Abstract
Background
We evaluated the clinical relevance of pretransplant donor-specific HLA antibodies (DSA) in renal transplantation patients who had negative T-cell cytotoxicity crossmatches.
Methods
From 328 consecutive renal transplant recipients, we selected 28 patients who had positive pretransplant (historical or at the time of transplantation) flow cytometry crossmatches, but negative T-cell cytotoxicity crossmatches at the time of transplantation. The presence of DSA and its level at the time of transplantation were retrospectively tested using Luminex single antigen assays.
Results
DSA was present in 16 (57.1%) of 28 patients. Biopsy-proven acute rejection (9 patients) occurred more frequently in patients with DSA than in those without DSA (56.3% vs. 0.0%; P=0.003). The positivity rate of class II DSA was significantly higher in patients with antibody-mediated rejection (AMR) than in those without AMR (100% vs. 21.7%; P=0.003). However, the positivity rate of class I DSA was not different between the two groups (40% vs. 40.9%). Among patients with class II DSA, those with AMR tended to have higher antibody levels (median fluorescence intensity, MFI) than those without AMR (16,359 vs. 5,910; P=0.056). A cut-off MFI value of 4,487 for class II DSA predicted the occurrence of AMR with good sensitivity and specificity (100% and 87.0%).
Conclusions
In patients with negative T-cell cytotoxicity crossmatches, the presence of class II DSA and its level at the time of transplantation were associated with the occurrence of AMR. Pretransplant DSA measurement with Luminex single antigen assay would be useful in renal transplantation.
Keywords: Donor-specific HLA antibodies, Renal transplantation, Antibody-mediated rejection
INTRODUCTION
Identifying acceptable donors for recipients with anti-HLA antibodies is one of the most difficult problems in kidney transplantation. Recently, solid-phase assays, such as the Luminex assay, have become available. These assays detect and quantify donor-specific HLA antibodies (DSA) to a degree that was not possible using cell-based crossmatch tests [1, 2]. When single HLA antigen-coated beads are used, these assays provide a relative indication of DSA level through the median fluorescence intensity (MFI) of the beads. Although the presence of pretransplant DSA has generally been considered to be a risk factor for antibody-mediated rejection (AMR) and graft loss [3-6], the clinical relevance of low levels of DSA is still debated [7, 8]. The clinical significance of DSA detected solely by using more sensitive assays, such as solid phase assays, or flow cytometry crossmatch (FCXM), are not certain. Some researchers have reported that high levels of DSA at baseline (prior to initiation of desensitization), in historical peak sera, or after desensitization therapy are associated with increased risk of AMR [9-11]. However, other researchers found no association between the level of DSA and occurrence of AMR [4, 7, 8].
Renal transplantation is usually performed in patients with a negative T-cell complement-dependent cytotoxicity (CDC) crossmatch at the time of transplantation. The aim of this study was to investigate whether the risk of acute graft injury and graft loss is related to the level of DSA in these patients at the time of transplantation.
METHODS
1. Study design
We selected 28 patients with positive FCXM (T and/or B) results at the time of transplantation or in their historical sera from 328 consecutive ABO-compatible renal transplants performed between June 2005 and May 2009 at the Seoul National University Hospital. The demographics and clinical characteristics of the study patients are shown in Table 1. Twelve of the 28 patients underwent pretransplant desensitization therapy, and all 28 patients had negative T-cell CDC crossmatch at the time of transplantation. Serum samples taken at the time of transplantation were retrospectively screened for the presence of class I and class II HLA antibodies with Luminex screening assay kits. Serum from the 12 patients who received desensitization therapy was also tested with Luminex screening assays using samples taken just before the initiation of desensitization therapy and samples taken at the time of transplantation.
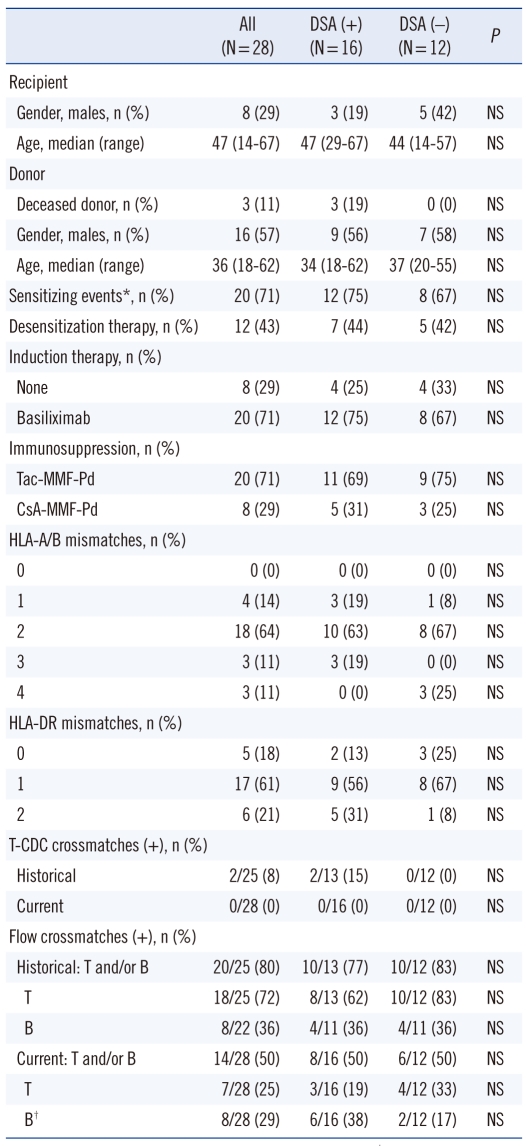
Table 1.
Demographics and clinical characteristics of the study patients
*Prior transplants, blood transfusion, or pregnancies; †including 2 cases in DSA (+) group and 2 cases in DSA (-) group, which were not interpretable due to the interference of rituximab.
Abbreviations: DSA, donor-specific HLA antibodies; NS, not significant (P > 0.05); CDC, complement-dependent cytotoxicity.
Sera that were obtained at the time of transplantation and tested positive in the Luminex screening assay (19 HLA class I and 13 HLA class II) were tested with Luminex single antigen assay kits to determine the level of class I and class II DSA. We evaluated the relationship between the presence of DSA and its level and the occurrence of biopsy-proven acute rejection (AR) and graft survival. All clinical data available through June 2010 were included in this analysis, and patients were followed for a median of 33.3±14.6 months (1-58 months).
2. HLA crossmatch test
T-cell CDC crossmatch was performed according to the National Institutes of Health Basic and additional Antiglobulin-enhanced Methods. FCXM was performed using a pronase-treated T/B single tube assay, as previously described [12]. T and B lymphocytes were stained with peridinin chlorophyll protein (PerCP)-conjugated and phycoerythrin (PE)-conjugated mouse monoclonal antibodies specific for human CD3 and CD19, respectively (BD Bioscience, San Jose, CA, USA). The presence of bound antibodies was determined using fluorescein isothiocyanate (FITC)-conjugated anti-human IgG (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA). Fluorescence was analyzed using a FACSCalibur flow cytometer (BD Bioscience). FCXM results are expressed as the ratio of test serum MFI to normal human AB type serum MFI. T-cell and B-cell FCXMs were considered to be positive when the MFI ratio was ≥2.0. At the time of transplantation, all patients had negative T-cell CDC crossmatches, and FCXM assays indicated, 6 patients were positive for T-cell only, 7 patients for B-cell only, and 1 patient for both T- and B-cell antibodies. The B-cell FCXM results of 2 patients who were positive for DSA and 2 patients negative for DSA were not interpretable due to rituximab interference (Table 1).
3. Detection of DSA
HLA antibody screening was performed with LIFECODES Life-Screen Deluxe kit (Gen-probe, Stamford, CT, USA). We performed HLA antibody identification in 28 HLA antibody-positive sera that were obtained at the time of transplantation (19 HLA class I and 13 HLA class II), using LIFECODES LSA class I and class II kits (Gen-probe) according to the manufacturer's instructions. Briefly, 10 µL of serum sample were added to microplate well, then 40 µL of HLA class I or class II single antigen Luminex beads were added, and incubated in the dark for 30 min at room temperature. After washing with wash buffer, 50 µL of goat anti-human IgG secondary antibody conjugated with phycoerythrin was added to the beads and samples were again incubated for 30 min in the dark at room temperature. After washing, the samples were read using the Luminex 200™ system (Luminex Corp. Austin, TX, USA). The cut-off MFI of positive reaction for each DSA bead was defined as 500. DSA levels were determined by the sum of MFI values for each DSA class: class I (HLA-A, HLA-B), class II (HLA-DR, HLA-DQ), and total (class I+II). HLA-DQ typing of donors was performed to determine donor-specificity when DQ antibodies were detected.
4. Desensitization protocol
The 12 patients who received desensitization treatment began taking mycophenolate mofetil (MMF, 750 mg twice daily, p.o.) and tacrolimus (0.05 mg/kg twice daily, p.o., target trough level 10-12 ng/mL) two days before their first plasmapheresis treatment (one plasma volume exchange with 4% albumin and/or fresh frozen plasma). Plasmapheresis was performed 3 to 7 times preoperatively, every other day. Intravenous immunoglobulin (100 mg/kg) was administered immediately after each plasmapheresis treatment. Methylprednisolone 1,000 mg i.v. was started at the time of the surgery, and the steroid dose was tapered to an oral dose of prednisolone. Combined immunosuppression with MMF, tacrolimus and prednisolone was continued through the post-transplantation period. The first 2 desensitization patients received 10 days of OKT3 (muromonab-CD3; 5 mg daily, i.v.) after transplantation. The remaining 10 patients received rituximab (375 mg/m2 of body surface area, i.v.) instead of OKT3. Rituximab was administered 3 days before the first plasmapheresis treatment and again 1 day before transplantation.
5. Detection and treatment of rejection
Biopsies were performed for all recipients with suspected rejection episodes. The biopsies were graded using the Banff 97 classification [13]. AMR was defined by C4d deposition [14]. Acute cellular rejection (ACR) was treated with methylprednisolone pulse therapy (1,000 mg/day for 3 days). AMR was treated with plasmapheresis followed by intravenous immunoglobulin (100 mg/kg), rituximab (375 mg/m2 of body surface area, i.v.), and methylprednisolone pulse therapy.
6. Statistical analysis
Quantitative variables were expressed as the mean (standard deviation) for normally distributed data or the median (interquartile range, IQR) for non-normally distributed data. Categorical variables were expressed as absolute and relative frequencies. Continuous variables were compared using Mann-Whitney U tests and categorical variables using chi-square tests or Fisher's exact tests, as appropriate. ROC curve analysis was computed for each predictor. All tests were 2-sided and used a significance level of 0.05. Data handling and analysis were performed with SPSS software for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The HLA antibody levels of patients undergoing desensitization therapy were tested before therapy and at the time of transplantation using the MFI values of pooled antigen beads from Luminex screen tests. Patients had significantly lower levels of HLA class I after therapy than before the start of desensitization therapy (median [IQR], 1,971 [1,355-3,599] vs. 645 [461-2,754]; P < 0.01). There was a similar, though non-significant, tendency for HLA class II levels to be lower at the time of transplantation than they were before desensitization therapy began (median [IQR], 3,604 [1,652-5,788] vs. 1,711 [1,141-3,471]).
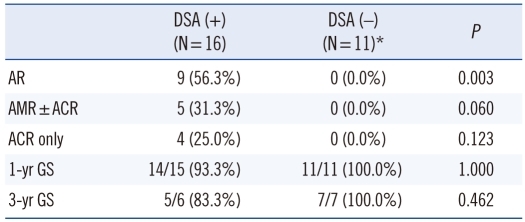
Of the 28 patients tested, 16 (57.1%) had DSA at the time of transplantation. The relationship between pretransplant DSA and occurrence of acute rejection and graft survival was analyzed in 27 patients (Table 2). One patient negative for DSA was excluded from the analysis, because the graft loss that occurred within 24 hr of transplantation was due to a clinically suspected vascular problem rather than to antibody mediated hyperacute rejection. Pathologic findings of extensive ischemic necrosis, subcapsular hemorrhage and congestion were observed in this patient. Nine patients had biopsy-proven acute rejection: 4 ACR, 3 AMR, 2 ACR+AMR. There was a significantly higher rate of biopsy-proven acute rejection among patients with DSA at the time of transplantation than among those without DSA (56.3% vs. 0.0%; P=0.003). The incidence of AMR (±ACR) tended to be higher in patients with DSA than in those without DSA (31.3% vs. 0.0%; P=0.060). However, 3 yr graft survival was not significantly different between the two groups of patients (83.3% vs. 100.0%).
Table 2.
Relationship between DSA at the time of transplantation and biopsy-proven acute rejection
*One case in the DSA (-) group was excluded, because graft loss within 24 hr of transplantation was due to clinically suspected vascular problem rather than antibody-mediated hyperacute rejection.
Abbreviations: DSA, donor-specific HLA antibodies; AR, acute rejection; ACR, acute cellular rejection; AMR, antibody-mediated rejection; GS, graft survival.
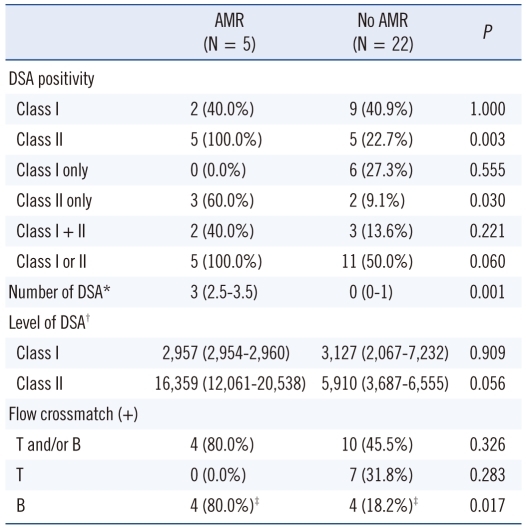
The relationship between pretransplant DSA and occurrence of AMR is shown in Table 3. Among the 27 patients evaluated, 6 patients had only class I DSA, 5 patients had only class II DSA, and 5 patients had both class I and class II DSA. The proportion of patients positive for class II DSA at the time of transplantation was significantly higher in patients with AMR than in those without AMR (100% vs. 22.7%; P=0.003). However, the proportion of patients positive for class I DSA was not different between the two groups (40% vs. 40.9%). The number of DSA was significantly higher in patients with AMR than in those without AMR (median [IQR], 3 [2.5-3.5] vs. 0 [0-1]; P=0.001).
Table 3.
Relationship between DSA at the time of transplantation and antibody-mediated rejection
*Median (interquartile range); †MFI sum value, median (interquartile range); ‡Including 2 cases that were not interpretable due to the interference of rituximab.
Abbreviations: AMR, antibody-mediated rejection; DSA, donor-specific HLA antibodies.
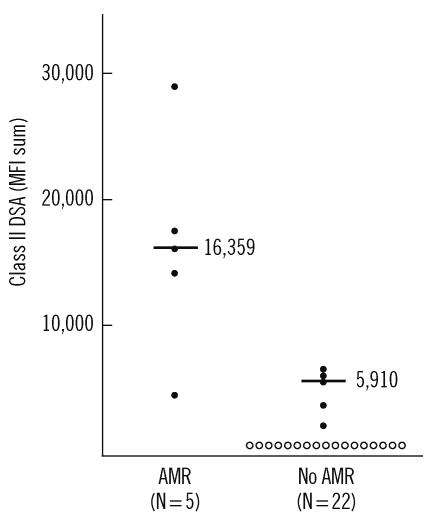
In patients with class II DSA, the antibody level (MFI sum value) tended to be higher in patients with AMR than in those without AMR (median [IQR], 16,359 [12,061-20,538] vs. 5,910 [3,687-6,555]; P=0.056; Table 3, Fig. 1). However, class I DSA level was not significantly different between the two groups.
Fig. 1.
Class II donor-specific HLA antibody (DSA) levels at the time of transplantation in patients with or without antibody mediated rejection (AMR). Filled and open circles represent DSA positive and negative cases, respectively. MFI sum values of DSA are plotted. Bars indicate median DSA value of the positive cases in each group.
Pretransplant (at the time of transplantation) class II DSA levels that were associated with the occurrence of AMR were evaluated by ROC curve analysis. The occurrence of AMR was predicted with good sensitivity and specificity (100% and 87.0%) using a cut-off MFI value of 4,487 for class II DSA. The area under the curve (AUC) was 0.974 (95% CI, 0.915-1.033).
DISCUSSION
In this study, pretransplant DSA was significantly associated with the occurrence of acute rejection in T-cell cytotoxicity crossmatch- negative patients (Table 2). Specifically, the presence of HLA class II DSA at the time of transplantation was significantly associated with the occurrence of AMR (Table 3). This finding is in accordance with the results of several recent studies, which have shown the importance of class II DSA in the outcome of renal transplantation in T-cell crossmatch (flow cytometry or cytotoxicity) negative patients [15-17]. The presence of HLA class II DSA in patients with negative T-cell FCXM was associated with the occurrence of AMR or poor graft survival [15, 16]. Higher levels of class II DSA in patients with negative T-cell cytotoxicity (AHG) crossmatches were related to increased risk of developing transplant glomerulopathy and the presence of C4d in peritubular capillaries [17].
In the present study, positive rate of class I DSA or its level was not different between patients with AMR and those without AMR (Table 3). Although a high level of class I DSA is detrimental to allograft survival, low levels of anti-HLA class I antibodies result in signaling cascades in endothelial cells that include survival proteins and induce accommodation of human allografts [18-20]. All studies showing the clinical impact of class II DSA, including the present study, were performed using patients with negative T-cell crossmatches (flow cytometry or cytotoxicity). Therefore, patients with high levels of class I antibodies were probably not included in any of these studies. Even if some of the patients in these studies had low levels of HLA class I antibodies, the antibodies might have assisted allograft accommodation, rather than adversely affect allografts. Such an effect may partially explain the lack of association of HLA class I antibodies with poor graft outcome in the patients in our study. Further studies of the effects of low levels of HLA class II antibodies on signaling cascades in endothelial cells are needed to clarify the roles of both HLA class I and class II antibodies in renal transplantation.
In the present study, the level of pretransplant class II DSA was associated with the occurrence of AMR (Table 3, Fig. 1). In ROC curve analysis, the cut-off MFI value of 4,487 for class II DSA at the time of transplantation predicted the occurrence of AMR with good sensitivity and specificity. In patients undergoing desensitization therapy, high levels of DSA both before and after desensitization therapy, have been reported to be associated with increased risk of AMR [9, 11]. Very high levels of baseline DSA has been reported to be associated with an increased risk of AMR and poor long-term allograft survival [9]. After desensitization therapy, patients who have DSA with a standard fluorescence intensity of >100,000 and with FCXM median channel shift >200 were found to be at higher risk of AMR than those who have lower DSA values [11]. However, the clinical relevance of lower levels of DSA is still debated [7, 8]. Recently, the presence of weak DSA in historical peak sera has been shown to be associated with AMR and poor graft survival [10]. MFI cut-off values of 465 for the highest single, or 820 for total, DSA showed maximal sensitivity and specificity for predicting the occurrence of AMR [10]. Further, 8-yr graft survival decreased progressively with increasing DSA levels: 82.5% in patients with MFI <465; 78.4% with MFI 466-3,000; 60.6% with MFI >3,000 (P <0.001).
DSA levels are not directly comparable between different studies and the differences between the reported levels of DSA with clinical significance may result from many factors. These include lack of standardization of MFI values in Luminex assays, differences in the method of DSA calculation, the extent (DQ, DP) and resolution of HLA typing in donors and recipients, and the density of HLA molecules on single antigen beads [21]. Further efforts are needed to standardize these sources of variability in order to determine the level of DSA that has clinical significance.
In conclusion, the presence of class II DSA and its level at the time of transplantation were associated with the occurrence of AMR in renal transplantation patients with negative T-cell cytotoxicity crossmatches. Pretransplant DSA measurement using single antigen Luminex bead assays would be useful for preventing AMR and post-operative follow up in renal transplantation.
Acknowledgement
This study was supported by a grant number 0420090900 from the SNUH research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000;69:1370–1374. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 2.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 4.Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681–1688. doi: 10.1097/TP.0b013e3181a5e034. [DOI] [PubMed] [Google Scholar]
- 5.Eng HS, Bennett G, Tsiopelas E, Lake M, Humphreys I, Chang SH, et al. Anti-HLA donor-specific antibodies detected in positive B-cell crossmatches by Luminex predict late graft loss. Am J Transplant. 2008;8:2335–2342. doi: 10.1111/j.1600-6143.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Iveson V, Varagunam M, Bodger S, Sinnott P, Thuraisingham RC. Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: are they relevant? Transplantation. 2008;85:1200–1204. doi: 10.1097/TP.0b013e31816b1c37. [DOI] [PubMed] [Google Scholar]
- 7.Aubert V, Venetz JP, Pantaleo G, Pascual M. Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol. 2009;70:580–583. doi: 10.1016/j.humimm.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, et al. Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol. 2009;70:589–594. doi: 10.1016/j.humimm.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86:820–825. doi: 10.1097/TP.0b013e3181856f98. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya S, Cooper TY, Avandsalehi J, Barnes T, Brooks K, Hymel P, et al. Improved flow cytometric detection of HLA alloantibodies using pronase: potential implications in renal transplantation. Transplantation. 2001;71:422–428. doi: 10.1097/00007890-200102150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 14.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 15.Pollinger HS, Stegall MD, Gloor JM, Moore SB, Degoey SR, Ploeger NA, et al. Kidney transplantation in patients with antibodies against donor HLA class II. Am J Transplant. 2007;7:857–863. doi: 10.1111/j.1600-6143.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 16.Stastny P, Ring S, Lu C, Arenas J, Han M, Lavingia B. Role of immunoglobulin (Ig)-G and IgM antibodies against donor human leukocyte antigens in organ transplant recipients. Hum Immunol. 2009;70:600–604. doi: 10.1016/j.humimm.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008;86:681–685. doi: 10.1097/TP.0b013e3181837626. [DOI] [PubMed] [Google Scholar]
- 18.Jin YP, Fishbein MC, Said JW, Jindra PT, Rajalingam R, Rozengurt E, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–2312. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 20.Salama AD, Delikouras A, Pusey CD, Cook HT, Bhangal G, Lechler RI, et al. Transplant accommodation in highly sensitized patients: a potential role for Bcl-xL and alloantibody. Am J Transplant. 2001;1:260–269. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 21.Warner PR, Youngs D, Nelson KA. HLA density on microparticles versus antibody detection sensitivity: implications for crossmatch prediction. Hum Immunol. 2007;68(S):S28. [Google Scholar]