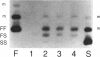
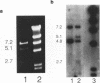
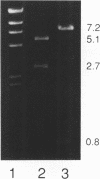
Abstract
The ability of a segment of the Drosophila Adh gene to produce ADH activity in larvae is dependent upon the presence of a 53 bp sequence (called NS1) located between 289 and 341 bp upstream of the larval transcription start site. This sequence behaves like an enhancer in that it can stimulate gene activity when it is placed at various distances from, or on either side of, an Adh gene. Like a typical enhancer, NS1 does not ordinarily function in trans. However, when an Adh gene lacking NS1 is placed on one plasmid, and a second gene carrying NS1 is placed on another, and the two plasmids are interlocked in a catenane, both genes are active. This finding supports the mechanism of loop-mediated enhancer action.
Full text
PDF
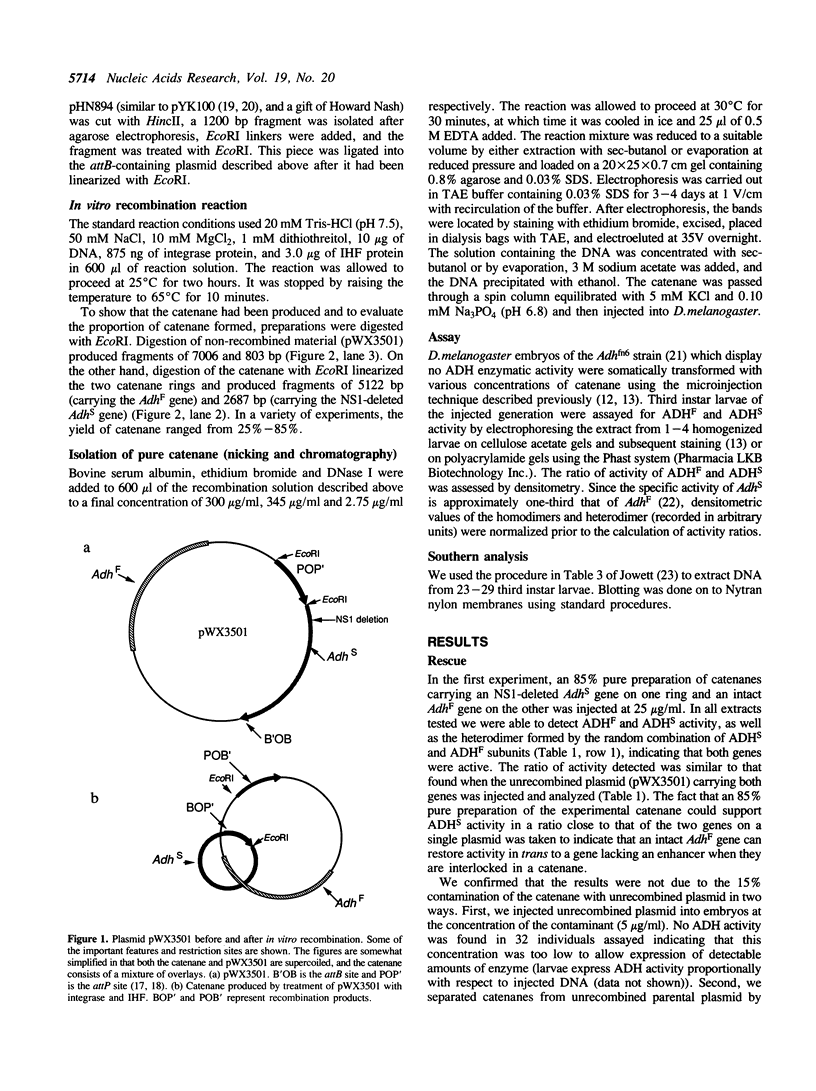


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C., Place A. R., Sofer W. Formaldehyde mutagenesis in Drosophila. Molecular analysis of ADH-negative mutants. Mutat Res. 1983 Sep;111(1):1–7. doi: 10.1016/0027-5107(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Bickel S., Benson M., Pirrotta V., Tjian R. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell. 1988 Jun 3;53(5):713–722. doi: 10.1016/0092-8674(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Dunaway M., Dröge P. Transactivation of the Xenopus rRNA gene promoter by its enhancer. Nature. 1989 Oct 19;341(6243):657–659. doi: 10.1038/341657a0. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 1990 Jul;9(7):2247–2256. doi: 10.1002/j.1460-2075.1990.tb07395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Bacterial gene regulation from distant DNA sites. Cell. 1989 Apr 21;57(2):193–195. doi: 10.1016/0092-8674(89)90955-0. [DOI] [PubMed] [Google Scholar]
- Greenfield L., Simpson L., Kaplan D. Conversion of closed circular DNA molecules to single-nicked molecules by digestion with DNAase I in the presence of ethidium bromide. Biochim Biophys Acta. 1975 Oct 15;407(3):365–375. doi: 10.1016/0005-2787(75)90104-5. [DOI] [PubMed] [Google Scholar]
- Guarente L. UASs and enhancers: common mechanism of transcriptional activation in yeast and mammals. Cell. 1988 Feb 12;52(3):303–305. doi: 10.1016/s0092-8674(88)80020-5. [DOI] [PubMed] [Google Scholar]
- Judd B. H. Transvection: allelic cross talk. Cell. 1988 Jun 17;53(6):841–843. doi: 10.1016/s0092-8674(88)90209-7. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. A. Nicking-closing activity associated with bacteriophage lambda int gene product. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3760–3764. doi: 10.1073/pnas.76.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. Integrative recombination of bacteriophage lambda: requirement for supertwisted DNA in vivo and characterization of int. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1099–1109. doi: 10.1101/sqb.1979.043.01.122. [DOI] [PubMed] [Google Scholar]
- Martin P., Martin A., Osmani A., Sofer W. A transient expression assay for tissue-specific gene expression of alcohol dehydrogenase in Drosophila. Dev Biol. 1986 Oct;117(2):574–580. doi: 10.1016/0012-1606(86)90326-x. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Detection of DNA looping due to simultaneous interaction of a DNA-binding protein with two spatially separated binding sites on DNA. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6287–6291. doi: 10.1073/pnas.85.17.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Enhancer-origin interaction in plasmid R6K involves a DNA loop mediated by initiator protein. Cell. 1988 Feb 12;52(3):375–383. doi: 10.1016/s0092-8674(88)80030-8. [DOI] [PubMed] [Google Scholar]
- Müeller-Storm H. P., Sogo J. M., Schaffner W. An enhancer stimulates transcription in trans when attached to the promoter via a protein bridge. Cell. 1989 Aug 25;58(4):767–777. doi: 10.1016/0092-8674(89)90110-4. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Gerster T., Schaffner W. Enhancer sequences and the regulation of gene transcription. Eur J Biochem. 1988 Oct 1;176(3):485–495. doi: 10.1111/j.1432-1033.1988.tb14306.x. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Richet E., Abcarian P., Nash H. A. The interaction of recombination proteins with supercoiled DNA: defining the role of supercoiling in lambda integrative recombination. Cell. 1986 Sep 26;46(7):1011–1021. doi: 10.1016/0092-8674(86)90700-2. [DOI] [PubMed] [Google Scholar]
- Shen N. L., Subrahmanyam G., Clark W., Martin P., Sofer W. Analysis of Adh gene regulation in Drosophila: studies using somatic transformation. Dev Genet. 1989;10(3):210–219. doi: 10.1002/dvg.1020100310. [DOI] [PubMed] [Google Scholar]
- Sofer W., Martin P. F. Analysis of alcohol dehydrogenase gene expression in Drosophila. Annu Rev Genet. 1987;21:203–225. doi: 10.1146/annurev.ge.21.120187.001223. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Wedel A., Weiss D. S., Popham D., Dröge P., Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990 Apr 27;248(4954):486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- Winberg J. O., Hovik R., McKinley-McKee J. S. The alcohol dehydrogenase alleloenzymes AdhS and AdhF from the fruitfly Drosophila melanogaster: an enzymatic rate assay to determine the active-site concentration. Biochem Genet. 1985 Apr;23(3-4):205–216. doi: 10.1007/BF00504319. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]