Abstract
Objective
To determine the association between diabetes mellitus (DM) and marijuana use.
Design
Cross-sectional study.
Setting
Data from the National Health and Nutrition Examination Survey (NHANES III, 1988–1994) conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
Participants
The study included participants of the NHANES III, a nationally representative sample of the US population. The total analytic sample was 10 896 adults. The study included four groups (n=10 896): non-marijuana users (61.0%), past marijuana users (30.7%), light (one to four times/month) (5.0%) and heavy (more than five times/month) current marijuana users (3.3%). DM was defined based on self-report or abnormal glycaemic parameters. We analysed data related to demographics, body mass index, smoking status, alcohol use, total serum cholesterol, high-density lipoprotein, triglyceride, serum 25-hydroxy vitamin D, plasma haemoglobin A1c, fasting plasma glucose level and the serum levels of C reactive protein and four additional inflammatory markers as related to marijuana use.
Main outcome measures
OR for DM associated with marijuana use adjusted for potential confounding variables (ie, odds of DM in marijuana users compared with non-marijuana users).
Results
Marijuana users had a lower age-adjusted prevalence of DM compared to non-marijuana users (OR 0.42, 95% CI 0.33 to 0.55; p<0.0001). The prevalence of elevated C reactive protein (>0.5 mg/dl) was significantly higher (p<0.0001) among non-marijuana users (18.9%) than among past (12.7%) or current light (15.8%) or heavy (9.2%) users. In a robust multivariate model controlling for socio-demographic factors, laboratory values and comorbidity, the lower odds of DM among marijuana users was significant (adjusted OR 0.36, 95% CI 0.24 to 0.55; p<0.0001).
Conclusions
Marijuana use was independently associated with a lower prevalence of DM. Further studies are needed to show a direct effect of marijuana on DM.
Article summary
Article focus
We hypothesised that the prevalence of DM would be reduced in marijuana users due to the presence of one or more cannabinoids because of their immunomodulatory and anti-inflammatory properties.
Key messages
Marijuana use was associated with a decreased prevalence of DM.
Prospective studies in rodents and humans are needed to determine a causal relationship between cannabinoid receptor activation and DM.
Until those studies are performed, we do not advocate the use of marijuana in patients at risk for DM.
Strengths and limitations of this study
Population-based national representative sample of the USA.
Cross-sectional data.
Marijuana use was based on self-report, and self-report of illicit substances is often underestimated on self-reports. Self-report is subjected to recall bias. However, we expect that recall bias would be similar in those with DM as those without DM and would be unlikely to bias our results.
Although current marijuana users were divided into heavy and light users based on the number of times they reported using marijuana per month, the amount of marijuana consumed, route of consumption (inhaled vs oral), duration of use and time when they quit were not reported.
Introduction
The prevalence of type 2 diabetes mellitus (DM) is increasing, and it is projected that in the USA alone, type 2 DM will increase to 48.3 million by 2050.1 In addition to defects in pancreatic β-cell function and insulin sensitivity, systemic inflammation is thought to be involved in its pathogenesis.1 2
Marijuana is the most commonly used illicit drug in the USA and is currently used by 14.4 million Americans.3 The Cannabis sativa (marijuana) plant contains bioactive components termed cannabinoids (CB). The major psychoactive CB is delta 9-tetrahydrocannabinol (THC) whose effect is mediated through the CB1 and the CB2 subtypes of CB receptors found in the brain and lymphoid tissues.4 The endocannabinoids, a group of neuromodulatory lipids also bind to these receptors.5 Cannabis, THC and other CBs have been shown to have both beneficial6 and detrimental effects.7 Marijuana users have higher caloric intake while eating less nutrient-rich foods,8 yet have similar8 or slightly lower9 body mass index (BMI) than non users.
We hypothesised that the prevalence of DM would be reduced in marijuana users due to the presence of one or more CBs because of their immunomodulatory and anti-inflammatory properties.4 We assessed the association between DM and marijuana use among adults aged 20–59 years in a national sample of the general population.
Methods
Study population
The study included participants of the National Health and Nutrition Examination Survey (NHANES III),10 conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. NHANES III used a highly stratified multistage probability sampling (total N=39 695) and employed oversampling of the older people (N=2273), non-Hispanic blacks (N=11 061) and Mexican Americans (N=11 110). Descriptions of the survey, sampling procedures and details of the laboratory tests evaluated can be found on the Centers for Disease Control and Prevention website (http://www.cdc.gov/nchs/nhanes/nh3rrm.htm#refman).
We limited the analysis to adults aged 20–59 years, as those <20 years did not have plasma glucose testing and only participants up to 60 years old were asked about marijuana use. In addition, we excluded those with missing laboratory data. The total analytic sample was 10 896 adults (complete data for marijuana use and DM). Subjects with missing data for the laboratory variables (n=2769) were excluded, and the number for the final model was 8127 adults. A flow diagram showing the number of subjects selected and reasons for exclusion is listed in supplement figure 1.
Study variables
Data on marijuana use were collected by self-report. Non-marijuana users included never users (n=6667) and those who reported ever having used marijuana, but who had not used marijuana in the past month (ie, past users) (n=3346). We classified participants who reported using marijuana in the past month by frequency of use as either light current users (≤4 days per month (n=557)) or heavy current users (≥5 days per month (n=326)) as previously described.9 The definition of marijuana for purposes of this survey includes ‘hash,’ ‘pot’ or ‘grass’ or any other references to the Cannabis plant. The phrase ‘used marijuana’ refers to either smoking or ingesting marijuana.
Subjects were defined as having DM if they answer ‘yes’ to the question ‘Have you ever been told you have sugar/diabetes?’ (n=525) or had a fasting blood glucose level ≥126 mg/dl (n=194). Of the 719 patients with DM, 418 answered the question about whether they take insulin and 116 reported that they do take insulin. Of those, nine reported that they began using insulin at age ≤20 years, the majority being likely to have type 1 DM, although a few may have had type 2 DM. Thus, we estimate that 1.5% of patients with DM (unadjusted) had type 1 DM, and because of this low number, we analysed all subjects with DM together. There was no difference in any of our analyses if the nine patients of age ≤20 years were excluded.
The study included 151 pregnant women (1.5%). Of them, eight women had diabetes. There was no difference in the use of marijuana by DM. Because of the low number in the diabetes category, we included them in the analysis. A series of sensitivity analyses excluding the pregnant women showed no difference.
Plasma glucose and whole blood haemoglobin A1c (HbA1c) were measured at the University of Missouri-Columbia School of Medicine Department of Child Health, Diabetes Reference Laboratory, Columbia, Missouri, by David Goldstein, MD, director.11
Subjects were classified as obese/non-obese according to the BMI level using a cut-off of 30 kg/m2.
We analysed data related to DM, age, gender, race/ethnicity, education level, family history of DM, physical activity, BMI, cigarette smoking, cocaine use, alcohol use, total serum cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, serum 25-hydroxy vitamin D (vitamin D), HbA1c, fasting plasma glucose level, C reactive protein (CRP) level and the serum levels of less robust inflammatory markers (ferritin, fibrinogen, white blood cell (WBC) count and uric acid) that have been previously used in NHANES III analysis.12 Physical activity was assessed using self-report to several questions (http://www.cdc.gov/nchs/nhanes/nh3rrm.htm#refman). For the physical activity variable, subjects were classified as inactive if they did not report engaging in any of the following activities during the previous month: walking, jogging, bike riding, swimming, aerobics, dancing, calisthenics, gardening, lifting weights or other physical activity outside their occupation. Physical activity was classified as moderate or vigorous intensity based on metabolic equivalent intensity levels. Individuals were considered to fulfil national recommendations for physical activity if they reported five or more episodes per week of moderate-intensity physical activity or three or more episodes per week of vigorous-intensity physical activity.
Statistical analysis
Descriptive statistics were used to characterise the subjects (mean and SD for continuous variables, and percentages for categorical variables). To test the statistical difference between the groups, we used χ2 test for categorical variables and two-sided t tests for continuous variables. A p value of <0.05 was considered significant.
Univariate and multivariate logistic regression analyses (for categorical outcome—DM/no DM) were used to determine the relationship between DM and marijuana use. We used multivariate logistic regression to adjust for confounding variables and reported the OR and the 95% CI. Variables considered as possible confounders in the multivariate analysis were age, gender, race/ethnicity, BMI, education level, cigarette smoking, alcohol use, physical activity, serum total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, vitamin D, CRP, ferritin, fibrinogen, WBC count and uric acid. In order to confirm that marijuana use was associated with DM and not due to confounders, we analysed how each potential confounder changed the OR of having DM. Variables that changed the OR by ≥10% were considered as confounders and included in the multivariate model.
We performed stratified analysis to test for effect modification. For effect modifier variable, multivariate logistic regression model was constructed for each subgroup.
In addition, to help adjust for selection bias, we analysed the data using the propensity score matching and estimated the average treatment effect for the treated, bootstrap SE and t statistics. We added the propensity score to the logistic regression model as inverse weight, blocks (N=8) that satisfy the balancing property and quartiles.
Data were analysed using SAS (Release V.9.1.3, 2002; SAS, Inc.) and the survey module of STATA (Release V.10, 1984–2007 Statistics/Data Analysis; StataCorp). Sample weights, provided by the National Center for Health Statistics, were used to correct for differential selection probabilities and to adjust for non-coverage and non-response.13
Results
Among NHANES III participants aged 20–59 years, there were 6667 (54.5%) non-marijuana users, 3346 (36.7%) past marijuana users, 557 (5.5%) light current users and 326 (3.3%) heavy current users. As shown in table 1, current and past marijuana users tended to be <40 years old, be male, had a BMI of <30 kg/m2, smoked cigarettes and used alcohol and cocaine more frequently compared to non-marijuana users. Compared to non-marijuana users, past users tended to be white and to have a college education, while current users included more white and black subjects and were more likely to have a high school education or less. Non-marijuana users, past and current marijuana users had a similar percentage of family history of DM (p>0.05) but significantly different percentage of physical activity levels (p<0.001), with past and current marijuana users being more active than non-marijuana users.
Table 1.
Diabetes mellitus (DM), socio-demographic characteristics, body mass index, general laboratory characteristics and select inflammatory markers of adults aged 20–59 years who were self-identified as marijuana users or non-marijuana users (N=10 896)
| Non-marijuana users, weighted % or mean (SD)* | Past users, weighted % or mean (SD)* | Current users |
||
| 1–4 times/month, weighted % or mean (SD)* | ≥5 times/month, weighted % or mean (SD)* | |||
| DM (yes) | 6.3 | 2.9† | 1.9† | 3.0† |
| Age (years) | ||||
| ≤40 | 46.2 | 73.4† | 86.6† | 84.0† |
| >40 | 53.8 | 26.6† | 13.4† | 16.0† |
| Gender | ||||
| Male | 42.9 | 53.6† | 64.4† | 73.0† |
| Female | 57.1 | 46.5† | 35.6† | 27.0† |
| Race | ||||
| White | 70.6 | 81.2† | 72.6† | 74.9† |
| Black | 11.0 | 11.3† | 17.3† | 14.6† |
| Hispanic | 7.4 | 4.1† | 3.6† | 4.4† |
| Asian/other | 11.0 | 3.5† | 6.5† | 6.2† |
| Education | ||||
| ≤High school | 21.4 | 15.3† | 23.1† | 25.7‡ |
| High school | 34.8 | 33.4† | 41.2† | 39.2‡ |
| College | 43.8 | 51.3† | 35.7† | 35.1‡ |
| Family history of DM | ||||
| Yes | 46.2 | 48.3 | 52.5 | 48.3 |
| No | 53.8 | 58.3 | 47.5 | 51.7 |
| BMI (kg/m2) | ||||
| ≥30 | 25.0 | 18.8† | 16.6† | 12.5† |
| <30 | 75.0 | 81.2† | 83.4† | 87.5† |
| Physical activity | ||||
| Inactive | 17.0 | 10.3† | 8.0† | 10.9† |
| Insufficient activity | 46.0 | 47.5† | 48.6† | 37.3† |
| Recommended level of activity | 37.1 | 42.2† | 43.4† | 51.8† |
| Cigarette smoke | ||||
| Yes | 22.0 | 40.1† | 60.3† | 68.0† |
| No | 78.0 | 59.9† | 39.7† | 32.0† |
| Alcohol | ||||
| Yes | 57.3 | 74.7† | 90.4† | 87.9† |
| No | 42.7 | 25.3† | 9.6† | 12.1† |
| Cocaine | ||||
| Yes | 0.7 | 25.8† | 47.2† | 58.8† |
| No | 99.3 | 74.2† | 52.8† | 41.2† |
| BMI (kg/m2) | 28.0 (0.2) | 26.9 (0.3)† | 24.8 (0.7)† | 24.1 (0.4)† |
| Prevalence of select laboratory characteristics | ||||
| HDL (mg/dl) | ||||
| ≤40 | 42.6 | 39.5§ | 29.8§ | 22.5§ |
| >40 | 57.4 | 60.6 | 70.2 | 77.5 |
| LDL (mg/dl) | ||||
| ≥160 | 16.1 | 13.9 | 9.6¶ | 3.5¶ |
| <160 | 83.9 | 86.1 | 90.4 | 96.5 |
| Total cholesterol (mg/dl) | ||||
| ≥240 | 18.7 | 12.3§ | 8.1§ | 8.6§ |
| <240 | 81.3 | 87.7 | 91.9 | 91.4 |
| Triglyceride (mg/dl) | ||||
| ≥200 | 17.7 | 13.5† | 13.1† | 12.7† |
| <200 | 82.3 | 86.5 | 86.9 | 87.3 |
| CRP (mg/dl) | ||||
| ≥0.5 | 18.9 | 12.7§ | 15.8§ | 9.2§ |
| <0.5 | 81.1 | 87.3 | 84.2 | 90.8 |
| 25-Hydroxy vitamin D (nmol/l) | ||||
| ≤70 | 51.9 | 41.1§ | 56.4§ | 59.5§ |
| >70 | 48.1 | 58.9 | 43.6 | 40.5 |
| HbA1c | ||||
| ≥6.0 | 8.7 | 4.1§ | 4.2§ | 3.2§ |
| <6.0 | 91.3 | 96.0 | 95.8 | 96.8 |
| Serum levels of select laboratory values | ||||
| Glucose (mg/dl) | 97.8 (0.6) | 93.1 (0.7)† | 92.7 (0.6)† | 92.3 (0.7)† |
| HDL (mg/dl) | 50.1 (0.5) | 50.5 (0.8) | 49.8 (1.8)‡ | 50.5 (1.4) |
| LDL (mg/dl) | 126.4 (1.2) | 122.3 (1.5)† | 116.6 (3.1)† | 113.1 (3.7)† |
| Total cholesterol (mg/dl) | 201.6 (1.4) | 195.2 (1.6)† | 188.7 (3.3)† | 186.9 (3.3)† |
| Triglycerides (mg/dl) | 125.4 (2.2) | 112.1 (2.9)† | 111.7 (6.9)† | 116.6 (7.3)† |
| 25-Hydroxy vitamin D (nmol/l) | 73.0 (1.2) | 80.5 (1.8)† | 78.0 (3.5)† | 85.0 (4.8)† |
| Select inflammatory markers | ||||
| CRP (mg/dl) | 0.43 (0.01) | 0.36 (0.02)† | 0.42 (0.09) | 0.44 (0.11) |
| Ferritin (ng/ml) | 138.1 (2.9) | 149.5 (9.1)† | 132.5 (19.3) | 157.8 (24.1)¶ |
| Fibrinogen (mg/dl) | 296.1 (4.1) | 283.5 (3.7)† | 287.8 (15.3) | 285.2 (12.4) |
| Uric acid (mg/dl) | 5.3 (0.04) | 5.5 (0.08)† | 5.1 (0.3)† | 5.1 (0.2)† |
| White blood cell count (×103/μl) | 7.2 (0.06) | 7.3 (0.07) | 7.6 (0.19)† | 8.0 (0.21)† |
We used the sample weight provided by the National Center for Health Statistics to weigh the data.
p<0.001 compared with non-marijuana users.
p<0.05 compared with non-marijuana users.
p<0.0001 compared with non-marijuana users.
p<0.01 compared with non-marijuana users.
BMI, body mass index; HbA1c, haemoglobin A1c; LDL, low-density lipoproteins; CRP, C reactive protein; HDL, high-density lipoproteins.
As shown in supplement table 1, marijuana users (past and current) had a lower adjusted prevalence of DM, but not hypertension, stroke, myocardial infarction or heart failure compared to non-marijuana users.
The unadjusted prevalence of DM for non-marijuana users, past marijuana users, current light marijuana users and current heavy marijuana users was 6.3%, 2.9%, 1.9% and 3.0%, respectively, and there was a statistically significant difference between the groups (p<0.0001) (table 1). For subjects without DM (n=10 165), 46.4% were marijuana users and 53.6% were non-marijuana users (p<0.0001) (supplement table 2). For subjects with DM (n=719), 26.9% were marijuana users and 73.1% were non-marijuana users (p<0.0001). The difference in % of marijuana users between those with and without DM was highly significant (p<0.0001).
As shown in table 1, all marijuana users had a higher prevalence of serum HDL cholesterol >40 mg/dl, total cholesterol <240 mg/dl and triglycerides <200 mg/dl compared to non-users (p<0.0001). Current marijuana users had a higher prevalence of LDL cholesterol <160 mg/dl (p<0.05). All marijuana users had a higher prevalence of CRP <0.5 mg/dl (p<0.0001). Past users, but not current users, had a lower prevalence of vitamin D level <70 nmol/l compared to non-users (p<0.0001). All marijuana users had a higher prevalence of plasma HbA1c <6.0% (p<0.0001). Serum glucose levels and BMI were lower in all marijuana user groups compared to non-marijuana users (table 1).
We then examined the variation of markers of inflammation with marijuana use (table 1). Serum CRP and fibrinogen were significantly (p<0.001) lower in past marijuana users compared to current and non-marijuana users suggesting lower inflammation in past marijuana users. In contrast, serum ferritin levels were higher in past and current heavy users, and lower in light users, compared to non-users. Serum uric acid levels were higher in past and lower in current users compared to non-users. WBC count was higher among current users relative to non-users and past users.
In order to confirm that marijuana use was associated with a decreased prevalence of DM and not due to confounders, we analysed how each potential confounder changed the OR of having DM. Variables that changed the OR by ≥10% were considered as confounders (addition of age, BMI, alcohol use, total cholesterol, triglyceride, CRP and hypertension changed the OR by ≥10% from 0.42 to 0.60, 0.49, 0.50, 0.46, 0.47, 0.46, 0.46, respectively) (supplement table 3). Table 2 shows the unadjusted as well as the cumulative effect of the confounders, including race/ethnicity, physical activity and those variables that showed changes of ≥10% in the OR of having DM among all marijuana users relative to non-users in a series of regression models. Of note, race/ethnicity and physical activity did not change the OR by ≥10%, but we included them in the model because they are known risk factors. The interaction effect of the marijuana use and age was significant in the model indicating that age is an effect modifier (ie, the association between DM and marijuana use was modified by age and the association differed for different age groups) (OR for interaction=1.83, 95% CI 1.2 to 2.9). Stratified analysis by age group found an association between marijuana use and DM among subjects aged >40 years (p<0.01) and no association among subjects aged ≤40 years (table 2). The association of DM and marijuana was significant in both the overall and older age group even after adjusting for social variables (race/ethnicity, physical activity, alcohol use and BMI), laboratory variables (total cholesterol and triglyceride), inflammatory marker (CRP) and the comorbidity variable (hypertension) to the previous model.
Table 2.
Multivariate logistic model for the change in the association between marijuana use and DM (total and by age group) (N=8127)
| Outcome: DM | Total | Age group 41–59 years | Age group 20–40 years |
| Model | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Unadjusted | 0.42 (0.33 to 0.55)* | 0.51 (0.36 to 0.73)* | 0.74 (0.48 to 1.14) |
| Model 1: adjusted for social variables | 0.31 (0.20 to 0.48)* | 0.34 (0.20 to 0.59)* | 0.80 (0.43 to 1.50) |
| Model 2: adjusted for social variables and laboratory variables | 0.33 (0.22 to 0.50)* | 0.35 (0.21 to 0.61)* | 0.87 (0.47 to 1.63) |
| Model 3: adjusted for social variables, laboratory variables and inflammatory marker | 0.36 (0.24 to 0.54)* | 0.37 (0.21 to 0.63)* | 0.95 (0.50 to 1.78) |
| Model 4: adjusted for social variables, laboratory variables, inflammatory marker and comorbidity | 0.36 (0.24 to 0.55)* | 0.37 (0.22 to 0.62)* | 0.93 (0.51 to 1.70) |
OR for having DM among marijuana users compared to non-users and 95% CI.
Social variables: race/ethnicity, physical activity, alcohol use, interaction of alcohol and marijuana use and body mass index.
Laboratory variables: total cholesterol and triglyceride.
Inflammatory marker: C reactive protein.
Comorbidity: hypertension.
p<0.0001 compared to non-marijuana users.
Using the propensity score matching, we found similar results showing a lower prevalence of DM among marijuana users relative to non-users. The average treatment effect for the users (total sample)=−0.024, bootstrap SE=0.005 and t=−4.46, p<0.05 (ie, marijuana users had lower prevalence of DM). When we added the propensity score to the logistic regression model, marijuana users still had lower odds of DM than non-users (OR=0.54, 95% confidence level=0.40 to 0.73, p=0.001). Adding it as inverse weight, yielded an OR=0.52 (95% confidence level=0.39 to 0.71, p=0.001). We also added it as blocks (N=8) and found an OR=0.53 (95% confidence level=0.40 to 0.73, p=0.001). Adding it as quartiles yielded an OR=0.51 (95% confidence level=0.38 to 0.69, p=0.001). All still revealed a lower odds of DM with marijuana use. For age group 41–59 years, adding the propensity score as quartiles to the model, we found an OR=0.55 (95% confidence level=0.35 to 0.88, p=0.012), whereas for age group 20–40 years, OR=0.88 (95% confidence level=0.53 to 1.47, p>0.05).
We examine whether DM as diagnosed by self-report as compared to laboratory evidence of hyperglycaemia was correlated with different prevalence of marijuana use. As shown in the supplement table 2, there was no difference in marijuana use among those with DM by self-report and those with DM who were included based on an elevated fasting glucose (p=0.43). Patients with DM by self-report who were hyperglycaemic (fasting glucose ≥126 mg/dl) at the time of sampling had a statistically similar rate of marijuana use as those whose DM was well controlled (fasting glucose <126 mg/dl) at the time of sampling (p=0.06), although there was a trend for patients with a history of DM by self-report who were euglycaemia at the time of sampling to be associated with a lower rate of non-marijuana use. Those with DM by self-report and those with DM who were included based on an elevated fasting glucose had similar rates of the type of marijuana use (heavy current users, light current users and past users). Additionally, for subjects who did not have DM by self-report and did not have an elevated fasting glucose level but had an elevated HbA1c (>7.0%) (n=22), their prevalence of non-marijuana use (72.2%) was similar to the prevalence of non-marijuana use among subjects with DM (73.1%).
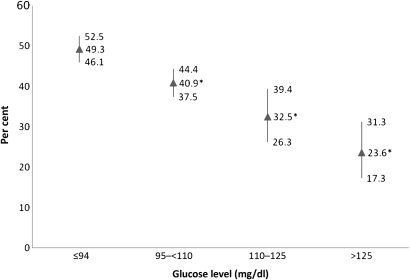
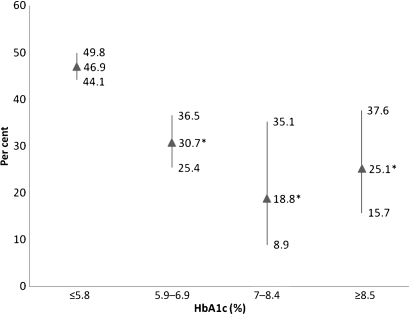
We then examined the prevalence of all marijuana users among subjects with different fasting glucose levels. As shown in figure 1, the highest prevalence of marijuana users was found in those with the lowest glucose levels. As the glucose levels increased, the prevalence of marijuana users decreased. For subjects with DM (fasting glucose >125 mg/dl), the prevalence of marijuana users was 23.6%. Similarly, the highest prevalence of marijuana users was found in those subjects with the lowest plasma HbA1c values (figure 2). As the HbA1c levels increased, the prevalence of marijuana users decreased.
Figure 1.
The prevalence of marijuana users (past and current) among subjects according to fasting glucose levels (in milligrams per decilitre). Per cent and 95% CI are depicted. *p<0.05 compared to glucose level <94 mg/dl.
Figure 2.
The prevalence of marijuana users (past and current) among subjects according to plasma haemoglobin A1c (HbA1c) levels. Per cent and 95% CI are depicted. *p<0.05 compared to <5.8%.
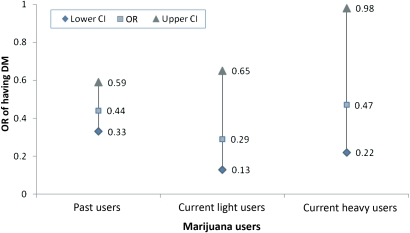
Furthermore, we analysed the data using logistic regression to assess the odds of having DM, an elevated glucose value or an elevated HbA1c for the categories of marijuana use. The OR for all marijuana users to have DM was 0.42 (95% CI 0.33 to 0.55), which was statistically significant (ie, marijuana non-users were 2.4 times more likely to have DM relative to all marijuana users). Relative to non-marijuana users, past marijuana users had an OR of having DM of 0.44 (95% CI 0.33 to 0.59), current light marijuana users had an OR of 0.29 (CI 0.13 to 0.65) and current heavy marijuana users had an OR of 0.47 (95% CI 0.22 to 0.98), all were statistically significant from non-marijuana users (p<0.001) (figure 3). Relative to non-marijuana users, marijuana users had significantly lower odds of having glucose level of >125 mg/dl (OR=0.36, 95% CI 0.24 to 0.52) and HbA1c level >7.0% (OR=0.35, 95% CI 0.22 to 0.54) (p<0.0001).
Figure 3.
OR and 95% CI of having diabetes mellitus (DM) among past and current marijuana users relative to non-marijuana users.
Discussion
Our analyses of adults aged 20–59 years in the NHANES III database showed that participants who used marijuana had lower prevalence of DM and had lower odds of DM relative to non-marijuana users. We did not find an association between the use of marijuana and other chronic diseases, such as hypertension, stroke, myocardial infarction and heart failure. This could be due to the smaller prevalence of stroke, myocardial infarction and heart failure in the examined age group.
We noted the lowest prevalence of DM in current light marijuana users, with current heavy marijuana users and past users also having a lower prevalence of DM than non-marijuana users. The finding that past marijuana users had lower odds of prevalent DM than non-users suggests that early exposure to marijuana may affect the development of DM and a window of time of marijuana exposure earlier in life could be a factor to study. Similarly, our findings of a significant association between marijuana use and DM was only found in those aged ≥40 years suggest that the possibility of some protection from marijuana use may require many years before they become manifested. By contrast, it could reflect the increased prevalence of DM with age and the ability to detect an association with a lesser sample size when there is a greater cohort at risk for DM. The possible association of light marijuana use with decreased DM is similar to that of alcohol on DM and the metabolic syndrome, in which mild alcohol use was associated with lower prevalence of DM and the metabolic syndrome,14 15 and higher alcohol use associated with higher prevalence of DM and the metabolic syndrome.14 16
Smit and Crespo9 used the NHANES III population to examine dietary factors of non-marijuana users and marijuana users among adults aged 20–59 years. Similar to our data, they found that 45% reported used marijuana in their lifetime and 8.7% used marijuana in the past month. Current marijuana users had higher intakes of energy and nutrients and consumed more soft drinks but had slightly lower BMI than non-current marijuana users. Thus, it is unlikely that a healthier diet contributed to the decreased prevalence of DM among marijuana users found in our study. In our study, all marijuana users had lower BMI than non-users, with heavy marijuana users having the lowest BMI. The lower BMI may be protective for DM, although when we controlled for BMI, the prevalence of DM was not significantly changed suggesting additional BMI-independent pathways. Smit and Crespo9 did not record glycaemic parameters or prevalence of DM.
Using NHANES III data, marijuana users had lower rates of obesity (BMI ≥30) and lower mean BMI, with current heavy marijuana users having the lowest BMI, in agreement with a recent report using National Epidemiologic Survey on Alcohol (2001–2002) and the National Comorbidity Survey–Replication (2001–2003) databases.17 Correcting for the effect of BMI, the association between marijuana use and DM was reduced by 17% but remained highly significant.
We postulate that the decreased prevalence of DM and marijuana use may be due to the anti-inflammatory properties of marijuana. CBs found in marijuana favourably modify inflammation probably through the inhibitory actions on prostaglandins and COX-2.18 Hu and colleagues2 reported that CRP, but not interleukin-6 and tumour necrosis factor-α receptor-2, was associated with the risk of developing DM. In our study, serum level of CRP, fibrinogen ferritin, uric acid and WBC counts revealed varied associations with marijuana use. Of note, the CRP assay used in NHANES III was not a highly sensitive assay11 and is unlikely to pick up small changes in an inflammatory state in a single individual; however, it is still a robust measure of inflammation and is useful in population studies.12 However, we did find a U-shaped association between the CRP levels and marijuana use groups.
Rodent studies using CBs have shown significant benefits against diabetic complications and atherosclerosis.19 20 Additionally, lower doses of CBs appear to be anti-inflammatory in rodents.19 CBs, including the non-psychoactive cannabidiol, have also been shown to attenuate progression of type 1 DM in animal models.21 22 We have not identified any study in human subjects or animals examining marijuana or its active ingredients and the incidence of type 2 DM, although one study found similar glucose levels in marijuana users as non-users.8 In a prospective study using a cannabis-based medicinal extract compared to placebo to treat diabetic neuropathy, glycaemic indices were not mentioned.23 We examined physical activity in patients using marijuana and found that it did not confound the association between marijuana and DM.
Although the CB1 antagonist, rimonabant has been used successfully to treat DM,24 we are not surprised at the association between marijuana use and decreased prevalence of DM. Marijuana contains a variety of CBs, of which some, such as cannabidiol and delta9-tetrahydrocannabivarin, have antagonist properties that may mediate the anti-inflammatory properties of marijuana.25
A limitation of our study was its cross-sectional nature. Despite the efforts of NHANES to enrol a random representative sample of the US population, persons attending the study visits may differ from those not attending in subtle ways that may affect the results of this study. We are unable to conclude that marijuana use does not lead to DM nor do we suggest that marijuana should be a treatment for DM. Although we controlled for major confounders, it is possible that non-marijuana users and subjects with DM share some, as yet unknown, characteristic accounting for the relationship between DM and non-marijuana use.
An additional limitation is that the marijuana use was based on self-report and self-report of illicit substances is often underestimated on self-reports.26 27 Self-report is subjected to recall bias. However, we expect that recall bias would be similar in those with DM as those without DM and would be unlikely to bias our results. Although current marijuana users were divided into heavy and light users based on the number of times they reported using marijuana per month, the amount of marijuana consumed, route of consumption (inhaled vs oral), duration of use and time when they quit were not reported.
A potential limitation was that most patients with DM were identified by self-report, with a smaller number of patients identified by having an elevated fasting blood glucose levels. Because some patients with DM receiving treatment are euglycaemic, blood glucose levels alone cannot be used to identify those patients with DM. However, the percentage of marijuana user was similar in those patients with DM identified by self-report as that of those with DM identified by fasting glucose testing. While we analysed all patients with DM together, we estimated that over 98% of the patients had type 2 DM, and therefore, our results are likely to apply only to patients with type 2 DM. Another limitation is the possibility of a cohort effect since those who use marijuana may have other factors that may predispose decreased prevalence of diabetes compared to non-users besides lower BMI.
In conclusion, marijuana use was associated with a decreased prevalence of DM. Prospective studies in rodents and humans are needed to determine a potential causal relationship between cannabinoid receptor activation and DM. Until those studies are performed, we do not advocate the use of marijuana in patients at risk for DM.
Supplementary Material
Acknowledgments
We thank Dr Mayer Davidson (Charles Drew University) for his helpful comments on this manuscript.
Footnotes
To cite: Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012;2:e000494. doi:10.1136/bmjopen-2011-000494
Contributors: TBR conceived and designed project, and drafted and reviewed manuscript. MS designed project, drafted and reviewed manuscript, analysed and interpreted data, provided statistical expertise and collected data. KCN drafted and reviewed manuscript. DP analysed data, provided statistical expertise and collected data. SKS drafted manuscript. JO drafted manuscript. TCF conceived and designed project, drafted and reviewed manuscript, provided statistical expertise, analysed and interpreted data. MS and TCF are guarantors.
Funding: This work was supported in part by the following grants: R24DA017298, R01 HL59180, U54 RR026138, P20 MD000182, S06 GM068510, U54 HD41748 and R25 RR019488.
Competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare the following: TBR is the owner of Omics Biotechnology, Inc, a company with an interest in using modifications of cannabinoids-mediated cell signalling to treat diabetes mellitus and other diseases of inflammation. TBR was not involved in the data collection or statistical analyses. MS, KCN, DP, SKS, JO and TCF have no relationships with companies that might have an interest in the submitted work in the previous 3 years. TBR, MS, KCN, DP, SKS, JO and TCF have no non-financial interests that may be relevant to the submitted work and their spouses, partners or children have no financial relationships that may be relevant to the submitted work.
Ethics approval: This study was exempt from Institutional Review Board (IRB) review. This exemption complied with the policy of the Charles Drew University IRB related to the use of publically available data for research and publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The NHANES data are publically available. Statistical code and working data set are available from the corresponding author at magdashaheen@cdrewu.edu.
References
- 1.Narayan KM, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 2006;29:2114–16 [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration Results from the 2006 National Survey On Drug Use and Health: National Findings, NSDUH Series H-32 (DHHS Publication No. SMA 07–4293). Rockville, MD: Substance Abuse and Mental Health Services Administration, 2007. http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.pdf (accessed 18 Aug 2009). [Google Scholar]
- 4.Carrier EJ, Patel S, Hillard CJ. Endocannabinoids in neuroimmunology and stress. Curr Drug Targets CNS Neurol Disord 2005;4:657–65 [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946–9 [DOI] [PubMed] [Google Scholar]
- 6.Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry 2001;178:107–15 [DOI] [PubMed] [Google Scholar]
- 7.Polen MR, Sidney S, Tekawa IS, et al. Health care use by frequent marijuana smokers who do not smoke tobacco. West J Med 1993;158:596–601 [PMC free article] [PubMed] [Google Scholar]
- 8.Rodondi N, Pletcher MJ, Liu K, et al. ; Coronary Artery Risk Development in Young Adults (CARDIA) Study Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol 2006;98:478–84 [DOI] [PubMed] [Google Scholar]
- 9.Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public Health Nutr 2001;4:781–6 [DOI] [PubMed] [Google Scholar]
- 10.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1 1994;32:1–407 [PubMed] [Google Scholar]
- 11.Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. 1996. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf (accessed 18 Aug 2009). [Google Scholar]
- 12.Chen J, Wildman RP, Hamm LL, et al. ; Third National Health and Nutrition Examination Survey Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2960–5 [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988-94). Hyattsville, MD: US Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics, 1996. http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf (accessed 13 Aug 2009). [Google Scholar]
- 14.Criqui MH, Golomb BA. Should patients with diabetes drink to their health? JAMA 1999;282:279–80 [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7 [DOI] [PubMed] [Google Scholar]
- 16.Fan AZ, Russell M, Naimi T, et al. Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab 2008;93:3833–8 [DOI] [PubMed] [Google Scholar]
- 17.Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol 2011;174:929–33 [DOI] [PubMed] [Google Scholar]
- 18.Patrignani P, Tacconelli S, Sciulli MG, et al. New insights into COX-2 biology and inhibition. Brain Res Brain Res Rev 2005;48:352–9 [DOI] [PubMed] [Google Scholar]
- 19.Steffens S, Veillard NR, Arnaud C, et al. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005;434:782–6 [DOI] [PubMed] [Google Scholar]
- 20.Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 2007;293:H610–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss L, Zeira M, Reich S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006;39:143–51 [DOI] [PubMed] [Google Scholar]
- 22.Weiss L, Zeira M, Reich S, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008;54:244–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvarajah D, Gandhi R, Emery CJ, et al. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care 2010;33:128–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheen AJ, Finer N, Hollander P, et al. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660–72 [DOI] [PubMed] [Google Scholar]
- 25.Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 2007;150:613–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain 2002;18(4 Suppl):S76–82 [DOI] [PubMed] [Google Scholar]
- 27.Litten RZ, Fertig J. Self-report and biochemical measures of alcohol consumption. Addiction 2003;98(Suppl 2):iii–iv [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.