Although typically associated with transcriptional repression, sumoylation was recently found linked to active gene transcription. Manley and colleagues now probe the role of sumoylation during transcription. They find that the Gcn4 transcription factor is sumoylated upon recruitment to active promoters and that, with the involvement of Mediator component Srb10, this marks Gcn4 for promoter clearance, preventing excessive transcription of the gene.
Keywords: Gcn4, Srb10, sumoylation, transcription
Abstract
The small ubiquitin-related modifier (SUMO) is a conserved factor that post-translationally regulates proteins involved in many cellular processes, including gene transcription. We previously demonstrated that promoter-bound factors become sumoylated during activation of inducible genes in yeast, but the identity of these factors, and the role of sumoylation in their function, was unknown. Here we show that the transcriptional activator Gcn4 is sumoylated on two specific lysine residues and in a manner that depends on its ability to bind DNA, indicating that sumoylation occurs after Gcn4 binding to target promoters. Importantly, this functions to facilitate the subsequent removal of the activator from these promoters after recruitment of RNA polymerase II, which can prevent inappropriate transcription of target genes. Furthermore, we show that clearance of sumoylated Gcn4 requires the protein kinase and Mediator complex subunit Srb10, linking activator removal with target gene transcription. Our study demonstrates an unexpected role for protein sumoylation in the process of transcriptional activation.
Protein sumoylation is emerging as an important mechanism of regulating many cellular processes. Sumoylation involves the covalent post-translational modification of specific Lys residues on target proteins with the SUMO (small ubiquitin-related modifier) polypeptide. The functional consequences of sumoylation vary, generally resulting from altered protein–protein interactions, and regulation by SUMO is widespread. Orthologs of the SUMO protein are found in all eukaryotes, and sumoylated proteins are involved in a wide range of processes, including DNA repair, chromosome segregation, and gene expression (Zhao 2007; Makhnevych et al. 2009). One of the largest groups of SUMO-modified proteins in both yeast and mammals is transcription factors, whose sumoylation is usually associated with transcriptional repression (Gill 2005; Zhao 2007). This is thought to occur by recruitment of transcriptional corepressor complexes specifically to SUMO-modified transcription factors bound to promoters (Gill 2005; Garcia-Dominguez and Reyes 2009). However, in a few cases, sumoylation of gene-specific transcription factors is associated with activating transcription (Lyst and Stancheva 2007; Guo and Sharrocks 2009 and references therein), indicating that SUMO does not have solely a repressive role in transcription.
Supporting a role for SUMO at transcriptionally active genes, we previously demonstrated that the SUMO-conjugating enzyme Ubc9 is recruited to promoters of activated genes and promoter-bound factors become sumoylated during activation (Rosonina et al. 2010). However, the identity of these proteins and the effect of SUMO modification on their specific function during activation were not determined. Nonetheless, we found that reducing overall sumoylation at the promoter of the induced ARG1 gene did not affect its activation, but instead resulted in elevated transcription levels and impaired ability to shut down ARG1 transcription, with prolonged detection of RNA polymerase II (RNAP II) at the promoter after activation had ceased (Rosonina et al. 2010). This pointed to a possible role for SUMO in facilitating clearance of promoter-bound transcription factors, which can help shut off transcription or possibly reset promoters for further activation.
Here we report that the yeast transcriptional activator Gcn4 is sumoylated at the promoter of target genes. Interestingly, this sumoylation, which occurs on two specific Lys residues, facilitates Gcn4 removal during ongoing transcription. We further demonstrate that the protein kinase and Mediator subunit Srb10 also acts to facilitate clearance of Gcn4 from promoters, and this function is strongly stimulated by Gcn4 sumoylation. We show that removal of sumoylated Gcn4 through the Srb10 pathway is important for preventing excessive accumulation of RNAP II and elevated transcription levels when Gcn4 sumoylation is blocked and a redundant, but sumoylation-independent, pathway for removal of Gcn4 is disabled. Together, our data support a model in which SUMO marks promoter-bound Gcn4 for clearance through Srb10 phosphorylation-mediated degradation after RNAP II has engaged in transcription, and this is important to ensure tight control of target gene expression.
Results and Discussion
Chromatin-associated Gcn4 is sumoylated at Lys 50 and Lys 58
To identify specifically sumoylated proteins at the ARG1 promoter and other genes induced by the transcriptional activator Gcn4 during amino acid starvation, we looked for sumoylated factors in Gcn4-containing complexes isolated from induced cells by coimmunoprecipitation (co-IP) with Gcn4 (as a 6xHA fusion protein, Gcn4-HA). Expression of Gcn4 was induced by addition of sulfometuron-methyl (SM) to growth medium, which depletes cellular levels of Val and Ile (Falco and Dumas 1985). Anti-HA immunoblotting of the Gcn4-HA immunoprecipitations showed multiple modified forms of Gcn4, at least four of which comigrated with sumoylated species detected by anti-SUMO immunoblotting of the immunoprecipitations (Fig. 1A; Supplemental Fig. S1A). These sumoylated species were not disrupted in immunoprecipitations performed in elevated levels of NaCl (0.5 M), but were, in fact, stabilized (Fig. 1A), indicating that they are not derived from interacting proteins, but are very likely sumoylated forms of Gcn4 itself. Our data support previous proteomic studies that identified Gcn4 as a SUMO substrate (Wohlschlegel et al. 2004; Denison et al. 2005) and point to sumoylation as a means of regulating Gcn4 function.
Figure 1.
Gcn4 is sumoylated at Lys 50 and Lys 58. (A) HA immunoprecipitations from untreated cells or cells induced for expression of Gcn4-HA by addition of SM were analyzed by HA and SUMO immunoblots (cf. Supplemental Fig. S1). (Asterisks) Putative sumoylated Gcn4 isoforms; (open circles) unsumoylated Gcn4 isoforms. (B) Analysis of HA immunoprecipitations performed from induced cells expressing indicated Gcn4-HA mutants or controls, as in A. (ΔCT40) Gcn4 lacking its 40 C-terminal amino acids; (Ig) immunoglobulin band. (C) Diagram of Gcn4 structure, with probable sites of sumoylation indicated (encircled S). (DBD) DNA-binding domain.
To confirm that Gcn4 is, in fact, sumoylated and to identify possible Gcn4 sumoylation sites, we analyzed the effects of a number of Lys-to-Arg mutations in Gcn4 on sumoylation (Fig. 1B). Double Lys-to-Arg mutation of residues K50 and K58, both of which fall within consensus sumoylation motifs (Fig. 1C; Denison et al. 2005), completely abolished the SUMO-modified forms of Gcn4-6HA, identifying these residues as the probable sites of sumoylation (Fig. 1B). Although chromatin immunoprecipitation (ChIP) analysis of SUMO occupancy on the ARG1 promoter suggests that multiple promoter-bound factors become sumoylated during activation (Supplemental Fig. S1B,C), our data indicate that Gcn4 is sumoylated during activation of its target genes.
We next investigated whether Gcn4 is sumoylated specifically when bound to DNA. Gcn4 sumoylation was eliminated by deleting 40 C-terminal amino acids required for DNA binding (Hope and Struhl 1985), but not necessary for nuclear import (Fig. 1B; Pries et al. 2002). Similarly, mutation of a single Asn residue (N235; invariant among bZIP domain-containing proteins) to either Lys or Thr, which abolishes DNA binding in vitro (Pu and Struhl 1991), greatly reduced Gcn4 sumoylation (Fig. 1B). Finally, by chromatin fractionation, we found that sumoylated Gcn4 is stably associated with chromatin, implying that this modification plays a role in regulating the promoter-associated functions of Gcn4 (Supplemental Fig. S1D). Furthermore, essentially all nonsumoylatable Gcn4-mt was also associated with chromatin, indicating that sumoylation is not required for Gcn4 recruitment to DNA. Together, these data demonstrate that sumoylated Gcn4 is associated with chromatin, and Gcn4 sumoylation most likely occurs after it binds target gene promoters.
Sumoylation of Gcn4 facilitates its clearance from promoters after recruitment of RNAP II
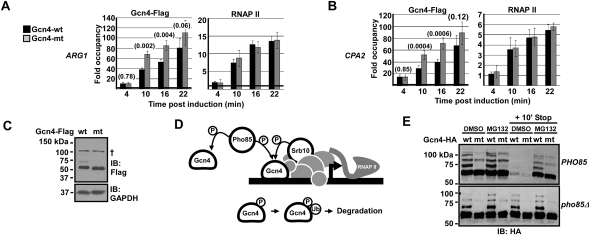
Next, to determine the function of Gcn4 sumoylation, we characterized yeast strains expressing nonsumoylatable, K50,58R mutant Gcn4 (Gcn4-mt) as the only source of Gcn4. Yeast expressing Gcn4-mt showed no discernible growth defect, compared with isogenic yeast expressing wild-type Gcn4 (Gcn4-wt), on medium depleted for amino acids (Supplemental Fig. S2A), nor did the mutations noticeably affect expression of the Gcn4 target genes ARG1 and CPA2, at least under these conditions (Supplemental Fig. S2B; but see below). However, using ChIP time-course analyses, we detected significantly higher levels of Gcn4-mt than Gcn4-wt on the ARG1 and CPA2 promoters ∼10–16 min post-induction (Fig. 2A,B), even though both Gcn4-wt and Gcn4-mt were expressed at equal levels (Fig. 2C). At a later time point (22 min), Gcn4-wt and Gcn4-mt occupancies were no longer statistically different (Fig. 2A,B), probably as activator occupancy of target promoters approached saturation, indicating that Gcn4 sumoylation regulates its occupancy levels under conditions in which promoter occupancy is not maximal. At an earlier time point (4 min), Gcn4-wt and Gcn4-mt occupancy levels were also equivalent (Fig. 2A,B), indicating that Gcn4 recruitment was not affected by the mutation, but that, over time, Gcn4-mt accumulated on target promoters to greater levels than did Gcn4-wt. Furthermore, the elevated levels of nonsumoylatable Gcn4 at the ARG1 and CPA2 promoters did not result in increased RNAP II levels (Fig. 2A,B), consistent with the lack of effect on transcript levels and pointing to a possible defect in removal of Gcn4-mt from promoters after RNAP II recruitment. Providing support for the idea that this defect was specifically due to blocked sumoylation, we found that Gcn4 occupancy on target promoters also increased in conditions in which global protein sumoylation was impaired (i.e., in yeast expressing a mutant form of Ubc9) (Supplemental Fig. S2C). Although we cannot entirely rule out the possibility that sumoylation affects recruitment of Gcn4 to promoters only after the first few minutes of induction, together these data are highly consistent with sumoylation being involved in clearance of Gcn4 from target promoters after RNAP II recruitment, such that blocking sumoylation causes prolonged association of Gcn4 with promoters.
Figure 2.
Gcn4 sumoylation facilitates its clearance from activated target promoters. (A,B) ChIP analysis of wild-type (wt) or K50,58R/sumoylation site mutant (mt) Gcn4-Flag and RNAP II occupancy on ARG1 (A) and CPA2 (B) promoters at indicated times after induction. The heavy gridline indicates background level (i.e., onefold over background). P-values (see the Materials and Methods) are indicated in parentheses above paired bars where relevant. (C) Flag and control GAPDH immunoblots of whole-cell extracts from cells induced for expression of Gcn4-Flag-wt or Gcn4-Flag-mt for 10 min. At the exposure shown and with a Flag tag, a single SUMO isoform can be detected in the wild-type sample (∼70 kDa). (†) Nonspecific band. (D) Model for regulation of promoter-bound or unbound Gcn4 by CDK (Pho85 or Srb10) phosphorylation-dependent, ubiquitin-mediated degradation. See the text for description. (E) HA immunoblot analysis of extracts from PHO85 or pho85Δ cells treated with DMSO or MG132, induced for expression of Gcn4-HA-wt or Gcn4-HA-mt, then treated with Stop Mix, as indicated.
Sumoylation of Gcn4 enhances its promoter clearance through Mediator component Srb10
We next wished to investigate how sumoylation might lead to removal of Gcn4 at target promoters. Two pathways are known to result in Gcn4 degradation through ubiquitin-mediated proteolysis, each involving a different cyclin-dependent kinase (CDK): (1) Pho85, which phosphorylates Gcn4 when amino acid levels are restored (Shemer et al. 2002; Bomeke et al. 2006), thereby marking it for ubiquitination and subsequent degradation through the 26S proteasome (see Fig. 2D,E; Kornitzer et al. 1994; Meimoun et al. 2000); and (2) Srb10, a component of the RNAP II Mediator complex that is recruited to activated promoters, including those activated by Gcn4 (Qiu et al. 2004). Like Pho85, Srb10 phosphorylates Gcn4, which targets it for ubiquitination in assays performed in cell extracts (Chi et al. 2001). However, deletion of PHO85 has a greater effect on Gcn4 stabilization than SRB10 deletion, which has an undetectable effect when Gcn4 is expressed at high levels (Meimoun et al. 2000; Chi et al. 2001), suggesting that Srb10 acts only on a fraction of total Gcn4 (Shemer et al. 2002; Irniger and Braus 2003). Thus, these Gcn4 degradation pathways serve different functions, either of which might be regulated by sumoylation.
We next examined whether Gcn4 sumoylation might be involved in regulating its clearance from target promoters by stimulating its degradation through Pho85 or Srb10. We confirmed that restoring amino acid levels by addition of concentrated Val and Ile (“Stop Mix”) (Rosonina et al. 2010) caused 26S proteasome-dependent (i.e., MG132-sensitive) Gcn4 degradation through Pho85 (Fig. 2E). Blocking the proteasome with MG132 likely had little or no effect on Gcn4 levels prior to addition of the Stop Mix because, within 20 min of induction, Gcn4 expression is expected to continue until amino acid levels are restored. No differences in degradation (Fig. 2E) or clearance from target gene promoters (Supplemental Fig. S2D) in response to the Stop Mix were observed between Gcn4-wt and Gcn4-mt, indicating that Gcn4 sumoylation is not involved in this Pho85-mediated degradation pathway. Additionally, since mutation of Lys 50 and Lys 58 did not affect ubiquitin-dependent proteasome-mediated degradation of Gcn4 (Fig. 2E), increased promoter occupancy of Gcn4-mt was not caused by blocking possible ubiquitination of these specific Lys residues (which, in any case, do not lie within previously reported Gcn4 degradation domains) (Meimoun et al. 2000).
Next, we determined whether SRB10 deletion affects levels of Gcn4 on the ARG1 and CPA2 promoters during induction. Upon induction of amino acid starvation, Gcn4 was expressed at somewhat reduced levels in srb10Δ cells compared with wild-type cells (Fig. 3A). In spite of this, time-course ChIP analysis demonstrated that after initial recruitment (4 min), as activation continued, significantly more Gcn4 was detected at target promoters in srb10Δ cells (Fig. 3B,C). This was not due to a defect in clearance of Gcn4 through the Pho85-mediated pathway as amino acid levels were restored, since addition of the Stop Mix effectively cleared Gcn4 in both SRB10 and srb10Δ strains (Supplemental Fig. S3B). Although blocking Gcn4 sumoylation and deletion of SRB10 had a similar effect on Gcn4 promoter retention, cells lacking Srb10 additionally showed reduced recruitment of RNAP II to Gcn4 target promoters (Fig. 3B,C; Supplemental Fig. S3A). This is consistent with previous observations (Qiu et al. 2004) and likely reflects the fact that, as a component of the Mediator complex present at many active genes, Srb10 has general functions in activation of transcription. Nonetheless, our results establish a specific role for Srb10 in clearance of promoter-bound Gcn4.
Figure 3.
Srb10 functions in clearance of Gcn4 from target promoters during activation, which is facilitated by Gcn4 sumoylation. (A) Flag and control GAPDH immunoblot analysis of extracts from SRB10 (+) or srb10Δ (Δ) cells induced for the indicated durations and after addition of Stop Mix. (B,C) ChIP analysis of Gcn4-Flag or RNAP II occupancy on the ARG1 (B) and CPA2 (C) promoters in SRB10 or srb10Δ cells after induction for indicated time. (D) ChIP analysis as in B and C, performed in srb10Δ cells expressing either Gcn4-wt or Gcn4-mt, as indicated.
Our data have demonstrated that both SUMO modification and Srb10 act in clearing Gcn4 from target promoters. We next examined whether both processes function in the same pathway. Deletion of SRB10 did not prevent Gcn4 sumoylation (Supplemental Fig. S3C), indicating that phosphorylation by Srb10 is not a prerequisite for Gcn4 sumoylation. However, we detected increased Gcn4 sumoylation in srb10Δ cells (increase of ∼30%) (Supplemental Fig. S3C,D), suggesting an accumulation of sumoylated Gcn4 in the absence of Srb10. This would be expected if Srb10 preferentially targets sumoylated Gcn4 for degradation. In strong support of this notion, Gcn4-wt and Gcn4-mt levels on target gene promoters were equivalent during a time course in srb10Δ cells (Fig. 3D), indicating that Srb10 is required for the increased clearance of wild-type (sumoylatable) Gcn4 from target promoters described above. Extending this result, mutation of all five possible Srb10 phosphorylation sites in Gcn4 (Chi et al. 2001) also caused increased Gcn4 promoter occupancy (Supplemental Fig. 3SE), providing evidence that it is indeed phosphorylation of Gcn4 by Srb10 that enhances Gcn4 clearance. Together, our data indicate that Gcn4 sumoylation facilitates its clearance from target promoters through an Srb10-dependent pathway.
Blocking Gcn4 sumoylation causes elevated transcription of ARG1 in the absence of Pho85
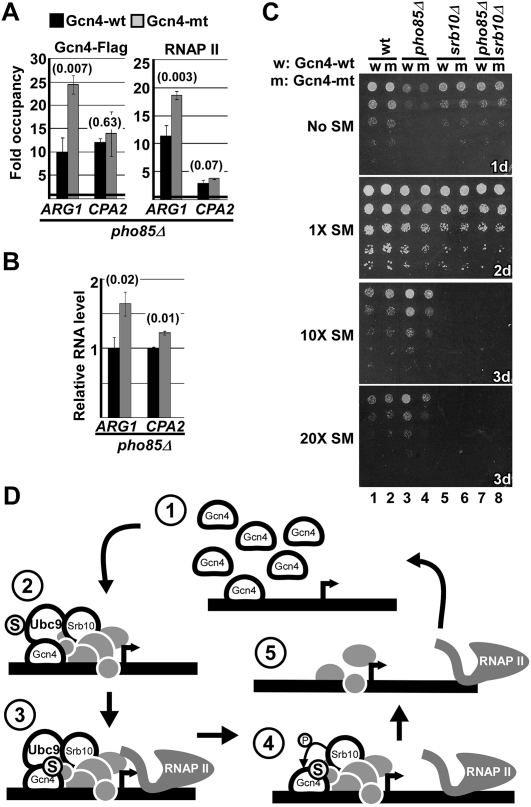
In light of the above, we next considered the possibility that the consequences of Gcn4 sumoylation on Srb10-mediated Gcn4 promoter clearance were being masked by Pho85, reflecting its prominent, SUMO-independent role in clearing of Gcn4 from cells. To address this, we determined the effects of Gcn4 sumoylation in the absence of the Pho85 pathway. Overall levels of Gcn4 occupancy were reduced in pho85Δ cells (cf. Figs. 2A,B and 4A), which grow slowly compared with wild type (see Fig. 4C). Nonetheless, blocking Gcn4 sumoylation in pho85Δ cells (by the K50,58R mutation) caused not only increased promoter occupancy of the activator, but also a corresponding increase in RNAP II accumulation and higher transcription of the ARG1 gene (Fig. 4A,B). Although no significant effect was observed for CPA2 in pho85Δ cells, whose activation was low compared with ARG1 (see RNAP II occupancy in Figs. 2–4), these results demonstrate that blocking Gcn4 sumoylation can, in fact, affect transcription of at least some target genes, but this effect is normally masked by Pho85, likely through Pho85-mediated degradation of Gcn4 independent of its sumoylation status.
Figure 4.
Blocking Gcn4 sumoylation causes increased transcription of ARG1 in the absence of Pho85. (A) ChIP analysis of Gcn4-Flag or RNAP II occupancy on the ARG1 and CPA2 promoters in pho85Δ cells 16 min after inducing expression of either Gcn4-wt or Gcn4-mt, as indicated. (B) RT–PCR analysis of ARG1 and CPA2 transcript levels in pho85Δ cells after induction of either Gcn4-wt or Gcn4-mt expression, as indicated. Values are normalized to the level of the indicated gene transcript for Gcn4-wt-expressing cells. (C) Comparison of growth for the indicated strains. Strains were spotted on medium containing the indicated level of SM (1× is 0.5 μg/mL) in fivefold dilution series and were grown for the indicated durations. (D) Model indicating the role of Gcn4 sumoylation in its Srb10-mediated clearance from target promoters. (1,2) Promoter-bound Gcn4 recruits transcription complexes, including Srb10 as part of the Mediator, Ubc9, and SUMO, to activated target promoters. Upon transcription initiation, Ubc9 sumoylates Gcn4 (3), marking it for clearance through an Srb10 phosphorylation-mediated pathway (4), clearing the promoter of Gcn4 and possibly other factors (5), which allows for reactivation of the promoter (1).
Previously, we showed that globally reduced sumoylation caused increased transcription of ARG1 as well as a growth defect in cells exposed to amino acid starvation (Rosonina et al. 2010). This is reminiscent of observations by others demonstrating that overexpression of Gcn4 inhibits cell growth, an effect that is exacerbated by deletion of PHO85 (Meimoun et al. 2000; Shemer et al. 2002). Consistent with this, specifically blocking Gcn4 sumoylation (i.e., in Gcn4-mt) also resulted in a growth defect in pho85Δ cells under more severe amino acid starvation conditions (10×–20× SM) (Fig. 4C, cf. growth in lanes 3 and 4). This growth defect is possibly a consequence of overactivity of Gcn4 (by overexpression or blocking of sumoylation-mediated promoter clearance), resulting in extended expression of amino acid biosynthesis genes beyond their need, which is energetically costly (Wagner 2005). This growth defect demonstrates a synthetic genetic interaction between Gcn4 sumoylation and PHO85, and, together with our observations in pho85Δ cells described above, reflects redundant or compensatory pathways for elimination of Gcn4 (see Hartman et al. 2001; Boone et al. 2007). This explains the necessity of eliminating Pho85 in order to observe effects of Gcn4 sumoylation on target gene transcription. Interestingly, we found that Srb10 is essential for growth under these conditions (Fig. 4C, lanes 5–8), raising the possibility that the Srb10–Gcn4 sumoylation pathway that we uncovered can be critical for appropriate transcription of Gcn4 target genes. Together, these data indicate that sumoylation of Gcn4 enables its efficient clearance from target promoters, thereby preventing aberrant and detrimental expression of target genes, specifically under conditions in which tight control of expression of these genes is necessary, such as in the absence of Pho85.
The experiments described above have provided new insights into the mechanism of transcriptional activation and identified an unexpected role for protein sumoylation in this process. These findings give rise to a model (Fig. 4D) whereby Gcn4 is sumoylated at target gene promoters after RNAP II has been recruited, and this modification specifically activates Srb10-mediated degradation of the activator. The functional consequence of SUMO-enhanced Gcn4 degradation at promoters, which is apparent when the dominant mechanism of Gcn4 degradation (Pho85 pathway) is blocked, is the prevention of unregulated and elevated transcription of target genes. In Pho85-expressing cells, blocking sumoylation causes retention of Gcn4 at promoters through the Srb10-dependent pathway, but the redundant Pho85-dependent pathway acts to remove Gcn4 before it can affect transcription levels. In the absence of Pho85, nonsumoylatable Gcn4 accumulates on promoters to a greater degree compared with wild-type Gcn4, causing increased RNAP II recruitment and higher levels of transcription, since neither the Srb10 nor Pho85 pathway can remove it efficiently. The rate of removal of an activator from target promoters, therefore, is not only linked to the condition of the cell (e.g., through Pho85 upon amino acid level restoration), but also tightly coupled to transcription itself through both the Pho85 and SUMO/Srb10-mediated pathways. This affords the cells tightly controlled mechanisms to adjust gene expression levels rapidly in response to changing growth conditions and connects target gene transcription levels with the abundance of an activator. Thus, we uncovered novel roles for Srb10 and SUMO in regulating transcription activation through a highly dynamic system of activator recruitment and removal, ensuring tight control of gene expression.
We provided evidence that Srb10 plays a key role in resetting promoters during transcription by clearing Gcn4. Srb10 phosphorylates Gcn4 in vivo and stimulates its ubiquitination in in vitro assays (Chi et al. 2001), indicating that Srb10 likely functions through phosphorylation-dependent ubiquitin-mediated degradation of Gcn4 (Fig. 4D). Because both Srb10 and Gcn4 are recruited to promoters of target genes during activation (Qiu et al. 2004), it had been speculated that Srb10 acts specifically on promoter-bound Gcn4 (Chi et al. 2001). Our data now directly demonstrate that Srb10 indeed controls Gcn4 levels on promoters of target genes, thereby linking Gcn4 clearance with transcription of target genes. In vitro binding assays showed that Srb10 interacts with the activation domains of Gcn4, Gal4, and other activators (Ansari et al. 2002), indicating that Srb10 has the potential to be involved in resetting additional inducible genes. In support of this, the yeast Gal4 activator was shown to associate dynamically with the GAL1 promoter during activation (Lipford et al. 2005; Collins et al. 2009), and Srb10 is required for full activation of that gene (Hirst et al. 1999 and references therein). Further supporting a role for Srb10 in resetting promoters for continued recruitment of RNAP II, in vitro transcription assays showed that Srb10 functions in disrupting the preinitiation complex, which is necessary for formation of a scaffold that sustains transcriptional reinitiation (Yudkovsky et al. 2000; Liu et al. 2004). By specifically targeting promoter-bound factors such as Gcn4 after RNAP II has initiated transcription, we showed that an important, likely general, function of Srb10 is to regulate promoter activity by facilitating multiple cycles of activation.
SUMO-mediated promoter clearance of transcriptional regulators is likely to be a general and evolutionarily conserved mechanism. For example, sumoylation of promoter-bound PARP-1 has been shown to facilitate its ubiquitin-mediated clearance from the activated HSP70.1 promoter in mammalian cells (Martin et al. 2009). A human transcription factor that shares homology with Gcn4, Atf4, contains a consensus sumoylation motif within a region that is highly homologous to the region surrounding Gcn4 K58, indicating that sumoylation might play a conserved role in regulating both factors. Consistent with our observations with Gcn4, a melanoma- and renal carcinoma-associated missense substitution found within a sumoylation site of transcription factor MITF was recently found to impair MITF sumoylation while increasing its promoter occupancy and the transcription of many target genes (Bertolotto et al. 2011). Furthermore, an unidentified post-translational modification of promoter-bound ERα was suggested to be necessary to signal its degradation, allowing cycling of activators on ERα target promoters (Reid et al. 2003). ERα was later shown to be sumoylated, and blocking its sumoylation was found to impair ERα-induced transcription (Sentis et al. 2005). It will be interesting to determine whether SUMO in fact marks promoter-bound ERα for degradation, demonstrating that SUMO acts to clear and reset promoters for reactivation across species. After decades of studying the coordinated recruitment of transcription factors to activated gene promoters that leads to transcriptional initiation, the dynamics involved in maintaining and terminating transcriptional activation are emerging, and our results indicate that protein sumoylation plays a central role.
Materials and methods
The yeast strains used are listed in Supplemental Table S1. Construction of plasmid pGCN4-Flag was previously reported (Rosonina et al. 2010). Strains expressing Flag-tagged Gcn4 (Gcn4-Flag) were used for ChIP experiments and accompanying control immunoblots, while strains expressing Gcn4-HA were used for detecting modified forms of Gcn4. At long exposures, immunoblots of Gcn4-Flag showed the same modified forms seen with Gcn4-HA (data not shown). Plasmid pGCN4-HA was constructed by replacing the Flag tag sequence with the 6xHA sequence in pGCN4-Flag, and derived mutants were generated by site-directed mutagenesis.
For induction of Gcn4 target genes by amino acid starvation, yeast were grown in minimal medium lacking Ile and Val to an optical density A260 (O.D.) of 0.6–0.8, then induced for 20 min (unless otherwise specified) with SM (Sigma) at a final concentration of 0.5 μg/mL. To stop induction, Ile and Val were added to 10 times their normal concentration (“Stop Mix”) for 10 min. ChIP, RT–PCR RNA analysis, ChIP and RT–PCR quantification, and yeast growth spot assays were performed as previously described (Rosonina et al. 2010). For protein immunoprecipitations, cells were grown as above and lysed by agitation with glass beads. Washes, lysis, and immunoprecipitations were performed in the presence of 2.5 mg/mL N-ethylmaleimide and 150 mM NaCl, except as indicated in Figure 1, A and B, where 500 mM NaCl was used. Proteasomal inhibition was performed as previously described (Liu et al. 2007) by addition of MG132 or mock-treated with DMSO for 45 min prior to induction. Quantitative immunoblotting was performed using the Odyssey system (Li-Cor). Where statistical analysis was performed, a two-tailed Student's t-test was used, with P-values indicated in parentheses above paired bars within graphs. Antibodies used for ChIP, immunoprecipitation, and immunoblotting were anti-Flag M2 (Sigma), rabbit anti-HA (abm), mouse anti-HA (HA.11; Covance), and anti-GAPDH (Sigma).
Acknowledgments
We thank Alan Hinnebusch for sharing yeast strains. We also thank Patricia Richard and Dafne Campigli Di Giammartino for discussions and comments on the manuscript. This work was supported by grant GM097174 from the National Institutes of Health to J.L.M.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.184689.111.
References
- Ansari AZ, Koh SS, Zaman Z, Bongards C, Lehming N, Young RA, Ptashne M 2002. Transcriptional activating regions target a cyclin-dependent kinase. Proc Natl Acad Sci 99: 14706–14709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotto C, Lesueur F, Giuliano S, Strub T, de Lichy M, Bille K, Dessen P, d'Hayer B, Mohamdi H, Remenieras A et al. 2011. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 480: 94–98 [DOI] [PubMed] [Google Scholar]
- Bomeke K, Pries R, Korte V, Scholz E, Herzog B, Schulze F, Braus GH 2006. Yeast Gcn4p stabilization is initiated by the dissociation of the nuclear Pho85p/Pcl5p complex. Mol Biol Cell 17: 2952–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C, Bussey H, Andrews BJ 2007. Exploring genetic interactions and networks with yeast. Nat Rev Genet 8: 437–449 [DOI] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev 15: 1078–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GA, Lipford JR, Deshaies RJ, Tansey WP 2009. Gal4 turnover and transcription activation. Nature 461: E7–E8 doi: 10.1038/nature08406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison C, Rudner AD, Gerber SA, Bakalarski CE, Moazed D, Gygi SP 2005. A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol Cell Proteomics 4: 246–254 [DOI] [PubMed] [Google Scholar]
- Falco SC, Dumas KS 1985. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics 109: 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Reyes JC 2009. SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim Biophys Acta 1789: 451–459 [DOI] [PubMed] [Google Scholar]
- Gill G 2005. Something about SUMO inhibits transcription. Curr Opin Genet Dev 15: 536–541 [DOI] [PubMed] [Google Scholar]
- Guo B, Sharrocks AD 2009. Extracellular signal-regulated kinase mitogen-activated protein kinase signaling initiates a dynamic interplay between sumoylation and ubiquitination to regulate the activity of the transcriptional activator PEA3. Mol Cell Biol 29: 3204–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman JLt, Garvik B, Hartwell L 2001. Principles for the buffering of genetic variation. Science 291: 1001–1004 [DOI] [PubMed] [Google Scholar]
- Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I 1999. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell 3: 673–678 [DOI] [PubMed] [Google Scholar]
- Hope IA, Struhl K 1985. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: Implications for general control of amino acid biosynthetic genes in yeast. Cell 43: 177–188 [DOI] [PubMed] [Google Scholar]
- Irniger S, Braus GH 2003. Controlling transcription by destruction: The regulation of yeast Gcn4p stability. Curr Genet 44: 8–18 [DOI] [PubMed] [Google Scholar]
- Kornitzer D, Raboy B, Kulka RG, Fink GR 1994. Regulated degradation of the transcription factor Gcn4. EMBO J 13: 6021–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford JR, Smith GT, Chi Y, Deshaies RJ 2005. A putative stimulatory role for activator turnover in gene expression. Nature 438: 113–116 [DOI] [PubMed] [Google Scholar]
- Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S 2004. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol Cell Biol 24: 1721–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Apodaca J, Davis LE, Rao H 2007. Proteasome inhibition in wild-type yeast Saccharomyces cerevisiae cells. Biotechniques 42: 158–162 [DOI] [PubMed] [Google Scholar]
- Lyst MJ, Stancheva I 2007. A role for SUMO modification in transcriptional repression and activation. Biochem Soc Trans 35: 1389–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhnevych T, Sydorskyy Y, Xin X, Srikumar T, Vizeacoumar FJ, Jeram SM, Li Z, Bahr S, Andrews BJ, Boone C, et al. 2009. Global map of SUMO function revealed by protein–protein interaction and genetic networks. Mol Cell 33: 124–135 [DOI] [PubMed] [Google Scholar]
- Martin N, Schwamborn K, Schreiber V, Werner A, Guillier C, Zhang XD, Bischof O, Seeler JS, Dejean A 2009. PARP-1 transcriptional activity is regulated by sumoylation upon heat shock. EMBO J 28: 3534–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimoun A, Holtzman T, Weissman Z, McBride HJ, Stillman DJ, Fink GR, Kornitzer D 2000. Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCF(CDC4) ubiquitin-ligase complex. Mol Biol Cell 11: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries R, Bomeke K, Irniger S, Grundmann O, Braus GH 2002. Amino acid-dependent Gcn4p stability regulation occurs exclusively in the yeast nucleus. Eukaryot Cell 1: 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu WT, Struhl K 1991. Highly conserved residues in the bZIP domain of yeast GCN4 are not essential for DNA binding. Mol Cell Biol 11: 4918–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Hu C, Yoon S, Natarajan K, Swanson MJ, Hinnebusch AG 2004. An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p. Mol Cell Biol 24: 4104–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F 2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11: 695–707 [DOI] [PubMed] [Google Scholar]
- Rosonina E, Duncan SM, Manley JL 2010. SUMO functions in constitutive transcription and during activation of inducible genes in yeast. Genes Dev 24: 1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L 2005. Sumoylation of the estrogen receptor α hinge region regulates its transcriptional activity. Mol Endocrinol 19: 2671–2684 [DOI] [PubMed] [Google Scholar]
- Shemer R, Meimoun A, Holtzman T, Kornitzer D 2002. Regulation of the transcription factor Gcn4 by Pho85 cyclin PCL5. Mol Cell Biol 22: 5395–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A 2005. Energy constraints on the evolution of gene expression. Mol Biol Evol 22: 1365–1374 [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Johnson ES, Reed SI, Yates JR III 2004. Global analysis of protein sumoylation in Saccharomyces cerevisiae. J Biol Chem 279: 45662–45668 [DOI] [PubMed] [Google Scholar]
- Yudkovsky N, Ranish JA, Hahn S 2000. A transcription reinitiation intermediate that is stabilized by activator. Nature 408: 225–229 [DOI] [PubMed] [Google Scholar]
- Zhao J 2007. Sumoylation regulates diverse biological processes. Cell Mol Life Sci 64: 3017–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]