Abstract
Background
Differentiation of atypical pathogens is important for community-acquired pneumonia (CAP). In this study, we compared sputum and nasopharyngeal swabs (NPS) for use in detection of Mycoplasma pneumoniae (MP), Chlamydophila pneumoniae (CP), and Legionella pneumophila (LP), using Seeplex PneumoBacter ACE Detection Assay (PneumoBacter; Seegene).
Methods
Sputum and NPS specimens were collected from patients in 15 hospitals. DNA was extracted from sputum using QIAamp DNA Stool Mini Kit (Qiagen) and from NPS using easyMAG (bioMérieux). Both types of specimens were evaluated by multiplex PCR using PneumoBacter. To determine the diagnostic performance of this assay, sputum samples were also tested using BD ProbeTec ET Atypical Pneumonia Assay (APA; Becton Dickinson).
Results
Among 217 sputum and NPS, 20 (9.2%), 2 (0.9%), and 0 sputum were positive for MP, LP, and CP, respectively, whereas 8 (3.7%) NPS were positive for MP. The sputum APA test yielded 186, 206, and 204 interpretable results for MP, LP, and CP, respectively. Of these, 21 (11.3%) were positive for MP, 2 (1.0%) were positive for LP, and 0 samples were positive for CP. Compared to APA, the sensitivity and specificity of the sputum assay for MP were 95.2% and 100.0%, respectively, whereas for the NPS assay, these were 38.1% and 93.9%. Sputum testing was more sensitive than NPS testing (P=0.002). For LP and CP diagnosis, PneumoBacter and APA tests agreed 100%.
Conclusions
Specimen type is crucial and sputum is preferred over NPS for simultaneous detection of MP, LP, and CP using multiplex PCR in CAP.
Keywords: Atypical pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Polymerase chain reaction, Nasopharyngeal swab, Sputum
INTRODUCTION
Mycoplasma pneumoniae (MP), Chlamydophila pneumoniae (CP), and Legionella pneumophila (LP) are the most common causes of atypical pneumonia [1, 2], which represents approximately 15% of all cases of community-acquired pneumonia (CAP) [2]. MP is second only to Streptococcus pneumoniae as the most common bacterial agent of CAP [3]. These atypical pathogens do not respond to β-lactam antimicrobial therapy, a commonly used empirical treatment for bacterial CAP [4]. Therefore, appropriate treatment of CAP requires the identification of the infecting pathogens [2, 5].
Currently available methods for diagnosis of MP, CP, and LP include conventional culture, serology, and nucleic acid-based tests. Although culture and serological tests are traditionally recommended for confirmatory diagnosis of pneumonia caused by MP, CP, or LP, such tests are of limited clinical utility because of the fastidious growth characteristics of the pathogens and the long turnaround time of the tests [6, 7]. As nucleic acid-based tests are rapid, highly sensitive, and specific [8, 9], and as MP, CP, and LP rarely colonize the respiratory tract [10, 11], molecular diagnostic tests for these pathogens are expected to be important tools in the clinical laboratory [12]. Because it is difficult to differentiate among MP, CP, LP, and other pathogens that cause CAP when using clinical and conventional laboratory tests, simultaneous detection of all pathogens that cause CAP is desirable [2, 11].
We recently had a chance to use Seeplex PneumoBacter ACE Detection Assays (PneumoBacter; Seegene, Seoul, Korea) to detect MP, CP, and LP for research. The appropriate specimen type for molecular diagnosis of atypical pneumonia remains controversial [9, 13, 14], and there have not yet been any reports describing the adequacy of different CAP specimen types for simultaneously detecting several atypical pathogens using multiplex PCR.
We therefore compared the detection of MP, CP, and LP in sputum and NPS samples using the PneumoBacter assay. The BD ProbeTec ET Atypical Pneumonia Assay (APA; Becton Dickinson, Sparks, MD, USA) was used as a control test.
METHODS
1. Patients and specimens
As part of a nationwide survey of CAP pathogens, sputum and NPS samples were collected from CAP patients diagnosed by previously reported criteria [3] at the time of initial assessment in 15 hospitals between May 2010 and February 2011. All specimens were collected using the same protocol and collection devices, which were distributed via the clinical microbiology laboratory of the Asan Medical Center. Expectorated sputum specimens were collected in sterile containers with screw caps, and NPS samples were obtained by use of flocked swabs and transported in universal transport medium (Copan Diagnostics, Corona, Italy). All specimens were transported to the central laboratory at 4℃ within 24 hr of collection.
2. DNA extraction
Sputum specimens (350 µL) were pre-treated with proteinase K, ASL buffer, and buffer AL, and incubated for 15 min at 70℃. DNA extraction was achieved using QIAamp DNA Stool Mini Kits (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions; the final elution volume of each sample was 200 µL. NPS specimens were vortexed briefly, and 500-µL aliquots of the 3-mL samples of universal transport media were processed using NucliSens easyMAG Kits (bioMérieux, Marcy l'Etoile, France); the final elution volume of each sample was 50 µL.
3. Seeplex PneumoBacter ACE Detection Assay
This multiplex PCR kit can detect MP, CP, LP, Streptococcus pneumoniae (SP), Haemophilus influenzae (HI), and Bordetella pertussis (BP). The SP, HI, and BP data were not analyzed. PCR was performed in a total volume of 20 µL containing 3 µL of a DNA sample, 4 µL 5× PneumoBacter primer, 3 µL 8-methoxypsoralen, and 10 µL 2× Multiplex Master Mix, as per the manufacturer's protocol. After heating at 94℃ for 15 min, PCR reaction mixutres underwent 40 cycles of amplification consisting of denaturation at 94℃ for 0.5 min, annealing at 60℃ for 1.5 min, and elongation at 72℃ for 1.5 min. At the end of the last cycle, the final elongation step was extended for 10 min. Amplicons were visualized after agarose gel electrophoresis and GelRed staining (Biotium, Hayward, CA, USA). The estimated sizes of amplicon characteristic of MP, LP, and CP were 583 bp, 472 bp, and 146 bp, respectively. Each amplification reaction contained plasmid DNA as an internal control, and each run was accompanied by a positive control containing plasmids of the 6 target pathogens, and sterilized distilled water as a negative control. If the results of internal controls were negative, the PCR reaction was considered to have failed due to the presence of inhibitory substances.
4. BD ProbeTec ET Atypical Pneumonia Assay
APA uses a strand-displacement amplification technique to directly (qualitatively) detect DNA from MP, LP, and Chlamydiaceae in separate reactions. The manufacturer recommends the use of throat swabs or specimens from the lower respiratory tract for detection of MP and CP, and only specimens from the lower respiratory tract for detection of LP. We followed the manufacturer's instructions to perform the assay. Briefly, 150 µL of a DNA sample was placed in the priming well at room temperature for 20 min. The priming well was next heated to 72℃ for 10 min. One hundred microliters of sample was transferred to an amplification plate and incubated in the BD ProbeTec ET instrument for 1 hr. Amplification and detection were automatically performed by the instrument. One positive control and one negative control were included in each assay run. If the control results were not as expected, we considered the assay data to be invalid.
5. Statistics
All statistical analyses were performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) and Excel 2007 (Microsoft, Redmond, WA, USA). We calculated agreement, sensitivity, specificity, and 95% confidence intervals.
RESULTS
Both sputum and NPS specimens were available from 217 patients and we successfully performed the PneumoBacter assay on all samples. Some samples failed APA quality control criteria, resulting in 186 interpretable MP results, 206 interpretable LP results, and 204 interpretable CP results.
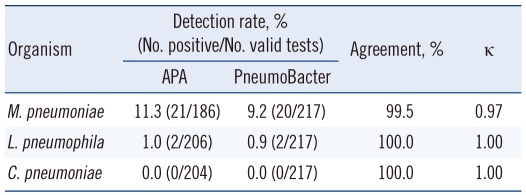
When the PneumoBacter assay was used to assess the 217 sputum specimens, 20 (9.2%) were positive for MP and 2 (0.9%) were positive for LP. Using the APA assay, 21 (11.3%) samples were positive for MP and 2 (1.0%) were positive for LP (Fig. 1). Agreement between the PneumoBacter and APA data in detection of MP, LP, and CP was 99.5%, 100%, and 100%, respectively. The kappa values were 0.97 for MP and 1.00 for both LP and CP (Table 1).
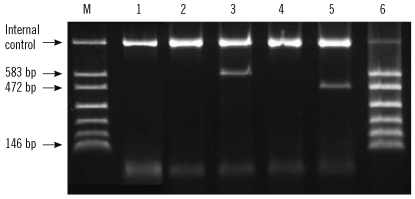
Fig. 1.
Results of the multiplex PCR Seeplex PneumoBacter ACE Detection Assay. Lane M, amplicon size marker. Bands of 583 bp, 472 bp, and 146 bp are characteristic of Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydophila pneumoniae, respectively. Lane 1, negative control; Lanes 2 and 3, nasopharyngeal swab (NPS) and sputum samples from an Mycoplasma pneumoniae-positive patient; Lanes 4 and 5, NPS and sputum samples from a Legionella pneumophila-positive patient; Lane 6, positive control.
Table 1.
Comparison of BD ProbeTec ET Atypical Pneumonia Assay and Seeplex PneumoBacter ACE Detection Assay results, using sputum specimens
Abbreviations: APA, BD ProbeTec ET Atypical Pneumonia Assay; Pneumo-Bacter, Seeplex PneumoBacter ACE Detection assay; M. pneumoniae, Mycoplasma pneumoniae; L. pneumophila, Legionella pneumophila; C. pneumoniae, Chlamydophila pneumoniae.
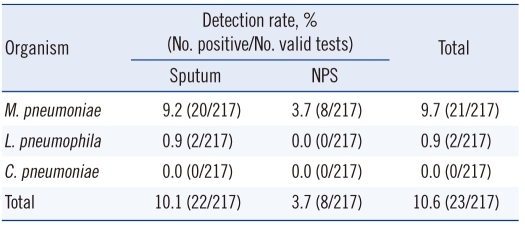
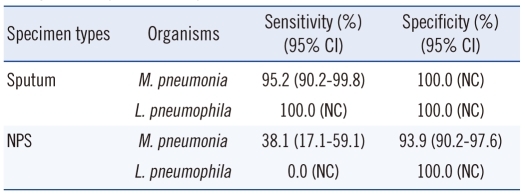
There were only 8 (3.7%) samples positive for MP among the 217 NPS samples. There were no NPS samples positive for LP. When results from the sputum and NPS samples were combined, MP was detected in 21 patients. Seven of these 21 patients were positive in both sputum and NPS tests; 13 were sputum-positive only, and 1 was NPS-positive only. Overall, sputum resulted in more PCR-positive (10.1% vs. 3.7%; Table 2). Compared to APA, the sensitivity and specificity of sputum tests for MP were 95.2%, and 100.0%, respectively, and those of NPS were 38.1% and 93.9%, respectively. Sputum tests were more sensitive than NPS tests (P=0.002; Table 3).
Table 2.
Comparison of M. pneumoniae, L. pneumophila, and C. pneumoniae detection in sputum and NPS using the Seeplex PneumoBacter ACE Detection Assay
Abbreviations: NPS, nasopharyngeal swab M. pneumoniae, Mycoplasma pneumoniae; L. pneumophila, Legionella pneumophila; C. pneumoniae, Chlamydophila pneumoniae.
Table 3.
Sensitivity and specificity of Seeplex PneumoBacter ACE Detection Assay compared to BD ProbeTec ET Atypical Pneumonia Assay when sputum samples were evaluated
Abbreviations: M. pneumoniae, Mycoplasma pneumoniae; L. pneumophila, Legionella pneumophila; NPS, nasopharyngeal swab; CI, confidence interval; NC, not calculated.
DISCUSSION
Our results reveal that specimen type is crucial for PCR-based diagnosis of MP and LP in CAP patients, and that sputum produces superior results than NPS. Throat swabs are the traditionally preferred sample collection method for detection of MP [15]. However, the optimal sample type for molecular diagnosis remains unclear [9, 12, 16-19]. In the studies evaluating specimen types for detection of serologically confirmed MP pneumonia, detection rates from sputum samples were higher than those from NPS samples or throat swabs (62.5% vs. 41.0% with nested PCR [16] and 69.0% vs. 37.5% [9] with PCR-hybridization).
In an animal study, quantitative culture of MP resulted in a number of colony-forming units from lung samples 100-1,000 times the number from throat samples [20]. MP was also more abundant in sputum than in upper respiratory tract (URT) samples in clinical specimens examined using nested PCR [21] and culture [22]. The higher number of MP organisms in pulmonary alveoli compared to the epithelium of the URT may explain the superiority of sputum samples for the MP tests in the present study. Compared to previous studies [9, 16], the sensitivity of tests using sputum samples were much higher than those using NPS in the present study. As only adult CAP patients were included in this study, the difference in sensitivity between URT and lower respiratory tract specimens may be more pronounced than in the studies that included patients with both upper and lower respiratory tract infections of MP [9]. Indeed, British Thoracic Society Guidelines from 2009 recommend sputum for PCR detection of MP in CAP patients [4].
The PneumoBacter assay had comparable sensitivity to APA when testing sputum samples. The 2 tests yielded near-perfect kappa values for MP and LP. However, we obtained valid results from all PneumoBacter sputum assays, whereas 14% of the APA sputum MP assays failed quality-control tests. PneumoBacter appears to be less vulnerable to defects in specimen quality. In a previous study using capillary PCR for analysis of throat swab, sputum, and bronchoalveolar lavage (BAL) samples, the lowest detection rate was from sputum samples (14.2%), compared to 28.6% from throat swabs and 20.0% from BAL. However, the authors did not analyze specimens in parallel and did not control for the existence of PCR inhibitors. In the present study, DNA from sputum specimens was extracted by QIAamp DNA Stool Mini Kits that included a supplement for removing PCR inhibitors. This helped to ensure valid results for all specimens in the PneumoBacter test. Thus, extraction methods yielding high-quality DNA are also important for molecular testing [23].
The difference in detection rates between sputum and URT samples in the present study was much larger than the differences reported previously [9, 16]. Efficient removal of PCR inhibitors can enhance the detection sensitivity in sputum specimens and exaggerates the difference in sensitivity between sputum and NPS samples. Flocked swabs collect more samples from patients than fibrous swabs [24]. While we used flocked swabs to obtain NPS samples, they were placed in universal transport medium. The effects of this medium on the results of molecular tests for MP, LP, and CP have not yet been evaluated. As this transport system may cause a dilution effect, the utility of NPS samples collected using flocked swabs for diagnosis of MP, CP, and LP requires further evaluation. Because 2 kinds of extraction kits were used in this study, the difference of sensitivity between types of specimen may result from extraction efficiency. However, in a subsequent experiment, easyMAG was 100 times more efficient for DNA extraction from sputum and 1,000 times more efficient for extraction from NPS than QIAamp DNA Stool Mini Kit (data not shown). Even though we used an extraction method for sputum that was less efficient than for NPS, sputum assays had higher sensitivity than NPS assays.
Only 2 sputum-positive LP samples were detected, and we found no CP-positive samples. Nucleic acid-based detection of Legionella spp. has been successful in sputum, urine, and blood specimens [25]. A few commercial assay kits are available, but only APA is approved by the U.S. Food and Drug Administration (FDA) [25]. The sensitivity of molecular tests has been estimated to be 80-100% when lower respiratory tract secretions are analyzed, and the specificity is estimated to be >90% that of culture [26]. Throat swabs have been used for diagnosis of LP pneumonia in only a few studies [27, 28] and in a more recent study, the sensitivity of swab samples was inadequate to permit detection of LP [29]. We did not detect LP in any NPS samples, supporting the preferred use of lower respiratory tract specimens for detection of LP. No optimal specimen type has been defined for diagnosis of CP infection. The clinical utility of nucleic acid-based tests for CP is limited by the absence of a reliable gold standard method [30]. The sensitivities of sputum culture and PCR using sputum have been reported to be as high as 95% and 100%, respectively, compared to 35% and 30% for NPS samples [31]. The results of a previous study measuring the DNA levels in various respiratory specimens also supported the superiority of sputum for detection of CP by real-time PCR. In that study, sputum contained the highest concentration of CP (8.6×105 copies/mL) compared to 1.5×104 copies/mL in samples from the nasopharynx and 3.1×103 copies/mL in throat samples [32]. Further studies using larger samples are required to evaluate molecular methods of LP and CP diagnosis.
In conclusion, the PneumoBacter assay for detection of MP, LP, and CP from sputum samples yielded the results comparable to those from APA. Specimen type is crucial in molecular diagnosis, and sputum is preferred over NPS for simultaneous detection of MP, LP, and CP from CAP patients.
Acknowledgement
We would like to thank Seegene (Seoul, Korea) for providing us with Seeplex PneumoBacter ACE Detection Assay Kits for this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Blasi F. Atypical pathogens and respiratory tract infections. Eur Respir J. 2004;24:171–181. doi: 10.1183/09031936.04.00135703. [DOI] [PubMed] [Google Scholar]
- 2.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12(Suppl 3):12–24. doi: 10.1111/j.1469-0691.2006.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, et al. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010;42:397–403. [Google Scholar]
- 4.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 5.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70:1–9. doi: 10.1016/j.diagmicrobio.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socan M, Marinic-Fiser N, Kraigher A, Kotnik A, Logar M. Microbial aetiology of community-acquired pneumonia in hospitalised patients. Eur J Clin Microbiol Infect Dis. 1999;18:777–782. doi: 10.1007/s100960050400. [DOI] [PubMed] [Google Scholar]
- 8.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Räty R, Rönkkö E, Kleemola M. Sample type is crucial to the diagnosis of Mycoplasma pneumoniae pneumonia by PCR. J Med Microbiol. 2005;54:287–291. doi: 10.1099/jmm.0.45888-0. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch DR. Molecular genetic methods in the diagnosis of lower respiratory tract infections. APMIS. 2004;112:713–727. doi: 10.1111/j.1600-0463.2004.apm11211-1202.x. [DOI] [PubMed] [Google Scholar]
- 11.Strålin K, Bäckman A, Holmberg H, Fredlund H, Olcén P. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples. APMIS. 2005;113:99–111. doi: 10.1111/j.1600-0463.2005.apm1130203.x. [DOI] [PubMed] [Google Scholar]
- 12.Loens K, Beck T, Ursi D, Overdijk M, Sillekens P, Goossens H, et al. Development of real-time multiplex nucleic acid sequence-based amplification for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella spp. in respiratory specimens. J Clin Microbiol. 2008;46:185–191. doi: 10.1128/JCM.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loens K, Van Heirstraeten L, Malhotra-Kumar S, Goossens H, Ieven M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J Clin Microbiol. 2009;47:21–31. doi: 10.1128/JCM.02037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reznikov M, Blackmore TK, Finlay-Jones JJ, Gordon DL. Comparison of nasopharyngeal aspirates and throat swab specimens in a polymerase chain reaction-based test for Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis. 1995;14:58–61. doi: 10.1007/BF02112622. [DOI] [PubMed] [Google Scholar]
- 15.Baron EJ, Thomson RB., Jr . Specimen collection, transport, and processing: Bacteriology. In: Versalovic J, Carroll KC, et al., editors. Manual of clinical microbiology. 10th ed. Washington DC: ASM press; 2011. pp. 255–256. [Google Scholar]
- 16.Dorigo-Zetsma JW, Verkooyen RP, van Helden HP, van der Nat H, van den Bosch JM. Molecular detection of Mycoplasma pneumoniae in adults with community-acquired pneumonia requiring hospitalization. J Clin Microbiol. 2001;39:1184–1186. doi: 10.1128/JCM.39.3.1184-1186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleemola SR, Karjalainen JE, Räty RK. Rapid diagnosis of Mycoplasma pneumoniae infection: clinical evaluation of a commercial probe test. J Infect Dis. 1990;162:70–75. doi: 10.1093/infdis/162.1.70. [DOI] [PubMed] [Google Scholar]
- 18.Loens K, Beck T, Ursi D, Overdijk M, Sillekens P, Goossens H, et al. Evaluation of different nucleic acid amplification techniques for the detection of M. pneumoniae, C. pneumoniae and Legionella spp. in respiratory specimens from patients with community-acquired pneumonia. J Microbiol Methods. 2008;73:257–262. doi: 10.1016/j.mimet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Honda J, Yano T, Kusaba M, Yonemitsu J, Kitajima H, Masuoka M, et al. Clinical use of capillary PCR to diagnose Mycoplasma pneumoniae. J Clin Microbiol. 2000;38:1382–1384. doi: 10.1128/jcm.38.4.1382-1384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner H. Adherence and pathogenicity of mycoplasma pneumoniae: a review. In: Ron E, Rottem S, editors. Microbial surface components and toxins in relation to pathogenesis. New york, NY: Plenum; 1991. pp. 81–89. [Google Scholar]
- 21.Dorigo-Zetsma JW, Zaat SA, Vriesema AJ, Dankert J. Demonstration by a nested PCR for Mycoplasma pneumoniae that M. pneumoniae load in the throat is higher in patients hospitalised for M. pneumoniae infection than in non-hospitalised subjects. J Med Microbiol. 1999;48:1115–1122. doi: 10.1099/00222615-48-12-1115. [DOI] [PubMed] [Google Scholar]
- 22.Kenny GE, Kaiser GG, Cooney MK, Foy HM. Diagnosis of Mycoplasma pneumoniae pneumonia: sensitivities and specificities of serology with lipid antigen and isolation of the organism on soy peptone medium for identification of infections. J Clin Microbiol. 1990;28:2087–2093. doi: 10.1128/jcm.28.9.2087-2093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bencini MA, van den Brule AJ, Claas EC, Hermans MH, Melchers WJ, Noordhoek GT, et al. Multicenter comparison of molecular methods for detection of Legionella spp. in sputum samples. J Clin Microbiol. 2007;45:3390–3392. doi: 10.1128/JCM.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernes SS, Quarsten H, Hagen E, Lyngroth AL, Pripp AH, Bjorvatn B, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011;30:159–165. doi: 10.1007/s10096-010-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelstein PH. Versalovic J, Carroll KC, et al. Manual of Clinical Microbiology. 10th ed. Washington DC: ASM press; 2011. Legionella; pp. 774–775. [Google Scholar]
- 26.Den Boer JW, Yzerman EP. Diagnosis of Legionella infection in Legionnaires' disease. Eur J Clin Microbiol Infect Dis. 2004;23:871–878. doi: 10.1007/s10096-004-1248-8. [DOI] [PubMed] [Google Scholar]
- 27.McDonough EA, Metzgar D, Hansen CJ, Myers CA, Russell KL. A cluster of Legionella-associated pneumonia cases in a population of military recruits. J Clin Microbiol. 2007;45:2075–2077. doi: 10.1128/JCM.02359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez JA, Ahkee S, Tolentino A, Miller RD, Summersgill JT. Diagnosis of Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae lower respiratory infection using the polymerase chain reaction on a single throat swab specimen. Diagn Microbiol Infect Dis. 1996;24:7–14. doi: 10.1016/0732-8893(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 29.Diederen BM, Peeters MF. Are oropharyngeal swabs suitable as samples for Legionella-specific PCR testing? J Clin Microbiol. 2007;45:3482–3483. doi: 10.1128/JCM.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaydos C, Essig A. Chlamydiaceae. In: Versalovic J, Carroll KC, et al., editors. Manual of Clinical Microbiology. 10th ed. Washington DC: ASM press; 2011. pp. 990–991. [Google Scholar]
- 31.Boman J, Allard A, Persson K, Lundborg M, Juto P, Wadell G. Rapid diagnosis of respiratory Chlamydia pneumoniae infection by nested touchdown polymerase chain reaction compared with culture and antigen detection by EIA. J Infect Dis. 1997;175:1523–1526. doi: 10.1086/516492. [DOI] [PubMed] [Google Scholar]
- 32.Kuoppa Y, Boman J, Scott L, Kumlin U, Eriksson I, Allard A. Quantitative detection of respiratory Chlamydia pneumoniae infection by realtime PCR. J Clin Microbiol. 2002;40:2273–2274. doi: 10.1128/JCM.40.6.2273-2274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]