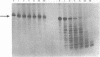
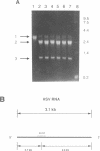
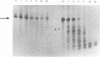
Abstract
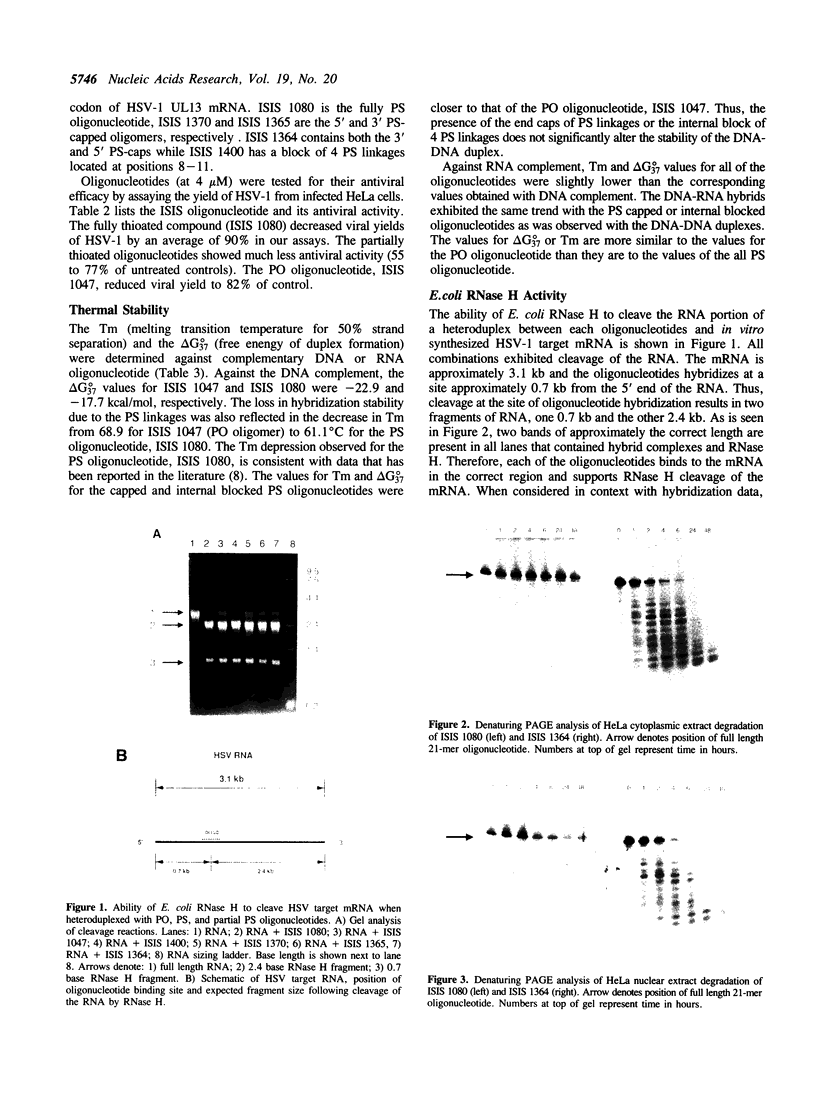
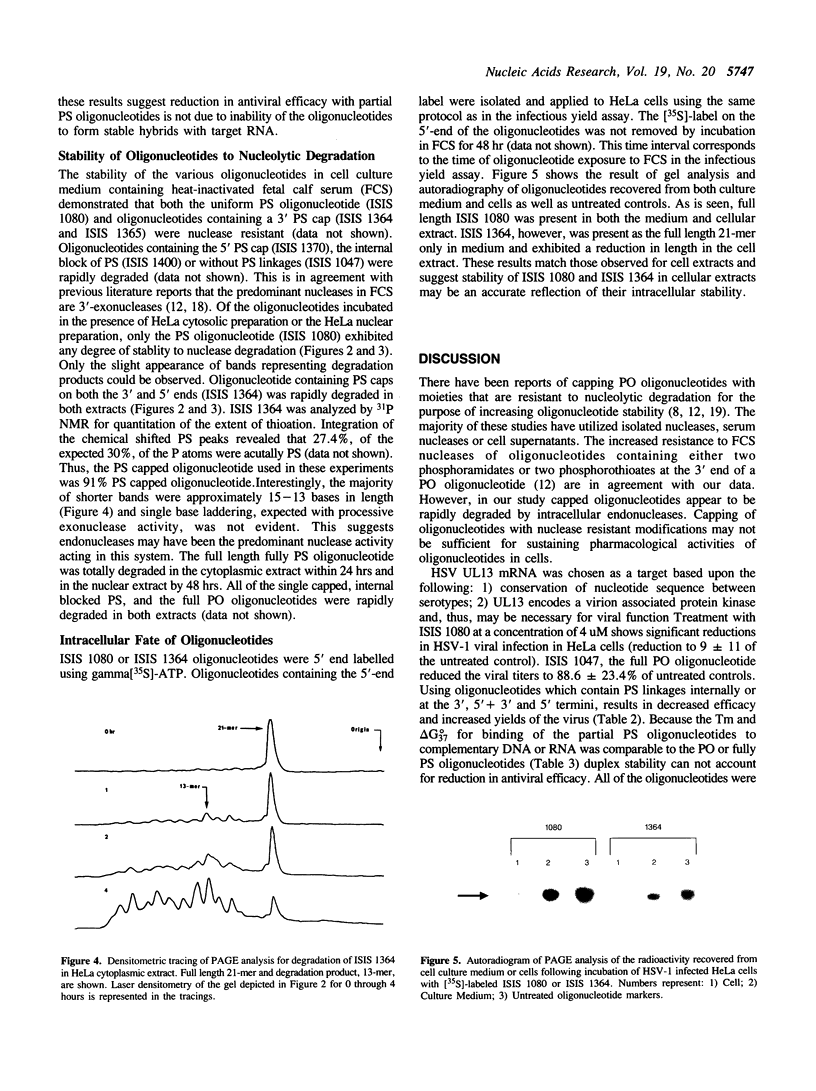
Efforts have been made to improve the biological stability of phosphodiester (PO) oligonucleotides by the addition of various modifications to either the 3', 5' or both the 3' and 5' ends of an oligonucleotide. ISIS 1080, a phosphorothioate (PS) 21-mer oligonucleotide complementary to the internal AUG codon of UL13 mRNA in HSV-1, reduces the infectious yield of HSV-1 in HeLa cells to 9.0% +/- 11%. PO analogs of ISIS 1080 containing three PS linkages placed on the 3' (ISIS 1365), 5' (ISIS 1370), both the 3' and 5' (ISIS 1364) ends or with four linkages in the middle (ISIS 1400) demonstrated reduced antiviral efficacy compared to fully PS ISIS 1080. Thermal denaturation profiles demonstrated that these oligonucleotides hybridized to complementary DNA or RNA with equivalent binding affinities. All were able to support E. coli RNAse H cleavage of the HSV mRNA to which they were targeted. The stability of the congeners in cell culture medium containing 10% fetal calf serum (FCS), HeLa cytosolic extract, HeLa nuclear extract and in intact HeLa cells revealed that ISIS 1080 was most resistant to nucleolytic digestion through 48 hours. Partial PS oligonucleotides exhibited increased degradation compared to the fully thioated oligonucleotide by exonuclease activity in FCS and endonuclease activity in cell extracts or intact cells. Thus, the reduced efficacy of partial compared to fully PS oligonucleotides against HSV-1 in HeLa cells may result from increased degradation of the mixed PO/PS oligonucleotides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease H: from discovery to 3D structure. New Biol. 1990 Sep;2(9):771–777. [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Hanes R. N., Vazquez-Padua M. A., Stein C. A., Cohen J. S., Cheng Y. C. Inhibition of herpes simplex virus type 2 growth by phosphorothioate oligodeoxynucleotides. Antimicrob Agents Chemother. 1990 May;34(5):808–812. doi: 10.1128/aac.34.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Stein C. A., Cohen J. S., Dutschman G. E., Cheng Y. C. Effect of phosphorothioate homo-oligodeoxynucleotides on herpes simplex virus type 2-induced DNA polymerase. J Biol Chem. 1989 Jul 5;264(19):11521–11526. [PubMed] [Google Scholar]
- Miller P. S., Dreon N., Pulford S. M., McParland K. B. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones. Synthesis and physical studies. J Biol Chem. 1980 Oct 25;255(20):9659–9665. [PubMed] [Google Scholar]
- Ott J., Eckstein F. Protection of oligonucleotide primers against degradation by DNA polymerase I. Biochemistry. 1987 Dec 15;26(25):8237–8241. doi: 10.1021/bi00399a032. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Base-stacking and base-pairing contributions to helix stability: thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):256–263. doi: 10.1021/bi00271a004. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tinoco I., Jr Absorbance melting curves of RNA. Methods Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Kent K., Bird J., Fishback J., Froehler B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucleic Acids Res. 1991 Feb 25;19(4):747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M., Warenius H. M. Partial protection of oncogene, anti-sense oligodeoxynucleotides against serum nuclease degradation using terminal methylphosphonate groups. Br J Cancer. 1989 Sep;60(3):343–350. doi: 10.1038/bjc.1989.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]