Abstract
The Common Data Elements (CDEs) initiative is a National Institutes of Health (NIH) interagency effort to standardize naming, definitions, and data structure for clinical research variables. Comparisons of the results of clinical studies of neurological disorders have been hampered by variability in data coding, definitions, and procedures for sample collection. The CDE project objective is to enable comparison of future clinical trials results in major neurological disorders, including traumatic brain injury (TBI), stroke, multiple sclerosis, and epilepsy. As part of this effort, recommendations for CDEs for research on TBI were developed through a 2009 multi-agency initiative. Following the initial recommendations of the Working Group on Demographics and Clinical Assessment, a separate workgroup developed recommendations on the coding of clinical and demographic variables specific to pediatric TBI studies for subjects younger than 18 years. This article summarizes the selection of measures by the Pediatric TBI Demographics and Clinical Assessment Working Group. The variables are grouped into modules which are grouped into categories. For consistency with other CDE working groups, each variable was classified by priority (core, supplemental, and emerging). Templates were produced to summarize coding formats, guide selection of data points, and provide procedural recommendations. This proposed standardization, together with the products of the other pediatric TBI working groups in imaging, biomarkers, and outcome assessment, will facilitate multi-center studies, comparison of results across studies, and high-quality meta-analyses of individual patient data.
Key words: clinical studies, common data elements, data coding, data collection, pediatric, standardization, traumatic brain injury
Introduction
Childhood traumatic brain injury (TBI) is the most common cause of death for children over the age of 1 year. While there are currently no specific therapies demonstrated to improve long-term outcome, aggressive intensive protocol-driven algorithms have been shown to improve mortality. The International Mission on Prognosis and Clinical Design in TBI (IMPACT) studies highlighted the hurdles and potential benefits to meaningful comparisons between clinical TBI studies. Merging individual patient data from eight randomized and three observational studies in preparation for analyses took over 10 person-years of work (Maas et al., 2007; Marmarou et al., 2007). A prospective unified approach to data collection and coding procedures would reduce variability in data collection and thereby facilitate comparisons between studies and meta-analyses. Given the heterogeneity of TBI studies inherent to the multiple mechanisms of injury and patient populations, this approach is attractive both scientifically and financially because it facilitates merging of data sets from small studies of specific populations.
Common Data Elements Overview
The Common Data Elements (CDE) is a National Institutes of Health (NIH) interagency effort to standardize naming, definitions, and data structure for clinical research variables. The goals of the overall CDE project are: (1) to disseminate standards for the collection of data from participants enrolled in studies of neurological diseases; (2) to create accessible data-collection tools for investigators that are ready to use “off-the-shelf”; (3) to encourage focused and simplified data collection to reduce the burden on investigators and practice-based clinicians to increase clinical research participation; (4) to improve study quality and reduce costs of data entry, cleaning, and analysis, by providing uniform data descriptions and tools across National Institute of Neurological Disorders and Stroke (NINDS)-funded clinical studies of treatment for neurological diseases (National Institute of Neurological Disorders and Stroke; http://www.commondataelements.ninds.nih.gov/default.aspx).
The TBI Working Group (WG) sought to address these issues by prioritizing and standardizing data collection elements for adult TBI patients to facilitate comparison of research findings across studies and encourage high-quality meta-analyses of individual patient data (Maas et al., 2010, 2011). The WG recognized that the types of data elements and assignment of priority in children (<18 years old) differ from adults. The causes and mechanisms of injury, risk factors in the medical history, contribution of birth injury, and impact of socioeconomic factors on the child's development following TBI are important pediatric considerations. In contrast to adult TBI, there are limited data on age-dependent values for physiological variables used to guide therapy for children with severe TBI, including intracranial pressure (ICP) and cerebral perfusion pressure (CPP) (Chambers et al., 2005,2006; Adelson et al., 2005).
Overcoming such limitations will require structured data collection that accommodates pediatric physiology and brain development. Thus, the goal of this pediatric “Demographics and Clinical Assessment” WG was to develop recommendations on coding and terminology of demographics and clinical assessments for studies across the spectrum of pediatric TBI as part of the larger multi-agency effort (Whyte et al., 2010) described by Thurmond and associates (2010). The primary objectives were to identify data elements specific to pediatric TBI, to prioritize these elements following the criteria established for adult TBI, to present the data elements in a clear format, and to use a structure consistent with that established for TBI by the original WG (Maas et al., 2010). Following this method, various elements and modules can be used as plug-in elements and used multiple times in clinical data collections. For example, the module on “Glasgow Coma Scale (GCS) and pupils” may be recorded only on admission, or also pre-hospital, as well as daily during the acute care phase.
This group followed the approach and structure of the precedent established by the original Demographics and Clinical Assessment WG for the broad spectrum of TBI, including adult, military, and pediatric cases (Maas et al., 2010, 2011; Menon et al., 2010). This publication reports the generation of pediatric-specific CDE for TBI. This is the first step in an iterative process of defining CDEs that harmonize the definitions of key variables and the collection of data in clinical studies of TBI. Other pediatric-specific WGs developed data elements with recommendations for imaging, biomarkers, and outcomes assessment.
Process for selecting common data elements
Two members of the pediatric WG (P.D.A. and M.S.W.) participated in the development of CDEs for TBI that focused on adult injury, but included limited recommendations for pediatric CDEs (Maas et al., 2010, referred to as the original WG in this article). A larger pediatric WG was then formed and the progress and recommendations of the WG were discussed during teleconferences in 2009–2010. In-depth discussions were conducted during face-to-face meetings in September 2009 and March 2010. The final recommendations of the WG were incorporated into a beta version of the pediatric TBI CDEs, reviewed by all WG members, and structured to ensure compatibility with the NINDS broad CDE project.
Distinguishing core, supplemental, and emerging data elements
In accordance with the criteria used by the other CDE WGs, the elements should be applicable across the spectrum of mild to severe TBI, for acute to long-term studies, and for studies including patients early after injury and those enrolling patients at later time periods. Following the nomenclature adopted by the CDE project, CDEs were classified by priority as core, supplemental, and emerging. Core elements are intended to encompass the minimal set of measures to characterize the broad spectrum of subjects. Core data elements are data considered essential for every study, and following the consensus of the CDE Steering Committee, are limited in number. Supplemental elements provide greater depth/breadth of exploration and/or may be useful for more specialized subpopulations. Emerging elements may require further validation, but they may fill gaps in currently validated measures, and/or substitute for recommended measures once validation is complete. Supplemental and emerging elements are specific to a research hypothesis or TBI subpopulations (i.e., children with concussion or abusive head trauma). Both may include a higher number of data elements because of the need for high data granularity and high resolution. As supplemental and emerging elements are refined and validated, some may need reclassification as core data elements, as well as future grouping of core data elements for particular types of studies (i.e., acute care clinical trials).
Categorizing elements as core/supplemental/emerging as proposed by the planning committee (Thurmond et al., 2010) is an unresolved issue for the CDE project, including pediatric TBI. The definition of a core element for an acute phase study may differ from what is considered a core element for an epidemiological or rehabilitation-oriented study. The broad range of settings and types of studies within TBI, including pediatric TBI, therefore precludes a large number of core clinical data elements that would be appropriate to all studies. Consensus did exist that as a minimum, the most relevant measures of injury severity and predictors for outcome should be collected in studies of severe and moderate TBI in the acute setting. Consistent with the consensus of the CDE Steering Committee, the number of core elements is small. Supplemental and emerging data elements can be selected appropriate to the aims of each study.
The level of detail required can vary greatly with the design and aim of a specific study. Observational studies or large pragmatic clinical trials would require less detail than highly-focused Phase II or Phase III trials. It is therefore necessary to incorporate flexibility into the supplemental recommendations so that data elements can be selected to accommodate different levels of complexity and research questions. This approach is consistent with objectives of the CDE initiative to facilitate comparisons of results or merging of data across multiple studies, in this case, pooling results from small pediatric studies or comparing pediatric to adult studies.
The product: Pediatric TBI demographics and clinical evaluation common data elements
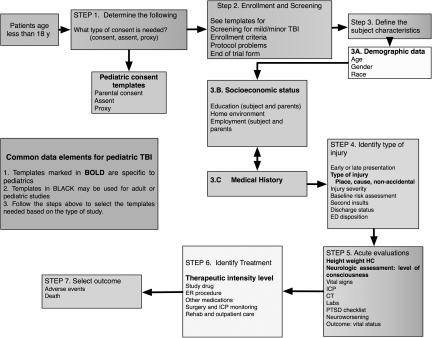
The structure of the adult CDEs (Maas et al., 2010) was modified to accommodate the CDEs specific to pediatric TBI as summarized in Figure 1. Importantly, these initial recommendations of the WG represent a beta version. The longer-term intent is to make this CDE a global initiative (Maas, 2009). The recommendations should be field tested in studies of pediatric TBI prior to general acceptance. This field testing may also serve to provide evidence for categorizing elements as core/supplemental/emerging.
FIG. 1.
Schema of sequential approach to selection of common data elements for pediatric studies (TBI, traumatic brain injury; ICP, intracranial pressure; ED, emergency department; CT, computed tomography; PTSD, post-traumatic stress disorder; HC, head circumference).
The data elements presented here were considered to be specific to pediatric TBI. For data elements not discussed here (e.g., gender, race, and ethnicity; Bhopal et al., 1998; Wynia et al., 2010), the elements and their classification did not differ between the adult and pediatric set, and therefore no changes were made to those already assigned (Maas et al., 2010). In many instances there were no differences between the adult and pediatric CDEs and no changes were made to the CDEs created for the adult studies. The intent was to keep the relevance of the CDEs as broad as necessary for the different types of investigators likely to use them (i.e., epidemiological/observational studies and acute/rehabilitation clinical trials). Different formats for data collection may, however, be appropriate in different circumstances. As an example, the WG proposes different formats for coding early details of injury and referral details for patients presenting acutely versus those presenting late. For patients who present early, referral policy and time of arrival, as well as mode of transport and emergency services provision, are relevant. For patients presenting late, the main reason for presentation and more general information on delivery of initial care and the specifics of such care are more appropriate. Capturing information on the reason for presentation is important also for later characterization of the population captured. For example, mild TBI may be overreported by individuals or families with possible financial gain, but underreported by individuals highly motivated to return to team play.
A consensus on the coding of CDEs for use in pediatric TBI was achieved. The CDEs are grouped into modules which are grouped into categories. For example, the data elements “age, gender, and race” are contained in the module “demographics,” under the category “subject characteristics.” The four main categories relevant to this manuscript are: (1) Participant or Subject Characteristics (Table 1), (2) Participant History and Family History (Table 2), (3) Injury- and Disease-Related Events (Table 3), and (4) Assessments and Examinations (Table 4). A complete overview of the data elements and definitions together with the templates may be found at the NINDS website www.commondataelements.ninds.nih.gov, and additional information is available at www.tbi-impact.org.
Table 1.
Participant or Subject Characteristics
|
CDE variable |
CDE definition |
Recommended instrument |
Description of measure |
Code list/permissible variable |
Classification: core supplemental/emerging |
|---|---|---|---|---|---|
| Module and data element name | Phrase or sentence to define the concept being measured | Name or acronym for the selected measure | Number and description of terms and subscale structure | Total range of scores | Indicate core/supplemental/emerging |
| Demographics | |||||
| Age at presentation | Age | Age_Presentation_ | 00.000≤1 y 00.0>1 y Adjust age to gestational age up to 1 y |
0 to <18 y | Core |
| Date of injury | Date of injury | DOI | Date that injury occurred; mm-dd-yyyy Verified/Unverified/Unknown |
Core | |
| Date of admission | Date of admission | DOA | mm-dd-yyyy | Date of enrollment to the study | Core |
| Time of injury | Time of injury | TOI | HH:MM Verified/Estimated/Unknown |
Verified-actually witnessed and time provided Estimated: It needs to be defined for each study: Time that the child became symptomatic; time of first trauma activation; time of presentation to ED Unknown |
Supplemental |
| Age at injury | Age at injury | Age at injury | 00.000≤1 y 01.0>1 y Adjust age to gestational age up to 1 y |
0 to <18 years | Core |
| Head circumference | Head circumference | Occipitofrontal circumference | Cm Percentile |
Core | |
| Height Weight Language |
Height Weight Primary and secondary languages of child at time of injury |
Height Weight Language |
cm kg English Spanish Other |
Core Core Supplemental |
|
| Educational level, current or highest completed | Present grade level or completed at time of injury | School level | Pre-school, K–12, college 13–16 Early intervention/Special Ed/Regular Ed |
Supplemental | |
| Age-appropriate grade level | Grade level for age | Correct grade | Behind for age Correct for age Ahead for age |
Supplemental | |
| Handedness | Hand preference by child at time of injury | Handedness | Right Left Equal Left & Right Unknown |
Supplemental | |
ED, emergency department.
Table 2.
Participant History and Family History
|
CDE variable |
CDE definition |
Recommended instrument |
Description of measure |
Code list/permissible variable |
Classification: core/supplemental/emerging |
|---|---|---|---|---|---|
| Module and data element name | Phrase or sentence to define the concept being measured | Name or acronym for the selected measure | Number and description of terms and subscale structure | Total range of scores | Indicate core/supplemental/emerging |
| Past Medical and Family History | |||||
| Prematurity/prenatal/antenatal or postnatal disorders | Prenatal/Antenatal/Postnatal | Supplemental | |||
| Congenital or acquired | Congenital/Acquired | Supplemental | |||
| Perinatal brain injury | Perinatal brain injury | Perinatal brain injury | HIE ICH PROM Abruption Cephalohematoma Other |
Emerging | |
| Epilepsy, congenital heart disease. sickle cell disease, metabolic, learning disability, behavioral disorders, cerebral palsy; familial macrocephaly; familial microcephaly | Supplemental | ||||
| Previous brain injury | Specify perinatal or postnatal: Encephalitis or meningitis Stroke ICH Cardiac arrest Perinatal asphyxia Near-drowning None |
Supplemental | |||
HIE, hypoxic-ischemic encephalopathy; ICE, intracerebral hemorrhage; PROM, premature rupture of membranes; ICH, intracerebral hemorrhage.
Table 3.
Injury- and Disease-Related Events
|
CDE variable |
CDE definition |
Recommended instrument |
Description of measure |
Code list/permissible variable |
Classification: core/supplemental/emerging |
|---|---|---|---|---|---|
| Module and data element name | Phrase or sentence to define the concept being measured | Name or acronym for the selected measure | Number and description of terms and subscale structure | Total range of scores | Indicate core/supplemental/emerging |
| Scene of Injury and Pre-Hospital Assessment | |||||
| Type and Mechanism of Injury | |||||
| Type of injury | Type of injury | Injury type | Closed/penetrating/blast/crush | Core | |
| Mechanism | Mechanism | Mechanism | Accidental/abusive inflicted/non-inflicted | Core | |
| Abusive head trauma | Abusive head trauma Incidental trauma Neglect |
AHT | Definite/suspected | Core | |
| Perinatal brain injury | Perinatal brain injury | Radiographic Signs/symptoms |
Emerging Core |
||
| Reported onset of symptoms | Date of onset of symptoms | DOS | mm-dd-yyyy HH:mm |
||
| Evidence of prior injury | Evidence of prior injury found either historically, at previous ED visits, radiographic findings, or signs or symptoms at time of presentation | Prior injury | Yes/no Brain injury Extracranial injury Both Spine injury |
Historical radiographic signs/symptoms Historical Radiographic signs/symptoms |
Core |
| Protective devices | Protective devices utilized at time of primary injury mechanism | Protective devices | Yes/no Type: Helmet/car seat/booster/seat belt |
Supplemental | |
ED, emergency department.
Table 4.
Assessments and Examinations
|
CDE variable |
CDE definition |
Recommended instrument |
Description of measure |
Code list/permissible variable |
Classification: core/supplemental/emerging |
|---|---|---|---|---|---|
| Module and data element name | Phrase or sentence to define the concept being measured | Name or acronym for the selected measure | Number and description of terms and subscale structure | Total range of scores | Indicate core/supplemental/emerging |
| Clinical Severity | |||||
| Classification | |||||
| Glasgow Coma Score (post-resuscitation) pediatric preferred | Glasgow Coma Score, best GCS obtained following resuscitation in the ED | GCS-I | 3–15 | Post-resuscitation | Core |
| Glasgow Coma Score (daily) | Glasgow Coma Score obtained on a daily basis during hospital stay. It should be best GCS on any given day | GCS-D | 3–15 | Best GCS during the last 24 h | Supplemental |
| Extracranial injuries | Extracranial injuries based on the Abbreviated Injury Severity Score | Extracranial injury | AIS ISS |
AIS and calculated ISS on admission | Supplemental |
| Spine/spinal cord injury Pediatric Risk of Mortality (PRISM) score |
Spinal column and or spinal cord injury Pediatric |
Spinal injury PRISM |
Radiographic signs/symptoms |
Supplemental Supplemental |
|
| Second Insults | (Self-populating) | ||||
| Hypoxia | Hypoxia | Hypoxia | Yes/no/unknown | Pao2 mm Hg O2 Saturation Oxygen percentage Time of measure First assessed vs. hourly |
Core |
| Hypotension | Hypotension | Hypotension | Yes/no/unknown | SBP mm Hg | Core |
| Hyperthermia | Hyperthermia | Hyperthermia | Yes/no/unknown | oC | Core |
| Hypothermia | Hypothermia | Hypothermia | Yes/no/unknown | oC | Core |
| Hyperventilation | Hyperventilation | Hyperventilation | Yes/no/unknown | Paco2 mm Hg | Core |
| Cardiac arrest requiring CPR | Cardiac arrest | Cardiac arrest | Yes/no/unknown | Core | |
| Seizures | Seizures | Seizures | Yes/no/unknown | Core | |
| Clinical seizures Status epilepticus |
Clinical | Clinical/Non-convulsive | Supplemental | ||
| Status epilepticus | Yes/no/unknown | Supplemental | |||
| Infection | Infection | Infection | Culture positive/suspected/unknown | Supplemental | |
| Laboratory Data | |||||
| Glucose | Glucose | Glucose | Serum values mg/dL |
Thresholds: Hyperglycemia 180 mg/dL Hypoglycemia 80 mg/dL Critical 40 mg/dL Time of assessment: First Lowest |
Supplemental |
| Hemoglobin | Hemoglobin | Hgb | Serum values g/dL |
First Time of assessment: First Lowest |
Supplemental |
| Vital Signs | |||||
| Blood pressure | Blood pressure | MAP | MAP | Highest and lowest pressure over a given period; hourly recommended | Core |
| Intracranial pressure | Intracranial pressure | ICP | ICP | Highest and lowest pressure over a given period; hourly recommended | Core |
| Cerebral perfusion pressure | CPP | CPP | MAP, ICP | Core | |
| Brain oxygenation, brain temperature, jugular venous oxygen | Hourly recording or high-frequency data capture | Emerging | |||
| Treatment | |||||
| Hospital treatment | Amount of therapy and treatment necessary for control of ICP | Pediatric Intensity Level of Therapy Scale (PILOT) | PILOT | Scale 0–38, daily recording | Core |
AIS, Abbreviated Injury Scale; ISS, Injury Severity Score; SBP, systolic blood pressure; Paco2, partial arterial carbon dioxide pressure; MAP, mean arterial pressure.
1. Subject Characteristics (Demographics and Pre-Injury Baseline)
Core data elements
Age
Recording age in TBI studies is of great importance. Causes of injury differ by age group and lead to different types of injury. Age is one of the strongest predictors of outcome in TBI, and younger patients may be at greater risk for poor outcome (Anderson et al., 2005).
Age is a core element and should be collected for all pediatric studies. Accurate recording of the date of birth (DOB) provides the most detailed and source-verifiable information, but collection of such data could lead to privacy concerns. Age should be calculated on the nominal scale. For children younger than 1 year, age should be expressed to three decimal places to allow calculation of age in days if needed. In addition, for those born at less than 36 weeks' gestation, age should be adjusted for gestational age. For children above 1 year, age should be expressed to two decimal places. When reporting the relationship between age and outcome, a continuous analysis is recommended over the use of threshold values.
Date of injury
Date of injury is recommended as a core element. This should be specifically recorded as verified, estimated, or unknown.
Age at injury
Again reflecting the importance of age-dependent responses to injury, and changes in key physiological variables including blood pressure and cerebral blood flow, the WG considered the age at injury to be a core variable. This should also be expressed on the nominal scale to three decimal places below 1 year of age, and one place above 1 year.
Head circumference
Head circumference (occipitofrontal circumference: OFC) is a quantifiable measure of brain development (Pryor and Thelander, 1968). The WG determined that OFC would serve as a core variable. Abnormalities in OFC may identify children who were not neurologically normal at the time of injury in acute injury studies, or serve as a baseline for following developmental outcome in studies with longer follow-up. To enable interpretation of the OFC, the percentile, height. and weight should also be recorded as core variables. The WG did not specify a source for calculating percentiles, but recommended that the source of the normative percentile data be included.
Supplemental data elements
Time of injury
Timing of injury may be a critical determinant of response to a given therapy. As discussed above, this consideration may not be relevant to all studies, and the WG therefore determined this to be a supplemental element. When recorded, it should be classified as verified by a witness, estimated, or unknown. In addition, the data should differentiate between times when the child was injured, became symptomatic, the time of trauma activation, and/or the time of presentation to the emergency department.
Language
The primary language of the child at the time of injury was determined to be a supplemental element. For multilingual children, recording the specific secondary language is recommended.
Educational level
For adults and children, educational level is a basic descriptor and an important component of socioeconomic status. Educational attainment is a strong correlate of income level and highly relevant to outcome. Educational level is associated with outcome following TBI (Mushkudiani et al., 2007). The WG agreed that documenting at least some information on education prior to injury is relevant. Various approaches exist for documenting educational level, and the WG recognized that attendance and achievement are not identical aspects. Achievement is probably the more relevant as a descriptor or predictor of outcome. For these reasons, the WG recommended that both the number of years of education completed as well as the highest level of education at the time of injury be recorded. If the child required special education services prior to injury, this should be specified. As an additional measure of pre-injury intellectual abilities, whether the child is behind, ahead of, or in the correct grade for age may also be recorded.
Handedness
Early selection of a dominant hand or change in dominant hand may reflect prior or new brain injury, respectively, and may be a sequela of brain trauma. In some studies, these data may be useful and this element was considered supplemental by the WG.
2. Subject and Family Medical History
Core data elements
No recommendations for core data elements were made for this module. Details on medical history, family history, and use of medications are collected in nearly every TBI study. However, medical history data are typically the least reliable data collected and are almost universally collected in a free text format, thus prohibiting any meaningful analysis. Nevertheless, pre-existing conditions may influence the disease course and chances of recovery, and information on medical history is essential for interpretation of adverse events occurring during clinical trials. It is therefore highly relevant to accurately record medical history and medications. In order to facilitate better use of such data, the WG agreed with the approach of the original WG and recommends pre-specified categories. Consistent with these recommendations, these data are supplemental.
Supplemental data elements
Past medical and family history
To accommodate medical disorders that are more relevant to children, the WG added categories that included type and timing of disorders (e.g., prenatal, antenatal, and post-natal; acquired or congenital disorders; and other specific types, such as epilepsy, congenital heart disease, sickle cell disease, metabolic, learning disabilities, familial macro- or microcephaly, and behavioral disorders). The WG also classified specific prior brain injuries.
Emerging data elements
Recognizing that the definition of perinatal brain injury is controversial (vide infra), a history of such injury is also included in this category as an emerging data element.
3. Injury-Related Elements and Pre-Hospital Assessment and Treatment
Core data elements
Type and mechanism of injury
Recording details on the type, place, nature, and mechanism of injury is highly relevant, both from an epidemiologic perspective (with implications for prevention programs), and because different pathophysiologic mechanisms occur in different types of injury. The WG followed the consensus of the original WG and agreed with classifying the type of injury into four categories: closed, penetrating, blast, and crush. Blast injuries (any form of TBI occurring in association with a blast explosion) are a negligible cause of pediatric TBI outside of combat theatres (Ling et al., 2009; Wolf et al., 2009). In combat theatres, up to 50% of civilian hospitalizations due to blast injury may be pediatric (Dr. C. Giza, personal communication). The WG considered it important to maintain a consistent classification system with the original WG, and to distinguish between data related to the place and cause of injury. The “place of injury” is intended to capture information on the location of injury, such as street, home/domestic, work/school, or sports/recreation. In distinction, “cause of injury” is directed towards causative factors such as road or traffic incident or fall. Indirectly, these imply a certain element of mechanism, but more detailed information on the mechanism of injury can be recorded separately.
Abusive head trauma (AHT)
The WG discussed at length the issues of classification related to AHT. This is a significant cause of head injury in infants and young children (Barlow et al., 2005; Berger et al., 2002; Keenan et al., 2004; Maguire et al., 2009). Accordingly this mechanism was added to the pediatric data elements. When identified as a potential mechanism, other relevant information including evidence of prior trauma, timing of the insult, and whether the insult was confirmed as inflicted (non-accidental), or classified as suspected, should be recorded.
Evidence of prior brain injury
In distinction to the past medical and family data (Table 2), which may be unreliable, the WG recommended that evidence of prior brain injury be recorded as a core data element.
Supplemental data elements
Pediatric protective devices
The use of protective devices (car seats or helmets) may affect outcome after pediatric TBI. The WG considered such data supplemental.
Emerging data elements
Perinatal brain injury
Brain injury as a result of the birth process is a common and well-described phenomenon (Holden et al., 1999; Pollina et al., 2001; Looney et al., 2007; Towner et al., 1999; Whitby et al., 2004). Birth-related brain injuries/abnormalities are most frequently characterized by intracranial hemorrhages (ICH) of multiple types, including subdural, subarachnoid, intraparenchymal, and intraventricular hemorrhages. Unlike TBI due to other causes, the locations of the ICH associated with birth are almost exclusively posterior fossa/occipital lobes (Looney et al., 2007; Whitby et al., 2004). Presenting symptoms due to birth-related injury can range from none to severe, and include apnea, bradycardia, and/or seizures (Looney et al., 2007). Furthermore, whether such imaging findings are related to neurologic injury is controversial, since they may occur in asymptomatic infants with normal developmental outcomes (Rooks et al., 2008).
The unique features of birth-related brain injury lead to the recommendation for inclusion as an emerging data element. This injury occurs only in neonates at the time of birth. The fact that the majority of neonates with such injury are asymptomatic, that the location of ICH is most commonly the posterior fossa, and the fact that there appears to be no effect of the injury on long-term development (Holden et al., 1999), also make this type of injury unique. The clinical scenario during which the possibility of undiagnosed birth trauma is invariably discussed is during the evaluation of possible inflicted childhood neurotrauma (e.g., AHT).
4. Assessments and Examinations
4.1 Classification of Clinical Severity
Core data elements
Traditionally, TBI has been classified by mechanism (closed versus penetrating), by clinical severity (GCS score, length of loss of consciousness, and/or length of post-traumatic amnesia), or by assessment of structural damage (neuroimaging). A substantial limitation of all these approaches is that they categorize patients artificially. For example, in classifying patients by clinical severity, patients are somewhat arbitrarily grouped into three distinct categories: severe (GCS score 3–8), moderate (GCS score 9–12), or mild (GCS score 13–15). This approach insufficiently recognizes that the severity of TBI lies along a continuum, the GCS score may fluctuate, and there exist additional reliability issues for using GCS in the young/preverbal/developmentally-delayed pediatric population. Furthermore, classification of TBI by clinical severity is increasingly limited in the acute setting by confounders, such as medical sedation, neuromuscular blockade, or intoxication, and does not account for anatomical features that may greatly influence outcome, such as brainstem injury. In addition, the extent of injury, treatment, prognosis, and outcome of patients at the same injury level differs markedly due to differences in the actual pathology. Finally, inaccurate or incomplete clinical classification of injury severity in the medical record is common. Advances in modern neuroimaging techniques and the emerging technology of biomarkers offer new opportunities towards development of a multidimensional classification for TBI. We agree with the original WG regarding the priority for further research in this field. Classification of injury severity in pediatric TBI studies will benefit from careful documentation of factors that may influence the GCS, timing of the evaluation (i.e., scene of the accident or post-resuscitation), and neuroradiological classification of the injury (computed tomography [CT] or magnetic resonance imaging [MRI] scan in severe TBI patients). It is important to acknowledge that methods for neuroradiological classification of TBI in children have not been validated. A GCS score (daily) was added as a supplemental element to provide for clinical severity assessment during the hospital stay, and is best GCS score on any given day.
Neurological assessment
The GCS score is widely used in clinical practice, and research and has evolved into a universal classification system for the severity of TBI. It consists of the sum score (range 3–15) of three components (eye, motor, and verbal scales). The GCS has good inter-rater reliability in adults, and in the acute phase can identify children with intracranial pathology who are at risk of needing significant interventions with acceptable levels of accuracy (Holmes et al., 2005). In patients with severe TBI, the GCS is frequency influenced by the presence of sedatives and neuromuscular blocking agents, and adult studies show that the GCS may overestimate severity of injury in this group of patients (Stocchetti et al., 2004). Documentation of these confounders is considered a core data element. Strategies to overcome this limitation include reporting the evolution of GCS scores in the first 24 h post-injury (Pineda et al., 2007).
In light of these limitations, the WG recommends that standardized assessments of severity of injury be performed using the pediatric GCS (Marcin and Pollack, 2002). While alternative scores for children younger than age 2 have been described, such methods have not been widely adopted at present (Durham et al., 2000). For assessment of injury severity in individual patients, the three components of the pediatric GCS should be reported separately. For intubated patients, nomenclature such as the use of the letter “T” is not recommended, as it cannot be statistically analyzed and may introduce variability in the assessment of the verbal response component of the GGS.
Supplemental data elements
Classifying extracranial injuries
In the past, relatively little attention has been paid in TBI to assessment of the occurrence and severity of extracranial injuries. Nevertheless, extracranial injuries occur frequently in combination with TBI and may affect short and long-term outcome. Practicality dictates that any scoring system used to quantify systemic injury must be widely disseminated and easily understood. For these reasons, the Abbreviated Injury Scale (AIS) is the most logical candidate. The AIS is defined as an anatomically-based, consensus-driven, global severity scoring system that classifies each injury by body region according to its relative importance on a six-point ordinal scale (Gennarelli and Wozdin, 2006).
For expressing the overall severity of injuries, the Injury Severity Score (ISS) can be calculated from the AIS (Baker et al., 1974). The spine is not considered separately in the original ISS classification, but given the association between TBI and in particular cervical spine injuries, the WG considers it important to record spinal injuries separately.
4.2 Second Insults
Core data elements
Second insults may aggravate processes of secondary damage in a brain already rendered vulnerable by the primary injury. The main physiologic insults relevant to TBI are hypotension, hyperthermia, hypoxia, seizures, and hypocapnia due to hyperventilation. The adverse effects of the occurrence of such insults both pre- and in-hospital is well established (Chesnut et al., 1993; McHugh et al., 2007; Signorini et al., 1999; Bullock et al., 2000). Second insults are commonly defined by threshold values, but these values are not well established in pediatrics, and when applied are based on limited data (Adelson et al., 2003a). However, given the consistent association between second insults and significantly worse outcome, the WG considered these to be core elements. The group recognized that these elements may not be available for studies performed late after injury. Similar considerations for biochemical second insults may also apply in the future for glucose, sodium [hyponatremia], and hemoglobin.
Supplemental data elements
The WG concluded that there is insufficient evidence to make recommendations on the frequency of recording and format for analysis (mean values versus min/max values, or present versus not present). Again, the WG recognized that definitions of normal or abnormal based on a threshold value were not satisfactory.
If seizures are present, the WG recommended recording the classification as (1) clinical versus non-convulsive, and (2) status epilepticus versus not status epilepticus. Some studies may need to classify the type of electroencephalography (EEG) monitoring used.
Emerging data elements
The WG recognized that the use of thresholds was unsatisfactory in the long-term. The approach of different groups to define the severity of insult based on the duration and deviation from the norm is clearly preferable (Barlow et al., 2005, Chambers et al., 2005, 2006; Jones et al., 2003). The WG sees a great need for further development and implementation of dedicated software to this purpose in existing monitoring systems, similarly to the approach taken for applying informatics to multivariate data in adult TBI (Cohen et al., 2010). Whether current methods (such as data extraction of documented vital signs) or emerging methods are used (high-resolution automated data collection), careful attention must be given to definitions such as frequency of data collection, use of average values versus time spent under a critical threshold, use of highest/lowest documented values, and the definition of adequate filters for non-physiological values. As the technology becomes available, the WG viewed high-resolution data collection and area under the curve (AUC) analysis as the most promising emerging methods, but agreed that it is premature to recommend collection of data in this format at present.
4.3 Laboratory Data
Supplemental data elements
Based on the available data for pediatric TBI, thresholds of 80–180 mg/dL for glucose are recommended (Adelson et al., 2003b). A threshold for hemoglobin is more difficult to define given emerging data on the lower limit of hemoglobin safely tolerated by critically ill children in general, and the variable effect of blood transfusion in children with severe TBI specifically. Considering the complexity of data collection, the WG concluded that these data elements would more commonly be included in supplemental datasets and should be well defined in each study using this element as to how the data element was utilized.
4.4 Recording and Documentation of Vital Signs
Core data elements
In the analysis phase, the WG recommended that all physiologic data are referenced to date and time of injury, since this represents the only fixed time event that is common to all patients.
Documentation of blood pressure, heart rate, temperature, and oxygen saturation is recommended for all TBI patients admitted to hospital directly after injury. This is important for two reasons. First, therapeutic interventions in a trial may increase the incidence of abnormal physiology, and such adverse effects need to be recorded on safety grounds. Second, regardless of whether or not physiological insults are due to trial interventions, systemic hypotension, low cerebral perfusion pressure (CPP), hypoxia, or hyperthermia may aggravate damage to the injured brain. Conversely, a high blood pressure may lead to a protracted course of increased ICP, and therapeutically induced hypertension carries an increased risk of cardiopulmonary complications.
As a minimum, vital signs should be recorded on admission, and also on a daily basis during the acute phase after injury. For the core datasets, the WG recommends recording the average and lowest blood pressure over a given period. In the intensive care unit (ICU) environment, recording blood pressure on an hourly basis is recommended, especially when ICP is monitored in order to permit determination of CPP, calculated as mean arterial pressure (MAP) – ICP (supplemental data set). As the technology and corresponding data analysis methods become available, high-resolution data collection of vital signs (every minute or even every second) will allow trend analysis and accurate AUC calculations (estimation of secondary insult “dose”).
Systolic blood pressure (SBP) was selected in addition to MAP as a data element for a number of reasons. First, there are well-established age-based normative values of SBP for children. Second, a single study in pediatrics of the effect of blood pressure on outcome from pediatric TBI (White et al., 2001) demonstrated that SBP greater than a given threshold was independently associated with survival in 136 subjects. To our knowledge there is only one published report of age-related normative MAP values (Haque and Zaritsky, 2007). Last, in the initial phase of care (pre-hospital and emergency department), measurements of MAP are also subject to significant error since diastolic blood pressure is only estimated by commonly used non-invasive blood pressure measuring devices.
Intracranial pressure
Monitoring of ICP is recommended in pediatric patients with severe TBI (Adelson et al., 2003a). For the supplemental data set, hourly documentation of ICP values is recommended at a minimum, because it allows a summary measure and the highest value on a daily basis. The emerging data set includes high-resolution data collection at frequencies that are not routinely documented and require specialized equipment and software. For both the supplemental and emerging data sets, periods of artifactually high ICP (for example, during calibration of the monitor), or short-duration ICP increases due to patient care interventions, coughing, and/or straining should be excluded when determining the highest ICP.
For valid comparison of results between patients and across studies, a common approach towards zeroing the ICP monitor should be agreed upon. For fluid-coupled systems, the WG suggests that the ICP monitor be zeroed to the level of the foramen of Monro. The format recommended for recording ICP can also be applied for other monitoring modalities and emerging pediatric data elements, such as brain tissue oxygen tension, brain temperature, or jugular venous oxygen saturation.
The WG realizes that the current approaches to analysis of hourly values is often rather crude. Therefore, the WG strongly advocates further development of software aimed at capturing the frequency distribution and AUC calculations of measured values during continuous monitoring, and further research into the benefits of such an approach relative to calculation of mean values, or the percentage of time measured hourly values are above or below a certain threshold (e.g., for ICP above or below 20 or 15 mm Hg).
The module on ICP monitoring includes capturing information on procedures and problems encountered. Recording the duration of ICP monitoring is essential. Documentation of the reason for stopping monitoring (e.g., clinically no longer required, device failure, or for reasons of futility) is relevant when interpreting measured values and their relation to therapy intensity. Identification of possible device malfunction (e.g., partial blockage of a ventricular catheter), and revisions of the monitoring device are highly relevant for accurate interpretation of values.
4.5 Treatment
Core data elements
To record in-hospital treatment of pediatric TBI, the WG recommended the use of the Pediatric Intensity Level of Therapy Scale (PILOT; Shore et al., 2006).
Supplemental data elements
For children with mild TBI the WG recommended collecting data specific to the signs and symptoms most commonly associated with this insult (Comper et al., 2005; Halstead et al., 2010;). Documentation of the method used to obtain vital signs is also important. In particular, temperature recording methods are important. The correlation of different temperature recording methods and brain temperature is just beginning to be explored in children. This relationship appears to be temperature-dependent, and is likely also influenced by age and brain hemodynamics. Depending on the purpose of the study, additional details may be required on the procedures and medications employed.
Emerging data elements
No recommendations were made for this module.
Next Steps
A number of gaps in knowledge specific to pediatric TBI may serve to focus future research. While this creates significant challenges to the development of CDEs for pediatric TBI research, the recommended structure of core, supplemental, and emerging data elements addresses these limitations. A number of valuable data elements can be currently recommended for the supplemental data sets. The emerging data sets can be used to address current limitations, but will require carefully designed studies for validation. For example, research is needed to better define mild to moderate TBI in newborns and infants, since assessments of level of consciousness are not reliable. This is related to the current lack of consensus on the measures to assess and classify the initial level of neurologic injury in infants and children. There are limited data on normal values for, and age-dependence of, key physiologic parameters including ICP and CPP. There is a need for long-term outcome studies to assess the impact of the sequelae of TBI on school performance, self-esteem, ability to enter the work force, and the impact of this injury on the child's family (Hoge et al., 2008). Longitudinal studies are complex and expensive, emphasizing the need for consensus on the measures needed to assess long-term functional outcome. Consideration is also given to the fact that consent must be obtained from parents or from a proxy. Validation of future data elements will require large prospective data sets, and consideration could be given to exempt consent in pediatric TBI studies that do not involve patient interventions (for example, a prospective registry that further evaluates the validity of core, supplemental, and emerging data elements).
This approach to defining CDEs for pediatric TBI is the first step in an iterative process. The elements selected here and priorities assigned represent the consensus of broad and expert input with representation from different disciplines and stakeholder organizations. In addition, they were selected since there was a basis for their use in clinical trials in pediatric TBI to date. Nevertheless, these currently recommended elements will require further refinement and validation. The processes for feedback from users and modification of CDEs are not yet fully defined. The expectation of the CDE project is that these first tools will be refined based on recommendations from researchers who make use of them in clinical trials and epidemiologic studies. There are clearly issues to resolve and gaps in the current CDE versions, including varying definitions of core variables, and the need for better integration between adult and pediatric CDEs. The next generation of CDEs will also need to be integrated with the electronic medical record. The current version of the pediatric CDEs will be successful if they: (1) are widely adopted by the pediatric TBI community as a standard of care, (2) enable efficient pooling of harmonized data from multiple studies, registries, and databases, and (3) yield aggregated patient samples powered to answer questions that could otherwise be studied only by large clinical studies.
The WG would like to see this initiative evolve as an international effort that has the potential to set global standards for data collection in TBI. To accomplish this goal, the following steps are proposed:
Refinement and validation of recommendations in collaboration with international partners and ratification by stakeholders and international scientific bodies.
Translation of the modules into a web-based data entry format with pull-down menus and automated data checks.
Use of relational database architecture to minimize duplicate data entry across research projects.
Software development for direct data entry and coordinated time stamping of the data to improve analysis and cross-study comparisons.
Acknowledgments
The meetings and activities of the pediatric WG Demographics and Clinical Assessment group were supported by funding in the context of the interagency initiative towards an integrated approach to Research in Psychological Health and Traumatic Brain Injury (NIH-NINDS; the National Institute on Disability and Rehabilitation Research; the Department of Veterans Affairs; the Defense and Veterans Brain Injury Center, and the Defense Centers of Excellence). The development of CDEs was further supported by a supplemental grant from NIH-NINDS (NS 042691).
The views expressed are those of the authors and do not necessarily reflect those of the agencies or institutions with which they are affiliated, including the U.S. Department of Veterans Affairs, the U.S. Department of Education, and the NIH. This work is not an official document, guidance, or policy of the U.S. government, nor should any official endorsement be inferred.
Note: With the exception of the first through the third authors and the last (corresponding) author, all other working group members have been listed in alphabetical order, and each has contributed significantly to the preparation of this manuscript.
This project was jointly supported by the NIH/NINDS and the U.S. Department of Education/National Institute on Disability and Rehabilitation Research (DOE/NIDRR).
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson P. Bratton S. Carney N. Chesnut R. du Coudray H. Goldstein B. Kochanek P. Miller H. Partington M. Selden N. Warden C. Wright D. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr. Crit. Care Med. 2003b;4:S12–S18. [PubMed] [Google Scholar]
- Adelson P. Bratton S. Carney N. Chesnut R. du Coudray H. Goldstein B. Kochanek P. Miller H. Partington M. Selden N. Warden C. Wright D. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Threshold for treatment of intracranial hypertension. Pediatr. Crit. Care Med. 2003a;4:S25–S27. [PubMed] [Google Scholar]
- Adelson P. Ragheb J. Muizelaar J. Kanev P. Brockmeyer D. Beers S. Brown S. Cassidy L. Chang Y. Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- American Congress of Rehabilitation Medicine; Mild Traumatic Brain Injury Committee. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- Anderson V. Catroppa C. Morse S. Haritou F. Rosenfeld J. Functional plasticity or vulnerability after early brain injury. Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Baker S. O'Neill B. Haddon W. Long W. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- Barlow K. Thomson E. Johnson D. Minns R. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:e174–e185. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- Berger R. Pierce M. Wisniewski S. Adelson P. Clark R. Ruppel R. Kochanek P. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:e31. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- Bhopal R. Donaldson L. White, European, Western, Caucasian, or What? Inappropriate labelling in research on race, ethnicity, and health. Am. J. Publ. Health. 1998;88:1303–1307. doi: 10.2105/ajph.88.9.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R. Chesnut R. Clifton G. Ghajar J. Marion D. Narayan R. Newell D. Pitts L. Rosner M. Walters B. Wilberger J. Management and prognosis of severe traumatic brain injury. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma. 2000;17:451–627. [Google Scholar]
- Chambers I. Jones P. Lo T. Forsyth R. Fulton B. Andrews P. Mendelow A. Minns R. Critical thresholds of intracranial pressure and cerebral perfusion pressure related to age in paediatric head injury. J. Neurol. Neurosurg. Psych. 2006;77:234–240. doi: 10.1136/jnnp.2005.072215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I. Stobbart L. Jones P. Kirkham F. Marsh M. Mendelow A. Minns R. Struthers S. Tasker R. Age-related differences in intracranial pressure and cerebral perfusion pressure in the first 6 hours of monitoring after children's head injury: association with outcome. Childs Nerv. Syst. 2005;21:195–199. doi: 10.1007/s00381-004-1060-x. [DOI] [PubMed] [Google Scholar]
- Chesnut R. Marshall L. Klauber M. Blunt B. Baldwin N. Eisenberg H. Jane J. Marmarou A. Foulkes M. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Cohen M. Grossman A. Morabito D. Knudson M. Butte A. Manley G. Identification of complex metabolic states in critically injured patients using bioinformatic cluster analysis. Critical Care. 2010;14:R10. doi: 10.1186/cc8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper P. Bisschop S. Carnide N. Tricco A. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19:863–880. doi: 10.1080/02699050400025042. [DOI] [PubMed] [Google Scholar]
- Durham S. Clancy R. Leuthardt E. Sun P. Kamerling S. Dominguez T. Duhaime A. CHOP infant coma scale (“Infant Face Scale”) a novel coma scale for children less than two years of age. J. Neurotrauma. 2000;17:729–737. doi: 10.1089/neu.2000.17.729. [DOI] [PubMed] [Google Scholar]
- Gennarelli T. Wodzin A. AIS 2005: A contemporary injury scale. Injury. 2006;37:1083–1091. doi: 10.1016/j.injury.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Halstead M. Walter K. for The Council on Sports Medicine and Fitness. Clinical report—sport-related concussion in children and adolescents. Pediatrics. 2010;126:587–615. doi: 10.1542/peds.2010-2005. [DOI] [PubMed] [Google Scholar]
- Haque I. Zaritsky A. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr. Crit. Care Med. 2007;8:138–144. doi: 10.1097/01.PCC.0000257039.32593.DC. [DOI] [PubMed] [Google Scholar]
- Hoge C. McGurk D. Thomas J. Cox A. Engel C. Castro C. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Holden K. Titus M. Van Tassel P. Cranial magnetic resonance imaging examination of normal term neonates: a pilot study. J. Child Neurol. 1999;14:708–710. doi: 10.1177/088307389901401104. [DOI] [PubMed] [Google Scholar]
- Holmes J. Palchak M. MacFarlane T. Kupperman N. Performance of the pediatric Glasgow Coma Scale in children with blunt head trauma. Acad. Emerg. Med. 2005;12:814–819. doi: 10.1197/j.aem.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Jones P. Andrews P. Easton V. Minns R. Traumatic brain injury in childhood: Intensive care time series data and outcome. Br. J. Neurosurg. 2003;17:29–39. [PubMed] [Google Scholar]
- Keenan H. Runyan D. Marshall S. Nocera M. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114:633–639. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. Jaffee M. Leskin G. Stokes J. Leal F. Fitzpatrick P. Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J. Rehabil. Res. Dev. 2009;44:895–920. doi: 10.1682/jrrd.2006.12.0166. [DOI] [PubMed] [Google Scholar]
- Ling G. Bandak F. Armonda R. Grant G. Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Looney C. Smith J. Merck L. Wolfe H. Chescheir N. Hamer R. Gilmore J. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242:535–541. doi: 10.1148/radiol.2422060133. [DOI] [PubMed] [Google Scholar]
- Maas A. Marmarou A. Murray G. Teasdale S. Steyerberg E. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Maas A. Standardisation of data collection in traumatic brain injury: key to the future? Critical Care. 2009;13:1016. doi: 10.1186/cc8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. Harrison-Felix C. Menon D. Adelson P. Balkin T. Bullock R. Engel D. Gordon W. Langlois-Orman J. Lew H. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D. Schwab K. Common data elements for traumatic brain injury: Recommendations from the Interagency Working Group on Demographics and Clinical Assessment. Arch. Phys. Med. Rehabil. 2010a;91:1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- Maas A. Harrison-Felix C. Menon D. Adelson P. Balkin T. Bullock R. Engel D. Gordon W. Langlois-Orman J. Lew H. Robertson C. Temkin N. Valadka A. Verfaellie M. Wainwright M. Wright D. Schwab K. Standardizing data collection in traumatic brain injury. J. Neurotrauma. 2011;28:177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire S. Pickerd N. Farewell D. Mann M. Tempest V. Kemp A. Which clinical features distinguish inflicted from non-inflicted brain injury? A systematic review. Arch. Dis. Child. 2009;94:860–867. doi: 10.1136/adc.2008.150110. [DOI] [PubMed] [Google Scholar]
- Marcin J. Pollack M. Triage scoring systems, severity of illness measures, and mortality prediction models in pediatric trauma. Crit. Care. Med. 2002;30:S457–S467. doi: 10.1097/00003246-200211001-00011. [DOI] [PubMed] [Google Scholar]
- Marmarou A. Lu J. Butcher I. McHugh G. Mushkudiani N. Murray G. Steyerberg E. Maas A. IMPACT database of traumatic brain injury: design and description. J. Neurotrauma. 2007;24:239–250. doi: 10.1089/neu.2006.0036. [DOI] [PubMed] [Google Scholar]
- McHugh G. Engel D. Butcher I. Steyerberg E. Lu J. Mushkudiani N. Hernández A. Marmarou A. Maas A. Murray G. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- Menon D. Schwab K. Wright D. Maas A. Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Mushkudiani N. Engel D. Steyerberg E. Butcher I. Lu J. Marmarou A. Slieker F. McHugh G. Murray G. Maas A. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke, NINDS Common Data Elements. http://www.commondataelements.ninds.nih.gov/default.aspx http://www.commondataelements.ninds.nih.gov/default.aspx
- Pineda J. Lewis S. Valadka A. Papa L. Hannay H. Heaton S. Demery J. Liu M. Aikman J. Akle V. Brophy G. Tepas J. Wang K. Robertson C. Hayes R. Clinical significance of II-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Pollina J. Diasm M. Li V. Kachurek D. Arbesman M. Cranial birth injuries in term newborn infants. Pediatr. Neurosurg. 2001;35:113–119. doi: 10.1159/000050403. [DOI] [PubMed] [Google Scholar]
- Pryor H. Thelander H. Abnormally small head size and intellect in children. J. Pediatr. 1968;73:593–598. doi: 10.1016/s0022-3476(68)80275-6. [DOI] [PubMed] [Google Scholar]
- Rooks V. Eaton J. Ruess L. Petermann G. Keck-Wherley J. Pedersen R. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. Am. J. Neuroradiol. 2008;29:1082–1089. doi: 10.3174/ajnr.A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P. Adelson P. Kochanek P. Hand L. Roy L. Reliability and validity of the Pediatric Intensity Level of Therapy (PILOT) Scale: A measure of the use of intracranial pressure-directed therapies. Crit. Care Med. 2006;34:1981–1987. doi: 10.1097/01.CCM.0000220765.22184.ED. [DOI] [PubMed] [Google Scholar]
- Signorini D. Andrews P. Jones P. Wardlaw J. Miller J. Adding insult to injury: the prognostic value of early secondary insults for survival after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 1999;66:26–31. doi: 10.1136/jnnp.66.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E. Mushkudiani N. Perel P. Butcher I. Lu J. McHugh G. Murray G. Marmarou A. Roberts I. Habbema J. Maas A. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchetti N. Pagan F. Calappi E. Canavesi K. Beretta L. Citerio G. Cormio M. Colombo A. Inaccurate early assessment of neurological severity in head injury. J. Neurotrauma. 2004;21:1131–1140. doi: 10.1089/neu.2004.21.1131. [DOI] [PubMed] [Google Scholar]
- Towner D. Castro M. Eby-Wilkens E. Gilbert W. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N. Engl. J. Med. 1999;341:1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- Thurmond V. Hicks R. Gleason T. Miller A. Szuflita N. Orman J. Schwab K. Advancing integrated research in psychological health and traumatic brain injury: Common Data Elements. Arch. Phys. Med. Rehab. 2010;91:1633–1636. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Vasterling J. Proctor S. Amoroso P. Kane R. Heeren T. White R. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296:519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- Whitby E. Griffiths P. Rutter S. Smith M. Sprigg A. Ohadike P. Davies N. Rigby A. Paley M. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363:846–851. doi: 10.1016/S0140-6736(04)15730-9. [DOI] [PubMed] [Google Scholar]
- White J. Farukhi Z. Bull C. Christensen J. Gordon T. Paidas C. Nichols D. Predictors of outcome in severely head-injured children. Pediatr. Crit. Care Med. 2001;29:534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- Whyte J. Vasterling J. Manley G. Common data elements for research on traumatic brain injury and psychological health: Current status and future development. Arch. Phys. Med. Rehabil. 2010;91:1692–1696. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Wilde E. Whiteneck G. Bogner J. Bushnik T. Cifu D. Dikmen S. French L. Giacino J. Hart T. Malec J. Millis S. Novack T. Sherer M. Tulsky D. Vanderploeg R. von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91:1650–1660. doi: 10.1016/j.apmr.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Wolf S. Bebarta V. Bonnett C. Pons P. Cantrill S. Blast injuries. Lancet. 2009;374:405–415. doi: 10.1016/S0140-6736(09)60257-9. [DOI] [PubMed] [Google Scholar]
- Wynia M. Ivey S. Hasnain-Wynia R. Collection of data on patients' race and ethnic group by physician practices. N. Engl. J. Med. 2010;362:846–850. doi: 10.1056/NEJMsb0910799. [DOI] [PubMed] [Google Scholar]