Abstract
Objectives
According to a large-scale international survey, Peru has one of the highest prevalences of asthma worldwide; however, data from this survey were limited to participants from urban Lima. The authors sought to characterise the epidemiology of asthma in Peru in two regions with disparate degrees of urbanisation. In this manuscript, the authors summarise the study design and implementation.
Design
A cross-sectional study.
Participants
Using census data of 13–15-year-old adolescents from two communities in Peru, the authors invited a random sample of participants in Lima (n=725) and all adolescents in Tumbes (n=716) to participate in our study.
Primary and secondary outcome measures
The authors asked participants to complete a questionnaire on asthma symptoms, environmental exposures and socio-demographics and to undergo spirometry before and after bronchodilator, skin allergy testing and exhaled nitric oxide testing. The authors obtained blood samples for haematocrit, total IgE levels, vitamin D levels and DNA in all participants and measured indoor particulate matter concentrations for 48 h in a random subset of 70–100 households at each site.
Results
Of 1851 eligible participants, 1441 (78%) were enrolled and 1159 (80% of enrolled) completed all physical tests. 1283 (89%) performed spirometry according to standard guidelines, of which 86% of prebronchodilator tests and 92% of postbronchodilator tests were acceptable and reproducible. 92% of allergy skin tests had an adequate negative control. The authors collected blood from 1146 participants (79%) and saliva samples from 148 participants (9%). Overall amounts of DNA obtained from blood or saliva were 25.8 μg, with a 260/280 ratio of 1.86.
Conclusions
This study will contribute to the characterisation of a variety of risk factors for asthma, including urbanisation, total IgE levels, vitamin D levels and candidate genes, in a resource-poor setting. The authors present data to support high quality of survey, allergic, spirometric and genetic data collected in our study.
Article summary
Article focus
We sought to characterise the epidemiology of asthma in Peru by studying two regions with disparate degrees of urbanisation.
We summarise the study design, implementation and standard operating procedures and provide quality control data for important outcome and exposure variables.
Key messages
We present data to support high quality of survey, allergic, spirometric and genetic data collected in our study.
Strengths and limitations of this study
This study will contribute to the characterisation of a variety of risk factors for asthma, including urbanisation, total IgE levels, vitamin D levels and candidate genes, in a resource-poor setting.
This study is cross-sectional and therefore does not track symptoms over time to directly determine causality. In addition, we did not collect stool samples to assess parasitic infections nor do we have information on respiratory infections in early childhood.
Introduction
As poorer countries undergo rapid urbanisation and development, rates of asthma have increased. These increases have been reported across continents.1 It has become clear that asthma is no longer a developed country disease. Worldwide, it is reported that the prevalence of asthma is approximately 300 million.2 Asthma is currently the 25th leading cause of disability-adjusted life-years lost, and its burden is comparable to that of diabetes mellitus. In comparative studies, asthma rates have been higher in urban areas than in rural areas,3 4 with reasons for this varying from outdoor air pollution5 6 to the ‘hygiene hypothesis.’7
However, limited research on asthma in Peru has been published. A MEDLINE search of ‘ASTHMA’ and ‘PERU’ revealed only 16 published articles. According to the questionnaire from the International Study of Asthma and Allergies in Childhood (ISAAC), 48% of children in Peru reported lifetime wheeze and 26% reported wheezing in the last year. A Peru-based study reported that 23% of children in a Lima shanty-town showed decreases in forced expiratory volume in one second (FEV1) of >15% following exercise testing.8 Both these studies, however, were limited to urban Lima. Other studies in Peru have shown elevated rates of diagnosis with more traffic flow,6 sought to validate exercise challenge as a diagnostic method at high altitude9 and shown elevated nitrite levels in sputum of patients with asthma.10
Other developing counties with rapid urbanisation have equally high rates of asthma. For example, in Kenya, researchers found that exercise-induced bronchoconstriction increased from 13% to 23% in a rural versus an urban setting.11 Another group found that in Ethiopia, the odds of asthma was 3 to 1 in an urban versus a rural setting.4
A number of studies in developed countries have linked asthma to allergies in the form of eczema allergic rhinitis and elevated circulating IgE.12–15 The ISAAC study showed high levels of allergic rhinitis and eczema in populations that also had high levels of wheezing. Environmental exposures have also been connected to asthma risk or severity, particularly exposure to particulate matter (PM) of 10 and 2.5 μm in size, carbon monoxide, nitrogen oxide species, sulphur dioxide and environmental tobacco smoke.16–19 Finally, there is a growing but limited body of evidence on the connection between genetics and asthma risk, as some antioxidant and immune system gene polymorphisms (SNPs) may increase susceptibility.20–23
The diagnosis of asthma in population-based studies, however, is challenging. Asthma is a clinical syndrome that is associated with airways inflammation, airflow limitation, bronchial hyper-responsiveness and symptoms of episodic wheeze and cough. Therefore, the need to derive a practical definition of asthma for epidemiological research has led to a focus on asthma symptoms. To complement or validate the questionnaire-based definitions of asthma, researchers have used measures of airflow limitation24 and bronchial hyper-responsiveness as markers of asthma. However, both presence of airflow limitation and bronchial hyper-responsiveness, even in known patients with asthma, can be negative on the day of testing. More recently, exhaled nitric oxide (eNO) has been used as a marker of inflammation in asthma, but results in epidemiological studies have been mixed25 and eNO may better serve as a means to assess treatment effectiveness or compliance. eNO may be elevated in a variety of lung diseases, which are common in developing countries, and its utility as a screening tool in this setting has not been evaluated.
Prompted by the paucity of published information on the epidemiology of asthma in Peru, we established the Peru Urban versus Rural Asthma Study to investigate differences in prevalence, severity and risk factors for asthma in two regions with disparate degrees of urbanisation. To investigate risk factors, we compared an urban site where rates were likely to be high (Lima) with a rural site where rates were likely to be low (Tumbes). Both regions are coastal and at sea level. Whereas Lima is highly urbanised, has high levels of outdoor air pollution and low levels of household biomass burning, Tumbes has low levels of outdoor air pollution and higher levels of household biomass burning. In this manuscript, we summarise the study design, implementation and standard operating procedures and provide quality control data for important outcome and exposure variables.
Methods
Study objectives
The primary objective of this study was to compare the prevalence and severity of asthma in adolescence in an urban and rural community in Peru. Secondary objectives were to determine differences in: proportions of atopic and non-atopic asthma, indoor air pollution concentrations, contribution of genetic admixture to asthma risk in these two populations; and the prevalence of SNPs of asthma identified by genome-wide association studies. An additional objective for data collected in Lima was to determine the risk of asthma according to household distance from the major roadway in the community.
Study design
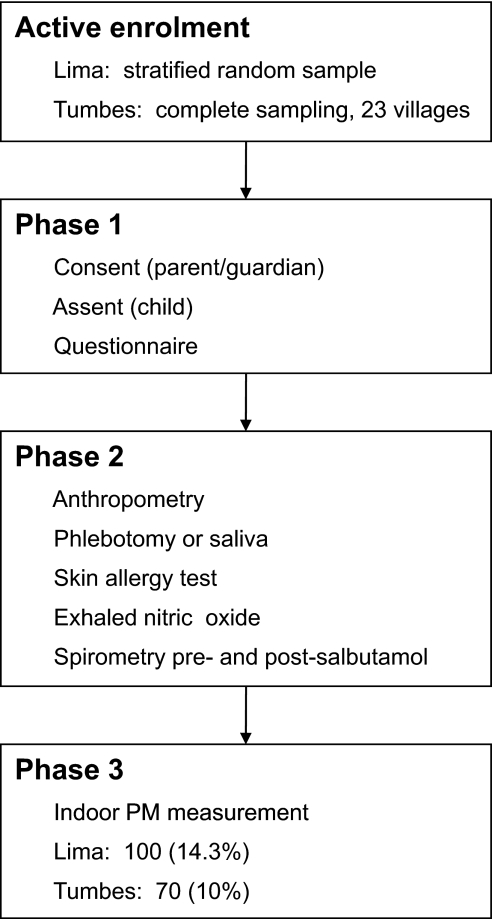
Our study was a cross-sectional study of asthma symptoms in 13–15-year-old adolescents in two regions in Peru. We carried out the study in two phases (figure 1). In the first phase of the study, we obtained written informed consent from parents and assent from children. At this visit, we asked the parent and adolescent to answer our survey. In the second phase, we revisited children at their homes or schools to measure anthropometry, to draw a blood sample, to perform an allergy skin test, to measure eNO and to perform spirometry before and after bronchodilator. We collected saliva from participants who refused to give blood. In a random subset of houses, we measured indoor PM concentrations over 48 h.
Figure 1.
Procedure flow chart. Flow chart shows course of procedures, in order, for Peru Urban versus Rural Asthma Study participants. As testing is ongoing, we opted not to include numbers as they are subject to change. PM, particulate matter.
Study sites
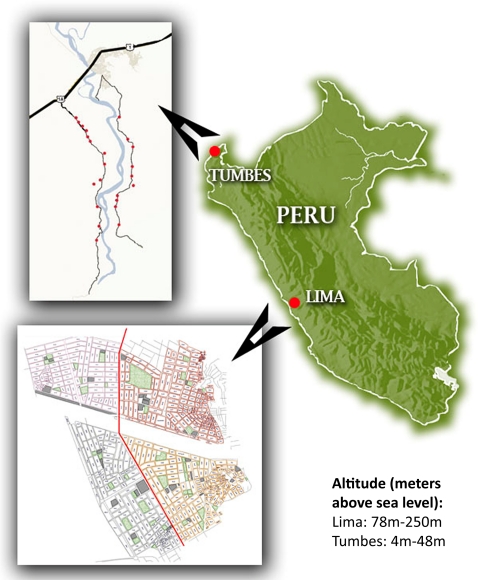
We show the location and altitude of both regions in figure 2. Lima, the highly urbanised capital city of Peru, is located on the central coast and has a population of approximately 10 million. The site for our study in Lima was Pampas de San Juan de Miraflores, a peri-urban shanty-town located 25 km south of central Lima. This community is described in detail elsewhere.26 27 The population mostly comprises highland immigrants. Homes are made mostly of concrete or plywood, clustered tightly with paved and unpaved roads separating each city block. A four-lane highway divides the community in half. Average ambient temperature in Lima ranges between 17°C and 30°C year-round, and relative humidity (RH) ranges between 55% and 80%. Annual precipitation in Lima is 50 mm per year.
Figure 2.
Map of Peru and study sites. Lima, Peru (n=725) and Tumbes, Peru (n=716) are coastal regions and thus of low altitude. Inset of Lima shows Pampas de San Juan neighbourhood divided into quadrants by main thoroughfare (vertical red line). Pampas de San Juan is set on a large hill, with base to the west and peak to the east. Inset of Tumbes shows 23 rural study towns set along small roadways on east and west margins of the Tumbes River. City of Tumbes at crossing of major highway (thick black line) and river (light blue).
The second site for our study was the rural area of Tumbes, located in the northern region of Peru bordering with Ecuador. There are two main roads that run through our study towns, which line the banks of the Tumbes River. Average ambient temperature in Tumbes ranges between 25°C and 33°C, RH ranges between 55% and 80% and precipitation is much higher, with annual rainfall up to 2200 mm per year.
Study population
Our target population was adolescents aged 13–15 years. We identified participants from census data. The census data in Lima was conducted originally in 1997 and subsequently repeated in 2000, which was updated several times thereafter. For these censuses, fieldworkers employed by A.B. PRISMA obtained the full name, age and sex of each permanent household member. Census data collection in Tumbes was completed in 2008, immediately prior to the start of our study, by the Proyecto de Eliminación de Cysticercosis (PEC). Maps were made throughout the Tumbes region of Peru, and again the full name, age and sex of each permanent household member was obtained. For more information on A.B. PRISMA and PEC, please see the ‘Study organisation’ section.
In Lima, we invited an age- and sex-random stratified sample of subjects from the census to participate in our study. In Tumbes, we invited all 13–15-year-old children in the first 23 villages along the Tumbes River to participate. Adolescents were eligible to participate if they were capable of understanding and performing procedures, if their parents or guardians were capable of providing written informed consent and they were capable of providing assent; if they had no ocular, abdominal or thoracic surgery in the last 3 months and if they were not hospitalised for cardiac reasons in the last 3 months. Children were ineligible to participate if they had a chronic respiratory condition other than asthma, such as cystic fibrosis or chronic lung disease of prematurity, if they were pregnant or if they had pulmonary tuberculosis or were currently receiving treatment for pulmonary tuberculosis. We recruited only one adolescent per household.
Outcomes
The primary outcome for this study was self-report of asthma symptoms. Current asthma symptoms were defined as wheezing or asthma medication use in the previous year, while we defined atopy as a positive test to ≥1 skin test allergens. We consider a skin test as positive if the sum of the vertical and horizontal dimensions of the induration was ≥3 mm more than that of the negative control or if the sum of the vertical and horizontal dimensions of erythema was ≥5 mm larger than that of the negative control. Meanwhile, we defined reversibility as a ≥12% increase in FEV1 after bronchodilator administration. We classified severity as per the National Asthma Education Prevention Program guidelines.24 Other important outcomes include asthma risk due to household distance from a highly trafficked avenue, differences in indoor air pollution, differences in genetic admixture and differences in the distributions of SNPs associated with asthma between these two populations.
Exposures
We asked about household characteristics and structures. We also asked about type of cooking fuels, lifetime exposure to biomass fuels, tobacco exposure in the house and trash burning. We measured indoor PM levels via in-house monitoring, and we obtained outdoor levels in Lima from the Peruvian governmental agency, DIGESA (http://www.digesa.sld.pe). In Lima, an additional exposure was distance from the major four-lane avenue that divides the community in half.
Sample size
The prevalence of asthma symptoms in the past 12 months in Peru was estimated at 20%–26% according to the previous studies.1 8 To estimate a prevalence of current asthma symptoms of 20% with a precision of 3% and 95% confidence, we required 683 participants at each site. Our initial assumption for loss to follow-up between visits was approximately 5%. Therefore, we aimed to enrol approximately 720 participants at each site, which we achieved as noted below.
Study organisation
A.B. PRISMA in Lima, Peru and the Johns Hopkins University in Baltimore, USA, provided administrative oversight for the study. In Lima, the Pampas office of A.B. PRISMA provided operations and logistic support for fieldwork. In Tumbes, the PEC provided additional operational and logistical support. There was a study centre coordinator at each site (CLR and LMB) that was responsible for protocol design and daily management of questionnaire and physical testing teams. Each site provided a data manager for database design, double-data entry and database management. Additionally, at Johns Hopkins were collaborating geneticists, environmental scientists and epidemiologists with experience in the design and conduct of population-based studies. All investigators were involved in protocol design and technical support and remain involved in ongoing analyses.
Personnel, training and logistics
For each site, the local team was trained by the study coordinators and a physician with expertise in spirometry. Questionnaire interviewer training lasted a minimum of 3 days in addition to field piloting. Study coordinators supervised interviewer performance in the field. Spirometry training lasted at least 2 weeks and included observed pilot subjects outside the study cohort.
In the first phase of our study, we recruited participants into our study. We obtained written informed consent from parents or guardians and assent from adolescents and administered our survey at the time of that encounter. This work was completed by nine trained interviewers, four in Lima and five in Tumbes. In the second phase of our study, we visited participants at their homes or schools and measured anthropometry; performed skin allergy testing, eNO and spirometry and obtained a blood or saliva sample. In Lima, this was done by two field teams, each with one physician and one trained health worker (nurse or nurse technician). In Tumbes, all physical evaluations were done by a team of one physician and at least one of the three health workers (two nurse technicians and one lab technician). In Tumbes, most tests were administered in schools, while in Lima, most tests were done by home visitation. All results were returned and explained to the participants, and we referred to a physician those whose results suggested a diagnosis of asthma.
Questionnaire
We administered questionnaires (online supplement S1) in the presence of the study participant and his or her parent. The basis of the questionnaire was the validated Spanish version of the ISAAC study.28 We scrutinised this translation with local health professionals at A.B. PRISMA to ensure cultural appropriateness. In addition to ISAAC questions, our questionnaire contained questions on asthma severity, food and medicine allergies, household tobacco and combustible exposures, trash burning, ventilation, household materials, household animal exposure, time spent in home and family socioeconomic status. Assessment of asthma severity was based on National Asthma Education and Prevention Program guidelines.24 We maintained a codebook to outline the response to each question and facilitate quick data reviews and cleaning.
Anthropometry
We measured participants in triplicate for height, weight and blood pressure on the day of the appointment. We measured height with a locally made stadiometer, weight with a digital scale (Seca, Hamburg, Germany) and blood pressure with an automated sleeve (OMRON HEM712-C, Kyoto, Japan). We used the averages of the three heights and weights as the final values. Data sheets for anthropometry, eNO and spirometry testing are found in online supplement S2.
eNO testing
We used the NIOXMINO handheld portable eNO monitor (Aerocrine New Providence, New Jersey, USA) to measure eNO values in parts per billion. We performed short daily and weekly calibrations as per manufacturer's instructions. Children performed a full inhalation followed by a full steady exhalation at 50 ml/s. All eNO tests were performed prior to spirometry to avoid washout. Values were recorded manually.
Allergy skin testing
We performed allergy tests with the Multi-Test II system (Lincoln Diagnostics, Decatur, Illinois, USA) and allergens made by ALK-Abello (Round Rock, Texas, USA). We used a total of 10 allergens: cockroach (Blattella germanica), dust mite mix (Dermatophagoides farinae and D pteronyssinus), cat hair, dog epithelium, mouse epithelium and mixed molds (Alternaria, Cladosporium, mixed Aspergillus and mixed Penicillium). As per manufacturer's instructions, we recorded vertical and horizontal measurements of induration and erythema, alongside 0–2 scales of itchiness and pseudopodia. At each site, a maximum two fieldworkers, trained by the same coordinator, read and recorded the reactions for consistency (online supplement S3). We double-entered measurements and used an algorithm based on the manufacturer's interpretation instructions to compare dimensions of erythema and induration with negative and positive controls (online supplement S5).
Spirometry
We performed spirometry with a portable, battery-operated, handheld spirometer (SpiroPro, Jaeger/Cardinal Health, Hoechberg, Germany). SpiroPro uses disposable, single-use factory-calibrated pneumotachometer tubes (pneumotachs). We asked participants to withhold any short-acting bronchodilators within 8 h and long-acting bronchodilators for 24–48 h of testing unless clinically necessary; however, we did not have instances where this occurred. Our criteria were based on those European Respi/European Respiratory Society American Thoracic Society and European Respiratory Society (ATS/ERS)29 which specify 4 h for short-acting bronchodilators and 12 h for long-acting bronchodilators. We revisited participants who reported having a respiratory infection in the last 2 weeks on a later date. Participants with heart rate >140, systolic blood pressure >185 or diastolic blood pressure >105 would not have been eligible for testing on that day, although this did not occur. We obtained information on smoking, alcohol and caffeine consumption in the immediate period before testing, although these were not considered exclusion criteria.
We asked each participant to perform a maximum of eight prebronchodilator tests to attain three acceptable error-free tests as per standard criteria.30 We required each expiration to last a minimum of 6 s, to reach an end-expiratory plateau of 25 ml/s, to be without an unsatisfactory start defined by a back-extrapolation volume that was either 5% or 150 ml of the forced vital capacity (FVC) and to be free of cough or glottis closure artefacts. Spirometry values were stored on the spirometer, but we also recorded these values on a paper form. The participant was then administered four doses of salbutamol (100 μg/dose). Spirometry was repeated 15 min later. All manoeuvres were performed seated upright with a nose clip. Field spirometry methods were pilot tested at each site at least 50 times by each spirometrist prior to field implementation.
To ensure reproducibility, the two highest values of prebronchodilator FEV1 and of prebronchodilator FVC could not have intra-test variability >150 ml.30 The same applied for postbronchodilator FEV1 and FVC. In addition, the highest single postbronchodilator FEV1 and FVC values could not be 150 ml less than the highest prebronchodilator FEV1 and FVC values, respectively. Spirometrists immediately reviewed all curves for quality of inhalation and expiration curve shape. Participants who did not meet these criteria were asked to repeat spirometry testing on a different day, with up to three opportunities. Additional grading according to slightly modified standard criteria31 were performed at the end of the study. Spirometry data were sent electronically to Johns Hopkins University (WC) for regular assessment and feedback. We held regular conferences (CLR, LMB and WC) to review flow curves and provide feedback.
Blood draw and processing
All blood was drawn by standard phlebotomy techniques into non-heparinised 10-ml Vacutainer tubes (Becton-Dickinson, Franklin Lakes, New Jersey, USA). Blood was drawn either prior to allergy testing or more than 24 h following this test to not influence IgE levels. Small amounts of blood were immediately gathered into heparinised capillary tubes for haematocrit processing. Blood in Vacutainer tubes was refrigerated at 4°C for <48 h prior to separation into coagulate and serum. After separation, all samples were subsequently stored at −20°C. DNA was later extracted from the coagulate using a salt-wasting (ie, phenol–chloroform extraction) protocol in our laboratory at UPCH in Lima. Serum and DNA samples from each patient were sent overnight to the USA for processing and storage, while a back-up copy of each remains stored at −20°C in Lima.
We will analyse blood sera for total human serum IgE using a United States Food and Drug Administration cleared fluorescent enzyme immunoassay (ImmunoCAP250; Phadia, Kalamazoo, Michigan, USA). The assay involves binding IgE in the test specimen to an anti-IgE, which has been immobilised on a solid-phase matrix. Following removal of unbound proteins with a buffer wash, bound IgE is quantitatively detected with an enzyme-labelled anti-human IgE reagent. The assay is calibrated using a standard curve that has been cross-validated to known quantities of total IgE in the WHO 75/502 IgE International Reference Preparation. Results are reported in kilo IU of IgE per litre (kU/l). The serum samples will also be processed for vitamin D levels using the LIAISON 25 OH vitamin D total assay (DiaSorin Inc, Stillwater, Minnesota, USA). The assay is a direct competitive chemiluminescence immunoassay for determination of metabolised 25-hydroxyvitamin D quantities in serum. The process involves disassociation of 25-OH vitamin D from its serum binding protein and subsequent binding to a specific antibody on a solid-phase matrix. Following removal of unbound proteins with a buffer wash, antibody-bound 25-OH vitamin D levels are detected by initiating a flash chemiluminescent reaction and measurement of light signals. The LIAISON assay is calibrated using a predefined master curve that has been adjusted according to instrument-specific calibration. Results are reported in units of nanograms of 25-OH vitamin D per millilitre (ng/ml).
Saliva DNA collection
In the event blood draw was refused, we collected saliva. The patient was instructed to spit 2 ml of sputum-free saliva into the Oragene DNA Self Collection Kit (DNA Genotek, Ontario, Canada) and stored at room temperature. DNA was then extracted as per manufacturer's instructions.
Genetic analysis
We will perform genetic analysis of several well-replicated genes associated with asthma and total IgE in other populations.32 33 Our main interest is to characterise the frequency of variants of those genes among patients with and without asthma across the two study sites. Genotyping of SNPs for ADAM33, CD14, GSTM1, GSTP1, IL4, IL13, IL1RL1, TSLP and IL33 (table 1) will be performed using the custom-designed Illumina (San Diego, California, USA) oligonucleotide pool assay for the BeadXpress Reader System, which combines the GoldenGate Assay with VeraCode Bead technology (http://www.illumina.com).
Table 1.
Single nucleotide polymorphisms of candidate genes associated with asthma
| Gene | Loci | Polymorphism |
| ADAM33 | rs570269 | C/G |
| rs677044 | A/G | |
| rs11905870 | C/T | |
| rs2787095 | C/G | |
| rs12479696 | A/T | |
| rs6115987 | C/T | |
| rs3918395 | A/C | |
| rs2853210 | C/T | |
| rs487377 | C/T | |
| rs554743 | C/T | |
| rs7354032 | A/G | |
| rs511898 | C/T | |
| rs598418 | A/G | |
| CD14 | rs3776138 | C/G |
| rs2569190 | A/G | |
| rs5744456 | A/T | |
| GSTM1 | rs2239892 | A/G |
| GSTP1 | rs8191439 | A/C |
| rs1138272 | C/T | |
| rs1695 | A/G | |
| rs749174 | A/G | |
| rs1871042 | C/T | |
| rs4147581 | C/G | |
| IL4 | rs2243258 | C/T |
| rs2243283 | C/G | |
| rs2243261 | G/T | |
| rs2243252 | C/T | |
| rs2243276 | C/T | |
| rs2070874 | C/T | |
| rs2243266 | A/G | |
| rs734244 | C/T | |
| rs2243267 | C/G | |
| rs2243288 | A/G | |
| rs2227282 | C/G | |
| rs2243263 | C/G | |
| rs2243268 | A/C | |
| rs2243270 | A/G | |
| rs2243274 | A/G | |
| rs2243279 | A/G | |
| rs2243282 | A/C | |
| rs2243290 | A/C | |
| IL13 | rs2069744 | C/T |
| rs20541 | A/G | |
| rs2069745 | C/G | |
| rs1295687 | C/T | |
| rs1295686 | C/T | |
| rs1295685 | A/G | |
| rs848 | A/C | |
| ILR1 | rs10197862 | A/G |
| rs10204137 | A/G | |
| rs10206753 | C/T | |
| rs11123918 | C/T | |
| rs12712142 | A/C | |
| rs12905 | A/G | |
| rs12999517 | C/T | |
| rs13431828 | C/T | |
| rs1921622 | A/G | |
| rs2160203 | A/G | |
| rs3771175 | A/T | |
| rs4988958 | C/T | |
| TSLP | rs2289276 | C/T |
| rs10062929 | A/C | |
| rs11466741 | C/T | |
| rs11466744 | G/T | |
| rs11466749 | A/G | |
| rs11466750 | A/G | |
| rs6864123 | A/G | |
| IL-33 | rs4742170 | C/T |
| rs10975514 | A/G | |
| rs7037276 | C/T | |
| rs10975516 | A/G | |
| rs1317230 | A/C | |
| rs1330383 | G/T | |
| rs10975519 | C/T | |
| rs10975520 | C/G | |
| rs1412420 | A/G | |
| rs7047921 | A/G | |
| rs7019575 | A/T | |
| rs1332290 | G/T | |
| rs16924241 | C/G | |
| rs1048274 | A/G | |
| rs16924243 | C/T | |
| rs12000491 | C/T | |
| rs7036053 | C/T | |
| GC | rs115563 | T/C |
| rs22822679 | A/C | |
| rs12512631 | T/C | |
| VDR | rs2228510 | G/A |
| rs10783219 | A/T | |
| rs71339166 | C/G | |
| rs4516035 | T/C | |
| CYP2B1 | rs703842 | T/C |
| rs4646536 | T/C | |
| rs10877012 | G/T | |
| CYP2R1 | rs10741657 | G/A |
| rs10766197 | G/A | |
| CYP24A1 | rs2244719 | T/C |
| rs2296241 | A/G | |
| rs2426496 | T/G | |
| rs17219315 | A/G |
We will also genotype for vitamin D receptor polymorphisms GC, VDR, CYP2B1, CYP2R1 and CYP24A1 using the same approach (table 1). We will explore population structure in these two groups as the basis for future admixture mapping studies. To evaluate genetic structure of the ethnic groups from the three ancestral populations in the HAPMAP (http://www.hapmap.org), we will genotype an additional 384 SNPs identified as ancestry informative markers selected for maximal difference between Native American, European and African populations.
Indoor PM concentration
In Lima, we allocated all participating households into 11 geographic zones based on 300-m increments from the main avenue. We randomly selected a geographic zone-stratified sample of 100 households for monitoring, approximately nine from each zone. In Tumbes, we randomly selected households proportional to the total population for each of the 23 towns for a total of 70 households. Passive PM concentrations, collected only on weekdays, were measured over 48 h using a portable nephelometer (pDR-1000; Thermo Scientific, Franklin, Massachusetts, USA). Empirical evidence suggests that the pDR-1000 detects particles in the size range of 0.3–2 μm more efficiently than those of 2–10 μm.34–36 Quintana et al35 reported a high degree of correlation between the PM determined by the pDR-1000 and PM2.5 measurements. Thus, PM measured with the pDR-1000 is an accurate approximation for PM2.5.
We collected RH and temperature data concurrently using the HOBO Data Logger (Onset Computer Corporation, Bourne, Massachusetts, USA). We calibrated all pDR-1000s to a zero measurement before each trial and then placed them between 1.5 and 2.0 m in height in the main living room (figure 3). We adjusted all PM measurements to consider effects of high RH using the following correction factor CF=1 + 0.25×RH2/(1−RH). The crude PM value was then divided by the CF to provide an adjusted PM measurement.37 We recorded dimensions of the room in which the machine was placed in all households (online supplement S4). We also recorded the location and size of all windows and doors, as well as whether windows and doors were opened or closed. DIGESA measured outdoor PM of 10 and 2.5 μm in a high-traffic area in the southern region of Lima approximately 1.5–2.0 km from our study community.
Figure 3.
Particulate matter measurement device in place. pDR-1000 in place in study household, accompanied by HOBO Data Logger and protected by open cage for air flow.
Geographic information systems mapping
We measured global positioning system coordinates for each participating household in Lima. We calculated the distance from each home to the main avenue (Avenida Miguel Iglesias) using the program ArcGis 9.3 (ESRI Corporation, Redlands, California, USA).
Data management
All questionnaire data were double-entered at each site via Microsoft ACCESS (Microsoft Corp. Redmond, Washington, USA), and these two data sets were cross-compared for validity and errors. Spirometry data were collected electronically and on paper forms. Data from the spirometers were downloaded daily or every other day into a computer database. An extensive combined data set was created using the data extracted from SpiroPro and cross-referenced with data double-entered on paper forms. Other testing data, such as anthropometry, allergy testing, eNO and other environmental data, were written on the paper forms and also double-data entered. PM concentration and RH data were collected electronically. We developed an ACCESS program to merge the PM and RH data using the date and time stamp and calculated the adjusted PM as described above. Detailed data codebooks were created for each data set to ensure consistency between the two sites and facilitate database management.
Specific aims and statistical considerations
The specific aims of this study were as follows: (1) determine differences in prevalence and risk factors of asthma between peri-urban and rural adolescents in Peru, (2) determine the relationship of total serum IgE to asthma and lung function in peri-urban and rural adolescents in Peru, (3) determine the effects of serum vitamin D concentrations and vitamin D receptor polymorphisms on the prevalence of asthma in peri-urban and rural adolescents in Peru, (4) determine the effect of genetic differences by gene ancestry mapping and single nucleotide polymorphisms on the prevalence of asthma between peri-urban and rural adolescents in Peru, (5) determine genetic differences by gene ancestry mapping and single nucleotide polymorphisms that may be associated with lung function in peri-urban and rural adolescents in Peru. In this manuscript, we summarise quality control data with simple means and percentages. Statistical methods used to test differences in the prevalence and risk factors of asthma between Lima and Tumbes were presented in detail in two previous publications.38 39 Subsequent publications will also present detailed statistical methods.
Ethical considerations
We obtained approval from the Ethics Committees of A.B. PRISMA in Lima, Peru, and Johns Hopkins University, Bloomberg School of Public Health, in Baltimore, Maryland. Since all participants were younger than 18 years, written informed consent was obtained from parents or guardians and assent was obtained from the participant. All subjects were offered fruit or crackers and a juice box upon completion of the examination. Test results were delivered at a subsequent visit, at which time a fieldworker or team physician explained the results to the parents. Those with asthma symptoms or other medical concerns were offered a consultation with our study physicians and referred to a local physician. Subjects with anaemia were given 30 days of iron supplements and recommended to follow-up with their physicians. All data files and sensitive information were kept on password-protected devices that were only accessible to study coordinators for confidentiality.
Results
Of the 1851 potential participants listed on our censuses and approached across both sites, a total of 1441 adolescents were recruited to participate in the study, 725 in Lima and 716 in Tumbes. In Lima, 321 (30.4%) refused participation or were unable to be contacted, while 10 (0.9%) were ineligible, generally because their ages fell out of our study age range. In Tumbes, 67 (8.4%) refused participation or were unable to be contacted, while 12 (1.5%) were ineligible for similar reasons. Of those not enrolled from census, there were no significant differences in distribution by age or sex (data not reported).
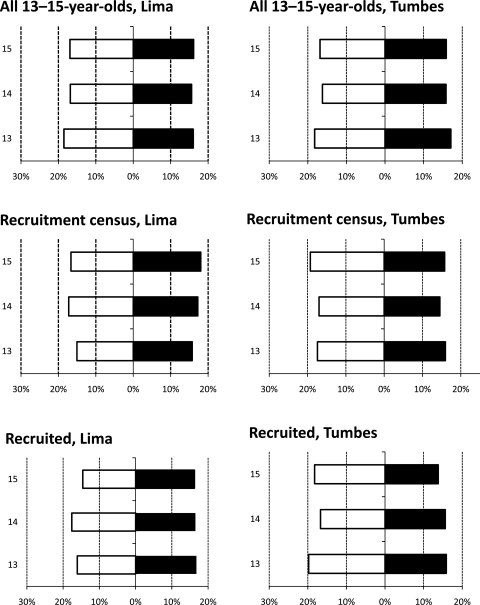
Participants were 52% boys overall, with 49% boys in Lima and 54% boys in Tumbes. At the start of the project, ages were evenly distributed in both sexes (figure 4). At recruitment, 20% were 13 years old, 34% were 14 years old, 34% were 15 years old and 12% of participants had just turned 16 years. Of the 1441 participants, physical tests were fully completed on 80.4% (1159/1441): 75.2% (545/725) in Lima and 85.8% (614/716) in Tumbes. In addition, at least one test was completed on 91.7% (1321/1441): 90.0% in Lima (653/725) and 93.2% in Tumbes (668/716).
Figure 4.
Age and sex distributions by site. Study demographic distribution shows few changes in sex and age distributions from all individuals in both sites (top two panels), all included in census (middle two panels) and all recruited (bottom two panels). Thus, sampling and recruitment shows no bias. Also, very few differences in distribution are seen between sites, comparing left and right. The x-axis refers to per cent contribution of each population to the total population. The y-axis refers to age of participants. White bars refer to boys and black bars refer to girls.
Questionnaire
All participants completed the questionnaire in full in the presence of at least one parent or guardian. There were few missing data. In reviewing 35 random surveys from each site, we found that 0.1% and <0.1% of survey fields were filled out incorrectly in Lima and Tumbes, respectively. We also found rates of 0.02% and 0.1% for data entry errors in Lima and Tumbes, respectively. These two sets of values represent errors following daily review of new questionnaires and quality checks of double data entry.
Spirometry
In Lima, 86% (625/725) of recruited participants completed spirometry according to our prespecified criteria, while in Tumbes, that value was 90% (646/716). Overall, this represents 88% of all recruited children (1271/1441). Of all children who were approached for spirometry at all, 97.2% (1271/1308) successfully completed the testing within three visits: 96.7% (625/646) in Lima and 97.6% (646/662) in Tumbes.
The overall percentage of spirometry tests that resulted in both acceptable and reproducible prebronchodilator and postbronchodilator tests was 77%. Our acceptability criteria were based directly on the ATS/ERS and were as follows: expiration ≥6.0 s; back-extrapolation volume <5.0% of the FVC or <150 ml, whichever is larger; the end-expiratory plateau <25 ml/s and no evidence of cough, glottis closure or leaks upon curve review.30 Reproducibility was determined per previously described guidelines.
Using these criteria, 85.5% of all prebronchodilator spirometry appointments were acceptable and reproducible and 91.7% of postbronchodilator efforts were acceptable (79.8% of all appointments). When applying prebronchodilator to postbronchodilator reproducibility criteria, 76.8% of all appointments were entirely acceptable and reproducible.
Concerned that the ATS/ERS reproducibility criteria were too stringent, we decided to analyse reproducibility as per a modified version of spirometry criteria published by Ferguson et al31 In brief, the criteria are as follows: Grade 1 (≥3 curves acceptable per the ATS/ERS criteria with the best two repeatable within 150 ml for FEV1 or FVC); Grade 2 (≥2 acceptable curves, repeatable within 150 ml for FEV1 or FVC); Grade 3 (≥2 acceptable curves, repeatable within 250 ml FEV1 or FVC); Grade 4 (one acceptable curve); and, Grade 5 (no acceptable curves). We only considered Grades 1–3 to be acceptable and reproducible. Using these criteria, 97.4% of all prebronchodilator spirometry appointments were acceptable and reproducible and 99.2% of postbronchodilator efforts were acceptable (86.4% of all appointments). Thus, employing Ferguson criteria would have included a slightly greater number of tests in our study and reduced the number of return visits.
Allergy skin testing
We placed 1244 skin allergy tests (Lima=614, Tumbes=630). In Lima, 96.9% of positive control tests resulted in positive reactions, while 85.8% of negative controls resulted in negative reactions. In Tumbes, a similar 95.1% of positive control tests were interpreted as positive, while 98.7% of negative controls showed up as negative. This disparity between negative control testing in Lima and Tumbes may reflect increased baseline skin irritability in Lima, given higher observed rates of atopy in Lima.
Prevalence of asthma
We found current asthma prevalence to be 12% (84/725) in Lima and 3% (22/716) in Tumbes. Current asthma was defined as wheeze symptoms and/or use of asthma medication in the past 12 months. In Lima, 52% (44/84) of patients with asthma presented with mild intermittent asthma, and in Tumbes, 55% (12/22) of patients with asthma were classified as mild intermittent, as shown in table 2. Only 5% (4/84) of patients with asthma in Lima presented with severe persistent symptoms compared with 14% (3/22) in Tumbes.
Table 2.
Asthma severity by site
| Asthma severity classification, N (%) | Lima (N=84) | Tumbes (N=22) |
| Mild intermittent | 44 (52.4) | 12 (54.5) |
| Mild persistent | 18 (21.4) | 4 (18.2) |
| Moderate persistent | 18 (21.4) | 3 (13.6) |
| Severe persistent | 4 (4.8) | 3 (13.6) |
Environmental testing
We achieved our goal of 100 household 48-h PM measurements in Lima and 70 in Tumbes. Outdoor PM10 levels recorded by the Peruvian agency DIGESA during the study, calendar year 2009, were 78.6 μg/m3 and showed highest levels during the cooler months of May, June and July. Outdoor PM2.5 levels were much lower at 40.2 μg/m3 and did not show the same peak during the cooler months. While no international outdoor PM2.5 concentration standard exists, these are 2.7-fold higher than US Environmental Protection Agency standard.
DNA collection
Overall, 1326/1441 (92%) of study participants agreed to give a blood sample. We successfully collected blood from 568 participants in Lima (78%) and 578 participants (81%) in Tumbes. We also collected saliva samples in 65 participants in Lima (9%) and 83 participants in Tumbes (12%) who did not perform or refused blood draw. Overall amounts of DNA obtained from blood extractions have been 25.6 μg, with a 260/280 ratio of 1.86. Overall average amounts of DNA extracted from saliva samples have been slightly higher at 28.0 μg, with a comparable 260/280 ratio of 1.89 (table 3).
Table 3.
Quality and quantity of DNA extraction
| Lima | Tumbes | Overall | |
| Blood, mean (SD) | n=568 | n=578 | n=1146 |
| Mass (μg) | 13.6 (6.7) | 36.3 (29.3) | 25.6 (24.6) |
| 260/280 | 1.86 (0.04) | 1.87 (0.14) | 1.86 (0.11) |
| Saliva, mean (SD) | n=65 | n=83 | n=148 |
| Mass (μg) | 36.2 (32.9) | 23.1 (17.1) | 28.0 (24.9) |
| 260/280 | 1.88 (0.08) | 1.89 (0.07) | 1.89 (0.08) |
Discussion
Our study portends to offer a comprehensive understanding of asthma in two regions of Peru. It will provide prevalence, exposure and risk factor data and highlight differences in asthma between rural and urban areas. We employed questionnaires on asthma symptoms, spirometry with reversibility, skin allergy testing, eNO testing and blood collection. In collecting this breadth of data, we will not only address our initial study question as to the disparity in asthma rates across the two regions but we hope to contribute information regarding differences between genetics and environmental exposures and to explore interactions between the two. There is potential for a longitudinal cohort built into the Peru Urban versus Rural Asthma Study, as nearly all families granted permission for future contact.
Our study implemented large-scale organisation and quality control methods across separate study sites. Available census data at both study sites were an invaluable tool for our work. Standardisation and regular sharing of information across sites proved a successful method to ensure seamless data collection and facilitate ease of comparing data. All fieldworkers were trained under the supervision of the same project administrators, and field visits by all administrators at each site over the course of the study helped to maintain consistency.
While we have successfully collected a range of data on both populations, this did not come without difficulties and shortcomings. The most difficult task was to find participants in by home visitation in the second phase of our study, especially in Lima. In Tumbes, most participants studied at the school within their own or neighbouring village and were thus accessible for testing. However, it was not possible to apply the same strategy in Lima, given the higher number of schools attended by the study children and the greater distances of these schools from the neighbourhood. In addition, because of the community-based nature of our study, which included home visitations in Lima, we found that it was not logistically feasible to perform exercise challenge testing as an additional marker of asthma. Transporting a stationary bike and necessary accessories would have been very difficult in Lima, where we visited children in their homes and could not work in schools. Administering running exercise tests would have also been quite difficult in the urban environment of Pampas de San Juan de Miraflores, given that many of the streets in this community are on an incline.
It was more common in Tumbes for at least one parent to be at home at any point during the day. Therefore, coordinating second appointments in Lima after initial recruitment was difficult in many cases and impossible in others. We attempted to optimise our time by contracting transportation workers in Lima to work with the team, decreasing travel time between appointments, but we were still limited by participant availability. In future population-based work that includes physical testing in our community and in similar studies, we recommend that all questionnaires and testing be performed during these home visits on the same day of enrolment to minimise loss to follow-up. Finally, we required several return visits to meet the ATS/ERS spirometry reproducibility criteria. Had we used less stringent standards, namely the Ferguson criteria,31 we would have been able to include a slightly greater number of tests while saving time by not having to return to participants whose first appointments did not meet the ATS/ERS criteria.
Nonetheless, our study shows that we can collect extensive high-quality data by home visitation in a resource-poor setting, and therefore, this type and quality of data may be collected in other, though not necessarily all, resource-poor settings as well. To this point, based on the data collected by the methods described in this paper, we have published two initial manuscripts.38 39 Because of the quality of data that we describe, we can be confident in the quality and fidelity of our findings in these separate analyses. We are currently working on side projects that more closely target identified patients with asthma, assess quality of life as a result of asthma or even elucidate the value of eNO for the identification of new-onset asthma in a population-based study.40 Most importantly, we hope to combine the asthma symptoms and environmental results of our study with extensive genetic analyses via candidate gene and genome-wide association studies. Once the results are collected for our study and other projects, we will be able to design targeted interventions to at-risk children via health presentations in schools, meetings with community leaders or even provide the data to the Peruvian government to begin to affect change in pollution regulations.
Conclusions
In conclusion, we successfully conducted a cross-sectional asthma prevalence study in two diverse settings in Peru. We were able to show that high-quality data on asthma symptoms, measures of airflow obstruction and markers of airway inflammation can be successfully collected by home visitation in a resource-poor setting. From preliminary data, we are confident that this testing will reliably demonstrate differences in asthma rates as well as the characteristics of patients with asthma between the two sites. While we hope to better describe asthma in the context of a developing country, we also seek to better characterise the various factors contributing to a burdensome health issue that is growing internationally.
Supplementary Material
Acknowledgments
Lincoln Diagnostics (Decatur, Illinois, USA) and ALK-Abello (Round Rock, Texas, USA) generously donated all skin prick atopy kits and antigens, respectively, used in this study. Aerocrine (New Providence, New Jersey, USA) provided us, at discount, materials for eNO testing.
Footnotes
To cite: Robinson CL, Baumann LM, Gilman RH, et al. The Peru Urban versus Rural Asthma (PURA) Study: methods and baseline quality control data from a cross-sectional investigation into the prevalence, severity, genetics, immunology and environmental factors affecting asthma in adolescence in Peru. BMJ Open 2012;2:e000421. doi:10.1136/bmjopen-2011-000421
Contributors: All authors were involved in the study design and writing of the manuscript and reviewed the final manuscript before submission. CR directly contributed to the study design, supervision of field activities in Tumbes and Lima, is responsible for data management, data analysis and the writing of this manuscript. LB contributed equally to the study design, administration of fieldwork and data management and analysis. RG is the main investigator at the Lima site and provided technical support during the conduct of the study. KR, JMC and AG were the on-site study physicians who performed most physical testing and were extensively involved in administering fieldwork. LC is the fieldwork supervisor at the Lima site, provided census data and technical support. GG was the physician supervisor for research activities at the Tumbes site and provided census data and technical support. NH and RAW contributed to the study design and provided technical support for the study. KB contributed to the study design and provided logistical support for the study. PB contributed to study design of the environmental measurements and provided both technical support and equipment for our study. WC had ultimate oversight over study design and administration and was equally responsible for the analysis and writing of the manuscript. He also served as a mentor to CR and LB throughout the conduct of the study.
Funding: Funding for the study was provided primarily by the Johns Hopkins Center for Global Health and the Fogarty International Center (Grant R24 TW007988). WC was further supported by a Clinician Scientist Award from the Johns Hopkins University and a K99/R00 Pathway to Independence Award (K99HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health and by a contract (HHSN268200900033C) with the National Heart, Lung and Blood Institute, National Institutes of Health. CR was a Fogarty International Clinical Research Scholar during the time of the study and was further supported by Tufts University School of Medicine. LB was supported by a NIH T35 Training Grant (T35AI065385). Additional support came from A.B. PRISMA and collaborators at JHU.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by JHU, PRISMA.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional unpublished data are currently available for public use.
References
- 1.Anonymous. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998;351:1225–32 [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78 [DOI] [PubMed] [Google Scholar]
- 3.Odhiambo JA, Ng'ang'a LW, Mungai MW, et al. Urban-rural differences in questionnaire-derived markers of asthma in Kenyan school children. Eur Respir J 1998;12:1105–12 [DOI] [PubMed] [Google Scholar]
- 4.Yemaneberhan H, Bekele Z, Venn A, et al. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet 1997;350:85–90 [DOI] [PubMed] [Google Scholar]
- 5.Romieu I, Meneses F, Sienra-Monge JJ, et al. Effects of urban air pollutants on emergency visits for childhood asthma in Mexico City. Am J Epidemiol 1995;141:546–53 [DOI] [PubMed] [Google Scholar]
- 6.Carbajal-Arroyo L, Barraza-Villarreal A, Durand-Pardo R, et al. Impact of traffic flow on the asthma prevalence among school children in Lima, Peru. J Asthma 2007;44:197–202 [DOI] [PubMed] [Google Scholar]
- 7.Wong GW, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol 2008;43:107–16 [DOI] [PubMed] [Google Scholar]
- 8.Penny ME, Murad S, Madrid SS, et al. Respiratory symptoms, asthma, exercise test spirometry, and atopy in schoolchildren from a Lima shanty town. Thorax 2001;56:607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazo-Velásquez JC, Lozada AR, Cruz HM. Evaluation of severity of bronchial asthma through an exercise bronchial challenge. Pediatr Pulmonol 2005;40:457–63 [DOI] [PubMed] [Google Scholar]
- 10.Recabarren A, Apaza C, Castro-Rodriguez JA. Nitrites in induced sputum as a simple and cheap non-invasive marker of airway inflammation for asthmatic schoolchildren. Pediatr Allergy Immunol 2008;19:433–7 [DOI] [PubMed] [Google Scholar]
- 11.Ng'ang'a LW, Odhiambo JA, Mungai MW, et al. Prevalence of exercise induced bronchospasm in Kenyan school children: an urban-rural comparison. Thorax 1998;53:919–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet 2008;372:1049–57 [DOI] [PubMed] [Google Scholar]
- 13.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007;120:565–9 [DOI] [PubMed] [Google Scholar]
- 14.Sherrill DL, Stein R, Halonen M, et al. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol 1999;104:28–36 [DOI] [PubMed] [Google Scholar]
- 15.Burgess JA, Dharmage SC, Byrnes GB, et al. Childhood eczema and asthma incidence and persistence: a cohort study from childhood to middle age. J Allergy Clin Immunol 2008;122:280–5 [DOI] [PubMed] [Google Scholar]
- 16.Brauer M, Hoek G, Van Vliet P, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 2002;166:1092–8 [DOI] [PubMed] [Google Scholar]
- 17.Lee YL, Lin YC, Hsiue TR, et al. Indoor and outdoor environmental exposures, parental atopy, and physician-diagnosed asthma in Taiwanese schoolchildren. Pediatrics 2003;112:e389. [DOI] [PubMed] [Google Scholar]
- 18.Stone V. Environmental air pollution. Am J Respir Crit Care Med 2000;162:S44–7 [DOI] [PubMed] [Google Scholar]
- 19.Henderson AJ. The effects of tobacco smoke exposure on respiratory health in school-aged children. Paediatr Respir Rev 2008;9:21–7; quiz 27–8. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan D, Meade G, Foley VM, et al. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 2001;360:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLeish S, Turner SW. Gene-environment interactions in asthma. Arch Dis Child 2007;92:1032–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenten M, Gauderman WJ, Berhane K, et al. Functional variants in the catalase and myeloperoxidase genes, ambient air pollution, and respiratory-related school absences: an example of epistasis in gene-environment interactions. Am J Epidemiol 2009;170:1494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz DA. Gene-environment interactions and airway disease in children. Pediatrics 2009;123(Suppl 3):S151–9 [DOI] [PubMed] [Google Scholar]
- 24.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007;120(5 Suppl):S94–138 [DOI] [PubMed] [Google Scholar]
- 25.Rodway GW, Choi J, Hoffman LA, et al. Exhaled nitric oxide in the diagnosis and management of asthma: clinical implications. Chron Respir Dis 2009;6:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilman RH, Marquis GS, Miranda E, et al. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet 1988;1:343–5 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Concha D, Gilman RH, Gilman JB. A home nutritional rehabilitation programme in a Peruvian peri-urban shanty town (pueblo joven). Trans R Soc Trop Med Hyg 1991;85:809–13 [DOI] [PubMed] [Google Scholar]
- 28.Mata Fernandez C, Fernandez-Benitez M, Perez Miranda M, et al. Validation of the Spanish version of the Phase III ISAAC questionnaire on asthma. J Investig Allergol Clin Immunol 2005;15:201–10 [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38 [DOI] [PubMed] [Google Scholar]
- 30.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax 2006;61:744–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferguson GT, Enright PL, Buist AS, et al. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest 2000;117:1146–61 [DOI] [PubMed] [Google Scholar]
- 32.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun 2006;7:95–100 [DOI] [PubMed] [Google Scholar]
- 33.Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 2011;43:887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu LJ, Slaughter JC, Larson TV. Comparison of light scattering devices and impactors for particulate measurements in indoor, outdoor, and personal environments. Environ Sci Technol 2002;36:2977–86 [DOI] [PubMed] [Google Scholar]
- 35.Quintana PJ, Samimi BS, Kleinman MT, et al. Evaluation of a real-time passive personal particle monitor in fixed site residential indoor and ambient measurements. J Expo Anal Environ Epidemiol 2000;10:437–45 [DOI] [PubMed] [Google Scholar]
- 36.Howard-Reed C, Rea AW, Zufall MJ, et al. Use of a continuous nephelometer to measure personal exposure to particles. J Air Waste Manag Assoc 2000;50:1125–3 [DOI] [PubMed] [Google Scholar]
- 37.Chakrabarti B, Fine PM, Delfino R, et al. Performance evaluation of the active-flow personal DataRAM PM2.5 mass monitor (Thermo Anderson pDR-1200) designed for continuous personal exposure measurements. Atmos Environ 2004;38:3329–40 [Google Scholar]
- 38.Robinson CR, Baumann LM, Romero K, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 2011;66:1051–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumann LM, Robinson CR, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J Allergy Clin Immunol 2011;127:875–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olin AC, Rosengren A, Thelle DS, et al. Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population. Am J Respir Crit Care Med 2010;181:324–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.