Abstract
Rationale
Behavior occurring during cocaine self-administration can be classified as either consummatory or appetitive. These two concepts are usually addressed independently using separate reinforcement schedules. For example, appetitive behavior can be assessed with a progressive ratio schedule, whereas consummatory behavior is typically measured using a fixed ratio schedule.
Objectives
Depending on the schedule used, it is often difficult to determine whether a particular drug pretreatment is affecting self-administration through an effect on appetitive responding, consummatory responding, or perhaps both. In the present study, we tested the effect of pretreating rats with four different drugs on appetitive and consummatory behaviors.
Materials and methods
We recently developed a technique that provides an independent assessment of both behavioral concepts within the same experimental session. In this threshold procedure, rats are offered a descending series of 11 unit doses (422–1.3 μg/injection) during consecutive timed intervals under a fixed-ratio schedule. Consummatory behavior can be analyzed by assessing intake at high unit doses; an estimate of appetitive responding can be determined from responding occurring at the threshold dose. Applying behavioral economics to these data provides dependent measures of consumption when minimally constrained by price and the maximal price paid (Pmax) for cocaine.
Results
Haloperidol increased cocaine consumption when minimally constrained by price but decreased Pmax. In contrast, d-amphetamine increased Pmax. Fluoxetine decreased Pmax and consumption when minimally constrained by price. Baclofen selectively decreased Pmax.
Conclusions
These data suggest that drug pretreatments can alter consummatory and appetitive behavior differently because each concept involves distinct neural mechanisms.
Keywords: Cocaine, Self-administration, Behavioral economics, Dopamine, Serotonin, GABA consummatory, Appetitive, Motivation, Reinforcing strength
Introduction
The ethological descriptors “appetitive” and “consummatory” (Craig 1918) have been used to distinguish specific classes of behavior associated with biologically important acts such as eating (Foltin 2005) and mating (Balthazart et al. 1998). The appetitive/consummator distinction has been helpful in characterizing the separate neural systems involved (Gan et al. 2010; Nicola and Deadwyler 2000; Pettit and Justice 1989). Consummatory behaviors are directly related to ingestion or sexual consummation and are viewed as somewhat reflexive or regulatory. In contrast, appetitive behaviors involve exploration of the environment and gaining access to the desired commodity. Appetitive behaviors are therefore considered to be more complex and influenced by learning and motivational processes (Godsil et al. 2003).
In the context of the drug abuse literature, appetitive and consummator behaviors have been used synonymously with “drug seeking” and “drug taking” (Cowen et al. 2007; Czachowski and Samson 1999; Foltin 2001; Sharpe and Samson 2001). The assumption is that the mechanisms that serve to regulate drug taking may be somewhat different from those that motivate drug seeking.
In order to study the neural bases of appetitive and consummator aspects of cocaine self-administration, it becomes important that animal models provide ways to study the two separately and in combination. This has been accomplished to a large extent with different schedules of reinforcement. Cocaine self-administration on a fixed ratio (FR) schedule offers a way to investigate the factors that regulate drug consumption. Under low price constraints, it has been suggested that the rate of cocaine consumption reflects a titration of cocaine concentrations in brain within a preferred range (Ahmed and Koob 1999; Lynch and Carroll 2001; Oleson et al. 2008). In contrast, studies designed to address appetitive influences have manipulated ratio requirements (e.g., progressive ratio, PR) in order to assess the cost an animal might pay to obtain drug. The observation that some drug pretreatments produce very different effects on cocaine self-administration depending on whether an FR1 or PR schedule is used suggests that cocaine taking and cocaine seeking can be influenced differentially. For example, both the GABAB agonist baclofen and the hypocretin/orexin 1 receptor antagonist SB334867 significantly reduce breakpoints on a PR schedule (Borgland et al. 2009; Brebner et al. 2000; Espana et al. 2010); however, neither of these drugs appeared to affect responding on an FR1 schedule (Brebner et al. 2000; Espana et al. 2010; Smith et al. 2009), suggesting that the appetitive but not the consummatory responses are affected by these pretreatments.
Unfortunately, caution must be exercised in interpreting data obtained under different experimental conditions. For example, it is well accepted that many variables—including environmental context (Caprioli et al. 2007), rat strain (Kosten et al. 1997), the experimenter-imposed inter-injection interval (Martelle et al. 2008), and the pharmacological history of the animal (Orio et al. 2009)—can influence cocaine self-administration. A method allowing for the testing of a drug pretreatment on both appetitive and consummatory responding in the same subject during the same experimental session would obviate some of these concerns.
We have recently developed a procedure that provides an independent assessment of both appetitive and consummatory aspects of cocaine self-administration across multiple self-administration sessions (Oleson and Roberts 2009). The technique is an adaptation of a threshold procedure (see Zittel-Lazarini et al. 2007). Rats are offered a descending series of 11 unit doses (422–1.3 μg/injection) on an FR1 schedule of reinforcement during consecutive daily 2-h sessions. Regulated consummatory behavior can be observed as the available unit injection dose decreases until a minimally reinforcing dose (i.e., threshold dose) is reached. Behavioral economics provides a useful framework for data analysis. A measure of consummatory behavior can be determined at high unit doses when response cost is low and a metric of appetitive behavior (maximal price paid, Pmax) can be determined by assessing the highest unit price that maintains consumption. This between-sessions approach has been adapted in the present series of studies; instead of offering rats a descending series of cocaine doses across sessions, all doses were presented consecutively within a single session in timed intervals. Thus, the effect of a drug pretreatment can be evaluated on the entire demand curve generated in a single daily session.
The sensitivity of this procedure to detect differential effects on appetitive and consummatory responding was tested by evaluating the effects of pretreatments with haloperidol, amphetamine, fluoxetine, or baclofen. These four drugs have been extensively characterized using schedules related to either consummator (e.g., FR) or appetitive (e.g., PR) behavior (Barrett et al. 2004; Borgland et al. 2009; Brebner et al. 2000; Campbell et al. 1999; Espana et al. 2010; Richardson and Roberts 1991; Richardson et al. 1994; Roberts et al. 1989; Smith et al. 2009) and are therefore useful for the validation of the current procedure.
Materials and methods
Animals, surgery, and housing
Male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing approximately 350 g at the time of surgery were used. All experiments were approved by the Wake Forest University Institutional Animal Care and Use Committee. Before entering the study, rats were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg) and implanted with chronically indwelling cannulae (CamCaths, Cambridgshire, UK) as previously described (Roberts et al. 1989). Briefly, a 2.5-cm length of cannula tubing was implanted into the right jugular vein while exiting dorsally through the skin in the region of the scapulae.
Upon recovery, animals were housed individually in 30× 30×30-cm experimental chambers. Each cannula was connected to tygon tubing enclosed within a stainless steel tether, which was then connected to a counterbalanced fluid swivel (Instech Laboratories Inc., Plymouth Meeting, PA, USA) mounted above the experimental chamber. An infusion pump (Razel Scientific Instruments Inc., Stamford, CT, USA) was connected to the opposite side of the fluid swivel using tygon tubing. Each cannula was flushed daily with heparinized saline to help maintain patency, although a cannula-related attrition rate resulted in the inclusion of data from 37 rats. Food and water were available ad libitum. Experimental chambers were housed in a temperature-controlled room (20–21°C) maintained on a 12-h light/dark cycle (lights on at 1500 hours).
General self-administration methods and acquisition
Animals were given 3–5 days to recover from surgery before entering the study. Each session began with the extension of a lever into the experimental chamber which, when depressed, initiated an intravenous injection of cocaine. Sessions occurred 7 days/week and began in the middle of the dark cycle (1000 hours). The beginning of each session was signaled by the extension of the lever into the experimental chamber.
During acquisition, all animals were given daily access to cocaine (0.75 mg/kg) under an FR1 schedule of reinforcement. Sessions were terminated after a maximum of 20 infusions or a period of 24 h had occurred. An animal was considered to have acquired if 20 injections were self-administered beginning at the onset of an experimental session and a stable pattern of post-infusion pauses between injections was apparent.
Threshold procedure
Following acquisition, animals were given access to cocaine in the within-session threshold procedure. Pump times were decreased across 11 intervals according to a quarter-log scale as follows: 3,156, 1,780, 1,000, 562, 310, 178, 100, 56, 31, 18, and 10 ms. A timeout period corresponded only to the duration of the pump infusion. The calculated dose equivalents (5 mg/ml×1.6 ml/min × pump duration) are as follows: 421, 237, 133, 75, 41, 24, 13, 7.5, 4.1, 2.4, and 1.3 μg/ infusion. See supplemental material of Oleson and Roberts (2009) for a full characterization of this approach and validation that the appropriate quantity of drug is delivered across all pump durations. The duration of the pump infusion was the only experimenter-imposed inter-injection period. The available cocaine dose decreased every 10 min for all drug pretreatments except d-amphetamine, for which the available dose decreased every 5 min. The threshold, or minimally reinforcing dose, was defined as the final dose presented that maintained stable responding (Zittel-Lazarini et al. 2007). Drug pretreatment times and doses for all drugs were chosen based on preliminary data. All injections, including vehicle, were administered intraperitoneally and assigned using a Latin square design with a minimum of 3 days between treatments. Pretreatment times were 15 min for d-amphetamine and 30 min for haloperidol, fluoxetine and baclofen.
Behavioral economic analysis
Behavioral economic theory has previously been applied to drug self-administration (Bickel et al. 1990; Hursh 1991). In most studies, the concept of unit price has been manipulated by increasing the response requirement for a set drug dose across daily sessions (Cosgrove and Carroll 2002; Wade-Galuska et al. 2007). However, as the unit price ratio (responses per milligram of drug) might suggest, unit price can be manipulated by fixing the response requirement and decreasing the available unit dose (Bickel et al. 1990). Therefore, by reducing the available unit injection dose throughout each session, as was done in the present study, rats were given access to cocaine across the following 11 ascending unit prices: 2.4, 4.2, 7.5, 13.3, 23.7, 39.9, 75, 133.9, 241.9, 416.7, and 750 responses/mg.
The behavioral economic analysis of data from the within-session threshold procedure provided two main dependent measures, the maximal price paid (Pmax) for cocaine and the average level of cocaine consumption within the low price range (i.e., high dose range). The mean level of consumption within the low price range was derived by averaging cocaine intake across the second through fourth available unit price (Fig. 1c, gray ellipse). The maximal price expended to maintain consumption (Pmax) is defined as the point, or unit price, at which maximal responding occurs (Hursh 1991). Pmax values were graphically determined in the present study, an approach that has been previously validated (Greenwald and Hursh 2006; Oleson and Roberts 2009). Therefore, Pmax was determined to be the unit price corresponding to the apex of the price response function. To verify this data analysis approach, Pmax values and a measure of consumption at minimal price were also derived mathematically using a demand curve. Demand curves were generated by curve fitting individual animal's dose intake data using an equation: log(Q) = log(Q0) + k × (e–α×Q0×C – 1) (Hursh and Silberberg 2008; Christensen et al. 2008). In this equation, the value Q0 represents initial consumption at a minimally constraining price, whereas Pmax was determined to be the unit price at which the first derivative point slope of the function = –1 (Hursh and Winger 1995). The value k, which represents the range of the dependent measure in logarithmic units, was set to 2 for all animals, while Q0 and α, which represent the acceleration of the function in response to changing price, were estimated to achieve best fit (Hursh and Silberberg 2008; Christensen et al. 2008). For all experiments, consumption and Pmax values were reported as a percent change from the preceding 2–3 days. The preceding 3 days were used in all cases unless one of the animal's baseline values was determined to be a statistical outlier.
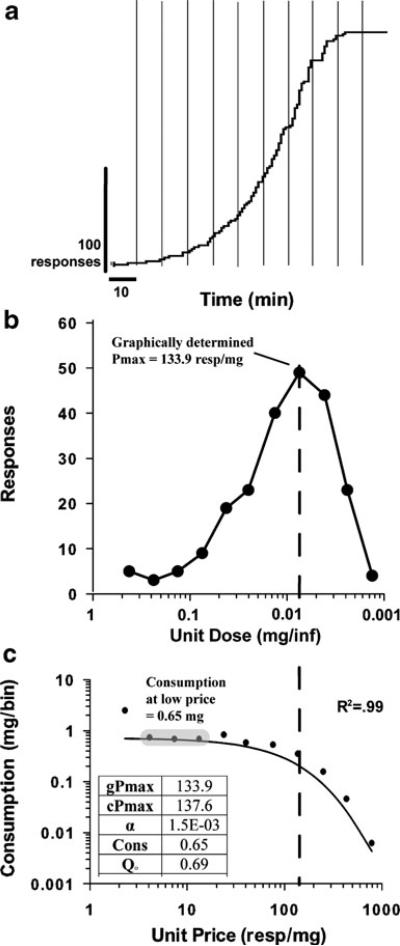
Fig. 1.
Representative within-session threshold data and explanation of behavioral economics measures. a A cumulative record from one animal responding in a single session of the current threshold procedure is shown. Note that the available unit injection dose decreases across 11 consecutive 10-min bins. b The same animal's data are replotted to show the dose–response relationship as a function of unit price. c The same animal's data are replotted to show consumption (intake/10 min bin) as a function of unit price. A demand curve is fitted to the data. The maximal price paid can be graphically distinguished (gPmax) by assessing the apex of the price response function or using an equation (cPmax). Mean cocaine consumption when minimally constrained by price (gray ellipse) can be calculated by averaging intake across bins 2–4 or by using an equation (Q0)
Statistics
All statistics were performed using SigmaPlot (version 11). Pmax and consumption data were analyzed using one-way ANOVA and Holm–Sidak post hoc analysis.
Drugs
Cocaine HCl, obtained from the National Institute on Drug Abuse (Research Triangle Institute, NC, USA), was dissolved in a solution of sterilized saline 0.9% and passed through a microfilter (0.45-μm pore size). A 5 mg/ml cocaine solution was used for threshold experiments; a 2.5 mg/ml solution was used for acquisition. Pre-dissolved injectable haloperidol (Bedford Labs, Bedford, OH, USA), d-amphetamine (Sigma-Aldrich, St. Louis, MO, USA), and a racemic mixture of baclofen (Sigma-Aldrich) were dissolved, or diluted in the case of haloperidol, in sterile saline and injected prior to the beginning of self-administration sessions. Sterile saline was used for vehicle injections for these three drugs. Fluoxetine (Sigma-Aldrich) was dissolved in sterile water and injected 30 min prior to self-administration sessions. Sterile water was used for vehicle injections in fluoxetine-treated rats. All dosages are expressed as the salt.
Results
Figure 1 illustrates a cumulative record, a dose–response function, and a demand curve from one representative animal responding in the within-session threshold procedure. Note that the available unit dose of cocaine decreased every 10 min and this decrease in dose corresponded to an increase in the unit price of cocaine. As shown in the cumulative record (Fig. 1a), responding increased every 10 min as the available cocaine dose decreased until a threshold dose (7.5 μg/injection) was reached. In Fig. 1b, these data are plotted as a dose–response function with doses shown in descending order. Plotting the data in such a manner allows for the relationship between unit dose to unit price to be observed. As would be expected, response rate increased as the available cocaine dose decreased until a threshold dose was reached, after which responding sharply declined. Maximal responding occurred at the threshold dose, which was determined to be the maximal price (Pmax) the animal paid for cocaine. Note that the apex of the dose–response function coincides with the final point at which cocaine intake is maintained in Fig. 1c. These data were converted to cocaine intake per bin and plotted as a function of unit price in Fig. 1c. Cocaine intake was higher in the first 10-min interval (1.26 mg) than at any other dose, which is presumably due to the effect of drug loading. This interval was therefore omitted from the analysis in which consumption within the low price range was assessed. After the first bin, the level of cocaine intake stabilized over the next several doses, and the mean level of consumption within the low price range (0.65 mg) was taken by averaging cocaine intake across intervals 2–4. The solid line shows a demand curve fitted to the price intake data. Using the demand curve, a value of consumption at a minimally constraining price (Q0) and a mathematically calculated Pmax value (cPmax) were calculated. It should be noted that the mathematically derived dependent measures of maximal price paid (cPmax) and consumption at minimal price (Q0) are similar to those determined graphically (gPmax and Cons) for this representative animal.
To further assess the validity of the graphical data analysis approach presented in Fig. 1, additional behavioral economics analyses were performed. The supplemental information contains demand curves showing how mathematically calculated Pmax and Q0 values compare to graphically determined Pmax and consumption values. Here, demand curves were generated for each individual animal from one experimental group at every dose tested. Baseline intake (filled circles) was plotted as the average cocaine intake per bin occurring on the 3 days preceding treatment (open circles). Each animal's graphically determined Pmax (gPmax), mathematically calculated Pmax (cPmax), averaged consumption value at minimal price (Cons), and mathematically derived value of consumption at minimal price (Q0) were reported following saline treatment (Electronic supplementary material (ESM) Fig. 1a, b), 100 μg/kg baclofen (ESM Fig. 2a, b), 180 μg/kg baclofen (ESM Fig. 3a, b), and 310 μg/kg baclofen (ESM Fig. 4a, b). Additional correlations were performed on these data. ESM Fig. 5 shows that graphically determined Pmax values correlate highly with mathematically derived Pmax values (r = 0.99). ESM Fig. 6 shows that the averaged values of cocaine consumption within the low price range correlate with Q0 (r=0.81). Supplemental Fig. 7 illustrates that Pmax (determined graphically) and average cocaine consumption within the low dose range are unrelated values (r=0.15).
The results of haloperidol pretreatment on Pmax and cocaine consumption are shown in Fig. 2. Figure 2a illustrates the effect of haloperidol (56, 100, 178, or 310 μg/kg IP) on Pmax. A one-way ANOVA revealed a significant effect of haloperidol dose on Pmax (F(4,32)=3.75, p=0.015). Holm–Sidak post hoc analysis revealed that doses of 100 (t=2.8, p=0.01), 178 (t=3.0, p=0.05), and 310 (t=3.1, p<0.05)μg/kg significantly decreased Pmax values relative to vehicle. Figure 2b illustrates the effect of haloperidol on cocaine consumption. A one-way ANOVA revealed a significant effect of haloperidol dose on consumption (F(4,32) =4.90, p<0.01). Holm–Sidak post hoc analysis revealed that doses of 56 (t=2.4, p=0.02), 100 (t=3.9, p<0.05), 178 (t=3.2, p<0.05), and 310 (t=3.1, p<0.05)μg/kg significantly increased consumption relative to vehicle.
Fig. 2.
Effects of four doses of haloperidol on Pmax (top) and cocaine consumption of high doses (bottom). a Haloperidol produced a significant decrease in the maximal price (Pmax) paid for cocaine. b In contrast, haloperidol produced a significant increase in cocaine consumption when minimally constrained by price. Data are expressed as mean (±SEM) Pmax or consumption, and asterisks indicate a significant difference between a haloperidol dose to vehicle (p≤0.05)
Figure 3 illustrates the effect of d-amphetamine pretreatment on Pmax and cocaine consumption. Figure 3a shows the effect of amphetamine (0.31, 0.56, and 1.0 mg/kg IP) on Pmax. A one-way ANOVA revealed a significant effect of amphetamine dose on Pmax (F(3,30)=3.07, p=0.045). Holm–Sidak post hoc analysis revealed that 1 mg/kg amphetamine significantly increased Pmax relative to vehicle (t=2.7, p=0.01). Figure 3b shows the effect of amphetamine on consumption. Amphetamine did not significantly change the rate of cocaine intake (F(3,30)=0.88, n.s.).
Fig. 3.
Effects of three doses of d-amphetamine on Pmax (top) and cocaine consumption when minimally constrained by price (bottom). a Amphetamine produced a significant increase Pmax. b However, amphetamine did not produce a significant change in cocaine consumption when minimally constrained by price. Data are expressed as mean (±SEM) Pmax or con sumption, and asterisks indicate a significant difference between amphetamine dose to vehicle (p≤0.05)
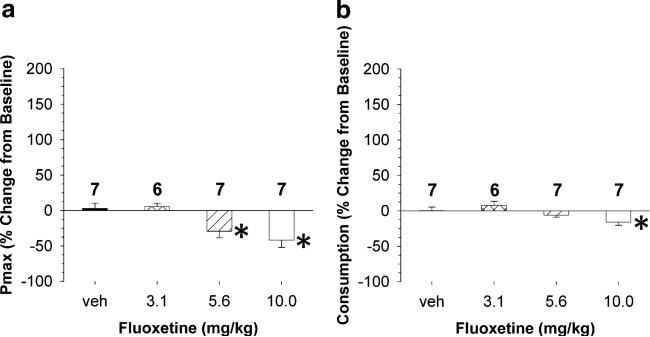
The effect of fluoxetine pretreatment on Pmax and cocaine consumption is shown in Fig. 4. Figure 4a illustrates the effect of fluoxetine (3.1, 5.6, and 10 mg/kg, IP) on Pmax. A one-way ANOVA revealed a significant effect of fluoxetine dose on Pmax (F(3,26)=4.82, p=0.01). Holm–Sidak post hoc analysis revealed that 5.6 mg/kg (t=2.5, p=0.02) and 10 mg/kg (t=3.5, p<0.05) fluoxetine significantly decreased Pmax relative to vehicle. Figure 4b shows the effect of fluoxetine on consumption. A one-way ANOVA revealed a significant effect of fluoxetine dose on consumption (F(3,26)=4.60, p=0.012). Holm–Sidak post hoc analysis revealed that 10 mg/kg fluoxetine significantly decreased cocaine intake relative to vehicle (t=2.8, p<0.011).
Fig. 4.
Effects of three doses of fluoxetine on Pmax (top) and cocaine consumption when minimally constrained by price (bottom). a Fluoxetine produced a significant decrease in Pmax for cocaine. b Fluoxetine also produced a significant decrease in cocaine consumption when minimally constrained by price. Data are expressed as mean (±SEM) Pmax or consumption, and asterisks indicate a significant difference between a fluoxetine dose to vehicle (p≤0.05)
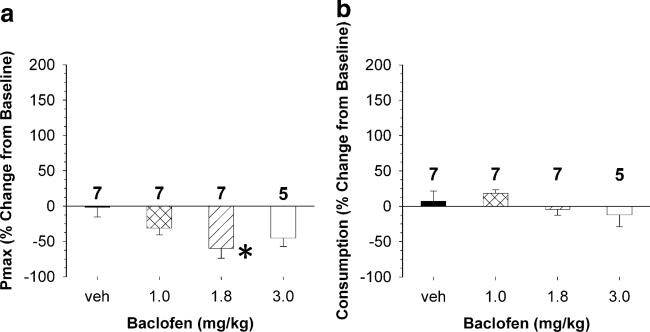
Figure 5 illustrates the effect of baclofen pretreatment on Pmax and consumption. Figure 5a shows the effect of baclofen (1.0, 1.8, and 3.0 mg/kg, IP) on Pmax. A one-way ANOVA revealed a significant effect of baclofen dose on Pmax (F(3,25)=4.08, p=0.019). Holm–Sidak post hoc analysis revealed that 1.8 mg/kg baclofen significantly decreased Pmax relative to vehicle (t=3.4, p<0.05). It should also be noted that despite failing to produce a significant change versus vehicle, the 3.0-mg/kg dose produced a 45% mean decrease in Pmax; however, the power of this dose comparison was limited because two rats did not start following pretreatment. Baclofen did not significantly change cocaine consumption (F(3,25)=1.60, n.s.).
Fig. 5.
Effects of three doses of baclofen on Pmax (top) and cocaine consumption when minimally constrained by price (bottom). a Baclofen produced a significant decrease in the maximal price paid (Pmax) for cocaine. b However, baclofen did not significantly change cocaine consumption when minimally constrained by price. Data are expressed as mean (±SEM) Pmax or consumption, and asterisks indicate a significant difference between a baclofen dose to vehicle (p≤0.05)
Discussion
Here, we describe a novel cocaine self-administration procedure that, within a single test session, allows for the assessment of both consummatory and appetitive responses. Each daily session consisted of 11 timed intervals. The unit dose of cocaine offered during each interval decreases through a log series (421, 237, 133, 75, 41, 24, 13, 7.5, 4.1, 2.4, and 1.3 μg/injection). The early intervals allow for an evaluation of cocaine consumption under relatively unrestrained conditions. The latter part of the session allows for the determination of the threshold dose that supports cocaine self-administration. This within-session technique is an adaptation of a between-sessions threshold procedure previously described (Zittel-Lazarini et al. 2007; Oleson and Roberts 2009).
Figure 1b illustrates that through a broad supra-threshold range, a decrease in unit injection dose produces an increase in the number of injections self-administered within each interval. Below the threshold unit dose, the rate of responding declines precipitously. Behavioral economics offers useful analytical tools to assess these data. Figure 1c illustrates the demand curve generated from the within-session threshold procedure. Note that our manipulation of dose is also a manipulation of price. Unit price is a ratio (responses per milligram of drug) which can be increased either by augmenting the numerator (response requirement) or by decreasing the denominator (reinforcer magnitude; Bickel et al. 1990). Fitting a line to the demand curve generates two useful dependent variables, Pmax (maximum price paid) and Q0 (consumption at a minimally constraining price), that can help in the assessment of appetitive and consummator responding.
The demand curve illustrated in Fig. 1c shows that cocaine consumption is relatively constant at high unit injection doses (i.e., low price). Note that a change in dose has virtually no effect on consumption. This section of the curve is therefore said to be “inelastic.” It has long been recognized that the rate of cocaine intake is inversely related to the available unit dose along the descending limb of the FR dose effect function (Pickens and Thompson 1968; Wilson et al. 1971). This observation has led various investigators to speculate that cocaine intake is titrated around a preferred blood or brain level (Ahmed and Koob 1999; Lynch and Carroll 2001; Oleson et al. 2008), variously referred to as a trigger point (Wise et al. 1995), set point (Ahmed and Koob 1999), or priming threshold (Tsibulsky and Norman 1999). A simple measure of consumption at a minimally constraining price used here is the average of the second through fourth intervals. The first interval is omitted since consumption in the first 10 min is almost always higher, reflecting a loading phase during which blood levels of cocaine escalated to a preferred level (Ahmed and Koob 1999; Lynch and Carroll 2001). Behavioral economic analysis provides Q0, which is derived from the fitted curve. We have found that the two measures of consumption at a minimally constraining price are highly correlated (r=0.81, see ESM), suggesting that the two can be used interchangeably.
Measures of maximal price paid (Pmax) can be determined in two ways. Appetitive responding can be derived from the section of the demand curve which is sensitive to price. Figure 1b illustrates how a Pmax value can be determined directly from the graph as the unit injection dose that supports the highest rate of responding. Alternatively, curve fitting can also be used to mathematically derive a Pmax value based on the change in slope on the descending limb of the demand curve. The graphical and mathematical methods of determining Pmax are almost perfectly correlated (r=0.99, see ESM). We find that the graphical method provides an intuitive and precisely defined measure. It should be noted, however, that the modeling method is particularly valuable if the demand curve has fewer data points than reported here (see ESM for additional information and comparison of analytical methods).
The utility of the within-session threshold procedure to detect changes in cocaine consumption and appetitive responding was investigated using four drug pretreatments from different pharmacological classes. The present results suggest that consummatory and appetitive behaviors are differentially affected by the four drug classes. The most robust effects were found following pretreatment with haloperidol, a dopamine D2 receptor antagonist. Haloperidol produced a dose-dependent increase in cocaine consumption at a minimally constraining price and a decrease in maximal price paid. Measures of consumption within the inelastic part of the demand curve are comparable to measures of drug intake in traditional FR studies. It has been repeatedly confirmed that dopamine antagonists increase the rate of cocaine intake on an FR schedule (Barrett et al. 2004; De Wit and Wise 1977; Richardson et al. 1994; Roberts et al. 1977), an effect that is theorized to be a compensatory response to pharmacological competition at post-synaptic dopamine receptors (De Wit and Wise 1977; Yokel and Wise 1975). The present finding showing a haloperidol-induced decrease in Pmax is also consistent with reports of haloperidol-induced decreases in break point on a PR schedule (Depoortere et al. 1993; Roberts et al. 1989). It would appear that blockade of D2 receptors has an opposite effect on cocaine consumption versus appetitive responding.
The effect of amphetamine pretreatment was generally the converse of the haloperidol effect. Amphetamine increased Pmax, suggesting an augmentation in the reinforcing effects of cocaine. It should be noted that although an apparent decrease in consummatory behavior was observed, this decrease did not reach statistical significance. It is likely that using 5-min bins decreased our sensitivity to detect changes in consummatory behavior. However, based on pilot studies, shorter bins were necessary to detect changes in Pmax following amphetamine pretreatment. These data are consistent with previous reports showing that amphetamine decreases the rate of cocaine intake on an FR1 schedule of reinforcement (Barrett et al. 2004) and increases the price paid for cocaine on a progressive ratio schedule (Läck et al. 2006).
In the present study, we found that the selective serotonin uptake inhibitor fluoxetine decreased both cocaine consumption when minimally constrained by price and the maximal price paid for cocaine. This represents an important example wherein the effect on consumption and Pmax are not inversely related but in fact positively correlated. These findings are in agreement with previous self-administration studies in which brain serotonin levels were manipulated using serotonin uptake inhibitors, dietary tryptophan and lesions. For example, fluoxetine decreases final ratios for cocaine on a PR schedule (Richardson and Roberts 1991) and rate of cocaine intake on an FR schedule (Carroll et al. 1990a). When brain serotonin levels are increased by dietary tryptophan, decreases in cocaine maintained responding under both a PR schedule (McGregor et al. 1993) and an FR schedule (Carroll et al. 1990b) are observed. Importantly, dietary tryptophan decreases final ratios for cocaine on a PR schedule without decreasing final ratios for food reinforcement (McGregor et al. 1993), suggesting that the serotonin-induced decrease in the maximal price paid for cocaine is not due to a generalized reduction in response rate. The pharmacological evidence that serotonin produces an inhibitory effect on cocaine reinforcement is further supported by lesion studies, which show that infusing the neurotoxin 5,7-dihydroxytryptamine into the intracerebroventricular space (Roberts et al. 1994), amygdala, or medial forebrain bundle (Loh and Roberts 1990) increases final ratios on a PR schedule of reinforcement. The finding that animals work harder for cocaine following depletion of brain serotonin levels supports the assertion that serotonin systems produce aversive effects during cocaine self-administration (Roberts et al. 1994) and therefore antagonize the reinforcing effects of cocaine.
The GABAB agonist baclofen modulates cocaine reinforcement by selectively decreasing appetitive responding for cocaine (Roberts et al. 1996; Brebner et al. 2000). Here, we found that baclofen reduced the maximal price paid for cocaine without affecting cocaine consumption when minimally constrained by price. These findings are reminiscent of an explanation of baclofen's effectiveness offered by Carroll and colleagues (Campbell et al. 1999). These authors proposed that drugs such as baclofen are most effective at suppressing cocaine self-administration at high unit prices—regardless of whether unit price is manipulated by increasing the response requirement or by decreasing the available unit dose. This explanation is supported by several additional self-administration studies using PR and FR schedules. When high unit injection doses (1.5 mg/kg per injection) are available under an FR schedule, baclofen does not affect the rate of cocaine self-administration (Brebner et al. 2000; Roberts et al. 1996). In contrast, the rate of cocaine intake is significantly decreased by baclofen (Campbell et al. 1999; Shoaib et al. 1998) when rats are given access to low unit injection doses (0.2–0.66 mg/kg per injection) under an FR schedule, an effect that is potentially explained by the unit price requirement imposed on animals to titrate cocaine intake at these low unit injection doses (Campbell et al. 1999). Furthermore, cocaine self-administration is reduced across a wide range of doses (0.18–1.5 mg/kg per injection; Roberts et al. 1996; Brebner et al. 2000) when rats are required to meet high response requirements for cocaine under a PR schedule. While some evidence suggests that baclofen can produce sedation and suppress locomotor activity (Paredes and Agmo 1989), baclofen only produces marginal effects on food-maintained responding under both FR (Shoaib et al. 1998) and PR schedules (Roberts et al. 1996) at doses that reduce cocaine self-administration (Roberts et al. 1996), which suggests that the reduction in the behavioral price paid for cocaine is not due to a generalized suppression of responding.
Advantages, pitfalls, and caveats
The within-session threshold procedure is an improvement over a technical approach presented in a previous study (Oleson and Roberts 2009) in which we performed behavioral economics analyses on data obtained using a threshold procedure occurring across 11 daily FR sessions. Providing access to cocaine at multiple unit prices within a single session allows for the investigation of drug pretreatments on both consumption and maximal price paid simultaneously. Furthermore, this approach allows the experimenter to vary the exact timing that each unit price is available to accommodate for the pharmacokinetics and pharmacodynamics of the specific drug pretreatment. An additional advantage of the current approach is that cocaine consumption is assessed across many different independent measures of price. In this case, a clear representation of the maximal behavioral price the animal pays to maintain cocaine intake can be observed directly. Although many useful equations have been developed over the years that provide information about maximal price paid when few price assessments are made (Bickel et al. 1990; Hursh 1991; Hursh and Silberberg 2008), the present approach allows for a direct analysis of the maximal price paid for cocaine that has previously been verified using similar equations (Greenwald and Hursh 2006; Oleson and Roberts 2009).
Despite the many positives, several limitations are associated with the current technique. For example, providing access to cocaine at unit prices that ascend throughout each session in fixed intervals requires that all animals begin to self-administer at the start of the session. In the present study, this issue resulted in the exclusion of data from several animals that failed to start following high-dose pretreatments of either baclofen or haloperidol. A solution to this problem would be to provide access to cocaine at the first presented unit price until stable responding occurs, although this would result in rats receiving different pretreatment times depending on when responding commences. Another major issue is that each animal's threshold (i.e., Pmax) can vary over days; therefore, establishing stable baselines must be done immediately prior to drug pretreatments. A further disadvantage of the current approach is that it might be limited to self-administration studies in which animals are given access to drugs that produce response rates that are inversely related to drug dose, but maintain high rates of responding. For example, the current approach may not be useful for studying consummatory and appetitive aspects of feeding behavior.
Behavioral economic theory regards two unit prices as equivalent if the ratios are the same. For example, a 1.0 mg/kg per injection on an FR 100 would be seen as equivalent to 0.1 mg/kg per injection on an FR10. However, unit prices may not be neurochemically and pharmacologically equivalent. For example, Gan et al. (2010) reported that dopamine signaling may encode the individual variables that define unit price (i.e., response cost vs. reinforcer magnitude) separately. Specifically, it was shown that phasic dopamine encodes information about the magnitude of reinforcement rather than the required response cost as animals gain experience about the presented cost–benefit relationship (Gan et al. 2010). This latter finding suggests that behavioral responses to changes in unit price may vary depending on whether the response requirement is increased or reinforcer magnitude is decreased, a subject that has been behaviorally addressed but not completely resolved (cf. Nader et al. 1993; Woolverton and English 1997). If the components of unit price are indeed neurochemically and behaviorally dissociable, the implication would be that final ratios and Pmax values determined by increasing response requirements are distinct from Pmax values determined by decreasing the available unit injection dose.
Finally, it should be emphasized that procedural differences between the within-session to the between-session threshold approaches may affect Pmax values in a theoretically important manner. In the present study, doses were presented in a descending sequence within a single session, whereas in our previous work (Oleson and Roberts 2009), doses were presented in a descending order across daily sessions. Such differences necessarily result in different cocaine levels on board at the time point when Pmax is determined. Animals tested in the within-session procedure are given access at the beginning of the session to relatively large unit doses of cocaine on an FR1 schedule. This virtually guarantees that subjects establish high blood levels of cocaine within the first 10 min. By increasing the cost (i.e., lowering the dose), the procedure is essentially measuring the price animals might pay in order to maintain an established level of cocaine consumption; that is to say, Pmax values derived from the within-session procedure reflect the motivation to continue a drug binge. In contrast, there is no guarantee that an animal will be able to “load up” on cocaine in the between-sessions procedure. As the dose of cocaine is decreased across days and the threshold is approached, the high cost of cocaine prevents the animal from ‘loading-up’. This may explain why Pmax values observed using the current within-session approach are higher than those previously reported with the between-sessions approach (Oleson and Roberts 2009). These data support the idea that high cocaine levels serve to sustain a binge.
Conclusion
Consumption of cocaine when minimally constrained by price and appetitive responding for cocaine are dissociable concepts, both of which are regulated by multiple neurotransmitter systems. As expected, dopamine antagonists were found to increase cocaine consumption but decrease appetitive responding. Consummatory and appetitive behaviors are not always inversely related, however. For example, serotonin appears to antagonize the reinforcing effects of cocaine by decreasing both the regulation of consumption and the behavioral price paid for cocaine, whereas other pharmacological targets such as GABAB receptors and hypocretin/orexin 1A receptors appear to affect these processes independently. These findings suggest that drug pretreatments can alter consummatory and appetitive behaviors differently because each process probably involves distinct neural mechanisms; therefore, more encompassing screening tools should be used to assess the effectiveness of potential pharmacotherapies for drug addiction. Furthermore, caution should be used in drawing encompassing conclusions about drug treatments from data obtained using a single schedule of reinforcement.
Supplementary Material
Acknowledgments
Funding for this study was provided by grants R01DA14030 (DCSR) and F31DA024525 (EBO). We would like to thank Leanne N. Thomas for technical assistance and Benjamin Zimmer for helpful comments in the preparation of this manuscript. We would especially like to thank Dr. Steve Hursh for providing spreadsheets and mathematical equations that aided in the mathematical behavioral economic analyses.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-010-2058-6) contains supplementary material, which is available to authorized users.
Contributor Information
Erik B. Oleson, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA Department of Neurobiology and Anatomy, University of Maryland School of Medicine, 20 Penn St., Baltimore, MD 21201, USA eboleson@gmail.com.
Jasmine M. Richardson, Winston-Salem State University, Winston-Salem, NC 27110, USA
David C. S. Roberts, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA
References
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacol Berl. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacol Berl. 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacol Berl. 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Paolone G, Celentano M, Testa A, Nencini P, Badiani A. Environmental modulation of cocaine self-administration in the rat. Psychopharmacol Berl. 2007;192:397–406. doi: 10.1007/s00213-007-0717-z. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1990a;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Intravenous cocaine self-administration in rats is reduced by dietary l-tryptophan. Psychopharmacology. 1990b;100:293–300. doi: 10.1007/BF02244596. [DOI] [PubMed] [Google Scholar]
- Christensen C, Silberberg A, Hursh S, Huntsberry M, Riley A. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology. 2008;198:221–229. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J Pharmacol Exp Ther. 2002;301:993–1002. doi: 10.1124/jpet.301.3.993. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacol Berl. 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcohol Clin Exp Res. 1999;23:1580–1586. [PubMed] [Google Scholar]
- De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW. Effects of amphetamine, dexfenfluramine, diazepam, and other pharmacological and dietary manipulations on food “seeking” and “taking” behavior in non-human primates. Psychopharmacol Berl. 2001;158:28–38. doi: 10.1007/s002130100865. [DOI] [PubMed] [Google Scholar]
- Foltin RW. Effects of dietary and pharmacological manipulations on appetitive and consummatory aspects of feeding in non-human primates. Appetite. 2005;45:110–120. doi: 10.1016/j.appet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil B, Tinsley M, Fanselow M. Why do theories of behavior need motivational constructs? In: Weiner I, editor. Handbook of psychology. 4th edn. Wiley; New Jersey: 2003. pp. 33–55. [Google Scholar]
- Greenwald MK, Hursh SR. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend. 2006;85:35–48. doi: 10.1016/j.drugalcdep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Läck CM, Oleson EB, Roberts DC. Constant subcutaneous amphetamine decreases the motivation to self-administer cocaine, Program No. 591.3 Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. (Online) [Google Scholar]
- Loh EA, Roberts DC. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacol Berl. 1990;101:262–266. doi: 10.1007/BF02244137. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Martelle JL, Czoty PW, Nader MA. Effect of time-out duration on the reinforcing strength of cocaine assessed under a progressive-ratio schedule in rhesus monkeys. Behav Pharmacol. 2008;19:743–746. doi: 10.1097/FBP.0b013e3283123c6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Lacosta S, Roberts DC. l-Tryptophan decreases the breaking point under a progressive ratio schedule of intravenous cocaine reinforcement in the rat. Pharmacol Biochem Behav. 1993;44:651–655. doi: 10.1016/0091-3057(93)90181-r. [DOI] [PubMed] [Google Scholar]
- Nader MA, Hedeker D, Woolverton WL. Behavioral economics and drug choice: effects of unit price on cocaine self-administration by monkeys. Drug Alcohol Depend. 1993;33:193–199. doi: 10.1016/0376-8716(93)90060-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin K, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 2008;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes R, Agmo A. Stereospecific actions of baclofen on sociosexual behavior, locomotor activity and motor execution. Psychopharmacol Berl. 1989;97:358–364. doi: 10.1007/BF00439451. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo micro-dialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Smith AM, Roberts DC. A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug Alcohol Depend. 1994;36:23–25. doi: 10.1016/0376-8716(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran M, Fibiger H. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose–response relationship and effect of haloperidol pretreatment. Psychopharmacol Berl. 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh E, Baker G, Vickers G. Lesions of central serotonin systems affect responding on a progressive ratio schedule reinforced either by intravenous cocaine or by food. Pharmacol Biochem Behav. 1994;49:177–182. doi: 10.1016/0091-3057(94)90473-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin Exp Res. 2001;25:1006–1011. [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav Pharmacol. 1998;9:195–206. [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacol Berl. 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacol Berl. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, English JA. Further analysis of choice between cocaine to food using the unit price model of behavioral economics. Drug Alcohol Depend. 1997;49:71–78. doi: 10.1016/s0376-8716(97)00145-2. [DOI] [PubMed] [Google Scholar]
- Yokel R, Wise R. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- Zittel-Lazarini A, Cador M, Ahmed SH. A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacol Berl. 2007;192:337–346. doi: 10.1007/s00213-007-0724-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.