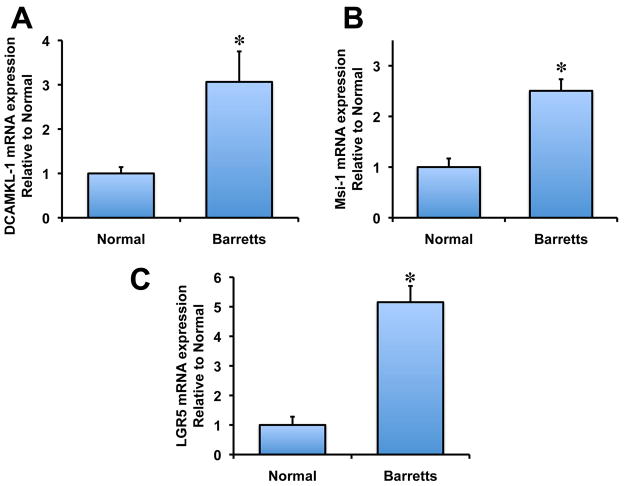
DCAMKL-1 mRNA is upregulated in BE
Recently, it has been demonstrated that RNA binding protein and putative stem cell marker, Musashi1 (Msi-1), is upregulated in BE and EAC 13. Furthermore, LGR5, a putative gut stem cell marker, is also upregulated in BE and EAC 18,19. To determine whether DCAMKL-1 mRNA is overexpressed in human BE, we performed real-time RT-PCR analysis on total RNA isolated from human BE and paired normal tissues. We observed a significant 3 fold increase of DCAMKL-1 mRNA in human BE compared to normal (Figure 4A). Similarly, we also observed a 2.5 fold increase in Msi-1 mRNA (Figure 4B) and 5 fold increase in LGR5 mRNA in human BE compared to its paired normal tissues (Figure 4C). Additionally, we observed an increased expression of LGR5 in patients with BE compared to normal tissues following immunohistochemical analysis (Supplementary Figure 1). These data taken together suggest that several key stem cell markers are upregulated in human BE compared to normal esophageal tissues.
Figure 4.
DCAMKL-1 is overexpressed in BE. (A) Increased DCAMKL-1 mRNA expression in BE compared to normal. (B–C) Increased Msi-1 (B) and LGR5 (C) mRNA expression in BE compared to normal. Values in the bar graphs are given as average ± SEM, and asterisks denote statistically significant differences (* p<0.01) compared to normal.
DISCUSSION
Here we report for the first time, to our knowledge, that immunoreactive DCAMKL-1 although minimally expressed in normal distal esophageal squamous mucosa, is markedly expressed in BE epithelium. Furthermore, an increased epithelial and stromal expression pattern was observed in patients with progression of dysplasia. Moreover, a marked increase in stromal DCAMKL-1 was observed in EAC. Despite the tremendous increase in EAC incidence over the past 3 decades, identification of the cellular origin of BE and the role of this cell during progression to EAC remains elusive. BE is a premalignant lesion detected in the majority of patients with EAC, conferring increased risk for cancer development 20. EAC is associated with a very low rate of survival once detected clinically. BE progression is associated with increasing severity of dysplasia prior to the development of cancer 10. Therefore, development of biomarkers that can assist in evaluating the risk of histologic progression toward EAC from BE are essential in limiting cancer development.
Surveillance programs are the mainstay for monitoring the progression of BE from no dysplasia to HGID. Generally, patients with HGID are offered a surgical option, after confirmation of the diagnosis by two expert pathologists 21. Newer experimental ablative therapies may be an option for patients at high surgical risk or for those who decline surgery. The limitations of these approaches and the lack of medical therapy illustrate the need for additional techniques that estimate the risk of progression and confirm the presence of dysplasia either within the specimen or potentially in the bloodstream.
The recent emerging stem cell hypothesis of solid tumor cancers has only recently been explored in esophageal cancer 22. The squamous epithelium of the normal esophagus undergoes metaplasia to form intestinal type mucosa in patients with BE. Recent challenges to this hypothesis suggest that the cell of origin may be a proximal gastric stem cell that migrates across the EG junction and crosses the squamocolumnar junction 23. These stem cells then proliferate and give rise to the intestinal type epithelia known as Barrett’s epithelium. Recently, in animal models it has been shown that the Barrett’s epithelium may be driven by bone marrow derived stromal cells that convert to epithelium and promote aggressive growth of BE 23–25. Thus, identification of the cell(s) of origin is key to gaining a more complete understanding of the molecular features of BE, including initiation and progression to EAC. In this manuscript we have developed immunohistochemical evidence of a unique cellular expression pattern of the novel intestinal stem cell marker DCAMKL-1. Although expressed in many gut tissues and pancreas, up regulation of DCAMKL-1 has been particularly noted in the stromal desmoplastic compartment in many solid tumors 16, 17. Although DCAMKL-1 staining is primarily epithelial, there is also clear evidence in early BE, of distinct stromal expression. In addition to the epithelial and stromal staining, evidence of endothelial and blood vessel expression of DCAMKL-1 was observed in some patients. As BE progresses to dysplasia, an increase in stromal and epithelial DCAMKL-1 is observed. Many more patients with varying degrees of dysplasia need to be examined to confirm the present observations. Nevertheless, we contend that even in this small sample set there is a clear, statistically significant increase in the expression of DCAMKL-1.
These findings suggest that DCAMKL-1 is expressed in pre-malignant tissues, which makes it an intriguing candidate for investigation as a tissue specific biomarker for BE. If validated, it could potentially be used as a surrogate marker after diagnostic confirmation using conventional pathologic techniques. Finally, this marker could be used in the confirmation of focal lesion eradication following endoscopic ablative therapies. Endoscopic techniques have been developed recently to eradicate BE with HGID, and EAC with varying levels of success 26–28. However, eradication does not always occur and its durability remains in question. Therefore, identification of cellular markers indicating the presence of neoplastic stem/progenitor cells either before or after endoscopic ablation would enhance the clinical and endoscopic management of individuals who undergo this therapy.
Previous efforts attempting to categorize patients at increased risk for progression to EAC using genetic markers have yielded limited results. These have included markers for tumor suppressor genes, aneuploidy, or tetraploidy. To date, there are no studies identifying an increased esophageal stem cell cohort during the progression from normal squamous mucosa through dysplasia to EAC. Identification of such a marker in serum could allow for a noninvasive assessment of general EAC risk in patients with gastroesophageal reflux disease.
Recently, epidermal growth factor receptor (EGFR) was reported to increase during the transition from BE to EAC29. However, use of EGFR as a molecular marker for transition of BE toward EAC is problematic for several reasons. EGFR was noted to stain squamous tissue at a higher intensity than BE or EAC. The optimal biomarker would be either absent or low in the normal state prior to the development of metaplasia or cancer as a negative control, limiting potential sample contamination. In addition, EGFR was not observed to increase in the stroma during transition of BE toward EAC. The absence of increased stromal staining does not allow for assessment of the stromal compartment as seen with DCAMKL-1, decreasing the potential usefulness of EGFR as a biomarker.
We hypothesize that a gastrointestinal type stem cell originating from the proximal stomach, EG junction, or bone marrow may represent the precursor cell type for the intestinal metaplasia associated with BE and EAC. In nude mice, knockdown of DCAMKL-1 results in cessation of HCT-116-mediated tumor xenograft growth. Furthermore, reduction of DCAMKL-1 correlates with increased expression of the tumor suppressor microRNA Let-7a and a reduction in oncogenic c-Myc RNA and protein 14. These data support a functional role for DCAMKL-1 in cancer progression.
Using a series of tissue microarray slides obtained from the Tissue Array Network, we performed immunohistochemical analysis to investigate DCAMKL-1 protein levels in human patients. Given the potential for interactions between epithelial stem cells and stroma during tumor progression, we predict that DCAMKL-1 may play a key role in the initiation and progression of BE and EAC. Our observations of increased accumulation of DCAMKL-1 provide a potential mechanistic link between esophageal injury/inflammation and carcinogenesis risk in proximal gastric epithelial cells.
In order to further evaluate the expression patterns of stem/progenitor markers in BE, we evaluated mRNA expression of DCAMKL-1, Msi-1 and LGR5 in patients with BE compared to normal. Relative mRNA expression determined using quantitative real-time RT-PCR demonstrated upregulation of all of these mRNAs in patients with BE. These data provide strong support for the involvement of stem/progenitor proteins in BE.
Overall, these data present a detailed immunohistochemical analysis of the putative gastrointestinal stem cell marker, DCAMKL-1, in the distal esophagus of patients with BE and EAC. These findings suggest a potential candidate for further study to evaluate the role of stem/progenitor cells in BE initiation and progression to adenocarcinoma, as well as a novel hypothesis to explain the intestinal metaplasia associated with this pre-malignant condition.
Supplementary Material
Immunohistochemical expression of LGR5 in Normal and BE. (A) Minimal LGR5 staining in normal squamous epithelium. (B) Increased expression of LGR5 in epithelial and stroma of BE.
Acknowledgments
Grant Support: NIH DK-065887, DK-002822, DK-085508, CA-137482, VA Merit Award and OCAST-AR101-030 to CWH
Footnotes
Authors have no conflict of interest
References
- 1.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Krishnadath KK, Reid BJ, Wang KK. Biomarkers in Barrett esophagus. Mayo Clin Proc. 2001;76:438–46. doi: 10.4065/76.4.438. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Kubo A, Levin TR, Block G, et al. Alcohol types and sociodemographic characteristics as risk factors for Barrett’s esophagus. Gastroenterology. 2009;136:806–15. doi: 10.1053/j.gastro.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett’s esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3:1–10. doi: 10.1016/s1542-3565(04)00602-0. [DOI] [PubMed] [Google Scholar]
- 9.van Dekken H, Hop WC, Tilanus HW, et al. Immunohistochemical evaluation of a panel of tumor cell markers during malignant progression in Barrett esophagus. Am J Clin Pathol. 2008;130:745–53. doi: 10.1309/AJCPO31THGVEUIDH. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–56. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 11.Mendelson J, Song S, Li Y, et al. Dysfunctional transforming growth factor-beta signaling with constitutively active notch signaling in Barrett’s esophageal Adenocarcinoma. Cancer. 2011 doi: 10.1002/cncr.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grotenhuis BA, Dinjens WN, Wijnhoven BP, et al. Barrett’s oesophageal adenocarcinoma encompasses tumour-initiating cells that do not express common cancer stem cell markers. J Pathol. 2010;221:379–89. doi: 10.1002/path.2733. [DOI] [PubMed] [Google Scholar]
- 13.Bobryshev YV, Freeman AK, Botelho NK, Tran D, Levert-Mignon AJ, Lord RV. Expression of the putative stem cell marker Musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23:580–9. doi: 10.1111/j.1442-2050.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 14.Sureban SM, May R, Ramalingam S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137:649–59. 59, e1–2. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sureban SM, May R, Lightfoot S, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–7. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 17.May R, Sureban SM, Lightfoot SA, et al. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299:G303–10. doi: 10.1152/ajpgi.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Rahden BH, Kircher S, Lazariotou M, et al. LgR5 expression and cancer stem cell hypothesis: clue to define the true origin of esophageal adenocarcinomas with and without Barrett’s esophagus? J Exp Clin Cancer Res. 2011;30:23. doi: 10.1186/1756-9966-30-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker L, Huang Q, Mashimo H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23:168–74. doi: 10.1111/j.1442-2050.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 20.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 21.Rossi M, Barreca M, de Bortoli N, et al. Efficacy of Nissen fundoplication versus medical therapy in the regression of low-grade dysplasia in patients with Barrett esophagus: a prospective study. Ann Surg. 2006;243:58–63. doi: 10.1097/01.sla.0000194085.56699.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobryshev YV, Freeman AK, Botelho NK, Tran D, Levert-Mignon AJ, Lord RV. Expression of the putative stem cell marker Musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010 doi: 10.1111/j.1442-2050.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbera M, Fitzgerald RC. Cellular origin of Barrett’s metaplasia and oesophageal stem cells. Biochem Soc Trans. 2010;38:370–3. doi: 10.1042/BST0380370. [DOI] [PubMed] [Google Scholar]
- 24.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–8. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson L, Stenstrom B, Chen D, et al. Human Barrett’s adenocarcinoma of the esophagus, associated myofibroblasts and endothelium can arise from bone marrow derived cells after allogeneic stem cell transplant. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118:670–7. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 27.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–6. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 29.Cronin J, McAdam E, Danikas A, et al. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett’s esophagus (BE) Am J Gastroenterol. 2011;106:46–56. doi: 10.1038/ajg.2010.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemical expression of LGR5 in Normal and BE. (A) Minimal LGR5 staining in normal squamous epithelium. (B) Increased expression of LGR5 in epithelial and stroma of BE.