Abstract
Forkhead box P3 (FOXP3)+ regulatory T (TReg) cells prevent autoimmune disease, maintain immune homeostasis and modulate immune responses during infection. To accomplish these tasks, TReg cell activity is precisely controlled, and this requires TReg cells to alter their migratory, functional and homeostatic properties in response specific cues in the immune environment. We review progress in understanding the diversity of TReg cells, TReg cell function in different anatomical and inflammatory settings, and the influence of the immune environment on TReg cell activity. We also consider how these factors impact immune-mediated disease in the contexts of infection, autoimmunity, cancer and transplantation.
Forkhead box P3 (FOXP3)+ regulatory T (TReg) cells function to maintain immune tolerance and prevent inflammatory diseases1. This is best exemplified by the severe systemic autoimmunity and lymphoproliferative disease observed in TReg cell-deficient mice and humans carrying non-functional or hypomorphic alleles of the FOXP3 gene. The impaired function and/or homeostasis of TReg cells have also been implicated in development of several common autoimmune and inflammatory diseases, including type-1 diabetes, rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus2–5. In addition to preventing autoimmunity, TReg cells can also regulate immunity to infections of viral, bacterial or parasitic origin as well as restrain anti-tumor and anti-transplant immune responses6, 7. Thus, TReg cells must ‘walk the line’ by allowing protective anti-tumor and anti-pathogen immunity while preventing autoimmune disease by restraining aberrant responses to self or to innocuous antigens.
To function properly, TReg cells must modulate the activities of a wide variety of cellular components of both the innate and adaptive immune systems, and this depends on their ability to come into physical proximity with their targets by migrating to specific tissues and microenvironments. Additionally, it is critical that TReg cells elaborate an appropriate immunomodulatory mechanism that will effectively inhibit the targeted cell population. Recently, it has become clear that TReg cells can be divided into several distinct subsets displaying unique functional and homeostatic properties that work in concert to maintain normal immune homeostasis8. In this Review, we will summarize recent advances in our understanding of the relationship between TReg cell trafficking and function in lymphoid and non-lymphoid tissues, TReg cell specialization during different types of immune responses, and the impact of the cytokine milieu on the phenotype, function and stability of TReg cells.
Widespread distribution of TReg cells
TReg cells localize to lymphoid and non-lymphoid sites
The cells and tissues of the immune system are anatomically organized to facilitate cellular interactions required for the development, activation, function and regulation of diverse leukocyte populations 9. The organization of the immune system is the result of tissue- and microenvironment-specific lymphocyte homing, which in turn is mediated by lymphocyte expression of surface adhesion and chemoattractant receptors. Although TReg cells were initially identified in the secondary lymphoid tissues of mice and peripheral blood of humans, they express a dizzying array of adhesion molecules and chemoattractant receptors expected to target them to both lymphoid and non-lymphoid sites (Table 1). Indeed, in addition to their constitutive presence in secondary lymphoid tissues, TReg cells can be found in most non-lymphoid tissues, even in the absence of any overt inflammation10. Additionally, TReg cells can be found in abundance within tumors, where they are thought to help blunt effective tumor clearance7. In recent years, genetic studies have revealed the importance of several homing receptors for the appropriate tissue distribution and function of TReg cells. For instance, expression of the αE integrin chain (also known as CD103) and the chemokine receptor CC-chemokine receptor 4 (CCR4), as well as the ability to generate carbohydrate ligands for P- and E-selectin by the action of the α(1,4)-fucosyltransferase VII enzyme are all important for the migration and/or retention of TReg cells within the skin. Accordingly, deletion of any of these molecules on TReg cells results in development of skin-specific autoimmunity and altered pathogen clearance during cutaneous infection10–13. Similarly, loss of CCR7 blocks TReg cell migration to the lymph nodes, and inhibits TReg cell function in an experimental model of colitis14.
Table 1.
A summary of important homing receptors expressed by TReg cells and their functions.
| Site of TReg cell migration |
Receptor | Function | Key References |
|---|---|---|---|
| Lymphoid tissues | CD62L | Migration to lymph nodes | Function in TReg cell migration to lymph nodes112 Importance in NOD/SCID model of diabetes113 |
| CCR7 | Migration to lymph nodes and spleen | CCR7−/− TReg cells fail to prevent colitis14 Importance in TReg cell migration during allograft rejection18 |
|
| Non-lymphoid tissues | E-/P-selectin ligand | Migration to skin and other inflamed tissues | Expression by human TReg cells114 Function in cutaneous hypersensitivity response115 Importance in cutaneous tolerance11 |
| αEβ7 integrin | Epithelial localization | Expression by mouse TReg cells20 TReg cell retention during infection with Leishmania major 12 |
|
| α4β7 integrin | Migration to gut-associated lymphoid tissues | Function in TReg cell migration to the intestine116 | |
| CCR2 | Migration to inflamed tissues | Expression by human and mouse TReg cells19, 117 Importance in TReg cell migration during allograft rejection18 |
|
| CCR4 | Migration to skin and other inflamed tissues | Expression by human TReg cells114 Importance in cutaneous/pulmonary tolerance10 CCR4−/− TReg cells fail to prevent colitis118 |
|
| CCR5 | Migration to inflamed tissues | Importance in TReg cell migration during allograft rejection18 Function in migration to the inflamed intestine119 Directing TReg cell localization infection with Leishmania major120 |
|
| CCR6 | Migration to sites of TH17-mediated inflammation | Expression by IL-10-producing TReg cells121 Function in TReg cell migration during Th17-mediated autoimmunity15, 122 |
|
| CCR8 | Migration to skin and sites of Th2-mediated inflammation | Expression by TReg cells123 | |
| CCR9 | Migration to small intestine | Expression by TReg cells in the intestinal lamina propria124 | |
| CCR10 | Migration to mucosal tissues and skin | Expression by TReg cells in inflamed human liver125 | |
| CXCR3 | Migration to sites of TH1-mediated inflammation | Expression by TReg cells is T-bet dependent47 Importance in TReg cell localization to inflamed liver16, 126 |
|
| CXCR5 | Migration to B cell follicles and germinal centers | Expression by human TReg cells127 Inhibition of B cell responses by TReg cells128 |
|
| CXCR6 | Migration to the liver | Expression by human TReg cells127 | |
| Both lymphoid and non-lymphoid tissues | CXCR4 | Migration to bone marrow, Peyer’s patches and tumour sites | Expression by naïve phenotype TReg cells127 Association with tumor infiltrating TReg cells129, 130 |
In addition to their constitutive recirculation, TReg cell recruitment to non-lymphoid tissues is substantially enhanced during inflammation. However, the contributions of individual homing receptors to this ‘inflammation-induced’ TReg cell migration vary considerably depending on the tissue involved and the type of inflammatory response. For instance, interleukin-17 (IL-17) produced during T helper 17 (TH17) cell-mediated inflammation promotes epithelial cell expression of the CCR6 ligand, CC-chemokine 20 (CCL20), and CCR6 is essential for the optimal recruitment of TReg cells to sites of TH17-mediated inflammation during experimental autoimmune encephalomyelitis (EAE)15. Similarly, interferon-γ (IFNγ) induces expression of the CXC-chemokine receptor 3 (CXCR3) ligands CXC-chemokine ligand 9 (CXCL9), CXCL10 and CXCL11 and the subsequent recruitment of CXCR3+ TReg cells to the liver during conA-induced hepatitis16. Additionally, CXCR3 may influence the microenvironmental positioning of TReg cells in the central nervous system during EAE17. In this fashion, recruitment of CCR6+ and CXCR3+ TReg cells may act downstream of the key effector cytokines IL-17 and IFNγ in a feedback loop to limit TH17- and TH1-induced inflammatory responses.
In addition to CCR6 and CXCR3, many other homing receptors have been implicated in inflammatory recruitment of TReg cells in different immunological settings, including CCR1, CCR2, CCR4, CCR5, CCR8, CCR9, CXCR4, CXCR5, CXCR6, α4β1 integrin (also known as VLA4), αEβ7 integrin, α4β7 integrin and the P- and E-selectin ligands. Indeed, TReg cells most likely use a combination of homing molecules that can function redundantly to control their migration during inflammatory responses. For example, in an islet allograft model, TReg cells used CCR2, CCR5, CCR4 and P- and E-selectin ligands to migrate to the transplant site, whereas CCR2, CCR5 and CCR7 were required for trafficking from the inflamed allograft to the draining lymph node18. Interestingly, this sequential migration of TReg cells from the graft site to the draining lymph node was required for prevention of graft rejection, highlighting the complex and dynamic nature of TReg cell migration during inflammation. Thus, therapeutically targeting TReg cell migration in the contexts of autoimmunity, transplantation, chronic infection and cancer remains a daunting task that will require significantly more study in both animal models and patient populations.
Differences in TReg cells in lymphoid vs. non-lymphoid tissues
Although TReg cells can be found throughout the body, there is substantial phenotypic and functional variation between TReg cells in lymphoid and non-lymphoid tissues. Developing TReg cells in the thymus are a relatively homogenous population of CD25hiCD62L+CCR7+ cells that resemble conventional naive T cells and preferentially migrate to secondary lymphoid tissues. However, upon entering the periphery, a subset of TReg cells rapidly acquires phenotypic features of effector or memory T cells, becoming CD44hi and upregulating expression of homing receptors that allow them to access non-lymphoid sites19, 20. Moreover, similar to conventional naive T cells and effector and memory T cells, CD44lo and CD44hi TReg cell subsets have distinct homeostatic characteristics, with CD44hi TReg cells proliferating at a significantly higher rate in the steady-state21. This suggests that there is a ‘division of labor’ between distinct TReg cell subsets specialized for functioning in lymphoid or in non-lymphoid tissues. In addition, the phenotype of the CD44hi effector/ memory-like TReg cell population indicates that it arises as a result of TReg cell activation, presumably due to recognition of self-antigens in secondary lymphoid tissues (Box 1).
BOX 1: Influence of the T cell receptor on TReg cell phenotype and function.
Thymically-derived regulatory T (TReg) cells are thought to be largely autoreactive106. However, the identity of the natural self-antigens thought to drive TReg cell development is still completely unknown. Despite the fact that TReg cell specificity is still poorly characterized, there is evidence that T cell receptor (TCR) recognition has a key role in influencing the phenotype, function and localization of TReg cells in vivo. For instance, activation of antigen-specific TReg cells isolated from TCR transgenic mice alters their expression of several lymphocyte homing receptors, resulting in their subsequent redistribution to non-lymphoid tissues10, 19. Moreover, TReg cells isolated from different tissues have distinct TCR repertoires, indicating that TCR-driven activation helps impart cells with tropism for specific sets of lymphoid and non-lymphoid tissues107. This likely occurs as TReg cells specific for tissue-restricted self-antigens encounter signals during their activation that drive expression of tissue-specific homing receptors62. Although together these data suggest a key role for TCR recognition of specific self-antigens in driving the phenotypic diversity present among TReg cells, a better understanding of the self-antigens recognized by TReg cells is necessary to clarify precisely how their specificity impacts their localization, phenotype, homeostasis and function.
Although the functional mechanisms used by TReg cells are complex and still incompletely understood, there is increasing evidence that TReg cells use different mechanisms to regulate immune responses in lymphoid and in non-lymphoid tissues. This concept is best exemplified by the distinct phenotypes in mice lacking expression of either IL-10 or cytotoxic T lymphocyte antigen 4 (CTLA4) selectively in TReg cells (Figure 1). IL-10 is a cytokine that is produced by CD44hi TReg cells and can both directly and indirectly inhibit effector T cell responses during infection, autoimmunity and cancer22–24. Although it is also expressed by other leukocyte populations, deletion of IL-10 selectively in TReg cells resulted in the development of spontaneous colitis, and exaggerated immune responses at other environmental interfaces such as the skin and lungs25. However, these animals did not develop the systemic autoimmunity and dysregulated T cell activation profiles characteristic of mice with a complete loss of TReg cell function. By contrast, loss of CTLA4 expression in TReg cells resulted in severe lymphoproliferative disease characterized by massive lymphadenopathy and splenomegaly associated with accumulation of CD4+CD44hi effector T cells, spontaneous multi-organ autoimmunity, and early death26.
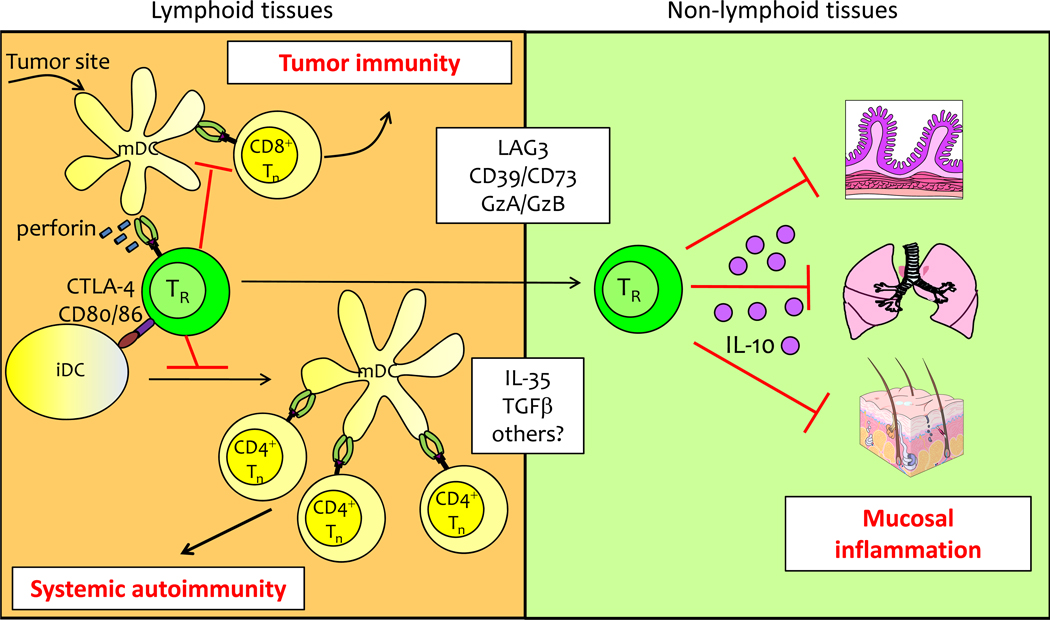
Figure 1. Differing immunosuppressive mechanisms used by TReg cells in lymphoid vs. non-lymphoid tissues.
(Left) TReg cells in secondary lymphoid tissues use multiple mechanisms to inhibit DC function and block initiation of autoimmunity or prevent tumor clearance. (Right) TReg cell production of IL-10 is essential for immunoregulation at mucosal tissues such as the intestines, lungs and skin. The relative importance of other immunosuppressive mechanisms used by TReg cells (shown in white box) in lymphoid vs. non-lymphoid tissues remains to be established
Immunoregulation by TReg cell-expressed CTLA4 is due, at least in part, to the ability of CTLA4 to render dendritic cells (DCs) in lymphoid tissues less immunostimulatory by downregulating their surface expression of the co-stimulatory ligands CD80 and CD8626. Additionally, CTLA4 ligation of CD80 and CD86 on DCs can induce expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO)27, 28. Together, these activities can raise the threshold required for T cell activation, thereby preventing the priming of autoreactive T cells. Indeed, time-lapse imaging studies have revealed that TReg cells form long-lasting, stable contacts with DCs, and this has led to the hypothesis that a primary mode of TReg cell-mediated suppression within lymphoid tissues may be through inhibition of DC activation and/or function29, 30. TReg cells can also induce perforin-dependent cytolysis of DCs in tumor draining lymph nodes31. Thus, TReg cells use multiple mechanisms to limit DC activity in secondary lymphoid tissues, thereby quelling effector T cell activation and promoting functional tolerance. Accordingly, acute depletion of TReg cells in mice leads to the rapid development of systemic autoimmunity, associated with increases in both the number and activation state of DCs in lymphoid tissues32. The importance of TReg cell function in secondary lymphoid tissues extends beyond their integral role in preventing autoimmunity. For instance, depletion of TReg cells following intravaginal infection with herpes simplex virus 2 (HSV2) accentuated T cell priming and proliferation in the draining lymph node, but prevented effector T cell mobilization from the lymph node to the vaginal epithelium, resulting in uncontrolled viral replication and death. These results highlight a previously unappreciated function of TReg cells in down-modulating immune responses in the lymphoid tissues to allow for efficient effector T cell migration to sites of infection33.
The spontaneous phenotypes of mice lacking either IL-10 or CTLA-4 in TReg cells demonstrate that these molecules are essential for proper TReg cell function in vivo. However, numerous other mechanisms have been implicated in TReg cell function in both lymphoid and non-lymphoid tissues in various experimental settings34. These include production of additional immunosuppressive cytokines such as transforming growth factorβ (TGFβ) and IL-35, along with metabolic inhibition of effector T cells through adenosine or cyclic AMP. Thus, TReg cells use a variety of immunosuppressive mechanisms to modulate both the initiation of the immune response in secondary lymphoid tissues, and the progression and termination of inflammatory responses at non-lymphoid sites, and the suppressive module used by TReg cells appears to be a function of both the tissue site and character of the inflammatory response. Indeed, it has been difficult to determine the importance of specific immunoregulatory mechanisms used by TReg cells, indicating that although TReg cells are clearly essential for establishing and maintaining tolerance, substantial functional redundancy may exist in the means they employ to do so. Further unraveling the complex relationship between TReg cell localization and function will likely yield important new insights into the functional diversity of TReg cells, and in understanding how specific immunomodulatory functions are delivered to different tissues during the course of the immune response.
TReg cell control of distinct immune responses
Functional specialization of TReg cells
CD4+ effector T cells can adopt one of several functional fates and elaborate distinct effector mechanisms depending on the cytokines present during their initial activation35. Production of these polarizing cytokines is dictated by the type of pathogen encountered, such that TGFβ and IL-6 direct the development of IL-17-producing TH17 cells during extracellular bacterial or fungal infection, IFNγ and IL-12 drive the differentiation of IFNγ-producing TH1 cells that help combat intracellular pathogens, and IL-4 induces IL-4-producing TH2 cells during infection with large mucosal parasites36–38. The functional specialization of these various CD4+ T cell subsets is due to the differential expression of ‘master’ transcription factors, namely retinoic acid receptor-related orphan receptor-γt (RORγt), T-bet and GATA-binding protein 3 (GATA3), which turn on distinct programs of gene expression controlling T cell function and migration39–41. However, each of these responses is pro-inflammatory and potentially harmful to host tissues. Accordingly, aberrant TH1, TH2 and TH17 responses can all contribute to immunopathology in the contexts of infection, autoimmunity, allergy and other inflammatory conditions42, 43. Therefore, these distinct immune responses must be carefully regulated to ensure that they are initiated only when appropriate, and efficiently resolved upon pathogen eradication, or when the burden of tissue destruction outweighs the benefit of pathogen control. Indeed, defects in TReg cell function can result in TH1-, TH2- or TH17-mediated inflammatory disease, indicating that these cells are required for the proper regulation of multiple types of immune responses.
Several recent studies have demonstrated that TReg cells use canonical TH cell-associated transcription factors in order to maintain or restore immune homeostasis during polarized TH1-, TH2- and TH17-driven immune responses. For instance, in addition to controlling the differentiation, migration and function of IFNγ-producing TH1 cells, T-bet influences the generation of effector and memory CD8+ T cells, and regulates the homeostasis and function of natural killer (NK) cells, thereby coordinating the cellular immune response to intracellular infection.40, 44–46. Interestingly, T-bet is also expressed by a subset of TReg cells, and is required for TReg cell homeostasis and function during polarized TH1 inflammatory responses47. T-bet+ TReg cells accumulate at sites of TH1-type inflammation, and T-bet deficient- TReg cells display impaired proliferation during TH1-mediated immune responses, ultimately failing to control expansion of IFNγ-producing TH1 cells when transferred into FOXP3-deficient scurfy mice.
Similarly, TReg cell expression of interferon regulatory factor 4 (IRF4), a transcription factor involved in control of IL-4 production by CD4+ T cells and TH2 differentiation, is required for TReg cell mediated-control of TH2-type inflammatory responses48. Mice in which IRF4 is specifically deleted within FOXP3+ TReg cells develop a lymphoproliferative disease associated with a selective increase in the number and frequency of IL-4- and IL-5-producing CD4+ T cells and have elevated serum levels of IgG1 and IgE. Consistent with this increase in serum antibodies, these animals also contain dramatically increased numbers of plasma cells and spontaneously develop splenic germinal centers. Interestingly, IRF4 is also critical for the differentiation of T follicular helper cells (TFH), which provide help to B cells and help regulate antibody production in vivo49. Coupled with the dearth of eosinophilia and IL-13-producing CD4+ T cells, the profound increase in germinal center formation in these animals implies that TReg cell expression of IRF4 may be required to control a specific component of TH2-associated inflammation, namely aberrant TFH activation and high affinity antibody production. Finally, deletion of the transcription factor signal transducer and activator of transcription (STAT3) in TReg cells results in development of spontaneous fatal intestinal inflammation triggered by excessive IL-17 production without significant differences in TH1- or TH2-associated inflammatory cytokines, indicating a selective dysregulation of TH17 responses by STAT3-deficient TReg cells50.
The mechanisms by which T-bet, IRF4 and STAT3 control TReg cell activity during TH1, TH2 and TH17 responses are still unclear, but likely involve a combination of influences on TReg cell migration, function and homeostasis. For instance, TReg cells deficient in T-bet, IRF4 or STAT3 display impaired expression of chemokine receptors implicated in TReg cell localization during TH1- (CXCR3), TH2- (CCR8) or TH17- (CCR6) mediated inflammation, suggesting that altered TReg cell migration may therefore underlie some of the functional defects in these cells47, 48, 50. Additionally, loss of these transcription factors may impact the functional properties of TReg cells. Of note, TReg cells lacking T-bet, IRF4 or STAT3 all show reduced expression of Il1047, 48, 50. Moreover, IRF4 and STAT3-deficient TReg cells have reduced expression of other genes associated with TReg cell function such as Icos, Fgl2, Ebi3 (which encodes a component of the immunosuppressive cytokine IL-35), Prf1 and Gzb48, 50. By contrast, expression of Ctla4 is substantially increased in STAT3-deficient TReg cells, while Tgfb1 expression was unaltered in TReg cells lacking any of the aforementioned transcription factors. Finally, loss of T-bet expression resulted in the impaired proliferation and accumulation of TReg cells during TH1-type inflammatory responses and the failure of T-bet-deficient TReg cells to control TH1-driven inflammatory responses may in part be secondary to the inability of these cells to survive and proliferate in a highly polarized TH1-type environment47. From these data, a model emerges whereby selective expression or activation of transcriptional regulators associated with specific TH1, TH2 and TH17 cells drives the phenotypic and functional specialization of TReg cells, endowing them with the molecular machinery needed to restrain these different types of CD4+ T cell responses (Figure 2). This model has important implications for use of TReg cells therapeutically, as it implies that only specific subsets of TReg cells will be efficacious in treating TH-1, TH2- or TH17-driven inflammatory diseases. Thus, it will be critical in future studies to identify and characterize the cellular and molecular mechanisms that underlie the functional specialization of TReg cells, and to develop methods for selectively isolating and expanding different subsets of TReg cells.
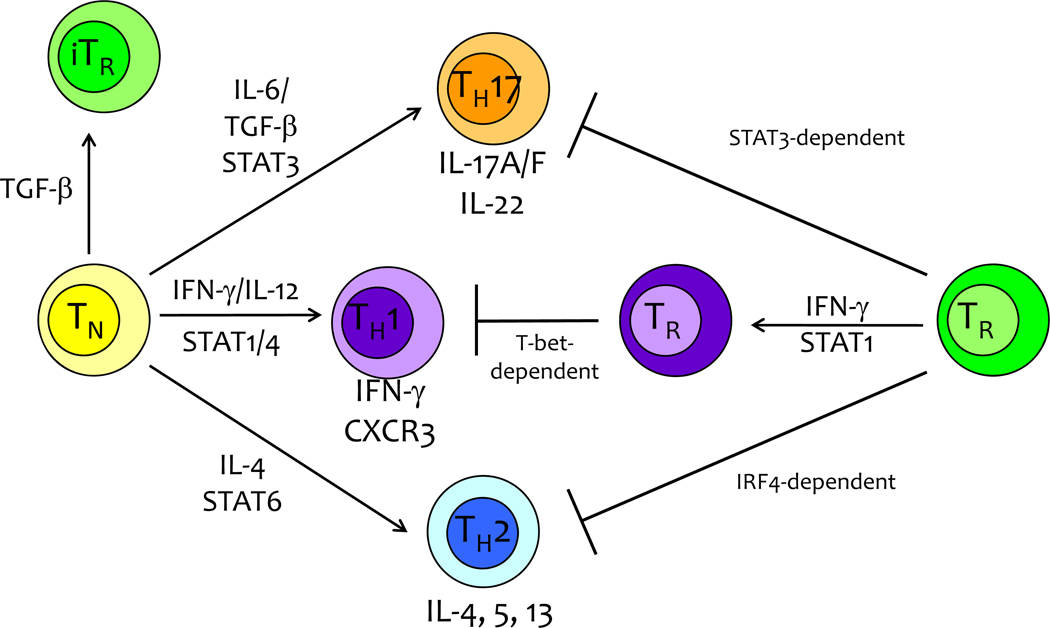
Figure 2. Functional Differentiation of Treg cells and Tconv cells.
The differentiation of naive TH cells into functionally distinct effector subsets (TH17, TH1, TH2, induced TR) is dependent on the induction of key transcriptional regulators (RORγT, T-bet, GATA3, Foxp3) following TCR stimulation in conjunction with cytokine signaling/STAT activation. Comparably, thymic-derived Treg cells utilize specific molecular programs driven by STAT3, T-bet, or IRF4 to restrain particular types of immune responses orchestrated by distinct effector T cell subsets.
Environmental control of TReg cells
The need to carefully modulate both the number and activity of TReg cells to maintain the balance between autoimmunity and immunosuppression suggests that TReg cells pay close attention to the immune environment, and alter their phenotype, migration and function in response to specific cues encountered in the periphery. Cytokines are a diverse group of generally secreted proteins that control nearly all aspects of leukocyte biology. They do so by binding to specific receptors on the surface of cells which transmit signals promoting cellular proliferation, activation, differentiation and/or death. Broadly speaking, cytokines can be divided into those that are constitutively expressed and promote normal lymphocyte development and homeostasis, and those whose expression is induced by specific inflammatory stimuli. Decades of research have demonstrated that both homeostatic and inflammatory cytokines have a profound influence on the phenotypic and functional differentiation of the various T cell populations. In addition to cytokines, non-protein small molecules such as steroids, sphingolipids and metabolites of vitamins A and D can also influence T cell differentiation, migration and function51–54. In the following sections, we will discuss recent progress in understanding how TReg cells respond to the cytokines, vitamin metabolites and other factors present in the immune environment, and how this controls their activity in health and disease (Figure 3).
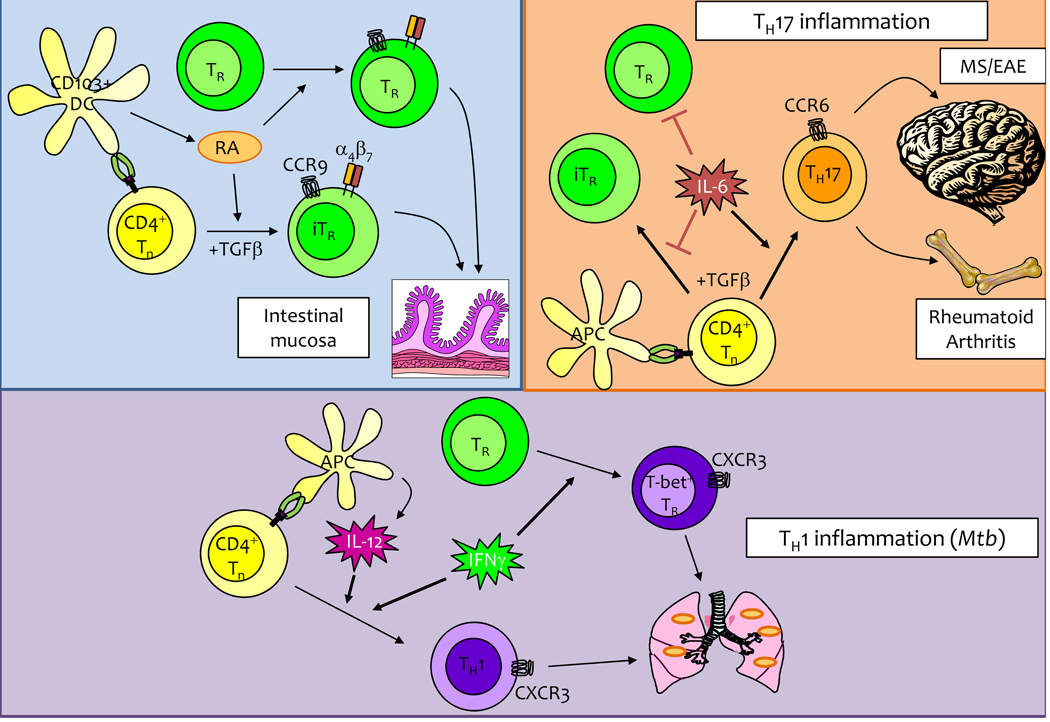
Figure 3. Modulation of TReg cell activity can be by different factors in the immune environment.
(Left) The vitamin-A metabolite retinoic acid (RA) can boost TReg cell activity within the intestine by inducing Foxp3 expression in naïve T cells and directing the expression of gut-homing receptors on both TReg and iTReg cells. (Right) During TH17-driven inflammation, IL-6 impairs TReg cell activity both by directly blocking TReg function as well as by promoting TH17 differentiation at the expense of iTReg generation. (Middle) During type-1 inflammatory responses, such as Mtb infection, IFNγ and IL-12 direct TH1 differentiation. Coordinately, IFNγ produced in response to infection directs the differentiation of T-bet+ TReg cells that are specialized to restrain pro-inflammatory TH1 cells.
Control of TReg cell homeostasis
Common-γ chain-signalling cytokines
By far, the best studied cytokine in terms of its impact on the development, homeostasis and function of TReg cells is IL-255. Indeed, TReg cells were initially identified based on their constitutive expression of the high-affinity IL-2 receptor component CD25. Moreover, it is clear that IL-2 has an essential and non-redundant role in controlling TReg cell function in the periphery, as evidenced by the lymphoproliferative disease and colitis that develop in either IL-2- or CD25-deficient mice. However, the precise manner by which IL-2 influences TReg cell function is still largely unknown. Several studies have demonstrated that TReg cells occupy a distinct homeostatic ‘niche’, and this is thought in part to be controlled by access to IL-256, 57. Accordingly, blocking IL-2 activity through the administration of IL-2-specific antibodies to mice decreased proliferation of CD4+CD25+ T cells, and impaired TReg cell activity58. These data led to a model in which IL-2 produced by activated CD4+FOXP3− T cells acts in a paracrine fashion to promote TReg cell proliferation and survival. However, both the frequency and absolute number of peripheral TReg cells are largely normal in mice lacking either IL-2 or CD25, indicating that the homeostasis of TReg cells is more complicated than previously appreciated59, 60 (Box 2). In addition, expression of CD25 varies substantially among different TReg cell populations, with activated CD44hi TReg cells generally being CD25low/−21. Interestingly, nearly all TReg cells in IL-2- and CD25-deficient mice belong to the CD44hi subset, and have increased expression of other activation markers such as CD69, CD103 and inducible T cell co-stimulator (ICOS)57. Although the activated phenotype of TReg cells in these mice is thought to be secondary to their inflammatory disease, an alternate interpretation of these results is that the critical function of IL-2 is to help sustain the CD44loCD25hi TReg cell subset, and that this population has a non-redundant function in preventing lymphoproliferative and autoimmune disease.
BOX 2: TReg cell homeostasis – beyond IL-2.
Lymphocyte homeostasis is the process by which various T and B cell populations are maintained at near constant frequencies in the periphery due to their balanced generation, proliferation and death. The major cytokine thought to control TReg cell homeostasis is interleukin-2 (IL-2). However, many other factors have been identified that influence TReg cell homeostasis. For instance, TReg cell expression of the co-stimulatory molecule CD28 is required for their peripheral maintenance, and TReg cell numbers are substantially reduced in CD28-deficient mice108. Accordingly, these animals show enhanced susceptibility to autoimmune diabetes109. Given that TReg cells form long-lasting contacts with DCs in the secondary lymphoid tissues, it is also not surprising that TReg cell proliferation and abundance are highly sensitive to changes in DC frequency110. This is likely due to the ability of DCs to present self-antigens to TReg cells, and to provide CD28-dependent co-stimulatory signals via CD80 and CD86 that can drive TReg cell activation and proliferation. Another molecule that influences TReg cell homeostasis is sphingosine 1-phosphate (S1P). S1P is best known for promoting lymphocyte egress form the thymus, spleen and LNs. However, TReg cells overexpressing the S1P receptor, S1P1, have a competitive disadvantage in the periphery, suggesting that S1P can also modulate the proliferation and/or survival of TReg cells via activation of the AKT–mTOR (mammalian target of rapamycin) pathway51. Other molecules implicated in TReg cell homeostasis include CD44, which can promote TReg cell proliferation upon binding to high molecular weight hyaluronan in the extracellular matrix111, and CC-chemokine receptor 4 (CCR4), which is required for the proper homeostasis of CD103hi cutaneous TReg cells10. Thus, TReg cell homeostasis is far more complex than currently appreciated, and much more work needs to be done to understand how TReg cell abundance is controlled in various tissues, both in the steady-state and during the course of different types of immune responses.
The abundance of functional CD44hi TReg cells in IL-2- and CD25-deficient indicates that either the maintenance of these cells does not normally depend on IL-2, or that other cytokines can compensate for loss of IL-2 in driving the homeostasis of this population. Like IL-2, IL-15 uses the common-γ chain and CD122 as the signalling components of its receptor. As such, they are thought to deliver very similar signals into cells, and thus can have redundant functions. For instance, thymic development of TReg cells proceeds normally in mice lacking IL-2, IL-15, CD25 or IL15Rα, but is severely disrupted in either CD122-deficient or IL-2/IL-15 double-deficient mice59, 60. In the periphery, IL-15 is ‘trans-presented’ in association with IL15Rα on the surface of DCs, where it supports the homeostasis of CD8+ memory T cells and NK cells61. As previously discussed, TReg cells functionally interact with DCs in lymphoid tissues29, 30, and this may grant them access to trans-presented IL-15 that could help support their peripheral survival and function. However, the precise role of IL-15 in the IL-2-independent peripheral homeostasis of different TReg cell populations has not been explored.
Vitamin A and D metabolites
At barrier tissues such as the intestine and skin, the immune system faces the daunting task of ignoring the large number of commensal organisms and harmless environmental antigens while responding vigorously to enteric and cutaneous pathogens. Recent studies have demonstrated that metabolites of vitamins A and D can help control this balance in by influencing the development and homeostasis of cutaneous and intestinal TReg cells. For example, retinoic acid produced from dietary vitamin A induces TReg cell expression of the intestinal homing receptors α4β7 integrin and CCR962. Additionally, retinoic acid promotes the peripheral differentiation of inducible TReg cells, and helps sustain TReg cell numbers and function during inflammatory responses63–66. Among DCs, the enzymes that convert vitamin A into retinoic acid are most prominently expressed in a population of CD103+ DCs found in the intestine and the gut-associated lymphoid tissues64, 65. Thus, under steady state conditions, retinoic acid produced by these DCs is thought to influence the balance of effector and regulatory T cells in the intestine, and help promote tolerance to commensal bacteria and food antigens.
Although retinoic acid is primarily thought to promote TReg cell activity in the intestine, the active form of vitamin D, 1,25-Dihydroxyvitamin D3, can have similar effects in the skin, where vitamin D is produced in response to sunlight. Like retinoic acid, 1,25-Dihydroxyvitamin D3 can both augment the function of existing TReg cells and promote de novo differentiation of TReg cells from naive CD4+ T cell precursors54, 67. Moreover, 1,25-Dihydroxyvitamin D3 produced by cutaneous DCs can induce T cell expression of CCR10, a chemokine receptor implicated in T cell localization to the epidermis68, although this has not been formally demonstrated in TReg cells. Nonetheless, these data raise the intriguing possibility that anatomical cues delivered by metabolites enriched in the skin vs. intestine can drive TReg cell specialization, endowing them with the migratory and functional properties needed for immunoregulation in these tissues.
Inflammatory cytokines and TReg cells
During infection, cytokines produced in response to pathogen recognition by cells of the innate immune system initiate the inflammatory response, which is subsequently amplified by products of the adaptive immune response. These inflammatory cytokines have broad effects on the phenotypes, functions and migration of T cells and other leukocyte populations, and ultimately dictate the course of the pathogen eradication. Additionally, dysregulated production of inflammatory cytokines underlies the pathogenesis of most autoimmune and inflammatory diseases. Because of their central function in driving inflammatory responses, it is not surprising that many of these cytokines can also act directly on TReg cells, influencing their phenotype and activity in complex ways such that vigorous immune responses are allowed to occur when necessary, but generally with the restraint needed to prevent collateral damage and immunopathology. Indeed, this complexity is only beginning to be appreciated and addressed experimentally, and although not exhaustive, the following discussion highlights the fact that many cytokines can have both positive and negative effects of TReg cell activity. Thus, careful analyses are still required to determine how TReg cell activity is augmented and inhibited by various inflammatory cytokines during different types of inflammatory responses, and this promises to be a fruitful area of future study that has substantial implications for the development of therapies aimed at manipulating TReg cell activity.
IFN-γ and IL-12
IFNγ and IL-12 function together to promote TH1 cell differentiation and function69. Additionally, IFNγ is the principle effector cytokine produced by TH1 cells, NK cells and CD8+ T cells, and it is required for clearance of intracellular pathogens such as Mycobacterium tuberculosis, Leishmania major and Listeria monocytogenes70–72. Like many inflammatory cytokines, IFNγ actively inhibits the peripheral generation of FOXP3+ TReg cells from naive CD4+ cells73. However, IFNγ signalling via STAT1 activation also drives T-bet expression by thymus-derived TReg cells, and as discussed in the preceding section, this endows them with the molecular machinery required for efficient control of TH1-type responses47. Thus, in addition to being a pro-inflammatory cytokine essential for combating intracellular pathogens, IFNγ also has an immunoregulatory function that may help limit the magnitude and duration of TH1-type inflammatory responses. However, although IFNγ-induced signalling within TReg cells may be beneficial for the differentiation of functionally specialized T-bet+ TReg cells, excessive STAT1 activation can have a deleterious effect on TReg cell function, resulting in excessive TH1 cell activity and inflammatory disease74. Similarly, a recent study demonstrated that when IL-2 availability is limited during oral infection with Toxoplasma gondii, TReg cells become IL-12 responsive, express high amounts of T-bet and acquire the ability to produce IFNγ, subsequently contributing to the fatal intestinal immunopathology that develops during infection75. Thus, IFNγ and IL-12 can either promote or inhibit TReg cell activity depending on the magnitude of the cytokine response and the context in which it is perceived. These studies emphasize the need to precisely regulate TReg cell responses to these cytokines during TH1-mediated inflammation, and underscore the complex and confounding impact cytokines can have on TReg cell activity in vivo.
IL-6
IL-6 is a widely expressed cytokine with multiple functions that can have a profound influence on TReg cell development and activity76, 77. For instance, IL-6 potently prevents the TGFβ-mediated development of inducible TReg cells, and instead acts with TGFβ to induce TH17 cell differentiation78. Thus, the presence or absence of IL-6 can regulate the induction of proinflammatory and tolerogenic T cell responses, respectively. Moreover, in addition to controlling the development of inducible TReg cells, IL-6 can also influence the stability and function of thymus-derived TReg cells. For instance, stimulation of TReg cells in the presence of IL-6 results in loss of FOXP3 expression and acquisition of a Th17 cell phenotype and function79, 80. Moreover, IL-6 produced downstream of Toll-like receptor ligation blocks TReg cell-mediated inhibition of T cell activation, and this is likely due to both direct effects of IL-6 on TReg cells, as well as the ability of IL-6 to render effector T cells resistant to TReg cell-mediated suppression81.
Although these studies emphasize the ability of IL-6 to inhibit TReg cell activity, the importance of STAT3 for TReg cell-mediated control of Th17 responses raises the intriguing question; what are the important STAT3-activating cytokines that act on TReg cells? IL-6 is a potent activator of STAT3, and thus it is tempting to speculate that as with IFNγ and STAT1 during TH1 cell responses, IL-6 simultaneously promotes TH17 cell differentiation while acting on TReg cells via STAT3 to maximize their ability to modulate TH17 responses. However, several other cytokines also activate STAT3, including IL-10, IL-27 and IL-21, and the impact of these cytokines on TReg cell activity during Th17 responses remains to be determined. Nonetheless, it is clear that the presence or absence of IL-6 can modulate TReg cell activity during inflammatory responses. As drugs targeting IL-6 reach the clinic82, it will be interesting to determine how IL-6 blockade impacts the abundance, phenotype and functional activity of TReg cells in the context of immune-mediated diseases such as rheumatoid arthritis and inflammatory bowel disease.
IL-4
IL-4 is a key effector cytokines produced by Th2 cells, and is also required for Th2 cell differentiation. However, although the effects of IL-4 on TH2 cell differentiation and function are well characterized, its impact on TReg cell stability and function are not well understood. Indeed, like many other inflammatory cytokines, IL-4 can both augment and inhibit TReg cell development and function in different experimental settings. For instance, similar to IL-6, IL-4 can inhibit TGFβ-induced peripheral TReg cell development, and act with TGFβ to drive development of a recently described population of IL-9-producing effector T cells83, 84. Additionally, IL-4 is thought to render Th2 cells resistant to TReg cell-mediated suppression85. By contrast, IL-4 stimulation of TReg cells can boost their expression of CD25 and FOXP3, prevent their apoptosis and increase their suppressive function in vitro85, 86. Thus, the effects of IL-4 on TReg cells clearly require further investigation, and likely depend on other factors such as the presence or absence of TGFβ in the inflammatory environment.
Type-1 IFNs
The type-1 IFNs are a group of more than a dozen closely related cytokines that are highly upregulated during viral infection and function to block viral replication and to qualitatively and quantitatively influence the anti-viral adaptive immune response. However, overproduction of type-1 IFN has been associated with a variety of autoimmune disorders87. Surprisingly, despite the dramatic effects type-1 IFNs can have on conventional CD4+ and CD8+ effector T cells, and the clear association between type-1 IFN production and development of autoimmunity, the effects of type-1 IFNs on the development, homeostasis and function of TReg cells are largely unexplored. Several studies have indicated that IFNβ treatment can help restore both the number and function of TReg cells in patients with multiple sclerosis88, 89. By contrast, stimulation with IFNα inhibited TReg cell generation in an in vitro culture system, though this was due largely to inhibition of IL-2 production by effector T cells90. Clearly, more careful studies are needed to determine how type-1 IFNs impact TReg cell homeostasis and function, and how this in turn modulates TReg cell activity during acute and chronic viral infection, and in type-1 IFN-associated autoimmune diseases.
TNF and IL-1
Tumour necrosis factor (TNF) and IL-1 are both pleiotropic cytokines that act on a wide range of cells and generally promote inflammation. Indeed, both TNFα and IL-1 have been successfully targeted therapeutically for the treatment of a number of inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriasis and inflammatory bowel disease91. It is not surprising therefore that these cytokines can significantly impact TReg cell function. Although TNFα is primarily known for its proinflammatory functions, most current evidence indicates that upon binding and signaling through TNFR2, this cytokine actually potentiates TReg cell activity92. Indeed, TNFR2 is expressed by a subset of effector or memory phenotype TReg cells that are highly suppressive, and in vitro treatment of mouse TReg cells with TNFα can augment their proliferation and suppressive activity93. Furthermore, blockade of TNFα actually exacerbates cutaneous inflammation in a mouse model of psoriasis, and this was associated with decreased expansion of populations of TReg cells94. Simialry, TNFα–TNFR2 interactions may control TReg cell population expansion during cecal ligation and puncture (CLP) in mice93, although the requirement for direct recognition of TNFα by TReg cells was not addressed in either the psoriasis or CLP studies. The impact of IL-1 on TReg cell function is still poorly understood. Although IL-1 could potentiate expansion of FOXP3+ cells from cultures of CD4+CD25+ cells, this was due to the effects of IL-1 stimulation of FOXP3− cells, and IL-1 did not directly augment proliferation of FOXP3+ cells95. In fact, in conjunction with IL-2, IL-1 acts to convert TReg cells into FOXP3− TH17 cells96.
Stability of Treg cells
The ability of cytokines such as IL-1, IL-6 and IL-12 to downregulate FOXP3 expression and convert TReg cells into proinflammatory effector cells suggests that TReg cells retain some functional plasticity. Indeed, a subject of recent controversy is the extent to which TReg cells can extinguish forkhead box P3 (FOXP3) expression and convert to conventional FOXP3− effector T cells. TReg cells induced by TGF-β in the periphery show incomplete demethylation of the Foxp3 locus that is associated with unstable Foxp3 expression97. However, several studies have demonstrated that even thymic-derived TReg cells can convert to an effector phenotype following transfer into lymphopenic recipients98–100, and during ex vivo stimulation with inflammatory cytokines79, 80, 96.
To determine if a portion of TReg cells converts to an effector phenotype in lymphoreplete mice, two groups recently generated mice engineered to track the fate of FOXP3+ cells in vivo. In these systems, mice expressing the cre recombinase in TReg cells under control of regulatory elements from the Foxp3 gene were crossed to mice in which cre-mediated recombination removes a stop codon in a fluorescent reporter protein knocked in to the ubiquitously expressed Rosa26 locus. Thus, in these animals, cells even transiently expressing Foxp3 are permanently marked, and their phenotypic and functional properties can be spatially and temporally examined. Using this system, Bluestone and colleagues observed that a portion of FOXP3+CD4+ cells downregulate FOXP3 expression and acquire the ability to produce effector cytokines such as interferon-γ (IFNγ) even in the absence of any experimental manipulation. Furthermore, the frequency of these ‘ex- TReg cells’ was increased in the pancreatic islets of non-obese diabetic (NOD) mice, indicating that they may contribute to autoimmune pathology101. These data are complemented by another recent study demonstrating that during infection with a lethal strain of Toxoplasma gondii, TReg cells can lose FOXP3 expression and acquire TH1 effector characteristics75. Together, these studies suggest that highly polarized inflammatory environments can subvert TReg cell function by converting them to FOXP3− effector T cells in vivo, and are consistent with a recent epigenetic analysis demonstrating that the loci encoding key transcription factors and cytokines associated with TH1, TH2 and TH17 cells are not fully repressed in TReg cells102. However, using a similar reporter mouse system to monitor TReg cell stability, Rudensky’s group recently demonstrated that FOXP3 expression by TReg cells is remarkably stable, even in highly inflammatory settings103. The discrepancies in these studies may be due to differences in the inflammatory systems used to examine TReg cell stability in vivo, or to subtle differences in the way the reporter mice were constructed. Bluestone’s group drove cre expression using a bacterial artificial chromosome (BAC) transgene, whereas Rudensky’s group knocked a cre expression cassette into the 3’ untranslated region of the endogenous Foxp3 locus, and this may lead to differences in the timing and extent of cre expression that result in the divergent conclusions of these studies.
In discussing the stability of TReg cells, it is also important to keep in mind differences in Foxp3 expression observed in mouse vs. human T cells. In mouse, Foxp3 appears to be a robust marker for either thymic-derived or induced TReg cells. However, nearly all human CD4+ T cells transiently express Foxp3 during activation, and this is not associated with acquisition of regulatory function. Thus, Foxp3 alone is not a reliable marker for human TReg cells, further complicating analyses of their function and stability. Moreover, because thymic output is severely curtailed following puberty, causing fewer TReg cells to emerge from the thymus, less-stable induced TReg cells may assume a greater role in maintaining immune homeostasis in adults104. Clearly, the degree to which TReg cells can acquire effector functions and contribute to the development of autoimmune and inflammatory diseases merits further study, with careful attention payed to potential differences observed in mouse and systems.
Concluding Remarks
TReg cells have emerged as potent anti-inflammatory cells, and this has generated considerable excitement for the potential to therapeutically manipulate TReg cell activity to treat autoimmune disease, prevent graft rejection, and boost immune responses during cancer and chronic infection105. However, it is now evident that TReg cells are a dynamic and diverse T cell population, composed of several phenotypically and functionally distinct subsets whose differentiation and function are controlled by specific signals in the immune environment. Thus, in order to optimally utilize TReg cells in the clinic, it is essential that we better understand how these subsets function together to ensure that robust immune responses can occur when needed without development of significant immunopathology and inflammatory disease. Key unresolved issues include defining the importance of each TReg cell subset in the control of different types of inflammatory responses, identifying the factors that govern the peripheral differentiation of these subsets, and determining how cytokines and other factors in the immune environment influence TReg cell activity and stability during the initiation, progression and termination of normal and pathological immune responses.
Reference List
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res. Ther. 2008;10:227. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindley S, et al. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int. J. Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 8.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 9.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol. Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 10.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J. Exp. Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 13.Freyschmidt EJ, et al. Skin inflammation arising from cutaneous regulatory T cell deficiency leads to impaired viral immune responses. J. Immunol. 2010;185:1295–1302. doi: 10.4049/jimmunol.0903144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2008 doi: 10.1002/hep.22761. [DOI] [PubMed] [Google Scholar]
- 17.Muller M, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J. Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J. Immunol. 2007;178:301–311. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 20.Huehn J, et al. Developmental Stage, Phenotype, and Migration Distinguish Naive- and Effector/Memory-like CD4+ Regulatory T Cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min B, et al. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur. J. Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 22.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 24.Loser K, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J. Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 25.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 27.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 28.Onodera T, et al. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J. Immunol. 2009;183:5608–5614. doi: 10.4049/jimmunol.0804116. [DOI] [PubMed] [Google Scholar]
- 29.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boissonnas A, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 33.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin. Immunol. 2007;19:400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 38.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 42.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 45.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan BM, Juedes A, Szabo SJ, von HM, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009 doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhry A, et al. CD4+ Regulatory T Cells Control TH17 Responses in a Stat3-Dependent Manner. Science. 2009 doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strober W. Vitamin A rewrites the ABCs of oral tolerance. Mucosal. Immunol. 2008;1:92–95. doi: 10.1038/mi.2007.22. [DOI] [PubMed] [Google Scholar]
- 53.Ghoreishi M, et al. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J. Immunol. 2009;182:6071–6078. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeffery LE, et al. 1, 25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malek TR. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 56.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 57.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 58.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 60.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur. J. Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 61.Burkett PR, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siewert C, et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 63.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 64.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gorman S, et al. Topically applied 1, 25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J. Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 68.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 69.Murphy KM, et al. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr. Top. Microbiol. Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- 70.Cooper AM, et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 72.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J. Exp. Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J. Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu LF, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kishimoto T. IL-6: from its discovery to clinical applications. Int. Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 77.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 78.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 79.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 80.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 81.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 82.Mima T, Nishimoto N. Clinical value of blocking IL-6 receptor. Curr. Opin. Rheumatol. 2009;21:224–230. doi: 10.1097/BOR.0b013e3283295fec. [DOI] [PubMed] [Google Scholar]
- 83.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veldhoen M, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 85.Pillemer BB, et al. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J. Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maerten P, et al. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J. Autoimmun. 2005;25:112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Trinchieri G. Type I interferon: friend or foe? J. Exp. Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study. J. Neuroimmunol. 2010;218:120–124. doi: 10.1016/j.jneuroim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Vandenbark AA, et al. Interferon-beta-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Golding A, Rosen A, Petri M, Akhter E, Andrade F. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 2010;131:107–117. doi: 10.1111/j.1365-2567.2010.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffman HM. Therapy of autoinflammatory syndromes. J. Allergy Clin. Immunol. 2009;124:1129–1138. doi: 10.1016/j.jaci.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen X, Oppenheim JJ. TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr. Dir. Autoimmun. 2010;11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 94.Ma HL, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–440. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- 95.Brinster C, Shevach EM. Costimulatory effects of IL-1 on the expansion/differentiation of CD4+CD25+Foxp3+ and CD4+CD25+Foxp3- T cells. J. Leukoc. Biol. 2008;84:480–487. doi: 10.1189/jlb.0208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin. Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 97.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS. Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 99.Komatsu N, et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 101.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 105.Allan SE, et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol. Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 106.Picca CC, et al. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol. Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 107.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 109.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 110.Darrasse-Jeze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bollyky PL, et al. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J. Leukoc. Biol. 2009;86:567–572. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Venturi GM, Conway RM, Steeber DA, Tedder TF. CD25+CD4+ regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J. Immunol. 2007;178:291–300. doi: 10.4049/jimmunol.178.1.291. [DOI] [PubMed] [Google Scholar]
- 113.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 114.Hirahara K, et al. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 115.Siegmund K, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 117.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 118.Yuan Q, et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J. Exp. Med. 2007;204:1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang SG, et al. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966–981. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Yurchenko E, et al. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleinewietfeld M, et al. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T cell subset. Blood. 2004 doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 122.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soler D, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J. Immunol. 2006;177:6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 124.Guo Z, et al. CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int. Immunol. 2008;20:307–315. doi: 10.1093/intimm/dxm143. [DOI] [PubMed] [Google Scholar]
- 125.Eksteen B, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J. Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- 126.Oo YH, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J. Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 127.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J. Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 128.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J. Clin. Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grauer OM, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int. J. Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 130.Wald O, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J. Immunol. 2006;177:6983–6990. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]