Abstract
This article identifies emerging neuroimaging measures considered by the inter-agency Pediatric Traumatic Brain Injury (TBI) Neuroimaging Workgroup. This article attempts to address some of the potential uses of more advanced forms of imaging in TBI as well as highlight some of the current considerations and unresolved challenges of using them. We summarize emerging elements likely to gain more widespread use in the coming years, because of 1) their utility in diagnosis, prognosis, and understanding the natural course of degeneration or recovery following TBI, and potential for evaluating treatment strategies; 2) the ability of many centers to acquire these data with scanners and equipment that are readily available in existing clinical and research settings; and 3) advances in software that provide more automated, readily available, and cost-effective analysis methods for large scale data image analysis. These include multi-slice CT, volumetric MRI analysis, susceptibility-weighted imaging (SWI), diffusion tensor imaging (DTI), magnetization transfer imaging (MTI), arterial spin tag labeling (ASL), functional MRI (fMRI), including resting state and connectivity MRI, MR spectroscopy (MRS), and hyperpolarization scanning. However, we also include brief introductions to other specialized forms of advanced imaging that currently do require specialized equipment, for example, single photon emission computed tomography (SPECT), positron emission tomography (PET), encephalography (EEG), and magnetoencephalography (MEG)/magnetic source imaging (MSI). Finally, we identify some of the challenges that users of the emerging imaging CDEs may wish to consider, including quality control, performing multi-site and longitudinal imaging studies, and MR scanning in infants and children.
Key words: adults, children, CT, MRI, neuroimaging, TBI
Introduction
The Common Data Elements (CDE) initiative for traumatic brain injury (TBI) is an attempt to promote integration in measures applied in TBI research. As previously reported, the original TBI CDE recommendations were formulated using three descriptive levels: “core” elements that are intended to encompass a minimal set of measures to characterize a broad spectrum of subjects, “supplemental” elements that are intended for greater depth or breadth of exploration or are intended for more specialized subpopulations, and “emerging” elements that represent measures with significant promise to expand knowledge in the field, but that currently require further validation or special consideration (Thurmond et al., 2010).
Emerging neuroimaging tools with the potential to inform TBI research have been previously identified, both by the original Neuroimaging Workgroup (Duhaime et al., 2010; Haacke et al., 2010) and the Pediatric Neuroimaging Workgroup (Duhaime, 2011). The recommendations for core and supplemental CDEs from the original Neuroimaging Workgroup were generally focused on conventional imaging, largely because of the need to establish radiological definitions for forms of injury identifiable on conventional imaging sequences, and also because these techniques are widely available in many TBI-related studies and across centers. However, the group acknowledged that a number of more advanced imaging modalities exist and have great potential to further our understanding of TBI. Although the group did specify some specific techniques and suggested parameters for optimal acquisition for some imaging techniques (see Appendix II of Haacke et al., 2010 or http://www.commondataelements.ninds.gov), both the original and pediatric workgroups acknowledged that prescription of specific protocols for some of the advanced modalities was problematic and required further consideration, as these forms of imaging are still evolving, require further validation or standardization, and continue to pose challenges for use across different groups of investigators.
This article attempts to provide additional detail regarding some of the potential uses of these more advanced forms of imaging in TBI as well as highlight some of the current considerations and unresolved challenges of each. It is intended for investigators who wish to consider applying advanced neuroimaging techniques in TBI-related studies. Although this article accompanies other recommendations for CDEs in pediatric TBI research, and places an emphasis on issues that may be important in early development, the following comments generally apply to the use of these modalities in adults as well as infants, children and adolescents.
Selection of Emerging Imaging Measures
Neuroimaging is a rapidly evolving field, and the potentially useful techniques discussed in this article are by no means exhaustive, even for modalities that are already in use in TBI research. Additionally, other forms of structural and functional imaging will also be developed or come into use in the near future as technology advances. However, we have attempted to summarize emerging elements that are most likely to gain more widespread use in the coming years, because of 1) their utility in diagnosis, prognosis, and understanding the natural course of degeneration or recovery following TBI, and potential for evaluating treatment strategies; 2) the ability of many centers to acquire these data with scanners and equipment that are readily available in existing clinical and research settings; and 3) advances in software that provide more automated, readily available, and cost-effective analysis methods for large scale data image analysis. We also include a brief introduction to a couple of new or more-specialized forms of advanced imaging that do require specialized equipment or software, for example, positron emission tomography (PET) and magnetoencephalography (MEG)/magnetic source imaging (MSI)). Finally, we identify some of the general and unique challenges that users of the emerging imaging CDEs may wish to consider. The emerging neuroimaging tools commented upon in this article include:
multi-slice CT
volumetric MRI analysis
susceptibility-weighted imaging (SWI)
diffusion tensor imaging (DTI)
magnetization transfer imaging (MTI)
arterial spin tag labeling (ASL)
functional MRI (fMRI), including resting state and connectivity MRI
MR spectroscopy (MRS)
PET and single photon emission computed tomography (SPECT)
MSI or MEG
hyperpolarization scanning
Several excellent reviews of the use of some of these techniques in TBI have been previously published, and may provide additional information (Ashwal et al., 2006a; Belanger et al., 2007; Coles, 2007; Gallagher et al., 2007; Hillary et al., 2002; Kou et al., 2010; Suskauer and Huisman, 2009; Tshibanda et al., 2009; Van Boven et al., 2009). As with all the CDE recommendations, the selection of recommended outcome measures is an evolving process and recommendations may change with additional evidence and ongoing discussion regarding the current CDEs. Therefore, the Workgroup advises the reader to consult the CDE website (http://www.nindscommondataelements.org) for any updates and information, particularly with respect to these emerging measures.
General Considerations in the Use of Emerging Imaging Measures
Quality control
Particularly for the advanced imaging MRI sequences, quality assurance (QA) testing is essential for ensuring the fidelity of the data acquired. Scanners should undergo qualification prior to the beginning of subject enrollment and be re-qualified following hardware or software upgrades, using a specific QA protocol (Friedman and Glover, 2006; Friedman et al., 2008) and a specific phantom to ensure optimal scanner performance (Van Boven et al., 2009). For example, in other large collaborative groups that have endeavored to use multi-site imaging, such as the Alzheimer's Disease Neuroimaging Initiative (ADNI), a phantom scan is acquired with each subject, allowing identification of scanning errors or correction of drifts or discontinuities in the decoupled human data. Frequent (weekly or more often) repeated QA with designated phantoms, for example, General Electric (GE), Biomedical Informatics Research Network (BIRN), gel or “doped” water bottle for Weisskoff testing (Weisskoff, 1996), or use of a “travelling” human phantom (i.e., the same individual is scanned on each of the different scanners) for multi-site studies is strongly recommended.
Once acquired (or during acquisition), all data should be reviewed for compliance with the protocol. Image quality, presence of any significant artifact that would compromise accuracy or reliability (including, where applicable, motion, susceptibility, eddy current, dental or metal artifact) and incidental or medically significant findings that would impact study inclusion or exclusion criteria, should all be assessed. Additional post-processing correction specific to each modality should also be applied in a uniform manner (preferably by a single center) to correct for issues such as gradient nonlinearity, intensity inhomogeneity, motion, or eddy current.
Multi-center studies
Several obstacles have prevented more widespread use of the advanced MRI modalities. The first of these is the broad range of hardware and software platforms that exist across different sites. Differences related to the scanner manufacturer (e.g., General Electric vs. Philips vs. Siemens) may introduce significant unwanted variability in the data, as each approaches the acquisition and standard parameters somewhat uniquely, leading to differences in contrast (important for volumetrics), regions prone to artifact or distortion (especially important to echo planar imaging sequences for DTI and fMRI), and level of noise. Establishing equivalency in sequences and parameters across different scanner manufacturers remains extremely challenging. Even when scanners of the same manufacturer are used, differences in the scanner model should be carefully considered, as this may also introduce variability in the data that are acquired. Site-specific issues including radio frequency (RF) shielding and vibration will also vary from one location to another. Field strength (e.g., 1.5 Tesla vs. 3 Tesla) may also introduce differences in data secondary to signal-to-noise ratio (SNR) and degree of resolution. Next, head coil differences may introduce variability (e.g., 8-channel vs. 16-channel vs. 32-channel). Finally, differences in scanner software release may alter quantitative measurement in imaging data, and this remains a challenge in most major medical centers where upgrades can occur frequently. Ideally, for very sensitive modalities such as diffusion tensor imaging, multi-site centers should use the same scanner manufacturer, scanner model, software release, head coil, field strength, and acquisition parameters, where possible, although this clearly limits the number of sites that can be included, and becomes problematic for clinical trials and large-scale studies. Alternatively, general comparability of data acquired at different sites should be demonstrated before data are pooled. Although there has been some discussion among researchers in the field related to creation of “standard scores” derived from actual values in modalities such as DTI, MRS, and MTI to address this issue, no optimal solution has yet been established. Additional obstacles have historically included significant variability in levels of expertise, experience, and access to equipment and software for comparable data post-processing and analysis.
Longitudinal studies
Perhaps the most significant challenge in the use of advanced imaging modalities in longitudinal research is simply the rapid pace at which technology evolves in this area. Within the life of a project sufficiently long enough to collect an adequate amount of longitudinal data, acquisition parameters (e.g., the number of gradient directions in DTI) or analysis methodology or software versions initially established at the outset may be considered “outdated” by the completion of a longer study (e.g., new software releases for freely available programs may occur yearly or even more often). As previously mentioned, scanner software upgrades also occur as technology advances, and the impact of these upgrades on certain advanced MRI sequences may be inevitable in longitudinal studies in which quantitative data analyses are involved. Therefore, longitudinal studies require careful consideration of these factors, and some formal testing and monitoring of the impact of these factors may be required throughout the study. In the case of upgrades in analysis software, comparison of analysis using subsets of data analyzed on each version may be advisable to ensure comparability in results. Obtaining pre- and post- scanner upgrade data on a limited number of subjects may be done to determine (and potentially adjust for) any impact of the upgrade.
Modality-specific challenges also exist in the analysis of longitudinal data, particularly for region-of-interest (ROI) approach techniques in structural imaging, where exact replication in placement of an ROI is difficult either because of changes in the placement of the slices or degenerative change that necessitates alteration in the size or placement of the ROI. The reproducibility of some advanced techniques, particularly in regard to functional imaging modalities, also deserves careful consideration.
Finally, the analysis of longitudinal data, especially in infants, children, and adolescents, requires careful consideration of the underlying developmental context and how TBI-related sequelae may interact with developmental changes that are co-occurring over time. Expected developmental trajectories may differ by age, gender, handedness, region of the brain, and tissue type, and some imaging modalities may be more impacted by developmental changes than others, and the direction of this change may alter by modality.
Scanning in children
Because of the increased speed of acquisition with modern CT scanners, most children do not require sedation for imaging of the brain on unenhanced CT. However, acquisition of lengthy sequences in MRI can present challenges because of the increased potential for motion artifact, particularly in children with acute or severe injury or in children with TBI-induced attentional difficulties or impulsivity. Motion during imaging may be mitigated to some degree by mock scanner training and repeated instruction to the child to remain still. Normally developing children, and even those with TBI, >8 years of age can typically be accommodated in the MR scanner without sedation. High functioning children <8 years of age may also be able to remain still in the scanner (often with a parent present in the scanner room). Some 3 Tesla scanners are physically noisier than conventional 1.5 Tesla instruments and may create more difficulty for the younger child to lie still. Although many institutional review boards will not approve protocols requiring sedation for the purposes of research, most conventional sequences can be acquired without data alteration with conscious sedation. However, it is recognized that certain anesthetic agents (e.g., propofol) may alter and decrease the activation seen with functional imaging paradigms. Moreover, functional imaging modalities (fMRI and MSI) may require increased monitoring of levels of alertness in children.
Description of Emerging Imaging Techniques and Unique Considerations
CT
CT scanning continues to form the mainstay for clinical screening of TBI, because of its speed and reproducibility in allowing identification of fresh blood and some acute parenchymal injury, in addition to giving information regarding the presence of bony injury in both the head and spine. However, early CT scanning immediately following injury may miss later development of hemorrhagic shear injury and enlargement of extra-axial collections, which are both typically apparent by 24 h post-TBI.
In recent years, multi-detector technology has been introduced that permits 4-sec scan times of the brain with a 64-slice scanner. This very fast scan time enables most patients' unenhanced scans to be performed without sedation. Recent introduction of a 320-row detector CT scanner holds the promise of even faster acquisition times (1–2 sec), and scans may be performed in either axial or helical mode, the latter being a volumetric examination. This latest technology is still being assessed in the field to ensure that radiation dosage for soft tissues such as the brain can be maintained at a low level without compromising the detection of pathology because of a reduction in signal to noise. The guiding principle is acquisition at the lowest acceptable radiation dose (ALARA). Helpful guidelines for clinicians and parents regarding reductions in radiation dosage may be found at www.imagegently.com. Because of the concern surrounding CT-related ionizing radiation exposure, particularly in children, use of CT solely as a research tool is not advised. However, clinically indicated CT results are often used in TBI research for characterization of the patient cohort(s), description of the size and nature of acute lesions or abnormalities, determination of participant eligibility (e.g., meeting inclusion or exclusion criteria), or for injury severity stratification purposes.
MRI
Conventional MRI imaging including T1-weighted, T2-weighted and T2 fluid-attenuated inversion recovery (FLAIR) sequences as well as T2* (gradient echo) and diffusion-weighted imaging (DWI) are used in routine clinical practice for patients with TBI. They may give some important information concerning visible hemorrhage and extra-axial collections as well as demonstration of early ischemia and possible detection of edema in sub-acute moderate-to-severe injury. However, detection of abnormalities in uncomplicated early acute mild TBI (as determined by normal CT) with conventional sequences is uncommon in the authors' experience and more moderate-to-severe brain-injured subjects may be too unstable to undergo MRI scanning acutely.
Volumetric analysis
Volumetric analysis allows for the detection or quantification of white and gray matter volumes, either globally or for specific regions, as well as cerebrospinal fluid and total intracranial volume, generally using three-dimensional (3-D) volumetric high resolution T1-weighted imaging or combinations of complementary MR sequences. Volumetric analysis using specific regions has demonstrated significant decreases in volume in both adult (Tomaiuolo et al., 2004; Warner et al., 2010a; Wilde et al., 2004, 2006a) and pediatric (Beauchamp et al., 2010; Fearing et al., 2008; Spanos et al., 2007; Wilde et al., 2005, 2006b, 2007; Wu et al., 2010a) subjects with TBI in the chronic post-injury interval. Additionally, voxel-based, whole-surface based, and tensor-based analyses have also demonstrated significant global reductions in cortical thickness (Merkley et al., 2008) and cortical gray matter volume (Bigler et al., 2010a; Ding et al., 2008; Gale et al., 2005; Warner et al., 2010a,b) as well as white matter volume (Ding et al., 2008; Sidaros et al., 2009; Vannorsdall et al., 2010). Late volumetric measures have demonstrated relation to measures of clinical severity (Levine et al., 2008; Schonberger et al., 2009; Trivedi et al., 2007), outcome, (Ding et al., 2008; Gale and Prigatano, 2010; Warner et al., 2010b), cognition (Bergeson et al., 2004; Fujiwara et al., 2008; Himanen et al., 2005; Serra-Grabulosa et al., 2005; Tomaiuolo et al., 2004; Warner et al., 2010a), and potential for benefit from rehabilitation intervention (Strangman et al., 2010), although other studies have reported no correlation between volumetric measures and functional outcome (Anderson et al., 1995; Sherer et al., 2006; Yount et al., 2002).
Whereas volumetric analysis is an emerging neuroimaging tool and has been successfully used in large-scale analyses of data acquired using different scanners and protocols such as those performed by the Alzheimer's Disease Neuroimaging Initiative (Chou et al., 2010; Hua et al., 2010; Mueller et al., 2005; Risacher et al., 2010; Simmons et al., 2011), challenges remain in its use in multi-site studies in TBI. First, volumetric changes can evolve over an extended time (Sidaros et al., 2009; Trivedi et al., 2007; Wu et al., 2010a) and may be evident later than changes seen on other advanced imaging techniques such as DTI (Bendlin et al., 2008; Groen et al., 2010; Hutchinson et al., 2010; Wu et al., 2010a). It should be kept in mind that the relation between tissue integrity, as measured by volumetry, and outcome may be dynamic and complex, particularly in the earlier phases of recovery, as trajectories of rapid cognitive recovery and incomplete degenerative tissue change may be progressing in opposing directions and to differing degrees. This relation becomes even more complex in infants, children, adolescents, and the elderly in whom TBI-related parenchymal and cognitive changes are occurring within a context of known developmental (or degenerative) brain changes (e.g., linear increases/decreases in white matter with myelination, regional reductions in regional cortical thickness and volume related to programmed apoptosis). Variability in severity of injury, nature and location of brain insult, mechanism of injury, and other co-morbidities present additional challenges for volumetric analysis in TBI. Semi-automated programs for volumetric measurement have emerged in recent years, several of which are freely available (e.g., FreeSurfer, FSL, SPM8), facilitating the potential for large-scale analyses using volumetric data. With the rapid evolution of technology in this area, both the accuracy of the results and the automation are increasing. Additionally, such programs allow for combination of volumetric data with data from other imaging modalities. However, such programs may still have some accuracy limitations in regions of the brain that are difficult to model (e.g., medial temporal areas) or that contain very large lesions or types of pathology (e.g., acute hemorrhage) that the software cannot accurately distinguish. The more automated methods of volumetric analysis generally only take into account a T1-weighted image; therefore, smaller lesions or certain kinds of pathology such as gliosis can be easily missed. Also, because of the dynamic changes that occur during infancy and early childhood in white matter secondary to myelination and the manner in which these are manifest on imaging (e.g., non-uniform signal intensity), many manual and semi-automated techniques produce inaccuracies in segmentation of white and gray matter in very young children. Finally, determination of TBI-related cortical change requires an appropriate normative comparison, given the dynamic developmental changes that occur throughout the life span and potential differences related to gender and handedness.
SWI
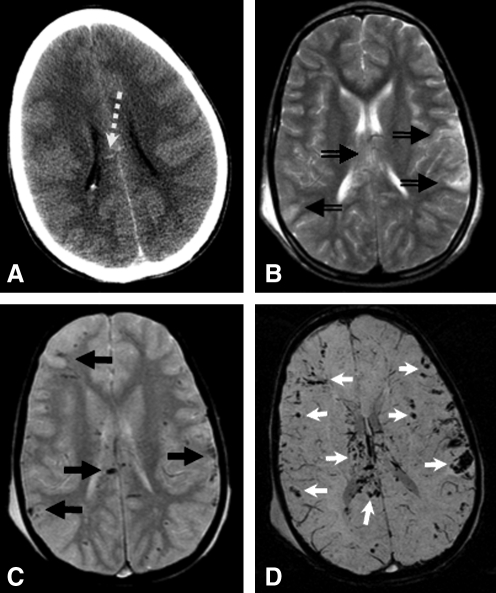
SWI is a modified high spatial resolution 3D gradient recalled-echo (GRE) MR technique that accentuates the magnetic properties of blood products, thereby rendering it useful in detecting small amounts of altered blood and blood product on neuroimaging (Haacke et al., 2004; Reichenbach et al., 1997; Sehgal et al., 2005). SWI has primarily been considered to be helpful in the identification of hemorrhagic axonal injury where smaller hemorrhages not visible on CT or conventional MRI sequences may be visualized with this technique (Ashwal et al., 2006b). SWI may be helpful in the detection of these “microhemorrhages,” especially in early acute and sub-acute phases of injury, and in detecting areas of hypoxia-ischemia induced secondary injury. Figure 1 demonstrates the increased visibility of small hemorrhagic abnormalities demonstrated in SWI as opposed to other more conventional imaging modalities.
FIG. 1.
CT (A) only shows a few small hyperdense hemorrhages in the corpus callosum (dashed white arrow) of a child with TBI. T2WI (B) is not sensitive to hemorrhage, although ill-defined T2 hyperintense areas of edema are detected in the corpus callosum and the periphery of the hemispheres (double line black arrows). Conventional T2*-WI (C) is routinely used to detect hemorrhage, which demonstrates small hypointense hemorrhagic contusions along the brain surface as well as hemorrhagic shearing injury in the corpus callosum (solid black arrows). However, susceptibility-weighted imaging (SWI) (D) is significantly more sensitive to hemorrhage, and shows many more hemorrhages (white arrows) than any of the other images.
SWI has demonstrated increased sensitivity to the number and volume of hemorrhagic traumatic axonal injury lesions as compared to conventional T2*-weighted two-dimensional (2D)-GRE imaging (Tong et al., 2003), and the extent of SWI-identified hemorrhage has been shown to correlate with initial severity of injury measured by Glasgow Coma Scale (GCS), duration of coma, long-term outcome measured at 6–12 months after injury (Tong et al., 2004), and specific neuropsychological deficits (Babikian et al., 2005). SWI is able to detect a larger number of lesions and to define smaller areas of damage than CT, T2-weighted imaging, and FLAIR imaging. However, SWI has not yet been shown to be superior to T2-weighted imaging or FLAIR in discriminating between good and poor outcomes (Chastain et al., 2009). There are currently conflicting results on the relationship between outcome and the number of lesions on MRI, given the few comparison studies available. Some studies report that lesions on T2WI (Chastain et al., 2009; Gerber et al., 2004; Sigmund et al., 2007), FLAIR (Chastain et al., 2009; Sigmund et al., 2007), T2*-GRE (Gerber et al., 2004; Yanagawa et al., 2000), and SWI (Sigmund et al., 2007; Tong et al., 2004), can discriminate or correlate with outcomes. Other studies report that there is no correlation between outcomes and lesions on T2WI (Yanagawa et al., 2000), T2*-GRE (Scheid et al., 2003), and SWI (Chastain et al., 2009). It is likely that a combination of imaging findings will improve the ability to predict clinical outcome, and more studies are needed to confirm this.
In secondary injuries, SWI may also demonstrate increased oxygen extraction in areas of tissue infarction or hypoxemia. Increased deep medullary veins can be observed surrounding an area of infarction, which may reflect impaired cerebral blood flow (CBF) in the penumbra around an infarct. In this zone, lower levels of oxygenated red blood cells, and therefore higher levels of deoxygenated red blood cells, may account for increased visibility of veins in these regions. In more severe global hypoxic injury, increased oxygen extraction may reflect catastrophic brain insults in those children who become brain dead. The evolution of injury after severe hypoxia-ischemia includes a period of time when increased oxygen extraction occurs as CBF dramatically decreases because of increasing cerebral edema and increased intracranial pressure (ICP).
A detailed explanation of the underlying physics and mathematical principles is described in depth in several recent publications (Haacke et al., 2004; Sehgal et al., 2005) and parameters for the original and modified sequences have been previously published. The sequence along with its automatic post-processing is currently available for Siemens 1.5 and 3.0 Tesla MRI scanner platforms, as well as on other recent scanner platforms from Toshiba and Philips. GE has a different version, known as SWAN (T2 Star Weighted Angiography), without the extensive post-processing. In the future, it is likely that this method, particularly a faster version, will replace conventional T2*-GRE imaging in clinical evaluation as well as research studies. Remaining challenges in using SWI in TBI research include its susceptibility to artifacts in air–tissue interfaces, including the orbital frontal areas, a region of distinct vulnerability in TBI. Adequate training and familiarity with the technique are needed to differentiate veins from small hemorrhages. In regards to the use of SWI in the detection of hemorrhage, SWI may not be as sensitive to subarachnoid hemorrhage in cisterns where bone structures are in close proximity (Wu et al., 2010b). It is currently unknown whether SWI can be used to differentiate subarachnoid or subdural hemorrhage along the tentorium (Wu et al., 2010b).
DTI
DTI allows a potentially useful in vivo estimation of the integrity of white matter, as changes in diffusion or anisotropy measured by DTI are thought to be associated with microstructural alteration resulting from loss or disorganization of fibers associated with breakdown of myelin and downstream nerve terminals, neuronal swelling or shrinkage, and increased or decreased extracellular space (Ducreux et al., 2005). DTI allows measurement of the directionality of anisotropic diffusion (restricted diffusion in one direction with enhanced direction in another) of water molecules within coherently organized white matter tracts. The speed and direction of this diffusion renders information about the microstructural environment of the brain. Diffusion anisotropy can be quantified within white matter tracts using the DTI-derived measure fractional anisotropy (FA) or other DTI-derived diffusion metrics such as apparent diffusion coefficient (ADC) or mean diffusivity (MD) or axial diffusivity (AD) or radial diffusivity (RD). Currently, this technique is considered to be most useful in the evaluation of axonal injury in white matter, although it has also been used to examine the microstructural properties of gray matter in TBI (Newcombe et al., 2008). Other advanced forms of DTI including diffusion spectrum imaging (DSI), high angle resolution diffusion imaging (HARDI), and q-ball are also demonstrating great promise and can resolve some of the known pitfalls of DTI for regions with crossing fibers, although these may require longer acquisition times and specific hardware requirements. Studies of TBI using DTI, as well as the software to analyze these data, have been steadily increasing in recent years. Significant differences in the FA, ADC, or MD, or other DTI-derived diffusivity metrics have been demonstrated in studies of TBI in both adults (Bigler et al., 2010b; Kraus et al., 2007; Lipton et al., 2008; Perlbarg et al., 2009; Warner et al., 2010a) and children (Ewing-Cobbs et al., 2008; Levin et al., 2008; McCauley et al., 2011; Wilde et al., 2006b, 2010; Wozniak et al., 2007; Wu et al., 2010a; Yuan et al., 2007), with decreases in FA and increases in measures of diffusivity often found in chronic post-injury intervals. More importantly, changes in DTI-derived measures have shown correlation with injury severity (Arfanakis et al., 2002; Benson et al., 2007; Wilde et al., 2010; Yuan et al., 2007), functional outcome (Huisman et al., 2004; Levin et al., 2008; Salmond et al., 2006; Wozniak et al., 2007), neurologic functioning (Caeyenberghs et al., 2010a,b), and cognitive ability (Bigler et al., 2010b; Ewing-Cobbs et al., 2008; Kraus et al., 2007; Kumar et al., 2009; Levin et al., 2008; McCauley et al., 2011; Niogi et al., 2008; Salmond et al., 2006; Warner et al., 2010a; Wilde et al., 2010). Longitudinal studies have also indicated that DTI might serve as a tool for revealing changes in the neural tissue during recovery from TBI (Bendlin et al., 2008; Sidaros et al., 2008; Wu et al., 2010a). DTI remains a promising tool in TBI research and clinical practice for 1) assisting in clinical diagnosis (particularly in mild TBI) (Bazarian et al., 2007; Mayer et al., 2010; Miles et al., 2008; Wilde et al., 2008) and prognosis (Newcombe et al., 2007; Perlbarg et al., 2009); 2) understanding the nature and time course of degenerative brain changes in vivo; 3) uncovering potential evidence for neuroplastic changes (e.g., reorganization or recovery); and 4) evaluating therapeutic interventions and rehabilitation. It should be kept in mind that further studies will be necessary to validate and more completely understand the neuropathological bases that underlie differences in DTI-derived metrics (Adams et al., 2011), particularly given the complexity of TBI-induced tissue changes. Additionally, particular challenges surround the potential for its use in large-scale, multi-site, or longitudinal studies. Perhaps most significantly, it is difficult to establish the equivalency of quantitative measures derived from protocols that use different acquisition parameters (including but not limited to number of gradient directions, b-value, voxel size), scanner manufacturer and/or software platform, and field strength, or head coil. At this time, caution is advised in combining data collected from different sites or using different protocols. Second, DTI data can also be quite vulnerable to distortion and artifacts (i.e., metal, eddy current, motion, susceptibility), and there is no widely used threshold for determining “acceptable” data quality. Third, there is no consensus on which DTI metric (e.g., FA, ADC/MD, AD, RD) is best used in TBI-related studies, as each may be sensitive to different forms of pathology co-occurring in TBI. Indeed, studies using multiple metrics examining the relation of DTI to outcome have often found disparate results among the metrics. Fourth, certain forms of analysis, such as a single-slice ROI approach are difficult to use in longitudinal studies because the placement of the slices between the studies will inevitably vary, leading to further difficulty in the placement of an identical ROI. Fifth, in TBI, inclusion of subjects with large focal lesions may also create challenges in many forms of analysis, particularly given field susceptibility from hemosiderin deposition, gliosis, and encephalomalacia which may skew diffusion parameters. Sixth, the time course of DTI-related changes remains unknown and may vary with injury severity, mechanism of injury, and age of the participants; therefore, these factors may require careful consideration. Finally, as with many advanced techniques, appropriate normative data are required because age-related differences can be substantial, particularly at the extremes of age. Given the sensitivity of DTI data to changes in just about any parameter, such normative data are ideally acquired on the same scanner using the same protocol.
Currently there are numerous commercially and freely available software packages for the post-processing and analysis of DTI data, and several different analysis methods exist, including (but not limited to) single- or multiple-slice ROI approaches, quantitative tractography, tract-based spatial statistics, voxel-based analyses, and histogram analyses. Each of these forms of analysis has advantages and disadvantages depending upon the question being addressed. A detailed listing of the limitations of each analytic technique is beyond the scope of this report, but investigators should be advised that differences in post-processing and analytic methods may produce differences in the results obtained.
MTI
MTI is a technique that uses an off-resonance saturation selective pulse to saturate protons associated with macromolecules. MT generates contrast based upon submolecular exchange processes in parenchyma, specifically, the interaction between free water protons and macromolecular protons such as proteins and phospholipids, which coat the axonal membranes and myelin sheaths (Duckworth and Stevens, 2010) in the brain's microstructure. The technique is achieved through magnetization interaction based on dipolar or chemical exchange (sometimes both). In order to do this, an off-resonance RF pulse is applied to macromolecular protons, and some of their excited magnetization is transferred to free water protons. The amount of this exchange can be quantified by calculation of the magnetization transfer ratio (MTR) (Wolff and Balaban, 1989). Exogenous cellular components are the major contributors to the MT effect in brain tissue, and therefore the concentration and integrity of myelin is reflected in MTR values (Dousset et al., 1992; Rademacher et al., 1999; Rocca et al., 2002). The MTR is the quantitative measure of the amount of MT that occurs between the bound and the mobile water molecules in a given ROI.
Studies have shown decreased MTR in white matter regions of patients with TBI (Bagley et al., 2000; McGowan et al., 2000) even when there was no observable pathology on conventional imaging (Duckworth and Stevens, 2010; Kimura et al., 1996; Mamere et al., 2009; Sinson et al., 2001). Furthermore, MTI has proven sensitive in detecting changes in patients with even mild brain injury (McGowan et al., 2000). It has been postulated that decreased MTR in TBI may reflect an increase in concentration of microglia, amyloid, phagocytic vacuoles, and/or other injury products, such as extracellular matrix components within both the gray and white matter. Decreased MTR has been correlated with worse overall outcome in TBI (Sinson et al., 2001) and also with specific neuropsychological deficits (McGowan et al., 2000).
Challenges for MTI include the fact that MTR reflects complex biological conditions that make it difficult to attribute change to a specific pathological process (e.g., edema, Wallerian degeneration or myelin loss). Second, the comparability of results obtained with different scanners or acquisition protocols is undetermined and may be somewhat difficult to establish in multi-site studies (Pagani et al., 2008). Third, MTI sequences can be lengthy and motion artifact can influence MTR values substantially. Fourth, as with all advanced sequences where manual region of interest approaches are used, inter- and intra-rater reliability should be established, particularly for cortical gray matter ROIs, given the thickness of the cortical mantle, because inclusion of white matter or cerebrospinal fluid (CSF) can distort values. Next, because thick slices are often used in ROI analytic approaches, studies using a longitudinal design should carefully asses the comparability of the level at which the ROIs are measured for each study. Finally, not all studies have MTR abnormalities associated with functional outcome (Bagley et al., 2000; Sinson et al., 2001), and more work needs to be done in this area. However, because of its relative sensitivity compared with conventional imaging, MTI has proven capable of demonstrating the white and gray matter changes associated with a traumatic insult to the head and could prove important in our understanding of changes in the brain's microstructure following TBI (Kumar et al., 2003; McGowan et al., 2000).
Perfusion imaging
CT perfusion
CT perfusion can be performed by tracking the passage of contrast-laden blood through the brain following injection of a bolus of water-soluble iodinated contrast medium. Data are typically acquired every 2–3 sec and a time-density curve is constructed. There is a linear relationship between iodine concentration and Hounsfield number, produced over the range of radiation dosages that are typically used to acquire a CT scan of the brain. Quantitative analyses can therefore be performed to estimate the amount of blood flow through the brain as well as other parameters, such as time of first arrival, time to half-max, time to peak, and transit time of the first bolus of contrast-laden blood. These allow for some semi-quantitative estimates of CBF on a voxel-by-voxel basis. However, there is a radiation penalty to be paid for this frequent scanning as well as a (albeit low) morbidity/mortality rate associated with the administration of iodinated contrast media. A few recent studies have assessed regional cerebral hemodynamics using perfusion CT in relation to clinical variables such as post-traumatic amnesia in patients with mild brain injury (Metting et al., 2010), and assessment, monitoring, and prognostication in patients with more severe injury (Soustiel et al., 2008; Wintermark et al., 2004a,b).
MRI perfusion
Historically, MR perfusion imaging has been performed using a first pass T2* “dye dilution” technique using a bolus of gadolinium (Gd) contrast and plotting change in signal induced by the contrast-laden blood against time. Integration of the area under the curve gives a measure of the volume of blood passing through an ROI. Other parameters that can be measured include time of arrival, time to peak, and transit time. Alternatively, T1 techniques with a small preparatory dose of Gd prior to the bolus of contrast, to remove T2* effects, can be used in conjunction with compartmental analysis to better understand breakdown of the blood–brain barrier (Wintermark et al., 2005). Because of the need for a contrast agent, MR perfusion is less commonly used in research.
ASL
ASL perfusion MRI is a newer technique for measuring cerebral perfusion that uses arterial blood water as an endogenous contrast agent, negating the need for Gd. In ASL, the protons in arterial blood water are electromagnetically labeled proximal to the tissue of interest and the effects of pre-labeling are determined by pair-wise comparison with images acquired using control labeling. The rate of decay of the signal from a known band proximal to the tissue of interest can be used qualitatively to compare perfusion of different areas of the brain on the same slice as well as calculate CBF using known parameters of protons in the blood, based on numerical values abstracted from the literature (Buxton, 2005). There are now at least four different variations on ASL including continuous ASL (CASL) (Detre and Alsop, 1999), pulsed ASL (PASL), cPASL (continuous PASL), and velocity selected ASL (VSASL) (Wong et al., 2006).
ASL has been used to study rodent models of TBI (Forbes et al., 1997a,b; Hendrich et al., 1999; Kochanek et al., 2002, 2005; Robertson et al., 2000), and has recently also been used in patient studies (Kim et al., 2010). Several promising potential applications for ASL in the study of TBI have been proposed, including characterization of regional brain function in severe TBI in which task-evoked responses are difficult to obtain, determination of the relationship between changes in regional CBF and cognitive deficits to identify potential targets for pharmacological therapy or other intervention, and use as a biomarker for pharmaceutical trials (Van Boven et al., 2009). ASL should be especially robust in children as the smaller head size, higher intrinsic brain water, faster inherent blood flow rate, and typical absence of stenoses or atherosclerosis all lead to an increase in intensity of tracer signal. Perfusion parameters that can be developed from ASL include quantitative CBF as well as transit times and the possibility of measuring extraction fractions. ASL has also been used to perform fMRI. ASL may be of interest both in acute mild TBI as well as during the sub-acute and later phases of recovery from more moderate-to-severe TBI.
Other advanced forms of MRI
fMRI; including resting state
The mainstay of fMRI is based on the blood oxygen level dependent (BOLD) technique resulting in the observation that when neurons are activated, there is an oversupply of arterial blood diverted to that region. This results in an overabundance of oxygenated blood in the venous effluent coming away from this area of the brain. The resulting alteration in oxy- to deoxy-hemoglobin changes the paramagnetic/diamagnetic properties of the tissue, which can be recorded using a T2* acquisition. Using an appropriate paradigm (e.g., an 8 Hz flashing and rotating checkerboard in the case of stimulation of the visual cortex), the stimulus may be applied using a box car (or event-related) design with appropriate alternating periods of rest and stimulation, which can later be subtracted to remove background noise. The acquired results of a series of repeated stimuli are summed and subtracted to increase the robustness of the data and statistical parametric maps derived (using a program such as statistical parametric mapping [SPM] or analysis of functional neuroimages [AFNI]) which can then be re-applied to a high spatial resolution matching T1-weighted anatomic slice. fMRI has been used to examine functional activation patterns in patients with TBI at all levels of severity in both adults (Cazalis et al., 2006; Christodoulou et al., 2001; Maruishi et al., 2007; McAllister et al., 1999, 2001; Newsome et al., 2007b; Perlstein et al., 2004; Rasmussen et al., 2008; Scheibel et al., 2003, 2007; Schmitz et al., 2006; Soeda et al., 2005) and children (Lovell et al., 2007; Newsome et al., 2007a; Scheibel et al., 2003). fMRI may also be important in understanding recovery from mild TBI (Chen et al., 2004, 2007, 2008; Jantzen et al., 2004; Lovell et al., 2007) or in rehabilitation efforts in more severe forms of TBI (Kim, Y. H. et al., 2009; Laatsch, L et al., 2004a,b; Strangman et al., 2005, 2008).
More recently, there has been increasing interest in the concept of what the brain does at rest, and data have been collected with a subject in the scanner, using the BOLD techniques but without any stimulus. This resting fMRI (rfMRI) data have been analyzed to look for neural networks or areas of connectivity (fcMRI) within the brain. There are currently two published ways in which this has been performed: 1) by identifying clusters of voxels with high “activation” using a priori knowledge of 8 pre-designated nodes (James et al., 2009). and 2) by applying independent components analysis (ICA) (Calhoun et al., 2002) to look for connectivity within different areas of the brain. Functional connectivity has been recently applied in both mild TBI (Mayer et al., 2011) and more severe TBI (Marquez de la Plata et al., 2011) and understanding the coherence of functional networks may provide important insights into recovery and therapeutic interventions. Graph theory has also now been applied to this area of research. Of interest is a recent study on thalamic resting state networks purporting to demonstrate disruption in patients with mild TBI (Tang et al., 2011).
Although there does appear to be some commonality in background patterns of activity in the brain when data are acquired using the BOLD technique in the resting state, it remains unclear which, if either, of the current techniques mentioned previously is optimal in demonstrating connectivity and displaying neural networks. Some early data in patients with altered levels of consciousness have already been acquired, and all the previously stated general pitfalls of motion and other etiologies of artifact apply, including the particular problem of T2* artifact generated at air/bone/fluid/soft tissue interfaces, as well as the T2* artifact inherent in blood and calcium, which may be present in patients who have sustained recent or prior trauma.
MRS
MRS provides a sensitive assessment of post-injury neurometabolite alterations, particularly in tissue with no visible injury on conventional imaging, and has shown potential for providing early prognostic information regarding clinical outcome in pediatric patients with accidental and non-accidental TBI (Aaen et al., 2010; Ashwal et al., 2000; Babikian et al., 2006; Brenner et al., 2003; Holshouser et al., 1997, 2005; Hunter et al., 2005; Makoroff et al., 2005; Yeo et al., 2006). Studies using proton MRS in children have found similar neurometabolite alterations after injury. Typically, N-acetyl aspartate (NAA), an amino acid synthesized in mitochondria, is a neuronal and axonal marker that is reduced as a result of neuronal loss or dysfunction after injury. Several studies have shown a direct correlation of reduced NAA and impaired long-term neuropsychological function in children (Babikian et al., 2005; Brenner et al., 2003; Hunter et al., 2005; Yeo et al., 2006). Total choline (Cho) primarily consisting of phosphoryl and glycerophosphoryl Cho is elevated because of shearing of myelin and cellular membranes (diffuse axonal injury) and/or repair. Total creatine (Cr) composed of Cr and phosphocreatine, is assumed to be fairly constant, however recent studies have shown that Cr changes in various disease states (Hattingen et al., 2008; Inglese et al., 2003; Munoz Maniega et al., 2008) including TBI (Gasparovic et al., 2009). Lactate (Lac) accumulates as a result of anaerobic glycolysis and in the setting of TBI may be a response to release of glutamate (Alessandri et al., 2000). Studies have shown that lactate presence is more common in children after non-accidental TBI than after accidental TBI (Aaen et al., 2010; Holshouser et al., 1997) and is strongly correlated with poor outcome in both groups. Glutamate and immediately formed glutamine (Glx) are excitatory amino acid neurotransmitters released to the extracellular space after brain injury that play a major role in neuronal death (Ashwal et al., 2004a; Bullock et al., 1998). Myoinositol (Ins), an organic osmolyte located in astrocytes, increases as a result of glial proliferation and has been correlated with poor outcome after TBI (Ashwal et al., 2004b; Garnett et al., 2001). Lipids and macromolecules (LipMM) produce broad peaks in a spectrum and may increase as a result of severe brain injury, because of a breakdown of cell membrane and release of fatty acids (Panigrahy et al., 2010).
MRS has benefited in the last few years from advances in technology and software development. Scanner manufacturers provide spectroscopy packages with standard sequences such as point resolved spectroscopy (PRESS) and stimulated echo acquisition mode (STEAM) to be used with techniques such as single voxel spectroscopy (SVS), which allows acquisition of a single spectrum from one volume element (voxel), and 2- or 3-D magnetic resonance spectroscopic imaging (2D-MRSI/3D-MRSI), also called chemical shift imaging (CSI), which allows simultaneous acquisition of multiple spectra in a defined ROI through multiple slabs in the brain. MRSI provides more information than SVS but also requires more acquisition time, which must always be considered when imaging children. However, higher field strengths with better signal-to-noise, multi-channel head coils, and advanced sequences are being used to shorten acquisition times. Manufacturers have also incorporated automatic water suppression and shimming, outer volume fat saturation bands, and post-processing software into their spectroscopy packages to facilitate clinical use of MRS.
The field strength, the type of sequence and the parameters used such as the TR (repetition time) and the echo delay time (TE) determine the appearance of the spectrum and the metabolites that can be detected. In order to compare findings from one institution to another, strict protocols must be used not only for spectral acquisition, but also for the post-processing, which greatly affects the information displayed in the spectrum. Spectral processing identifies metabolites according to their chemical shift resonance and measures the area under each peak, proportional to their concentration. The findings are often reported as peak area metabolite ratios such as NAA/Cr or Cho/Cr. The distribution of metabolite levels or ratios acquired with MRSI displayed as signal intensities for each voxel and overlayed onto anatomic images, are known as metabolite maps or images. Previously, it was thought that Cr (the creatine: phosphocreatine [Cr:PCr] pool) is maintained at a constant level in the brain, and is therefore used in many studies as an internal reference standard. Although it is known that Cr levels can change in certain conditions, ratios continue to be useful for reporting and comparing serial measurements or data among institutions using similar acquisition techniques, and has the advantage that correction for partial volume signal intensity loss and signal intensity calibrations are not required (Govind et al., 2010). Quantitation of MR spectra to determine absolute metabolite concentrations, however, is being used more commonly. Quantitative methods use water as an internal reference (Barker et al., 1993) or phantoms containing known metabolite concentrations to quantify peak areas and report absolute or relative metabolite concentrations rather than ratios (Barker et al., 2010; Danielsen and Henriksen, 1994; Provencher, 1993). It is also becoming more common to use segmentation (McLean et al., 2000) and linear regression (Schuff et al., 2001) techniques to estimate the contributions to metabolite signal arising from both gray and white matter within the same voxel, as well as CSF that mimics signal loss.
P31 phosphorus MRS has been used historically to look at energy metabolites, but suffers from long acquisition times. Phosphorus MRS can also be used to calculate pH of tissue. In the future, third party vendor, tuneable head coils will expand the multi-nuclear capability of MRS in conventional scanners and also allow for emerging techniques such as hyperpolarization imaging (described subsequently).
Other considerations for study design to be aware of before using MRS, are that metabolite levels vary by anatomic region (Frahm et al., 1989) and change rapidly as the brain develops (Kreis et al., 1993) requiring the use of normal age-matched reference data for interpreting MR spectra from children. At birth, NAA and Cr levels are low, whereas Cho and Ins are high. NAA and Cr increase rapidly with normal brain maturation in the first 18–24 months of life at the same time that Cho and Ins are decreasing rapidly, with all metabolites leveling off at near adult levels by 24 months (Kreis et al., 1993; Ross et al., 1998). However, NAA continues to rise slightly, peaking at ∼10–15 years, and then decreases slightly over time as the number of neurons and axons gradually declines with normal aging, whereas Cr continues to slightly increase after reaching adult levels (Kreis et al., 1993). Although normal age-related metabolite levels and ratios have been published (Holshouser et al., 1997; Huppi et al., 1991; Pouwels et al., 1999), most institutions establish their own control values for comparison to patient studies, as values may vary with specific scanners, field strengths, and protocols.
MRS holds great promise for TBI evaluation as it provides a sensitive, noninvasive assessment of neurochemical alterations after brain injury, and has shown metabolic abnormalities in regions of the brain that appear normal on conventional MRI sequences (Garnett et al., 2000; Holshouser et al., 2005). However, similar to DTI, the post-processing and analysis of MRS is more complicated, and the expertise needed to establish an MRS program may not be available at all institutions. The challenge is to establish standard, automated techniques and protocols so that MRS can be routinely incorporated into the evaluation of patients with TBI. To this end, manufacturers are already providing proton MRS software packages that require minimal training to use with standard sequences and fully automated shimming, water suppression, and reference scanning. In addition, commercially available packages for automated spectral fitting and processing are already available and widely used. An initial approach for multi-center studies may be to use single-voxel MRS with a standard sequence (such as PRESS with short echo time) that ensures high signal-to-noise and enables acquisition of short T2 relaxation time metabolites with relatively short acquisition times. These sequences can be acquired in minutes followed immediately by a water reference spectrum requiring only seconds, to enable metabolite quantification. Spectral fitting and automated output of metabolite results can then be done with a commercially available fully automated, user-independent spectral fitting package. LCModel is one of the most widely used packages and includes automated baseline and phase correction, and allows absolute quantification with appropriate calibration as well as an estimate of the uncertainty of the fit (Provencher, 1993). The automated output of metabolite concentrations (i.e., NAA, Cr, Cho, Ins, Glx), the fit estimate that would be used as an indication of the quality of the spectra, and the pertinent acquisition information (i.e., TR, TE, voxel size, brain region) could then become a set of data elements added to the existing set already established. Although MRSI allows mapping of the regional distribution of metabolites, the technique may not be suitable immediately for multi-center trials because of the longer acquisition times, the homogeneity issues with shimming larger volumes, the need to process and review hundreds of spectra, and the challenge of quantification. Despite these disadvantages, automated, fast 3D-MRSI software with whole brain coverage is under development and may be available in the future as a standardized package for routine use (Maudsley et al., 2006). Until then, there are many academic institutions that are already capable of providing the single-voxel spectroscopic set of data elements described previously for evaluation of TBI.
Hyperpolarization scanning
Although MRI currently provides superb soft tissue contrast, the inherent low sensitivity of the instrument has limited its use to the imaging of water protons that abound in the human brain. Hyperpolarization techniques enable the signal from a given number of nuclear spins to be raised by >100,000 times, allowing imaging of nuclei other than protons, such as C13, N15 and Xe29. Feasibility has already been demonstrated in a clinical setting (Kaushik et al., 2010). Because the natural occurrence of C13 in the human body is so low, there is essentially no background noise. Hyperpolarized nuclei generate the signal themselves rather than being moderated. As with SPECT and PET, however, in which the scanner detects the radiation from an injected contrast agent containing gamma-emitting nuclei, the strength of the signal with hyperpolarized MRI is directly proportional to its concentration. The availability of an injectable hyperpolarized C13 opens a new field in MRI. The coil and receiver system need to be re-tuned to the resonance frequency of the hyperpolarized nucleus. The signal strength is a linear function of the concentration of the polarized nucleus in question. MRI is capable of identifying signal from the tracer nuclei (e.g., C13) in different molecules, unlike PET/SPECT. Consequently, distribution patterns may be mapped by injection and imaging of several hyperpolarized C13 molecules simultaneously giving valuable information about membrane structure and permeability. There has already been proof of concept in the pulmonary system in adults using Xe29 (Kaushik et al., 2010) and translation into the pediatric population is anticipated, allowing us to enter the world of molecular imaging, proteomics, and genomics.
Nuclear medicine
SPECT
SPECT imaging is performed by collecting data from a gamma-emitting isotope (e.g., Tc99m), by use of a gamma camera to acquire multiple 2D images. This process typically takes ∼20 min in order to obtain enough signal-to-noise. Using a computer and applying a tomographic reconstruction algorithm to the multiple projections, a 3D data set is produced that can then be manipulated and reconstructed in any plane, producing images in a similar fashion to those produced by other tomographic techniques such as CT, MRI, and PET.
Following injection, Tc99m-labelled tracers such as hexamethylpropylene amine oxide (HMPAO) are taken up by brain tissue in a manner proportional to CBF and can therefore be used to assess brain blood flow. Because blood flow to the brain is tightly coupled to local brain energy use, this radioisotope-labeled tracer can be used to assess regional brain metabolism. Recent studies have shown the accuracy of SPECT in Alzheimer's disease to be as high as 88% (Bonte et al., 2006). Tc99m is a metastable nuclear isomer that is readily available in most hospitals, and easy and cheap to produce. SPECT has been used in TBI to examine recovery (Chiu Wong et al., 2006).
PET
Whereas SPECT uses radioactive tracer materials that emit gamma radiation directly, PET tracers (e.g., F19 deoxyglucose [FDG]) emit positrons that annihilate with electrons up to a few millimeters away, causing two gamma photons to be emitted at 180 degrees to each other. The PET scanner detects these emissions “coincident” in time, providing more radiation localization information and therefore higher spatial resolution images than SPECT (∼1 cm resolution).
FDG PET scanning of the brain assesses regional brain glucose metabolism and can provide information concerning local brain damage. The information extracted is not dissimilar to that obtained from Tc99m HMPAO SPECT imaging which is, however, more freely and cheaply available. Limited studies have used FDG-PET in TBI (Benzinger et al., 2009; Hattori et al., 2003, 2004; Kato et al., 2007). O15 has also been used to measure CBF and oxygen extraction fraction. As with all techniques involving radiation, consideration has to be given to the dose received by the patient, and therefore limits the performance of serial examinations
Mini-cyclotrons have been developed over the last 10 years that enable positron emitters to be produced locally, thereby removing dependence on a few major centers with a cyclotron. However the cost of the radiopharmaceuticals that necessitate a hot-lab, on-site chemist and selection of a special site (e.g., basement or ground floor) for extraction of any radioactive gases such as O15, make this an expensive exercise. PET-CT scanners have also been introduced in this time frame and combine both pieces of equipment to further improve localization of isotope activity by better enabling registration of images from both modalities. More recently, early work with PET/MRI scanners has been performed, but there remain numerous obstacles to overcome before these will be available for routine clinical use.
Other techniques
Electroencephalography (EEG)
EEG and amplitude EEG (aEEG) are the current techniques used in clinical practice to monitor effects on the electrical activity of the brain following TBI, and for the earliest detection of seizure activity. For the purposes of epilepsy surgery that may occur in the post-traumatic setting, EEG has been co-registered with fMRI for the purposes of improved localization of foci of seizure activity. EEG has also been used to examine TBI-related disruption of the blood–brain barrier (Korn et al., 2005; Tomkins et al., 2011).
MEG/MSI
MEG is a method of recording magnetic flux on the surface of the head that is associated with intracranial electrical currents produced between synapses or within the axons or dendrites of neurons. Similar to EEG and evoked potential (EPs) recordings, it can be used to detect abnormalities in spontaneous brain activity. However, MEG can also be used for localization and estimation of the order and time course for these kinds of signals, and allows construction of images of brain activity, a process that is often referred to as MSI. Because of its high degree of resolution of normal and abnormal brain physiology for both spatial and temporal resolution, MSI has been considered a potentially useful tool in TBI-related research (Bigler, 1999), particularly for diagnosis of milder TBI for which MR findings are unrevealing (Huang et al., 2009; Lewine et al., 1999, 2007).
However, significant challenges remain in the use of MEG/MSI. Most importantly, few medical centers have access to magnetometers or gradiometers, and analysis of the data requires specialized expertise, thereby limiting its widespread use as either a clinical or a research tool. Second, although the temporal resolution of MEG is excellent, variations in signaling can be introduced through a number of sources (e.g., external stimulation, movement, unrelated mental activity); therefore, challenges exist related to its use in acute or severe TBI, because drowsiness, suboptimal levels of cooperation, and states of altered consciousness may limit the robustness of findings. MSI is best used for measurement of surface cortical activity; depending upon the modeling used, regions such as the basal temporal regions or the sub-cortical regions may be substantially more difficult to accurately measure.
Future Issues and Research Needs
The Pediatric CDE Workgroup identified several challenges and areas in which additional research would enhance the use of advanced imaging techniques in TBI. First, the pace of technological advance has provided both tools that allow significant advances in the understanding of TBI, and also with time- and resource-efficient analysis tools for large data sets. However, it is precisely the rapidity at which advanced forms of neuroimaging evolve that has also decreased the likelihood that a single, common protocol or set of analysis tools would remain in use for many years at a time. Reevaluation of the emerging elements may need to reflect the pace at which things change to optimize certain kinds of discovery, but may also limit the amount of data that can be collected under any given version of the recommendations made by the Workgroup. Additional work is needed to consider adjustments that can be made to better “equate” measurements derived from older data. At this time, specific protocols for most of the advanced imaging techniques have not been established, and this remains an area of future need.
At present, significant challenges remain in ensuring comparability between data acquired on magnets of different manufacturers or using different scanner parameters, equipment, or software. Creation of large normative data sets would be a tremendous contribution to understanding TBI-related changes, particularly in the context of development, but this remains a challenging issue for some of the advanced modalities, given the issues previously mentioned. The acquisition of advanced imaging data can also be a significant expense, and data storage requirements should be considered, which also limits the amount of data that are currently collected for clinical trials and large multi-site studies in patients. However, technology has also aided in providing more cost-effective solutions to data acquisition, storage, and processing, and the evolution of these may result in further reduction in cost and the availability of these resources to a greater number of investigators.
Conclusions
The diversity of imaging for use in TBI is evidenced by this article. Although MRI is currently the mainstay of instrumentation for both current and emerging techniques, other technologies are also available. CT and nuclear medicine techniques have the drawback of an accompanying radiation dose, but at present offer some complementary information regarding the detection of acute subarachnoid hemorrhage and bone injury, and real-time metabolism, respectively. Electrophysiological techniques such as MEG and MSI (when combined with MRI) are already revealing new information concerning acute mild TBI, which is the most ubiquitous of all brain injuries. New and emerging hyperpolarization experiments performed on current MR scanners offer a fascinating potential insight into the world of molecular imaging that has yet to be fully realized with respect to the brain. Multimodal imaging studies are becoming more common, as different forms of imaging may provide unique insights into the nature of injury to the brain and the course of recovery. More advanced methods of integrating different imaging modalities, together with genetic information and the results of neurocognitive testing, should provide a very powerful set of tools for better understanding the pathophysiology underlying TBI, allowing for more accurate prognostication and the possibility of reliable testing for new and future interventions with ultimate improvement in patient outcomes. Currently, the Workgroup is developing a “toolbox” that allows for the establishment of potentially reproducible quantitative measures that can be used to determine the extent of injury as well as assess new interventions and therapies for the sequelae of TBI as they arise. Additionally, efforts are underway to evaluate the utility of different commercially available software and public domain tools that may be very helpful in processing data, as these may provide an excellent basis for data sharing and standardization in processing imaging data. Clearly, however, ongoing discussion is required to address some of the remaining challenges highlighted in this article, and imaging recommendations will continue to evolve.
Acknowledgments
We gratefully acknowledge the valuable assistance of Alyssa P. Ibarra with manuscript preparation.
Views expressed are those of the authors, and do not necessarily reflect those of the agencies or institutions with which they are affiliated, including the United States Department of Veterans Affairs, the United States Department of Education, the United States Department of Defense, and the National Institutes of Health. This work is not an official document, guidance, or policy of the United States government, nor should any official endorsement be inferred.
This project was jointly supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke; NIH/NINDS), the United States Department of Education/National Institute on Disability and Rehabilitation Research (DOE/NIDRR), the Department of Veterans' Affairs, and the United States Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- Aaen G.S. Holshouser B.A. Sheridan C. Colbert C. McKenney M. Kido D. Ashwal S. Magnetic resonance spectroscopy predicts outcomes for children with nonaccidental trauma. Pediatrics. 2010;125:295–303. doi: 10.1542/peds.2008-3312. [DOI] [PubMed] [Google Scholar]
- Adams J.H. Jennett B. Murray L.S. Teasdale G.M. Gennarelli T.A. Graham D.I. Neuropathological findings in disabled survivors of a head injury. J. Neurotrauma. 2011;28:701–709. doi: 10.1089/neu.2010.1733. [DOI] [PubMed] [Google Scholar]
- Alessandri B. Basciani R. Langemann H. Lyrer P. Pluess D. Landolt H. Gratz O. Chronic effects of an aminosteroid on microdialytically measured parameters after experimental middle cerebral artery occlusion in the rat. J. Clin. Neurosci. 2000;7:47–51. doi: 10.1054/jocn.1998.0139. [DOI] [PubMed] [Google Scholar]
- Anderson C.V. Bigler E.D. Blatter D.D. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain– injured patients. J. Clin. Exp. Neuropsychol. 1995;17:900–908. doi: 10.1080/01688639508402438. [DOI] [PubMed] [Google Scholar]
- Arfanakis K. Haughton V.M. Carew J.D. Rogers B.P. Dempsey R.J. Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Ashwal S. Babikian T. Gardner-Nichols J. Freier M.C. Tong K.A. Holshouser B.A. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch. Phys. Med. Rehabil. 2006a;87:S50–58. doi: 10.1016/j.apmr.2006.07.275. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B. Tong K. Serna T. Osterdock R. Gross M. Kido D. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J. Neurotrauma. 2004a;21:1539–1552. doi: 10.1089/neu.2004.21.1539. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B. Tong K. Serna T. Osterdock R. Gross M. Kido D. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr. Res. 2004b;56:630–638. doi: 10.1203/01.PDR.0000139928.60530.7D. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B.A. Shu S.K. Simmons P.L. Perkin R.M. Tomasi L.G. Knierim D.S. Sheridan C. Craig K. Andrews G.H. Hinshaw D.B. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr. Neurol. 2000;23:114–125. doi: 10.1016/s0887-8994(00)00176-4. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B.A. Tong K.A. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev. Neurosci. 2006b;28:309–326. doi: 10.1159/000094157. [DOI] [PubMed] [Google Scholar]
- Babikian T. Freier M.C. Ashwal S. Riggs M.L. Burley T. Holshouser B.A. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J. Magn. Reson. Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Babikian T. Freier M.C. Tong K.A. Nickerson J.P. Wall C.J. Holshouser B.A. Burley T. Riggs M.L. Ashwal S. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr. Neurol. 2005;33:184–194. doi: 10.1016/j.pediatrneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bagley L.J. McGowan J.C. Grossman R.I. Sinson G. Kotapka M. Lexa F.J. Berlin J.A. McIntosh T.K. Magnetization transfer imaging of traumatic brain injury. J. Magn. Reson. Imaging. 2000;11:1–8. doi: 10.1002/(sici)1522-2586(200001)11:1<1::aid-jmri1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Barker P. Bizzi A. De Stefano N. Gullapalli R. Lin D. Clinical MR Spectroscopy: Techniques and Applications. Cambridge University Press; New York: 2010. Spectral analysis methods, quantitation and common artifacts; pp. 39–42. [Google Scholar]
- Barker P.B. Soher B.J. Blackband S.J. Chatham J.C. Mathews V.P. Bryan R.N. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6:89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.H. Ditchfield M. Maller J.J. Catroppa C. Godfrey C. Rosenfeld J.V. Kean M.J. Anderson V.A. Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 2011;24:137–143. doi: 10.1016/j.ijdevneu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bendlin B.B. Ries M.L. Lazar M. Alexander A.L. Dempsey R.J. Rowley H.A. Sherman J.E. Johnson S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson R.R. Meda S.A. Vasudevan S. Kou Z. Govindarajan K.A. Hanks R.A. Millis S.R. Makki M. Latif Z. Coplin W. Meythaler J. Haacke E.M. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J. Neurotrauma. 2007;24:446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Benzinger T.L. Brody D. Cardin S. Curley K.C. Mintun M.A. Mun S.K. Wong K.H. Wrathall J.R. Blast-related brain injury: imaging for clinical and research applications: report of the 2008 St. Louis workshop. J. Neurotrauma. 2009;26:2127–2144. doi: 10.1089/neu.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeson A.G. Lundin R. Parkinson R.B. Tate D.F. Victoroff J. Hopkins R.O. Bigler E.D. Clinical rating of cortical atrophy and cognitive correlates following traumatic brain injury. Clin. Neuropsychol. 2004;18:509–520. doi: 10.1080/1385404049052414. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Neuroimaging in pediatric traumatic head injury: diagnostic considerations and relationships to neurobehavioral outcome. J. Head Trauma Rehabil. 1999;14:406–423. doi: 10.1097/00001199-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Abildskov T.J. Wilde E.A. McCauley S.R. Li X. Merkley T.L. Fearing M.A. Newsome M.R. Scheibel R.S. Hunter J.V. Chu Z. Levin H.S. Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage. 2010a;50:1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. McCauley S.R. Wu T.C. Yallampalli R. Shah S. MacLeod M. Chu Z. Hunter J.V. Clifton G.L. Levin H.S. Wilde E.A. The temporal stem in traumatic brain injury: preliminary findings. Brain Imaging Behav. 2010b;4:270–282. doi: 10.1007/s11682-010-9105-0. [DOI] [PubMed] [Google Scholar]
- Bonte F.J. Harris T.S. Hynan L.S. Bigio E.H. White C.L., 3rd. Tc-99m HMPAO SPECT in the differential diagnosis of the dementias with histopathologic confirmation. Clin. Nucl. Med. 2006;31:376–378. doi: 10.1097/01.rlu.0000222736.81365.63. [DOI] [PubMed] [Google Scholar]
- Brenner T. Freier M.C. Holshouser B.A. Burley T. Ashwal S. Predicting neuropsychologic outcome after traumatic brain injury in children. Pediatr. Neurol. 2003;28:104–114. doi: 10.1016/s0887-8994(02)00491-5. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Woodward J.J. Myseros J. Choi S.C. Ward J.D. Marmarou A. Young H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Buxton R.B. Quantifying CBF with arterial spin labeling. J. Magn. Reson. Imaging. 2005;22:723–726. doi: 10.1002/jmri.20462. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K. Leemans A. Geurts M. Taymans T. Linden C.V. Smits–Engelsman B.C. Sunaert S. Swinnen S.P. Brain–behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Hum. Brain Mapp. 2010a;31:992–1002. doi: 10.1002/hbm.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K. Leemans A. Geurts M. Taymans T. Vander Linden C. Smits–Engelsman B.C. Sunaert S. Swinnen S.P. Brain–behavior relationships in young traumatic brain injury patients: fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia. 2010b;48:1472–1482. doi: 10.1016/j.neuropsychologia.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D. Adali T. Pearlson G.D. van Zijl P.C. Pekar J.J. Independent component analysis of fMRI data in the complex domain. Magn. Reson. Med. 2002;48:180–192. doi: 10.1002/mrm.10202. [DOI] [PubMed] [Google Scholar]
- Cazalis F. Feydy A. Valabregue R. Pelegrini–Issac M. Pierot L. Azouvi P. fMRI study of problem-solving after severe traumatic brain injury. Brain Inj. 2006;20:1019–1028. doi: 10.1080/02699050600664384. [DOI] [PubMed] [Google Scholar]
- Chastain C.A. Oyoyo U.E. Zipperman M. Joo E. Ashwal S. Shutter L.A. Tong K.A. Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. J. Neurotrauma. 2009;26:1183–1196. doi: 10.1089/neu.2008.0650. [DOI] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Collie A. McCrory P. Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J. Neurol. Neurosurg. Psychiatry. 2007;78:1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Frey S. Petrides M. Worsley K. Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Petrides M. Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch. Gen. Psychiatry. 2008;65:81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- Chiu Wong S.B. Chapman S.B. Cook L.G. Anand R. Gamino J.F. Devous M.D., Sr. A SPECT study of language and brain reorganization three years after pediatric brain injury. Prog. Brain Res. 2006;157:173–185. doi: 10.1016/s0079-6123(06)57011-6. [DOI] [PubMed] [Google Scholar]
- Chou Y.Y. Lepore N. Saharan P. Madsen S.K. Hua X. Jack C.R. Shaw L.M. Trojanowski J.Q. Weiner M.W. Toga A.W. Thompson P.M. Alzheimer's Disease Neuroimaging I. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiol. Aging. 2010;31:1386–1400. doi: 10.1016/j.neurobiolaging.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou C. DeLuca J. Ricker J.H. Madigan N.K. Bly B.M. Lange G. Kalnin A.J. Liu W.C. Steffener J. Diamond B.J. Ni A.C. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J.P. Imaging after brain injury. Br. J. Anaesth. 2007;99:49–60. doi: 10.1093/bja/aem141. [DOI] [PubMed] [Google Scholar]
- Danielsen E.R. Henriksen O. Absolute quantitative proton NMR spectroscopy based on the amplitude of the local water suppression pulse. Quantification of brain water and metabolites. NMR Biomed. 1994;7:311–318. doi: 10.1002/nbm.1940070704. [DOI] [PubMed] [Google Scholar]
- Detre J.A. Alsop D.C. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur. J. Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Ding K. Marquez de la Plata C. Wang J.Y. Mumphrey M. Moore C. Harper C. Madden C.J. McColl R. Whittemore A. Devous M.D. Diaz–Arrastia R. Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J. Neurotrauma. 2008;25:1433–1440. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousset V. Grossman R.I. Ramer K.N. Schnall M.D. Young L.H. Gonzalez–Scarano F. Lavi E. Cohen J.A. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology. 1992;182:483–491. doi: 10.1148/radiology.182.2.1732968. [DOI] [PubMed] [Google Scholar]
- Duckworth J.L. Stevens R.D. Imaging brain trauma. Curr. Opin. Crit. Care. 2010;11:92–97. doi: 10.1097/MCC.0b013e3283374900. [DOI] [PubMed] [Google Scholar]
- Ducreux D. Huynh I. Fillard P. Renoux J. Petit–Lacour M.C. Marsot–Dupuch K. Lasjaunias P. Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology. 2005;47:604–608. doi: 10.1007/s00234-005-1389-1. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Gean A.D. Haacke E.M. Hicks R. Wintermark M. Mukherjee P. Brody D. Latour L. Riedy G. Common Data Elements Neuroimaging Working Group Members PWGM. Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1661–1666. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Holshouser B. Hunter J.V. Tong K. Common data elements for neuroimaging of traumatic brain injury: pediatric consideration. J. Neurotrauma. 2011 doi: 10.1089/neu.2011.1927. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing–Cobbs L. Prasad M.R. Swank P. Kramer L. Cox C.S., Jr. Fletcher J.M. Barnes M. Zhang X. Hasan K.M. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearing M.A. Bigler E.D. Wilde E.A. Johnson J.L. Hunter J.V. Xiaoqi L. Hanten G. Levin H.S. Morphometric MRI findings in the thalamus and brainstem in children after moderate to severe traumatic brain injury. J. Child Neurol. 2008;23:729–737. doi: 10.1177/0883073808314159. [DOI] [PubMed] [Google Scholar]
- Forbes M.L. Hendrich K.S. Kochanek P.M. Williams D.S. Schiding J.K. Wisniewski S.R. Kelsey S.F. DeKosky S.T. Graham S.H. Marion D.W. Ho C. Assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact by perfusion magnetic resonance imaging using arterial spin-labeling in rats. J. Cereb. Blood Flow Metab. 1997a;17:865–874. doi: 10.1097/00004647-199708000-00005. [DOI] [PubMed] [Google Scholar]
- Forbes M.L. Hendrich K.S. Schiding J.K. Williams D.S. Ho C. DeKosky S.T. Marion D.W. Kochanek P.M. Perfusion MRI assessment of cerebral blood flow and CO2 reactivity after controlled cortical impact in rats. Adv. Exp. Med. Biol. 1997b;411:7–12. doi: 10.1007/978-1-4615-5865-1_2. [DOI] [PubMed] [Google Scholar]
- Frahm J. Bruhn H. Gyngell M.L. Merboldt K.D. Hanicke W. Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magn. Reson. Med. 1989;11:47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]
- Friedman L. Glover G.H. Report on a multicenter fMRI quality assurance protocol. J. Magn. Reson. Imaging. 2006;23:827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- Friedman L. Stern H. Brown G.G. Mathalon D.H. Turner J. Glover G.H. Gollub R.L. Lauriello J. Lim K.O. Cannon T. Greve D.N. Bockholt H.J. Belger A. Mueller B. Doty M.J. He J. Wells W. Smyth P. Pieper S. Kim S. Kubicki M. Vangel M. Potkin S.G. Test–retest and between-site reliability in a multicenter fMRI study. Hum. Brain Mapp. 2008;29:958–972. doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara E. Schwartz M.L. Gao F. Black S.E. Levine B. Ventral frontal cortex functions and quantified MRI in traumatic brain injury. Neuropsychologia. 2008;46:461–474. doi: 10.1016/j.neuropsychologia.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D. Baxter L. Roundy N. Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D. Prigatano G.P. Deep white matter volume loss and social reintegration after traumatic brain injury in children. J. Head Trauma Rehabil. 2010;25:15–22. doi: 10.1097/HTR.0b013e3181c39960. [DOI] [PubMed] [Google Scholar]
- Gallagher C.N. Hutchinson P.J. Pickard J.D. Neuroimaging in trauma. Curr. Opin. Neurol. 2007;20:403–409. doi: 10.1097/WCO.0b013e32821b987b. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Corkill R.G. Cadoux–Hudson T.A. Rajagopalan B. Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123:2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Corkill R.G. Blamire A.M. Rajagopalan B. Manners D.N. Young J.D. Styles P. Cadoux–Hudson T.A. Altered cellular metabolism following traumatic brain injury: a magnetic resonance spectroscopy study. J. Neurotrauma. 2001;18:231–240. doi: 10.1089/08977150151070838. [DOI] [PubMed] [Google Scholar]
- Gasparovic C. Yeo R. Mannell M. Ling J. Elgie R. Phillips J. Doezema D. Mayer A.R. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J. Neurotrauma. 2009;26:1635–1643. doi: 10.1089/neu.2009.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber D.J. Weintraub A.H. Cusick C.P. Ricci P.E. Whiteneck G.G. Magnetic resonance imaging of traumatic brain injury: relationship of T2*SE and T2GE to clinical severity and outcome. Brain Inj. 2004;18:1083–1097. doi: 10.1080/02699050410001672341. [DOI] [PubMed] [Google Scholar]
- Govind V. Gold S. Kaliannan K. Saigal G. Falcone S. Arheart K.L. Harris L. Jagid J. Maudsley A.A. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen W.B. Buitelaar J.K. van der Gaag R.J. Zwiers M.P. Pervasive microstructural abnormalities in autism: a DTI study. J. Psychiatry Neurosci. 2010;36:32–40. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E.M. Duhaime A.C. Gean A.D. Riedy G. Wintermark M. Mukherjee P. Brody D.L. DeGraba T. Duncan T.D. Elovic E. Hurley R. Latour L. Smirniotopoulos J.G. Smith D.H. Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging. 2010;32:516–543. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- Haacke E.M. Xu Y. Cheng Y.C. Reichenbach J.R. Susceptibility weighted imaging (SWI) Magn. Reson. Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- Hattingen E. Raab P. Franz K. Lanfermann H. Setzer M. Gerlach R. Zanella F.E. Pilatus U. Prognostic value of choline and creatine in WHO grade II gliomas. Neuroradiology. 2008;50:759–767. doi: 10.1007/s00234-008-0409-3. [DOI] [PubMed] [Google Scholar]
- Hattori N. Huang S.C. Wu H.M. Liao W. Glenn T.C. Vespa P.M. Phelps M.E. Hovda D.A. Bergsneider M. Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J. Nucl. Med. 2004;45:775–783. [PubMed] [Google Scholar]
- Hattori N. Huang S.C. Wu H.M. Yeh E. Glenn T.C. Vespa P.M. McArthur D. Phelps M.E. Hovda D.A. Bergsneider M. Correlation of regional metabolic rates of glucose with glasgow coma scale after traumatic brain injury. J. Nucl. Med. 2003;44:1709–1716. [PubMed] [Google Scholar]
- Hendrich K.S. Kochanek P.M. Williams D.S. Schiding J.K. Marion D.W. Ho C. Early perfusion after controlled cortical impact in rats: quantification by arterial spin-labeled MRI and the influence of spin-lattice relaxation time heterogeneity. Magn. Reson. Med. 1999;42:673–681. doi: 10.1002/(sici)1522-2594(199910)42:4<673::aid-mrm8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hillary F.G. Steffener J. Biswal B.B. Lange G. DeLuca J. Ashburner J. Functional magnetic resonance imaging technology and traumatic brain injury rehabilitation: guidelines for methodological and conceptual pitfalls. J. Head Trauma Rehabil. 2002;17:411–430. doi: 10.1097/00001199-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Himanen L. Portin R. Isoniemi H. Helenius H. Kurki T. Tenovuo O. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005;19:93–100. doi: 10.1080/02699050410001720031. [DOI] [PubMed] [Google Scholar]
- Holshouser B.A. Ashwal S. Luh G.Y. Shu S. Kahlon S. Auld K.L. Tomasi L.G. Perkin R.M. Hinshaw D.B., Jr. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology. 1997;202:487–496. doi: 10.1148/radiology.202.2.9015079. [DOI] [PubMed] [Google Scholar]
- Holshouser B.A. Tong K.A. Ashwal S. Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR Am. J. Neuroradiol. 2005;26:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Hua X. Hibar D.P. Lee S. Toga A.W. Jack C.R., Jr. Weiner M.W. Thompson P.M. Alzheimer's Disease Neuroimaging I. Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol. Aging. 2010;31:1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X. Theilmann R.J. Robb A. Angeles A. Nichols S. Drake A. D'Andrea J. Levy M. Holland M. Song T. Ge S. Hwang E. Yoo K. Cui L. Baker D.G. Trauner D. Coimbra R. Lee R.R. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma. 2009;26:1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Huisman T.A. Schwamm L.H. Schaefer P.W. Koroshetz W.J. Shetty–Alva N. Ozsunar Y. Wu O. Sorensen A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- Hunter J.V. Thornton R.J. Wang Z.J. Levin H.S. Roberson G. Brooks W.M. Swank P.R. Late proton MR spectroscopy in children after traumatic brain injury: correlation with cognitive outcomes. AJNR Am. J. Neuroradiol. 2005;26:482–488. [PMC free article] [PubMed] [Google Scholar]
- Huppi P.S. Posse S. Lazeyras F. Burri R. Bossi E. Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr. Res. 1991;30:574–578. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- Hutchinson E. Pulsipher D. Dabbs K. Myers y Gutierrez A. Sheth R. Jones J. Seidenberg M. Meyerand E. Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–214. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M. Li B.S. Rusinek H. Babb J.S. Grossman R.I. Gonen O. Diffusely elevated cerebral choline and creatine in relapsing–remitting multiple sclerosis. Magn. Reson. Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- James G.A. Kelley M.E. Craddock R.C. Holtzheimer P.E. Dunlop B.W. Nemeroff C.B. Mayberg H.S. Hu X.P. Exploratory structural equation modeling of resting-state fMRI: applicability of group models to individual subjects. Neuroimage. 2009;45:778–787. doi: 10.1016/j.neuroimage.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen K.J. Anderson B. Steinberg F.L. Kelso J.A. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am. J. Neuroradiol. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Kato T. Nakayama N. Yasokawa Y. Okumura A. Shinoda J. Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J. Neurotrauma. 2007;24:919–926. doi: 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- Kaushik S.S. Cleveland Z.I. Cofer G.P. Metz G. Beaver D. Nouls J. Kraft M. Auffermann W. Wolber J. McAdams H.P. Driehuys B. Diffusion-weighted hyperpolarized (129)Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn. Reson. Med. 2010;65:1154–1165. doi: 10.1002/mrm.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Whyte J. Patel S. Avants B. Europa E. Wang J. Slattery J. Gee J.C. Coslett H.B. Detre J.A. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J. Neurotrauma. 2010;27:1399–1411. doi: 10.1089/neu.2009.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.H. Yoo W.K. Ko M.H. Park C.H. Kim S.T. Na D.L. Plasticity of the attentional network after brain injury and cognitive rehabilitation. Neurorehabil. Neural Repair. 2009;23:468–477. doi: 10.1177/1545968308328728. [DOI] [PubMed] [Google Scholar]
- Kimura H. Meaney D.F. McGowan J.C. Grossman R.I. Lenkinski R.E. Ross D.T. McIntosh T.K. Gennarelli T.A. Smith D.H. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: characterization by magnetization transfer ratio with histopathologic correlation. J. Comput. Assist. Tomogr. 1996;20:540–546. doi: 10.1097/00004728-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Kochanek P.M. Hendrich K.S. Dixon C.E. Schiding J.K. Williams D.S. Ho C. Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma. 2002;19:1029–1037. doi: 10.1089/089771502760341947. [DOI] [PubMed] [Google Scholar]
- Kochanek P.M. Hendrich K.S. Jackson E.K. Wisniewski S.R. Melick J.A. Shore P.M. Janesko K.L. Zacharia L. Ho C. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2005;25:1596–1612. doi: 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- Korn A. Golan H. Melamed I. Pascual–Marqui R. Friedman A. Focal cortical dysfunction and blood–brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- Kou Z. Wu Z. Tong K.A. Holshouser B. Benson R.R. Hu J. Haacke E.M. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J. Head Trauma Rehabil. 2010;25:267–282. doi: 10.1097/HTR.0b013e3181e54793. [DOI] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kreis R. Ernst T. Ross B. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J. Magn. Reson. 1993;102:9–19. [Google Scholar]
- Kumar R. Gupta R.K. Rao S.B. Chawla S. Husain M. Rathore R.K. Magnetization transfer and T2 quantitation in normal appearing cortical gray matter and white matter adjacent to focal abnormality in patients with traumatic brain injury. Magn. Reson. Imaging. 2003;21:893–899. doi: 10.1016/s0730-725x(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Kumar R. Husain M. Gupta R.K. Hasan K.M. Haris M. Agarwal A.K. Pandey C.M. Narayana P.A. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J. Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Laatsch L. Little D. Thulborn K. Changes in fMRI following cognitive rehabilitation in severe traumatic brain injury: A case study. Rehabil. Psychol. 2004a;49:262–267. [Google Scholar]
- Laatsch L.K. Thulborn K.R. Krisky C.M. Shobat D.M. Sweeney J.A. Investigating the neurobiological basis of cognitive rehabilitation therapy with fMRI. Brain Inj. 2004b;18:957–974. doi: 10.1080/02699050410001672369. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Wilde E.A. Chu Z. Yallampalli R. Hanten G.R. Li X. Chia J. Vasquez A.C. Hunter J.V. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 2008;23:197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Kovacevic N. Nica E.I. Cheung G. Gao F. Schwartz M.L. Black S.E. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Lewine J.D. Davis J.T. Bigler E.D. Thoma R. Hill D. Funke M. Sloan J.H. Hall S. Orrison W.W. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 2007;22:141–155. doi: 10.1097/01.HTR.0000271115.29954.27. [DOI] [PubMed] [Google Scholar]
- Lewine J.D. Davis J.T. Sloan J.H. Kodituwakku P.W. Orrison W.W., Jr. Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. Am. J. Neuroradiol. 1999;20:857–866. [PMC free article] [PubMed] [Google Scholar]
- Lipton M.L. Gellella E. Lo C. Gold T. Ardekani B.A. Shifteh K. Bello J.A. Branch C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Lovell M.R. Pardini J.E. Welling J. Collins M.W. Bakal J. Lazar N. Roush R. Eddy W.F. Becker J.T. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61:352–359. doi: 10.1227/01.NEU.0000279985.94168.7F. discussion 359–360. [DOI] [PubMed] [Google Scholar]
- Makoroff K.L. Cecil K.M. Care M. Ball W.S., Jr. Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr. Radiol. 2005;35:668–676. doi: 10.1007/s00247-005-1441-7. [DOI] [PubMed] [Google Scholar]
- Mamere A.E. Saraiva L.A. Matos A.L. Carneiro A.A. Santos A.C. Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. AJNR Am. J. Neuroradiol. 2009;30:947–952. doi: 10.3174/ajnr.A1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata C.D. Garces J. Shokri Kojori E. Grinnan J. Krishnan K. Pidikiti R. Spence J. Devous M.D., Sr. Moore C. McColl R. Madden C. Diaz–Arrastia R. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch. Neurol. 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruishi M. Miyatani M. Nakao T. Muranaka H. Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: a functional magnetic resonance imaging study. J. Neurol. Neurosurg. Psychiatry. 2007;78:168–173. doi: 10.1136/jnnp.2006.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley A.A. Darkazanli A. Alger J.R. Hall L.O. Schuff N. Studholme C. Yu Y. Ebel A. Frew A. Goldgof D. Gu Y. Pagare R. Rousseau F. Sivasankaran K. Soher B.J. Weber P. Young K. Zhu X. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19:492–503. doi: 10.1002/nbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R. Ling J. Mannell M.V. Gasparovic C. Phillips J.P. Doezema D. Reichard R. Yeo R.A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R. Mannell M.V. Ling J. Gasparovic C. Yeo R.A. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011 doi: 10.1002/hbm.21151. (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W. Saykin A.J. Flashman L.A. Sparling M.B. Johnson S.C. Guerin S.J. Mamourian A.C. Weaver J.B. Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Sparling M.B. Flashman L.A. Guerin S.J. Mamourian A.C. Saykin A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. Wilde E.A. Bigler E.D. Chu Z. Yallampalli R. Oni M.B. Wu T. Ramos M.A. Pedroza C. Vasquez A.C. Hunter J.V. Levin H. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J. Neurotrauma. 2011;28:503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J.C. Yang J.H. Plotkin R.C. Grossman R.I. Umile E.M. Cecil K.M. Bagley L.J. Magnetization transfer imaging in the detection of injury associated with mild head trauma. AJNR Am. J. Neuroradiol. 2000;21:875–880. [PMC free article] [PubMed] [Google Scholar]
- McLean M.A. Woermann F.G. Barker G.J. Duncan J.S. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magn. Reson. Med. 2000;44:401–411. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Merkley T.L. Bigler E.D. Wilde E.A. McCauley S.R. Hunter J.V. Levin H.S. Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. J. Neurotrauma. 2008;25:1343–1345. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metting Z. Rodiger L.A. de Jong B.M. Stewart R.E. Kremer B.P. van der Naalt J. Acute cerebral perfusion CT abnormalities associated with posttraumatic amnesia in mild head injury. J. Neurotrauma. 2010;27:2183–2189. doi: 10.1089/neu.2010.1395. [DOI] [PubMed] [Google Scholar]
- Miles L. Grossman R.I. Johnson G. Babb J.S. Diller L. Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Mueller S.G. Weiner M.W. Thal L.J. Petersen R.C. Jack C.R. Jagust W. Trojanowski J.Q. Toga A.W. Beckett L. Ways toward an early diagnosis in Alzheimer's disease: the Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz Maniega S. Cvoro V. Armitage P.A. Marshall I. Bastin M.E. Wardlaw J.M. Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke. 2008;39:2467–2469. doi: 10.1161/STROKEAHA.107.507020. [DOI] [PubMed] [Google Scholar]
- Newcombe V.F. Williams G.B. Nortje J. Bradley P.G. Chatfield D.A. Outtrim J.G. Harding S.G. Coles J.P. Maiya B. Gillard J.H. Hutchinson P.J. Pickard J.D. Carpenter T.A. Menon D.K. Concordant biology underlies discordant imaging findings: diffusivity behaves differently in grey and white matter post acute neurotrauma. Acta Neurochirurg. 2008;102:247–251. doi: 10.1007/978-3-211-85578-2_47. [DOI] [PubMed] [Google Scholar]
- Newcombe V.F. Williams G.B. Nortje J. Bradley P.G. Harding S.G. Smielewski P. Coles J.P. Maiya B. Gillard J.H. Hutchinson P.J. Pickard J.D. Carpenter T.A. Menon D.K. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br. J. Neurosurg. 2007;21:340–348. doi: 10.1080/02688690701400882. [DOI] [PubMed] [Google Scholar]
- Newsome M.R. Scheibel R.S. Hunter J.V. Wang Z.J. Chu Z. Li X. Levin H.S. Brain activation during working memory after traumatic brain injury in children. Neurocase. 2007a;13:16–24. doi: 10.1080/13554790601186629. [DOI] [PubMed] [Google Scholar]
- Newsome M.R. Scheibel R.S. Steinberg J.L. Troyanskaya M. Sharma R.G. Rauch R.A. Li X. Levin H.S. Working memory brain activation following severe traumatic brain injury. Cortex. 2007b;43:95–111. doi: 10.1016/s0010-9452(08)70448-9. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E. Bizzi A. Di Salle F. De Stefano N. Filippi M. Basic concepts of advanced MRI techniques. J. Neurol. Sci. 2008;29(Suppl. 3):290–295. doi: 10.1007/s10072-008-1001-7. [DOI] [PubMed] [Google Scholar]
- Panigrahy A. Nelson M.D., Jr. Bluml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr. Radiol. 2010;40:3–30. doi: 10.1007/s00247-009-1450-z. [DOI] [PubMed] [Google Scholar]
- Perlbarg V. Puybasset L. Tollard E. Lehericy S. Benali H. Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum. Brain Mapp. 2009;30:3924–3933. doi: 10.1002/hbm.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein W.M. Cole M.A. Demery J.A. Seignourel P.J. Dixit N.K. Larson M.J. Briggs R.W. Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. J. Int. Neuropsychol. Soc. 2004;10:724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Pouwels P.J. Brockmann K. Kruse B. Wilken B. Wick M. Hanefeld F. Frahm J. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr. Res. 1999;46:474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rademacher J. Engelbrecht V. Burgel U. Freund H. Zilles K. Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. Neuroimage. 1999;9:393–406. doi: 10.1006/nimg.1998.0416. [DOI] [PubMed] [Google Scholar]
- Rasmussen I.A. Xu J. Antonsen I.K. Brunner J. Skandsen T. Axelson D.E. Berntsen E.M. Lydersen S. Haberg A. Simple dual tasking recruits prefrontal cortices in chronic severe traumatic brain injury patients, but not in controls. J. Neurotrauma. 2008;25:1057–1070. doi: 10.1089/neu.2008.0520. [DOI] [PubMed] [Google Scholar]
- Reichenbach J.R. Venkatesan R. Schillinger D.J. Kido D.K. Haacke E.M. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- Risacher S.L. Shen L. West J.D. Kim S. McDonald B.C. Beckett L.A. Harvey D.J. Jack C.R., Jr. Weiner M.W. Saykin A.J. Alzheimer's Disease Neuroimaging I. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol. Aging. 2010;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C.L. Hendrich K.S. Kochanek P.M. Jackson E.K. Melick J.A. Graham S.H. Marion D.W. Williams D.S. Ho C. Assessment of 2-chloroadenosine treatment after experimental traumatic brain injury in the rat using arterial spin-labeled MRI: a preliminary report. Acta Neurochirurg. 2000;76:187–189. doi: 10.1007/978-3-7091-6346-7_37. [DOI] [PubMed] [Google Scholar]
- Rocca M.A. Falini A. Colombo B. Scotti G. Comi G. Filippi M. Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann. Neurol. 2002;51:330–339. doi: 10.1002/ana.10120. [DOI] [PubMed] [Google Scholar]
- Ross B.D. Ernst T. Kreis R. Haseler L.J. Bayer S. Danielsen E. Bluml S. Shonk T. Mandigo J.C. Caton W. Clark C. Jensen S.W. Lehman N.L. Arcinue E. Pudenz R. Shelden C.H. 1H MRS in acute traumatic brain injury. J. Magn. Reson. Imaging. 1998;8:829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- Salmond C.H. Menon D.K. Chatfield D.A. Williams G.B. Pena A. Sahakian B.J. Pickard J.D. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Scheibel R.S. Newsome M.R. Steinberg J.L. Pearson D.A. Rauch R.A. Mao H. Troyanskaya M. Sharma R.G. Levin H.S. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabil. Neural Repair. 2007;21:36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- Scheibel R.S. Pearson D.A. Faria L.P. Kotrla K.J. Aylward E. Bachevalier J. Levin H.S. An fMRI study of executive functioning after severe diffuse TBI. Brain Inj. 2003;17:919–930. doi: 10.1080/0269905031000110472. [DOI] [PubMed] [Google Scholar]
- Scheid R. Preul C. Gruber O. Wiggins C. von Cramon D.Y. Diffuse axonal injury associated with chronic traumatic brain injury, evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am. J. Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Schmitz T.W. Rowley H.A. Kawahara T.N. Johnson S.C. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia. 2006;44:762–773. doi: 10.1016/j.neuropsychologia.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Schonberger M. Ponsford J. Reutens D. Beare R. O'Sullivan R. The Relationship between age, injury severity, and MRI findings after traumatic brain injury. J. Neurotrauma. 2009;26:2157–2167. doi: 10.1089/neu.2009.0939. [DOI] [PubMed] [Google Scholar]
- Schuff N. Ezekiel F. Gamst A.C. Amend D.L. Capizzano A.A. Maudsley A.A. Weiner M.W. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn. Reson. Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal V. Delproposto Z. Haacke E.M. Tong K.A. Wycliffe N. Kido D.K. Xu Y. Neelavalli J. Haddar D. Reichenbach J.R. Clinical applications of neuroimaging with susceptibility-weighted imaging. Journal of Magnetic Resonance Imaging. 2005;22:439–450. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- Serra–Grabulosa J.M. Junque C. Verger K. Salgado–Pineda P. Maneru C. Mercader J.M. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2005;76:129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M. Stouter J. Hart T. Nakase–Richardson R. Olivier J. Manning E. Yablon S.A. Computed tomography findings and early cognitive outcome after traumatic brain injury. Brain Inj. 2006;20:997–1005. doi: 10.1080/02699050600677055. [DOI] [PubMed] [Google Scholar]
- Sidaros A. Engberg A.W. Sidaros K. Liptrot M.G. Herning M. Petersen P. Paulson O.B. Jernigan T.L. Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Sidaros A. Skimminge A. Liptrot M.G. Sidaros K. Engberg A.W. Herning M. Paulson O.B. Jernigan T.L. Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44:1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Sigmund G.A. Tong K.A. Nickerson J.P. Wall C.J. Oyoyo U. Ashwal S. Multimodality comparison of neuroimaging in pediatric traumatic brain injury. Pediatr. Neurol. 2007;36:217–226. doi: 10.1016/j.pediatrneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Simmons A. Westman E. Muehlboeck S. Mecocci P. Vellas B. Tsolaki M. Kloszewska I. Wahlund L.O. Soininen H. Lovestone S. Evans A. Spenger C. The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer's disease: experience from the first 24 months. Int. J. Geriatr. Psychiatry. 2011;26:75–82. doi: 10.1002/gps.2491. [DOI] [PubMed] [Google Scholar]
- Sinson G. Bagley L.J. Cecil K.M. Torchia M. McGowan J.C. Lenkinski R.E. McIntosh T.K. Grossman R.I. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. Am. J. Neuroradiol. 2001;22:143–151. [PMC free article] [PubMed] [Google Scholar]
- Soeda A. Nakashima T. Okumura A. Kuwata K. Shinoda J. Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Soustiel J.F. Mahamid E. Goldsher D. Zaaroor M. Perfusion-CT for early assessment of traumatic cerebral contusions. Neuroradiology. 2008;50:189–196. doi: 10.1007/s00234-007-0337-7. [DOI] [PubMed] [Google Scholar]
- Spanos G.K. Wilde E.A. Bigler E.D. Cleavinger H.B. Fearing M.A. Levin H.S. Li X. Hunter J.V. Cerebellar atrophy after moderate-to-severe pediatric traumatic brain injury. Am. J. Neuroradiol. 2007;28:537–542. [PMC free article] [PubMed] [Google Scholar]
- Strangman G.E. O'Neil–Pirozzi T.M. Goldstein R. Kelkar K. Katz D.I. Burke D. Rauch S.L. Savage C.R. Glenn M.B. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch. Phys. Med. Rehabil. 2008;89:974–981. doi: 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Strangman G.E. O'Neil–Pirozzi T.M. Supelana C. Goldstein R. Katz D.I. Glenn M.B. Regional brain morphometry predicts memory rehabilitation outcome after traumatic brain injury. Front. Hum. Neurosci. 2010;4:182. doi: 10.3389/fnhum.2010.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G.M. O'Neil–Pirozzi T.M. Burke D. Cristina D. Goldstein R. Rauch S.L. Savage C.R. Glenn M.B. Functional neuroimaging and cognitive rehabilitation for people with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2005;84:62–75. doi: 10.1097/01.phm.0000150787.26860.12. [DOI] [PubMed] [Google Scholar]
- Suskauer S.J. Huisman T.A. Neuroimaging in pediatric traumatic brain injury: current and future predictors of functional outcome. Dev. Disabil. Res. Rev. 2009;15:117–123. doi: 10.1002/ddrr.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. Ge Y. Sodickson D.K. Miles L. Zhou Y. Reaume J. Grossman R.I. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260:831–840. doi: 10.1148/radiol.11110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond V.A. Hicks R. Gleason T. Miller A.C. Szuflita N. Orman J. Schwab K. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch. Phys. Med. Rehabil. 2010;91:1633–1636. doi: 10.1016/j.apmr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F. Carlesimo G.A. Di Paola M. Petrides M. Fera F. Bonanni R. Formisano R. Pasqualetti P. Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins O. Feintuch A. Benifla M. Cohen A. Friedman A. Shelef I. Blood–brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc. Psychiatry Neurol. 2011 doi: 10.1155/2011/765923. (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K.A. Ashwal S. Holshouser B.A. Nickerson J.P. Wall C.J. Shutter L.A. Osterdock R.J. Haacke E.M. Kido D. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann. Neurol. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- Tong K.A. Ashwal S. Holshouser B.A. Shutter L.A. Herigault G. Haacke E.M. Kido D.K. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- Trivedi M.A. Ward M.A. Hess T.M. Gale S.D. Dempsey R.J. Rowley H.A. Johnson S.C. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. J. Neurotrauma. 2007;24:766–771. doi: 10.1089/neu.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshibanda L. Vanhaudenhuyse A. Galanaud D. Boly M. Laureys S. Puybasset L. Magnetic resonance spectroscopy and diffusion tensor imaging in coma survivors: promises and pitfalls. Prog. Brain Res. 2009;177:215–229. doi: 10.1016/S0079-6123(09)17715-4. [DOI] [PubMed] [Google Scholar]
- Van Boven R. Harrington G. Hackney D. Ebel A. Gauger G. Bremner J. D'Esposito M. Detre J. Haacke E. Jack C., Jr. Jagust W. Le Bihan D. Mathis C. Mueller S. Mukherjee P. Schuff N. Chen A. Weiner M. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. J. Rehabil. Res. Dev. 2009;46:717–757. doi: 10.1682/jrrd.2008.12.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall T.D. Cascella N.G. Rao V. Pearlson G.D. Gordon B. Schretlen D.J. A morphometric analysis of neuroanatomic abnormalities in traumatic brain injury. J. Neuropsychiatry Clin. Neurosc. 2010;22:173–181. doi: 10.1176/jnp.2010.22.2.173. [DOI] [PubMed] [Google Scholar]
- Warner M.A. Marquez de la Plata C. Spence J. Wang J.Y. Harper C. Moore C. Devous M. Diaz–Arrastia R. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J. Neurotrauma. 2010a;27:2121–2130. doi: 10.1089/neu.2010.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A. Youn T.S. Davis T. Chandra A. Marquez de la Plata C. Moore C. Harper C. Madden C.J. Spence J. McColl R. Devous M. King R.D. Diaz–Arrastia R. Regionally selective atrophy after traumatic axonal injury. Arch. Neurol. 2010b;67:1336–1344. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskoff R.M. Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magn. Reson. Med. 1996;36:643–645. doi: 10.1002/mrm.1910360422. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Bigler E.D. Gandhi P.V. Lowry C.M. Blatter D.D. Brooks J. Ryser D.K. Alcohol abuse and traumatic brain injury: quantitative magnetic resonance imaging and neuropsychological outcome. J. Neurotrauma. 2004;21:137–147. doi: 10.1089/089771504322778604. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Bigler E.D. Hunter J.V. Fearing M.A. Scheibel R.S. Newsome M.R. Johnson J.L. Bachevalier J. Li X. Levin H.S. Hippocampus, amygdala, and basal ganglia morphometrics in children after moderate-to-severe traumatic brain injury. Dev. Med. Child Neurol. 2007;49:294–299. doi: 10.1111/j.1469-8749.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Bigler E.D. Pedroza C. Ryser D.K. Post-traumatic amnesia predicts long-term cerebral atrophy in traumatic brain injury. Brain Inj. 2006a;20:695–699. doi: 10.1080/02699050600744079. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Chu Z. Bigler E.D. Hunter J.V. Fearing M.A. Hanten G. Newsome M.R. Scheibel R.S. Li X. Levin H.S. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2006b;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Hunter J.V. Newsome M.R. Scheibel R.S. Bigler E.D. Johnson J.L. Fearing M.A. Cleavinger H.B. Li X. Swank P.R. Pedroza C. Roberson G.S. Bachevalier J. Levin H.S. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J. Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. McCauley S.R. Hunter J.V. Bigler E.D. Chu Z. Wang Z.J. Hanten G.R. Troyanskaya M. Yallampalli R. Li X. Chia J. Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. Ramos M.A. Yallampalli R. Bigler E.D. McCauley S.R. Chu Z. Wu T.C. Hanten G. Scheibel R.S. Li X. Vasquez A.C. Hunter J.V. Levin H.S. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 2010;35:333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark M. Chiolero R. van Melle G. Revelly J.P. Porchet F. Regli L. Meuli R. Schnyder P. Maeder P. Relationship between brain perfusion computed tomography variables and cerebral perfusion pressure in severe head trauma patients. Crit. Care Med. 2004a;32:1579–1587. doi: 10.1097/01.ccm.0000130171.08842.72. [DOI] [PubMed] [Google Scholar]
- Wintermark M. Sesay M. Barbier E. Borbely K. Dillon W.P. Eastwood J.D. Glenn T.C. Grandin C.B. Pedraza S. Soustiel J.F. Nariai T. Zaharchuk G. Caille J.M. Dousset V. Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36:e83–99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- Wintermark M. van Melle G. Schnyder P. Revelly J.P. Porchet F. Regli L. Meuli R. Maeder P. Chiolero R. Admission perfusion CT, prognostic value in patients with severe head trauma. Radiology. 2004b;232:211–220. doi: 10.1148/radiol.2321030824. [DOI] [PubMed] [Google Scholar]
- Wolff S.D. Balaban R.S. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn. Reson. Med. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Wong E.C. Cronin M. Wu W.C. Inglis B. Frank L.R. Liu T.T. Velocity-selective arterial spin labeling. Magn. Reson. Med. 2006;55:1334–1341. doi: 10.1002/mrm.20906. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R. Krach L. Ward E. Mueller B.A. Muetzel R. Schnoebelen S. Kiragu A. Lim K.O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C. Wilde E.A. Bigler E.D. Li X. Merkley T.L. Yallampalli R. McCauley S.R. Schnelle K.P. Vasquez A.C. Chu Z. Hanten G. Hunter J.V. Levin H.S. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 2010a;32:361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. Li S. Lei J. An D. Haacke E.M. Evaluation of traumatic subarachnoid hemorrhage using susceptibility-weighted imaging. Am. J. Neuroradiol. 2010b;31:1302–1310. doi: 10.3174/ajnr.A2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y. Tsushima Y. Tokumaru A. Un–no Y. Sakamoto T. Okada Y. Nawashiro H. Shima K. A quantitative analysis of head injury using T2*-weighted gradient-echo imaging. J. Trauma. 2000;49:272–277. doi: 10.1097/00005373-200008000-00013. [DOI] [PubMed] [Google Scholar]
- Yeo R.A. Phillips J.P. Jung R.E. Brown A.J. Campbell R.C. Brooks W.M. Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. J. Neurotrauma. 2006;23:1427–1435. doi: 10.1089/neu.2006.23.1427. [DOI] [PubMed] [Google Scholar]
- Yount R. Raschke K.A. Biru M. Tate D.F. Miller M.J. Abildskov T. Gandhi P. Ryser D. Hopkins R.O. Bigler E.D. Traumatic brain injury and atrophy of the cingulate gyrus. J. Neuropsychiatry Clin. Neurosci. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Yuan W. Holland S.K. Schmithorst V.J. Walz N.C. Cecil K.M. Jones B.V. Karunanayaka P. Michaud L. Wade S.L. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am. J. Neuroradiol. 2007;28:1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]