Abstract
Background
Hyperlipidemia increases the level of blood plasminogen activator inhibitor-1 (PAI-1) that is responsible for regulating fibrinolysis by inhibiting both urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (t-PA). While this fibrinolytic pathway is well known, the role of PAI-1 in venous thrombosis (VT) in hyperlipidemic conditions has not been fully established. We sought to determine the effects of PAI-1 in an in vivo hyperlipidemic model of VT.
Method
C57BL/6 wild type (WT) mice, Apolipoprotein E gene-deleted mice (ApoE-/-), having hyperlipidemia, and PAI-1 gene-deleted (PAI-1-/-) mice were used in this study. Inferior vena cava (IVC) ligation below the level of the renal veins was performed to create a stasis VT. Endpoints included measuring acute thrombosis (day 2) and chronic thrombosis (days 6 and 14). At euthanasia, blood samples were collected for plasmin activity, and PAI-1 activity. In addition, the IVC and its thrombus were evaluated for thrombus weight (TW), u-PA activity, differential leukocyte count while the vein wall only was analyzed for monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase (MMP) 2 and MMP-9.
Results
Compared to WT at day 2, ApoE-/- mice demonstrated a statistically significant increase by 14% in TW (P<.05), and a significant 41% increase in circulating PAI-1 activity (P<.05), while showing a trend of decreased plasmin activity. In addition, TW in ApoE-/- mice was 45% higher than PAI-1-/- mice at day 2 (P<.05), 33% at day 6 (P<.01) and 41% at day 14 (P<.01).
ApoE-/- mice exhibited undetectable levels of u-PA in both vein wall and thrombus, compared to WT, at all time points. Also, vein wall MMP-2 was significant decreased by 64% at day 6 (P<.01) and 58% at day 14 (P<.05). MMP-9 was significantly decreased by 71% at day 2 (P<.01) and 48% at day 6 (P<.01), in ApoE-/- mice compared to WT.
In addition, in ApoE-/- mice, MCP-1 was significantly decreased by 38% at day 2 (P<.01) and 67% at day 6 (P<.01), versus WT mice. As expected in ApoE mice, following a decrease in MCP-1, monocyte recruitment was significantly decreased at days 6 (P<.01) and 14 (P<.05).
Conclusions
A significant increase of circulating PAI-1 levels, in hyperlipidemic mice, correlated with an early increase in TW, due to impaired fibrinolysis. The undetectable levels of u-PA in ApoE-/- mice correlated to a decrease in vein wall MMP-2, MMP-9, MCP-1 and a decrease in monocyte recruitment diminishing thrombus resolution.
Introduction
The primary role of the fibrinolytic system is to prevent the formation of or lyse existing thrombus through plasmin, the enzyme responsible for breaking down fibrin that forms in layers within a thrombus. This role is maintained through a constant dynamic balance between activators and inhibitors of fibrinolysis. The leading activators are tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA) and one of the key inhibitors is plasminogen activator inhibitor-1 (PAI-1). PAI-1 is produced mainly by the endothelium, but is also secreted in an active form by the liver and adipose tissue. Increased PAI-1 levels are found in various disease states such as cancer, obesity and metabolic syndrome1,2. Thus, it has been suggested that the increased incidence of thrombosis in patients with these conditions may be associated with elevated PAI-1 levels1,2. A secondary role of the fibrinolytic system is tissue remodeling, and u-PA mainly orchestrates this function. Tissue remodeling in blood vessels is the reorganization or renovation of existing tissues after an injury, such as the changes that occur in the vein wall after deep vein thrombosis (DVT)3. Tissue remodeling involves the matrix metalloproteinases (MMPs), general proteolytic enzymes, and monocyte chemotactic protein-1 (MCP-1) that are responsible for monocyte recruitment3-5. u-PA regulates both gene expression and protein synthesis of MMP-2, MMP-9 and MCP-16,7.
Hyperlipidemia has been established as a hypercoagulable state as demonstrated in mice. Using a carotid artery ferric chloride (FeCl3) model, ApoE-/- mice have a faster occlusion time and require shorter times of FeCl3 application in order to produce complete occlusion, compared to controls 8,9. In addition, some investigators have found a connection between hyperlipidemia and PAI-110-12, and others have established a connection between PAI-1 and DVT13-15. The objective of this study was to characterize the Apolipoprotein E gene deleted (ApoE-/-) mice with hyperlipidemia in the context of DVT. We hypothesized that an increase in PAI-1 in ApoE-/- hyperlipidemic mice would decrease fibrinolysis and promote venous thrombosis in a mouse model of this disease.
Materials and methods
Animals
Male C57BL/6 wild type (WT) mice (Charles River Laboratories, Wilmington, MA), ApoE-/- mice (Stock #2052, Jackson laboratories) and PAI-1-/- mice (Daniel Lawrence Ph. D. University of Michigan) were utilized in this study. The animals were all 8-10 weeks in age and had an average weight of 24.3 grams (g) across the groups (WT: 24.0 g, ApoE-/-: 24.9 g, PAI-1-/-: 24.1 g). All work was approved by the University of Michigan, University Committee on Use and Care of Animals and was performed in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The University of Michigan maintains accreditation by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Inferior Vena Cava (IVC) Stasis Model
A DVT model of IVC ligation, or stasis, was performed as previously described5. Briefly, mice were anesthetized via inhalation of 2% isoflurane with 100% oxygen. A midline laparotomy was performed with exteriorization of the intestines in order to expose the IVC. Dorsal branches were cauterized and side branches were ligated with 7-0 Prolene (Ethicon, Inc, Somerville, NJ.). The IVC was also ligated just below the level of the renal veins. The abdominal cavity was closed in a bi-layer fashion using 5-0 Vicryl for abdominal muscles and Vetbond tissue adhesive (3M Animal Care Products, St. Paul, MN) for the skin. Mice were euthanized at specific time points, post-thrombosis, for tissue harvest and blood collection5,16,17.
Experimental design
We used ApoE-/- as the hyperlipidemic experimental mice (4-fold increase in cholesterol and 2-fold increase in triglycerides compared to WT, data not shown) and WT mice as controls for the majority of the assays in this work. In addition, a limited number of PAI-1-/- mice were used for thrombus weight determinations only. All animals were maintained on a normal rodent chow diet for the duration of the study. The total numbers of mice utilized in this study included ApoE-/- mice (n=126), WT (n=122), and PAI-1-/- mice (n=35). Mice in each group were studied on either day 2 (acute time point), 6, or 14 (chronic time points) post-surgery18,19. Mice that did not receive surgery were used as true controls (TC), for baseline comparisons. Mice distribution used per each experiment included: n=5 for TC and at least n=10 for each DVT time point (Table I). The number of animals per time point was calculated based on power analysis of previous studies with this model 20. At the time of euthanasia, blood was collected by cardiocentesis and the IVC, with its associated thrombus, was harvested together for thrombus weight. The thrombus was then removed from the IVC, each frozen individually and then processed separately for the molecular assays. For histology, the harvested specimen included the IVC, its associated thrombus and the surrounding tissues to ensure the complete visualization of the vein wall and thrombus. Additional processing of the samples is described further in the following paragraphs.
Table I.
Mice distribution.
| Blood | Tissue | C57BL/6 (n122) | ApoE-/- (n126) | PAI-1-/- (n35) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | D2 | D6 | D14 | TC | D2 | D6 | D14 | TC | D2 | D6 | D14 | ||
| PAI-1 | u-PA (W/T) | 5 | 10 | 10 | 10 | 5 | 10 | 10 | 10 | ||||
| Plasmin | MCP-1 (W) | 5 | 10 | 10 | 10 | 5 | 12 | 10 | 12 | ||||
| MMP2/9 (W) | 5 | 12 | 10 | 10 | 5 | 11 | 10 | 11 | |||||
|
| |||||||||||||
| Thrombus weight | 15 | 32 | 30 | 30 | 15 | 33 | 30 | 33 | 5 | 10 | 10 | 10 | |
|
| |||||||||||||
| Histology | 5 | 5 | 5 | 5 | 5 | 5 | |||||||
TC: True control or no DVT mice. D2, D6, D14: Days after surgery. PAI-1: Plasminogen activator inhibitor-1. u-PA: Urokinase. MCP-1: Monocyte chemoattractant protein-1. MMP: Matrix metalloproteinase. W/T: Vein wall and thrombus. W: Vein wall. C57BL/6: wild type mice. ApoE-/-: Apolipoprotein E null mice. PAI-1-/-: PAI-1 null mice.
Thrombus Weight
On harvest day, the IVC and its associated thrombus were removed from anesthetized mice, weighed in grams (g), and measured for length in centimeters (cm). Thrombus weight, that included the weight of the thrombus and the weight of vein wall combined, was used as a reference of thrombosis5.
Fibrinolytic system
PAI-1 activity assay
To measure active murine PAI-1 concentrations in plasma samples, human uPA (rheotromb) was coupled to carboxylated beads (Luminex, Austin, TX) and used to capture active PAI-1. A standard curve was generated using known concentrations of murine PAI-1 in PAI-1 depleted mouse plasma (Molecular Innovations, Novi, MI). The assay was performed by adding 10 μl of either the standards or samples to a 96 well plate filter plate (Millipore, Billerica, MA). Each well then received 10uL PBS-1% BSA and 5000 beads in 30μL of PBS-1% BSA. The wells were incubated overnight in the dark at 4°C. The solution from each well was removed by vacuum suctioning and each well was washed twice with 200μL PBS-0.05 % Tween-20. The beads were mixed with continuous shaking in the darkat room temperature for 1 hour with 50 μl of 2μg/mL biotin-labeled rabbit anti-mouse PAI-1 (Molecular Innovations, Novi, MI). The wells were vacuum washed and 25 μl of 4 μg/mL streptavidin-R-phycoerythrin (Molecular Probes, Eugene, OR) was added to each well, and the mixture incubated in the dark with continuous agitation at room temperature for 30 minutes. Finally, the solution was removed from each well by vacuum suctioning, the beads were washed three times with 200 μl of PBS-0.05 % Tween-20, and 150 μl of sheath fluid was added for 5-10 minutes. The beads were then read with a Luminex 100 (Luminex, Austin, TX), median setting, 100 μl sample size, 100 events/bead.
Plasmin and u-PA activity assays
These assays quantify the amount of active plasmin in mouse plasma and the amount of active u-PA in mouse IVC wall samples and in separate thrombus samples. Functionally active mouse plasmin in citrated plasma samples bind to alpha 2-antiplasmin coated on the microtiter plate and only free active plasmin enzyme reacts with the anti-plasmin on the plate. Functionally active mouse u-PA in homogenized IVC or thrombus samples bind to biotinylated human PAI-1 that is coated on the microtiter plate. Then, monoclonal mouse anti-murine u-PA primary antibody binds to the captured u-PA and only free active u-PA reacts with the PAI-1 on the plate. Samples were detected by a biotinylated antibody, followed by the addition of tetramethylbenzadine (TMB) substrate for color development, according to the manufacturer’s protocol (Molecular Innovations, Novi, MI). The plates were read on an Elx808 plate reader (Biotek, Winooski, VT) at a 450 nm wavelength. In order to normalize the results, total protein was measured by using a bicinchoninic acid protein assay kit assessing colorimetric detection and quantification of total protein, read at 590 nm (Pierce, Rockford, IL).
Antigen determination
An enzyme-linked immunosorbent assay (ELISA) for MCP-1 (R&D Systems, Minneapolis, MN) was performed on individual homogenized IVC wall specimens. Samples were captured and then detected by a biotinylated antibody, followed by tetramethylbenzadine (TMB) substrate according to the manufacturer’s protocol. The results were read on an Elx808 plate reader (Biotek, Winooski, VT) at a 450 nm wavelength. In order to normalize the results, total protein was measured by using a bicinchoninic acid protein assay kit assessing colorimetric detection and quantification of total protein, read at 590 nm (Pierce, Rockford, IL).
Analysis of the Vein Wall Cellular Components (Morphometrics)
Histology samples were paraffin-embedded, cut in 4 mm sections, processed as slides, stained with hematoxylin and eosin (H&E), and examined under high-power (1,000 X) oil immersion light microscopy5. Five representative high-power fields (5HPFs) were examined around the vein wall for inflammatory cell counts and averaged. Inflammatory cells were identified as neutrophils, monocytes/macrophages, or lymphocytes on the basis of standard histological criteria, including nuclear size, cytoplasmic content, and total cell size, in a blinded manner by a board-certified veterinary pathologist.
Zymography
Determination of MMP-2 and MMP-9: Gelatin substrate zymography was performed using pre-cast 10% SDS-polyacrylamide gels containing 1 mg/ml of gelatin (unless otherwise stated, all zymography supplies were from Novex, San Diego, CA) 21-23. IVC wall specimens were homogenized in SDS, centrifuged for 10 minutes at 2000 × g, and equal volumes of the supernatants were diluted with tris-glycine-SDS sample buffer and electrophoretically separated under non-reducing conditions. Separated proteins were re-natured in 2.7% Triton X-100 for 2 hours to induce gelatin lysis by re-natured MMPs. The gels were developed for 48 hours at 37°C in 50 mM Tris-HCL, 5 mM CaCl2 and 0.2% Brij 35 followed by staining with Coomassie Blue and destaining in 10% acetic acid. Gelatinase or MMP activity was evident by clear bands against a dark stained background where the substrate has been degraded by the enzyme.
Samples containing human recombinant MMP-2 and MMP-9 (Oncogene, Boston, MA) were included as standards. Concentrations of the densitometry analysis were performed using a FOTO/Analyst CCD CAMERA (Fotodyne, Hartland, WI) and GEL-Pro Analyzer software version 3.1 (Media Cybernetics, Silver Springs, MD). Ratios of optical densities/total protein were calculated. In order to normalize the results, total protein was measured by using a bicinchoninic acid protein assay kit assessing colorimetric detection and quantification of total protein, read at 590 nm (Pierce, Rockford, IL).
Statistical analyses
All statistical analyses were performed using Graph Pad Prism version 5.01 for Windows (Graph Pad Software, San Diego, CA). The statistical differences between groups were determined by a Student-t test and a one-way ANOVA analysis of variance with a Tukey’s multiple comparison test performed between groups for additional comparisons. A value of P≤.05 was considered significant and all data is reported as mean ± standard error of the mean (SEM).
Results
Thrombussize was increased in mice with hyperlipidemia
The thrombus weight was significantly increased by 14%, in ApoE-/- mice at day 2, versus WT (0.036±0.001g vs. 0.032±0.001g; P<.05) and by 24% at day 14 (0.017±0.001g vs. 0.013±0.001g; P<.01). No differences were observed at day 6. In PAI-1-/- mice, the thrombus weight was significantly decreased at all time points, compared to ApoE-/- mice. Thrombus weight was 45% less at day 2 (0.020±0.005 vs. 0.036±0.001g; P<.01); 33% at day 6 (0.015±0.001g vs. 0.030±0.001g; P<.01) and 41% at day 14 (0.010±0.001g vs. 0.017±0.001g; P<.01) (Figure 1).
Figure 1. Thrombus size was increased in mice with hyperlipidemia.
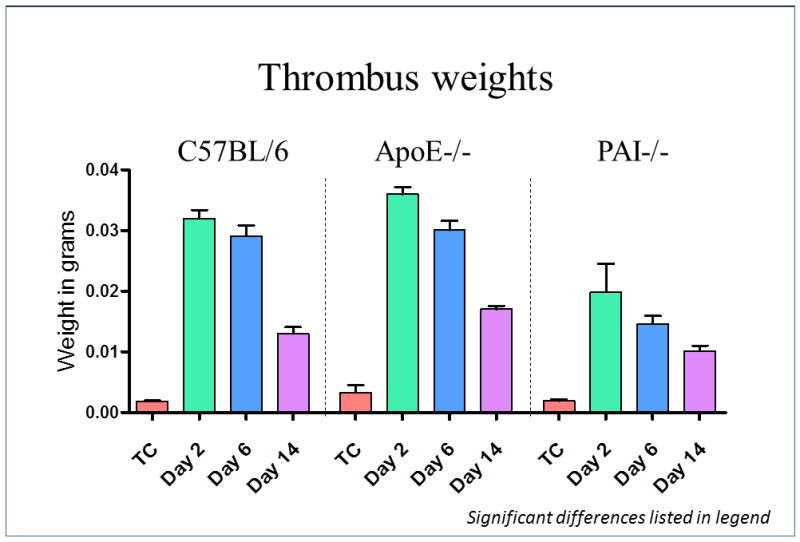
The thrombus weight was significantly increased by 14%, in ApoE-/- mice at day 2 (P<.05), and by 24% at day 14 (P<.01), vs. WT. No differences were observed at day 6 between ApoE-/- and WT mice. In PAI-1-/- mice, the thrombus weight was significantly decreased at all-time points compared to ApoE-/-, 45% at day 2 (P<.01); 33% at day 6 (P<.01) and 41% at day 14 (P<.01).
Fibrinolysis was impaired during acute DVT in mice with hyperlipidemia
No significant differences were observed in plasmin levels between ApoE-/- mice and WT. However, a trend showed a decrease in plasmin levels of 19% in ApoE-/- mice at day 2, compared to WT (523±15ng/mg vs. 644±90ng/mg); 13% at day 6 (252±18ng/mg vs. 291±13ng/mg) and 22% at day 14 (227±18ng/mg vs. 291±28ng/mg) (Figure 2A). Levels of PAI-1 were significantly increased by 41%, in ApoE-/- mice, compared to controls at day 2 (261±23pg/ml vs. 159±21pg/ml; P<0.05). No significant differences were observed at days 6 and 14, post DVT (Figure 2B).
Figure 2. The fibrinolytic system was impaired mainly at acute time point in mice with hyperlipidemia.
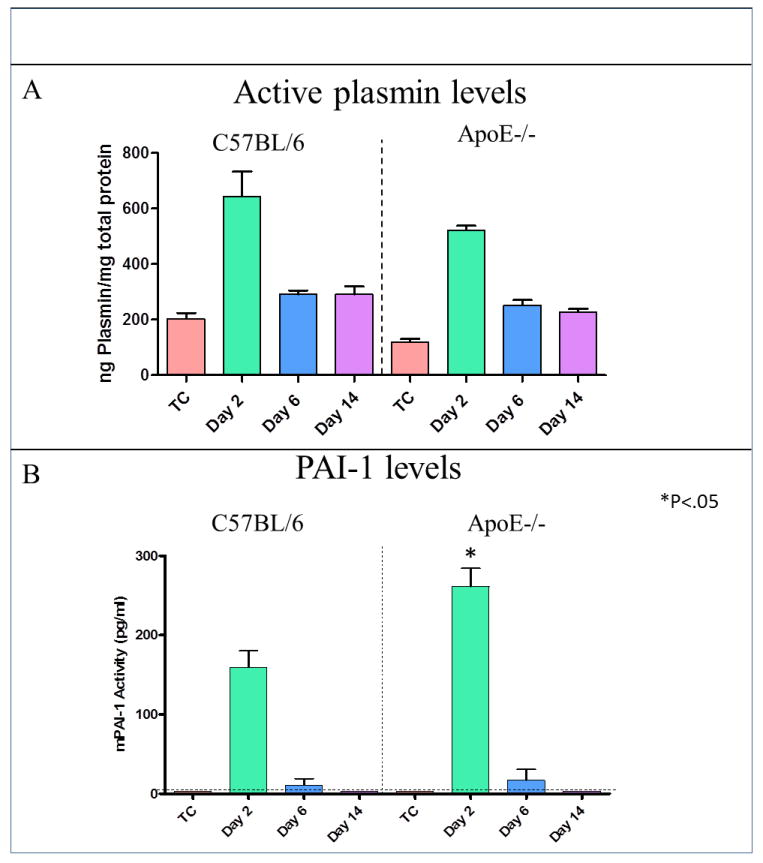
(A) A trend of decreased plasmin levels in ApoE-/- mice, compared to WT, was noted; 19% at day 2, 13% at day 6 and 22% at day 14. (B) Levels of PAI-1 were significantly increased by 41% in ApoE-/- mice, vs. WT at day 2 (P<.05). No significant differences were observed at days 6 and 14, post DVT.
Impaired urokinase-plasminogen activator (u-PA) decreased thrombus resolution
u-PA was detected in both vein wall and thrombus samples in C57BL/6 mice. The highest u-PA levels in vein wall were found at day 2, while the highest level of u-PA in the thrombus was on day 6 in the controls. Undetectable levels of u-PA were found in ApoE-/- at all-time points in both vein wall and thrombus (Figure 3). In ApoE-/- mice, vein wall MMP-2 was significantly decreased by 64% at day 6 post thrombosis (105±13ng/mg vs. 304±17ng/mg, P<.01) and 58% at day 14 (125±31ng/mg vs. 299±50ng/mg, P<.05), compared to WT (Figure 4A). Also, vein wall MMP-9 was significantly reduced by 71% in ApoE-/- mice at day 2 (188±5ng/mg vs. 619±46ng/mg, P<.01) and 48% at day 6 (128±32ng/mg vs. 248±16ng/mg, P<.01), compared to controls (Figure 4B).
Figure 3. The u-PA system was impaired in mice with hyperlipidemia.
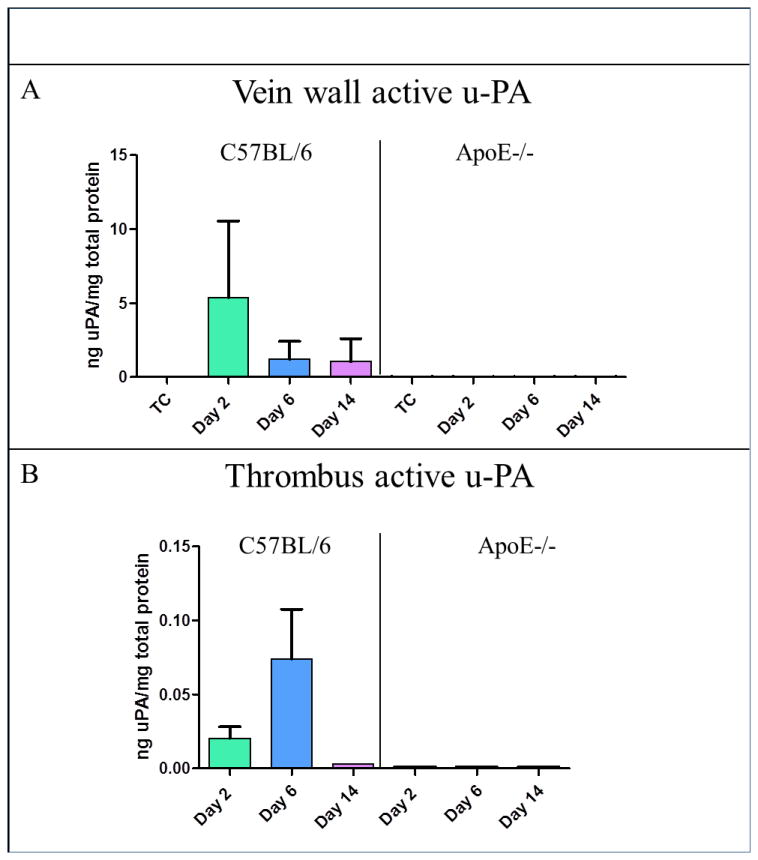
(A) This graph showed that it was possible to quantify levels of u-PA in both vein wall and thrombus in C57BL/6 mice. (B) Using the same assay for ApoE-/- mice, undetectable levels of u-PA were found at all time points in both the vein wall and thrombus.
Figure 4. The decreased u-PA system activity was reflected by MMPs in ApoE-/- mice.
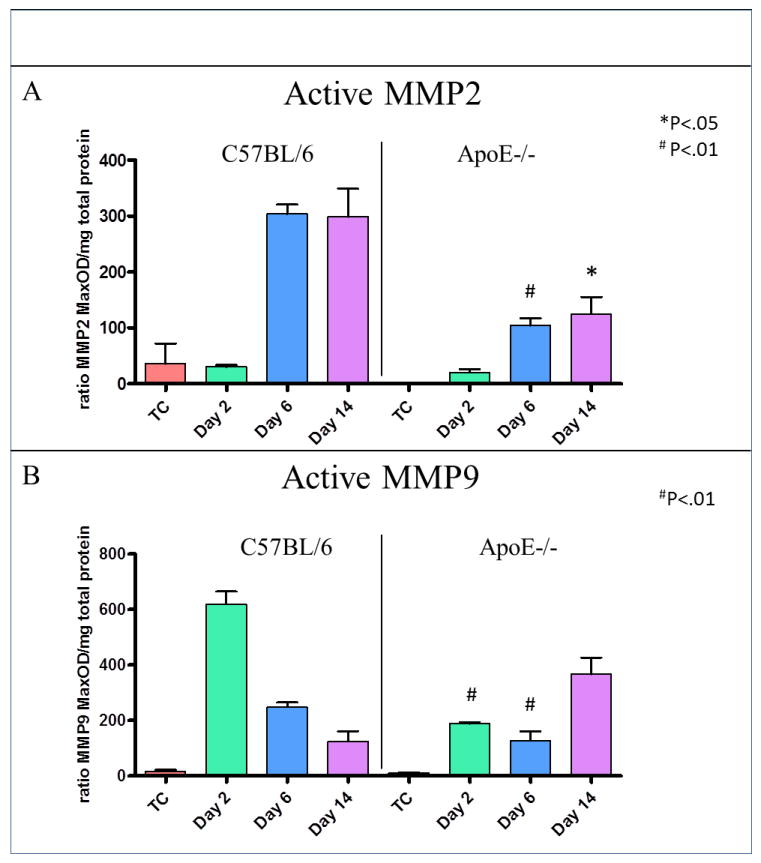
Zymography Results: (A) Graph showing that the protein concentration of MMP-2 was significantly decreased at days 6 and 14 in the vein wall (B) Graph showing the protein concentration of MMP-9 in the vein wall. It was significantly decreased at day 2 and day 6.
Vein wall MCP-1 was found to be significantly decreased by 38% in ApoE-/- mice, 2 days post DVT (1450±220ng/mg vs. 2293±172ng/mg, P<.01), and 67% at day 6 (88±12ng/mg vs. 264±28ng/mg, P<.01), versus WT (Figure 5A). Finally, the monocyte cell counts were significantly reduced by 41% at day 6 (4.5±0.8 monocytes/5HPF vs. 7.6±0.9 monocytes/5HPF, P<.01) and 39% at day 14 (6±0.7 monocytes/5HPF vs. 9.8±1 monocytes/5HPF, P<.05) in ApoE-/- mice with hyperlipidemia, versus controls (Figure 5B).
Figure 5. The decreased u-PA system activity was reflected by MCP-1 and monocyte recruitment in mice with hyperlipidemia.
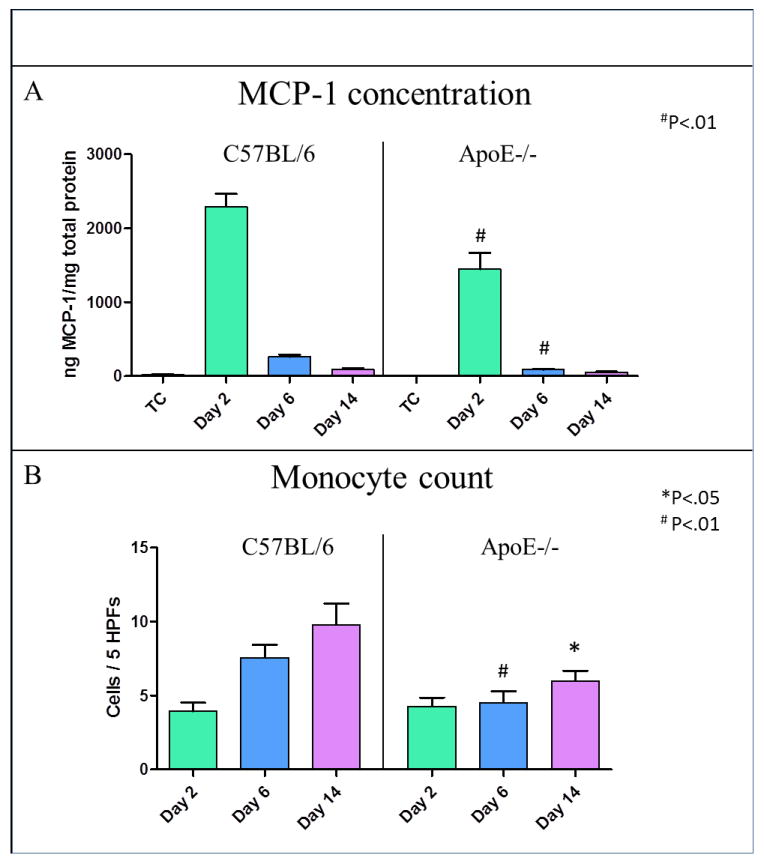
(A) This graph shows the vein wall protein concentration of MCP-1 to be significantly decreased by 38% at day 2 (P<.01) and by 67% at day 6 (P<.01) after DVT in ApoE-/- mice vs. WT. (B) Monocyte cell counts, average counts of 5 high power fields (HPF) in at least 5 specimens per each time point, were significantly reduced at day 6 (P<.01) and day 14 (P<.05) in mice with hyperlipidemia vs. controls.
Discussion
The JUPITER trial, published in 2009, documented a significant decrease in the incidence of DVT in patients treated with statins. Even though it brought to light the role of statins, hyperlipidemia and inflammation in DVT 24, the mechanism behind these findings was not addressed. Despite the fact that it is not considered a risk factor of DVT, hyperlipidemia is associated with a hypercoagulable state as demonstrated in mice studies8,9. In addition, some investigators have reported a link between hyperlipidemia and increased levels of PAI-1 in humans and mice10-12 and others have found a link between PAI-1 and DVT13-15, but up to this point no connection between hyperlipidemia, PAI-1 and DVT had been established. Thus, our aim was to determine if hyperlipidemic animals are more prone to developing a DVT through the effects of PAI-1. The balance between plasminogen activators (t-PA and u-PA) and PAI-1 determines the overall activity of the fibrinolytic system. The presence or activity of fibrinolytic proteins is able to be detected in circulation. In general, an increase of PAI-1 concentrations in plasma is associated with impaired fibrinolysis, decreased plasmin activity, an increase in the accumulation of fibrin and a decreased activation of MMPs 25. Here, we present the first work documenting the kinetics of DVT in a model of hyperlipidemia in mice.
We used the IVC ligation model that has been well-established and successful in producing consistent thrombus formation within the IVC in order to study DVT. It has been broadly used in multiple mouse strains and rats to study both acute venous thrombosis (2 days after ligation) and chronic venous thrombosis (days 6 and 14 after IVC ligation)3,18,19. Thrombus weight, a measurement of thrombus size in our mouse model of DVT, was assessed at all time points. The ApoE-/- mice produced the largest thrombus weights at days 2 and 14. This was an important initial finding in this work since, across the mouse strains, we performed the identical surgery on mice that had similar average body weights, were comparable in age, housed under the same conditions, and fed the same normal rodent diet.
Then we investigated the fibrinolytic system. Our findings during acute DVT (2 days after the IVC was ligated) demonstrated a deficit in the functioning of the fibrinolytic system when DVT occurs in hyperlipidemic mice. The levels of PAI-1, the main inhibitor of the fibrinolytic system, were significantly increased during acute thrombosis in mice with hyperlipidemia. This correlated with a decreased level of plasmin, the enzyme responsible for fibrin degradation, that was found in ApoE-/- mice at the same acute time point. When coagulation is triggered, multiple events occur simultaneously, including fibrinolysis. If fibrinolysis does not respond according to the normal thrombogenesis in this mouse model of DVT, the result is an increase in thrombus weight.
Thus, elevated levels of PAI-1 suggest impaired fibrinolysis in ApoE-/- mice that had a direct impact on thrombus weight, as observed with the increase in thrombus size documented in mice with hyperlipidemia. To further demonstrate that the increase in thrombus weight in ApoE-/- mice was due to the increase in PAI-1 levels, similar surgeries were performed on PAI-1-/-mice. PAI-1-/- mice had the smallest thrombus sizes at all time points, compared to ApoE-/- mice. This significant reduction in the thrombus weights of PAI-/- mice, suggest a relationship between PAI-1, DVT, and hyperlipidemia.
PAI-1 is the main inhibitor of u-PA and recent information regarding the u-PA system has shown that degradation of fibrin is not the only function of the fibrinolytic system. It has been demonstrated that, in rat cortical astrocytes, u-PA induces a rapid dose-dependent upregulation in MMP-2 and MMP-9 (by zymography) and also induces elevations in secreted MCP-16, providing evidence for the role of u-PA in orchestrating tissue remodeling. However, the role of the u-PA system in “vein wall” remodeling is an understudied area in the context of hyperlipidemia associated with DVT.
We investigated the u-PA system in hyperlipidemic mice using the IVC ligation model. Undetectable levels of u-PA were found in both the vein wall and thrombus of ApoE-/-. MMP-2, whose main effect is tissue remodeling at chronic time points, was significantly decreased at days 6 and 14. In addition, MMP-9 was significantly decreased at days 2 and 6. The participation of MMP-2 and MMP-9 in cell migration and thrombus resolution has been previously established3. The significant decrease that we found in MMP-2 and 9, suggest impaired thrombus resolution in the ApoE-/- mice, in comparison to the controls.
The main function of MCP-1 is to attract monocytes to the vein wall in order to aid in the thrombus resolution process. We found that the protein levels of MCP-1 were significantly decreased in ApoE-/- mice, compared to WT mice. This correlated with a significant decrease in monocyte recruitment at chronic time points. Monocytes are the main cellular players for thrombus resolution, as was previously demonstrated in rodents4,26 and its significant reduction may contribute to the increased thrombus weights in ApoE-/- mice that were, observed at the most chronic time point.
These results suggest that during chronic DVT, the ApoE -/- mice lack the ability to recruit monocytes to the area of thrombosis and to produce MMPs, well-known and key players in thrombus resolution, and correlates with the decreased thrombus resolution observed in ApoE-/- mice during the latter chronic time point. Since the only difference between ApoE-/- and WT mice in this work was hyperlipidemia, the significant decreases observed in the markers of tissue remodeling and thrombus resolution in ApoE-/- reflects an impairment of thrombus resolution.
A limitation of this study includes the fact that there was no manipulation of PAI-1 levels in the hyperlipidemic mice. In order to further demonstrate the PAI-1 mechanism, in the context of hyperlipidemia, the use of ApoE/PAI-1double -/- mice would be appropriate or the use of a PAI-1 inhibitor in the ApoE -/- mice. Also, using other hyperlipidemic mice such as LDLr -/- mice would be beneficial in order to further evaluate this mechanism. These are all part of our future direction.
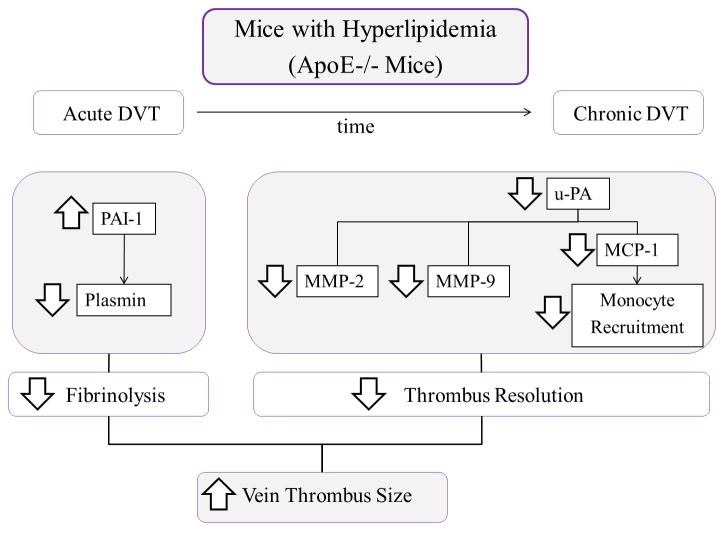
To our knowledge, this is the first time that a dysfunction in the fibrinolytic system has been characterized in mice with hyperlipidemia, undergoing venous thrombosis. During acute DVT, increased PAI-1 correlated with an early increase in thrombus weight, due to impaired fibrinolysis suggesting that these hyperlipidemic mice are more prone to developing DVT (Figure 6). In addition, undetectable levels of u-PA correlated with the decreases in MMP-2, MMP-9, MCP-1 and monocyte recruitment observed during chronic DVT, all key players in thrombus resolution, suggesting that this late mechanism was impaired (Figure 6). In summary, the elevation of PAI-1 activity found in mice with hyperlipidemia, promoted thrombus amplification and initiated a sequence of biological events that resulted in diminished thrombus resolution.
Figure 6. Schematic representation of our findings.
Fibrinolysis and thrombus resolution were found to be impaired in mice with hyperlipidemia undergoing DVT. Increased PAI-1 contributes to an early increase in thrombus size, due to impaired fibrinolysis. Additionally, undetectable levels of u-PA led to decreased vein wall MMP-2 and MMP-9, and lower levels of MCP-1 affecting monocyte recruitment, thus impairing thrombus resolution.
Acknowledgments
Supported by NIH 1PO1HL089407
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trost S, Pratley R, Sobel B. Impaired fibrinolysis and risk for cardiovascular disease in the metabolic syndrome and type 2 diabetes. Curr Diab Rep. 2006;6:47–54. doi: 10.1007/s11892-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 2.Berberoglu M, Evliyaoglu O, Adiyaman P, et al. Plasminogen activator inhibitor-1 (PAI-1) gene polymorphism (-675 4G/5G) associated with obesity and vascular risk in children. J Pediatr Endocrinol Metab. 2006;19:741–8. doi: 10.1515/jpem.2006.19.5.741. [DOI] [PubMed] [Google Scholar]
- 3.Sood V, Luke C, Miller E, et al. Vein wall remodeling after deep vein thrombosis: differential effects of low molecular weight heparin and doxycycline. Ann Vasc Surg. 2010;24:233–41. doi: 10.1016/j.avsg.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers DD, Jr, Henke PK, Wrobleski SK, et al. P-selectin inhibition enhances thrombus resolution and decreases vein wall fibrosis in a rat model. J Vasc Surg. 2002;36:928–38. doi: 10.1067/mva.2002.128636. [DOI] [PubMed] [Google Scholar]
- 5.Myers D, Jr, Farris D, Hawley A, et al. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 2002;108:212–21. doi: 10.1006/jsre.2002.6552. [DOI] [PubMed] [Google Scholar]
- 6.Lee SR, Guo SZ, Scannevin RH, et al. Induction of matrix metalloproteinase, cytokines and chemokines in rat cortical astrocytes exposed to plasminogen activators. Neurosci Lett. 2007;417:1–5. doi: 10.1016/j.neulet.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Minisini AM, Fabbro D, Di Loreto C, et al. Markers of the uPA system and common prognostic factors in breast cancer. Am J Clin Pathol. 2007;128:112–7. doi: 10.1309/M0GXVXA89BVLJ5C9. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh A, Li W, Febbraio M, et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest. 2008;118:1934–43. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–95. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lira FS, Rosa JC, Lima-Silva AE, et al. Sedentary subjects have higher PAI-1 and lipoproteins levels than highly trained athletes. Diabetol Metab Syndr. 2010;2:7. doi: 10.1186/1758-5996-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouwers MC, Govers-Riemslag J, Schalkwijk CG, et al. Plasma PAI-1 levels are independently related to fatty liver and hypertriglyceridemia in familial combined hyperlipidemia, involvement of apolipoprotein E. Thromb Res. 2008;122:466–72. doi: 10.1016/j.thromres.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Kudo T, Nakayama E, Suzuki S, Akiyama M, Shibata S. Cholesterol diet enhances daily rhythm of Pai-1 mRNA in the mouse liver. Am J Physiol Endocrinol Metab. 2004;287:E644–51. doi: 10.1152/ajpendo.00095.2004. [DOI] [PubMed] [Google Scholar]
- 13.Meissner MH, Zierler BK, Bergelin RO, Chandler WL, Strandness DE., Jr Coagulation, fibrinolysis, and recanalization after acute deep venous thrombosis. J Vasc Surg. 2002;35:278–85. doi: 10.1067/mva.2002.121066. [DOI] [PubMed] [Google Scholar]
- 14.Akhter MS, Biswas A, Ranjan R, et al. Plasminogen activator inhibitor-1 (PAI-1) gene 4G/5G promoter polymorphism is seen in higher frequency in the Indian patients with deep vein thrombosis. Clin Appl Thromb Hemost. 2010;16:184–8. doi: 10.1177/1076029609333673. [DOI] [PubMed] [Google Scholar]
- 15.Segui R, Estelles A, Mira Y, et al. PAI-1 promoter 4G/5G genotype as an additional risk factor for venous thrombosis in subjects with genetic thrombophilic defects. Br J Haematol. 2000;111:122–8. doi: 10.1046/j.1365-2141.2000.02321.x. [DOI] [PubMed] [Google Scholar]
- 16.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–94. [PubMed] [Google Scholar]
- 17.Wrobleski SK, Farris DM, Diaz JA, Myers DD, Jr, Wakefield TW. Mouse Complete Stasis Model of Inferior Vena Cava Thrombosis. J Vis Exp. 2011 doi: 10.3791/2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers DD, Jr, Rectenwald JE, Bedard PW, et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J Vasc Surg. 2005;42:329–36. doi: 10.1016/j.jvs.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg. 2011;25:229–39. doi: 10.1016/j.avsg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan VV, Hawley AE, Farris DM, et al. Decrease in fibrin content of venous thrombi in selectin-deficient mice. J Surg Res. 2003;109:1–7. doi: 10.1016/s0022-4804(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 21.Eagleton MJ, Peterson DA, Sullivan VV, et al. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res. 2002;104:15–21. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- 22.Dorffler-Melly J, Schwarte LA, Ince C, Levi M. Mouse models of focal arterial and venous thrombosis. Basic Res Cardiol. 2000;95:503–9. doi: 10.1007/s003950070028. [DOI] [PubMed] [Google Scholar]
- 23.Upchurch GR, Ford JW, Weiss SJ, et al. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by a rat aortic smooth muscle cells in vitro. J Vasc Surg. 2001;34:76–83. doi: 10.1067/mva.2001.115598. [DOI] [PubMed] [Google Scholar]
- 24.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–24. [PubMed] [Google Scholar]
- 26.Humphries J, Gossage JA, Modarai B, et al. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J Vasc Surg. 2009;50:1127–34. doi: 10.1016/j.jvs.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]