Abstract
Multislice computed tomography (MSCT) for the noninvasive detection of coronary artery stenoses is a promising candidate for widespread clinical application because of its noninvasive nature and high sensitivity and negative predictive value as found in several previous studies using 16 to 64 simultaneous detector rows. A multi-centre study of CT coronary angiography using 16 simultaneous detector rows has shown that 16-slice CT is limited by a high number of nondiagnostic cases and a high false-positive rate. A recent meta-analysis indicated a significant interaction between the size of the study sample and the diagnostic odds ratios suggestive of small study bias, highlighting the importance of evaluating MSCT using 64 simultaneous detector rows in a multi-centre approach with a larger sample size. In this manuscript we detail the objectives and methods of the prospective “CORE-64” trial (“Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography using 64 Detectors”). This multi-centre trialwas unique in that it assessed the diagnostic performance of 64-slice CT coronary angiography in nine centres worldwide in comparison to conventional coronary angiography. In conclusion, the multi-centre, multi-institutional and multi-continental trial CORE-64 has great potential to ultimately assess the per-patient diagnostic performance of coronary CT angiography using 64 simultaneous detector rows.
Keywords: Computed tomography, Coronary vessels, Multi-centre study, Methods, Design
Multislice computed tomography (MSCT) for the noninvasive detection of coronary artery stenoses is a promising candidate for widespread clinical application because of its non-invasive nature and its improving diagnostic accuracy as shown in previous studies comparing the results of 16- [1–5], 32- [6], and 64-slice scanners [7–15] with conventional coronary angiography (CCA). In addition, noninvasive coronary angiography with MSCT has significantly higher accuracy than MRI for the detection of coronary artery stenosis [3, 16]. Thus, despite radiation exposure and administration of an iodinated contrast agent, MSCT may be preferable to other noninvasive methods for the evaluation of patients with a low to intermediate likelihood of coronary artery disease [17]. A recent scientific statement of the American Heart Association concluded that CT coronary angiography may be clinically useful and may be reasonable for assessment of symptomatic patients with suspected coronary artery disease (evidence class IIA, level of evidence: B) [18], while the American College of Radiology has issued a similar practice guideline including appropriateness criteria for the performance of cardiac CT [19].
There have been several studies that have assessed the diagnostic accuracy of MSCT (4-, 16-, 32-, and 64-slice CT) in comparison with CCA. However, a vast majority of these studies were limited in sample size and were performed at single centres. Single-centre based studies may be limited due their ability to recruit a large sample size, but are particularly prone to study bias (selection, reporting, publication, or other bias) and the ability to apply the results to other centres or a broader patient population (variation in diagnostic parameters or definitions). For example, although multiple single-centre studies have shown good diagnostic performance of MSCT, the first multi-centre study of CT coronary angiography using 16 simultaneous detector rows (CATSCAN Study) has shown that this technology is limited by a high number of uninterpretable cases and a high false-positive rate [20]. In the meta-analysis by Hamon et al., there was a significant interaction between the size of the study sample and the diagnostic accuracy (diagnostic odds ratios) in the individual studies, with small studies being more likely to provide higher diagnostic odds ratios. This is suggestive of small study bias and highlights the importance of evaluating the technology in a multi-centre fashion. With the improvement of image quality with 64 detector rows as compared with 16-slice CT [21], and the increase in the percentage of assessable segments [22, 23], per-patient diagnostic accuracy should be improved to support the clinical usefulness of MSCT. However, up to now no study has analysed 64-slice CT coronary angiography in a multi-centre design, including rigorous methodology and centralised core labs. Results of a multi-centre trial could become the foundation for MSCT clinical application guidelines, due to its characteristics of reduced study bias and wide applicability to different centres, countries, and patient populations. Thus, we designed a multi-centre study called the “CORE-64” trial (“Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography using 64 Detectors”). This paper presents the objectives, methods, and approaches of this large diagnostic study conducted in seven countries in Europe, America, and Asia.
Study methods
Objectives
The “Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography using 64 Detectors” or “CORE-64” study was designed to evaluate the diagnostic accuracy of multislice spiral CT angiography using 64 detector rows for identifying coronary artery stenosis in patients with suspected or known significant coronary artery disease. The study was designed as a prospective, multi-centre (nine centres), international (seven countries) study examining the diagnostic accuracy of 64-slice CT in comparison with CCA. The primary hypothesis of the study was that 64-slice CT coronary angiography will be able to detect significant coronary artery disease in a patient with acceptable diagnostic accuracy as compared to CCA. Significant CAD is defined as ≥50% stenosis as determined by coronary angiography (QCA) of CCA. The primary diagnostic parameters were per-patient sensitivity and specificity compared with CCA. These were analysed using both point estimates and continuous measurements (using area under the receiver-operating characteristics curve) expressed with 95% confidence intervals for the corresponding true values to indicate the precision of the estimates. We also compared the diagnostic performance of CCA with that of MSCT for anatomy-based prediction of subsequent clinically driven revascularisation on a per-patient and per-vessel level. Other secondary objectives include the evaluation of diagnostic accuracy based on a per-vessel and per-segment unit of analysis, and defining significant stenosis at both ≥50% and ≥70% thresholds, with QCA as the reference standard.
Design
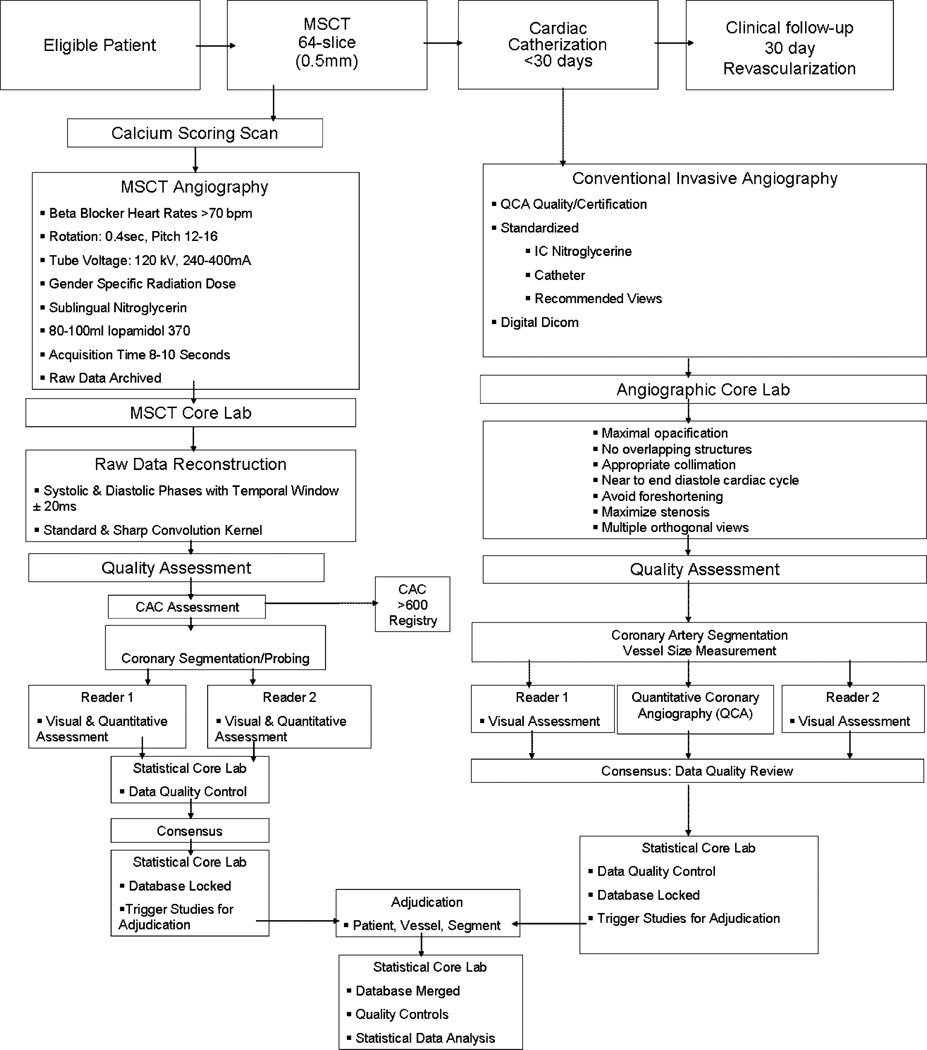
This was a prospectively designed, multi-centre, international, single-vendor study examining the diagnostic accuracy of MSCT-64 in the ability to detect coronary artery disease compared with CCA. The sponsor of the study was Toshiba Medical Systems. The CORE-64 study was conducted at nine centres, with three centres in the US, and one centre in Brazil, Germany, Japan, The Netherlands, Canada, and Singapore. All centres used the same multi-slice CT scanner using 64 simultaneous detector rows (Aquilion 64, Toshiba Medical Systems, Nasu, Japan). The study protocol was developed by the CORE-64 Steering Committee. Briefly, patients who were enrolled in the study first underwent the study-related MSCT, followed by CCA within the following 30 days, and clinical follow-up thereafter. CT and CCA studies were forwarded to individual core laboratories (MSCT and CCA) and were analysed by separate teams blinded to clinical parameters and the other imaging test. The overall study design of the CORE-64 trial is depicted in Fig. 1.
Fig. 1.
CORE-64 study design. Workflow of patient enrolment, data acquisition, and data handling. CAC = coronary artery calcium. MSCT = multislice CT. QCA = quantitative coronary angiography
MSCT was always performed before the clinically indicated conventional cardiac catheterisation to avoid selection bias and include patients typically referred for CCA. Investigators and patients were blinded to the MSCT results, and all further clinical decisions were based on the patient’s clinical standard of care and clinically indicated CCA. Core laboratories were blinded to each other and to clinical data. All enrolled patients provided informed consent approved by local institutional review boards. Patients then received a unique, randomly assigned subject study number that was used as the subject identifier for the data acquired for research purposes.
Population
CORE-64 was designed to prospectively include patients older than or equal to 40 years of age, who were referred for clinically driven CCA for suspected or known coronary artery disease and who were willing and able to sign written informed consent. Women of child-bearing potential required a negative pregnancy test within 24 h of the study MSCT. Patients were excluded from participation if they had any of the following: history of allergic reaction to iodinated contrast media, a history of contrast-induced nephropathy, history of multiple myeloma or previous organ transplantation, elevated serum creatinine (>1.5 mg/dl) or calculated creatinine clearance of <60 ml/min (using the Cockcroft-Gault formula), atrial fibrillation or uncontrolled tachyarrhythmia, or advanced atrioventricular block (second or third degree heart block), evidence of severe symptomatic heart failure (NYHA class III or IV), known or suspected moderate or severe aortic stenosis, previous coronary artery bypass or other cardiac surgery, coronary artery intervention within the last 6 months, known or suspected intolerance or contraindication to beta-blockers (including: known allergy to beta-blockers, history of moderate to severe bronchospastic lung disease including moderate to severe asthma, severe pulmonary disease), body mass index >40, or the presence of any other history or condition that the investigator judged to be a significant reason for exclusion. Patients with coronary artery stents were not excluded from participating in the study. However, stented coronary segments were excluded from the comparative analysis of CCA and MSCT. To control for enrolment selection bias, it was decided to enrol all patients, but only analyse patients with overall Agatston calcium scores of ≤600 for the primary cohort; those patients with Agatston calcium scores >600 were entered into a registry.
Sample size
The sample size computation was based on the precision of the diagnostic accuracy measurement. We estimated that a sample size of 350 was needed to determine the diagnostic accuracy of MSCT defined as an area under the receiver characteristics curve (AUC) of at least 0.85 with 95% confidence intervals of at most ±5% imprecision based on a disease prevalence rate of 35% and a drop out rate of 10% [24].
The primary outcome measure of the study was the determination of diagnostic performance of MSCT on a per-patient basis. Secondary analyses examining diagnostic accuracy based on a vessel unit of analysis were also planned. Diagnostic accuracy was defined by the area under the receiver-operating characteristic (ROC) curve and the point estimates of sensitivity and specificity as determined by standard methods [25]. The ROC curve is a plot of a diagnostic test’s sensitivity (plotted on the y axis) versus its false-positive rate (1-specificity) (plotted on the × axis). An ROC analysis plots the relationship between sensitivity and specificity across all cut-points of the test and calculates the area under the curve (AUC). Computation of confidence limits for vessel-level data took account of the clustering of vessels within a patient to adjust for interdependence, using either logistic regression or boot-strap resampling methods [26]. Confidence limits were calculated using the percentile method with B=2,000 samples. Additionally predictive values were determined by standard methods.
Quality assurance and safety
All technologists and investigators underwent study-related training prior to the initiation of the enrolment at their site. Technologists underwent specific 2-day training in the specific CORE-64 MSCT protocol and MSCT acquisition, and safety, to ensure protocol compliance and standardised data acquisition and data transfer. Before enrolment of patients into the study, each enrolling centre provided three qualifying MSCT examinations to the MSCT core laboratory and three CCA films to the angiography core laboratory, which were reviewed for protocol compliance and data quality. All centres had the study protocol and informed consent form approved by their local institutional review board, and reviewed also by a centralised institutional review board and the German Federal Department for Radiation Protection. Adverse events were tracked and reported to the Coordinating Centre in a timely manner, and were also reviewed by an independent Data Safety and Monitoring Board.
MSCT and CCA acquisition
Prescan preparation and positioning for CT
Patients were instructed to avoid caffeine 4 h before their CT examination. Fasting before CT was at the discretion of the local investigator. A pre-MSCT serum creatinine was obtained (if not already available) within 30 days before the MSCT. A review of inclusion/exclusion criteria, concomitant medications (i.e., metformin, beta-blockers), and allergies (i.e., history of contrast agent-related allergoid reactions) was performed before MSCT. Vital signs (blood pressure, heart rate) were obtained. Patients with resting heart rates ≥70 beats per minute (bpm) received beta-blockers in compliance with institutional standards. Subjects with heart rates <70 did not receive routine intravenous or oral beta-blockers, and vital signs were monitored according to local standards at each centre. If a heart rate ≤80 bpm could not be obtained, the patients were excluded from the primary analysis cohort and entered into a registry.
Nitroglycerin
Short-acting nitrates were administered just before MSCT scanning in all patients in whom this was not contraindicated (systolic blood pressure <110 mm Hg, intake of phosphodiesterase inhibitors, severe obstructive cardiomyopathy, known intolerance) in order to reduce vasospasm, standardise vasomotor tone, increase coronary artery diameters, and facilitate image assessment (percent stenosis) as recently described [27]. The choice of sublingual nitrate was determined per local standards.
CT
Patients were examined on a multislice CT system, using 0.5-mm slice thickness, with 64 simultaneous detector rows (Aquilion 64, Toshiba Medical Systems, Japan). The 320-mm scan field of view (M) of the CT scanner was used for image acquisition. First, an anteroposterior scanogram (120 kV, 50 mA) was obtained for further planning. Based on the cardiac dimensions on this scanogram, the z-axis length of the subsequent non-contrast calcium scoring CT was defined. In addition, a single axial slice was acquired at the largest diameter of the heart, which served to define the reconstruction field of view to be used for all subsequent reconstructions. The size of this field of view was in the range of 200 to 220 mm in order to achieve an in-plane pixel size of approximately 0.4*0.4 mm2 at an image matrix of 512 by 512. The reconstruction field of view was set to cover the entire heart and the descending aorta and was used for both calcium scoring and CT coronary angiography. Calcium analysis was performed using prospective electrocardiogram gating with a gantry rotation time of 0.4 s, a detector collimation of 4 by 3.0 mm, a tube voltage of 120 kV, and a tube current of 300 mA. The trigger delay for prospective gating in the calcium CT study was adjusted to the heart rate in order to acquire CT data during the rest period of the coronary arteries (Table 1).
Table 1.
Prospective trigger positions for different heart rates in CT calcium scanning
| Heart rate | Prospective (%) trigger position in the RR interval* |
|---|---|
| 50 | 79 |
| 60 | 75 |
| 70 | 71 |
| 80 | 67 |
| 90 | 63 |
Based on the unenhanced CT images used for calcium scoring, the start and end positions of subsequent CT coronary angiography were determined to minimise radiation exposure of the CTA by reducing the z-axis coverage as much as possible. The start position was defined as the plane 10 mm above the first cranial slice that no longer showed any coronary structures. Similarly, the end position was defined as the plane 10 mm below the last caudal slice without cardiac structures. The start and end positions determined in this way were entered into the CT coronary angiography protocol for each patient. The CT coronary angiography protocol was performed using 64 by 0.5-mm detector collimation and a minimum gantry rotation time of 0.4 s, and the CT system’s settings were adjusted according to the patient’s sex and weight and the pitch (Tables 2 and 3) in order to keep the radiation dose within the CORE 64 protocol’s pre-specified limit of 20 mSv for all patients.
Table 2.
Scanner settings for coronary CT angiography in men
| Weight | kV | mA | |
|---|---|---|---|
| Pitch of 0.2 to <0.225* | Pitch of ≥0.225 | ||
| <60 kg (<130 lb) | 120 | 300 | 300 |
| 60–79 kg (130–179 lb) | 120 | 340 | 360 |
| >80 kg (>180 lb) | 120 | 360 | 400 |
The pitch was adjusted according to heart rate and heart rate variability during the preceding breath-hold examination
Table 3.
Scanner settings for coronary CT angiography in women
| Weight | kV | mA | |
|---|---|---|---|
| Pitch of 0.2 to <0.225* | Pitch of ≥0.225 | ||
| All weights | 120 | 240 | 270 |
The pitch was adjusted according to heart rate and heart rate variability during the preceding breath-hold examination. The tube current was lower in women than in men and was the same for all weights in order to ensure an effective dose for CT coronary angiography below 17 mSv for women (together with calcium scanning below 20 mSv) as requested by the German Federal Department for Radiation Protection. The effective dose used for the “CORE-64” trial protocol will be the objective of a separate dosimetric measurement study.
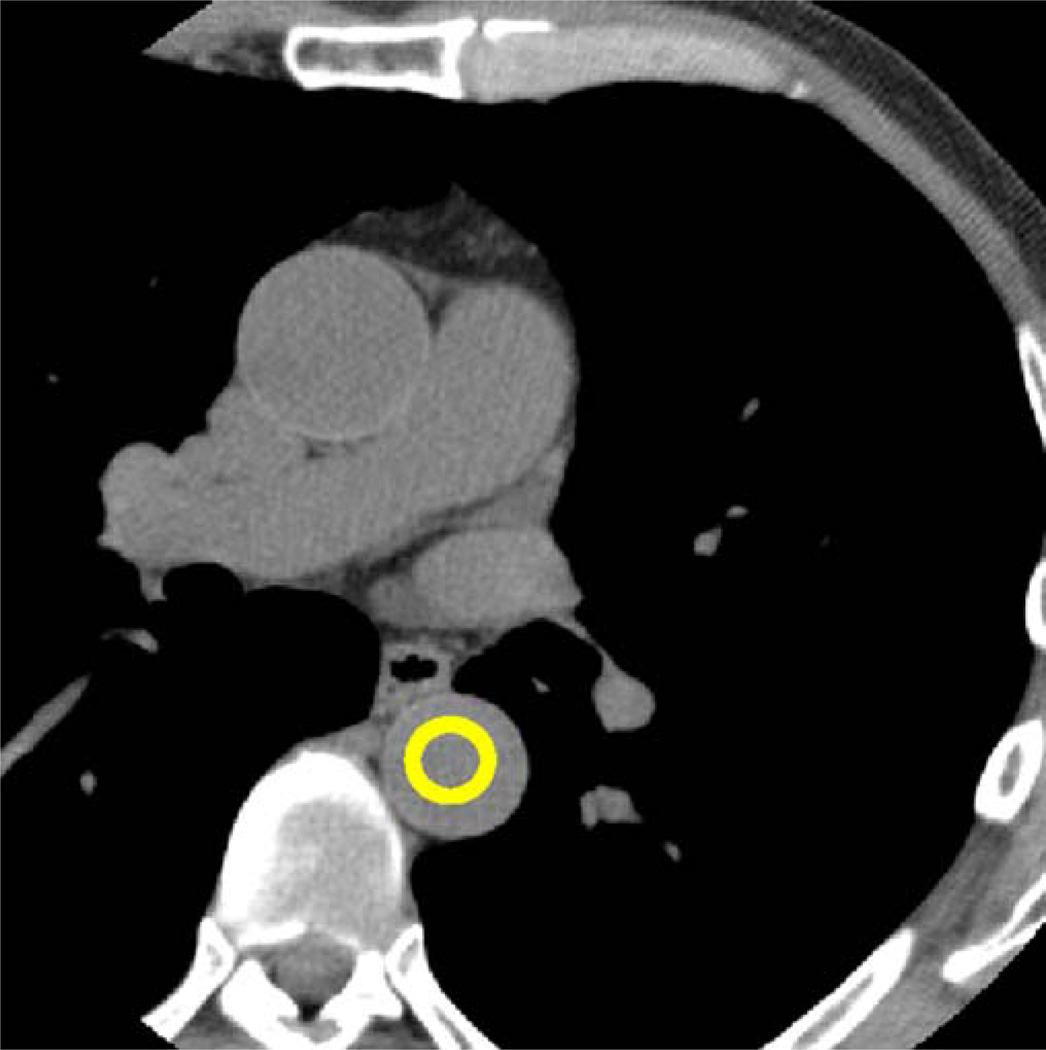
Another single axial CT image was obtained at the start point of the helical acquisition. The image obtained in this position served to measure HU densities in a region-of interest in the descending aorta beginning 15 s after contrast agent administration and to trigger the initiation of helical CT once a threshold of 180 HU was reached (SUREStart, Toshiba Medical Systems, Fig. 2). Immediately before injecting the contrast agent, starting this SUREStart CT run and the helical CT coronary angiography acquisition, a breath-hold trial (“mock examination”) was performed by simulating these processes with a single breath-hold command (“breathe in and hold your breath”). An electrocardiogram tracing was recorded during the mock examination, and heart rate was plotted against time by a software plug-in (SURECardio 3.0, Toshiba Medical Systems). As with the true helical CT, electrocardiogram recording commenced 2–3 s after the breath-hold command to minimise breathing-related fluctuation in heart rate during acquisition. The breath-hold trial was repeated in patients with extrasystoles or heart rate variability of more than 10%. The software determined the optimum scanning parameters (gantry rotation time and pitch) depending on the minimum and maximum heart rate during the breath-hold trial and the expected image reconstruction window width for this heart rate range. The tube current was modified according to this pitch (Tables 2 and 3) to adhere to the “as low as reasonably achievable” paradigm and to achieve constant high image quality regardless of body mass.
Fig. 2.
Single axial scan at the level of the start position single axial CT image at the level of the start position of the helical scan with a region of interest in the descending aorta. HUs were measured in this region, and the helical CT data acquisition was started when the densities were consistently above 180
CT contrast agent administration
An iodinated contrast agent (Solutrast, Isovue, iopamidol, 370 mg iodine/ml, Bracco Diagnostics) was injected using an 18- or 20-gauge intravenous line (preferably in right brachial veins). The flow of the contrast agent was adjusted according to patient weight (Table 4). The volume of the contrast agent to be administered for the helical CT acquisition was also calculated individually for each patient using the following formula:
Table 4.
Contrast agent flow rates for coronary CT angiography according to weight
| Weight | Flow rate (ml/s) |
|---|---|
| <60 kg (<130 lb) | 3.5 |
| 60–100 kg (130–219 lb) | 4.0 |
| >100 kg (>220 lb) | 5.0 |
During administration of contrast agent a physician or technician was available to ensure that no contrast agent extravasation occurred at the site of injection. Fifteen seconds after starting contrast administration, the above-described SUREStart CT acquisition began tracking the density in the descending aorta. When a threshold of 180 HU was achieved, a single automated breath-hold command (“breathe in and hold your breath”, breath-hold for 5 s) was given. Helical scan acquisition commenced 2–3 s after the breath-hold command to minimise breathing-related fluctuation in heart rate during acquisition.
Conventional coronary angiography (CCA) acquisition
A clinically driven coronary angiography was performed within the 30 days following MSCT scan. All centres were to follow standardised coronary angiography techniques to obtain optimum images for comprehensive quantitative coronary analysis. Intracoronary nitroglycerin was administered (150–200 mcg) before the first image of the left coronary artery system and right coronary artery to standardise the vasomotor state of the coronary artery and eliminate any potential for catheter-induced spasm. Coronary angiographic images were saved in the universal DICOM format and forwarded to the Angiographic Core Laboratory for Quantitative Coronary Angiographic Analysis. All image data were anonymised for transfer to the core laboratory with the unique study number. QCA was preformed using standard, validated analysis software from Pie Medical Imaging, CASS II Research version 2.0.1 software.
Data handling, analysis, and follow-up
Image reconstruction and transfer
Raw data were protected on the scanner console and were transferred to a raw-data workstation for storage on an advanced intelligence tape (AIT). All raw data were anonymised for transfer to the MSCT core laboratory with the unique study number assigned at the time of enrolment. CT raw data were also saved on an AIT tape for storage at each centre.
Non-cardiac findings
Evaluation for the presence of non-cardiac findings on CT coronary angiography was performed locally at each centre by a radiologist within a maximum of 3 weeks but within local institutional standards. For these non-cardiac safety assessments, images were reconstructed with a 320-mm field of view at 80% of the RR interval with a slice thickness of 4.0 mm at 3.0-mm intervals using mediastinal and lung kernels. These were the only data clinically available to the patient and his/her physician.
MSCT coronary angiography reconstruction
Raw image data were transferred by AIT tape to the CT core laboratory for analysis (Fig. 1). Raw data were reconstructed at 0.5-mm slice thickness by an adaptive multisegment reconstruction algorithm [28]. An image reconstruction increment of 0.3 mm was used to assure optimal image quality. The initial cardiac phase selected for reconstruction was centred at 75% of the RR interval [29, 30]. Using ImageXact® software (Toshiba Medical Systems) one minimal cardiac motion phase [31] each in systole and diastole, in which the least cardiac motion was present, was also selected for reconstruction using both a standard and a hard convolution kernel. A temporal window of ±20 ms was used for both the systolic and diastolic reconstruction to permit for better assessment of proximal and distal coronary arteries in the case of minor variations in movement. Adaptive multisegment reconstruction was used to improve temporal resolution [28, 32] in patients with heart rates above 50 bpm. All acquisition ECGs were reviewed for arrhythmias prior to phase selection. In the event of an arrhythmia ECG editing was employed to maintain regular R-to-R intervals while minimising loss of data. Several reconstructions with and without edits were performed to identify the optimal data set for the final images. Readers were informed of cases in which an arrhythmia occurred during acquisition. Optimal reconstruction was identified not only on a per-patient basis but also on a per-vessel basis. Therefore, images from at least six different phases were provided for image analysis. In cases with severe coronary calcification, a sharp convolution kernel was also reconstructed when requested by the readers.
Overall image quality was assessed for each CTA study on a nominal scale: good, adequate, poor, and unevaluable. Protocol compliance in regards to mA, kV, estimated radiation dose, contrast flow rate and dose, triggering, heart rate, heart rhythm, scan field of view, coronary opacification, nitroglycerin, and beta-blocker administration was reviewed. Overall image quality was assessed for each study. The central CT processing technician measured total and regional (for each individual coronary artery territory) Agatston [33] and calcium-volume [34] scores with careful avoidance of stents using standardised software (Vital Images, Vitrea 2 version 3.9.0.1).
Additionally, the central CT processing technician probed and segmented each coronary study. In addition to axial interrogation, an automated vessel probe on each portion of every segment was performed in vessels ≥1.5 mm in diameter by MSCT (estimated by Vitrea lesion tool). This semi-automated centreline detection [35] of the coronary vessel lumen on slabs of 3D cardiac volume rendering was used to generate curved multiplanar reformations and cross-sectional images. Both three-dimensional renderings and multiplanar reformation images were used to assure adequate interrogation of the artery. Following interrogation of all arteries of at least 1.5 mm in diameter, the coronary artery tree was segmented based on a modified 29-segment ACC/AHA model nomenclature condensed to 19 segments [36, 37]. Segments that were not visualised were recorded as such. Segments were labelled with the specific segment number based on the pre-specified definitions used in both core laboratories. Vessel diameter in the CT core laboratory was determined by measurement of the luminal diameter of the proximal portion of the segment, using the non-diseased portion of the segment if present. For tapering arteries in the distal part of the vessels, the segment was analysed until the segment diameter no longer measured 1.5 mm.
Coronary artery segmentation
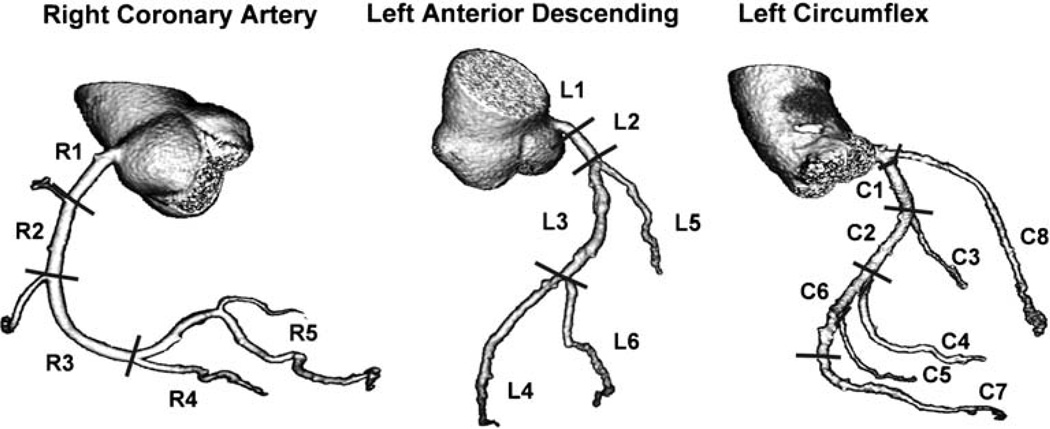
Previous studies describing MSCT have reported using the 15- or 17-segment AHA model of coronary artery segmentation [38]. CCA studies typically use a more expansive model (AHA/ACC 29 segment model) [39]. In order to accommodate a more comprehensive coronary artery tree description, without placing undue emphasis on small and very distal territories, we developed a modified 19-segment model, based on the standardised 29-segment nomenclature (Fig. 3). We excluded the acute marginal, first septal, and third diagonal. Additionally we grouped together the right posterior laterals and the distal circumflex with the left posterolaterals. For all defined 19 segments, the tightest lesion was analysed.
Fig. 3.
Coronary artery segmentation model modified from the BARI [32] and CASS [31] investigators used for the CORE-64 trial [with 19 segments: 5 in the right coronary artery, 6 in the left anterior descending coronary artery (including left main), 7 in the left circumflex coronary artery, and the intermediate branch]. The entire coronary artery tree is included in the model. All segments ≥1.5 mm were included in data analysis. R1=proximal right coronary artery, R2=mid-right coronary artery, R3=distal right coronary artery, R4=right posterior descending, R5=grouped right posterolateral (RPL): first RPL, second RPL, and third RPL (segments 5, 6, 7, 8 of the CASS model), L1 = left main, L2 = proximal left anterior descending, L3=mid-left anterior descending, L4=distal left anterior descending, L5=first diagonal branch, L6=second diagonal branch, C1=proximal left circumflex coronary artery, C2=mid-left circumflex coronary artery, C3=first obtuse marginal, C4=second obtuse marginal, C5=third obtuse marginal, C6=grouped: distal left circumflex coronary artery (segments 19.1, 23, 24, 25, 26 of the CASS model), first left posterolateral (LPL), second LPL, and third LPL, C7=left posterior descending, C8=intermediate branch
MSCT coronary angiography analysis
Image data were transmitted to two independent Vitrea™ workstations for independent and blinded reader analysis. Two independent readers blinded to each other, the invasive angiography data, as well as clinical data, performed the analysis. Each reader examined study quality, calcium burden, and lumen stenosis for each of the coronary artery segments independently. The percentage of diameter stenosis was derived from volume-rendered images, curved multiplanar reformations, maximum-intensity projections, and cross-sectional images in systolic or diastolic phases as well as standard and sharp kernels. The degree of stenosis in all coronary segments was visually and quantitatively assessed by both modalities. The degree of stenosis in all coronary segments was first visually performed and then later quantitatively assessed by both modalities. Visual assessment scores could not be modified following the quantitative assessment. Visual and quantitative datasets were separate and processed individually. Following final determination of segment scores, vessel-based datasets were constructed from segment data and patient-based datasets from vessel datasets.
Visual MSCT coronary angiography analysis
Visual assessments were performed by two independent readers, using a categorical scale: no disease, 1–29%, 30– 49%, 50–69%, 70–99%, and 100% diameter reduction before quantitative measurement, or as non-evaluable. All segments in which a significant difference in assessments between the two principal readers (i.e., crossing a threshold of 50% or 70%) or any segment in which readers deemed a segment non-evaluable, underwent a consensus process that incorporated a third experienced observer. The final segment score was given by the consensus process. A segment could only be considered non-evaluable by consensus. In segments in which there was no significant difference between the two principle readers, a final segment score was derived through averaging.
Quantitative visual MSCT coronary angiography analysis
Quantitative assessment of the degree of diameter stenosis was performed in a cross-sectional and a longitudinal projection following all visual identification with at least ≥30% stenosis by by two independent, blinded readers. Measurements were made with Vitrea 2 version 3.9.0.1 (Vital Images) semi-automatic contour detection algorithm to obtain preliminary diameter estimations. Manual measurements of reference diameters and lumen diameter were made using electronic callipers and rulers in cross-sectional and longitudinal projections. Final determinations of reference and lumen diameters were made only after manual contour editing.
The maximum percentage of diameter stenosis was determined for each segment. Proximal and distal reference measurements within the same segment at a disease-free site were made in addition to a measurement for minimal lumen diameter. The maximum percentage of diameter stenosis was derived by the following formula:
All segments in which a significant difference in assessments between the two principal readers, or any segment in which one of the readers deemed it unevaluable, underwent a consensus process that incorporated a third experienced observer. In segments in which the difference between the two principle readers was not significant, a consensus score was derived through averaging. A “significant difference” was defined as maximum percentage diameter stenosis crossing the 50% or 70% threshold. In addition, if any one reader judged a segment non-evaluable, the segment underwent consensus. Segments were only deemed non-evaluable if there was no quantitative measurement made by any of the readers. Vessel-based datasets were constructed from segment datasets and patient datasets from vessel datasets.
Analysis of CCA
Digital cinefluoroscopic images received on compact disks were analysed by quantitative coronary angiography (QCA) using the CAAS II QCA Research version 2.0.1 software (PIE Medial Imaging, Maastricht, The Netherlands). Quantitative coronary angiography analysis was performed by use of a computer-assisted, automated edge-detection algorithm. With the outer diameter of the contrast-filled catheter as the calibration standard, the minimum lumen diameter (MLD) in diastole or near end diastolic phase was measured from orthogonal projections. The percentage of stenosis was derived from the view with the greatest reduction of diameter (“worst view”) with the least foreshortening of the segment in question. The reference diameter is an interpolated diameter of normal segments proximal and distal to the lesion. Obstructive analysis is used with minimal user input. When a normal proximal segment could not be identified (e.g., ostial lesion location), a distal angiographically normal segment closest to the lesion (but not beyond major branches) was analysed. All vessels in the coronary artery tree were examined. Vessels or branches that were <1.5 mm were not analysed. In addition to quantitative assessments, visual assessments of coronary artery stenosis were performed for CCA by two independent readers, using the same categorical scale as the MSCT core laboratory: no disease, 1–29%, 30–49%, 50–69%, 70–99%, and 100%. Segments could be judged as nonevaluable by either visual or quantitative assessment due to incomplete visualisation. Segments distal to total occlusions with incomplete contrast filling were deemed none-valuable. Lesions that were too tight to quantify (i.e., >90% <100%) due to software limitations were imputed to a coronary stenosis of 95%.
The standard 29-segment model as defined by the AHA/ACC for classification of segmentation was first performed in order to assure standardisation of the dual-reader process and to use this tree to estimate vessel size and exclude those vessels <1.5 mm from the analysis. In addition, clear definitions and rules in regards to nomenclature, branching, and segmentation were prospectively designed and implemented to allow for prospective, yet blinded, segmentation and localisation of specific stenosis. Following this process, the 29-segment model was condensed into the modified 19-segment model (Fig. 3) to allow comparison of CCA analysis and the CT angiography analysis. Vessel size thresholds for inclusion in the final analysis were determined by the measurements from the CCA core laboratory.
Segment adjudication
After completion and locking of all qualitative and quantitative stenosis measurements, an adjudication process was performed to assure that segment scores recorded for both MSCT and QCA were for identical segments. The adjudication committee consisted of a member from the angiography core laboratory and two members of the computed tomography core laboratory, and was supervised by an independent overseer with expertise in both imaging modalities. Studies undergoing adjudication were derived from the database manager. The criterion used to trigger a study for adjudication was any segment in which there was a discrepancy between CCA and MSCT in the reporting of a lesion of ≥50% by visual and/or quantitative assessment. If the study had multiple lesions, all lesions were adjudicated. The adjudication committee compared MSCT and CCA images side by side to identify fiduciary landmarks and confirm that segment scores were recorded for identical segments. Scores were re-assigned to the appropriate segments in cases of misalignment. No visual assessment or quantitative assessment scores by either modality were changed: only the label of the attributed segment could be changed. Studies in which adjudication resulted in a lesion changing a vessel (e.g., an intermediate branch mislabelled as first diagonal branch) or a lesion traversing two segments were flagged for overview by a blinded senior MSCT core laboratory member and a blinded senior CCA core laboratory member; discrepancies were resolved through a consensus process.
Clinical follow-up
Clinical follow-up for patients enrolled in the CORE-64 study was scheduled for 7 days, 30 days, 6 months, and 1 year. Clinical status and interval events were reviewed via telephone with the patient, review of medical records, or written communication with the patient. Specific clinical outcomes and safety parameters to be captured included (but were not limited to) the occurrence of death, myocardial infarction, stroke, coronary revascularisation (including percutaneous coronary intervention or coronary artery bypass surgery), or hospitalisations. Of note, particular focus was placed on revascularisation data, including revascularisation type, location, and outcome. Other events included those related to the safety of contrast use, such as allergic reaction or clinical renal failure.
Discussion
The objectives and methods outlined above are unique in that the “CORE-64” trial assessed the diagnostic performance of 64-slice CT coronary angiography in nine centres worldwide using a clearly prescribed approach to patient recruitment, CT imaging, CCA, and data analysis. Statistical planning including a power analysis is of importance for any clinical trial. Despite numerous single-centre studies addressing the diagnostic accuracy of MSCT, a power analysis before initiating the trial has been performed in only one previous study [3]. The diagnostic accuracy results of the CORE-64 trial will be estimated and presented together with 95% confidence intervals to indicate the precision of the estimates. Other advantages of the CORE-64 trial include the extensive collection of clinical baseline characteristics and the conduction of clinical follow-up — an approach that has not been previously used. In contrast to a 16-detector multi-centre study [20] where reconstructed images were transferred to a core laboratory for evaluation, CORE-64 raw image data were transferred. This enabled ECG editing to be performed and unlimited images to be generated.
In comparison to multiple other studies we routinely administered nitroglycerin because this increases coronary artery diameters on average by 16% as recently shown in an intra-individual comparison [27]. We also recorded adverse events during CT and CCA to assess the safety of the two tests. Patients with cardiac pacemakers and defibrillators were not excluded from the CORE-64 trial, making the results more applicable. The radiation exposure of CT coronary angiography is a potential safety issue. However, it should be noted that the present study was approved by the central IRB, all local IRBs at each centre, and the German Federal Department for Radiation Protection. The CORE-64 Steering Committee determined the effective dose should be below 20 mSv in all patients, also consistent with the German Federal Department for Radiation Protection requirements. In order to achieve this it was decided to include only patients with a body mass index <40. Also, in contrast to most of the studies published on 64-slice technology [7–12, 40], the present study used true 64-detector row technology. The large sample size and the performance of CT at many institutions worldwide are important further advantages of the study detailed here.
The coronary artery segmentation model used for the study was adopted after intensive discussion by the CORE-64 steering committee. It was determined to employ a more comprehensive coronary segmentation scheme. The coronary artery segmentation model used for the study presents a more comprehensive approach to the analysis of CT coronary angiography in that it tries to parallel the updated invasive angiography AHA model (29 segment) instead of the older 15-segment AHA segmentation model [38, 39]. We opted for the more comprehensive approach to allow for a more complete analysis of the coronary tree in locations where not only surgical, but also percutaneous revascularisation may be performed. The goal of the 19-segment model is to make it consistent with the established AHA/ACC conventional coronary angiography reporting guidelines [39] while taking into account the commonly used CT model, thus making the new scheme more accepted by the interventional cardiology community. No other CT study has used this comprehensive segmentation model and, despite its advantages, the new approach might limit the comparability of our results with those obtained by other investigators. However, our comprehensive approach is more reflective of the increasing need to evaluate a greater proportion of the coronary tree, including branches and smaller vessels, which may require treatment.
For about 50 years CCA has been the favoured gold standard for the detection of significant coronary artery disease. CCA is known to underestimate [41] the degree of coronary atherosclerosis, and intravascular ultrasound may be a better cross-sectional gold standard for assessment of atherosclerotic burden, but was not used in the “CORE-64” trial due to its lack of global applicability in clinical practice along with increased risks and costs. Moreover, quantitative analysis of CCA is more accurate and reliable than visual interpretation [42, 43], and intravascular ultrasound is not a mandatory test in clinical routine [44]. For these reasons, similar to other non-invasive imaging studies assessing the diagnostic accuracy for detecting coronary artery disease, we selected QCA performed in a core laboratory as the reference standard.
The CORE-64 trial has some limitations. It was a single-vendor study, and the conclusions to be drawn will only pertain to current technology as the study presents a “snapshot” in time and new developments may further increase clinical utility and applicability of CT coronary angiography. Recently, a 320-slice CT scanner was introduced, which has great potential to increase image quality of noninvasive coronary angiography while at the same time reducing radiation exposure [45, 46]. Furthermore, dual-source CT technology is available now [13, 47–49], which has led to relevant improvement in temporal resolution. Patients were excluded from the CORE-64 trial if they were not in sinus rhythm. This approach was also used in the vast majority of previous studies of CT coronary angiography [50] since atrial fibrillation or other arrhythmias are very likely to result in nondiagnostic examinations on the per-patient level [51]. Nevertheless, our results will only be valid for patients who are in sinus rhythm and do not apply to patients with arrhythmias. Other exclusion criteria such as high heart rates (above 80 bpm during scanning) also represent a limitation because patients with higher heart rates are not uncommon in clinical practice and are less likely to benefit from CT coronary angiography [52–56]. The same limitation holds also true for patients with a high body mass index (above 40) who were also not eligible for the study. On the other hand, a lower body mass index threshold may have been more suitable because it might have improved image quality and diagnostic accuracy of coronary CT angiography, but would also have made the study results less applicable to obese patient. However, given the radiation dose necessary to obtain diagnostic-quality images in the morbidly obese patient, alternative diagnostic modalities may more appropriately be able to balance the risk versus benefits of a diagnostic study. Finally, patients with stents were not excluded from our study, but stented segments were excluded from analysis since previous reports [57–60] have shown that current CT technology lacks the diagnostic accuracy necessary for stent assessment.
In summary, the multi-centre, multi-institutional, and multi-continental “CORE-64” trial is designed to rigorously assess the per-patient diagnostic accuracy of CT coronary angiography using 64 simultaneous detector rows.
Acknowledgments
This work was supported in part by the Doris Duke Charitable Foundation (Julie M. Miller, Clinical Scientist Development Program). Joao A.C. Lima is funded by NHLBI RO1-HL66075-01 and HO1-HC95162-01, the National Institutes on Aging RO1-AG021570-01 and the Donald W. Reynolds Foundation.
Footnotes
Conflicts of interest (Other authors: none reported)
Marc Dewey:
Research grants: Amersham Buchler (now: GE Healthcare Biosciences), Bracco-Altana, and Toshiba Medical Systems.
Speakers Bureau: Toshiba Medical Systems and Schering (now: Bayer).
Workshops: www.herz-kurs.de
Narinder Paul:
Research grants: Toshiba Medical Systems.
John Hoe:
Research grants: Toshiba Medical Systems.
Speakers Bureau: Toshiba Medical Systems and GE Healthcare Biosciences.
Workshops: regional training centre for cardiac CT for Toshiba users.
Pedro Lemos:
Consulting fee/Advisory Board: Scietch, Boston Scientific.
Honoraria for lectures: Boston Scientific, Biotronik, Cordis.
Albert Lardo:
Research grants: Toshiba Medical Systems, Medrad.
Speakers Bureau: Toshiba Medical Systems.
Consulting fee: Medrad.
Julie M. Miller:
Research grants: Dr. Miller was primarily funded by the Doris Duke Foundation during the entire study but is also funded in part by a grant from Toshiba Medical Systems and NHLBI.
Narinder Paul:
Research grants: Toshiba Medical Systems.
Speakers Bureau: Toshiba Medical Systems.
Advisory Board: Vital Images, Inc.
David Bush:
Research grants: Toshiba Medical Systems.
Speakers Bureau: Toshiba Medical Systems, Bristol-Myers Squibb and Sanofi-Adventis.
João A. C. Lima:
Research grants: Principal Investigator of the grant from Toshiba Medical Systems that funded all Core64 activities based at the Johns Hopkins Hospital.
Speakers Bureau: Toshiba Medical Systems, Siemens Medical Systems, GE Medical Systems as well as Bracco Inc., Astellas Inc. and Abbott Laboratories.
Contributor Information
Julie M. Miller, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA, jmmiller@jhmi.edu, Tel.: +1-410-6148970, Fax: +1-410-6149990
Marc Dewey, Department of Radiology, Charité -Universitätsmedizin Berlin, Medical School, Humboldt-Universität und Freie Universität zu Berlin, Charitéplatz 1, 10117 Berlin, PO Box 10098, Germany, marc.dewey@charite.de, Tel.: +49-30-450527296, Fax: +49-30-450527996.
Andrea L. Vavere, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA
Carlos E. Rochitte, Heart Institute (InCor), University of São Paulo Medical School, São Paulo, Brazil
Hiroyuki Niinuma, Department of Cardiology, Iwate, Medical University, Morioka, Japan.
Armin Arbab-Zadeh, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA.
Narinder Paul, Department of Medical Imaging, Toronto General Hospital, Toronto, Canada.
John Hoe, Medi-Rad Associates Ltd, CT Centre, Mt Elizabeth Hospital, Singapore, Singapore.
Albert de Roos, Department of Radiology, Leiden, University Medical Center, Leiden, The Netherlands.
Kunihiro Yoshioka, Department of Radiology, Iwate, Medical University, Morioka, Japan.
Pedro A. Lemos, Heart Institute (InCor), University of São Paulo Medical School, São Paulo, Brazil
David E. Bush, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA
Albert C. Lardo, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA
John Texter, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA.
Jeffery Brinker, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA.
Christopher Cox, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Melvin E. Clouse, Department of Radiology, Beth Israel Deaconess, Harvard University, Boston, MA, USA
João A. C. Lima, Department of Medicine, Division of Cardiology, Johns Hopkins Hospital, Johns Hopkins University, 600 N. Wolfe Street, Blalock 536, Baltimore, MD, USA
References
- 1.Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast sub-millimeter multislice spiral computed tomography. Circulation. 2002;106:2051–2054. doi: 10.1161/01.cir.0000037222.58317.3d. [DOI] [PubMed] [Google Scholar]
- 2.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation. 2003;107:664–666. doi: 10.1161/01.cir.0000055738.31551.a9. [DOI] [PubMed] [Google Scholar]
- 3.Dewey M, Teige F, Schnapauff D, et al. Noninvasive detection of coronary artery stenoses with multislice computed tomography or magnetic resonance imaging. Ann Intern Med. 2006;145:407–415. doi: 10.7326/0003-4819-145-6-200609190-00004. [DOI] [PubMed] [Google Scholar]
- 4.Mollet NR, Cademartiri F, Nieman K, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–2270. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann MH, Shi H, Schmitz BL, et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005;293:2471–2478. doi: 10.1001/jama.293.20.2471. [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro MA, Miller JM, Schmidt A, et al. Non-invasive half millimetre-32 detector row computed tomography angiography accurately excludes significant stenoses in patients with advanced coronary artery disease and high calcium scores. Heart. 2006;92:589–597. doi: 10.1136/hrt.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leschka S, Alkadhi H, Plass A, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–1487. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 8.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 9.Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–557. doi: 10.1016/j.jacc.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 10.Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of noninvasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–582. doi: 10.1007/s00330-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 11.Schuijf JD, Pundziute G, Jukema JW, et al. Diagnostic accuracy of 64-slice multislice computed tomography in the noninvasive evaluation of significant coronary artery disease. Am J Cardiol. 2006;98:145–148. doi: 10.1016/j.amjcard.2006.01.092. [DOI] [PubMed] [Google Scholar]
- 12.Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64- × 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97:343–348. doi: 10.1016/j.amjcard.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Leber AW, Johnson T, Becker A, et al. Diagnostic accuracy of dual-source multi-slice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J. 2007;28:2354–2360. doi: 10.1093/eurheartj/ehm294. [DOI] [PubMed] [Google Scholar]
- 14.Weustink AC, Meijboom WB, Mollet NR, et al. Reliable high-speed coronary computed tomography in symptomatic patients. J Am Coll Cardiol. 2007;50:786–794. doi: 10.1016/j.jacc.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 15.Herzog C, Zwerner PL, Doll JR, et al. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology. 2007;244:112–120. doi: 10.1148/radiol.2441060332. [DOI] [PubMed] [Google Scholar]
- 16.Schuijf JD, Bax JJ, Shaw LJ, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J. 2006;151:404–411. doi: 10.1016/j.ahj.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Greenland P. Who is a candidate for noninvasive coronary angiography? Ann Intern Med. 2006;145:466–467. doi: 10.7326/0003-4819-145-6-200609190-00012. [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JE, Boxt LM, Desjardins B, Fishman EK, Larson PA, Schoepf J. ACR practice guideline for the performance and interpretation of cardiac computed tomography (CT) J Am Coll Radiol. 2006;3:677–685. doi: 10.1016/j.jacr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Garcia MJ, Lessick J, Hoffmann MH. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 21.Dewey M, Hoffmann H, Hamm B. CT Coronary Angiography Using 16 and 64 Simultaneous Detector Rows: Intraindividual Comparison. Fortschr Röntgenstr. 2007;179:581–586. doi: 10.1055/s-2007-963112. [DOI] [PubMed] [Google Scholar]
- 22.Hamon M, Biondi-Zoccai GG, Malagutti P, Agostoni P, Morello R, Valgimigli M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48:1896–1910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Hamon M, Morello R, Riddell JW. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography-meta-analysis. Radiology. 2007;245:720–731. doi: 10.1148/radiol.2453061899. [DOI] [PubMed] [Google Scholar]
- 24.Obuchowski NA, McClish DK. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med. 1997;16:1529–1542. doi: 10.1002/(sici)1097-0258(19970715)16:13<1529::aid-sim565>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 27.Dewey M, Hoffmann H, Hamm B. Multislice CT coronary angiography: effect of sublingual nitroglycerin on the diameter of coronary arteries. Fortschr Röntgenstr. 2006;178:600–604. doi: 10.1055/s-2006-926755. [DOI] [PubMed] [Google Scholar]
- 28.Dewey M, Laule M, Krug L, et al. Multisegment and halfscan reconstruction of 16-slice computed tomography for detection of coronary artery stenoses. Invest Radiol. 2004;39:223–229. doi: 10.1097/01.rli.0000115201.27096.6e. [DOI] [PubMed] [Google Scholar]
- 29.Leschka S, Husmann L, Desbiolles LM, et al. Optimal image reconstruction intervals for non-invasive coronary angiography with 64-slice CT. Eur Radiol. 2006;16:1964–1972. doi: 10.1007/s00330-006-0262-x. [DOI] [PubMed] [Google Scholar]
- 30.Dewey M, Teige F, Rutsch W, Schink T, Hamm B. CT coronary angiography: Influence of different cardiac reconstruction intervals on image quality and diagnostic accuracy. Eur J Radiol. 2008;67(1):92–99. doi: 10.1016/j.ejrad.2007.07.012. Epub 2007 Sep 4. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann MH, Lessick J, Manzke R, et al. Automatic determination of minimal cardiac motion phases for computed tomography imaging: initial experience. Eur Radiol. 2006;16:365–373. doi: 10.1007/s00330-005-2849-z. [DOI] [PubMed] [Google Scholar]
- 32.Dewey M, Müller M, Teige F, et al. Multisegment and halfscan reconstruction of 16-slice computed tomography for assessment of regional and global left ventricular myocardial function. Invest Radiol. 2006;41:400–409. doi: 10.1097/01.rli.0000201233.42994.9b. [DOI] [PubMed] [Google Scholar]
- 33.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 34.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 35.Dewey M, Schnapauff D, Laule M, et al. Multislice CT coronary angiography: evaluation of an automatic vessel detection tool. Fortschr Röntgenstr. 2004:478–483. doi: 10.1055/s-2004-812991. [DOI] [PubMed] [Google Scholar]
- 36.Ringqvist I, Fisher LD, Mock M, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Invest. 1983;71:1854–1866. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderman E, Stadius M. The angiographie definitions of the Bypass Angioplasty Revascularization Investigation. Cor Art Dis. 1992;3:1189–1208. [Google Scholar]
- 38.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 39.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 40.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 41.Arnett EN, Isner JM, Redwood DR, et al. Coronary artery narrowing in coronary heart disease: comparison of cineangiographic and necropsy findings. Ann Intern Med. 1979;91:350–356. doi: 10.7326/0003-4819-91-3-350. [DOI] [PubMed] [Google Scholar]
- 42.Fleming RM, Kirkeeide RL, Smalling RW, Gould KL. Patterns in visual interpretation of coronary arteriograms as detected by quantitative coronary arteriography. J Am Coll Cardiol. 1991;18:945–951. doi: 10.1016/0735-1097(91)90752-u. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg RK, Kleiman NS, Minor ST, Abukhalil J, Raizner AE. Comparison of quantitative coronary angiography to visual estimates of lesion severity pre and post PTCA. Am Heart J. 1990;119:178–184. doi: 10.1016/s0002-8703(05)80098-5. [DOI] [PubMed] [Google Scholar]
- 44.Orford JL, Denktas AE, Williams BA, et al. Routine intravascular ultrasound scanning guidance of coronary stenting is not associated with improved clinical outcomes. Am Heart J. 2004;148:501–506. doi: 10.1016/j.ahj.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 45.Dewey M, Zimmermann E, Laule M, Rutsch W, Hamm B. Three-vessel coronary artery disease examined with 320-slice computed tomography coronary angiography. Eur Heart J. 2008;29(13):1669. doi: 10.1093/eurheartj/ehm626. Epub 2008 Feb 7. [DOI] [PubMed] [Google Scholar]
- 46.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 47.Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 48.Achenbach S, Ropers D, Kuettner A, et al. Contrast-enhanced coronary artery visualization by dual-source computed tomography-initial experience. Eur J Radiol. 2006;57:331–335. doi: 10.1016/j.ejrad.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography Coronary angiography. J Am Coll Cardiol. 2007;50:2393–2398. doi: 10.1016/j.jacc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Kovacs A, Probst C, Sommer T, et al. CT Coronary angiography in patients with Atrial Fibrillation. Fortschr Röntgenstr. 2005;177:1655–1662. doi: 10.1055/s-2005-858548. [DOI] [PubMed] [Google Scholar]
- 51.Dewey M, Kovacs A. CT Coronary Angiography in patients with Atrial Fibrillation. Fortschr Röntgenstr. 2006;178:721. author reply 721–722. [PubMed] [Google Scholar]
- 52.Hoffmann MH, Shi H, Manzke R, et al. Noninvasive coronary angiography with 16-detector row CT: effect of heart rate. Radiology. 2005;234:86–97. doi: 10.1148/radiol.2341031408. [DOI] [PubMed] [Google Scholar]
- 53.Greuter MJ, Dorgelo J, Tukker WG, Oudkerk M. Study on motion artifacts in coronary arteries with an anthropomorphic moving heart phantom on an ECG-gated multidetector computed tomography unit. Eur Radiol. 2005;15:995–1007. doi: 10.1007/s00330-004-2602-z. [DOI] [PubMed] [Google Scholar]
- 54.Greuter MJ, Flohr T, van Ooijen PM, Oudkerk M. A model for temporal resolution of multidetector computed tomography of coronary arteries in relation to rotation time, heart rate and reconstruction algorithm. Eur Radiol. 2007;17(3):784–812. doi: 10.1007/s00330-006-0228-z. Epub 2006 Apr 27. [DOI] [PubMed] [Google Scholar]
- 55.Leschka S, Wildermuth S, Boehm T, et al. Noninvasive coronary angiography with 64-section CT: effect of average heart rate and heart rate variability on image quality. Radiology. 2006;241:378–385. doi: 10.1148/radiol.2412051384. [DOI] [PubMed] [Google Scholar]
- 56.Dewey M, Teige F, Laule M, Hamm B. Influence of heart rate on diagnostic accuracy and image quality of 16-slice CT coronary angiography: comparison of multisegment and halfscan reconstruction approaches. Eur Radiol. 2007;17:2829–2837. doi: 10.1007/s00330-007-0685-z. [DOI] [PubMed] [Google Scholar]
- 57.Schuijf JD, Bax JJ, Jukema JW, et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol. 2004;94:427–430. doi: 10.1016/j.amjcard.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 58.Gaspar T, Halon DA, Lewis BS, et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol. 2005;46:1573–1579. doi: 10.1016/j.jacc.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 59.Gilard M, Cornily JC, Pennec PY, et al. Assessment of coronary artery stents by 16 slice computed tomography. Heart. 2006;92:58–61. doi: 10.1136/hrt.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rixe J, Achenbach S, Ropers D, et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J. 2006;27:2567–2572. doi: 10.1093/eurheartj/ehl303. [DOI] [PubMed] [Google Scholar]
- 61.He S, Dai R, Chen Y, Bai H. Optimal electrocardiographically triggered phase for reducing motion artifact at electron-beam CT in the coronary artery. Acad Radiol. 2001;8:48–56. doi: 10.1016/S1076-6332(03)80743-2. [DOI] [PubMed] [Google Scholar]
- 62.Lu B, Mao SS, Zhuang N, et al. Coronary artery motion during the cardiac cycle and optimal ECG triggering for coronary artery imaging. Invest Radiol. 2001;36:250–256. doi: 10.1097/00004424-200105000-00002. [DOI] [PubMed] [Google Scholar]