Abstract
Bacterial infections can be aggravated by antibiotic treatment that induces SOS response and vesiculation. This leads to a hypothesis concerning association of SOS with vesiculation. To test it, we conducted multiple analyses of outer membrane vesicles (OMVs) produced from the Pseudomonas aeruginosa wild type in which SOS is induced by ciprofloxacin and from the LexA noncleavable (lexAN) strain in which SOS is repressed. The levels of OMV proteins, lipids, and cytotoxicity increased for both the treated strains, demonstrating vesiculation stimulation by the antibiotic treatment. However, the further increase was suppressed in the lexAN strains, suggesting the SOS involvement. Obviously, the stimulated vesiculation is attributed by both SOS-related and unrelated factors. OMV subproteomic analysis was performed to examine these factors, which reflected the OMV-mediated cytotoxicity and the physiology of the vesiculating cells under treatment and SOS. Thus, SOS plays a role in the vesiculation stimulation that contributes to cytotoxicity.
1. Introduction
A Gram-negative bacterium, Pseudomonas aeruginosa has emerged as a prevalent nosocomial pathogen not only for hospital-acquired [1] and medical device-related infections [2, 3], but also for burn [4] and war-wound infections [5]. For such a wide spectrum of infections, the molecular pathogenesis is still incompletely understood. Mechanisms underlying the wide range of infections may entail the bacterial stress responses that help the microorganism to fit in new environments. The stress responses encompass the production of outer membrane vesicles (OMVs) in a process of vesiculation that occurs during all phases of growth of Gram-negative bacteria [6, 7]. OMVs also play a role in the pathogenesis as P. aeruginosa OMVs deliver multiple enzymes and virulence factors into the host cells [8]. Furthermore, bacteria produce OMVs in response to environmental and cellular stress factors [9–11], as vesiculation appears to increase survival of bacteria over stress. Environmental stress constitutes antibiotic treatments that have been found to affect vesiculation. Treatment of Shigella dysenteriae with mitomycin C, which activates the SOS response [12, 13], led to the increased level of Shiga toxin-associated OMV production [14] and toxin production [15]. Such stress-induced vesiculation seems to enhance survival, since the under-vesiculating mutants of Escherichia coli succumbed, whereas the overvesiculating mutants appeared more viable, when they were challenged with lethal envelope stressors [16]. With vesiculation identified as a bacterial stress response to environmental stimuli such as antibiotics, antibacterial treatment may aggravate the infections. Therefore, while mechanisms underlying vesiculation resulting from stress responses, especially the antimicrobial-triggered SOS response, remain poorly understood, it is imperative to investigate the connection so that effective intervention can be developed.
The SOS response [17] is a transcriptional response, in which LexA controls at least 40 SOS genes in E. coli [18–20] and 15 in P. aeruginosa [21]. SOS is triggered when bacteria are treated with DNA damage antibiotics, such as the quinolone antibiotic ciprofloxacin used in this work. The quinolone antibiotics target the type II topoisomerases including DNA gyrase (topoisomerase II) and topoisomerase IV [22]. These enzymes play an essential role in controlling superhelix density of chromosomal DNA to facilitate replication, recombination, repair, and transcription [22, 23]. Inhibiting these enzymes by ciprofloxacin leads to DNA strand breaks, the SOS signals. The mechanisms of the SOS response in P. aeruginosa and E. coli share the following steps (Figure 1). In the absence of the SOS signals, LexA blocks the transcription of the SOS genes [17]. When the SOS signals are generated during replication inhibition, RecA coprotease senses the signals and binds to the single-stranded DNAs to assume an active conformation [24]. Activated RecA stimulates the autocatalytic cleavage of LexA [25]. Consequently, LexA repression of the SOS genes is dismissed by this cleavage. Such derepression induces the SOS genes, leading to activation of the SOS response. One of them, sulA, is induced to inhibit and delay cell division transiently, resulting in cell filamentation, a sign of the SOS response, until DNA damage is ameliorated by the SOS proteins. The SOS proteins are involved in chromosome recombination, replication, repair, and segregation [26, 27]. As cell division is affected during SOS and is involved in OMV biogenesis [28], vesiculation may be linked to SOS. With antimicrobial agents inducing SOS and vesiculation, this link is quite likely as both SOS and vesiculation enhance bacterial survival [16, 29]. The purpose of this study is to investigate this link with multiple analyses.
Figure 1.
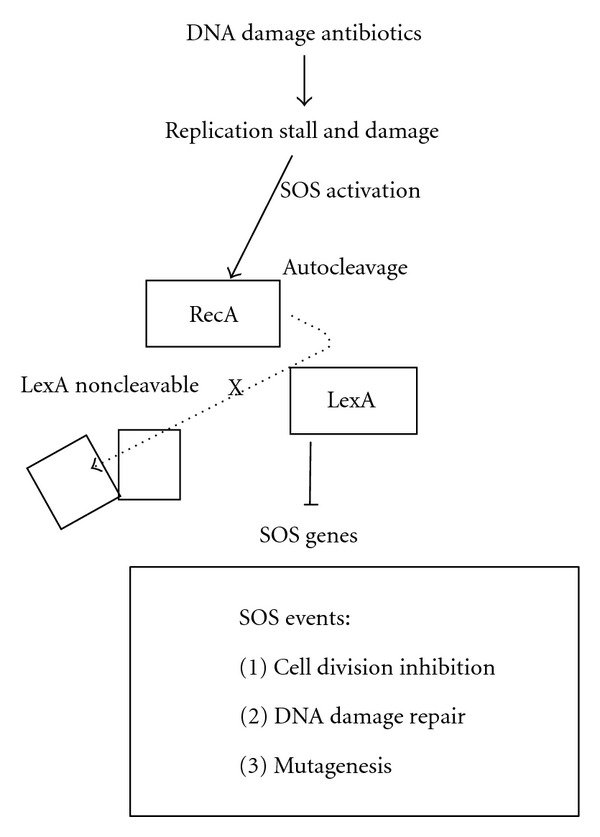
The bacterial SOS response. The response is triggered by DNA-damage antibiotics. This response is controlled by the RecA-LexA interplay, in which LexA represses the SOS genes. DNA damage activates RecA to simulate autocatalytic cleavage of LexA so that the SOS genes are depressed and expressed. X, the mutation rendering LexA noncleavable.
2. Materials and Methods
2.1. Bacterial Strains, Media, and Chemicals
P. aeruginosa PAO1 was obtained from the Pseudomonas Genetic Stock Center (strain PAO0001). The LexA noncleavable (lexAN) strain, gratefully from Dr. Floyd E. Romesberg, was constructed by the replacement of the catalytic serine of LexA with alanine as described in [21] so that the SOS regulon is repressed by lexAN in the presence of DNA damage. All the strains were grown at 37°C in Luria-Bertani (LB, purchased from Fisher Scientific) with 1-μg/mL ciprofloxacin (Sigma-Aldrich, minimal inhibitory concentrations or MIC = 0.125 μg/mL) as described before [21]. Experiments started with overnight cultures derived from the one-day-old single colonies grown on LB plates; experiments with colonies older than 3 days might not be reproducible.
2.2. Microscopic Analysis
Microscopy and measurement of cell length were performed as described [30, 31]. Briefly, the log-phase cells were fixed as described [30] and examined under a microscope (ZESS Axioshop 2 plus) equipped with CCD and computerized image analysis. The cell length was measured with NIH Image J. Significance levels (probability P values) in mean cell length were determined from a two-tailed Student's t-test.
2.3. OMV Extraction
Overnight cultures were diluted to the OD600 nm of 0.01 with 7 mL LB in a 25-mL flat-bottom glass tube. The subcultures were grown in a shaker at 37°C at the 250-rpm speed for 8 hrs. After the first two hours, ciprofloxacin was added to the subcultures to the final concentration of 1 μg/mL. OMVs were isolated by a standard method [32] with slight modifications. The cells in the subcultures were removed first by centrifugation at 10,000 rpm (12,000 g) for 10 minutes at 4°C and second by filtering the supernatant through a 0.2-μm filter. The filtered supernatant (6 mL) was ultracentrifuged in a fixed angle rotor (Ti-1270) for 3 hours at 4°C for 33,000 rpm (100,000 g). The supernatant was discarded, and the pellet was resuspended in 50 μL of phosphate buffered saline (PBS) by pipetting rather than by vortexing. The OMV samples could be stored at 4°C for 1 day for the macrophage assay and 3 days for proteomic analysis without losing activity.
2.4. Lipid Extraction and Quantification
The method was adopted from a protocol published previously [33] with slight modifications. The procedures included an extraction of the lipids with a mixture of methanol, chloroform, and water in a ratio of 2 : 2 : 0.8 (v/v). The OMV pellet collected after ultracentrifugation of the 7-mL cell-free culture was resuspended in 80 μL water in an eppendorf tube and then, to the OMV suspension, 200 μL methanol, and 200 μL chloroform were added and mixed. To the cell pellet, water was added to the final volume of 500 μL, and the cells were resuspended completely by vigorous vortexing. Then, an 80-μL volume of cell suspension was transferred to an effendorf tube and mixed with 200 μL methanol and 200 μL chloroform. After a 10-min vortexing, the sample was centrifuged for 10 min at 13,000 rpm at 4°C. The chloroform layer was transferred completely by gently inserting a pipette through the water-methanol phase and the interphase down to the bottom of the tube. To the tube containing the aquatic phases, 100 μL chloroform and 80 μL water were added and mixed by a vortexing. Centrifugation and chloroform layer transfer were repeated as above. The two fractions of chloroform layers were pooled; effort should be made to avoid losing any volumes of these fractions. For quantification, an empty eppendorf tube had been weighed, and then, the chloroform fractions were transferred to it and evaporated to dryness in a speed vac. The dry lipids were weighed, and the net weight was acquired by subtracting the tube weight.
2.5. Transmission Electron Microscope (TEM)
TEM was conducted according to a standard protocol [34] with modifications. A 10-μL volume of the OMV sample was placed on the lacey carbon film on 300-mesh copper grids. The grids were incubated at 25°C overnight. Then, they were negatively stained by 1% uranyl acetate (w/v, Sigma) for 20 seconds, washed three times with water, and air-dried. OMVs were examined on an Analytical Electron Microscope (JEOL JEM-2010F) with a Schottky field emission electron source. TEM was operated at accelerating voltage of 200 kV, with resolution at 0.1 nm lattice with 0.19 nm point-to-point, with magnification range from 2,0000x to 1,500,000x, with spot sizes of 2~5 nm at TEM mode, with EDS/NBD/CBD of 0.5~2.4 nm, and with specimen tilt at ±25 degrees (X, Y). Images were taken with a camera length of 80~2,000 mm and objective lens of Cs 0.5 mm and Cc 1.1 mm. For each sample, 5–10 images were recorded, and the diameter of each OMV was measured and compared for statistical significance as above.
2.6. Macrophage Cytotoxicity and MTT Assay
The indicated equal amount of OMVs (Figure 4) determined according to the OMV protein assay were suspended in DMEM medium and added into a 96-well plate which had been seeded with murine macrophage J774 cells (2 × 105/well). The plate was incubated at 37°C with 5% CO2 (v/v). The cytotoxicity was examined in the macrophage cytotoxicity assays as described previously [35] with modifications. The seeded macrophage was incubated with OMVs at the indicated concentrations. First, macrophage morphology was examined under a phase-contrast microscope with a 40-x lens [VZEISS AXIOVERT-200 with AXIOCAM camera equipped with CCD and computerized image analysis] after 0.5, 1, 2, 4, and 24 h. Approximately 10 fields were examined for each of duplicate wells. Second, the commercial kit (cyto 96 nonradioactive cytotoxicity kits from Promega) was used to measure OMV cytotoxicity to macrophage. The concept of this kit is to measure a stable cytosolic enzyme lactate dehydrogenase (LDH), which is released when macrophage cells are lysed. The released LDH then reacts with NAD, and protons are donated to NAD from LDH. The reducing NADH then reacts with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] and formazan (red) forms. By measuring the optical density of formazan at the wavelength of 490-nm, the cytotoxicity was quantified. The cytotoxicity is defined as the percentage of OMV-inflicted LDH release in contrast to the sum of detergent-lysed maximal release (positive control) and spontaneous release (negative control). The assay was carried out by following the manufacturer's protocol. The experiment wells were assembled to contain the 20-μL OMV sample, the 80-μL medium (DMEM plus 10% FBS, v/v and the 2 × 105target cells J774 cells), and the OMV effectors. The following control wells were arranged. The wells for effecter spontaneous LDH release contained medium and OMVs. The wells for target cell spontaneous LDH release included only target cells (macrophage) and medium. The wells for target cell maximum LDH release carried medium, target cells, and the Lysis Solution. A 10-μL volume of the Lysis Solution (10x) per 100 μL of culture medium was added, and the mixture was incubated for 45 minutes before the supernatant was harvested. The wells for volume correction control contained only medium and lysis solution. The wells for culture medium background were used to correct phenol red and LDH activity that might be present in serum-containing culture medium. The 96-well plate then was incubated at 37°C with 5% CO2 (v/v) for 4 hours. After incubation, the supernatant (40–50 μL) from each well (except for the Maximal LDH release control) was transferred into a well in a new 96-well plate and mixed with the same volume of substrate (tetrazolium salt, light-sensitive, so operate in dark). The 96-wells plate was incubated in the dark for 30 minutes at the room temperatures, and the reaction was terminated by adding the stop solution. The optical density was measured at the 490-nm wavelength.
Figure 4.
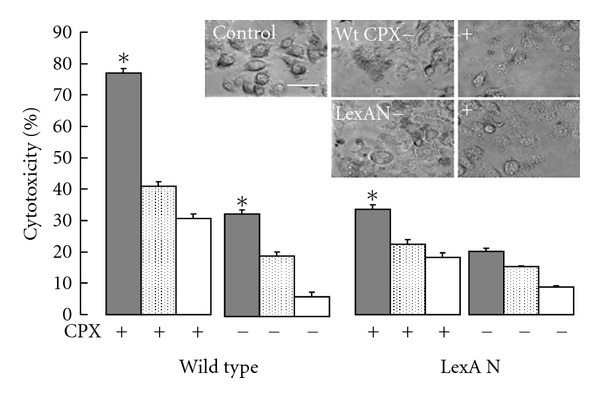
OMV-mediated macrophage cytotoxicity is SOS dependant. OMVs isolated from the wild-type (wt) and the LexA noncleavable (lexAN) cells as indicated were incubated with macrophage. Top: phase-contrast microscopy of macrophage incubated with OMVs at 1.3 μg/mL after a 1 hr incubation. Bar, 20 μm. Bottom: cytotoxicity was measured as the levels of released cytosolic lactate dehydrogenase after 4 hr incubation. It was set 0 for the macrophage-only control. OMV concentrations: grey, 1.3 μg/mL; doted, 0.65 μg/mL; blank, 0.325 μg/mL. (*P < 0.05, n = 4).
2.7. OMV Proteomic Analysis
The proteomic analysis was performed by following a standard method [36] with slight modifications. Specifically, the OMV proteins were fractionated by SDS-PAGE [37] (10%, w/v) and stained by Coomassie blue. The lanes in replicates containing the proteins were cut out, sliced into pieces (1 × 1 mm), and placed into Eppendorf tubes. The slices were subjected to in vitro proteolysis by trypsin as follows. The first step was SDS removal from the gel slices. A 100-μL volume of 25 mM NH4HCO3/50% acetonitrile (v/v, Fisher) was added to cover the gel slice. The mixture was vortexed for 10 minutes, and the supernatant was discarded. These steps were repeated until the gel became colorless. Acetonitrile (100%, v/v) was added to cover the gel slices, and the mixtures were incubated for a few minutes at room temperatures until the gel slices shrank and turned white. Acetonitrile in the gel slices was removed by spinning in a speed vacuum at room temperatures to complete dryness. The second step was reduction, alkylation, and proteolysis. For rehydration of the gel slices, an approximate 50-μL volume of 10-mM DTT (Sigma) in 25-mM NH4HCO3 was added to each tube to cover the gel slices followed by vortexing and brief spinning. The reagents were allowed to react with gel pieces at 56°C for 60 minutes, and the supernatant was discarded. A 40-μL volume of 55 mM iodoacetamide (Sigma) was added to the gel pieces, and the mixture was vortexed and then centrifuged briefly. The reaction was incubated in the dark for 30 min, and the supernatant was removed. The gels were washed in 100 μL (or enough to cover the gels) of 25-mM NH4HCO3/50% acetonitrile (v/v) followed by vortex and centrifugation for 5 min. A 200-μL volume of acetonitrile (100%, v/v) was added and removed as above. Trypsin (3 mg/mL, Promega) was added to just barely cover the gel pieces, and after a brief spinning, the mixture was incubated at 37°C for overnight. The third was extraction of peptides from the gel slices. The gel slices were centrifuged briefly, and the aquatic extract supernatant was collected into a 0.5-mL Eppendorf tube. To the gel pieces, a 30-μL volume of 0.1% formic acid (v/v, Burdic & Jacson) in 25-mM NH4HCO3 was added, followed by vortexing for 15 min and spinning briefly. The supernatant was harvested and pooled into the aquatic extract supernatant. The collected samples were then spun in a speed-vacuum to reduce the volume to approximately 10 μL (avoid complete dryness). The samples were stored at −20°C. Lastly, capillary liquid chromatography-tandem mass spectrometry (LC/MS/MS) was conducted at the RCMI Proteomics & Protein Biomarkers Cores to determine the peptides derived from the proteins in the gel slices. Capillary LC/MS/MS was performed with a linear ion trap tandem mass spectrometer (LTQ-XLS, ThermoFisher), where the top 7 eluting ions were fragmented by collision-induced dissociation.
Proteins were identified by searching MS/MS spectra against the NCBI nonredundant protein database (version 20100306; 10551781 sequences and 3596151245 residues). A probability-based database searching algorithm (Mascot, Matrixscience) was followed as described previously [38] with modifications. Briefly, database search criteria include taxonomy, bacteria (eubacteria, 3035644 sequences); enzyme, trypsin; variable modifications, carbamidomethylation of cysteines and oxidation of methionines; mass values, monoisotopic; protein mass, unrestricted; peptide mass tolerance, ±1000 ppm; fragment mass tolerance, ±0.8 Da; max missed cleavages, three instrument type, ESI-TRAP; number of queries, 87976. Peptide score distribution: Ions score is −10 log (P), where P is the probability that the observed match is a random event.
With the molecular weight search (MOWSE) peptide-mass database developed [39], the MOWSE scoring algorithm was used to calculate a score of each peptide entry. Briefly, the experimental mass values were searched across a calculated peptide mass database. Match of experimental mass values with calculated values were counted when the calculated value was in the range of a given mass tolerance of an experimental value. These matches were probability (P) based to ensure that the observed match is a random event. In a search for such random matches, the significance threshold was set for P to be ≤0.05, that is, a 1 in 20 chance of being a false positive. The matches were scored, based on the calculated P, that is, −10 log (P). The higher the score, the lower the P value. These ions scores were used to calculate protein score, which was the sum of the highest ions score for each distinct sequence. The proteins that were consistently detected in the replicates were counted. The inferred proteins were further categorized for functions and domains in amino acid sequences with the protein analysis software and with the published data. Functions and amino acid sequences were inferred by using http://www.uniprot.org/uniprot/O67077. Proteins with signal peptide were searched with http://www.signalpeptide.de/index.php?m=myproteinindex. Signal peptide in the proteins was predicted by using http://www.cbs.dtu.dk/services/SignalP/. Transmembrane domains were deduced with http://www.ch.embnet.org/software/TMPRED_form.html.
3. Results
3.1. Vesiculation Under Ciprofloxacin-Triggered SOS
The hypothesis concerning association of SOS with vesiculation was tested. The P. aeruginosa wild-type and the LexA noncleavable (lexAN) strains were treated with ciprofloxacin at 1 μg/mL. OMVs were extracted from these strains. The rationale for the antibiotic treatment was that this antibiotic was known to activate the SOS response in P. aeruginosa at 1 μg/mL, but SOS was noninducible in the lexAN strain [21]. Thus, testing of these strains with this drug would provide data relevant to SOS. When treated with the antibiotic, the wild-type cells became more filamented (cell length: 5.1 μm ± 1.2 and n = 169) than the lexAN cells (4.61 μm ± 1.2 and n = 89) (Figures 2(b) and 2(e), P < 0.0001). The significant cell filamentation is the manifestation of the SOS response [30, 40–43]. It is impossible to complement the lexAN mutant, because the lexAN phenotype is dominant; that is, in the lexAN background, the wild-type LexA would be cleaved, while the lexAN would remain not degraded during SOS. Besides, when both the wild-type and the lexAN cells were treated with ciprofloxacin at 1 μg/mL, lysed cells appeared imperceptible (<2%, n = 500), in contrast to treatment at 5 μg/mL (minimal bactericidal concentration, MBC = 3.25 μg/mL) that led to noticeable damaged and lysed cells (20%–30%, Figures 2(c) and 2(f)). With the growth conditions determined, OMVs were isolated by ultracentrifugation from the cell-free supernatants of the wild-type and the lexAN cultures shown in Figures 2(b) and 2(e). The presence of OMVs in the samples was confirmed with transmission electron microscopy (Figures 2(b) and 2(e) insets). The diameters of OMVs from both strains appeared similar (P = 0.2 and n = 70). Additionally, phage activity was not detected in the 1-μg/mL-drug-treated cell-free cultures and the OMV samples (data not shown). Hence, when the cells grew with the antibiotic at 1 μg/mL and produced OMVs, the likelihood of OMV contamination with the unrelated proteins from lysed cells appeared very small and was further addressed as below.
Figure 2.

Microscopy of P. aeruginosa under the ciprofloxacininduced SOS response. The wild-type (PAO1) and the LexA noncleavable (lexAN) strains were grown in LB with shaking for 2 hrs, and then ciprofloxacin (CPX) was added (1 μg/mL). The culture continued for 6 hrs. (a) PAO1 without and (b) with CPX. (c) PAO1 with 5 μg/mL CPX. (d) lexAN without and (e) with CPX. (f) lexAN with 5 μg/mL CPX. Arrows in (c) and (f) show damaged and lysed cells. Cell bar, 5 μm. Inset (b) shows transmission electron microscopy of OMVs from the treated wild-type cultures. Inset (e) shows lexAN OMVs. OMV bar, 0.1 μm.
3.2. Increase in OMV Protein Levels under SOS
OMVs were quantified from the wild-type and the lexAN strains treated with ciprofloxacin at 1 μg/mL. Briefly, both the wild-type and LexA noncleavable strains were grown with ciprofloxacin at 1 μg/mL. Both strains exhibited similar growth behaviors in the absence and in the presence of ciprofloxacin (Figure 3(a)). Since OMV protein quantity appeared to reflect the levels of OMVs [44], the OMV levels were determined from the same volume of culture containing the equal number of cells (Figure 3(a) at 480 min) by using Bradford assay of OMV proteins. With the ciprofloxacin treatment, the level of the wild-type OMV proteins increased more than 100-fold, as compared to that without (*P < 0.0001, Figure 3(a)). While the level of OMV proteins from the treated lexAN strain went up versus that of the untreated (P < 0.01), it did not reach the wild-type level, displaying 33% reduction reproducibly below the wild-type level (*P < 0.05, Figure 3(a)). These results demonstrate that vesiculation is stimulated by the antibiotic treatment. The data with lexAN suggest that the stimulation is attributed by LexA-dependent and independent mechanisms. The LexA-dependent mechanism of OMV stimulation involves SOS. The OMV protein level in the wild-type strain, which increased above that of the lexAN strain, was suppressed in the lexAN strain. Namely, the levels were increased when the SOS repressor LexA was autocleaved in the wild-type strain during SOS [25], but when LexA was made noncleavable in the lexAN strain [21], further augmentation appeared to cease. Therefore, these OMV proteins increased in the wild type but suppressed in the lexAN strain were termed the SOS-related. The LexA-independent mechanism may account for the increased levels of OMV proteins from the lexAN mutant over those from the untreated. These proteins were accordingly termed the SOS independent. Yet, the level was lower than that of the SOS-induced wild type (Figure 3(a)). In conclusion, the OMV protein level is increased from the cells treated with the antibiotic, and SOS contributes to the additional augmentation.
Figure 3.
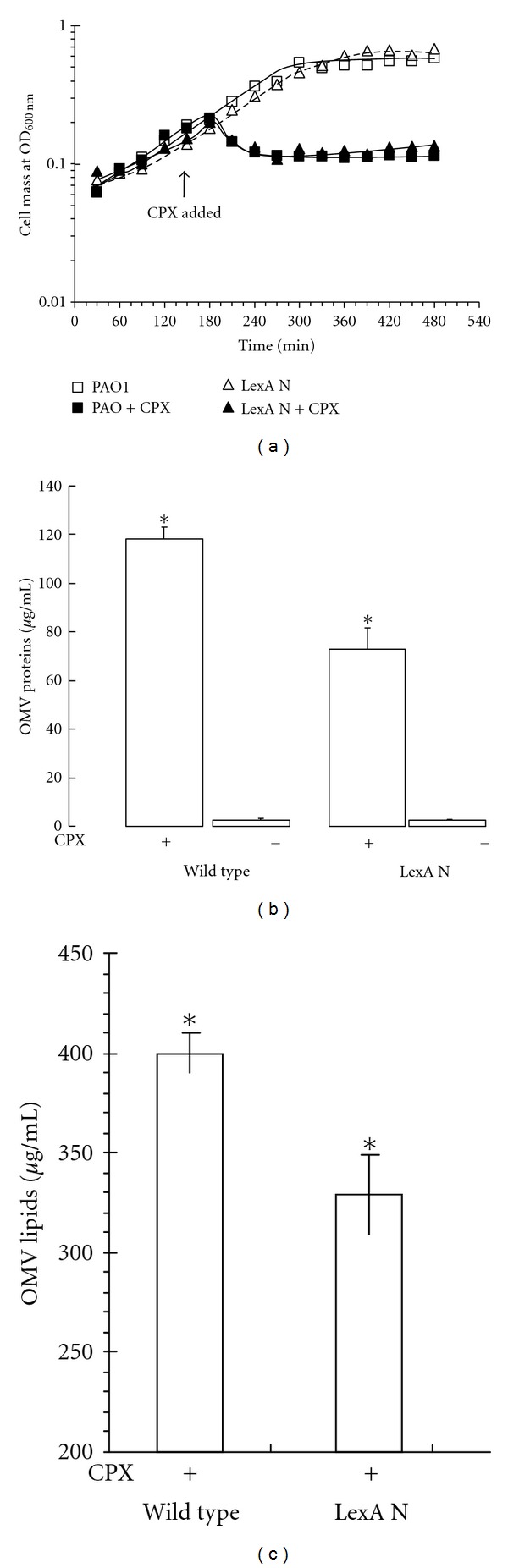
OMV production from P. aeruginosa under the ciprofloxacin-induced SOS response. (a) Growth of the wild-type (PAO1) and the LexA noncleavable strains. Both strain were grown in LB with shaking for 2 hrs, and then ciprofloxacin (CPX) was added. The culture continued for 6 hrs. OMVs were isolated from the wild-type and the lexAN cells treated with or without ciprofloxacin (CPX). (b) Quantification of OMV proteins by Bradford (n = 7) and (c) OMV lipids by weight (n = 3). (*P < 0.05).
3.3. Increase in OMV Lipid Levels under SOS
It seemed possible that the proteins unrelated to OMVs but produced during SOS might be co-centrifuged with OMVs. This possibility was excluded by OMV lipid quantification. From OMV protein quantification, given the observation that the increase in the OMV protein level in the treated wild-type strain was suppressed in the treated lexAN strain (Figure 3(a)), we wanted to confirm the increase in vesiculation with the lipid assay. The lipids were extracted from OMVs and cells, and the total dry lipids were weighed as described previously [33]. The OMV lipids of the wild-type strain treated with ciprofloxacin were heavier than those of the lexAN strain (*P < 0.05, Figure 3(b)). In contrast, the weights of the total lipids from cells did not change significantly, irrespective of strains and treatment (P ≥ 0.1, data not shown). Thus, the OMV lipid mass increased for the treated wild type, and the augmentation was suppressed in the treated lexAN strain, the results consistent with the OMV protein quantification. This consistency ruled out the possibility of OMV contamination with unrelated proteins but supported the notion of SOS involvement in vesiculation, yet, the SOS-unrelated factors contributing to the increase cannot be excluded.
3.4. OMV-Mediated Macrophage Cytotoxicity under SOS
Since OMVs act as a virulence factor [8, 45] and the OMV levels increase during the antibiotic-induced SOS, we wanted to investigate whether OMVs from the SOS strains differentially aggravates cytotoxicity. OMVs isolated from the wild-type and the lexAN cultures, either treated as above with ciprofloxacin or without, were added to macrophage in equal amounts. The cytotoxicity was assessed as described previously [35]. First, macrophage morphology was examined after incubation with OMVs from the untreated wild-type bacterial cells for 0.5, 1, 2, 4, and 24 h. Morphology changes appeared in the first h (Figure 4 Upper). OMVs from the ciprofloxacin-treated wild-type and the mutant cells caused dramatic alterations in the morphology of the macrophage, including cell shrinkage, detachment, and lysis, when compared with OMVs from the untreated bacterial cells. In contrast, treatment of macrophage with ciprofloxacin at 1 μg/mL did not cause the cytotoxic morphology, the result excluding a possibility of ciprofloxacin-inflicted toxicity presumably caused by the drug-carrying OMVs. Second, the cytotoxicity was quantified. It is defined as the percentage of the OMV-inflicted LDH release from macrophage in the detergent-lysed maximal release from macrophage (OMVs or macrophage alone did not lead to a LDH-increase). As shown in Figure 4 (lower), the OMV-inflicted macrophage toxicity appeared concentration-dependent. Cytotoxicity by OMVs from the treated wild-type strain increased over 55% versus that by OMVs from the untreated (P < 0.05). These results indicate that the OMV-mediated cytotoxicity is stimulated by OMVs from the antibiotic-treated bacterial cells. However, this stimulated cytotoxicity was not observed in OMVs from the treated lexAN culture (P < 0.05). Therefore, LexA appeared to suppress the increased cytotoxicity.
3.5. OMV Subproteomic Analysis
Under the ciprofloxacin treatment, the increased levels of the OMV proteins, lipids and the OMV-mediated cytotoxicity in the wild-type strain appeared to be suppressed in the lexAN strain. Many interesting questions were raised from these results as to what OMV proteins would be LexA-suppressed and what would be cytotoxicity-related. To address them, we examined the OMV subproteomes from the treated wild-type and the lexAN strains. The rationale for targeting the two treated strains was the following. For the treated wild-type strains, the OMV protein level was increased but suppressed for the lexAN strain. Thus, comparison of the OMV subproteomic data obtained from the two treated strains would provide information relevant to LexA or SOS. The comparison could help sort out the OMV proteins: the LexA-related and ciprofloxacin-specific or SOS-unrelated OMV proteins. When bacteria are treated with a certain antibiotic, drug-specific proteins were previously observed, such as OMPs [46, 47] and OMV proteins [48], which are unrelated to SOS. Most likely, the ciprofloxacin-specific OMV proteins would be found in both the wild-type and the lexAN OMV subproteomes, whereas the LexA-related OMV proteins would be detected in the OMV subproteome of the wild-type strain where LexA is autocleaved.
Experimentally, the OMV proteins from the antibiotic-treated wild-type and lexAN strains were characterized by the SDS-PAGE-based proteomic analysis (Figure 5). While small differences were observed in the OMV protein profiles for the treated wild-type and the lexAN strains (Figure 5), subtle distinctions were expected, based on LexA repression of gene expression, especially of the SOS regulons [18–21]. To unveil the differences, the in vitro trypsin proteolysis and capillary LC/MS/MS analysis was performed to determine the OMV proteins in the gel slices (Figure 5). The degraded peptide masses were determined and searched across the bacterial protein databases with the P < 0.05-based MOWSE scoring algorithm [39]. Totally, 145 proteins were identified in the OMV subproteomes from the treated wild-type and the lexAN strains (Tables 1–3). Many of the known OMV proteins, such as OstA (no. 1) [49] and OprE (no. 37) [50, 51], were detected in the wild-type OMVs (Figure 5 and Table 1), whereas GroEL (no. 75) [52] and OprF (no. 78) [50, 51] in both the wild-type and the lexAN OMVs (Table 2). Thus, the OMV subproteomes were confirmed to harbor some known OMV proteins. Moreover, with the SOS status of the wild-type and the lexAN strains used, the subproteomic analyses led to discovery and categorization of SOS- and cytotoxicity-related OMV proteins. The proteins detected only in OMVs from the drug-treated wild-type cells were termed the WT OMV proteins (74 proteins listed in Table 1 or 51% of 145). Since SOS was triggered in the wild type but repressed in the lexAN strain [21], the proteins produced during SOS were expected to emerge in the wild-type OMVs but not in the lexAN OMVs. These proteins were SOS-related. However, the proteins present in OMVs from both the treated wild-type and the lexAN strains were named the common OMV proteins (35 in Table 2 or 24%). The presence in both the OMV subproteomes implied that the appearance in OMVs was not affected by LexA; thus these proteins were called the SOS-unrelated. The OMV proteins present in OMVs from the lexAN cells alone were called the lexAN OMV proteins (36 in Table 3 or 26%). While the categorization provides insights into the antibiotic-stimulated vesiculation, it does not seem reconciled with the protein banding profiles that show slight differences in OMV proteins from the wild-type and the lexAN strains. The apparent discrepancy stems from the limited capacity of SDS-PAGE in resolving proteins with similar sizes in a certain band and inability to separate proteins of similar masses but of different pIs. For instance, when parallel bands in the wild-type and the lexAN OMV proteins were cut off for proteomic analysis, the subproteomic contents of the proteins carrying various pIs in one band were not completely identical to those in its counterpart (data not shown). Obviously, the OMV subproteomic analysis appears comprehensive, remedying the limitation of SDS-PAGE analysis.
Figure 5.
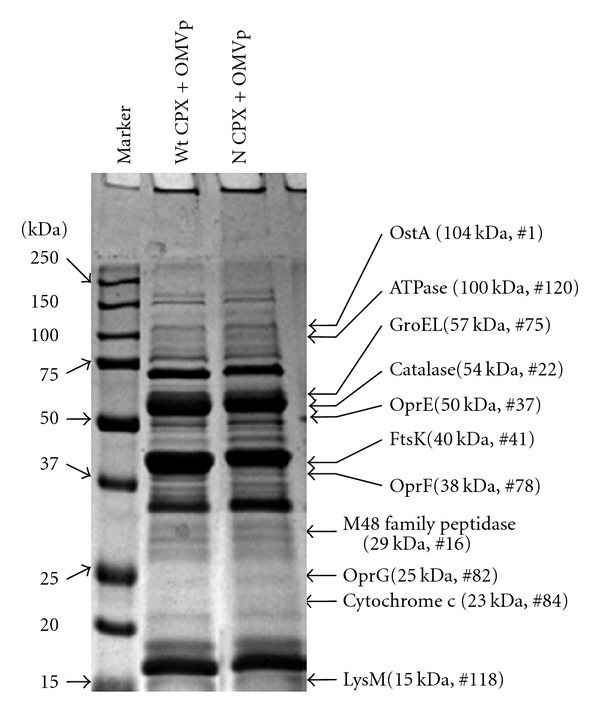
The SDS-PAGE based proteomic analyses of OMV proteins from the SOS-induced and the un-induced cells. CPX, ciprofloxacin. N, LexA noncleavable; and wt, wild-type. Illustrated to the right were the proteins known to associate with OMVs, cytotoxicity, and SOS (See text and Tables for details).
Table 1.
The WT OMV proteins.
| Protein access ID | Protein description | Score | pI | Mass | Predicted functions | Membrane domains |
|---|---|---|---|---|---|---|
| (1) gi∣15595792 | OstA precursor [P. aeruginosa PAO1] | 366 | 5.41 | 104207 | Organic solvent tolerance protein | SP, TM |
| (2) gi∣30525581 | ChaPs, heat-shock protein [Piscirickettsia salmonis] | 174 | 4.79 | 57310 | Protein folding, immunogenic protein | n, TM |
| (3) gi∣15595608 | PilJ [P. aeruginosa PAO1] | 165 | 4.65 | 72484 | Cell motility, twitching motility | SP, TM |
| (4) gi∣15597641 | Glycine dehydrogenase [P. aeruginosa PAO1] | 133 | 5.68 | 103853 | Degradation of glycine | n, TM |
| (5) gi∣254236694 | OprC precursor, putative [P. aeruginosa C3719] | 117 | 6.02 | 79268 | Copper transport outer membrane porin | SP, TM |
| (6) gi∣15599015 | SecF [P. aeruginosa PAO1] | 115 | 4.81 | 33021 | Protein translocation | SP, TM |
| (7) gi∣15599417 | Fe(III)-pyochelin OM receptor precursor [P. aeruginosa PAO1] | 107 | 5.86 | 79943 | Siderophore-iron transmembrane transporter activity | SP, TM |
| (8) gi∣309885 | Aspartate transcarbamoylase [P. aeruginosa PAO1] | 102 | 6.17 | 36521 | Pyrimidine biosynthesis | n, n |
| (9) gi∣83718562 | Peptide synthetase-like protein [Burkholderia thailandensis E264] | 96 | 5.91 | 349402 | Peptide synthesis | n, TM |
| (10) gi∣152986344 | YaeT [P. aeruginosa PA7] | 94 | 5.02 | 88222 | OMP assembly complex | SP, TM |
| (11) gi∣124022818 | Phosphate binding protein [Prochlorococcus marinus str. MIT 9303] | 90 | 9.51 | 18989 | ABC transport | SP, TM |
| (12) gi∣167840097 | Phospholipase D [Burkholderia thailandensis MSMB43] | 88 | 6 | 64431 | Lipid catalytic activity | n, TM |
| (13) gi∣167035883 | TonB-dependent copper receptor [P. putida GB-1] | 87 | 5.77 | 74399 | Copper receptor | SP, TM |
| (14) gi∣28871621 | Polyribonucleotide nucleotidyltransferase [P. syringae pv. tomato str. DC3000] | 81 | 5.20 | 74880 | mRNA degradation | n, TM |
| (15) gi∣15600748 | ATP synthase subunit γ [P. aeruginosa PAO1] | 73 | 7.70 | 31533 | ATP production | n,n |
| (16) gi∣70732441 | M48 family peptidase [P. fluorescens Pf-5] | 69 | 7.66 | 28833 | Protein dagradation | SP, TM |
| (17) gi∣50085971 | Acridine efflux pump [Acinetobacter sp. ADP1] | 67 | 6.36 | 114705 | Efflux pump | n, TM |
| (18) gi∣15596751 | Cytochrome oxidase subunit (cbb3-type) [P. aeruginosa PAO1] | 66 | 9.42 | 53073 | Electron transport chain | n, TM |
| (19) gi∣119713613 | SecD [uncultured marine bacterium EB0_39H12] | 62 | 5.63 | 67763 | Protein translocation | n, TM |
| (20) gi∣7715581 | PspA [Streptococcus pneumoniae] | 61 | 4.70 | 27305 | Pneumococcal surface protein A | n, n |
| (21) gi∣10945103 | PapA [P. aeruginosa] | 61 | 4.87 | 71649 | Lipid metabolic process | SP, TM |
| (22) gi∣121605556 | Catalase [Polaromonas naphthalenivorans CJ2] | 61 | 6.26 | 54212 | Hydrogen peroxide catabolic process | n, n |
| (23) gi∣94495248 | Rhizopine catabolism protein [Sphingomonas sp. SKA58] | 60 | 5.91 | 38825 | Oxidation reduction | n, TM |
| (24) gi∣116328093 | Zn-dependent hydrolase [Leptospira borgpetersenii serovar Hardjo-bovis L550] | 59 | 5.67 | 30997 | Hydrolase activity | SP, TM |
| (25) gi∣149378317 | PAS [Marinobacter algicola DG893] | 59 | 5.97 | 57059 | Signal transducer activity, chemotaxis | TM |
| (26) gi∣119356290 | Molybdate ABC transporter, ATPase subunit [Chlorobium phaeobacteroides DSM 266] | 59 | 9.23 | 38876 | ABC transport | n, n |
| (27) gi∣117619653 | Methyl-accepting chemotaxis transducer [P. stutzeri A1501] | 59 | 4.67 | 58670 | Methyl-accepting chemotaxis | SP, TM |
| (28) gi∣119899764 | MCP-domain-containing signal transduction protein [Azoarcus sp. BH72] | 59 | 5.80 | 53615 | Signal transduction | SP, TM |
| (29) gi∣887858 | DcrH [Desulfovibrio vulgaris str. Hildenborough] | 59 | 5.71 | 104664 | Signal transduction | SP, TM |
| (30) gi∣28869218 | Aerotaxis receptor [P. syringae pv. tomato str. DC3000] | 59 | 5.70 | 56965 | Aerotaxis | n, TM |
| (31) gi∣112004994 | Symbionin [Buchnera aphidicola] | 58 | 5.13 | 57904 | Protein folding | n, TM |
| (32) gi∣15597430 | PslD, biofilm proteins [P. aeruginosa PAO1] | 58 | 8.66 | 27891 | polysaccharide transmembrane transport | SP, TM |
| (33) gi∣154253615 | Molybdenum cofactor biosynthesis protein C [Parvibaculum lavamentivorans DS-1] | 58 | 9.64 | 16871 | Cofactor biosynthesis | n, n |
| (34) gi∣15600235 | PilO [P. aeruginosa PAO1] | 57 | 5.04 | 22805 | Cell motility, type 4 fimbrial biogenesis | n, TM |
| (35) gi∣29830660 | Oxidoreductase [Streptomyces avermitilis MA-4680] | 57 | 5.01 | 33162 | Electron transport chain | n, TM |
| (36) gi∣108803685 | Bifunctional homocysteine S-methyltransferase [Rubrobacter xylanophilus DSM 9941] | 57 | 6.33 | 65183 | Amino-acid biosynthesis | n, TM |
| (37) gi∣15595488 | OprE precursor [P. aeruginosa PAO1] | 54 | 8.67 | 49637 | Anaerobically-induced OM porin | SP, TM |
| (38) gi∣121997032 | Glycolate oxidase iron-sulfur subunit [Halorhodospira halophila SL1] | 53 | 8.55 | 43945 | Oxidation reduction | n, n |
| (39) gi∣161508096 | Biotin-acetyl-CoA-carboxylase ligase [Lactobacillus helveticus DPC 4571] | 53 | 9 | 37120 | Protein modification process | n, n |
| (40) gi∣119773234 | GTPase EngB [Shewanella amazonensis SB2B] | 52 | 6.97 | 24168 | Cell division | n, n |
| (41) gi∣75764772 | FtsK [Bacillus thuringiensis serovar israelensis ATCC 35646] | 50 | 4.33 | 40378 | Cell division | SP, TM |
| (42) gi∣3237312 | FimV [P. aeruginosa PAO1] | 50 | 4.33 | 98113 | Cell motility, twitching motility | SP, TM |
| (43) gi∣145588417 | Ferric uptake regulator family protein [Polynucleobacter necessarius subsp. asymbioticus QLW-P1DMWA-1] | 50 | 6.29 | 17176 | Repressor of the iron transport operon | n, n |
| (44) gi∣163846285 | Deoxyribose-phosphate aldolase [Chloroflexus aurantiacus J-10-fl] | 50 | 6.08 | 27440 | Deoxyribonucleotide catabolic process | n, n |
| (45) gi∣107099581 | Hypothetical+C448_01000594 [P. aeruginosa PACS2] | 267 | 5.13 | 101779 | Unknown | Unknown |
| (46) gi∣15598924 | Hypothetical PA3729 [P. aeruginosa PAO1] | 249 | 5.18 | 75836 | Unknown | n, TM |
| (47) gi∣15599685 | Hypothetical PA4489 [P. aeruginosa PAO1] | 179 | 5.48 | 167326 | Putative endopeptidase inhibitor activity | SP, TM |
| (48) gi∣156932378 | Hypothetical ESA_00154 [Enterobacter sakazakii ATCC BAA-894] | 167 | 4.83 | 57278 | Protein folding | n, TM |
| (49) gi∣15596261 | Hypothetical PA1064 [P. aeruginosa PAO1] | 116 | 5.38 | 24157 | Unknown | n, TM |
| (50) gi∣218890269 | Hypothetical PLES_15291 [P. aeruginosa LESB58] | 97 | 6.34 | 28636 | Unknown | SP, TM |
| (51) gi∣15595825 | Hypothetical PA0628 [P. aeruginosa PAO1] | 92 | 8.80 | 35848 | Unknown | n, n |
| (52) gi∣15600425 | Hypothetical PA5232 [P. aeruginosa PAO1] | 90 | 9.11 | 38586 | Protein transporter | SP, TM |
| (53) gi∣15596143 | Hypothetical PA0946 [P. aeruginosa PAO1] | 86 | 4.93 | 36754 | Unknown | SP, TM |
| (54) gi∣107103341 | Hypothetical PaerPA_01004410 [P. aeruginosa PACS2] | 84 | 9.02 | 67631 | Unknown | Unknown |
| (55) gi∣15599691 | Hypothetical PA4495 [P. aeruginosa PAO1] | 84 | 5.79 | 24864 | Unknown | SP, TM |
| (56) gi∣15600607 | Hypothetical PA5414 [P. aeruginosa PAO1] | 75 | 5.76 | 22533 | Unknown | SP, TM |
| (57) gi∣1162960 | Protein homologous to HI0366 in Haemophilus influenzae [P. aeruginosa PAO1] | 72 | 6.77 | 22403 | Unknown | SP, TM |
| (58) gi∣148980343 | Hypothetical VSWAT3_23674 [Vibrionales bacterium SWAT-3] | 59 | 4.85 | 56161 | Chemotaxis | SP, TM |
| (59) gi∣86146473 | Hypothetical MED222_12698 [Vibrio sp. MED222] | 59 | 4.76 | 68870 | Signal transducer activity | n, TM |
| (60) gi∣32266390 | Hypothetical HH0891 [Helicobacter hepaticus ATCC 51449] | 59 | 4.99 | 52013 | Signal transducer activity | n, n |
| (61) gi∣89893845 | Hypothetical DSY1099 [Desulfitobacterium hafniense Y51] | 59 | 5.00 | 54148 | Signal transducer activity | n, n |
| (62) gi∣18309647 | Hypothetical CPE0665 [Clostridium perfringens str. 13] | 59 | 4.91 | 28888 | Unknown | Unknown |
| (63) gi∣160938898 | Hypothetical CLOBOL_03792 [Clostridium bolteae ATCC BAA-613] | 59 | 4.79 | 60828 | Chemotaxis | SP, TM |
| (64) gi∣167772543 | Hypothetical ANACOL_03921 [Anaerotruncus colihominis DSM 17241] | 59 | 4.66 | 71478 | Signal transducer activity | SP, TM |
| (65) gi∣158334828 | Hypothetical AM1_1665 [Acaryochloris marina MBIC11017] | 59 | 4.54 | 28589 | Unknown | n, n |
| (66) gi∣146298741 | Hypothetical Fjoh_0980 [Flavobacterium johnsoniae UW101] | 58 | 8.90 | 168226 | Unknown | SP, TM |
| (67) gi∣126348240 | Conserved hypothetical [Streptomyces ambofaciens ATCC 23877] | 58 | 4.68 | 123551 | Unknown | Unknown |
| (68) gi∣162454210 | Hypothetical sce5933 [Sorangium cellulosum So ce 56] | 56 | 10.06 | 49450 | Unknown | SP, TM |
| (69) gi∣159184340 | Hypothetical Atu0493 [Agrobacterium tumefaciens str. C58] | 56 | 8.83 | 17570 | Unknown | Unknown |
| (70) gi∣120536835 | Hypothetical Maqu_4123 [Marinobacter aquaeolei VT8] | 54 | 6.02 | 36805 | Unknown | n, n |
| (71) gi∣88800650 | Hypothetical MED297_05259 [Reinekea sp. MED297] | 53 | 5.05 | 74044 | Unknown | Unknown |
| (72) gi∣107103648 | Hypothetical PaerPA_01004718 [P. aeruginosa PACS2] | 52 | 8.92 | 52714 | Unknown | Unknown |
| (73) gi∣124385398 | Hypothetical BMA10229_A0995 [Burkholderia mallei NCTC 10229] | 52 | 5.66 | 5192 | Unknown | n, n |
| (74) gi∣94986987 | Hypothetical LI0545 [Lawsonia intracellularis PHE/MN1-00] | 51 | 6.43 | 11768 | Unknown | n, n |
TM: transmembrane domains; SP: signal peptide; pI: isoelectric point.
Table 3.
The lexAN OMV proteins.
| Protein access ID | Protein description | Score | pI | Mass | Predicted functions | Membrane domains |
|---|---|---|---|---|---|---|
| (110) gi∣15599248 | 6,7-dimethyl-8-ribityllumazine synthase [P. aeruginosa PAO1] | 299 | 5.69 | 16403 | Riboflavin biosynthesis | n, n |
| (111) gi∣14573303 | PilA [P. aeruginosa] | 160 | 6.23 | 15488 | Major pilin subunit of type IV pili | n, TM |
| (112) gi∣15598049 | OprI precursor [P. aeruginosa PAO1] | 132 | 7.90 | 8829 | OM lipid-anchor. | SP, TM |
| (113) gi∣15596004 | AmpDh3 [P. aeruginosa PAO1] | 129 | 5.89 | 28703 | Peptidoglycan catabolic process | n, n |
| (114) gi∣15599856 | Lipid A 3-O-deacylase [P. aeruginosa PAO1] | 116 | 5.87 | 18382 | Modification of lipid A of LPS | SP, TM |
| (115) gi∣15596159 | DNA-binding stress protein [P. aeruginosa PAO1] | 100 | 4.96 | 17482 | Response to stress, iron ion homeostasis | n, n |
| (116) gi∣15599000 | PilF [P. aeruginosa PAO1] | 75 | 6.67 | 28520 | Type 4 fimbrial biogenesis | SP, TM |
| (117) gi∣15596133 | LpxO2 [P. aeruginosa PAO1] | 72 | 9.90 | 35737 | LPS biosynthesis | n, TM |
| (118) gi∣15600371 | LysM domain/BON superfamily protein [P. aeruginosa PAO1] | 69 | 5.45 | 15451 | Cleavage of septal peptidoglycan to allow cell separation | n, n |
| (119) gi∣15598026 | HtpX [P. aeruginosa PAO1] | 65 | 7.03 | 31573 | Heat shock protein, proteolysis | SP, TM |
| (120) gi∣15600018 | Mg(2+) transport ATPase, P-type 2 [P. aeruginosa PAO1] | 63 | 5.88 | 99987 | Magnesium-importing ATPase activity | n, TM |
| (121) gi∣15599764 | 50S ribosomal protein L21 [P. aeruginosa PAO1] | 62 | 9.85 | 11646 | Protein synthesis | n, n |
| (122) gi∣116620118 | HAD family hydrolase [Solibacter usitatus Ellin6076] | 57 | 5.37 | 22870 | Phosphoglycolate phosphatase activity | n, n |
| (123) gi∣21233204 | RhlB [Xanthomonas campestris pv. campestris str. ATCC 33913] | 51 | 9.25 | 62279 | ATP-dependent RNA helicase unwinding of double stranded RNA | n, n |
| (124) gi∣15599463 | 30S ribosomal protein S7 [P. aeruginosa PAO1] | 51 | 10.24 | 17493 | Protein synthesis | n, n |
| (125) gi∣73537822 | Twin-arginine translocation pathway signal [Ralstonia eutropha JMP134] | 50 | 9.37 | 36128 | Protein export through the cytoplasmic membrane | SP, TM |
| (126) gi∣71064880 | GltI [Psychrobacter arcticus 273-4] | 50 | 5.11 | 35348 | ABC glutamate/aspartate transporter | SP, n |
| (127) gi∣15596250 | HypotheticalPA1053 [P. aeruginosa PAO1] | 279 | 9.64 | 15639 | Unknown | SP, TM |
| (128) gi∣15599835 | HypotheticalPA4639 [P. aeruginosa PAO1] | 98 | 9.47 | 20723 | Unknown | SP, TM |
| (129) gi∣15600165 | HypotheticalPA4972 [P. aeruginosa PAO1] | 86 | 5.98 | 27836 | Unknown | SP, TM |
| (130) gi∣15598227 | HypotheticalPA3031 [P. aeruginosa PAO1] | 83 | 4.92 | 8007 | Unknown | SP, TM |
| (131) gi∣15598151 | HypotheticalPA2955 [P. aeruginosa PAO1] | 70 | 5.35 | 23677 | Unknown | SP, TM |
| (132) gi∣145635820 | HypotheticalCGSHiAA_01062 [Haemophilus influenzae PittAA] | 69 | 5.85 | 56041 | Unknown | n, n |
| (133) gi∣183222376 | Hypothetical LEPBI_I3030 [Leptospira biflexa serovar Patoc strain Patoc 1 (Paris)] | 61 | 8.40 | 51147 | Transporter activity | SP, TM |
| (134) gi∣57233652 | HypotheticalDET1586 [Dehalococcoides ethenogenes 195] | 61 | 8.80 | 21290 | Unknown | SP, TM |
| (135) gi∣29377408 | HypotheticalEF2944 [Enterococcus faecalis V583] | 58 | 4.99 | 19312 | Unknown | n, n |
| (136) gi∣15597823 | HypotheticalPA2627 [P. aeruginosa PAO1] | 57 | 10.35 | 23048 | Unknown | n, n |
| (137) gi∣116750341 | HypotheticalSfum_2918 [Syntrophobacter fumaroxidans MPOB] | 54 | 5.40 | 89798 | Carbohydrate binding | SP, TM |
| (138) gi∣167751139 | Hypothetical EUBSIR_02124 [Eubacterium siraeum DSM 15702] | 53 | 5.50 | 44267 | Metal ion binding | n, TM |
| (139) gi∣26250264 | Hypotheticalc4442 [E. coli CFT073] | 53 | 9.39 | 39090 | Unknown | n, TM |
| (140) gi∣167754381 | Hypothetical ALIPUT_02675 [Alistipes putredinis DSM 17216] | 53 | 6.89 | 18845 | Methyltransferase activity | n, n |
| (141) gi∣116048834 | Hypothetical PA14_52490 [P. aeruginosa UCBPP-PA14] | 52 | 6.19 | 17828 | Unknown | n, n |
| (142) gi∣83312716 | Hypothetical amb3617 [Magnetospirillum magneticum AMB-1] | 52 | 5.42 | 89219 | Cyclic nucleotide biosynthetic process | n, TM |
| (143) gi∣154503183 | Hypothetical RUMGNA_01007 [Ruminococcus gnavus ATCC 29149] | 51 | 5.29 | 49010 | Rhamnose metabolic process | n, n |
| (144) gi∣15598505 | Hypothetical PA3309 [P. aeruginosa PAO1] | 51 | 5.50 | 16486 | Response to stress | n, n |
| (145) gi∣148255416 | HypotheticalBBta_4029 [Bradyrhizobium sp. BTAi1] | 51 | 4.22 | 4004 | Unknown | n, n |
TM: transmembrane domains; SP: signal peptide. pI: isoelectric point.
Table 2.
The common OMV proteins.
| Protein access ID | Protein description | Score | pI | Mass | Predicted functions | Membrane domains |
|---|---|---|---|---|---|---|
| (75) gi∣576779 | GroEL [P. aeruginosa] | 784 | 5.04 | 57036 | Protein folding | n, n |
| (76) gi∣167855908 | 50S ribosomal protein L28 [Haemophilus parasuis 29755] | 242 | 4.90 | 57645 | Protein synthesis | n, n |
| (77) gi∣15596780 | Succinate dehydrogenase flavoprotein subunit [P. aeruginosa PAO1] | 175 | 6.04 | 63492 | Tricarboxylic acid cycle | n, TM |
| (78) gi∣15596974 | OprF precursor [P. aeruginosa PAO1] | 166 | 4.98 | 37616 | Major porin, ion transport | SP, TM |
| (79) gi∣15596375 | OprH precursor [P. aeruginosa PAO1] | 107 | 9.00 | 21561 | Response to Mg2+ starvation | SP, TM |
| (80) gi∣15598888 | OMP precursor [P. aeruginosa PAO1] | 103 | 9.45 | 28497 | OM | SP, TM |
| (81) gi∣2626833 | Chemotactic transducer [P. aeruginosaPAO1] | 98 | 4.88 | 68395 | Chemotaxis | SP, TM |
| (82) gi∣15599262 | OprG precursor [P. aeruginosa PAO1] | 93 | 4.85 | 25178 | OmpW family | SP, TM |
| (83) gi∣15598278 | Glycine betaine transmethylase [P. aeruginosa PAO1] | 74 | 4.74 | 71360 | Utilization of choline and glycine betaine as carbon and nitrogen sources | SP, TM |
| (84) gi∣15596750 | Cytochrome c oxidase subunit [P. aeruginosa PAO1] | 74 | 7.79 | 22744 | Electron carrier activity | n. TM |
| (85) gi∣37522034 | Glycosyltransferase [Gloeobacter violaceus PCC 7421] | 70 | 8.96 | 47611 | Biosynthesis of glycoproteins | n, n |
| (86) gi∣15596166 | TolQ [P. aeruginosa PAO1] | 67 | 5.96 | 25266 | Import of group A colicins for envelope integrity | n, TM |
| (87) gi∣15599941 | SecG [P. aeruginosa PAO1] | 65 | 5.21 | 13199 | Protein translocation | n, TM |
| (88) gi∣151545 | RNA polymerase subunit [P. aeruginosa] | 63 | 4.95 | 30372 | RNA synthesis | n, n |
| (89) gi∣115523809 | OmpA/MotB domain-containing protein [RhodoP. palustris BisA53] | 63 | 7.60 | 45685 | Major nonspecific porin | SP, TM |
| (90) gi∣114563330 | Phosphoglucomutase [Shewanella frigidimarina NCIMB 400] | 62 | 5.39 | 62288 | Carbohydrate metabolic process | n, n |
| (91) gi∣15596170 | OprL precursor [P. aeruginosa PAO1] | 61 | 5.95 | 17914 | Peptidoglycan-associated OM lipoprotein | SP, TM |
| (92) gi∣15598193 | Na(+)-translocating NADH-quinone reductase subunit C [P. aeruginosa PAO1] | 61 | 5.67 | 27763 | Reduction of ubiquinone-1 to ubiquinol and transport of Na+ ions | SP, TM |
| (93) gi∣15598107 | TonB-dependent receptor, putative [P. aeruginosa PAO1] | 60 | 6.24 | 80241 | High-affinity binding and energy-dependent uptake of specific substrates into the periplasmic space | SP, TM |
| (94) gi∣162455126 | Protein kinase [Sorangium cellulosum So ce 56] | 57 | 6.16 | 189825 | Kinase activity | n, n |
| (95) gi∣15597002 | Peptidyl-prolyl cis-trans isomerase D [P. aeruginosa PAO1] | 57 | 4.99 | 68699 | Protein folding | n, TM |
| (96) gi∣183602700 | Site-specific DNA-methyltransferase [Bifidobacterium animalis subsp. lactis HN019] | 56 | 6.24 | 51123 | DNA methylation | n, n |
| (97) gi∣15600134 | HflC [P. aeruginosa PAO1] | 50 | 9.48 | 33095 | Peptidase activity | SP, TM |
| (98) gi∣15599627 | Iron-sulfur protein [P. aeruginosa PAO1] | 50 | 6.07 | 20815 | Iron-sulfur cluster assembly | n, n |
| (99) gi∣15595268 | Hypothetica PA0070 [P. aeruginosa PAO1] | 114 | 8.93 | 31697 | Unknown | SP, TM |
| (100) gi∣15596030 | Hypothetica PA0833 [P. aeruginosa PAO1] | 104 | 8.89 | 24698 | OmpA family | SP, TM |
| (101) gi∣15597431 | Hypothetica PA2235 [P. aeruginosa PAO1] | 93 | 5.99 | 74519 | Lipopolysaccharide biosynthetic process | n, TM |
| (102) gi∣15595823 | Hypothetica PA0626 [P. aeruginosa PAO1] | 87 | 9.67 | 31273 | Unknown | n, n |
| (103) gi∣15599828 | Hypothetica PA4632 [P. aeruginosa PAO1] | 81 | 6.97 | 29141 | Proteolysis | SP, TM |
| (104) gi∣15599183 | Hypothetica PA3988 [P. aeruginosa PAO1] | 67 | 5.23 | 22870 | OM assembly | SP, TM |
| (105) gi∣152989513 | Hypothetica PSPA7_0777 [P. aeruginosa PA7] | 62 | 4.77 | 17484 | Unknown | n, n |
| (106) gi∣15595830 | Hypothetica PA0633 [P. aeruginosa PAO1] | 62 | 4.93 | 17528 | Unknown | n, n |
| (107) gi∣15595812 | Hypothetica PA0615 [P. aeruginosa PAO1] | 60 | 4.53 | 18939 | Unknown | n, n |
| (108) gi∣15595813 | Hypothetica PA0616 [P. aeruginosa PAO1] | 58 | 5.93 | 19410 | Unknown | n, TM |
| (109) gi∣15599619 | Hypothetica PA4423 [P. aeruginosa PAO1] | 51 | 6.69 | 65589 | Unknown | SP, TM |
TM: transmembrane domains; SP: signal peptide. pI: isoelectric point.
Interestingly, the OMV subproteomes seem to reflect the physiology of the cells under the antibiotic treatment and the cytotoxicity of OMVs to host cells. For instance, the known SOS-regulated proteins, such as FtsK (no. 41) [53] and catalase (no. 22) [54], were detected in the OMVs from the treated wild-type cells where SOS is induced. Proteins of efflux (no. 17) and cell motility (nos. 3, 34, and 42) were also found. Since efflux and cell mobility are involved in antibiotic resistance [55, 56], the presence of the related proteins in OMVs is likely to result from a response of the bacterial cells to the ciprofloxacin treatment. Furthermore, virulent proteins were detected in OMVs. In fact, cell mobility as mentioned above is known to enhance production of virulence factors [55]. An example in the WT OMV proteins is M48 family peptidase (no. 16 in Table 1) that contains Pseudomonas metalloproteases, elastase, and alkaline protease. These proteins are believed to mediate tissue penetration [57–59]. The examples in the common category include cytochrome c (no. 84 in Table 2) cytotoxic to macrophage [60], and OprG (no. 82) contributing to cytotoxicity toward human bronchial epithelial cells [61]. Examples in the lexAN group are the following: ATP-utilizing enzymes such as ATPase (no. 120 of Table 3) cytotoxic to macrophage [60] and the LysM domain carrying protein (no. 118) involved in pathogenesis [62]. Taken together, the OMV subproteomic results appeared aligned with the functional results pertinent to drug resistance, SOS, and cytotoxicity.
4. Discussion
Vesiculation from P. aeruginosa under ciprofloxacin treatment was investigated with multiple approaches. OMVs were isolated from the wild-type strain in which SOS is induced by ciprofloxacin and from the lexAN strain in which SOS is repressed. Cell morphology after the treatment showed cell filamentation, confirming SOS, while OMVs were not changed significantly in size during SOS. Vesiculation as determined chemically by the OMV protein and lipid levels and functionally by cytotoxicity is stimulated by the drug treatment, higher in the wild-type strain but suppressed in the lexAN strain. The overall increases for the wild-type and the lexAN strains suggest that the stimulation is attributed by the SOS-related and the independent factors; the suppression of further increase in the lexAN strain suggests that the additional augmentation involves SOS. The cytotoxicity of OMVs and the bacterial physiology under the antibiotic treatment and SOS were reflected by the results of the OMV subproteomic analysis.
An intriguing observation is the presence of cytosolic proteins in OMVs. Considering the hydrophobic nature of outer membrane, we were tempted to suspect contamination of OMVs with the cytosolic proteins. Nevertheless, the presence of the cytosolic proteins in OMVs is not just coincidental but consistently documented [63]. In fact, GroEL (no. 75), ribosomal proteins (nos. 76, 121, and 124), and DNA binding proteins (no. 96, 115) were detected in outer membrane [52, 64–66] and OMV fractions [52, 67]. The possible mechanisms for their OMV inclusion may involve association of the cytoplasmic proteins with membrane proteins that may bring the former to membrane proximity. For example, peptidyl-prolyl cis-trans isomerase, a membrane-associated protein (no. 95 in Table 2), is a trigger factor that is highly conserved in most bacteria [68, 69]. The presence of the trigger factor in the stressed cells is reasonable as the trigger factor is generally believed to play a central role in bacterial survival of environmental insult. Since the trigger factor in E. coli is associated with the 50S ribosomal subunit [70] and GroEL [71–73], the factor is likely to be translocated with 50S ribosomal protein L28 and GroEL to OMVs. Besides, because OMVs can package DNA [48, 74] and P. aeruginosa OMVs carry DNA [75], the DNA binding proteins, such as DNA-binding stress protein (no. 115) and DNA-methyltransferase (no. 96), may be delivered into OMVs through hitching onto DNA.
The molecular mechanisms behind the vesiculation stimulation during SOS remain poorly understood. OMVs are generated from living cells by budding from outer membrane bulges with subsequent fission [6, 7, 48, 74, 76]. Vesiculation does not concur with cell lysis, for OMVs package newly synthesized proteins [77–79]. These may be the reasons that OMV yields were too low when ciprofloxacin was used at and above MBC (data not shown), but the OMV levels were high when the drug was administered at 1 μg/mL. Therefore, the increase in the OMV protein levels observed in this work from the cultures treated with ciprofloxacin at 1 μg/mL is unlikely to result from cell lysis, especially as lysed cells barely were observed in the culture treated with the drug. The OMV protein levels are most likely to reflect the vesiculation stimulation during SOS. Indeed, the SOS-induced vesiculation is corroborated by the OMV lipid quantification. The OMV induction can be interpreted by combination of cell division delay and envelope alteration incurred in SOS. During SOS, sulA is induced, whose product inhibits and delays cell division transiently until DNA damage is ameliorated. In E. coli, this is achieved by SulA binding to FtsZ to block septum formation [42, 43, 80, 81]; similarly, a complex of P. aeruginosa SulA with FtsZ has been reported [82]. Inhibition of cell division was observed in P. aeruginosa treated with ciprofloxacin (Figure 2). According to the model of OMV biogenesis [28], such an episode of division inhibition may invoke temporary impact on the envelope structure, stimulating OMV generation.
Our finding of vesiculation stimulation during SOS is highly significant. On one hand, suppression of the SOS-repair network by LexA in E. coli with engineered bacteriophage increased bactericidal effects of SOS-inducing antibiotics in vitro and enhanced survival of infected mice in vivo [83], paving a way for the LexA-based therapeutic strategy. In parallel are our results that LexA represses OMV stimulation and cytotoxicity, yet the lexAN-based strategy fails to eliminate them (Figures 3 and 4), pointing to existence of LexA independent mechanisms. The OMV protein levels increased in the cultures of the ciprofloxacin-treated lexAN mutant; the noncleavable LexA even appeared to contribute to production of some OMV proteins (Figure 3(a) and Table 3). The observations lead to the SOS-independent mechanisms that demand future endeavor in investigation. On the other hand, SOS appears responsible for the antibiotic inducible-biofilm formation [30, 40] and vesiculation though the mechanisms behind the induction seem a mystery. Our data obtained with the OMV protein-, the lipid-, the cell- and the proteomic-based approaches suggest that SOS plays a role in the antibiotic-stimulated vesiculation and in OMV-mediated cytotoxicity to macrophage. The result may help develop guidelines for antibiotic practice to prevent such side effects as vesiculation and the related cytotoxicity to host defense cells.
Acknowledgments
The first two authors contributed equally to the work concerning the figures and the writing. All the authors have declared no conflict of interests. They thank Dr. Floyd E. Romesberg for the LexA noncleavable strain. They are also thankful to Anyu Tsai for assistance with OMV extraction, to Dr. Rodrigo A. Esparza-Munoz for TEM, to Shakinah Twinkle and Leigh Von Osselaer for proofreading of this paper, and Edward Rodriguez for protein data search. They thank Vidya Pericherla and the RCMI Proteomics & Protein Biomarkers Cores at UTSA (NIH G12 RR013646) for assistance with experiment design, sample preparation, data collection, results, and interpretation. They thank the Computational Biology Initiative (UTSA/UTHSCSA) for providing access and training to the analysis software used. This work was supported by the San Antonio Area Foundation and the UTSA Collaborative Research Seed Grant Program.
References
- 1.Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–1364. doi: 10.1592/phco.2005.25.10.1353. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielecki P, Glik J, Kawecki M, dos Santos VAPM. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnology Letters. 2008;30(5):777–790. doi: 10.1007/s10529-007-9620-2. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun JH, Murray CK, Manring MM. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clinical Orthopaedics and Related Research. 2008;466(6):1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beveridge TJ. Structures of Gram-negative cell walls and their derived membrane vesicles. Journal of Bacteriology. 1999;181(16):4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayrand D, Grenier D. Biological activities of outer membrane vesicles. Canadian Journal of Microbiology. 1989;35(6):607–613. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- 8.Bomberger JM, MacEachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by pseudomonas aeruginosa outer membrane vesicles. PLoS Pathogens. 2009;5(4) doi: 10.1371/journal.ppat.1000382. Article ID e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsui N, Tsuchido T, Hiramatsu R, Fujikawa S, Takano M, Shibasaki I. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. Journal of Bacteriology. 1982;151(3):1523–1531. doi: 10.1128/jb.151.3.1523-1531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikaido H. Isolation of outer membranes. Methods in Enzymology. 1994;235:225–234. doi: 10.1016/0076-6879(94)35143-0. [DOI] [PubMed] [Google Scholar]
- 11.Thompson SS, Naidu YM, Pestka JJ. Ultrastructural localization of an extracellular protease in Pseudomonas fragi by using the peroxidase-antiperoxidase reaction. Applied and Environmental Microbiology. 1985;50(4):1038–1042. doi: 10.1128/aem.50.4.1038-1042.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogoma T, Torrey TA, Connaughton MJ. Induction of UV-resistant DNA replication in Escherichia coli: induced stable DNA replication as an SOS function. Molecular and General Genetics. 1979;176(1):1–9. doi: 10.1007/BF00334288. [DOI] [PubMed] [Google Scholar]
- 13.Miller HI, Kirk M, Echols H. SOS induction and autoregulation of the himA gene for site-specific recombination in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(11):6754–6758. doi: 10.1073/pnas.78.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta S, Iida KI, Takade A, Meno Y, Nair GB, Yoshida SI. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiology and Immunology. 2004;48(12):965–969. doi: 10.1111/j.1348-0421.2004.tb03626.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner PL, Livny J, Neely MN, Acheson DWK, Friedman DI, Waldor MK. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Molecular Microbiology. 2002;44(4):957–970. doi: 10.1046/j.1365-2958.2002.02950.x. [DOI] [PubMed] [Google Scholar]
- 16.McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Molecular Microbiology. 2007;63(2):545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker GC. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiological Reviews. 1984;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158(1):41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Henestrosa ARF, Ogi T, Aoyagi S, et al. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Molecular Microbiology. 2000;35(6):1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 20.Khil PP, Camerini-Otero RD. Over 1000 genes are involved in the DNA damage response of Escherichia coli. Molecular Microbiology. 2002;44(1):89–105. doi: 10.1046/j.1365-2958.2002.02878.x. [DOI] [PubMed] [Google Scholar]
- 21.Cirz RT, O’Neill BM, Hammond JA, Head SR, Romesberg FE. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. Journal of Bacteriology. 2006;188(20):7101–7110. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiology and Molecular Biology Reviews. 1997;61(3):377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkey PM. Mechanisms of quinolone action and microbial response. Journal of Antimicrobial Chemotherapy. 2003;51(1):29–35. doi: 10.1093/jac/dkg207. [DOI] [PubMed] [Google Scholar]
- 24.Sassanfar M, Roberts JW. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. Journal of Molecular Biology. 1990;212(1):79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 25.Little JW. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie. 1991;73(4):411–421. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- 26.Cox MM. A broadening view of recombinational DNA repair in bacteria. Genes to Cells. 1998;3(2):65–78. doi: 10.1046/j.1365-2443.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- 27.Sherratt DJ. Bacterial chromosome dynamics. Science. 2003;301(5634):780–785. doi: 10.1126/science.1084780. [DOI] [PubMed] [Google Scholar]
- 28.Deatherage BL, Lara JC, Bergsbaken T, Barrett SLR, Lara S, Cookson BT. Biogenesis of bacterial membrane vesicles. Molecular Microbiology. 2009;72(6):1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dörr T, Lewis K, Vulić M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genetics. 2009;5(12) doi: 10.1371/journal.pgen.1000760. Article ID e1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotoh H, Zhang Y, Dallo SF, Hong S, Kasaraneni N, Weitao T. Pseudomonas aeruginosa, under DNA replication inhibition, tends to form biofilms via Arr. Research in Microbiology. 2008;159(4):294–302. doi: 10.1016/j.resmic.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Weitao T, Nordström K, Dasgupta S. Mutual suppression of mukB and seqA phenotypes might arise from their opposing influences on the Escherichia coli nucleoid structure. Molecular Microbiology. 1999;34(1):157–168. doi: 10.1046/j.1365-2958.1999.01589.x. [DOI] [PubMed] [Google Scholar]
- 32.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. Journal of Immunology. 2007;179(11):7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 33.Ames GF. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. Journal of Bacteriology. 1968;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon SO, Gho YS, Lee JC, Kim SI. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiology Letters. 2009;297(2):150–156. doi: 10.1111/j.1574-6968.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- 35.Venketaraman V, Lin AK, Le A, Kachlany SC, Connell ND, Kaplan JB. Both leukotoxin and poly-N-acetylglucosamine surface polysaccharide protect Aggregatibacter actinomycetemcomitans cells from macrophage killing. Microbial Pathogenesis. 2008;45(3):173–180. doi: 10.1016/j.micpath.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilm M, Shevchenko A, Houthaeve T, et al. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379(6564):466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd edition. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 38.Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O’Connor CD, Heckels JE. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infection and Immunity. 2007;75(3):1364–1372. doi: 10.1128/IAI.01424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappin DJC, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Current Biology. 1993;3(6):327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 40.Gotoh H, Kasaraneni N, Devineni N, Dallo SF, Weitao T. SOS involvement in stress-inducible biofilm formation. Biofouling. 2010;26(5):603–611. doi: 10.1080/08927014.2010.501895. [DOI] [PubMed] [Google Scholar]
- 41.Huisman O, D’Ari R, Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones C, Holland IB. Role of the SulB (FtsZ) protein in division inhibition during the SOS response in Escherichia coli: FtsZ stabilizes the inhibitor SulA in maxicells. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(18):6045–6049. doi: 10.1073/pnas.82.18.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutkenhaus JF. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. Journal of Bacteriology. 1983;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tashiro Y, Sakai R, Toyofuku M, et al. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. Journal of Bacteriology. 2009;191(24):7509–7519. doi: 10.1128/JB.00722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiology and Molecular Biology Reviews. 2010;74(1):81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng X, Xu C, Ren H, Lin X, Wu L, Wang S. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Pseudomonas aeruginosa responding to ampicilin, kanamycin, and tetracycline resistance. Journal of Proteome Research. 2005;4(6):2257–2265. doi: 10.1021/pr050159g. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Lin X, Ren H, Zhang Y, Wang S, Peng X. Analysis of outer membrane proteome of Escherichia coli related to resistance to ampicillin and tetracycline. Proteomics. 2006;6(2):462–473. doi: 10.1002/pmic.200500219. [DOI] [PubMed] [Google Scholar]
- 48.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. Journal of Bacteriology. 1995;177(14):3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nevot M, Deroncelé V, Messner P, Guinea J, Mercadé E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environmental Microbiology. 2006;8(9):1523–1533. doi: 10.1111/j.1462-2920.2006.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes and Infection. 2006;8(9-10):2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi DS, Kim DK, Choi SJ, et al. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics. 2011;11(16):3424–3429. doi: 10.1002/pmic.201000212. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari G, Garaguso I, Adu-Bobie J, et al. Outer membrane vesicles from group B Neisseria meningitidis Δgna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6(6):1856–1866. doi: 10.1002/pmic.200500164. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Lutkenhaus J. FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Molecular Microbiology. 1998;29(3):731–740. doi: 10.1046/j.1365-2958.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- 54.Imlay JA, Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. Journal of Bacteriology. 1987;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overhage J, Bains M, Brazas MD, Hancock REW. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. Journal of Bacteriology. 2008;190(8):2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. Journal of Bacteriology. 1993;175(22):7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cowell BA, Twining SS, Hobden JA, Kwong MSF, Fleiszig SMJ. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology. 2003;149(8):2291–2299. doi: 10.1099/mic.0.26280-0. [DOI] [PubMed] [Google Scholar]
- 58.Tang HB, Dimango E, Bryan R, et al. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infection and Immunity. 1996;64(1):37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Twining SS, Kirschner SE, Mahnke LA, Frank DW. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investigative Ophthalmology and Visual Science. 1993;34(9):2699–2712. [PubMed] [Google Scholar]
- 60.Zaborina O, Dhiman N, Chen ML, Kostal J, Holder IA, Chakrabarty AM. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology. 2000;146(10):2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- 61.McPhee JB, Tamber S, Bains M, et al. The major outer membrane protein OprG of Pseudomonas aeruginosa contributes to cytotoxicity and forms an anaerobically regulated, cation-selective channel. FEMS Microbiology Letters. 2009;296(2):241–247. doi: 10.1111/j.1574-6968.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- 62.Buist G, Steen A, Kok J, Kuipers OP. LysM, a widely distributed protein motif for binding to (peptido)glycans. Molecular Microbiology. 2008;68(4):838–847. doi: 10.1111/j.1365-2958.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- 63.Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in Gram-negative bacterial outer membrane vesicles. Mass Spectrometry Reviews. 2008;27(6):535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- 64.Davie JJ, Campagnari AA. Comparative proteomic analysis of the haemophilus ducreyi porin-deficient mutant 35000HP::P2AB. Journal of Bacteriology. 2009;191(7):2144–2152. doi: 10.1128/JB.01487-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frisk A, Ison CA, Lagergård T. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infection and Immunity. 1998;66(3):1252–1257. doi: 10.1128/iai.66.3.1252-1257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar G, Sharma P, Rathore G, Bisht D, Sengupta U. Proteomic analysis of outer membrane proteins of Edwardsiella tarda. Journal of Applied Microbiology. 2010;108(6):2214–2221. doi: 10.1111/j.1365-2672.2009.04627.x. [DOI] [PubMed] [Google Scholar]
- 67.Dallo SF, Zhang B, Denno J, et al. Association of Acinetobacter baumannii EF-Tu with cell surface, outer membrane vesicles and fibronectin doi: 10.1100/2012/128705. . In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hesterkamp T, Bukau B. The Escherichia coli trigger factor. FEBS Letters. 1996;389(1):32–34. doi: 10.1016/0014-5793(96)00582-0. [DOI] [PubMed] [Google Scholar]
- 69.Lyon WR, Gibson CM, Caparon MG. A role for Trigger factor and an Rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO Journal. 1998;17(21):6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vorderwülbecke S, Kramer G, Merz F, et al. Low temperature or GroEL/ES overproduction permits growth of Escherichia coli cells lacking trigger factor and DnaK. FEBS Letters. 2004;559(1–3):181–187. doi: 10.1016/S0014-5793(04)00052-3. [DOI] [PubMed] [Google Scholar]
- 71.Kandror O, Sherman M, Goldberg A. Rapid degradation of an abnormal protein in Escherichia coli proceeds through repeated cycles of association with GroEL. Journal of Biological Chemistry. 1999;274(53):37743–37749. doi: 10.1074/jbc.274.53.37743. [DOI] [PubMed] [Google Scholar]
- 72.Kandror O, Sherman M, Moerschell R, Goldberg AL. Trigger factor associates with GroEL in vivo and promotes its binding to certain polypeptides. Journal of Biological Chemistry. 1997;272(3):1730–1734. doi: 10.1074/jbc.272.3.1730. [DOI] [PubMed] [Google Scholar]
- 73.Kandror O, Sherman M, Rhode M, Goldberg AL. Trigger factor is involved in GroEL-dependent protein degradation in Escherichia coli and promotes binding of GroEL to unfolded proteins. EMBO Journal. 1995;14(23):6021–6027. doi: 10.1002/j.1460-2075.1995.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolling GL, Matthews KR. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Applied and Environmental Microbiology. 1999;65(5):1843–1848. doi: 10.1128/aem.65.5.1843-1848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Renelli M, Matias V, Lo RY, Beveridge TJ. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology. 2004;150(7):2161–2169. doi: 10.1099/mic.0.26841-0. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Clarke AJ, Beveridge TJ. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division, and secretion in surface membrane vesicles. Journal of Bacteriology. 1996;178(9):2479–2488. doi: 10.1128/jb.178.9.2479-2488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. Journal of Bacteriology. 2006;188(15):5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mug-Opstelten D, Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochimica et Biophysica Acta. 1978;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, Srisatjaluk R, Justus DE, Doyle RJ. On the origin of membrane vesicles in Gram-negative bacteria. FEMS Microbiology Letters. 1998;163(2):223–228. doi: 10.1111/j.1574-6968.1998.tb13049.x. [DOI] [PubMed] [Google Scholar]
- 80.Hill TM, Sharma B, Valjavec-Gratian M, Smith J. sfi-Independent filamentation in Escherichia coli is lexA dependent and requires DNA damage for induction. Journal of Bacteriology. 1997;179(6):1931–1939. doi: 10.1128/jb.179.6.1931-1939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trusca D, Scott S, Thompson C, Bramhill D. Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein. Journal of Bacteriology. 1998;180(15):3946–3953. doi: 10.1128/jb.180.15.3946-3953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cordell SC, Robinson EJH, Löwe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu TK, Collins JJ. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(12):4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]


