Abstract
Objective:
Necrotizing enterocolitis (NEC) is associated with high morbidity and mortality among infants admitted for intensive care. The factors associated with mortality and catastrophic presentation remain poorly understood. Our objective was to describe the factors associated with mortality in infants with NEC and to quantify the degree to which catastrophic presentation contributes to mortality in infants with NEC. Catastrophic NEC was defined before data analysis as NEC that led to death within 7 days of diagnosis.
Study Design:
We performed a retrospective review of the Pediatrix's Clinical Data Warehouse (1997 to 2009, n=560,227) to compare the demographic, therapeutic and outcome characteristics of infants who survived NEC vs those who died. Associations were tested by bivariate and multivariate analysis.
Result:
We compared the 5594 infants diagnosed with NEC and who were discharged home with 1505 infants diagnosed with NEC who died. In multivariate analysis, the factors associated with death (P<0.01 in analysis) were lower estimated gestational age, lower birth weight, treatment with assisted ventilation on the day of diagnosis of NEC, treatment with vasopressors at the time of diagnosis, and Black race. Patients who received only ampicillin and gentamicin on the day of diagnosis were less likely to die. Two-thirds of NEC deaths occurred quickly (<7 days from diagnosis), with a median time of death of one day from time of diagnosis. Infants who died within 7 days of diagnosis had a higher birth weight, more often were on vasopressors and high frequency ventilation at the time of diagnosis compared with patients who died at 7 or more days. Although mortality decreased with increasing gestational age, the proportion of deaths that occurred within 7 days was relatively consistent (65 to 75% of the patients who died) across all gestational ages.
Conclusion:
Mortality among infants who have NEC remains high and infants who die of NEC commonly (66%) die quickly. Most of the factors associated with mortality are related to immaturity, low birth weight and severity of illness.
Keywords: necrotizing enterocollitis, mortality, neonatal intensive care
Introduction
Necrotizing enterocolitis (NEC) is a common cause of morbidity in infants born prematurely.1 Our understanding of predictors of NEC outcomes has been limited to date and we do not have a full understanding of why some patients die and others do not. Rapidly progressive NEC that results in complete bowel necrosis in an otherwise well infant is also poorly understood and not well described in the literature.2 Some cases progress so quickly that they may never make it to surgery, making the distinctions of medical and surgical NEC inadequate surrogates for severity of illness. The purpose of this study was to investigate the factors associated with NEC mortality and specifically to delineate the difference between infants who die within 7 days of diagnosis from those that die later.
Methods
Research design
We performed a retrospective review of the Pediatrix Medical Group de-identified database to compare the demographic, therapeutic and outcome characteristics of infants who developed NEC and survived to those who developed NEC and died.
De-identified data set
Pediatrix Medical Group provides intensive care services in 275 hospitals in 33 states and Puerto Rico. Pediatrix Medical Group health care professionals providing care to infants admitted for intensive care use a proprietary software system to generate clinical admission, discharge and daily progress notes. Each day's notes are stored with diagnoses. The local data are consolidated within the Pediatrix Medical Group clinical data warehouse, de-identified and made compliant with Health Insurance Portability and Accountability Act of 1996 regulations.
Cases of patients with NEC were identified by searching the diagnosis table in our database for the term ‘Necrotizing Enterocolitis.' Patients with this diagnosis were included in our study cohort that evaluated the risk factors associated with death. Infants who died in the delivery room or those who were not admitted to the neonatal intensive care unit were not included in the data set. Data on estimated gestational age (EGA) represented the best estimates from both obstetrical data and neonatal examination findings. The Western Institutional Review Board has approved our research with the de-identified data set annually.
Our analytical approach to these data was descriptive in nature.
Definition of NEC
We defined NEC to be present if, in our database, a diagnosis of NEC was recorded. Infants with this diagnosis had one or more of the following clinical signs: bilious gastric aspirate or emesis, abdominal distention, blood in stool without evidence of a rectal fissure; and had one or more of the following radiographic findings: pneumatosis intestinalis, hepatobiliary gas or pneumoperitoneum. Either NEC-medical or NEC-surgical may be chosen at the time of data entry. Patients who had a diagnosis of NEC-medical who subsequently were treated with surgery (had both diagnoses) were assigned to the NEC-surgical group. All patients having a diagnosis of NEC-medical or NEC-surgical were included in our analysis of NEC. We also reviewed the surgical procedure to identify infants who had abdominal surgery for NEC or bowel perforation, and included them in the NEC-surgical if they were initially coded as NEC-medical. Infants with a diagnosis of isolated intestinal perforation and no evidence of NEC were excluded. Infants with a diagnosis of suspected NEC were also excluded. Catastrophic NEC was defined before data analysis as NEC that led to death within 7 days of diagnosis.
Mortality
Mortality was examined for all infants from the time of diagnosis during the initial hospitalization; we did not follow infants who were initially discharged or transferred and subsequently died. We also examined infants who were diagnosed with NEC and died within 7 days after diagnosis and compared these infants with patients who died more than 7 days following a diagnosis of NEC.
Statistical analysis
Before beginning our analysis, the available literature was reviewed to identify factors previously associated with the death related to NEC. Each of these prospectively identified factors was evaluated by bivariate and multivariate analysis. During the bivariate analysis, all of the numeric data (birth weight, EGA, APGAR score and length of hospital stay) were evaluated using both parametric (analysis of variance or two-sample t-test) and non-parametric test (Kruskal–Wallis non-parametric test and Mann–Whitney test). When the data were non-parametric, we used the Kruskal–Wallis non-parametric test when making more than two comparisons and the Mann–Whitney test for two-sample comparisons. After bivariate analysis, multivariate logistic regression was used to identify factors independently associated with death. We developed our model by incorporating the variables that were found to have significant interactions (P<0.1) with the rate of NEC. Variables were entered into the model using a stepwise selection (P-value for entry and retention P<0.1). Cases with missing values for any of the independent variables were excluded from the analysis. Only variables with adjusted odds ratio (AOR) 95% CI that did not cross 1 were considered to have an independent and significant association with mortality.
Results
Study population
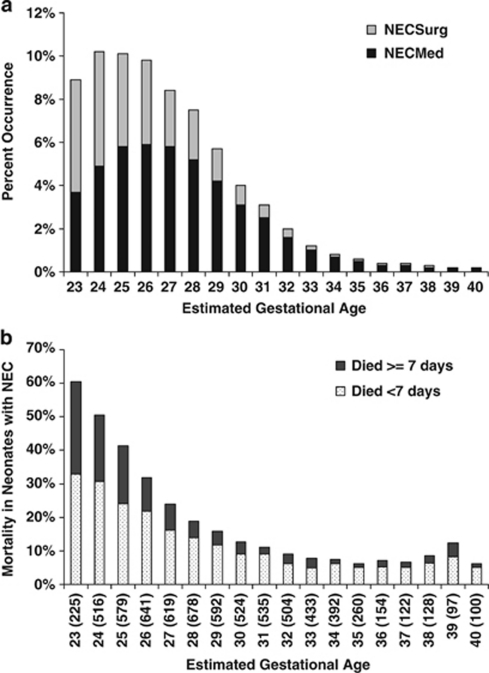
Between 1 January 1997 and 1 March 2009, there were 560 227 infants that were entered into the Pediatrix Clinical Data Warehouse. There were 6460 (1.2%) with a diagnosis of medically treated NEC and 2661 (0.5%) with a report of surgically treated NEC. There were 4346 (0.8%) with a diagnosis of suspected NEC and 1929 (0.3%) with spontaneous intestinal perforation; both of these groups of infants were excluded. NEC occurred at all EGA and the occurrence decreased with increasing EGA (Figure 1).
Figure 1.
(a) The occurrence of NEC by gestational age. (b) Mortality in infants who had a diagnosis of NEC by EGA. The number listed within parentheses represents the number of patients with NEC within each gestational age group. Mortality for infants with NEC decreases with increasing gestational age. The dark bars represent death at 7 or more days from diagnosis of NEC and the light bars represent deaths that occurred less than 7 days from diagnosis. Although mortality decreased with increasing gestational age, the proportion of deaths that occur within 7 days was relatively consistent (65 to 75% of the patients who died) across all gestational ages.
Of the 9121 infants with medically or surgically treated NEC, 5594 (61.3%) were discharged home, 1574 (17.3%) were transferred to another hospital before discharge, 1505 (16.5%) died, 403 (4.4%) were transferred to another service within the hospital of birth, and data were missing on discharge status in 38 (0.4%) infants. In this study, we compared the 5594 infants known to have lived and been discharged home with the 1505 infants known to have died. In addition, to better understand the factors associated with rapidly progressive NEC, we compared the 993 infants who died within 7 days of diagnosis to the 512 infants who died 7 or more days after diagnosis.
Differences between infants who survived and those that died
In bivariate analysis, infants who died were smaller, more immature, more often male, more often Black and less often White than infants who had NEC and lived (Table 1). Infants who died were more often outborn (transferred for care). During the first week after birth, infants who subsequently died from NEC more commonly were on mechanical ventilation and vasopressors than infants who lived (Table 2). In addition, they more often had a report of a patent ductus arteriosus and were more likely to have been treated with indomethacin and/or surfactant.
Table 1. Demographics.
| Variables | Lived | Died | P-value | Died <7 days | Died ⩾7 days | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 5594 | 1505 | 993 | 512 | ||
| Prenatal variables | ||||||
| Maternal age, median (10–90th percentile) | 26 (19–36) | 26 (18–36) | 0.3 | 26 (18–36) | 26 (18–36) | 0.3 |
| Inborn | 4350 (77.8) | 1090 (72.4) | 0.01 | 725 (73) | 365 (71.3) | 0.4 |
| Antenatal steroids | 3476 (62.1) | 972 (64.6) | 0.07 | 631 (63.5) | 341 (66.6) | 0.3 |
| Maternal tocolytics | 2339 (41.8) | 637 (42.3) | 0.7 | 420 (42.3) | 217 (42.4) | 0.9 |
| Maternal antibiotics | 2396 (42.8) | 667 (44.3) | 0.3 | 446 (44.9) | 221 (43.2) | 0.5 |
| Male | 3060 (54.7) | 891 (59.2) | 0.002 | 592 (59.6) | 299 (58.4) | 0.6 |
| Cesarean section | 3448 (61.6) | 952 (63.3) | 0.2 | 639 (64.4) | 313 (61.1) | 0.19 |
| Multiples | 1347 (24.1) | 298 (19.8) | 0.02 | 192 (19.3) | 106 (20.7) | 0.3 |
| Race | ||||||
| American/Alaska native | 56 (1) | 17 (1.1) | 0.7 | 12 (1.2) | 5 (1) | 0.8 |
| Asian | 123 (2.2) | 39 (2.6) | 0.8 | 26 (2.6) | 13 (2.5) | 0.8 |
| Black | 1269 (22.7) | 465 (30.9) | 0.01 | 312 (31.4) | 153 (29.9) | 0.5 |
| Hispanic | 1393 (24.9) | 397 (26.4) | 0.3 | 257 (25.9) | 140 (27.3) | 0.4 |
| White | 2478 (44.3) | 494 (32.8) | 0.01 | 333 (33.5) | 161 (31.4) | 0.5 |
| Other | 275 (4.9) | 93 (6.2) | 0.06 | 53 (5.3) | 40 (7.8) | 0.07 |
| Perinatal variables | ||||||
| Gestational age, median (10–90th percentile) | 30 (25–35) | 26 (24–32) | 0.001 | 26 (24–32) | 25 (23–31) | 0.01 |
| Birth weight, median (10–90th percentile) | 1.32 (0.72–2.5) | 0.82 (0.55–1.6) | 0.001 | 0.86 (0.6–1.7) | 0.74 (0.5–1.4) | 0.001 |
| APGAR 1 min, median (10–90th percentile) | 7 (2–8) | 5 (1–8) | 0.001 | 5 (1–8) | 5 (1–8) | 0.1 |
| APGAR 5 min, median (10–90th percentile) | 8 (6–9) | 7 (4–9) | 0.1 | 8 (5–9) | 7 (4–9) | 0.001 |
| Severe intraventricular hemorrhage (grades 3, 4) | 283 (5.1) | 264 (17.5) | 0.01 | 139 (14) | 125 (24.4) | 0.01 |
| Number of patients with major anomaliesa | 467 (8.3) | 138 (9.2) | 0.1 | 70 (7) | 68 (13.3) | 0.01 |
| Age at diagnosis, median (10–90th percentile) | 15 (4–40) | 18 (7–42) | 0.01 | 18 (7–40) | 19 (7–47) | 0.1 |
| Time diagnosis to death, median (10–90th percentile) | 2 (0–57) | 1 (0–4) | 30 (9–121) | 0.001 | ||
| Age at discharge, median (10–90th percentile) | 60 (25–125) | 27 (9–91) | 0.001 | 19 (8–42) | 53.5 (24.3–152) | 0.001 |
Most common anomalies were gastroschisis, trisomy 21 and heart anomalies.
Data are presented as N (%) except continuous data, which is presented as median (10–90th percentile).
Table 2. Antecedent events in the first week after birth.
| Variables | Lived | Died | P-value | Died <7 days | Died ⩾7 days | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 5594 | 1505 | 993 | 512 | ||
| On ventilator support in first 48 h | 2612 (46.7) | 1070 (71.1) | 0.001 | 670 (67.5) | 400 (78.1) | 0.001 |
| On high frequency ventilation in first 48 h | 494 (8.8) | 294 (19.5) | 0.001 | 177 (17.8) | 117 (22.9) | 0.3 |
| Antibiotics in the first week | 4830 (86.3) | 1321 (87.8) | 0.15 | 872 (87.8) | 449 (87.7) | 0.9 |
| Positive blood culture during first week of life | 304 (5.4) | 121 (8) | 0.001 | 78 (7.9) | 43 (8.4) | 0.7 |
| Surfactant | 2557 (45.7) | 982 (65.2) | 0.001 | 638 (64.2) | 344 (67.2) | 0.3 |
| Indomethacin any time | 1280 (22.9) | 560 (37.2) | 0.001 | 346 (34.8) | 214 (41.8) | 0.01 |
| PDA reported | 2185 (39.1) | 913 (60.7) | 0.001 | 556 (56) | 357 (69.7) | 0.001 |
| Vasopressors in the first weeka | 995 (17.8) | 604 (40.1) | 0.001 | 390 (39.3) | 214 (41.8) | 0.4 |
Abbreviation: PDA, patent ductus arteriosus.
Most commonly used vasopressors were dopamine, dobutamine and epinephrine.
Data are presented as N (%).
The diagnosis of NEC occurred later in infants who died compared with those that lived (18 vs 15 days, Table 1, but the difference is small and clinically insignificant). On the day of diagnosis of NEC, infants who eventually died were more often on mechanical ventilation, and when on assisted ventilation, they more often were supported with high frequency ventilation (Table 3). Infants who died more commonly had a positive blood culture (22.4 vs 14.7%, Supplementary Table) that was more often a Gram-negative organism (11.0 vs 5.2%, Supplementary Table). Infants who died more often received vancomycin, clindamycin, metronidazole or carbapenem and were less often on ampicillin or aminoglycoside than those that survived (Table 3). Specifically, survivors were more often treated with just ampicillin and gentamicin at the diagnosis of NEC than infants who died (11.9 vs 3.2%, Table 3). At the time of diagnosis, infants who died more often were treated with vasopressor support and more commonly were treated with more than one vasopressor (Table 3).
Table 3. Support and medications at diagnosis of NEC.
| Variables | Lived | Died | P-value | Died <7 days | Died ⩾7 days | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 5594 | 1505 | 993 | 512 | ||
| Any ventilator at diagnosis | 1702 (30.4) | 1274 (84.7) | 0.001 | 858 (86.4) | 416 (81.3) | 0.1 |
| High frequency ventilation | 224 (4) | 428 (28.4) | 0.001 | 313 (31.5) | 115 (22.5) | 0.01 |
| Vasopressor reporteda | 298 (5.3) | 560 (37.2) | 0.001 | 436 (43.9) | 124 (24.2) | 0.001 |
| Antibiotics on day of diagnosis | ||||||
| Any report of a specific antibiotic | ||||||
| Vancomycin | 2831 (50.6) | 839 (55.7) | 0.01 | 568 (57.2) | 271 (52.9) | 0.1 |
| Aminoglycosides | 3400 (60.8) | 846 (56.2) | 0.01 | 587 (59.1) | 259 (50.6) | 0.002 |
| Ampicillin | 1577 (28.2) | 287 (19.1) | 0.01 | 184 (18.5) | 103 (20.1) | 0.5 |
| Cefalosporins | 1325 (23.7) | 387 (25.7) | 0.1 | 255 (25.7) | 132 (25.8) | 0.9 |
| Clindamycin | 1473 (26.3) | 588 (39.1) | 0.001 | 424 (42.7) | 164 (32) | 0.001 |
| Metronidazole | 670 (12) | 215 (14.3) | 0.02 | 152 (15.3) | 63 (12.3) | 0.1 |
| Carbepeniums | 373 (6.7) | 151 (10) | 0.01 | 90 (9.1) | 61 (11.9) | 0.09 |
| Most common combinations | ||||||
| Vancomycin gentamicin | 891 (15.9) | 167 (11.1) | 0.01 | 110 (11.1) | 57 (11.1) | 0.9 |
| Ampicillin gentamicin | 667 (11.9) | 48 (3.2) | 0.001 | 30 (3) | 18 (3.5) | 0.6 |
| Vancomycin cefotaxime | 590 (10.5) | 108 (7.2) | 0.01 | 55 (5.5) | 53 (10.4) | 0.001 |
| Ampicillin clindamycin gentamicin | 389 (7) | 79 (5.2) | 0.02 | 57 (5.7) | 22 (4.3) | 0.27 |
| Vancomycin clindamycin gentamicin | 314 (5.6) | 149 (9.9) | 0.001 | 118 (11.9) | 31 (6.1) | 0.01 |
Abbreviation: NEC, necrotizing enterocolitis.
Most commonly used vasopressors were: dopamine, dobutamine and epinephrine.
Data are presented as N (%).
There were 605 (8.5%) infants in our study cohort who had major anomalies (Table 1). We were unable to identify any specific anomaly that was the primary cause of mortality and there was no association between anomalies and overall mortality. In subgroup analysis (presented below), the presence of a major anomaly did increase the likelihood that infants with NEC who were more than 32 weeks gestational age would die.
In multivariate analysis, the factors that remained independently associated with an increased risk of death (P<0.01 in analysis) were lower EGA (in the model as a continuous variable), lower birth weight (in the model as a continuous variable), on assisted ventilation on the day of diagnosis of NEC (AOR=5.3; 95% CI (4.5 to 6.4)), on vasopressors at the time of diagnosis (AOR=3.8 (3.2 to 4.6)), exposure to clindamycin (AOR=1.2 (1.0 to 1.4)), and Black racial/ethnicity (AOR=1.2 (1.1 to 1.5)). When interchanged with Black race, White race was associated with a decreased risk of dying (AOR=0.7 (0.6 to 0.8)). Patients who received only ampicillin and gentamicin on the day of diagnosis were less likely to die (AOR=0.5 (0.4 to 0.7)).
Differences between infants who died within 7 days of diagnosis and those who died 7 or more days after diagnosis of NEC
Of the 1505 infants who died, 993 (66%) died within 7 days of diagnosis and 512 (44%) died 7 days or more after diagnosis. In bivariate analysis, infants who died within 7 days of diagnosis were more mature and weighed more than infants who died after 7 days. During the first week after birth, infants who died within 7 days of NEC diagnosis less often were on assisted ventilation; less often had a report of a patent ductus arteriosus; and less often were treated with indomethacin. On the day of diagnosis of NEC, infants who died of their NEC within 7 days of diagnosis more often were treated with high frequency ventilation and more often were treated with vasopressors. Infants who died late were more likely to have a congenital anomaly (P<0.01) than infants with NEC who died early (but presence of a congenital anomaly was not associated with NEC death overall).
In multivariate analysis, infants who died within 7 days of diagnosis had a higher birth weight, more often were treated with vasopressors (OR=2.4 (1.8 to 3.0)) and more often were treated with high frequency ventilation at the time of diagnosis (OR=1.5 (1.2 to 2.0)).
Subgroup analysis
Of the 9121 infants with treated NEC, 6991 (77%) were 32 weeks or less and 2130 (23%) were more than 32 weeks EGA. Mortality was higher in the infants that were 32 weeks or less gestational age compared with infants with a gestational age of more than 32 weeks (20 vs 6%, P<0.01, also see Figure 1). As most of the infants in our study cohort were less than 32 weeks, the statistical findings in this subgroup of infants are very similar to the overall study cohort.
Infants 32 weeks or less EGA
In infants born at an EGA of 32 weeks or less, 4034 (57.7%) lived and were discharged home, 1555 (22%) were transferred, 1379 (20%) died and 23 (0.3%) had no data on discharge type. Using multivariate analysis, we compared the 4034 infants known to have lived and been discharged home with the 1379 infants known to have died to determine the independent risk factors associated with death.
The factors independently associated with an increased risk of death (P<0.01 in analysis) were lower EGA (in the model as a continuous variable), lower birth weight (in the model as a continuous variable), on assisted ventilation on the day of diagnosis of NEC (AOR=5.2; (4.3 to 6.3)), on vasopressors at the time of diagnosis (AOR=4; (3.3 to 4.8)), exposure to clindamycin (AOR=1.2; (1 to 1.4)), and Black racial/ethnicity (AOR=1.2; (1.1 to 1.4)). When interchanged with Black race, White race was associated with a decreased risk of dying (AOR=0.7 (0.6 to 0.8)). Patients who received only ampicillin and gentamicin on the day of diagnosis were less likely to die (AOR=0.5 (0.4 to 0.8)).
Of the 1379 infants who died, 900 (65%) died within 7 days of diagnosis and 479 (35%) died 7 days or more after diagnosis. The results of multivariate analysis produced identical AOR results to the overall group.
Infants more than 32 weeks EGA
In infants born at an EGA of more than 32 weeks, 1560 (73.3%) lived and were discharged home, 429 (20%) were transferred, 126 (6%) died, and 15 (0.7%) had no data on discharge type. Using multivariate analysis, we compared the 1560 infants known to have lived and been discharged home with the 126 infants known to have died to determine the independent risk factors associated with death.
The factors independently associated with an increased risk of death (P<0.01 in analysis) were lower birth weight (in the model as a continuous variable), on assisted ventilation on the day of diagnosis of NEC (AOR=7.2; (4.5 to 11.6)), on vasopressors at the time of diagnosis (AOR=8.3; (4.7 to 14.9)), and exposure to clindamycin (AOR=1.8; (1.1 to 2.8)). In this subgroup, decreasing gestational age and Black race were not independent risk factors associated with increased mortality. Exposure to only ampicillin and gentamicin was not associated with a decreased risk of mortality. The presence of an anomaly was associated with an increase risk of death (AOR=2.6; (1.6 to 4)).
Of the 126 infants who died, 93 (74%) died within 7 days of diagnosis and 33 (26%) died 7 days or more after diagnosis. The small number of patients prevented us from identifying any specific risk factors associated with death within 7 days of diagnosis.
Discussion
In a large data set of infants derived from neonatal intensive care units across the United States, we found the overall mortality associated with a diagnosis of NEC was high (16.5%). Mortality decreased with increasing gestational age (Figure 1). Two-thirds of these infants died within a week of diagnosis (with a median time of death, 1 day after diagnosis). Infants who died quickly (<7 days from diagnosis) were heavier than those infants who died later, but weighed considerably less than those who survived. They also were more often treated with vasopressors and high frequency ventilation at the time of diagnosis. Although overall mortality decreased with increasing gestational age, the likelihood of dying quickly was uniformly distributed across all gestational ages (Figure 1). The factors we found to be associated with mortality are similar to the results summarized in a recent review.3, 4
Several studies show an increased risk of NEC in Black infants; this is most often attributed to the high risk of prematurity in this group.5, 6 Holman et al.7 showed mortality associated with NEC was higher for Black infants than other groups. We also found racial differences associated with NEC mortality. Black infants were most likely to die from NEC than other races (mortality=27%), whereas Caucasians infants were less likely to die following NEC (mortality=17%). Comparison of the individual presentations of these demographic groups revealed that Black infants were smaller and presented later in life compared with White and Hispanic infants. It is thus unclear whether the primary risk factor that links race with death is NEC or extreme prematurity, as both are associated with African American race.
A positive blood culture was uncommon in infants with NEC, even in those who died. In multivariate analysis, positive cultures were not associated with an increased risk of death. We found that >75% of patients who died had negative cultures; only 11% of patients who died had a gram-negative bacterium grow from their blood culture. The culture findings included a paucity of anaerobic isolates, which are the only bacteria distinctly known to be capable of generating pneumatosis. This finding is consistent with an inability to capture and culture these organisms in the clinical setting.8 The absence of positive blood cultures in so many infants with NEC also indicates the possibility that infection may not be the precipitating etiological factor for many of these infants. The possibilities of viral infection, abnormal coagulation pathways and vascular/thrombotic triggers that initiate NEC need further consideration.
Cotten et al.9 reported that prolonged initial empirical antibiotic treatment in extremely low birth weight infants was associated with an increased risk of NEC and/or death. We found that broader spectrum antibiotic combinations used at the time of diagnosis of NEC were not associated with an increased likelihood of survival. In fact, the combination of ampicillin and gentamicin was associated with the best overall survival, whereas clindamycin was associated with an increased risk of death. Our observations may support previous reports that antibiotic choice10, 11 and duration influence outcome.9 Conversely, it is possible that physicians may have chosen broader spectrum antibiotics to give to sicker patients. Our study demonstrates an interesting association but cannot be used to determine causation.
Summary
Mortality among infants who have NEC remains high, and infants who die of NEC commonly (66%) die quickly. Most of the factors associated with mortality are related to immaturity, low birth weight and severity of illness. Our data also provide a testable hypothesis, namely, antibiotic choice at diagnosis of NEC may influence outcome. A prospective study of a limited vs broad antibiotic spectrum therapies in NEC might be valuable.
Acknowledgments
Dr Smith received support from NICHD 1K23HD060040-01.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
Supplementary Material
References
- Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half--today. Fetal Pediatr Pathol. 2010;29 (4:185–198. doi: 10.3109/15513815.2010.483874. [DOI] [PubMed] [Google Scholar]
- Henry MC, Lawrence MR. Surgical therapy for necrotizing enterocolitis: bringing evidence to the bedside. Semin Pediatr Surg. 2005;14 (3:181–190. doi: 10.1053/j.sempedsurg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32 (2:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368 (9543:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- Holman RC, Stehr-Green JK, Zelasky MT. Necrotizing enterocolitis mortality in the United States, 1979-85. Am J Public Health. 1989;79 (8:987–989. doi: 10.2105/ajph.79.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos AR, Moss ME, Pinzon MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol. 2002;16 (4:342–349. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87 (12:2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241 (6:984–989. doi: 10.1097/01.sla.0000164181.67862.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123 (1:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112 (3 part 1:543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117 (1:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.