Abstract
Effects of early ethanol exposure on later ethanol intake emphasize the importance of understanding the neurobiology of ethanol-induced reinforcement early in life. Infant rats exhibit ethanol-induced appetitive conditioning and ethanol-induced locomotor activation, which have been linked in theory and may have mechanisms in common. The appetitive effects of ethanol are significantly modulated by μ and δ opioid receptors, whereas μ but not δ receptors are involved in the motor stimulant effects of ethanol during early development. The involvement of the κ opioid receptor (KOR) system in the motivational effects of ethanol has been much less explored. The present study assessed, in preweanling (infant) rats, the modulatory role of the KOR system in several paradigms sensitive to ethanol-induced reinforcement. Kappa opioid activation and blockade was examined in second-order conditioned place preference with varied timing before conditioning and with varied ethanol doses. The role of KOR on ethanol-induced locomotion and ethanol-induced taste conditioning was also explored. The experiments were based on the assumption that ethanol concurrently induces appetitive and aversive effects and that the latter may be mediated by activation of kappa receptors. The main result was that blockade of kappa function facilitated the expression of appetitive ethanol reinforcement in terms of tactile and taste conditioning. The effects of kappa activation on ethanol conditioning seemed to be independent from ethanol's stimulant effects. Kappa opioid activation potentiated the motor depressing effects of ethanol but enhanced motor activity in control subjects. Overall, the results support the hypothesis that a reduced function of the KOR system in nondependent subjects should attenuate the aversive consequences of ethanol.
Keywords: ethanol, second-order conditioning, taste conditioning, κ opioid receptor
1. Introduction
Effects of early ethanol exposure on later ethanol intake [1] emphasize the importance of understanding the neurobiology of ethanol-induced reinforcement early in life. Ethanol seeking and intake are modulated by the appetitive and aversive effects of the drug [2], and preweanling rats have proven valuable for assessing these phenomena. Infants acquire ethanol-induced first- and second-order conditioned place preference (CPP) and readily express ethanol-induced taste conditioning (TC) and ethanol-induced locomotor activation (for review, see [3]).
In CPP and TC studies, animals are given pairings of a distinctive environment (CPP) or salient novel taste (TA) and ethanol’s postabsorptive consequences [3]. A change in preference for these conditioned stimuli is considered an index of ethanol-induced reinforcement. For second-order conditioning CPP subjects are administered ethanol followed by an intraoral infusion of a conditional intraoral taste stimulus (CS1). The CS1 is then paired with a distinctive tactile cue (CS2), such as rough sandpaper. Ethanol-mediated reinforcement is indexed by measuring subsequent preference for or aversion to the CS2. Preweanling rats typically display robust ethanol-mediated conditioned aversion in taste conditioning tasks. Ethanol-mediated conditioned preferences are more likely to be observed with the second-order CPP, particularly when low doses (e.g., 0.5 g/kg) are employed [3]. Ethanol-induced locomotor stimulation is another, albeit indirect, measure of ethanol’s motivational effects and has been considered a biomarker for predisposition to problematic alcohol consumption [4].
The objective of the present study was to assess the participation of the kappa opioid receptor (KOR) system in the sensitivity of preweanling, 2-week-old rats to ethanol’s reinforcing effects, using second-order CPP (Experiments 1, 2 and 3), TC (Experiment 4), and ethanol-induced locomotor stimulation (Experiment 5). Pups were tested after 5’-guanidinylnaltrindole dihydrochloride (GNTI) or nor-binaltorphimine (norBNI)-induced blockade of KOR function; or after spiradoline mesylate (U62,066E)-induced agonism of KOR. Blood alcohol levels (BALs) following agonism or antagonism of KOR were also measured.
Four classes of opioid receptors have been identified: μ, δ, κ and ORL1 [5, 6]. These subtypes are widely expressed in the nucleus accumbens and other brain areas associated with the reinforcing effects of drugs of abuse [7]. In the absence of ethanol, μ and κ receptor activation have opposite neurochemical and behavioral effects. Intracerebral administration of μ and κ receptor agonists respectively increase and decrease dopamine release in the ventral tegmental area [8] and lead to CPP and conditioned aversion [9, 10]. The administration of N/OFQ, the natural ligand of ORL1 receptors, also suppresses the activity of the mesocorticolimbic dopaminergic system, whereas central antagonism of ORL1 receptors induces anxiolyisis [11] and conditioned place preference [12]. Converging evidence indicates that the endogenous opioid system is involved in ethanol intake and reinforcement. Acute ethanol administration increases the release of β-endorphins, especially on the ascending limb of the blood ethanol curve [13]. Ethanol intake is reduced by selective κ opioid receptor (KOR) agonists and δ and μ receptor antagonists [14–16]. Moreover, ORL1 knockout mice exhibit lower preference for ethanol than wild-type counterparts [12]. The opioid system is also involved in ethanol-induced conditioned reinforcement. Administration of μ (CTOP) and δ (naltrindole) receptor antagonists inhibit ethanol-induced reinforcement in neonatal rats [17] and infant rats [18] and suppresses operant responding for ethanol in adult rats [14]. The opioid receptors for μ, but not δ, are involved in the psychomotor stimulant effects of ethanol [19, 20] and ORL1 knockout mice exhibit enhanced ethanol-induced LMA [12].
The involvement of the KOR system in mediating the motivational effects of ethanol has not been explored as extensively as other opioid systems. In adult rodents, specific κ agonists, such as U50-488H, can increase [21] or decrease [22–25] cocaine and ethanol-induced reinforcement, depending on the temporal relationship between the KOR agonist and conditioning. When the target drug and KOR agonist are administered close together in time, conditioning is usually abolished [26], but when the KOR agonist is administered 60–90 min before conditioning, potentiation of ethanol- [21] and cocaine- [26] induced CPP is observed. The explanation for this biphasic effect has been that activation of KORs 60–90 min prior to conditioning produces an aversive, stress-like state that, through mechanisms of negative reinforcement or successive contrast, enhances the reinforcing value of drugs that ameliorate this effect. Indeed, KOR agonism can substitute for stress exposure in adult mice [21]. The study of these phenomena is important because protracted activation of the KOR system may promote ethanol intake in dependent subjects [27]. The latter hypothesis has been supported by studies showing that treatment with the KOR antagonist nor-binaltorphimine (norBNI) does not affect ethanol intake in nondependent rats or monkeys [28, 29] but significantly reduces withdrawal-induced drinking in alcohol-dependent rats [30, 31].
The net effect of a given ethanol dose is the result of the interaction between ethanol’s appetitive and aversive effects. Acute ethanol treatment in the striatum increases the expression of dynorphin mRNA, the endogenous ligand of KOR, which in turn mediates aversion [32] and is involved in the down regulation of ethanol intake [33]. Moreover, KOR antagonism significantly reduces ethanol-induced hypothermia [34] and reverses the depressant effect of ethanol on open field activity [35]. These data suggest that the KOR system may help mediate the aversive effects of ethanol. Experiments 1 and 4 were based on this assumption and predicted that a reduced functionality of the KOR system in nondependent subjects should facilitate the expression of appetitive reinforcement. After establishing the role of the KOR system in ethanol reinforcement, Experiments 2 and 3 tested whether kappa agonists would have similar or opposing effects on ethanol reinforcement. Based on previous studies, the hypotheses were that treatment with a KOR agonist 15 min before conditioning would block conditioned reinforcement induced by 0.5 g/kg ethanol, and promote conditioned aversion to the 2.0 g/kg dose. On the other hand, we expected the agonist to promote conditioned ethanol reinforcement if injected 90 min before conditioning. The assumption was that pups given U62,066E 15 min before conditioning would experience an aversive state that should summate with the aversive effects of 2.0 g/kg ethanol and inhibit the appetitive effects of 0.5 g/kg ethanol. Rats given U62,066E 90 min before conditioning, on the other hand, were expected to enter a negative affective state, which would then be relieved by ethanol exposure. The last experiment analyzed the effects of kappa activation on ethanol-induced motor activation. The objective was to assess if the ability of KOR agonism to facilitate ethanol reinforcement was associated with an alteration in ethanol’s stimulant effects. Moreover, Arias et al. [19] suggested that KORs regulate behavior in a qualitatively different way in infant and adult subjects. Although the present study lacks an explicit comparison with adult subjects, it represents progress toward addressing this issue.
2. General Methods
2.1. Subjects
Three hundred ninety-five Sprague-Dawley rat pups born and reared in an AAALAC-accredited facility were used. These animals were derived from 54 litters born and reared in the vivarium of the Center for Development and Behavioral Neuroscience (Binghamton University, Binghamton, NY, USA). The litter representation and number of subjects used in each experiment were the following: Experiment 1 (55 animals, 8 litters), Experiment 2 (48 animals, 7 litters), Experiment 3a (56 animals, 7 litters), Experiment 3b (65 animals, 8 litters), Experiment 4 (60 animals, 8 litters), Experiment 5a (47 animals, 6 litters), Experiment 5b (64 animals, 10 litters). Births were examined every day, and the day of birth was considered postnatal day 0 (PD0). The colony was maintained at 22–24°C under a 12 h/12 h light/dark cycle. The experiments were approved by the Binghamton University Institutional Review Committee for the Use of Animal Subjects and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals [36]. Across experiments, animals were used in one and only one experiment. This served, unless specified, to control for order and carry-over effects.
2.2. Drug preparation and administration procedures
The ethanol doses of 0.0, 0.25, 0.5, and 2.0 g/kg were achieved by administering 0.015 ml of a 0.0, 2.1, 4.2, and 16.8% ethanol solution (190 proof ethanol, Pharmaco, Brookfield, CT; vehicle: tap water) per gram body weight, respectively. In accord with previous studies [37, 38, 39–41], ethanol in the second-order CPP and locomotor activity experiments was administered via the intragastric (i.g.) route, whereas the taste conditioning study (Experiment 4) used the intraperitoneal (i.p.) route to avoid the delay between CS exposure and the onset of ethanol’s effects [42, 43].
GNTI, nor-BNI, and U62,066E (Sigma-Aldrich, St. Louis, MO) were administered i.p. GNTI was administered at a dose of 1.0 mg/kg and was derived from a 0.1 mg/ml solution. The U62,066E doses of 1.0 and 10.0 mg/kg were derived from 0.1 and 1.0 mg/ml solutions, respectively. The norBNI doses of 1.0 and 2.5 mg/kg were derived from a 1 mg/ml solution. The injection volume was kept at 0.01 ml/g for all drugs and doses, and saline (0.9%) was used as the vehicle.
The i.g. administration involved introducing a 12 cm section of polyethylene tubing (PE-10; Clay-Adams, Parsippany, NJ) through the mouth and into the stomach of the animal. The tubing was connected to a 1 ml syringe through a 27½-gauge needle. The intubation took approximately 5 s, and ethanol was then delivered in 3–4 s. The i.p. administration was conducted as described in Pautassi et al. [44].
2.3. Conditioning and testing procedures
2.3.1. Second-order conditioning and testing
The second-order conditioning was conducted on PD14–15 and closely followed the procedure of Molina et al. in infant rats [37] and later used in adolescents by Pautassi et al. [38, 41, 45]. The rationale for choosing a second order CPP was that it has been difficult to observe first-order, ethanol-mediated CPP in the heterogeneous non-selected rat. Most studies have indicated conditioned place aversion (for review and references, see [3]). The second-order conditioning CPP procedure provides another model of ethanol-induced reinforcement. Infant rats exhibit higher-order stimulus processing capabilities that are readily expressed by second-order conditioning and this procedure has been useful to reveal conditioning when weakness in first-order conditioning training otherwise precluded evidence of learning [46]. It was suggested [47] that first-order CPP by ethanol may condition behaviors (e.g., behavioral activation expressed through locomotor activation or wall-climbing) that may interfere with expression of the target behavior at test, thus precluding expression of the CPP. This rationale prompted the development of second-order CPP. Since its inception this paradigm has been useful in detection of ethanol reinforcement in infants [37, 47] and adolescent rats [38, 41], and has aided in analyzing the role of opioid receptor antagonists [45] associated with the expression of ethanol reinforcement.
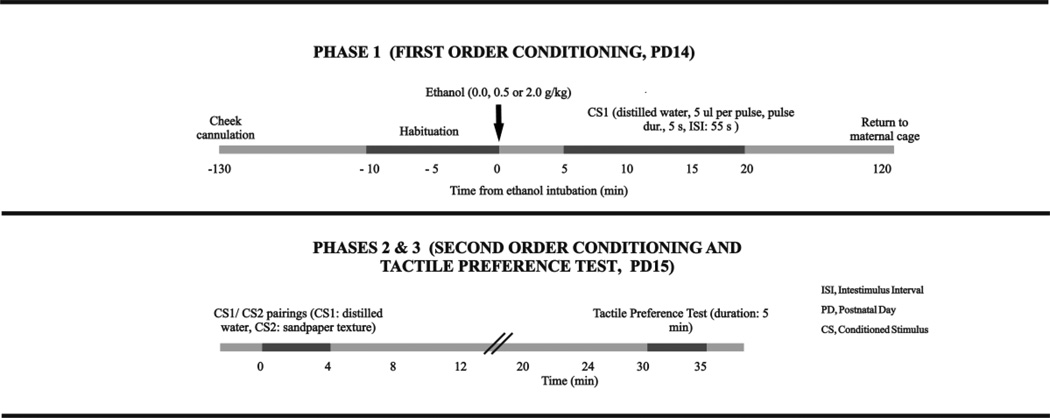
The procedure consisted of three phases (first-order conditioning, second-order conditioning and testing, see Figure 1).
Figure 1.
This illustrates the method of second-order conditioning for assessing ethanol’s reinforcing effects in in 2-week-old rats. Phase 1, first-order conditioning, postnatal day (PD) 14: Pups were removed from the maternal cage, cannulated and then briefly habituated to the experimental context. They were then given ethanol (0.0 or 2.0 g/kg, Experiments 1 and 2; 0.0 or 0.5 g/kg, Experiment 3) and stimulated with a conditioned stimulus (CS1) consisting of intraoral pulses of water. CS1 delivery occurred 5–20 min after ethanol. Phase 2, second-order conditioning, PD 15: Animals were stimulated with water pulses while placed in a sandpaper-lined compartment (CS2). Phase 3, place preference test, PD 15: Time spent on sandpaper was recorded during a 5 min preference test. The figure and legend were adapted with permission from Pautassi et al., 2011a.
Phase 1 (first-order conditioning, PD 14)
The pups were separated from their dams and implanted in the cheek with a small section of PE-10 tubing. The tubing was used to deliver the intraoral CS1. Two hours later, they were placed for 10 min into square Plexiglas chambers (10 × 10 × 12 cm) lined with cotton. The goal was to familiarize the animals to the chambers. The preweanling rats were then intragastrically administered 0.0 or 2.0 g/kg ethanol and returned to their holding chambers. We decided to use animals administered 0.0 g/kg ethanol instead of an unpaired control. We had employed unpaired controls in previous second-order CPP studies and found that ethanol-mediated second-order conditioning cannot be explained by pseudo-conditioning or by mere exposure (sensitization) to the unconditional stimulus (US) or the CS [7–9]. By using animals administered 0.0 g/kg instead of unpaired controls we were also able to minimize the time pups had to be isolated from the dam during conditioning. During conditioning, the preweanlings were stimulated with fifteen 5 s pulses (5 µl per pulse; interstimulus interval: 55 s) of distilled water (CS1). Conditioning occurred during post-administration minutes 5–20 in the square Plexiglas chambers. The cannulae were removed after the termination of conditioning. Every litter had animals with different drug histories and it was possible that dams treated such offspring differently. To control for this issue animals were not returned to their home cage immediately after conditioning but, instead, were kept in a warmed cage lined with pine shavings for 2 h. This procedure allowed for substantial metabolic processing of ethanol and the kappa agonist before being reunited with their dams. Preliminary statistical analysis also indicated that pups given the long-lasting GNTI or norBNI on PD13 exhibited similar body weight during conditioning.
Phase 2 (second-order conditioning, PD 15)
The animals received cannulae in the cheek and were kept in a warmed cage for 60 min. They were then placed into the square chambers used during Phase 1, which were now lined with sandpaper (50 grit; Gatorgrit, Fairborn, OH). While exposed to sandpaper (CS2), the animals received pulsed distilled water every 55 s (volume: 5 µl; pulse duration: 5 s). The animals were given pairings of sandpaper and the distilled water CS1.
Phase 3 (CPP test, PD 15)
A two-way, 5 min tactile preference test was conducted 30 min after the termination of Phase 2. The test animals were placed in a split-floor rectangular Plexiglas chamber (28 × 13 × 15.5 cm). Half of the surface was made of the sandpaper CS2 (50 grit; Gatorgrit), and the other half was lined with the smooth backside of a piece of sandpaper. The rats were individually tested under red light (provided by an overhead 40 watt bulb), and new sandpaper sheets were used for each test. The time spent in each section of the apparatus was manually recorded in 1 min bins by two experimenters blind to the conditioning treatments.
The intermediate section (15% of the entire surface) was considered a neutral area and not included for the data collection or analysis. Tactile preference scores are expressed as the percent time spent on sandpaper and was calculated as the following: (total time spent over sandpaper) / (total time spent over sandpaper + total time spent over smooth) × 100. Experiments conducted in our lab with two-week old rats have revealed equal preference for sandpaper and the smooth surface when evaluated in a two-way test [37, 47, 48]. Apparently preweanling pups do not have a significant preference for any of the textures employed nor find the smooth side anxiogenic. Just before the present Experiments we also ran additional experiments to determine that, when employing the two-way tactile preference test, naive animals showed approximately 50% selection of the sandpaper surface.
2.3.3. Assessment of ethanol-induced locomotor activity and blood alcohol levels
Ethanol-induced locomotor activity was assessed at ethanol post-administration time 5–20 min using a Plexiglas open field (42 × 42 × 30 cm) connected to an automatic VersaMax Animal Activity Monitoring System (Accuscan Instruments, Columbus, OH). The open field was dimly lit and enclosed into a sound-proof chamber (53 × 58 × 43 cm) made of wood and equipped with a fan that provided white noise. The open field was surrounded by photocell beams that allowed VersaMax software to record the distance traveled (cm) in 1 min bins. This output was automatically produced by the software.
Immediately following the locomotor activity test, a time period that corresponded with the termination of Phase 1 of the second-order CPP, trunk blood samples were obtained from ethanol-treated pups. The samples were obtained through decapitation and stored at −80°C for further processing with an AM5 Alcohol Analyzer (Analox Instruments, Lunenburg, MA; for more information about this procedure, see [18]). Blood alcohol levels are expressed as milligrams of ethanol per deciliter body fluid (mg/dl = mg%).
Preliminary statistical considerations
To prevent the confounding effect of litter, no more than one male and one female per litter were assigned to each particular treatment. Across variables and experiments, sex did not exert significant main effects or significantly interact with the remaining factors. Therefore, the data were collapsed across sex for all of the subsequent analyses and for representation in the figures. This lack of sex effects was not unexpected. Previous work found that ethanol-mediated taste and tactile conditioning [3, 18, 47] and ethanol-induced motor activation [39] is similar in male and female preweanling rats. In Experiment 1, six to eight animals were included in each group. Because sex did not exert main effects and was not involved in significant interactions, the subsequent second-order experiments had only 4–6 animals per group. The experimental groups in Experiments 4 and 5 had a minimum of five animals and maximum of seven animals each. The loci of significant main effects or significant interactions were analyzed using Fisher’s Least Significant difference post hoc test, with the alpha level set at 0.05.
3. Experiment 1
The present experiment examined second-order CPP with 2.0 g/kg ethanol after KOR antagonism. Pups were given GNTI (0.0 or 1.0 mg/kg) 24 h before conditioning. The interval between GNTI administration and conditioning was based on previous studies in which GNTI produced peak effects approximately 24 h after administration [49]. The ethanol dose was chosen because previous studies indicate that this dose exerts mild or no second-order CPP [50] and our expectation was that KOR antagonism would promote the emergence of ethanol-mediated appetitive conditioning. In other words, we expected ethanol to induce CPP only after blockade of KOR.
Twenty-four hours after CPP testing, the pups were given an ethanol challenge (2.0 g/kg) and tested for ethanol-induced locomotor stimulation during post-administration time 5–20 min. Trunk blood samples were obtained following the locomotor activity test. The aim of these additional tests was to determine whether KOR antagonism alters sensitivity to ethanol-induced locomotor stimulation and to control for possible alterations in metabolism of ethanol after GNTI administration. GNTI has a long duration of action and has been shown to retain some antagonistic effect 48 h after administration [51]. It should be noted, however, that pups were no longer naïve when tested for ethanol-induced locomotor stimulation. Therefore, the results of this test could be affected by the previous experience during second-order conditioning and test.
3.1. Methods
3.1.1 Experimental Design and Statistical Analysis
A 2 (sex: male or female) × 2 (ethanol dose: 0.0 or 2.0 g/kg) × 2 (GNTI dose: 0.0 or 1.0 mg/kg) factorial design was used. The dependent variable was the percent time spent on the rough sandpaper CS during Phase 3 of the CPP procedure, which was analyzed using a two-way analysis of variance (ANOVA). The between-group factors were ethanol dose and GNTI dose. The distance traveled in the open field after the ethanol challenge was divided into three 5 min bins (i.e., post-administration intervals of 5–9 min, 10–14 min, and 15–19 min) and then analyzed using a three-way mixed ANOVA (between-group factors were GNTI treatment and ethanol treatment; within-subjects factor was bin of evaluation). Blood alcohol levels were analyzed using a two-way ANOVA (GNTI treatment × ethanol treatment on PD14).
3.1.2 Procedures
Pups were given systemic administration of GNTI on PD13. Second-order conditioning and testing took place on PDs 14 and 15. On PD16 the animals were given 2.0 g/kg ethanol and locomotor activity was subsequently assessed for 15 min. Conditioning was defined by the three-phase, second-order place preference described in section 2.3.1 and Figure 1. Ethanol-induced LMA and blood ethanol levels were measured as described in section 2.3.3.
3.2 Results
CPP scores
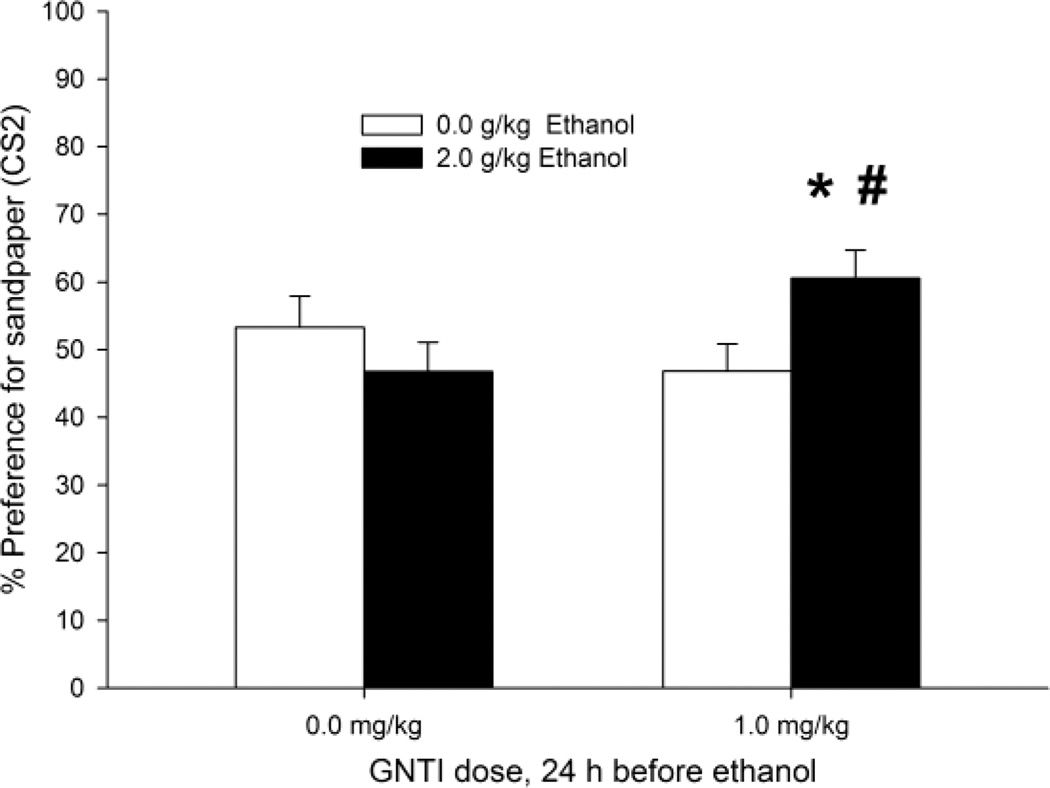
Conditioned place preference for ethanol-related stimuli is shown in Figure 2 in terms of percent preference for the sandpaper CS2. The ANOVA of the time spent on CS2 revealed a significant GNTI × ethanol treatment interaction (F1,51 = 6.18, p < 0.05). The post hoc tests indicated that pups treated with vehicle and then given CS1-ethanol pairings exhibited a similar predilection for CS2 as their controls. The pups pretreated with GNTI and subsequently exposed to CS1 while intoxicated with ethanol spent significantly more time on sandpaper than the pups that also underwent ethanol-CS1 pairings but received 0.0 mg/kg GNTI. The pups treated with GNTI and ethanol also spent significantly more time on CS2 than pups treated with 1.0 mg/kg GNTI and 0.0 g/kg ethanol. Ethanol appeared to induce second-order appetitive conditioning only after pretreatment with 1 mg/kg GNTI.
Figure 2.
Ethanol-induced second-order conditioned preference in infant rats, expressed as the percent time spent in the CS2 compartment during the test as a function of GNTI and ethanol treatment. Pups received GNTI, a KOR antagonist (0.0 or 1.0 mg/kg, i.p.) followed 24 h later by ethanol administration (2.0 or 0.0 g/kg, i.g.). Conditioning involved stimulation with intraoral pulses of water (CS1) during ethanol post-intubation time 5–20 min. The next day, the animals were subjected to CS1–CS2 pairings and then tested for CS2 preference. The asterisks (*) indicate significant differences between the group treated with 2.0 g/kg ethanol and its corresponding 0.0 g/kg control (p < 0.05). The pound signs (#) indicate a significant difference between groups treated with 0.0 and 1.0 mg/kg ethanol (p < 0.05). Vertical bars indicate the SEM.
Locomotion scores and blood alcohol levels
The ANOVA indicated that the distance traveled in the open field was not altered by either GNTI treatment on PD13 or ethanol treatment on PD14. Only a significant main effect of bin of testing was found, with locomotion scores decreasing from one bin to the next (F2,102 = 93.08, p < 0.001). The overall mean ± SEM (cm) locomotion scores for pups treated with 1.0 and 0.0 mg/kg GNTI was 868.92 ± 111.02 and 1070.01 ± 116.78, respectively. The ANOVA revealed that GNTI had no effect on BALs during SOC. The pups that received GNTI or its vehicle had BALs of 180.80 ± 3.46mg% and 173.34 ± 3.64 mg%, respectively.
4. Experiment 2
Once the role of the endogenous kappa system in ethanol-induced second order conditioning was established, the question was whether kappa agonists and ethanol would have similar effects. Experiment 2 pursued this question. This experiment tested CPP with 2.0 g/kg ethanol when the KOR agonist U62,066E was administered 90 min before conditioning. Based on previous work [21, 26] the expectation was that this manipulation of KOR, although opposite to that employed in Experiment 1, would similarly facilitate the emergence of ethanol-mediated appetitive conditioning. The rationale was that the aversive, stress-like state induced by KOR activation would enhance the reinforcing value of ethanol.
4.1. Methods
4.1.1 Experimental Design and Statistical Analysis
A 2 (sex: male or female) × 2 (ethanol dose: 0.0 or 2.0 g/kg) × 3 (U62,066E dose: 0.0, 1.0, or 10.0 mg/kg) factorial design was used. Percent preference for sandpaper was analyzed through a two-way ANOVA (ethanol dose × U62,066E dose).
4.1.2 Procedures
Pups underwent a second-order CPP procedure on PD14 and PD15 similar to that employed in Experiment 1. The difference was that the manipulation of the KOR system involved pharmacological activation by administering U62,066E (0.0, 1.0, and 10 mg/kg) 90 min before CS1-ethanol pairings. Ethanol dosage was kept at 2.0 g/kg. This dose did not induce CPP by itself in Experiment 1 and was chosen to assess if it would result in appetitive conditioning after 90 min of KOR activation.
4.2 Results
3.1.1. CPP scores
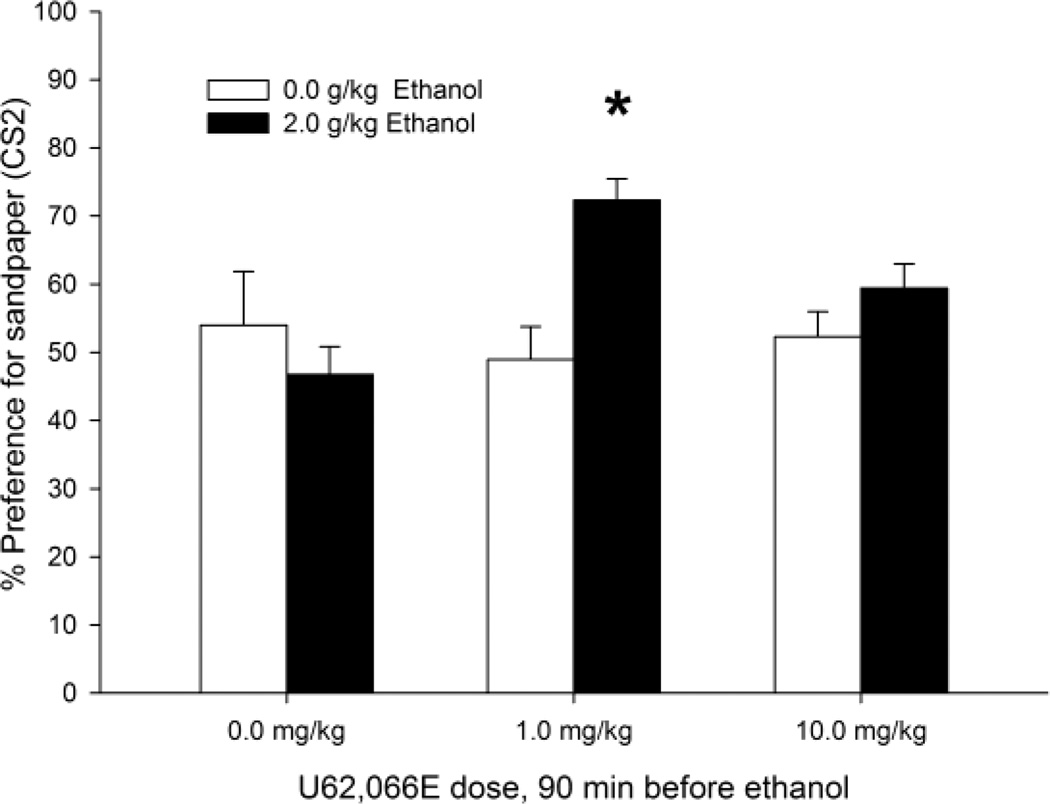
The percent preference for sandpaper during the test is shown in Figure 3. The dose of 2.0 g/kg ethanol induced appetitive reinforcement only in animals given 1 mg/kg U62,066E 90 min before conditioning. The pups pretreated with saline or 10.0 mg/kg U62,066E before ethanol-CS1 pairings did not differ from the basic control condition (i.e., animals exposed to CS1 after receiving i.p. saline and 0.0 g/kg ethanol). These results were confirmed by the ANOVA, which yielded a significant two-way interaction between ethanol and U62,066E treatment (F2,42 = 5.09, p < 0.05). The post hoc tests revealed that animals given 1.0 mg/kg U62,066E and later conditioned with ethanol spent significantly more time on the sandpaper side of the testing cage than any other group, which in turn did not differ between each other.
Figure 3.
Ethanol-induced second-order conditioned preference in infant rats, expressed as the percent time spent in the CS2 compartment during the test as a function of U62,066E and ethanol treatment. During conditioning, the pups received U62,066E, a KOR agonist (0.0, 1.0, or 10.0 mg/kg, i.p.) followed 90 min later by ethanol administration (2.0 or 0.0 g/kg, i.g.). Conditioning involved stimulation with intraoral pulses of water (CS1) during ethanol post-intubation time 5–20 min. The next day, the animals were subjected to CS1–CS2 pairings and then tested for CS2 preference. The asterisks (*) indicate significant differences between the 1 mg/kg U62,066E + 2.0 g/kg ethanol group and all of the remaining groups (p < 0.05), which in turn did not differ between each other. Vertical bars indicate the SEM.
5. Experiment 3
Experiment 3 continued analyzing the effects of kappa opioid activation on ethanol-induced CPP. In Experiment 2 activation of the KOR system 90 min before conditioning facilitated appetitive conditioning with an ethanol dose (2.0 g/kg) that normally did not produce reliable conditioning. This result could presumably be attributed to the anxiogenic state induced by KOR activation enhancing the subsequent motivational effect – i.e., negative reinforcement -- of ethanol. Previous work in adult rats [24] suggests, however, that if both treatments (i.e., ethanol and KOR activation) are given in close temporal proximity an opposite pattern should emerge. Specifically, we expected U62,066E – administered only 15 min prior to training -- to facilitate the emergence of aversive conditioning when 2.0 g/kg was used as US (Experiment 3a) and to block the expression of appetitive conditioned reinforcement induced by a lower ethanol dose (0.5 g/kg) (Experiment 3b). Previous studies indicate that 0.5 g/kg induces a reliable second-order CPP in preweanling rats [47, 50].
5.1 Methods
5.1.1 Experimental Design and Statistical Analysis
Experiments 3a and 3b assessed the effects of KOR activation 15 min before conditioning on ethanol-induced second-order CPP. They were similarly defined by the following factors: sex (male or female), U62,066E (0.0, 1.0, or 10.0 mg/kg), and ethanol (Experiment 3a: 0.0 or 2.0 g/kg; Experiment 3b: 0.0 or 0.5 g/kg). The dependent variable under consideration was percent preference for the CS1 texture and was analyzed by a two-way ANOVA.
5.1.2 Procedures
Fifteen minutes before ethanol administration pups were given U62,066E (0.0, 1.0, or 10.0 mg/kg). Pups were then administered the corresponding ethanol dose (2.0 or 0.5 g/kg, Experiments 3a and 3b, respectively) and conditioned as described earlier (see section 2.3.1.).
5.2. Results
5.2.1. Experiment 3a
Ethanol-mediated second-order conditioning was not observed with 2.0 g/kg ethanol. Rats given this ethanol dose spent as much time on sandpaper (CS2) as animals that received vehicle. The ANOVA indicated that, unlike our expectation, this pattern was similar in animals that received saline or either dose of U62,066E. The mean ± SEM percent CS2 preference in animals that received 0.0 g/kg ethanol was 50.65 ± 4.59, 49.32 ± 5.27, and 56.51 ± 5.05, whereas the mean ± SEM in subjects given 2.0 g/kg ethanol was 51.80 ± 5.58, 51.55 ± 5.90, and 41.95 ± 4.67 in pups that received 0.0, 1.0, and 10.0 mg/kg U62,066E, respectively.
5.2.2. Experiment 3b
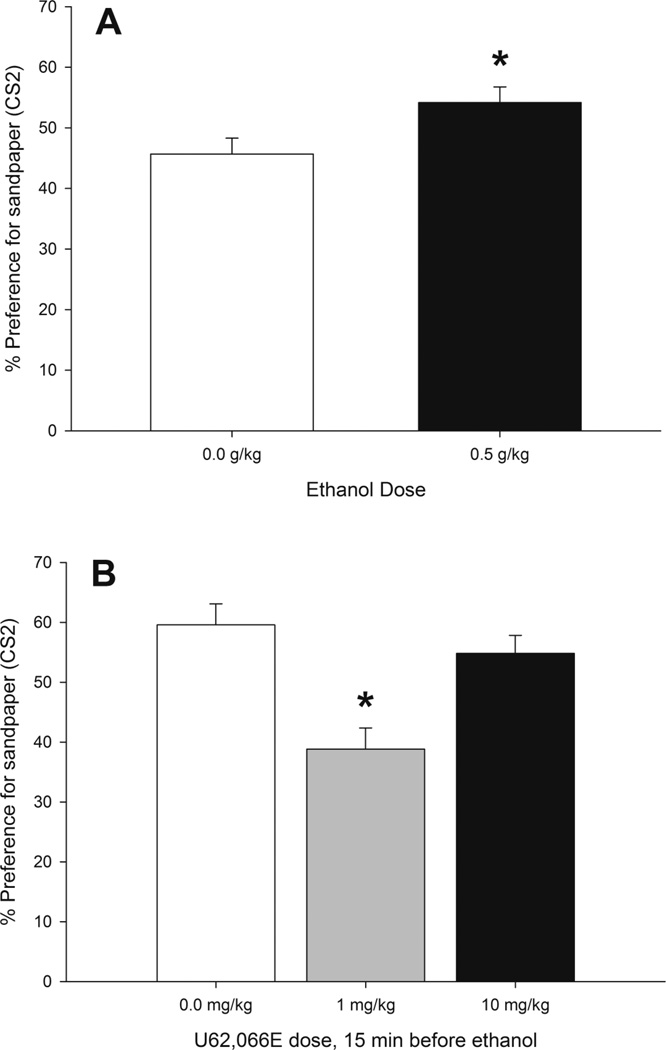
The ANOVA of the percent time preference for CS2 revealed independent significant main effects of U62,066E treatment (F2,50 = 12.68, p < 0.001) and ethanol (F1,50 = 5.30, p < 0.05). The pups that received pairings of 0.5 g/kg ethanol and CS1 spent significantly more time on the rough CS2 than control pups. This result indicates ethanol-induced reinforcement and was similar in animals that received U62,066E or its vehicle. The dose of 1 mg/kg U62,066E significantly reduced preference for sandpaper, independent of ethanol treatment, whether given with 0.5 g/kg ethanol or water as the US. These main effects are depicted in Figures 4A and B. It seems that, as expected, 0.5 g/kg induced appetitive CPP. The hypothesis of KOR activation blocking this effect, however, was not confirmed.
Figure 4.
Ethanol-induced second-order conditioning in infant rats, expressed as the percent time spent in the CS2 compartment during the test as a function of (A) ethanol treatment or (B) U62,066E treatment. During conditioning, the pups received U62,066E, a KOR agonist (0.0, 1.0, or 10.0 mg/kg, i.p.) followed 15 min later by ethanol administration (0.5 or 0.0 g/kg, i.g.). Conditioning involved stimulation with intraoral pulses of water (CS1) during ethanol post-intubation time 5–20 min. The next day, the animals were subjected to pairings of CS1 and CS2 (sandpaper) and then tested for CS2 preference. The ANOVA revealed independent significant main effects of ethanol treatment and U62,066E treatment. The asterisks (*) indicate significant differences between a given group and the remaining conditions (p < 0.05). Vertical bars indicate the SEM.
6. Experiment 4
Arguably the most important outcome of the preceding Experiments was that the endogenous kappa system seemed to be involved in ethanol reinforcement. This suggestion was provided through the second-order CPP technique. It was important, therefore, to assess the generalization of these findings. Experiment 4 tested the effects of KOR system blockade in a paradigm other than second-order CPP, specifically ethanol-mediated taste conditioning. This experiment tested whether the KOR antagonist norBNI [52] would promote appetitive conditioning when followed by a low ethanol dose (0.25 g/kg) that normally does not induce either appetitive or aversive taste conditioning [3]. The rationale for using norBNI -- instead of the GNTI compound employed in Experiment 1 -- was to facilitate the comparison with a previous study that analyzed the role of KOR in ethanol-mediated taste conditioning in rats [52]. Similar to Experiment 1, pups were given the KOR antagonist 24 before conditioning. Detectable doses of norBNI have a slow onset of action. During the first hours after its administration norBNI does not selectively antagonize KOR differently than μ receptors and achieves its peak selective antagonistic action approximately 24 h after administration [51].
6.1 Methods
6.1.1 Experimental Design and Statistical Analysis
The effects of KOR antagonism on ethanol-induced taste conditioning were assessed through a 2 (sex) × 2 (ethanol dose: 0.0 or 0.25 g/kg) × 3 (norBNI dose: 0.0, 1.0, or 2.5 mg/kg) factorial design. Saccharin consumption during conditioning and during the test sessions was expressed as percentage body weight gain (%BWG) and independently analyzed using a 2 (ethanol treatment) × 3 (nor-BNI treatment) factorial ANOVA.
6.1.2 Procedures
The experimental protocol was similar to that used by Broadbent et al. [53] and Arias et al. [54] in adult and infant rats, respectively. The pups were separated from their dams on PD11 and given injections of norBNI (0.0, 1.0, or 2.5 mg/kg). Following the injection, the pups were returned to their dams overnight. The following day, the pups were again separated from their dams, given cannula implantation, and placed in pairs in a maternity tub lined with pine shavings and warmed to approximately 35 ± 0.5°C. The animals remained in the tub for 3 h and were then voided and weighed. The mean weight of all subjects was calculated and used as a benchmark for the volume of the intraoral infusion of saccharin (0.1% w/v) during conditioning. Each subject’s cannula was connected to a length of PE50 tubing that in turn was connected to a 10 ml syringe operated by a computerized rotary device (designed by W. Kashinsky, Binghamton University). The subjects were then placed into a Plexiglas container divided into eight sections measuring 6 × 12 inches each. The bottoms of these containers were lined with cotton and slightly heated (26–27°C). The subjects then received an intraoral infusion of saccharin (2.5% of their body weight) over a 10 min period. These infusion parameters allowed the pups to either accept or reject the infused solution [55]. Immediately following the intraoral infusion, the pups were disconnected from the tubing, weighed to estimate saccharin consumption scores (the percentage of body weight gain was calculated as the following: [postinfusion weight - preinfusion weight] / preinfusion weight × 100), and intraperitoneally injected with ethanol (0.0 or 0.25 g/kg). This ethanol dose is the subthreshold dose in terms of inducing single-trial taste conditioning [56]. The cheek cannula was subsequently removed, and the pups were placed into the holding tub for 2 h for ethanol clearance. Following this period, the pups were returned to their dams. On PD13 (test day), the subjects underwent the same procedure as that on PD12 but without ethanol administration. During the testing day the experimenters were blind to the conditioning treatments. The use of unpaired controls was discarded based on recent studies [57] conducted in our lab. These studies revealed that saccharin consumption in preweanlings given unpaired exposure to saccharin and ethanol (dose range 0.25 –2.0 g/kg) was not different from that of counterparts given saccharin and 0.0 g/kg ethanol.
6.2. Results
Saccharin intake during the conditioning session on PD12 was fairly similar across groups. The ANOVA indicated a lack of significant main effects or significant interactions (all ps > .10). The mean ± SEM (%BWG) in animals that received 0.0 g/kg ethanol treatment was 1.62 ± 0.15, 1.87 ± 0.11 and 1.91 ± 0.05, whereas the mean ± SEM in subjects assigned to the 0.25 g/kg ethanol group was 1.72 ± 0.17, 1.60 ± 0.16, and 1.83 ± 0.08, in pups that received 0.0, 1.0, and 2.5 mg/kg nor-BNI, respectively. On the test day (PD13), the pups pretreated with 2.5 mg/kg norBNI followed by saccharin-ethanol pairings exhibited conditioned preference for saccharin. These observations were confirmed by the ANOVA. The analysis yielded a significant main effect of ethanol treatment (F1,54 = 4.39, p < 0.05) and a significant interaction between the norBNI and ethanol treatments (F2,54 = 3.38, p < 0.05). The post hoc tests revealed significantly greater saccharin consumption in pups that received 2.5 mg/kg norBNI and 0.25 g/kg ethanol than in any other group (all p < 0.02), except compared with pups that received 0.0 mg/kg norBNI and 0.25 g/kg ethanol, in which a borderline difference was observed (p = 0.07). All remaining groups exhibited similar levels of saccharin consumption during the test session. Saccharin preference scores during the test are shown in Fig. 5.
Figure 5.
Ethanol-induced taste conditioning in infant rats, with saccharin intake depicted as percent body weight gain in a 10 min test. Twenty-four hours prior to conditioning, the pups were treated with the KOR antagonist norBNI (0.0, 1.0, or 2.5 mg/kg, i.p). During conditioning, the pups were exposed to a saccharin solution and immediately following infusion intubated with ethanol (0.0 or 0.25 g/kg). The post hoc tests revealed significantly greater saccharin consumption in pups that received 2.5 mg/kg norBNI and 0.25 g/kg ethanol than in any other group (p < 0.02), except when compared with pups that received 0.0 mg/kg norBNI and 0.25 g/kg ethanol, in which a borderline difference was observed (p = 0.07). These significant differences are indicated by the asterisk (*). Vertical bars indicate the SEM.
7. Experiment 5
Ethanol-induced locomotor activity in rodents can be considered an indirect index of the reinforcing effects of the drug [39], similar to the increase in heart rate induced by alcohol in humans. Ethanol-induced increases in heart rate are associated with positive mood ratings during the ascending limb of the blood ethanol curve and are greater in subjects with a positive family history of alcoholism than in subjects not at risk for alcoholism [4]. Experiments 5a and 5b assessed the effects of U62,066E-induced KOR activation on ethanol-induced locomotion when the agonist was administered either 90 or 15 min before 2.0 g/kg ethanol administration, respectively; and BALs were measured after the locomotor activity test. This is, the experiments employed dosing and timing parameters similar to those of Experiments 1, 2 and 3, respectively.
Previous studies [39] indicated that preweanling rats do not exhibit ethanol-induced locomotor activation after the 0.5 g/kg dose used in Experiment 3. Therefore, the latter dose was not used in the present experiments. The 2.0 g/kg dose was selected because it was used in the second-order CPP procedure in Experiments 1 and 2. The aim was to assess if the ability of U62,066E to promote reinforcement with 2.0 g/kg ethanol was associated with a U62,066E-induced alteration of ethanol’s stimulant effects and to control for possible alterations in metabolism of ethanol after U62,066E administration.
7.1 Methods
7.1.1 Experimental Design and Statistical Analysis
Experiment 5 analyzed the modulatory effect of KOR agonism on ethanol-induced locomotor activity and tested the factors sex, ethanol dose (0.0 or 2.0 g/kg), and U62,066E dose (Experiment 5a: 0.0 or 1.0 mg/kg; Experiment 5b: 0.0, 1.0, or 10.0 mg/kg). Distance traveled in the open field was divided into three 5 min bins (i.e., post-administration intervals of 5–9 min, 10–14 min, and 15–19 min) and analyzed through a three-way mixed ANOVA (between-group factors were U62,066E treatment and ethanol treatment; within-subjects factor was bin of evaluation). Blood alcohol levels were analyzed using a one way ANOVA, with U62,066E as the comparative factor.
7.1.2 Procedures
On PD14, the pups were removed from their home cages and placed in pairs for 60 min in a holding chamber (30 × 20 × 20 cm) kept warm using of a heating pad. They were then weighed and given i.p. injections of U62,066E (Experiment 5a: 0.0 or 1.0 mg/kg; Experiment 5b: 0.0, 1.0, or 10.0 mg/kg). Experiment 5a did not employ a 10 mg/kg U62,066E dose. The rationale was that, in Experiment 2, this dose did not affect ethanol-induced CPP when administered 90 min before conditioning. Ninety or fifteen minutes after U62,066E administration (Experiments 5a and 5b, respectively), pups were given intubations of 0.0 or 2.0 g/kg ethanol. Ethanol-induced locomotor activity was assessed during ethanol post-intubation time. Trunk blood samples were taken after the test to determine BALs.
7.2. Results
Locomotor activity
The ANOVA for ethanol-induced locomotor activity after 90 min of KOR activation (Experiment 5a) revealed a significant main effect of bin of evaluation (F2,86 = 11.17, p < 0.001) and a significant ethanol treatment × bin of evaluation interaction (F2,86 = 3.36, p < 0.05). As shown in Fig. 6A, ethanol induced clear locomotor activation during the first bin (i.e., 5–10 min after ethanol), an effect that subsided as the testing progressed. This pattern was similar in animals treated with U62,066E or its vehicle.
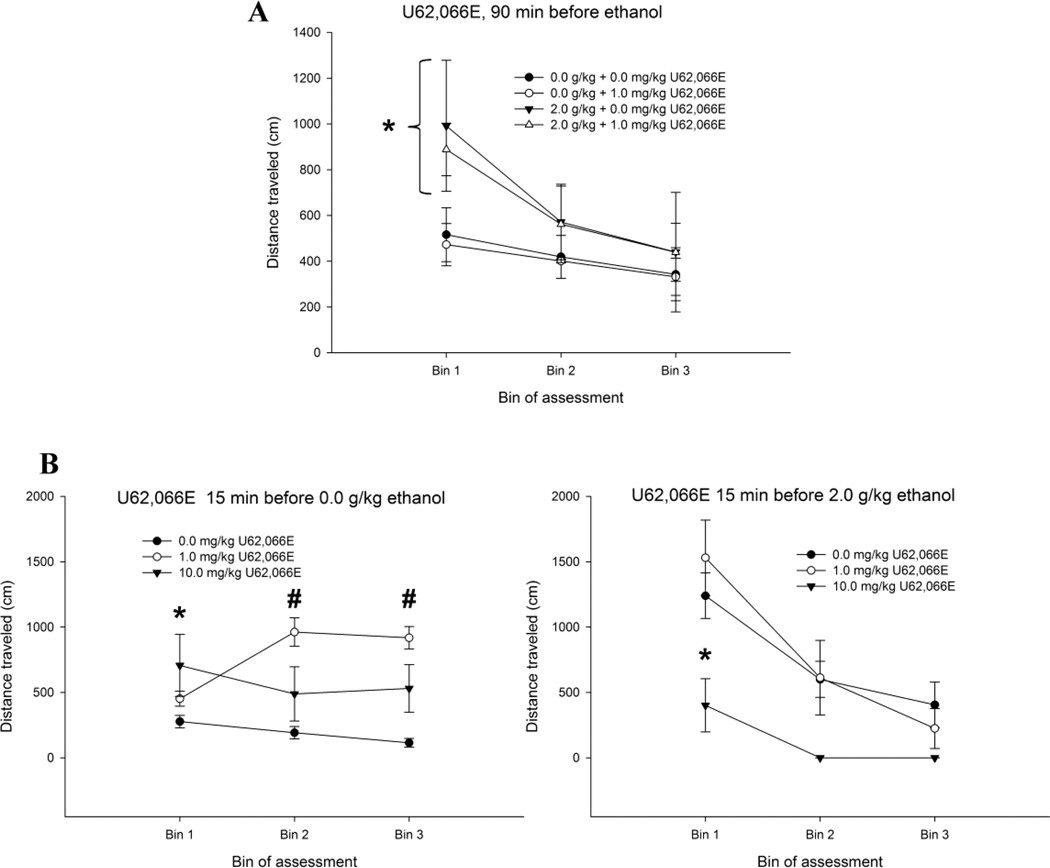
Figure 6.
Ethanol-induced locomotor activity in infant rats administered U62,066E, either 90 or 15 min before ethanol. Locomotor activity (distance traveled, cm) was assessed 5–9, 10–14, and 15–19 min after ethanol administration (bins 1, 2, and 3, respectively). Figure 6A. The animals received U62,066E (0.0 or 1.0 mg/kg) 90 min prior to ethanol administration (2.0 or 0.0 g/kg, i.g.). An ANOVA and subsequent post-hocs indicated that, during the initial bin of assessment, pups given 2.0 g/kg ethanol exhibited significantly greater distance traveled than controls given 0.0 g/kg ethanol. This significant effect of ethanol, which was independent of whether or not pups received U62,066E before test, is indicated by the asterisk (*). Figure 6B. The animals received U62,066E (0.0, 1.0, or 10.0 mg/kg) 15 min prior to ethanol administration (0.0 or 2.0 g/kg, left and right panels, respectively). Post-hocs indicated that, in the pups intubated with 0.0 g/kg ethanol (left panel), the animals that received U62,066E exhibited increased levels of locomotor activity compared with pups that received 0.0 mg/kg U62,066E. During the first testing bin, this effect [which is indicated by the asterisk (*)] was observed only after the highest U62,066E dose. During bins 2 and 3, both doses of U62,066E increased locomotion, and these differences are indicated by the pound signs (#). In the pups intubated with 2.0 g/kg ethanol (right panel), the post hoc tests indicated that ethanol induced locomotor activation during the first testing bin, and this stimulation effect was blocked by treatment with 10 mg/kg U62,066E. This significant difference is indicated by the asterisk (*). Vertical bars indicate the SEM.
Fig. 6B depicts locomotor activity in the pups that received U62,066E 15 min before 2.0 g/kg ethanol (Experiment 5b). The ANOVA yielded significant main effects of U62,066E (F2,72 = 4.38, p < 0.05) and bin of evaluation (F2,144 = 28.06, p < 0.005). The two-way U62,066E × ethanol treatment (F2,72 = 7.63, p < 0.001) and ethanol treatment × bin of evaluation (F2,144 = 36.28 p < 0.001) interactions also achieved significance. The three-way U62,066E × ethanol treatment × bin of evaluation interaction was also significant (F4,132 = 8.18, p < 0.001). The post hoc tests indicated that 2.0 g/kg ethanol induced locomotor activation during the first testing bin, and this stimulation effect was blocked by systemic treatment with 10 mg/kg U62,066E. Pups that received 1.0 mg/kg U62,066E before ethanol did not differ from their counterparts that received saline before ethanol (Fig. 6B, right panel). Similar to the previous experiments, ethanol no longer induced stimulant effects during testing bins 2 and 3, reflected by a lack of significant differences between ethanol- and vehicle-treated subjects that received 0.0 mg/kg U62,066E. Interestingly, in the pups intubated with 0.0 g/kg ethanol (Fig. 6B, left panel), U62,066E exerted independent effects on locomotor activity. The post hoc test revealed that pups that received U62,066E followed by vehicle exhibited increased levels of locomotor activity compared with the control condition (i.e., pups that received 0.0 mg/kg U62,066E followed by 0.0 g/kg ethanol). During the first testing bin, this effect was observed only after the highest U62,066E dose. During bins 2 and 3, both doses of U62,066E increased locomotion, although this effect was significantly greater in animals that received 1.0 mg/kg U62,066E than in peers that received 10.0 mg/kg.
Blood alcohol levels
The one-way ANOVA indicated that U62,066E administered 90 min before ethanol did not alter BALs. The mean ± SEM BALs in animals that received 0.0 and 1.0 mg/kg U62,066E was 179.47 ± 4.44 mg% and 170.84 ± 6.71 mg%, respectively. U62,066E, however, exerted a robust dose-response effect on BALs when administered 15 min before intubation with ethanol. The corresponding one-way ANOVA revealed a significant main effect of U62,066E treatment (F2,27 = 15.21, p < 0.0001). The post hoc test indicated that administration of the higher, but not lower, U62,066E dose significantly reduced BALs. The mean ± SEM BALs in animals that received 0.0, 1.0, and 10.0 mg/kg U62,066E was 164.03 ± 4.44 mg%, 174.84 ± 6.98 mg%, and 131.22 ± 5.61 mg%, respectively.
8. General Discussion
The present study indicated that ethanol induced significant appetitive reinforcement after blockade of KOR function with GNTI (Experiment 1, tactile conditioning) or norBNI (Experiment 4, taste conditioning). These experiments were based on the assumption that ethanol concurrently induces appetitive and aversive effects [3, 58], and that the latter may be mediated by KOR activation [32, 34]. According to these hypotheses, manipulations that reduce the function of the KOR system in nondependent subjects should attenuate the aversive consequences of ethanol and promote the expression of appetitive reinforcement.
Another result that helps underscore the role of the KOR system in ethanol reinforcement is that different and opposite manipulations of the KOR system promoted ethanol-mediated appetitive conditioning in infant rats, The administration of 2.0 g/kg ethanol alone did not induce conditioned preference. However, blockade of KOR function with GNTI (Experiment 1) or activation of the KOR system with U62,066E 90 min before conditioning with 2.0 g/kg ethanol (Experiment 2) resulted in appetitive reinforcement. The latter result is consistent with previous studies conducted in adult rats with ethanol [21] and stimulants [26]. This suggests that activation of KORs for the first 90 min has similar effects in two-week-old infant and adult rodents, inducing an aversive, stress-like state that enhances the reinforcing value of the upcoming drug-induced state through a negative reinforcement or successive contrast mechanism. The dose of 1.0 mg/kg U62,066E promoted conditioned preference with 2.0 g/kg ethanol, which contrasts with the findings of Experiment 5a, in which ethanol-induced locomotor activity was not affected by U62,066E administration 90 min before testing. This suggests that the ability of U62,066E to facilitate reinforcement with 2.0 g/kg ethanol does not rely on the ability of the agonist to alter ethanol’s stimulant effects. Furthermore, the agonist did not affect locomotion in vehicle-treated pups.
The apparent sensitivity of infants to pharmacologically induced stress may appear surprising when considering that the first 2 weeks of life are known as a stress hyporesponsive period [59, 60]. However, preweanling animals can exhibit stress responses, including the release of corticosterone, when exposed to stressors such as a novel environment in conditions of maternal isolation [61]. Moreover, we recently observed that in 13-day-old pups, ethanol increased the release of corticosterone [62].
It could be argued that it is unlikely that pups were able, with only one pairing, to associate ethanol’s motivational effects and then transfer this information to the texture CS2. Several previous experiments, however, have revealed that preweanling, adolescent and adult rats readily establish single trial, ethanol-induced second-order conditioning [37, 38, 45, 47]. The preweanling rat’s ability to acquire conditioned responses after a single trial can hardly be restricted to second-order schedules but, instead, has been observed when odor [63] taste [64–65] or tactile cues [66] were used as CSs for first-order conditioning.
Appetitive reinforcement by 0.25 g/kg ethanol (Experiment 4) was promoted by either of two different kappa opioid antagonists. An interpretation in terms of kappa antagonism reducing ethanol’s aversive effects seems less plausible for the taste conditioning experiment involving the very low ethanol dose (0.25 g/kg), although preweanling rats have in some circumstances shown ethanol-induced conditioned taste avoidance with ethanol doses as low as 0.4 g/kg [59]. Still another alternative is that norBNI facilitated ethanol-induced appetitive responding by blocking nonspecific, non-drug-related aversive states. We previously suggested that the prolonged, continuous intraoral infusion procedure employed in Experiment 4 may itself have aversive properties [56]. The appetitive reinforcement after nor-BNI might also be explained by kappa blockade potentiating ethanol-induced learning generally.
The finding that ethanol conditioned a taste preference is nevertheless an important addition to the literature because ethanol typically yields a conditioned aversion in flavor conditioning tasks [3; but see 68, 69]. Whether a kappa antagonist converts aversive effects of ethanol into appetitive effects probably depends on the dose of ethanol in relation to the animal’s age and strain. For instance, Roma et al. [52] found that norBNI was ineffective in altering ethanol-induced CTA (dose: 1.25 g/kg) in Fischer and Lewis rats. Altogether, these results are consistent with previous reports that ethanol-mediated aversion in infant or adolescent rats is more common at doses greater than 1.0 g/kg, whereas the expression of appetitive motivational consequences is associated with procedures that use relatively low ethanol doses [3].
Notably, the present study was conducted in naive subjects that were not ethanol-dependent. The KOR-ethanol interactions proposed here may not be applicable to alcohol-experienced subjects. Augmentation of KOR tone appears to be a major contributing factor in the maintenance of alcohol intake after alcohol dependence is established [27], and norBNI treatment has been shown to reduce alcohol avidity in alcohol-dependent rats [30, 31].
Unlike previous work in adult rats [24], treatment with a KOR agonist 15 min before conditioning did not alter reinforcement induced by a 0.5 g/kg ethanol dose or facilitate the emergence of aversive conditioning with a still higher dose. In Experiment 3, pups given U62,066E 15 min before sandpaper exhibited a significant decrease in preference for the CS, a behavior indicative of conditioned aversion. This result, however, was not replicated when a longer duration of agonist activity was permitted in Experiment 3b. In these circumstances the kappa agonist alone had little effect on the criterion behavior: U62,066E injected 90 min before conditioning failed to induce conditioned preference or aversion in subjects that received 0.0 g/kg ethanol. A similar lack of nonspecific effects was observed after the GNTI and norBNI treatments.
Experiment 5b analyzed the effects of a KOR agonist, given 15 min before ethanol intubation, on basal and ethanol-induced locomotor activity. A novel finding was that administration of U62,066E 15 min before testing dose-dependently blocked ethanol-induced locomotor activity. This effect was time and dose-specific, as ethanol induced locomotor activation only during the first testing bin and this stimulation was inhibited by the higher, but not the lower, U62,066E dose. The suppressant effect of U62,066E on ethanol-induced locomotor activity may be partially related to the U62,066E-induced decrease in BALs. Notably, however, BALs after the higher dose, 10 mg/kg U62,066E were similar (131.22 mg%) to those found in previous experiments with preweanling rats in which 1.25 g/kg ethanol-induced locomotor activation was reliably observed [70]. Intriguingly, U62,066E alone exerted significant effects on spontaneous open field exploration, and the direction of this effect (i.e., enhanced ambulation) was opposite to that observed when the pups were under the effects of ethanol.
Based on these results, it could be hypothesized that 10.0 mg/kg U62,066E selectively potentiated the motor depressing effects of ethanol. This may also help explain why 1.0 but not 10.0 mg/kg U62,066E altered the expression of second-order CPP in Experiments 2 and 3. The behavioral response in these experiments was not subject to orderly dose-response effects. The lack of a clear dose-response effect for the kappa agonist may be due to the motor activating and depressing effects of U62,066E interfering with the ability of pups to successfully acquire tactile conditioning. It has been suggested that behavioral activation can interfere with the acquisition or expression of conditioned responses in tests of ethanol-induced CPP [71]. Also, in Experiment 2 it was possible that 10.0 mg/kg U62,066E not only induced stress-like effects but also enhanced ethanol-induced aversion. This interaction would have counteracted any stress-related potentiation of CPP. An explanation in terms of an inverted u-shaped dose-response curve is also possible, although the study does not feature enough doses to conclusively warrant this assertion.
One weakness of the present study, albeit shared by most of the literature that employs long acting antagonists, is that pups were probably under the influence of GNTI or nor-BNI during the place preference and taste conditioning tests, respectively, as well as during conditioning There is, however, no evidence that these compounds alter basic sensory or cognitive abilities in a way that may have interfered with expression of learning at test. Moreover, testing under the effects of these compounds could be considered advantageous by preventing generalization decrements in learning expression due to differences between training and testing contexts [72].
In summary, the present study explored the role of the KOR system in several behavioral paradigms sensitive to ethanol-induced reinforcement. Kappa opioid activation and blockade was examined in second-order conditioned place preference with varied timing before conditioning and with varied ethanol doses. Additionally, the study explored the role of KOR on ethanol-induced locomotion and ethanol-induced taste conditioning. The kappa opioid system interacted with the effects of ethanol under some, but not all, the measures of reinforcement. The main results were that 1) kappa agonists and antagonists can exert a similar permissive effect on ethanol-induced reinforcement, depending on the time of administration, 2) the effects of kappa activation on ethanol conditioning seem to be independent of ethanol's stimulant effects, and 3) the kappa opioid system may mediate the aversive component of ethanol exposure early in life as well as later on. Evidence of a direct interaction between ethanol and the KOR system is still scarce, however, and more research is needed to confirm that activation of this system plays a role in mediating the aversive effects of ethanol and in shaping the balance between ethanol’s appetitive and aversive effects.
Highlights.
Prolonged activation of the KOR system promoted ethanol-induced conditioning.
Blockade of KOR function promoted ethanol-induced tactile conditioning.
Blockade of KOR function promoted the expression of taste conditioning by ethanol.
Ethanol-induced motor activation was blocked by spiradoline (U62,066E).
U62,066E affected spontaneous open field exploration in vehicle-treated controls.
Acknowledgments
Acknowledgments and Funding Sources
This work was a collaborative project between the Research Foundation of SUNY Binghamton and Instituto Ferreyra (INIMEC-CONICET) and was supported by National Institute on Alcohol Abuse and Alcoholism grants AA011960, AA01309, and AA017823 to NES and AA018164 and AA017823 to MN, grants PIP CONICET, PICT-PRH, and SECYT-UNC to RMP, and by a fellowship awarded by the Fulbright Commission to RMP. We would like to thank Teri Tanenhaus for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham CL. Drug conditioning and seeking behavior. In: O’Donohue WT, editor. Learning and behavior therapy. Boston: Allyn and Bacon; 1998. pp. 518–540. [Google Scholar]
- 3.Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev. 2009;33:953–974. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997;21:140–149. [PubMed] [Google Scholar]
- 5.Froehlich JC. Opioid peptides. Alcohol Health Res World. 1997;21:132–136. [PMC free article] [PubMed] [Google Scholar]
- 6.New DC, Wong YH. The ORL1 Receptor: Molecular Pharmacology and Signalling Mechanisms. Neurosignals. 2002;11:197–212. doi: 10.1159/000065432. [DOI] [PubMed] [Google Scholar]
- 7.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- 8.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. κ-Opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nader K, van der Kooy D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J Neurosci. 1997;17:383–390. doi: 10.1523/JNEUROSCI.17-01-00383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 11.Duzzioni M, Duarte FS, Leme LR, Gavioli EC, Lima TCMDe. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 2011;222:206–211. doi: 10.1016/j.bbr.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- 13.de Waele JP, Gianoulakis C. Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–709. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- 14.Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology. 1995;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- 16.Lindholm S, Werme M, Brené S, Franck J. The selective κ-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- 17.Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120:267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- 18.Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias C, Molina JC, Spear NE. Differential role of mu, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav. 2010;99:348–354. doi: 10.1016/j.physbeh.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastor R, Sanchis-Segura C, Aragon CMG. Effect of selective antagonism of mu(1)-, mu(1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug Alcohol Depend. 2005;78:289–295. doi: 10.1016/j.drugalcdep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology. 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- 22.Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- 23.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 24.Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43:359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wee S, Koob GF. The role of the dynorphin-κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. κ-Opioid receptor modulation of accumbal dopamine con- centration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the μ-, κ-, or δ-receptor. Alcohol Clin Exp Res. 1998;22:1634–1639. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]
- 30.Walker BM, Koob GF. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol- experienced rats. Psychopharmacology. 2000;153:93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- 32.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- 34.Pillai NP, Ross DH. Ethanol-induced hypothermia in rats: possible involvement of opiate kappa receptors. Alcohol. 1986;3:249–253. doi: 10.1016/0741-8329(86)90033-9. [DOI] [PubMed] [Google Scholar]
- 35.Pohorecky LA, Patel V, Roberts P. Effects of ethanol in an open field apparatus: modification by U50488H and WIN 44441-3. Physiol Behav. 1989;45:273–287. doi: 10.1016/0031-9384(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 36.National Research Council. Washington DC: National Academy Press; 1985. Guide for the care and use of laboratory animals [NIH publication no. 85-23] [Google Scholar]
- 37.Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol reinforcing properties during infancy in the rat: operant self-administration of the drug. Alcohol Clin Exp Res. 2006;30(6 suppl):186A. [Google Scholar]
- 41.Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol. 2011a;45:45–55. doi: 10.1016/j.alcohol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, 2nd, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Naloxone blocks ethanol-mediated appetitive conditioning and locomotor activation in adolescent rats. Behav Brain Res. 2010;216:262–269. doi: 10.1016/j.bbr.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JS, Molina JC, Spear NE. Ontogenetic differences in the expression of odor-aversion learning in 4- and 8-day-old rats. Dev Psychobiol. 1990;23:319–330. doi: 10.1002/dev.420230404. [DOI] [PubMed] [Google Scholar]
- 47.Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 48.Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol’s orosensory effects but are positively reinforced by ethanol's post-ingestive effects. Pharmacol Biochem Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negus SS, Mello NK, Jones DCLRM, Portoghese PS. Kappa opioid antagonist effects of the novel kappa antagonist 5 ′ -guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology. 2002:412–419. doi: 10.1007/s00213-002-1038-x. [DOI] [PubMed] [Google Scholar]
- 50.Pautassi RM, Nizhnikov ME, Fabio MC, Spear NE. Early maternal separation affects ethanol-induced conditioning in a nor-Bni insensitive manner, but does not alter ethanol-induced locomotor activity. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2011.11.005. (early online version); [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roma PG, Rinker JA, Serafine KM, Chen SA, Barr CS, Cheng K, Rice KC, Riley AL. Genetic and early environmental contributions to alcohol’s aversive and physiological effects. Pharmacol Biochem Behav. 2008;91:134–139. doi: 10.1016/j.pbb.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broadbent J, Linder HV, Cunningham CL. Ethanol-induced conditioned taste preference in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- 54.Arias C, Molina JC, Spear NE. Ethanol-mediated aversive learning as a function of locomotor activity in a novel environment in infant Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92:621–628. doi: 10.1016/j.pbb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behavioral neuroscience. 2006;120:710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- 56.Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Nizhnikov ME, Pautassi RM, Varlinskaya E, Rahmani P, Spear NE. Ontogenetic Differences in Ethanol’s Motivational Properties during Infancy. Alcohol. 2101 doi: 10.1016/j.alcohol.2011.09.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 60.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 61.Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- 62.Pautassi RM, Nizhnikov ME, Spear NE. Ethanol-mediated appetitive conditioning in infant rats, but not corticosterone release, is dependent on route of ethanol administration. Dev Psychobiol. 2011b doi: 10.1002/dev.20567. in press. [DOI] [PubMed] [Google Scholar]
- 63.Samama B, Boehm N. nhibition of nitric oxide synthase impairs early olfactory associative learning in newborn rats. Neurobiol Learn Mem. 1999;71:219–231. doi: 10.1006/nlme.1998.3869. [DOI] [PubMed] [Google Scholar]
- 64.Gemberling GA, Domjan M, Amsel AJ. Aversion learning in 5-day-old rats: taste-toxicosis and texture-shock associations. J Comp Physiol Psychol. 1980;94:734–745. doi: 10.1037/h0077706. [DOI] [PubMed] [Google Scholar]
- 65.Arias C, Pautassi RM, Molina JC, Spear NE. A comparison between taste avoidance and conditioned disgust reactions induced by ethanol and lithium chloride in preweanling rats. Dev Psych. 2010;52:545–557. doi: 10.1002/dev.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina JC, Bannoura MD, Chotro MG, McKinzie DL, Arnold HM, Spear NE. Alcohol-mediated tactile conditioned aversions in infant rats: devaluation of conditioning through alcohol-sucrose associations. Neurobiol Learn Mem. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- 67.Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16-day-old) rats. Behav Neural Biol. 1990;54:300–322. doi: 10.1016/0163-1047(90)90650-u. [DOI] [PubMed] [Google Scholar]
- 68.Cunningham CL, Niehus JS. Flavor preference conditioning by oral self-administration of ethanol. Psychopharmacology. 1997;134:293–302. doi: 10.1007/s002130050452. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Vidal JM, Spear NE, Molina JC. Adolescent rats discriminate a mild state of ethanol intoxication likely to act as an appetitive unconditioned stimulus. Alcohol. 2003;30:45–60. doi: 10.1016/s0741-8329(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 70.Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol's hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- 72.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]