Abstract
Background.
Substantial efforts have been made toward defining the dose threshold of continuous renal replacement therapy (CRRT) associated with improved survival in critically ill patients with acute kidney injury. Published studies have used prescribed effluent rates, expressed as total effluent volume (TEV) per weight and unit time (mL/kg/h), as a surrogate for dose. The purpose of this study was to compare differences in CRRT dose based on prescribed effluent rate, measured TEV and direct measurement of urea and creatinine clearance.
Methods.
We analyzed data that had been prospectively collected on 200 patients enrolled in a randomized trial comparing survival with a prescribed effluent rate of 20 mL/kg/h (standard dose) to 35 mL/kg/h (high dose) using pre-dilution continuous venovenous hemodiafiltration (CVVHDF). Filters were changed every 72 h. Blood urea nitrogen (BUN), serum creatinine (SCr), effluent urea nitrogen (EUN) and effluent creatinine (ECr) were collected daily. Actual delivered dose was calculated as: (EUN/BUN)*TEV for urea and (ECr/SCr)*TEV for creatinine. Data were available for 165 patients.
Results.
In both groups, prescribed dose differed significantly from the measured TEV dose (P < 0.001). In the standard dose group, there was no difference between the measured TEV dose and actual delivered urea and creatinine clearances. However, in the high-dose group, measured TEV dose differed significantly from delivered urea clearance by 7.1% (P < 0.001) and creatinine clearance by 13.9% (P < 0.001).
Conclusions.
Dose based on prescribed effluent rate or measured TEV is a poor substitute for actual CVVHDF creatinine and urea clearance.
Keywords: acute kidney injury, continuous renal replacement therapy, critical care nephrology, dialysis dose
Introduction
Acute kidney injury (AKI) occurs commonly in the intensive care unit (ICU) and is an independent predictor of mortality [1]. Despite improvements in dialysis technology over the past 50 years, the mortality rate of AKI in the ICU remains ∼50% [2, 3]. Continuous renal replacement therapy (CRRT) is the most common dialytic therapy utilized in management of AKI in the ICU worldwide [4]. Consequently, substantial efforts have been made to establish a dose–response relationship for CRRT [5]. Seven randomized controlled studies have examined outcomes in patients with AKI requiring CRRT in the ICU. Two trials demonstrated a survival benefit following more intense dialysis [6, 7]. Five trials demonstrated no survival benefit [8–12].
Each of these studies utilized weight-based effluent rates in mL/kg/h as a surrogate for dialysis dose. However, none of these studies reported direct measures of solute clearance to compare the prescribed versus delivered dose. Many variables may contribute to reduced solute clearance in CRRT, including time off the machine for procedures, machine alarms, filter clotting and the use of prefilter replacement fluid [13]. We retrospectively analyzed data from a prior prospective dose study performed at our medical center to determine the relationship between prescribed dose, dose based on measured effluent rate and dose based on measured urea and creatinine concentrations.
Materials and methods
Patients
Over a 32-month period (August 2003 to March 2006), 200 patients were recruited from the medical and surgical ICUs at the University of Alabama at Birmingham Hospital for a study designed to evaluate standard versus high dose continuous venovenous hemodiafiltration (CVVHDF) in patients who developed AKI. Patients were randomized to either standard dose (20 mL/kg/h) or high dose (35 mL/kg/h) CVVHDF. All patients had AKI in the ICU, as defined by having at least one of the following: (1) volume overload despite diuretics, (2) oliguria (urine output <200 mL/12 h) despite fluid resuscitation and diuretics, (3) anuria (urine output <50 mL/12 h), (4) azotemia [blood urea nitrogen (BUN) ≥ 80 mg/dL] or (5) hyperkalemia (K+ ≥ 6.5 mmol/L) and/or an increase in serum creatinine > 2.5 mg/dL from normal values or a sustained rise in serum creatinine of ≥1 mg/dL over baseline. Exclusion criteria were end-stage renal disease, a history of having previously had intermittent hemodialysis, >24 h of CRRT at the time of enrollment or weight >125 or <50 kg due to limitations of the CRRT machine to deliver doses required for the study at those weights. CVVHDF was initiated at the discretion of the consulting nephrologist, without consideration of patient’s eligibility for the study.
Each of the 200 patients enrolled had their hematocrit, BUN, serum creatinine (SCr), effluent urea nitrogen (EUN) and effluent creatinine (ECr) checked daily. Total CVVHDF effluent volume and total time of actual CVVHDF treatment (min/24-h period) were measured daily as well. Complete data were available for 1237 days from a total of 165 patients. The standard-dose arm yielded 587 observations from 83 patients. The high-dose arm yielded 650 observations from 82 patients.
CRRT technique
CVVHDF was performed with COBE Prisma (Lakewood, CO) M100 set and AN69 dialyzer (effective surface area 0.9 m2) through a double-lumen 12F catheter inserted into the internal jugular, subclavian or femoral vein. Hemodiafiltration was accomplished using blood flow rates of 100–150 mL/min and predilution replacement fluid. Regional citrate or no anti-coagulation was used according to the consulting nephrologist's clinical decision, with regional citrate anticoagulation employed ∼95% of the time and no anticoagulation in the remainder. Dialyzers were changed due to circuit failure from clotting, after 72 h of use or when the patient was off CVVHDF for >2 h due to a procedure or imaging study.
The patients were randomized to each arm based on the prescribed effluent rate. The prescribed effluent rate (mL/h) is the sum of the replacement fluid rate, dialyzate rate and fluid removal rate. For example, a 70-kg patient assigned to the high-dose arm would require an effluent rate of 2450 mL/h (70 kg × 35 mL/kg/h). The replacement fluid rate, dialyzate rate and fluid removal rate for that patient would be adjusted to achieve an effluent rate of 2450 mL/h/day for the study duration. Dose was calculated only once per patient and based on the patient’s actual body weight on the day of CVVHDF initiation. This dose remained constant throughout the treatment period and was not adjusted for body weight changes. Every attempt was made to divide the effluent rate equally between the replacement fluid and dialyzate.
Calculations
Urea clearance (KU, mL/kg/h) and creatinine clearance (KC, mL/kg/h) were calculated for prescribed, estimated and delivered doses. Units of mL/kg/h were utilized rather than mL/min because mL/kg/h is the most widely accepted method of measuring CRRT dose.
Prescribed K (KP).
Prescribed K (KP) was calculated from the programmed effluent rate from the initial prescription. It was corrected for the effect of pre-dilutional replacement fluid. All clearance formulas are demonstrated below [14–17].
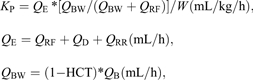 |
where KP is the prescribed clearance corrected for predilutional replacement fluid, QE is the prescribed effluent rate (mL/h), QRF is the prefilter replacement fluid flow rate (mL/h), QD is the dialyzate flow rate (mL/h), QRR is the fluid removal rate (mL/h), W is the patient’s weight at initiation of CVVHDF (kg), QBW is the blood water flow rate (mL/h), QB is the blood flow rate (mL/h) and HCT is the hematocrit.
Estimated clearance (KE).
Estimated clearance was calculated from the measured effluent volume over 24 h, adjusted for the effect of predilutional replacement fluid.
where KE is the estimated clearance and TEV is the measured effluent volume in a given 24-h period.
Solute clearance (KU and KC).
Solute clearance was calculated from the measured effluent rate over 24 h and the effluent to BUN ratio for urea clearance and the effluent to serum creatinine ratio for creatinine clearance.
where KU is the measured urea clearance and KC is the measured creatinine clearance.
Statistical analyses
Continuous variables were expressed as mean ± SD and analyzed using the unpaired t-test or Wilcoxon rank sum test, as indicated. Nonparametric variables were expressed as median and 25th–75th percentiles and analyzed using the Mann–Whitney test. Categorical variables were expressed as absolute (n) and relative (%) frequency and were analyzed using Chi-square or Fisher’s Exact, where indicated. All statistical tests were two-tailed and P < 0.05 was considered significant. Analyses were performed using JMP 8.0.1 Statistical Software (Cary, NC) and GraphPad InStat 3.0 (San Diego, CA).
Results
Baseline patient characteristics were similar between dose groups (Table 1). Mean (SD) age for the standard dose group was 62 ± 14 years, while that for the high-dose group was 59 ± 15 years. Approximately 60% of the study subjects in both groups were men. Roughly 95% of the study subjects in each arm received citrate anticoagulation, with the remainder receiving no anticoagulation. There was no significant difference in filter clotting between both dose groups. Mean (SD) serum creatinine was 4.2 ± 2.2 mg/dL in the standard-dose group and 4.2 ± 1.6 mg/dL in the high-dose group at time of CRRT initiation. Acute physiology and chronic health evaluation (APACHE) II scores at initiation of CRRT were similar for both groups.
Table 1.
Baseline patient characteristics at initiation of CVVHDF
| Characteristic | Standard dose (20 mg/kg/h) | High dose (35 mg/kg/h) | P |
| Patients (n) | 83 | 82 | |
| Citrate anticoagulation | 95 | 97 | 0.41 |
| Age (years) | 62 ± 14 | 59 ± 15 | 0.22 |
| Male gender (%) | 60 | 63 | 0.68 |
| Weight (kg) | 91 ± 18 | 94 ± 18 | 0.27 |
| Creatinine (mg/dL) | 4.2 ± 2.2 | 4.2 ± 1.6 | 0.92 |
| BUN (mg/dL) | 76 ± 40 | 77 ± 36 | 0.82 |
| Urine output (mL/day) | 611 ± 792 | 522 ± 574 | 0.40 |
| APACHE II | 26.0 ± 6.3 | 26.3 ± 5.9 | 0.76 |
Table 2 shows the prescribed clearance, estimated clearance and measured urea and creatinine clearances for the standard-dose and high-dose groups. Mean prescribed clearance was lower than the prescribed 20 versus 35 mL/kg/h dose due to the effect of predilutional replacement fluid. The standard and high dose cohorts differed significantly for each calculated clearance (P < 0.001 for all comparisons), demonstrating separation of dose between both groups for prescribed dose, estimated effluent dose and actual measured urea and creatinine clearance.
Table 2.
CVVHDF clearance comparisons
| Standard dose (20 mg/kg/h) | High dose (35 mg/kg/h) | P | |
| Prescribed clearance (KP) | 17.62 ± 0.96 | 28.10 ± 1.44 | <0.001 |
| Estimated clearance (KE) | 15.79 ± 2.47 | 25.10 ± 3.16 | <0.001 |
| Urea clearance (KU) | 15.55 ± 3.07 | 23.31 ± 5.30 | <0.001 |
| Creatinine clearance (KC) | 15.67 ± 3.88 | 21.62 ± 5.5 | <0.001 |
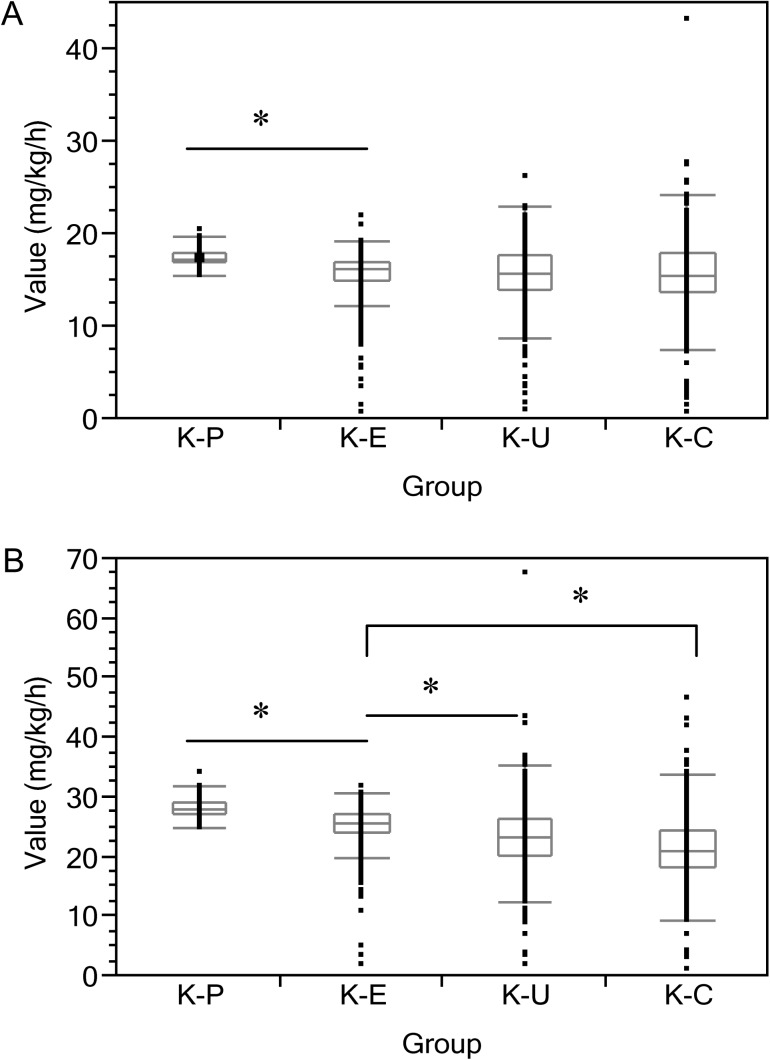
For the standard dose cohort, KP was 17.62 ± 0.96 mL/kg/h, KE was 15.79 ± 2.47 mL/kg/h, KU was 15.55 ± 3.07 mL/kg/h and KC was 15.67 ± 3.88 mL/kg/h. KP differed significantly from KE, KU and KC (P < 0.001 for all comparisons). KP over-estimated KU and KC by 11.7 and 11.1%, respectively. There was no statistically significant difference between KE, KU and KC (Figure 1).
Fig. 1.
Distribution of prescribed and measured clearance values for the (A) 20 mg/kg/h cohort and the (B) 35 mg/kg/h cohort. Value denotes serum measurement in mg/kg/h. KP = prescribed clearance. KE = estimated clearance. KU = measured urea clearance. KC = measured creatinine clearance. Each box and whisker plot shows the median value (line in the middle of each box) and 25th–75th quantile (box). Whiskers include 95% of measures. *P < 0.001 for designated comparison groups.
For the high dose cohort, KP was 28.10 ± 1.44 mL/kg/h, KE was 25.10 ± 3.16 mL/kg/h, KU was 23.32 ± 5.30 mL/kg/h and KC was 21.62 ± 5.50 mL/kg/h. KP, KE, KU and KC all differed significantly from each other (P < 0.001 for all comparisons). KE overestimated KU and KC by 7.1 and 13.9%, respectively. KP overestimated KU and KC by 17.0 and 23.1%, respectively (Figure 1).
Discussion
In all CRRT modalities, the ‘effluent’ represents the end product of filtration and comprises the ultrafiltrate in convective therapies, the spent dialyzate in diffusive therapies and the sum of both in combined therapies. CRRT solute clearance is determined by the ratio between the concentration of the solute in the effluent and in the plasma multiplied by the effluent rate. Because urea is a small molecular weight solute, it reaches complete equilibrium in the effluent; thus, the ratio of the concentration of urea in the effluent to plasma side of the dialysis membrane should be 1. Urea clearance becomes equal to the effluent rate, provided that the replacement fluid is given post-filter. For the published randomized trials regarding dose, utilization of TEV to estimate CRRT dose is based on this assumption that urea and other small solutes diffuse freely across the dialysis membrane.
For both dose cohorts in this study, the above assumption did not hold true; KP (accounting for the predilution effect of replacement fluid) overestimated KE, KU and KC. For the standard dose cohort, KE (accounting for the predilution effect of replacement fluid) was no different from KU or KC. However, for the high dose cohort, KE significantly overestimated both actual urea and creatinine clearance. The creatinine clearance was overestimated by an even larger margin than urea clearance. These results demonstrate that prescribed dose based on effluent rate significantly overestimates actual delivered dose based on measured solute clearances, even when correcting for predilution effect and time on therapy. As one would expect from previous research, this discrepancy increases with larger solute size and higher prescribed effluent rates [18].
Since the CRRT machine maintains a constant effluent generation rate, the reduced efficiency of solute removal must result directly from compromised filter permeability. One possible mechanism of compromised filter permeability is clotting of the filter due to insufficient anticoagulation. However, ∼95% of patients in each arm received citrate anticoagulation, with the remaining 5% receiving no anticoagulation. Other proposed mechanisms involve protein layering on the membrane, which would effectively reduce pore size and lead to preferential reduction in the clearance of larger molecules [19]. Over 15 years ago, Langsdorf and Zydney [20] showed a reduction in middle molecule clearance using the AN69 membrane attributed to a layer of plasma proteins on the membrane. The thickness of the protein layer increases with time, leading to further reduction in solute clearance, particularly those >10 000 D [21]. Messer et al. [22] recently demonstrated a reduction in middle molecule clearance in CRRT in vitro when convective clearance and higher ultrafiltration rates are utilized. Since the molecular weight of creatinine is almost twice that of urea (113.1 versus 60.1 D), the further reduction in creatinine clearance relative to urea clearance in the higher dose arm of our study suggests this protein layering as a possible mechanism despite the use of citrate anticoagulation and changing of filter every 72 h routinely.
In our dose study, despite the discrepancy in the prescribed dose and actual delivered dose, there was still a significant difference in small solute clearance in the standard-dose group versus the high-dose group. However, given that the measured creatinine clearance had a greater reduction in the high dose cohort as compared to the standard dose cohort, it is possible that the dose prescriptions in our dose study and the Acute Renal Failure Trial Network (ATN) study [10] and Randomized Evaluation of Normal versus Augmented Level Replacement Therapy (RENAL) study [11] may have delivered similar clearances for solutes larger than creatinine despite achieving nearly perfect dose separation in small solute clearance. This was seen in the in vitro study published by Hofman and Fissell [23]. They analyzed dialyzate-side clearances of tracer molecules from 10 to 100 KD molecular weights in an in vitro simulation of bovine blood using CVVHDF at 20 and 35 mL/kg/h effluent. Their results demonstrated that middle molecule clearance differed by <2 mL/min between the two dosing arms. They inferred from their results that the CRRT prescription used in our trial and the ATN study achieved dose ranging for small molecules while holding middle molecule clearance nearly constant due to protein polarization. This suggests that we may not yet know what the ‘ideal’ CRRT dose is to improve outcome. It is possible that higher clearances of middle molecular weight solutes may have a survival benefit.
Claure-Del Granado et al. [24] recently published a similar study to ours by comparing measured urea clearance to the prescribed CRRT dose based on effluent rate. Their results demonstrated that for urea clearance, even after accounting for the effects of predilution, the prescribed and estimated clearance overestimated the delivered dose by 26 and 25.7%, respectively. This is a much larger discrepancy than seen in our study. This discrepancy may be due to several reasons. Firstly, their study population had overall higher prescribed effluent rates (mean 30 mL/kg/h). Our study had two dosing arms: 20 versus 35 mL/kg/h. We noticed that the reduction in clearances for both urea and creatinine were greater in our high-dose arm at effluent rates comparable to the study by Claure-Del Granado. Secondly, we changed our filters every 72 h, while Claure-Del Granado et al. let their filters run much longer, allowing for more protein layering to occur. Finally, 18% of all their treatments were done with a Braun Diapact machine with a Fresenius NR60 filter. The rest of the treatments were done with a Prisma using an M100 filter. It is possible that the membrane characteristics of the NR60 differ from the M100 and result in differences in actual solute clearances.
Despite the published randomized dose trials in CRRT, controversy remains about the best way to measure and what constitutes optimal dose of CRRT for patients with AKI. The methods used for CRRT dose quantification in AKI have several limitations and have not been fully validated in this specific population. They have focused on urea clearance as the target clearance molecule for outcome. Even though adequate separation between small molecule clearance (as with urea) should be achieved in the dose studies, this does not necessarily apply to larger molecular weight molecules. Over the past few years, several articles have been published regarding the clearance of middle molecular weight molecules. Given our results regarding creatinine clearance, which is only a slightly larger molecule than urea, it becomes apparent that clearance of any larger molecules between the high- and standard dose cohorts is similar, without a dose separation. Since we do not know which molecule to target clearance for better outcomes, it is important to measure other molecules and design studies that look at other parameters for dose and ensure a dose separation with other solutes.
Our study has several limitations. It involves only a single center. Data were analyzed retrospectively. Thirty-five patients enrolled in the initial study had incomplete data and therefore were excluded from this analysis.
In conclusion, prescribed effluent rates overestimate solute removal in predilutional CVVHDF when utilizing doses commonly used today. Therefore, direct measurements of solute clearance are indicated if one is targeting a specific CRRT dose. Further research should focus on determining the optimal dose parameter in CRRT for AKI, including clearance of larger molecular weight solutes in AKI and its effect on outcomes. Furthermore, additional studies are needed to determine how different membrane properties affect solute clearance in CRRT.
Acknowledgments
This study was supported by a 2002 Pfizer Scholars in Clinical Epidemiology Grant and by General Clinical Research Center grant M01 RR-00032 from the National Center for Research Resources.
This work was presented in abstract form at the annual CRRT meeting, 22 February 2011 to 25 February 2011 in San Diego, CA and NKF spring clinical meeting, 26 April 2011 to 30 April 2011 in Las Vegas, NV.
Conflict of interest statement. None declared.
References
- 1.Barrantes F, Tian J, Vazquez R, et al. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 2.Liano F, Pascual J. Outcomes in acute renal failure. Semin Nephrol. 1998;18:541–550. [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol. 2010;6:521–529. doi: 10.1038/nrneph.2010.100. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard J, Macedo E, Mehta R. Dosing of renal replacement therapy in acute kidney injury: lessons learned from clinical trials. Am J Kidney Dis. 2010;55:570–579. doi: 10.1053/j.ajkd.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: A prospective randomized trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 7.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 8.Bouman C, Oudemans-van Straaten H, Tijssen J, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–2211. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tolwani AJ, Campbell RC, Stofan BS, et al. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 12.Vesconi S, Cruz DN, Fumagalli R, et al. Devlivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009;13:R57. doi: 10.1186/cc7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care. 2002;17:246–250. doi: 10.1053/jcrc.2002.36757. [DOI] [PubMed] [Google Scholar]
- 14.Clark WR, Turk JE, Kraus MA, et al. Dose determinants in continuous renal replacement therapy. Artif Organs. 2003;27:815–820. doi: 10.1046/j.1525-1594.2003.07288.x. [DOI] [PubMed] [Google Scholar]
- 15.Palevsky PM. Intensity of continuous renal replacement therapy in acute kidney injury. Semin Dial. 2009;22:151–154. doi: 10.1111/j.1525-139X.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- 16.Troyanov S, Cardinal J, Geadah D, et al. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18:961–966. doi: 10.1093/ndt/gfg055. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, Zhang W, Hardy PA, et al. Kinetic comparison of different acute dialysis therapies. Artif Organs. 2003;27:802–807. doi: 10.1046/j.1525-1594.2003.07282.x. [DOI] [PubMed] [Google Scholar]
- 18.Brunet S, Leblanc M, Geadah D, et al. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999;34:486–492. doi: 10.1016/s0272-6386(99)70076-4. [DOI] [PubMed] [Google Scholar]
- 19.Marshall MR. Current status of dosing and quantification of acute renal replacement therapy. Part 1: Mechanisms and consequences of therapy under-delivery. Nephrology (Carlton) 2006;11:171–180. doi: 10.1111/j.1440-1797.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 20.Langsdorf LJ, Zydney AL. Effect of blood contact on the transport properties of hemodialysis membrances: A two-layer membrane model. Blood Purif. 1994;12:292–307. doi: 10.1159/000170178. [DOI] [PubMed] [Google Scholar]
- 21.Morti SM, Zydney AL. Protein-membrane interactions during hemodialysis: effects of solute transport. ASAIO J. 1998;44:319–326. doi: 10.1097/00002480-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Messer J, Mulcahy B, Fissell WH. Middle-molecule clearance in CRRT: In vitro convection, diffusion and dialyzer area. ASAIO J. 2009;55:224–226. doi: 10.1097/MAT.0b013e318194b26c. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman CL, Fissell WH. Middle molecule clearance at 20 and 35 ml/kg/h in continuous venovenous hemodiafiltration. Blood Purif. 2010;29:259–263. doi: 10.1159/000266483. [DOI] [PubMed] [Google Scholar]
- 24.Claure-Del Granado R, Macedo E, Chertow G, et al. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011;6:467–475. doi: 10.2215/CJN.02500310. [DOI] [PMC free article] [PubMed] [Google Scholar]