Abstract
Background.
The purpose of this study was to identify determinants of renal disease progression in adults with Fabry disease during treatment with agalsidase beta.
Methods.
Renal function was evaluated in 151 men and 62 women from the Fabry Registry who received agalsidase beta at an average dose of 1 mg/kg/2 weeks for at least 2 years. Patients were categorized into quartiles based on slopes of estimated glomerular filtration rate (eGFR) during treatment. Multivariate logistic regression analyses were used to identify factors associated with renal disease progression.
Results.
Men within the first quartile had a mean eGFR slope of –0.1 mL/min/1.73m2/year, whereas men with the most rapid renal disease progression (Quartile 4) had a mean eGFR slope of –6.7 mL/min/1.73m2/year. The risk factor most strongly associated with renal disease progression was averaged urinary protein:creatinine ratio (UP/Cr) ≥1 g/g (odds ratio 112, 95% confidence interval (95% CI) 4–3109, P = 0.0054). Longer time from symptom onset to treatment was also associated with renal disease progression (odds ratio 19, 95% CI 2–184, P = 0.0098). Women in Quartile 4 had the highest averaged UP/Cr (mean 1.8 g/g) and the most rapid renal disease progression: (mean slope –4.4 mL/min/1.73m2/year).
Conclusions.
Adults with Fabry disease are at risk for progressive loss of eGFR despite enzyme replacement therapy, particularly if proteinuria is ≥1 g/g. Men with little urinary protein excretion and those who began receiving agalsidase beta sooner after the onset of symptoms had stable renal function. These findings suggest that early intervention may lead to optimal renal outcomes.
Keywords: alpha galactosidase, enzyme replacement therapy, Fabry disease, genetic renal disease, proteinuria
Introduction
Fabry disease is a rare X-linked systemic disorder, in which globotriaosylceramide (GL-3) and other glycosphingolipids accumulate within lysosomes, due to insufficient activity of α-galactosidase A (α-GalA). Progressive accumulation of GL-3, particularly in the vascular endothelium, is associated with chronic kidney disease, cerebrovascular and cardiovascular disease [1]. In the kidneys, GL-3 deposits have been described in all types of glomerular, tubular, vascular and interstitial cells [2–5]. Hemizygous males typically experience the most severe manifestations of Fabry disease [1], but heterozygous females can also develop major complications [6–9].
Most men and some women with Fabry disease exhibit deterioration of renal function [7, 10, 11], and many eventually develop end-stage renal disease (ESRD) [6, 12]. Although progression to ESRD is much less common in women, the median age at which patients reached ESRD (38 years) was the same in both genders [6, 12]. Studies of untreated patients with Fabry disease have identified proteinuria as a major risk factor for renal disease progression [7, 10].
Agalsidase beta, a form of recombinant human α-GalA, is approved for use as enzyme replacement therapy (ERT) for Fabry disease (Fabrazyme®; Genzyme Corporation, Cambridge, MA). In clinical studies, agalsidase beta cleared microvascular endothelial GL-3 deposits from the kidney, heart and skin [13, 14]. It also provided long-term stabilization of renal function in patients with mild renal involvement (serum creatinine <2.2 mg/dL) [14] and delayed time to renal, cardiovascular and cerebrovascular events in patients with more advanced Fabry disease [15]. Agalsidase beta treatment did not stabilize renal function in patients with severe renal involvement [i.e. proteinuria >1 g/24 h or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2] at the time treatment was started [14, 15].
Randomized clinical trials of ERT for patients with Fabry disease have been performed on a relatively limited number of subjects. The Fabry Registry is an observational database that compiles clinical and laboratory data from both treated and untreated patients; it was established to further investigate the long-term effects of ERT and the natural progression of Fabry disease in a larger population. The objective of these analyses was to identify determinants of renal disease progression among adult patients treated with agalsidase beta at a dose of 1 mg/kg/2 weeks.
Materials and methods
As of 3 July 2009, the Fabry Registry included 1429 men and 1458 women aged ≥18 years. Patients provide informed consent through local Institutional Review Boards/Ethics Committees and may decline to participate or withdraw consent at any time. Given the voluntary participation, patients’ ages at clinical assessments and time intervals between assessments are variable.
To be included in these analyses, patients must have received agalsidase beta at an average dose at or near the recommended licensed dose of 1 mg/kg/2 weeks (≥0.9 to ≤1.1 mg/kg/2 weeks). Patients must have had at least three serum creatinine values reported over a span of at least 2 years after their first agalsidase beta infusion, with at least one of the three eGFR assessments reported within 3 months before or after their first infusion and at least one urinary protein:creatinine ratio (UP/Cr) value reported within 3 months before or after their first infusion. Both spot and 24-h UP/Cr values were used to calculate UP/Cr values and the average of all UP/Cr values reported during the follow-up period was calculated for patients with multiple values.
Data were collected prior to any chronic dialysis or kidney transplantation. Cardiovascular clinical events were defined as myocardial infarction, congestive heart failure, arrhythmia, angina pectoris or a significant cardiac procedure (e.g. pacemaker or other implantable cardiac device placement, bypass, stent placement, valve replacement, transplantation). Cerebrovascular events were defined as stroke.
eGFR was calculated from serum creatinine values, using the Chronic Kidney Disease Epidemiology Collaboration equation [16]. Regression slopes representing an individual’s change in eGFR following initiation of treatment were calculated based on eGFR values reported after the first infusion. Patients were grouped into quartiles, based on eGFR slope.
Logistic regression analyses were used to identify factors associated with men in Quartile 1 (Q1) compared to men in Quartile 4 (Q4). Proportional odds regression analyses were used to identify predictors in men across quartiles. Baseline eGFR, averaged UP/Cr, the occurrence a cardiovascular or cerebrovascular clinical event prior to the initiation of treatment, age at first infusion, time from diagnosis to first infusion, time from symptom onset to first infusion and averaged systolic and diastolic blood pressure were used as predictor variables. Results from logistic regression and proportional odds regression are expressed as odds ratios, with corresponding 95% confidence intervals (95% CI) and P-values. Because there were fewer women in the cohort, a simplified logistic regression model was created by including fewer covariates and by combining data from women in Q1–Q3 and comparing these against data from women in Q4.
Statistical analyses were performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC). An alpha-level of 0.05 was used as the cut-point to determine statistical significance.
Results
One hundred fifty-one men and 62 women met the inclusion criteria, including a baseline UP/Cr value and three or more eGFR values (see Materials and methods); 95% of the men and 92% of the women had two or more UP/Cr values reported during the treatment period. The median age at initiation of agalsidase beta treatment was 38 years for men and 43 years for women. Men were followed for 5 ± 1.9 years (mean ± SD) and women for 4 ± 1.6 years.
The 38 men in Q1 (those with the most stable renal function) had a mean eGFR slope of −0.1 mL/min/1.73m2/year, whereas the 37 men in Q4 (those with the fastest renal disease progression) had a mean eGFR slope of −6.7 mL/min/1.73m2/year. Clinical characteristics for these patients are shown in Table 1. Men in Q1 had lower mean averaged UP/Cr levels than those in Q4: 0.7 versus 1.7 g/g. Men in Q1 also had higher mean baseline eGFR levels than men in Q4: 99 versus 72 mL/min/1.73m2 (i.e. eGFR at the time treatment was initiated). Men in Q1 initiated agalsidase beta treatment sooner after the onset of Fabry symptoms than men in Q4: median 20 versus 35 years. Mean averaged blood pressure levels were similar across quartiles.
Table 1.
Clinical characteristics of men during agalsidase beta treatmenta
| eGFR slope Quartile 1 (−1.2 to +5.3) | eGFR slope Quartile 2 (−2.8 to −1.3) | eGFR slope Quartile 3 (−4.8 to −2.9) | eGFR slope Quartile 4 (−15.5 to −4.9) | |
| eGFR slope (mL/min/1.73m2/year), n | 38 | 38 | 38 | 37 |
| Mean (SD) | −0.1 (1.20) | −2.1 (0.52) | −3.8 (0.61) | −6.7 (2.26) |
| Median (25th, 75th) | −0.5 (−0.9, 0.6) | −2.1 (−2.6, −1.5) | −3.6 (−4.3, −3.3) | −6.2 (−6.9, −5.5) |
| Averaged UP/Cr (g/g), n | 38 | 38 | 38 | 37 |
| Mean (SD) | 0.7 (0.80) | 0.7 (0.78) | 0.8 (0.86) | 1.7 (1.21) |
| Median (25th, 75th) | 0.3 (0.1, 0.9) | 0.4 (0.2, 1.1) | 0.6 (0.3, 1.1) | 1.5 (1.1, 2.0) |
| Pre-treatment (baseline) eGFR (mL/min/1.73m2), n | 37 | 37 | 38 | 36 |
| Mean (SD) | 99 (22.6) | 77 (32.9) | 88 (38.7) | 72 (34.3) |
| Median (25th, 75th) | 104 (89, 115) | 73 (46, 108) | 88 (51, 123) | 61 (47, 95) |
| Averaged SBP (mmHg), n | 38 | 38 | 38 | 37 |
| Mean (SD) | 119 (11.8) | 122 (10.0) | 121 (9.5) | 125 (10.8) |
| Median (25th, 75th) | 120 (110, 126) | 122 (114, 128) | 122 (116, 127) | 125 (115, 132) |
| Averaged DBP (mmHg), n | 38 | 38 | 38 | 37 |
| Mean (SD) | 71 (8.7) | 73 (7.7) | 73 (8.1) | 74 (6.6) |
| Median (25th, 75th) | 70 (64, 77) | 72 (68, 78) | 74 (67, 79) | 73 (71, 79) |
| Reported ACEi/ARB use, n (%) | 13 (34.2) | 19 (50.0) | 19 (50.0) | 19 (51.4) |
| Stroke or cardiovascular eventb prior to first infusion, n (%) | 3 (7.9) | 13 (34.2) | 13 (34.2) | 17 (45.9) |
| Time from symptom onset to first agalsidase beta infusion (years), n | 35 | 31 | 31 | 32 |
| Mean (SD) | 20.2 (10.92) | 22.4 (13.92) | 20.4 (12.63) | 31.9 (12.34) |
| Median (25th, 75th) | 19.9 (12.9, 28.2) | 20.3 (13.1, 31.6) | 18.9 (10.9, 30.1) | 34.6 (26.0, 41.7) |
| Time from diagnosis to first agalsidase beta infusion (years), n | 38 | 38 | 38 | 37 |
| Mean (SD) | 6.7 (7.50) | 7.5 (10.45) | 9.0 (11.05) | 10.7 (11.86) |
| Median (25th, 75th) | 3.2 (0.9, 13.3) | 2.1 (0.6, 7.5) | 2.4 (0.7, 18.5) | 4.0 (0.6, 22.2) |
| Age at first infusion (years), n | 38 | 38 | 38 | 37 |
| Mean (SD) | 35.3 (11.04) | 40.7 (11.12) | 37.0 (10.91) | 42.0 (9.22) |
| Median (25th, 75th) | 34.7 (27.5, 41.9) | 41.9 (33.2, 51.1) | 37.1 (27.9, 46.1) | 42.7 (35.7, 48.0) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; 25th, 25th percentile value; 75th, 75th percentile value. All data are from adult Fabry Registry patients (≥18 years) with three or more eGFR assessments (calculated by Chronic Kidney Disease Epidemiology Collaboration equation) over a period of ≥2 years (at least one eGFR assessment within 3 months of initiation of agalsidase beta treatment) and prior to any chronic dialysis or renal transplant events. ‘Averaged’ UP/Cr and blood pressure data reflect the average of all values reported within 3 months of the date of the first agalsidase beta infusion to the most recent assessment during treatment. Baseline eGFR is based on the assessment date closest to a patient’s first infusion of agalsidase beta (within 3 months of the first infusion).
Cardiovascular events were defined as described in ‘Materials and methods’.
Clinical characteristics of women are shown in Table 2. Like men, women in Q4 tended to have higher UP/Cr levels than women in Q1. There were no apparent differences across quartiles in baseline eGFR levels, time from symptom onset to first infusion or age at first infusion in women. Averaged blood pressure levels in women were within or slightly above the normal range across quartiles.
Table 2.
Clinical characteristics of women during agalsidase beta treatmenta
| eGFR slope Quartile 1 (+1.1 to +7.5) | eGFR slope Quartile 2 (−0.4 to +1.0) | eGFR slope Quartile 3 (−2.3 to −0.4) | eGFR slope Quartile 4 (−7.6 to −2.4) | |
| eGFR slope (mL/min/1.73m2/year), n | 15 | 16 | 16 | 15 |
| Mean (SD) | 2.7 (1.65) | 0.1 (0.41) | −1.3 (0.66) | −4.4 (1.58) |
| Median (25th, 75th) | 2.6 (1.3, 3.4) | 0.1 (−0.2, 0.4) | −1.4 (−1.7, −0.6) | −4.2 (−5.5, −3.0) |
| Averaged UP/Cr (g/g), n | 15 | 16 | 16 | 15 |
| Mean (SD) | 0.8 (1.47) | 0.9 (1.03) | 0.6 (0.86) | 1.8 (1.65) |
| Median (25th, 75th) | 0.2 (0.1, 0.6) | 0.5 (0.2, 1.1) | 0.3 (0.1, 0.7) | 1.3 (0.5, 2.9) |
| Pre-treatment (baseline) eGFR (mL/min/1.73m2), n | 14 | 14 | 16 | 14 |
| Mean (SD) | 88 (16.0) | 90 (23.9) | 95 (27.6) | 78 (35.6) |
| Median (25th, 75th) | 87 (75, 100) | 93 (72, 111) | 98 (76, 118) | 85 (36, 105) |
| Averaged SBP (mmHg), n | 14 | 16 | 16 | 15 |
| Mean (SD) | 124 (15.5) | 122 (10.7) | 117 (10.8) | 129 (16.0) |
| Median (25th, 75th) | 126 (111, 135) | 121 (114, 129) | 117 (113, 121) | 127 (120, 140) |
| Averaged DBP (mmHg), n | 14 | 16 | 16 | 15 |
| Mean (SD) | 74 (9.7) | 75 (6.3) | 73 (7.0) | 76 (11.1) |
| Median (25th, 75th) | 70 (69, 80) | 76 (71, 81) | 73 (67, 80) | 77 (65, 81) |
| Reported ACEi/ARB use, n (%) | 8 (53.3) | 10 (62.5) | 9 (56.3) | 11 (73.3) |
| Stroke or cardiovascular eventb prior to first infusion, n (%) | 3 (20.0) | 1 (6.3) | 5 (31.3) | 6 (40.0) |
| Time from symptom onset to first agalsidase beta infusion (years), n | 10 | 11 | 11 | 10 |
| Mean (SD) | 19.5 (14.00) | 24.7 (14.38) | 21.0 (14.57) | 18.2 (16.15) |
| Median (25th, 75th) | 24.2 (1.9, 31.3) | 31.4 (7.9, 34.5) | 13.4 (10.1, 34.9) | 10.8 (6.8, 33.4) |
| Time from diagnosis to first agalsidase beta infusion (years), n | 15 | 16 | 16 | 15 |
| Mean (SD) | 5.7 (6.71) | 4.3 (8.29) | 6.3 (8.58) | 8.0 (10.03) |
| Median (25th, 75th) | 3.2 (0.4, 6.6) | 0.8 (0.5, 2.6) | 2.4 (0.4, 11.3) | 3.8 (0.5, 13.0) |
| Age at first agalsidase beta infusion (years), n | 15 | 16 | 16 | 15 |
| Mean (SD) | 43.2 (11.30) | 41.0 (11.83) | 40.5 (15.12) | 47.4 (13.09) |
| Median (25th, 75th) | 45.8 (33.5, 51.6) | 35.5 (33.5, 52.5) | 43.8 (23.6, 51.6) | 43.6 (41.4, 57.6) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; 25th, 25th percentile value; 75th, 75th percentile value. All data are from adult Fabry Registry patients (≥18 years) with three or more eGFR assessments (calculated by Chronic Kidney Disease Epidemiology Collaboration equation) over a period of ≥2 years (at least one eGFR assessment within 3 months of initiation of agalsidase beta treatment) and prior to any chronic dialysis or renal transplant events. ‘Averaged’ UP/Cr and blood pressure data reflect the average of all values reported within 3 months of the date of the first agalsidase beta infusion to the most recent assessment during treatment. Baseline eGFR is based on the assessment date closest to a patient’s first infusion of agalsidase beta (within 3 months of the first infusion).
Cardiovascular events were defined as described in ‘Materials and methods’.
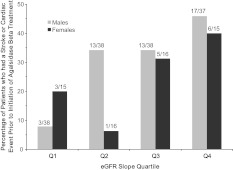
In both genders, fewer patients in Q1 had experienced a stroke or cardiovascular event (as defined in Materials and methods) prior to the initiation of agalsidase beta treatment compared to patients in Q4 (Figure 1). Patients in each quartile were categorized by mutation type and by specific genotype (Table 3). Men with nonsense mutations (n = 16) were more likely to be in Q3–Q4 (n = 12, 75%) than in Q1–Q2 (n = 4, 25%). Conversely, men with missense mutations (n = 71) were more likely to be in Q1–Q2 (n = 44, 62%) than Q3–Q4 (n = 27, 38%). The five most prevalent missense genotypes are shown in Table 3. Most patients had unique genotypes and there was no clear association between any specific genotype and renal function.
Fig. 1.
Patients with rapid renal disease progression experienced clinical events prior to the initiation of agalsidase beta treatment. Patients were grouped into quartiles (Q), based on eGFR slope. Data are expressed as the percentage of patients within each eGFR slope quartile who had a stroke or cardiovascular event (as defined in Materials and Methods section) prior to the initiation of agalsidase beta treatment. The numbers above each bar indicate number of patients who had clinical events prior to the initiation of treatment/total number of patients in each quartile. Data for males are shown in light grey bars and data for females are shown in dark grey bars.
Table 3.
Genotype and eGFR slope quartiles
| Males |
Females |
|||||||
| eGFR slope Quartile 1 (−1.2 to +5.3), N = 38 | eGFR slope Quartile 2 (−2.8 to −1.3), N = 38 | eGFR slope Quartile 3 (−4.8 to −2.9), N = 38 | eGFR slope Quartile 4 (−15.5 to −4.9), N = 37 | eGFR slope Quartile 1 (+1.1 to +7.5), N = 15 | eGFR slope Quartile 2 (−0.4 to +1.0), N = 16 | eGFR slope Quartile 3 (−2.3 to −0.4), N = 16 | eGFR slope Quartile 4 (−7.6 to −2.4), N = 15 | |
| Mutation type, n (%)a | ||||||||
| Nonsense | 3 (18.8) | 1 (3.6) | 5 (31.3) | 7 (43.8) | 3 (23.1) | 3 (23.1) | 2 (15.4) | 5 (38.5) |
| Missense | 23 (32.4) | 21 (29.6) | 15 (21.1) | 12 (16.9) | 5 (17.2) | 10 (34.5) | 9 (31.0) | 5 (17.2) |
| Splice site | 0 | 2 (40.0) | 2 (40.0) | 1 (20.0) | 0 | 0 | 1 (50.0) | 1 (50.0) |
| Frameshift | 3 (25.0) | 2 (16.7) | 4 (33.3) | 3 (25.0) | 3 (50.0) | 3 (50.0) | 0 | 0 |
| Intronic | 0 | 1 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Initiator codon | 0 | 1 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Small deletion (no frameshift) | 2 (66.7) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Small insertion (no frameshift) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100.0) | 0 |
| Other | 0 | 0 | 0 | 1 (100.0) | 0 | 0 | 1 (100.0) | 0 |
| Not available | 7 (17.1) | 9 (22.0) | 12 (29.3) | 13 (31.7) | 4 (40.0) | 0 | 2 (20.0) | 4 (40.0) |
| Five most prevalent missense genotypes, n (%)b | ||||||||
| N215S | 2 (2.8) | 1 (1.4) | 2 (2.8) | 0 | 0 | 0 | 0 | 1 (3.4) |
| L415P | 4 (5.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| R301Q | 2 (2.8) | 2 (2.8) | 0 | 0 | 0 | 0 | 0 | 0 |
| P259R | 1 (1.4) | 0 | 0 | 1 (1.4) | 1 (3.4) | 1 (3.4) | 0 | 0 |
| C52G | 1 (1.4) | 0 | 0 | 0 | 0 | 2 (6.9) | 0 | 0 |
For mutation type, percentages were calculated based on the total number of patients with each type of mutation, by gender.
These five missense mutations (N215S, L415P, R301Q, P259R and C52G) were the only genotypes reported by more than two patients in this cohort. Percentages for these five genotypes were calculated based on the total number of patients who reported missense mutations, by gender (n = 71 men and n = 29 women).
Results of the logistic regression model comparing men in Q1 to men in Q4 are shown in Table 4. Among the 75 men in Q1 and Q4, those who had averaged UP/Cr levels ≥1.0 g/g had a 112-fold increase in the risk of having more rapid renal disease progression (i.e. being in Q4) than did men who had averaged UP/Cr levels <0.3 g/g (odds ratio 112, 95% CI 4.0–3108.9, P = 0.0054). In addition to averaged UP/Cr, the time from symptom onset to first agalsidase beta infusion was also strongly associated with renal disease progression. Men in whom the time from symptom onset to first infusion was greater than the median (24 years) had a 19-fold increase in the risk of being in Q4 than men who initiated treatment sooner (odds ratio 19, 95% CI 2.0–183.9, P = 0.0098). When UP/Cr was removed from the regression model, lower baseline eGFR also emerged as a significant risk factor for renal disease progression in men; men with eGFR <60 mL/min/1.73m2 at baseline had a 12-fold increase in the risk of being in Q4 than men who had higher eGFR levels (odds ratio 12, 95% CI 1.2–114.0, P = 0.0316).
Table 4.
Logistic regression modeling of renal disease progression in men: eGFR slope Q1 versus eGFR slope Q4a
| Odds ratio (95% CI) |
||
| UP/CR included in model | No UP/Cr in model | |
| Higher averaged UP/Cr (≥0.3 to <1.0 versus ≥1.0 g/g) | 2.29 (0.35, 14.99) | – |
| Higher averaged UP/Cr (<0.3 versus ≥1.0 g/g) | 111.85 (4.02, 3108.89)* | – |
| Lower baseline eGFR (<60 mL/min/1.73m2) | 9.31 (0.74, 116.62) | 11.91 (1.24, 114.02)* |
| Had a stroke or cardiovascular eventb prior to first infusion | 1.41 (0.20, 10.08) | 4.57 (0.83, 25.22) |
| Older age at first infusion (<30 versus ≥50 years) | 0.51 (0.01, 20.83) | 0.23 (0.01, 0.521) |
| Older age at first infusion (30 to <40 versus ≥50 years) | 2.53 (0.13, 49.50) | 1.31 (0.12, 14.85) |
| Older age at first infusion (40 to <50 versus ≥50 years) | 1.13 (0.05, 25.84) | 0.64 (0.05, 8.23) |
| Longer time from Fabry diagnosis to first infusion (>median, 2.6 years) | 0.62 (0.11, 3.54) | 0.85 (0.22, 3.36) |
| Longer time from Fabry symptom onset to first infusion (>median, 24 years) | 19.39 (2.05, 183.88)* | 10.22 (1.57, 66.37)* |
| Higher averaged systolic blood pressure (>120 mmHg) | 0.36 (0.05, 2.75) | 1.48 (0.36, 6.01) |
| Higher averaged diastolic blood pressure (>80 mmHg) | 0.71 (0.05, 10.62) | 1.12 (0.10, 12.40) |
Analyses were based on n = 38 males in eGFR slope Q1 and n = 37 males in eGFR slope Q4 for all parameters except ‘time from first symptom to first infusion’. Because several patients did not have a date of symptom onset available, this parameter included n = 35 males in eGFR slope Q1 and n=32 males in eGFR slope Q4.
Cardiovascular events were defined as described in ‘Materials and Methods.
*P < 0.05 by the Wald test.
A proportional odds regression model was used to evaluate factors associated with renal disease progression in men across quartiles, as shown in Table 5. As in the logistic regression model of Q1 versus Q4, higher averaged UP/Cr (≥1 versus <0.3 g/g) was the variable most strongly associated with renal disease progression in men across quartiles. Among the 151 men in this cohort, those with averaged UP/Cr ≥1 g/g had a 4.5-fold higher risk of renal disease progression (i.e. more negative eGFR slope) compared to those with UP/Cr <0.3 g/g (odds ratio 4.5, 95% CI 1.9–10.7, P = 0.0006). The time from symptom onset to initiation of treatment was also a significant factor; men who initiated treatment >24 years after symptom onset had a 2.9-fold higher risk of renal disease progression compared to those who initiated treatment sooner (odds ratio 2.9, 95% CI 1.3–6.7, P = 0.0113). When UP/Cr was removed from the proportional odds regression model, lower baseline eGFR, the occurrence of a stroke or cardiovascular event prior to initiation of treatment and time from symptom onset to initiation of treatment were significant factors associated with renal disease progression in men (Table 5).
Table 5.
Proportional odds regression modeling of renal disease progression in men across eGFR slope quartilesa
| Odds ratio (95% CI) |
||
| UP/Cr included in model | No UP/Cr in model | |
| Higher averaged UP/Cr (≥0.3 to <1.0 versus ≥1.0 g/g) | 1.59 (0.69, 3.68) | – |
| Higher averaged UP/Cr (<0.3 versus ≥1.0 g/g) | 4.52 (1.90, 10.75)* | – |
| Lower baseline eGFR (<60 mL/min/1.73m2) | 2.42 (0.97, 6.03) | 2.86 (1.18, 6.94)* |
| Had a stroke or cardiovascular eventb during natural history period | 2.19 (0.99, 4.84) | 2.28 (1.05, 4.98)* |
| Older age at first infusion (<30 versus ≥50 years) | 0.41 (0.11, 1.57) | 0.55 (0.15, 1.99) |
| Older age at first infusion (30 to <40 versus ≥50 years) | 0.83 (0.27, 2.51) | 0.82 (0.28, 2.44) |
| Older age at first infusion (40 to <50 versus ≥50 years) | 1.27 (0.43, 3.73) | 1.22 (0.42, 3.51) |
| Longer time from Fabry diagnosis to first infusion (>median, 2.6 years) | 0.85 (0.42, 1.71) | 0.87 (0.44, 1.75) |
| Longer time from Fabry symptom onset to first infusion (>median, 24 years) | 2.92 (1.27, 6.68)* | 3.27 (1.47, 7.26)* |
| Higher averaged systolic blood pressure (>120 mmHg) | 1.07 (0.54, 2.14) | 1.03 (0.52, 2.04) |
| Higher averaged diastolic blood pressure (>80 mmHg) | 1.38 (0.49, 3.85) | 1.63 (0.61, 4.37) |
Analyses were based on N = 151 males for all parameters except ‘time from first symptom to first infusion’. Because some patients did not have a date of symptom onset available, this parameter included n = 129 males across eGFR slope quartiles.
Cardiovascular events were defined as described in ‘Materials and methods’.
*P < 0.05 by the Wald test.
It was more difficult to develop a suitable logistic regression model for predictors of eGFR slope among the smaller number of women in this cohort (n = 15 or 16 in each quartile). For this reason, women in Q1–Q3 were combined, as their mean averaged UP/Cr levels and baseline eGFR values were similar (see Materials and methods and Table 2). When covariates were limited to averaged UP/Cr, age at first infusion and time from symptom onset to initiation of treatment, averaged UP/Cr was found to be associated with renal disease progression in women (Table 6). Among all 62 women, those who had averaged UP/Cr levels ≥1.0 g/g had a 12-fold increased risk of having more rapid renal disease progression (i.e. being in Q4) than did women who had averaged UP/Cr levels <0.3 g/g (odds ratio 11.7, 95% CI 1.1–119.7, P = 0.0388). When UP/Cr was removed from the regression model, age at first infusion also appeared as a significant risk factor. Women who received their first infusion at age ≥40 years had a 7-fold increase in the risk of being in Q4 compared to women who received their first infusion at a younger age (odds ratio 7.4, 95% CI 1.12–48.3, P = 0.0375). Time from symptom onset to first infusion was not a significant predictor of renal disease progression in women; however, only a subset of women had reported the age at which they first experienced symptoms of Fabry disease (n = 42).
Table 6.
Logistic regression modeling of renal disease progression in women: eGFR slope Q1, Q2 and Q3 versus eGFR slope Q4a
| Odds ratio (95% CI) |
||
| UP/CR included in model | No UP/Cr in model | |
| Higher averaged UP/Cr (≥0.3 to <1.0 versus ≥1.0 g/g) | 2.09 (0.25, 17.46) | – |
| Higher averaged UP/Cr (<0.3 versus ≥1.0 g/g) | 11.66 (1.14, 119.73)* | – |
| Older age at first infusion (≥40 years) | 4.85 (0.66, 35.70) | 7.36 (1.12, 48.29)* |
| Longer time from Fabry symptom onset to first infusion (>median, 24 years) | 0.31 (0.047, 2.041) | 0.29 (0.055, 1.55) |
Analyses were based on n = 47 females in eGFR slope Q1–Q3 (combined) and n = 15 females in eGFR slope Q4 for all parameters except ‘time from first symptom to first infusion’. Because some patients did not have a date of symptom onset available, this parameter included n = 32 females in eGFR slope Q1–Q3 (combined) and n = 10 females in Q4.
*P < 0.05 by the Wald test.
Discussion
The purpose of these analyses was to evaluate renal function during agalsidase beta treatment and to identify determinants of renal disease progression during treatment. Our findings demonstrate that baseline clinical status plays a critical role in renal outcomes during agalsidase beta treatment. Higher urinary protein levels, poorer initial renal function and delayed initiation of agalsidase beta treatment after the onset of Fabry symptoms were independent predictors strongly associated with renal disease progression in men. In proportional odds regression analyses across quartiles, the occurrence of a stroke or cardiovascular event prior to the initiation of agalsidase beta treatment was also associated with more rapid renal disease progression in men.
Men generally have more severe manifestations of Fabry disease than women [1, 9] and a higher proportion of men are treated with ERT [9]. The smaller number of women who received treatment and met the inclusion criteria limited the analysis of women in this cohort. Renal function among the women in Q1–Q3 appeared to be similar to or more stable than the normal expected rate of renal decline of –1 mL/min/1.73m2/year in the general population [17, 18]. However, like men, women who had the most rapid renal disease progression also had the highest averaged UP/Cr levels.
These findings are consistent with results from the open-label extension of a placebo-controlled double-blind Phase 3 clinical study [14] and a placebo-controlled double-blind Phase 4 clinical study [15] of agalsidase beta at a dose of 1 mg/kg/2 weeks. The clinical studies demonstrated that men with urinary protein levels ≥1 g/24 h and men with lower baseline eGFR had a poorer response to agalsidase beta than those with lower urinary protein levels and better baseline eGFR [14, 15]. There were too few female patients in either study to make meaningful comparisons to the present analyses. Our findings are consistent with a recent analysis of data from untreated Fabry Registry patients, which identified proteinuria as the predominant risk factor for renal disease progression, especially in men [10]. Similar findings were reported in a retrospective chart review of clinical data from untreated Fabry patients [7].
These findings underscore the possible importance of controlling proteinuria in patients with Fabry disease. ERT alone does not appear to decrease proteinuria [14, 15, 19–21] and it has been recommended that patients receiving ERT also receive angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ACEi/ARBs) to reduce proteinuria [22–25]. Only 46% of men in this cohort and 61% of women reported receiving ACEi/ARBs at any time during agalsidase beta treatment. This may underestimate the actual percentage of patients treated with ACEi/ARBs, due to the voluntary nature of reporting data to the Fabry Registry. However, there may also be reluctance among physicians to prescribe ACEi/ARBs for Fabry patients who have normal or low baseline systemic blood pressure. A growing body of evidence suggests that the benefits of reducing proteinuria outweigh the risks of reducing blood pressure in patients receiving ERT [14, 15, 21, 26]. An open-label prospective evaluation specifically examined the effects of ACEi/ARB therapy in 11 patients with Fabry disease who received agalsidase beta at the prescribed dose of 1 mg/kg/2 weeks in conjunction with ACEi/ARB therapy [26]. Sustained reductions in proteinuria were associated with stable renal function over a 30-month follow-up period with no serious complications from hypotension; in fact, the measured blood pressure levels returned to the baseline levels after kidney function was stabilized during the course of anti-proteinuric therapy [26].
Although agalsidase beta treatment may have slowed renal disease progression in the men in Q3 and Q4, renal function continued to decline in these patients over the follow-up period. In contrast, the men in Q1, who had little urinary protein excretion, higher baseline eGFR levels, and who began receiving agalsidase beta sooner after the onset of Fabry symptoms had stable renal function over the course of their treatment (mean slope –0.1 mL/min/1.73m2). These findings are also consistent with those from the open-label Phase 3 extension study of agalsidase beta at a dose of 1 mg/kg/2 weeks [14]. In that study, patients who had baseline UP/Cr levels <1 g/24 h had stable renal function over a 54-month treatment period (eGFR slope –1.0 mL/min/1.73m2/year), whereas patients with baseline UP/Cr levels ≥1 g/24 h exhibited rapid renal disease progression (eGFR slope –7.4 mL/min/1.73m2/year).
Agalsidase alfa, another form of recombinant human α-GalA (Shire Human Genetic Therapies Inc., Cambridge, MA), has a recommended licensed dose of 0.2 mg/kg/2 weeks. In a secondary, pooled analysis of data from clinical studies, West et al. [21] reported that patients treated with agalsidase alfa who had baseline UP/Cr levels >1 g/24 h also had more rapid renal disease progression than patients with lower baseline UP/Cr levels (–6.4 versus –2.11 mL/min/1.73m2/year). Mehta et al. [27] reported an eGFR slope of –3 mL/min/1.73m2/year in men in the Fabry Outcome Survey observational database who were treated with agalsidase alfa at a dose of 0.2 mg/kg/2 weeks and who remained on this therapy for 5 years. However, urinary protein levels were not included as part of those analyses [27], making it difficult to interpret the eGFR slope data in this context [28].
Various limitations are associated with analysing observational data from a disease registry, particularly for rare diseases with small numbers of patients. Many patients who were treated with agalsidase beta were not included in these analyses because they did not have the required number of eGFR and UP/Cr assessments reported. Patients who received frequent renal assessments may have higher UP/Cr levels at baseline, which could lead to an over-representation of patients with more severe renal disease. Interpretation of these findings is also limited by the lack of an appropriate parallel control group, such as those included in randomized clinical trials with agalsidase beta [13, 15]. Many of the patients were enrolled in the Fabry Registry at the time they began receiving ERT, so it was not possible to compare the eGFR slopes before and after treatment for individual patients. A recent analysis of Fabry Registry data reported that during the natural history period, renal function declined at a rate of –5.6 mL/min/1.73m2/year in men with mean averaged UP/Cr >1.5 g/g [10]. However, the current findings might not be directly comparable with those of Wanner et al. [10] because patients in the natural history cohort were younger and had less severe renal involvement (i.e. higher baseline eGFR) than patients in the current cohort. If in fact, the eGFR slope was similar in ERT treated and untreated men with heavy proteinuria, this would speak strongly for studies to examine additional treatment strategies such as proteinuria lowering, earlier institution of therapy and ERT doses >0.2 mg/kg/2 weeks [29].
The timing of initiation of ERT could be confounded if there were a significant number of patients in the Fabry Registry cohort with ‘late-onset mutations’, such as N215S [30, 31]. Because the majority of mutations in Fabry disease are unique to individual family groups, there were very few patients within any single genotype. Six patients in this cohort had the N215S mutation (Table 3). Although these patients may have had later onset disease manifestations than others, there was no clear trend between this genotype and renal disease progression. The limited number of such cases did not affect the results or conclusions of these analyses. As expected, men with nonsense mutations generally had poorer renal function than men with missense mutations in the GLA gene.
These findings provide further evidence that it is critical to closely monitor urinary protein levels in patients treated with ERT and support the hypothesis that proteinuria should be aggressively managed with ACEi/ARBs to a pre-specified treatment target [11, 25]. ACEi/ARBs in conjunction with ERT can reduce proteinuria and stabilize renal function in Fabry patients [26]. This is a key issue, as ERT alone does not decrease overt proteinuria in Fabry disease [14, 15, 19]. These findings also highlight the importance of early initiation of ERT, before the first clinical signs of kidney dysfunction appear, because substantial organ damage can occur before proteinuria or loss of eGFR can be detected [32]. A shorter time from the onset of Fabry symptoms until the initiation of treatment was associated with the best renal outcomes among men. Finally, the fact that agalsidase beta was not an approved therapy at the time that most of these patients began experiencing disease symptoms must be considered when interpreting these findings. Men did not begin receiving agalsidase beta until an overall median of 24 years after their Fabry symptoms began (median 35 years among men in Q4). With increased awareness of Fabry disease and the availability of ERT, younger patients who begin agalsidase beta treatment sooner after the onset of Fabry symptoms would be expected to have the most favourable renal outcomes.
Acknowledgments
The authors would like to thank the many patients who have agreed to participate in the Fabry Registry as well as the physicians and research coordinators that have entered clinical data on these patients. We also acknowledge Badari Gudivada (Genzyme Corporation, Cambridge, MA) for statistical programming support.
Conflict of interest statement. Genzyme Corporation sponsors the Fabry Registry. Members of the Fabry Registry Board of Advisors include C.W., J.P.O., A.O., M.M., D.P.G., B.V., S.W. and D.G.W. Authors who have received research funds or travel support from Genzyme include C.W., J.P.O., A.O., M.M., D.P.G., A.S., L.M., R.M., E.S., B.V., S.W. and D.G.W. Authors who have received speaking fees from Genzyme include C.W., J.P.O., A.O., G.E.L., M.M., D.P.G., R.M., E.S., B.V. and S.W. All honoraria received by GEL are donated to the Gaucher Stichting, a national foundation that supports research in the field of lysosomal storage disorders. R.L., A.C. and D.B.J. are Genzyme employees. D.G.W. has served as a paid consultant to Genzyme and also has consultancies and has received travel funds from Abbot, Amgen, Amicus, Gilead, Parion, Relypsa and Shire.
References
- 1.Desnick RJ, Ioannou YA, Eng CM. Alpha-Galactosidase A deficiency: Fabry disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic Bases of Inherited Disease. 8th ed. New York, NY: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 2.Fogo AB, Bostad L, Svarstad E, et al. Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN) Nephrol Dial Transplant. 2010;25:2168–2177. doi: 10.1093/ndt/gfp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler MC, Lenoir G, Grunfeld JP, et al. Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978;13:223–235. doi: 10.1038/ki.1978.32. [DOI] [PubMed] [Google Scholar]
- 4.Thurberg BL, Rennke H, Colvin RB, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Valbuena C, Carvalho E, Bustorff M, et al. Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch. 2008;453:329–338. doi: 10.1007/s00428-008-0653-2. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz A, Cianciaruso B, Cizmarik M, et al. End-stage renal disease in patients with Fabry disease: natural history data from the Fabry Registry. Nephrol Dial Transplant. 2010;25:769–775. doi: 10.1093/ndt/gfp554. [DOI] [PubMed] [Google Scholar]
- 7.Schiffmann R, Warnock DG, Banikazemi M, et al. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24:2102–2111. doi: 10.1093/ndt/gfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sims K, Politei J, Banikazemi M, et al. Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: natural history data from the Fabry Registry. Stroke. 2009;40:788–794. doi: 10.1161/STROKEAHA.108.526293. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Oliveira JP, Ortiz A, et al. Prognostic indicators of renal disease progression in adults with Fabry disease: natural history data from the Fabry Registry. Clin J Am Soc Nephrol. 2010;5:2220–2228. doi: 10.2215/CJN.04340510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz A, Oliveira JP, Waldek S, et al. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant. 2008;23:1600–1607. doi: 10.1093/ndt/gfm848. [DOI] [PubMed] [Google Scholar]
- 12.Mignani R, Feriozzi S, Schaefer RM, et al. Dialysis and transplantation in Fabry disease: indications for enzyme replacement therapy. Clin J Am Soc Nephrol. 2010;5:379–385. doi: 10.2215/CJN.05570809. [DOI] [PubMed] [Google Scholar]
- 13.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 14.Germain D, Waldek S, Banikazemi M, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007;18:1547–1557. doi: 10.1681/ASN.2006080816. [DOI] [PubMed] [Google Scholar]
- 15.Banikazemi M, Bultas J, Waldek S, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86. doi: 10.7326/0003-4819-146-2-200701160-00148. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 19.Breunig F, Weidemann F, Strotmann J, et al. Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int. 2006;69:1216–1221. doi: 10.1038/sj.ki.5000208. [DOI] [PubMed] [Google Scholar]
- 20.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West M, Nicholls K, Mehta A, et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009;20:1132–1139. doi: 10.1681/ASN.2008080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oqvist B, Brenner BM, Oliveira JP, et al. Nephropathy in Fabry disease: the importance of early diagnosis and testing in high-risk populations. Nephrol Dial Transplant. 2009;24:1736–1743. doi: 10.1093/ndt/gfp105. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz A, Oliveira JP, Wanner C, et al. Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol. 2008;4:327–336. doi: 10.1038/ncpneph0806. [DOI] [PubMed] [Google Scholar]
- 24.Warnock DG, Daina E, Remuzzi G, et al. Enzyme replacement therapy and Fabry nephropathy. Clin J Am Soc Nephrol. 2010;5:371–378. doi: 10.2215/CJN.06900909. [DOI] [PubMed] [Google Scholar]
- 25.Mehta A, West ML, Pintos-Morell G, et al. Therapeutic goals in the treatment of Fabry disease. Genet Med. 2010;12:713–720. doi: 10.1097/GIM.0b013e3181f6e676. [DOI] [PubMed] [Google Scholar]
- 26.Tahir H, Jackson LL, Warnock DG. Antiproteinuric therapy and Fabry nephropathy: sustained reduction in proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol. 2007;18:2609–2617. doi: 10.1681/ASN.2006121400. [DOI] [PubMed] [Google Scholar]
- 27.Mehta A, Beck M, Elliott P, et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry's disease: an analysis of registry data. Lancet. 2009;374:1986–1996. doi: 10.1016/S0140-6736(09)61493-8. [DOI] [PubMed] [Google Scholar]
- 28.Waldek S, Germain DP, Wanner C, et al. Enzyme replacement therapy for Fabry's disease. Lancet. 2010;375:1523–1524. doi: 10.1016/S0140-6736(10)60653-8. [DOI] [PubMed] [Google Scholar]
- 29.Schiffmann R, Askari H, Timmons M, et al. Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol. 2007;18:1576–1583. doi: 10.1681/ASN.2006111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakao S, Kodama C, Takenaka T, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64:801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 31.Spada M, Pagliardini S, Yasuda M, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tondel C, Bostad L, Hirth A, et al. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–776. doi: 10.1053/j.ajkd.2007.12.032. [DOI] [PubMed] [Google Scholar]