Abstract
In healthy individuals, deep inspirations (DIs) have a potent bronchodilatory ability against methacholine (MCh)-induced bronchoconstriction. This is variably attenuated in asthma. We hypothesized that inability to bronchodilate with DIs is related to reduced airway distensibility. We examined the relationship between DI-induced bronchodilation and airway distensibility in 15 asthmatic individuals with a wide range of baseline lung function [forced expired volume in 1 s (FEV1) = 60–99% predicted]. After abstaining from DIs for 20 min, subjects received a single-dose MCh challenge and then asked to perform DIs. The effectiveness of DIs was assessed by the ability of the subjects to improve FEV1. The same subjects were studied by two sets of high-resolution CT scans, one at functional residual capacity (FRC) and one at total lung capacity (TLC). In each subject, the areas of 21–41 airways (0.8–6.8 mm diameter at FRC) were matched and measured, and airway distensibility (increase in airway diameter from FRC to TLC) was calculated. The bronchodilatory ability of DIs was significantly lower in individuals with FEV1 <75% predicted than in those with FEV1 ≥75% predicted (15 ± 11% vs. 46 ± 9%, P = 0.04) and strongly correlated with airway distensibility (r = 0.57, P = 0.03), but also with residual volume (RV)/TLC (r = −0.63, P = 0.01). In multiple regression, only RV/TLC was a significant determinant of DI-induced bronchodilation. These relationships were lost when the airways were examined after maximal bronchodilation with albuterol. Our data indicate that the loss of the bronchodilatory effect of DI in asthma is related to the ability to distend the airways with lung inflation, which is, in turn, related to the extent of air trapping and airway smooth muscle tone. These relationships only exist in the presence of airway tone, indicating that structural changes in the conducting airways visualized by high-resolution CT do not play a pivotal role.
Keywords: bronchoprotection, airway obstruction, high-resolution CT
the relationship between deep inspiration (DI), airway mechanics, and airway hyperresponsiveness has been a focus of asthma research in the past two decades (1–9). Several earlier studies showed that DIs have a beneficial effect on human airways (10–14). In healthy individuals, DIs taken prior to exposure to methacholine (MCh) protect the airways from bronchoconstriction, a property referred to as DI-induced bronchoprotection (3, 9, 15). This property is largely lost in asthma, even in mild disease (5). DIs can also reverse airway obstruction that has been experimentally induced with a direct spasmogen, and this is referred to as DI-induced bronchodilation (26, 32, 35). The bronchodilatory effect of DIs is minimally affected in individuals with mild asthma (5) but decreases with increasing severity of asthma (35), raising the possibility that the impairment of this physiological function of the lung is one of the determinants of severe obstructive disease.
Airway stretch has been implied as a necessary step to activate the beneficial properties of DI (16, 22). By virtue of the forces of interdependence between the external airway walls and the surrounding parenchyma, a DI maneuver transmits its distending forces to the airways, and as the lung volume increases, the airway caliber enlarges. This phenomenon has been demonstrated in animal models (8, 10–12) and in healthy humans (15) using high-resolution CT (HRCT). We define airway distensibility as the change in airway size with lung inflation. Scanning of airways at functional residual capacity (FRC) and total lung capacity (TLC) allows measurements of luminal areas of the same airway locations before and at the end of a DI. By doing so, the ability of the single DI to distend airways can be calculated. This procedure has been validated and extensively used by our group (25, 31–39). In our studies on the pathophysiology of asthma, we previously showed that DIs stretch the airways of individuals with mild asthma to the same degree as the airways of healthy individuals (4). However, it is not known whether airway distension is preserved in more severely obstructed asthmatics, particularly in those with airway obstruction, who, by definition, are experiencing alterations in airway physiology. The extension of the HRCT assessment of airway distensibility to the more severe forms of obstruction in asthma could elucidate the mechanisms underlying the loss of the beneficial effects of DI in asthma.
The observation that DI-induced bronchodilation was impaired with increasing severity of asthma led us to hypothesize that this phenomenon is dependent on the diminished ability of DIs to distend the airways. The study was designed to test this hypothesis by evaluating whether, in individuals with asthma with a range of baseline lung function, impaired DI-induced bronchodilation was related to reduced airway distensibility by DI, as assessed by HRCT.
METHODS
Subjects
We enrolled 15 subjects for the study. Inclusion criteria were as follows: male or female subjects between 18 and 65 yr, nonsmokers, with a history of asthma (Table 1). All subjects had typical asthma and allergy symptoms with bouts of mild exacerbations. No subject had a serious exacerbation requiring hospitalization or intubation within the previous 6 mo. All but two subjects were hyperresponsive to MCh [provocative concentration of MCh producing a 20% fall in forced expiratory volume in 1 s (FEV1), i.e., PC20, ≤8 mg/ml]. One subject did not have a baseline MCh challenge for logistical reasons, and one subject was not hyperresponsive to a standard dose-response challenge to MCh but had a very convincing history of asthma and responded to the single-dose challenge protocol when DIs were absent. All subjects were asked to refrain from use of inhaled bronchodilators for 12 h for short-acting β-agonists and 48 h for long-acting β-agonists. The protocol was approved by the Johns Hopkins Institutional Review Board, and written informed consent was obtained from each subject.
Table 1.
Demographic characteristics of all subjects
| Subj No. | Age, yr | Sex | PC20, mg/ml | FEV1, %Pred | FVC, %Pred | FEV1/FVC | RV, %Pred | FRC, %Pred | TLC, %Pred | Medications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | F | 2.11 | 75 | 79 | 0.78 | 61 | 77 | 73 | 1,2,3,6 |
| 2 | 41 | M | 1.83 | 84 | 113 | 0.62 | 118 | 116 | 114 | 1 |
| 3 | 53 | F | 2.93 | 89 | 87 | 0.82 | 94 | 77 | 90 | 1,3,5 |
| 4 | 38 | M | 69 | 87 | 0.67 | 119 | 84 | 96 | 1 | |
| 5 | 22 | M | 1.65 | 99 | 108 | 0.80 | 72 | 69 | 99 | 1 |
| 6 | 49 | F | 0.21 | 66 | 81 | 0.67 | 184 | 121 | 117 | 1,2,6 |
| 7 | 29 | F | 0.91 | 78 | 75 | 0.89 | 98 | 90 | 82 | 1,3,6 |
| 8 | 30 | M | 0.16 | 78 | 91 | 0.73 | 61 | 71 | 83 | 1 |
| 9 | 19 | F | 8.01 | 75 | 81 | 0.81 | 74 | 62 | 79 | 1,6 |
| 10 | 30 | F | 0.74 | 60 | 70 | 0.74 | 174 | 106 | 103 | 1,3,6 |
| 11 | 21 | M | 0.07 | 82 | 115 | 0.62 | 136 | 118 | 120 | 1,6 |
| 12 | 64 | M | 0.41 | 62 | 85 | 0.57 | 190 | 129 | 125 | 1,2,4,6 |
| 13 | 49 | F | 61.37 | 73 | 72 | 0.83 | 66 | 74 | 70 | 1,2,6 |
| 14 | 25 | M | 0.13 | 70 | 102 | 0.59 | 126 | 96 | 108 | 1,6 |
| 15 | 35 | F | 4.5 | 98 | 109 | 0.75 | 1,6 | |||
| Mean ± SE | 37 ± 3 | 6.07 ± 4 | 75 ± 3 | 87 ± 3 | 0.73 ± 0.03 | 112 ± 12 | 92 ± 6 | 97 ± 5 |
PC20, provocative concentration of methacholine that causes a 20% fall in forced expiratory volume in 1 s (FEV1) from baseline; %Pred, percent predicted. Subject 4 was not challenged with methacholine on the screening visit. Residual volume (RV), functional residual capacity (FRC), and total lung capacity (TLC) were not measured in subject 15. Medications are as follows: inhaled β-agonist bronchodilators (1), inhaled steroids (2), nasal steroids (3), oral steroids (4), theophylline (5), and antihistamines (6).
Study Design
Screening.
Screening included a respiratory questionnaire, epicutaneous skin testing with a panel of common aeroallergens, and baseline spirometry and plethysmographic thoracic gas volume followed by a conventional MCh challenge. We used Hankinson [National Health and Nutrition Examination Survey (NHANES) III] predicted values (24).
Evaluation of DI-induced bronchodilation.
DI-induced bronchodilation was assessed using multiple modified single-dose MCh challenges on separate days, as previously described (32). At every single-dose challenge, after baseline spirometry, subjects were instructed to abstain from taking DIs for 20 min. At the end of this period, a single dose of MCh (starting at 0.025 mg/ml) was delivered with five tidal inspirations from a deVilbiss 646 nebulizer attached to a model 2A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Fairfax, VA). After 3 min, a single full spirometric maneuver was performed, and the degree of airway obstruction was calculated by comparison of baseline with post-MCh FEV1. If the reduction in FEV1 was not >20%, the subject was invited to return on a separate day for another single-dose MCh challenge using the next-highest single dose of MCh (e.g., 0.075 mg/ml). This process was continued with additional single-dose challenges (0.25, 0.75, 2.5, and 7.5 mg/ml) on separate days until the PC20 was delivered. At the challenge where ≥20% reduction in FEV1 was achieved, the subject was instructed to continue the procedure by taking four DIs immediately after the single post-MCh spirometry. Then another spirometric maneuver was performed immediately after the four DIs to calculate the degree to which the subject was able to reverse the MCh-induced airway obstruction. By measuring the difference between the post-MCh FEV1 and the FEV1 obtained after the four DIs, we were able to calculate a measure of bronchodilation induced by the series of DIs, which we termed the bronchodilation index. This measure, which has been previously described (36), is calculated as follows
| (1) |
where percent reduction in FEV1 after MCh without DIs is the percent decrease in FEV1 after the single-breath MCh challenge, as measured by the first post-MCh spirometric maneuver, and percent reduction in FEV1 after MCh with DIs is the percent decrease in FEV1 after the single-dose MCh challenge and after four DI maneuvers. In addition, since FEV1 = FEV1/FVC × FVC (where FVC is forced vital capacity), we partitioned the bronchodilation index, calculated on the basis of FEV1, into its components of airway narrowing (FEV1/FVC) and airway closure (FVC) (21, 41). This was done to determine whether the impaired effects of DIs were related to an inability to increase airway caliber or an inability to influence air trapping by recruiting previously closed airways.
Evaluation of airway distensibility at baseline and after removal of airway tone with albuterol.
On a separate day, subjects underwent HRCT scanning for evaluation of baseline airway distensibility. Subjects were scanned twice in sequence, once at FRC and once at TLC. We also wanted to examine the intrinsic distensibility, i.e., airway wall stiffness secondary to components within the airway wall, without the potential confounder of stiffness due to airway smooth muscle tone. Therefore, we also measured airway distensibility after administering albuterol to remove the effect of airway tone. On a second visit, subjects underwent spirometry and plethysmographic lung volume measurements followed by treatment with consecutive albuterol nebulizations (up to 4 treatments) until their FEV1 did not change >5% (22). After albuterol bronchodilation plateau was reached, subjects repeated plethysmographic thoracic gas volume measurements and HRCT scanning of their chest at FRC and TLC.
Maintenance of constant lung volumes.
By controlling and adjusting the amount of inspired air, we maintained lung volume at each scan series approximately constant. Prior to each FRC series, while wearing nose clips, subjects exhaled to residual volume (RV) and then inspired all air delivered from a respiratory bag connected to the mouthpiece through a pneumatic valve. Once all air was inspired, the pneumatic valve was closed, so that the subject would not inadvertently exhale or inhale any air during scanning. The fixed amount of air delivered from the bag was equal to the expiratory reserve volume determined during the first visit by body plethysmography (6). For measurement of airway area at TLC, the subject was instructed to take a maximal deep breath and the pneumatic valve was closed, so that the subject would not inadvertently exhale or inhale any air during scanning (15).
Acquisition of CT data.
All scans were performed using the spiral CT (Somatom Plus 4, Siemens), with settings of 120 kVp, 170 mAs, 2-mm slice thickness, 2 mm/s rotation feed, and 1-mm reconstruction interval (total 61 scans per set), during a single breath hold for ∼24 s at FRC and moved caudally. A reference scan was acquired prior to each spiral CT scan set to ensure reproducible image location in the lung. The images were reconstructed as a 16-bit 512 × 512 matrix using a field of view of 200 mm. Images were reconstructed with the use of a high-spatial-frequency (resolution) algorithm that enhanced edge detection, at a window level of −450 Hounsfield units and a window width of 1,350 Hounsfield units. All airways visualized approximately perpendicular to the scan plane (long-to-short axis ratio <1.5:1) were measured. For repeated airway measurements in a given subject, within each experimental protocol, adjacent anatomic landmarks, such as airway or vascular branching points, were identified on the HRCT images obtained at FRC and again on the TLC scans.
Airway measurements with HRCT.
The airway luminal size measurement has previously been described (4) and validated in animals models (1, 5). Briefly, the HRCT images were transferred to a UNIX-based workstation and analyzed using the airway analysis module of the Volumetric Image and Display Analysis (VIDA) software package (Dept. of Radiology, Division of Physiologic Imaging, University of Iowa, Iowa City, IA). To measure the airway areas, the operator drew a rough isocontour estimate of the lumen of the airway. The VIDA program automatically located a precise isocontour perimeter of the airway lumen by sending out rays in a spoke-wheel fashion to a predesignated pixel intensity level that defined the luminal edge of that airway wall. The length of the rays was set at 6 pixels. The software program used an algorithm for edge detection based on the “full-width–half-maximum” principle. The edge of the wall was defined by the program by the points along the lines where the pixel density changed to half its maximum through the wall. All full and partial pixels (full pixel size = 0.1537 mm2 with our settings) within the adjusted isocontour were counted and represented the airway area. In addition, we divided the airways into arbitrary groups by size in their relaxed state after albuterol: small (<5 mm diameter), medium (5–8 mm diameter), and large (>8 mm diameter).
To measure airway wall thickness, we randomly drew at least three lines through the airway wall. The VIDA program displayed a histogram of pixel intensity along the line that was drawn through the airway wall. The inflection points of increasing intensity represented the inner and outer edges of the airway wall. The line and points were selected manually. The program then automatically measured the distance in pixels between the two points. The software is capable of measuring fractions of a pixel; therefore, the measurements of wall thickness were not necessarily quantized to multiples of the pixel dimension. This measurement of the number of pixels and any fraction of a pixel that was counted in the airway wall was then converted to millimeters by multiplying by the size of the pixel in millimeters based on the scanning parameter. The airway wall measurements were then averaged to give a mean airway wall thickness for each airway. Airway wall thickness was only measured in the scans taken at FRC. Airway wall thickness was expressed as a percentage of airway diameter after maximal airway relaxation with albuterol. This was done to eliminate the potentially confounding effect of airway constriction on the apparent airway wall thickness.
We also calculated a measure of the change in airway size with lung inflation. The subjects were scanned twice in sequence, once at FRC and once at TLC. The percent change in airway size from FRC to TLC was defined as the airway distensibility (see Eq. 2).
Calculation of airway distensibility.
To measure the ability to distend the airways with lung inflation, we calculated the distensibility of the airways from FRC to TLC.
By comparing the airway diameter between the HRCT scans obtained at FRC and TLC, we were able to calculate an airway distensibility. The formula used for this calculation is shown below. This was calculated as the percent change in the airway luminal diameter from FRC to TLC
| (2) |
The airway diameter at TLC is the individual airway diameter measured on HRCT at full lung inflation, and the airway diameter at FRC is the diameter of the same individual airway measured on HRCT at end expiration.
Data Analysis
Data analysis was performed using JMP 7.0.1 software (SAS Institute) and involved two-way ANOVA and simple linear regressions and stepwise linear regression where appropriate. Significance was accepted at P ≤ 0.05.
RESULTS
A total of 15 subjects were studied. The mean baseline FEV1 was 77 ± 3% predicted (mean ± SE) with a range of 60–99%. Mean age was 37 ± 3 yr. The subject population consisted of seven men and eight women. All subjects were skin test-positive and were using β-adrenergic agonists. Four subjects used inhaled corticosteroids regularly, four used nasal steroids, and one used oral steroids (Table 1). In 13 subjects, MCh PC20 was ≤8 mg/ml. One subject did not have a baseline MCh challenge for logistical reasons, and another had a PC20 of 61.4 mg/ml; however, this subject had a very convincing history of asthma. Both of these subjects reacted per protocol to the single-dose MCh challenge (see below); thus the bronchodilatory ability of DIs could be measured. One subject was not able to adequately perform the maneuvers in the body plethysmograph.
Bronchodilatory Effect of DI
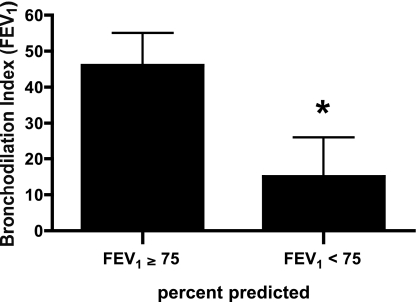
The median dose of MCh used for the single-dose challenge was 1.625 mg/ml (range 0.075–7.5 mg/ml). The mean fall in FEV1 after the single-dose MCh challenge was 33 ± 4% (range 17–66%). The bronchodilation index, calculated on the basis of FEV1, was 34 ± 8% (range −15% to 97%). No correlation was found between the bronchodilation index and airway obstruction as measured by the baseline FEV1 percent predicted (P = 0.27). However, when we divided the subjects by the median value of the entire group into those with baseline FEV1 ≥75% predicted (not obstructed group) and those subjects with baseline FEV1 <75% predicted (obstructed group), we found a significant difference in the bronchodilation index (46 ± 9% and 15 ± 11%, respectively, P = 0.04; Fig. 1). The bronchodilation index for those with FEV1 <75% predicted was not statistically different from zero (P = 0.21). Two individuals in this group had a negative bronchodilation index; i.e., DIs further worsened the degree of airway obstruction.
Fig. 1.
Bronchodilation index in study participants with baseline forced expired volume in 1 s (FEV1) ≥75% predicted and <75% predicted. Values are means ± SE. *P = 0.04.
The bronchodilation index calculated on the basis of FVC was 41 ± 9% (range −9% to 122%). No correlation was found between the DI bronchodilation index and baseline FVC percent predicted (P = 0.25). Again, we divided the subjects into obstructed and not-obstructed groups. The bronchodilation index for FVC for the obstructed group was not statistically different from zero (P = 0.09), whereas that for the not-obstructed group was statistically different from zero (P = 0.001); however, we did not find a significant difference in the FVC bronchodilation index between the not-obstructed and the obstructed group (52 ± 11% and 25 ± 12%, respectively, P = 0.12). The DI bronchodilation index calculated on the basis of FEV1/FVC was 38 ± 14% (range −62% to 189%). No correlation was found between the bronchodilation index and airway obstruction as measured by the baseline FEV1/FVC percent predicted (P = 0.66). The bronchodilation index for FEV1/FVC for the obstructed group was not statistically different from zero (P = 0.46), whereas that for the not-obstructed group was statistically different from zero (P = 0.02); however, we did not find a significant difference in the FEV1/FVC bronchodilation index between the not-obstructed and the obstructed group (56 ± 18% and 14 ± 17%, respectively, P = 0.13).
Airway Distensibility by HRCT
In each subject, the luminal diameters of 21–41 airways (2.2–17.4 mm diameter at FRC) were matched at FRC and TLC and measured. First, the distensibility for each individual airway was calculated as described by Eq. 2. Next, a within-subject mean distensibility value was calculated by averaging all individual airway distensibility measurements. Finally, mean distensibility values for all subjects were calculated. At baseline, overall mean airway distensibility was 16 ± 2% (range 0.4 ± 2.9% to 27 ± 4.4%). We did not detect differences in distensibility when airways were examined by size. Mean airway distensibility was 16 ± 3%, 16 ± 2%, and 15 ± 2% for the small, medium, and large airways, respectively (ANOVA, P = 0.91). After relaxation of the airways with albuterol, the overall mean airway distensibility was 7.7 ± 1% (range 1.1 ± 1.7% to 17.7 ± 3.9%) among the subjects. Again, we did not detect differences in distensibility when airways were examined by size. Mean airway distensibility after albuterol was 5 ± 3%, 9 ± 2%, and 7 ± 1% for the small, medium, and large airways, respectively (ANOVA, P = 0.49).
Assessment of Determinants of the Effect of DI
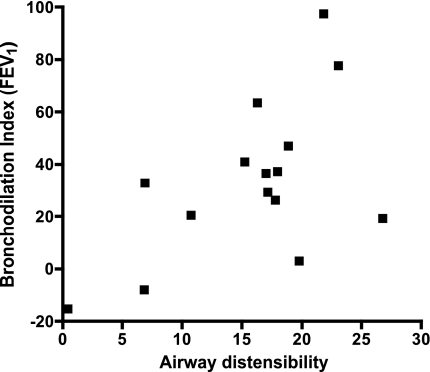
Our primary objective was to determine the relationship between airway distensibility and the bronchodilation index. Overall, we found a statistically significant correlation between airway distensibility by HRCT and the DI bronchodilation index calculated on the basis of FEV1 (r = 0.57, P = 0.03; Fig. 2), indicating that greater distensibility of the airways at baseline (preconstricted state) is associated with greater ability of DIs to dilate the MCh-constricted airways. When we examined this relationship by airway size, we found a significant correlation between the bronchodilation index and the distensibility of the large (r = 0.78, P = 0.03) and medium (r = 0.64, P = 0.03), but not small (r = 0.45, P = 0.11) airways. In addition, we examined the relationships between airway distensibility by HRCT and the bronchodilation index calculated on the basis of FVC (r = 0.66, P = 0.007) and FEV1/FVC (r = 0.30, P = 0.27), indicating that greater distensibility of the airways at baseline (preconstricted state) is associated with greater ability of DIs to reverse airway closure, and not with the ability to reverse airway narrowing, in the MCh-constricted airways.
Fig. 2.
Relationship between the change in mean airway size measured by high-resolution CT (HRCT) with lung inflation from functional residual capacity (FRC) to total lung capacity (TLC; airway distensibility) and the bronchodilation index (r = 0.57, P = 0.03).
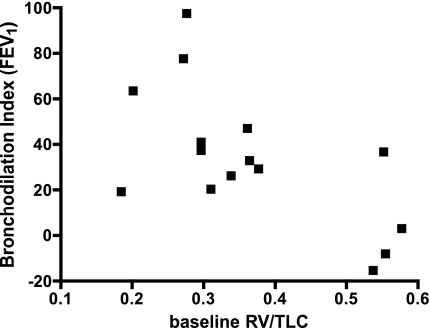
To further evaluate whether the ability of DIs to dilate constricted airways was dependent on features of air trapping/lung hyperinflation, we examined the relationship between the DI bronchodilation index calculated on the basis of FEV1 and baseline lung volumes. We found no correlation between the DI bronchodilation index and either the RV percent predicted (r = −0.31, P = 0.26) or the TLC percent predicted (r = −0.15, P = 0.58). However, we found a significant negative correlation between the DI bronchodilation index and RV/TLC (r = −0.47, P = 0.01; Fig. 3). The greater the RV/TLC, i.e., the more air trapping prior to MCh challenge, the less able were DIs to relieve constriction after MCh. We found similar relationships between the DI bronchodilation index calculated on the basis of FVC and RV (r = −0.40, P = 0.14), TLC (r = −0.23, P = 0.42), and RV/TLC (r = −0.71, P = 0.003), but no correlations between the DI bronchodilation index calculated on the basis of FEV1/FVC and RV (r = −0.09, P = 0.74), TLC (r = −0.04, P = 0.89), and RV/TLC (r = −0.30, P = 0.29).
Fig. 3.
Relationship between baseline residual volume (RV)/TLC (a measure of air trapping) and the bronchodilation index (r = −0.63, P = 0.01).
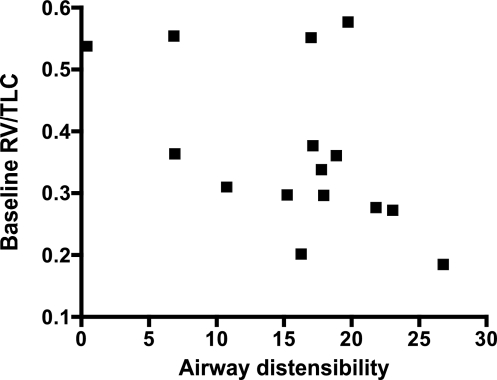
Since baseline RV/TLC was related to the DI bronchodilation index, we further explored whether airway distensibility was also influenced by RV/TLC. We found that higher RV/TLC was associated with lower airway distensibility (r = −0.51, P = 0.05; Fig. 4). When we examined this relationship by airway size, we found a significant correlation between baseline RV/TLC and airway distensibility for the large airways (r = −0.50, P = 0.02), but not for the medium (r = −0.36, P = 0.12) or small (r = −0.26, P = 0.37) airways.
Fig. 4.
Relationship between the change in mean airway size measured by HRCT with lung inflation from FRC to TLC (airway distensibility) and baseline RV/TLC as a measure of air trapping (r = −0.51, P = 0.05).
Furthermore, in a stepwise regression model with bronchodilation index calculated on the basis of FEV1 or FVC as the dependent outcome variable and baseline RV/TLC and distensibility as the independent variables, only the RV/TLC remained in the model (data not shown), supporting the finding that distensibility is dependent on air trapping and is not an independent predictor of the magnitude of DI-induced bronchodilation.
We also assessed the relationship between airway wall thickness and the DI bronchodilation index. Mean airway wall thickness as a percentage of airway diameter was 29 ± 1% (range 25 ± 2% to 37 ± 2%) among the subjects. As expected, we detected differences in airway wall thickness as a percentage of airway diameter when airways were examined by size. The airway wall thickness as a percentage of airway diameter was 39 ± 2%, 31 ± 1%, and 23 ± 1% for the small, medium, and large airways, respectively (ANOVA, P < 0.0001). We did not find significant correlations between the DI bronchodilation index calculated on the basis of FEV1, FVC, or FEV1/FVC and the airway wall thickness either overall or by airway size. On the other hand, we found a correlation between airway wall thickness and post-albuterol airway distensibility (r = 0.74, P = 0.002). The direction of this relationship indicated that thicker airway walls had greater distensibility. This correlation was present for the medium (r = 0.82, P = 0.0002) and small (r = 0.53, P = 0.05), but not the large (r = 0.28, P = 0.31), airways. After albuterol, there was no correlation between RV/TLC and airway wall thickness either overall or by airway size.
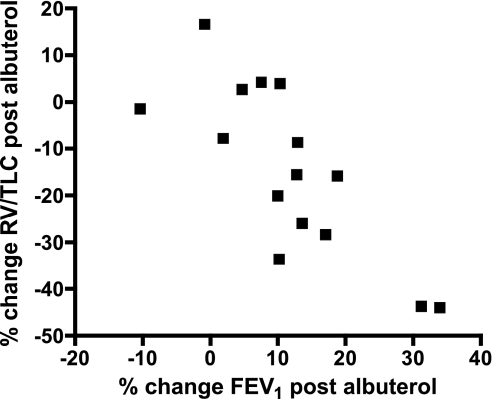
Next, we examined whether the relationship between airway distensibility and the DI bronchodilation index, as well as the relationship between air trapping and distensibility, remained after removal of airway tone with albuterol. The maximum effect of albuterol was a 12 ± 3% increase in FEV1, a 19 ± 6% decrease in RV, and a 15 ± 5% decrease in RV/TLC. The albuterol-induced improvement in FEV1 was strongly correlated with the decrease in RV/TLC (r = −0.79, P = 0.0005), i.e., with the reduction in air trapping (Fig. 5). Albuterol also caused mean airway diameters to increase by 24 ± 2% at FRC and by 14 ± 1% at TLC. We detected similar changes after albuterol in the small, medium, and large airways at FRC and TLC, respectively. After albuterol, the relationship between airway distensibility and the DI bronchodilation index calculated on the basis of FEV1, FVC, or FEV1/FVC, either overall or by airway size, was lost. Similarly, no relationship between post-albuterol RV/TLC and post-albuterol airway distensibility (r = 0.02, P = 0.93) or between post-albuterol RV/TLC and the DI bronchodilation index calculated on the basis of FEV1 (r = −0.47, P = 0.08) or FEV1/FVC (r = −0.25, P = 0.36), was present. However, we still found a significant correlation between the post-albuterol RV/TLC and the DI bronchodilation index calculated on the basis of FVC (r = −0.57, P = 0.03).
Fig. 5.
Relationship between the change in FEV1 after maximum relaxation with albuterol and the change in air trapping as measured by the change in RV/TLC after albuterol (r = −0.79, P = 0.0005).
DISCUSSION
In this study, we examined potential mechanisms of the reduction in the bronchodilatory effects of DI in asthma with the primary hypothesis that these effects may be directly associated with reduced airway distensibility as measured by HRCT. This hypothesis was supported by our findings (Fig. 2), which showed that reduced ability to expand the airways with a DI from FRC to TLC is associated with the loss of the ability to reverse MCh-induced airway constriction. The basis of our hypothesis was the concept that there is a minimum required stretch of the airway walls that initiates some biochemical process or neural activity that allows the airway to remain dilated, even after the stretch is removed. This concept is supported by ex vivo experimentation and by in vivo animal data (8–10, 23, 40). We previously used HRCT to assess airway distensibility in healthy individuals and patients with mild asthma and we showed that, in mild asthma, distensibility was intact. This is the first time that HRCT was applied to assess airway distensibility in individuals with more severe obstruction, many of whom have lost the ability to bronchodilate via DIs.
Airway distension depends on the forces generated during DI-induced inflation of the lungs. One of the factors that influence the magnitude of these forces is the extent to which the lungs can inflate. In the presence of hyperinflation, a common state in moderate-to-severe obstruction in asthma (2, 28, 30), further lung inflation by DI may be impaired. In the hyperinflated state, the ability to generate high transpulmonary pressure is severely decreased, thus reducing the amplitude of the applied stretch and, as a consequence, the distension of the airways. Indeed, our results indicate that airway distensibility depends on the state of air trapping as measured by RV/TLC (Fig. 3) and, in logical sequence, that the degree of air trapping was a major determinant of the bronchodilatory effect of DI (Fig. 4). Most importantly, airway distensibility appeared to be related to the bronchodilatory effect of DI through its association with RV/TLC, and not independently, as indicated by multiple regression analysis.
Interestingly, the relationship between reduced bronchodilation by DI and reduced distensibility was absent in the smaller (<3-mm-diameter) airways. One possibility is that this was due to methodological issues. First, a major limitation of HRCT is the inability to measure airways less than ∼1 mm in diameter. We are unable to make any direct inference about these small airways, which play a major role in airway closure and air trapping. If small airways are closed at FRC, then by protocol, they will not be measured at TLC. Also, given the limits of resolution with HRCT and the pixel size for the imaging we performed, 0.39 mm on a side, variability in the measurement would be greater for the smaller than the larger airways, leading to a non-statistically significant result. On the other hand, the correlation we found between the DI bronchodilation index and the distensibility of the large and medium, but not small, airways measured by HRCT may simply reflect the possibility that FEV1 (on the basis of which the DI bronchodilation index was measured) is more a measure of large or central airway function. We also observed that the relationship between airway distensibility and RV/TLC was driven by the large airways. This result is consistent with our previous finding of an association between large airway narrowing and dynamic hyperinflation (14). Closure of the large airways would not be necessary. Sufficient narrowing of the large airways that produced a ventilatory “choke point” could lead to dynamic hyperinflation, causing an increase in RV/TLC. Thus, if distension of the larger airways relieved dynamic hyperinflation, this would lead to a greater bronchodilatory response by DI.
Additional support for the notion that the reduction in the effects of DIs stems from its reduced effectiveness in relieving air trapping comes from the data we generated by partitioning the bronchodilation index calculated on the basis of FEV1 into the FEV1 components of airway narrowing (FEV1/FVC) and airway closure (FVC) (21, 41). Both components improved with DIs following MCh challenge in the mildly obstructed or not-obstructed subjects, whereas, in subjects with baseline FEV1 <75% predicted, DIs failed to improve either component. This indicates that DIs influence airway closure and airway narrowing. However, a striking difference was found when the DI effect based on FVC or FEV1/FVC was related to airway distensibility or to RV/TLC: the airway closure component of the DI effect strongly correlated with the distensibility and the air-trapping outcomes (r = 0.66 and −0.71, respectively), whereas the airway-narrowing component was not (r = 0.30 and −0.30 respectively). These findings support the concept that the loss of the ability of DIs to improve FEV1 after MCh challenge derives from its inability to improve airway closure, and not airway caliber.
In the present study, we did not detect a difference in airway distensibility by airway size as measured by HRCT; in previous work, we noted greater distensibility at baseline in small than in large airways (15). The difference between the two studies may have several explanations. 1) It may be a methodological issue, since we used only three size bins in the present study compared with four in our previous work. 2) The study populations are different. In our previous study, all participants with asthma had mild disease and no obstruction. In the present study, ∼50% of our subjects had moderate or severe obstruction. 3) While we did not find a statistically significant correlation between the overall airway distensibility and baseline FEV1 percent predicted (r = 0.45, P = 0.09), we did find a statistically significant correlation between small airway distensibility and baseline FEV1 (r = 0.58, P = 0.03). The lower the baseline FEV1 percent predicted, the lower was the distensibility. Therefore, the greater degree of obstruction could account for the lower distensibility in the small airways in the present study population than in our previous study.
Changes in the airway wall could also influence airway distensibility. While lung inflation drives the magnitude of the distending forces induced by DI, the efficacy of these forces can also be influenced by the degree of airway-parenchymal interdependence and by the state of the airways. These parameters may, in turn, be affected by airway wall thickness. With the use of HRCT, we were able to examine airway wall thickness (7, 14, 15). We measured airway wall thickness at baseline FRC and after maximal airway relaxation by albuterol. We chose this latter condition assuming that measurements at the lowest possible airway tone would be most representative of true thickness; airway wall folding resulting from airway tone can confound imaging of airway wall thickness (27). Our data indicate that airway wall thickness plays little or no role in the bronchodilatory effect of DI as measured with FEV1. This applies to all airway sizes that can be imaged by HRCT. Interestingly, we found a relationship between airway wall thickness and airway distensibility (both measured with HRCT), in that distensibility was greater in the subjects with the thicker airway walls. One could interpret this finding as suggestive of a protective effect of thicker airways against early airway closure and hyperinflation, resulting in maintained distensibility (29). However, we found no relationship between wall thickness and RV/TLC before or after albuterol. Alternatively, if airway wall thickness is increased as a result of edematous as opposed to fibrotic changes, distensibility of the wall may also increase.
An additional factor that could influence airway distensibility is lung elastic recoil. Gelb et al. (19, 20) described decreased lung elastic recoil in asthmatic individuals. Changes in lung elastic recoil, as measured by pressure-volume curves, describe changes in the parenchyma. A key issue, not directly addressed in the studies of Gelb et al. or other studies of which we are aware, is how the parenchyma affects airway size. The size of an airway with lung inflation will depend on the effective pressure around the airway, and this will be a function of the lung volume and the transpulmonary pressure. We directly examined the airway changes with lung inflation to TLC and, thus, only know about the lung volume changes. How changes in lung elastic recoil affect the airway size is not clear, but it is reasonable to assume that elastic recoil could contribute to the final size.
Airway tone may also influence airway distensibility through various mechanisms, possibly including its effects on air trapping and smooth muscle stretchability. For example, increased tone, especially if it is present concomitantly with reduced amplitude of respiratory oscillations (low frequency of DIs), may lead to a state of smooth muscle stiffness that could resist stretch (17, 18). Therefore, our study was also designed to examine whether maximal removal of airway tone with albuterol would influence the relationship between airway distensibility and the bronchodilatory effects of DI. We found that when the airways are maximally relaxed, the relationship between airway distensibility and the bronchodilatory ability of DI disappeared. Similarly, airway distensibility after albuterol did not correlate with post-albuterol RV/TLC, and the latter outcome did not correlate with the bronchodilatory ability of DI. These effects of airway relaxation indicate that airway tone is a modulator of the bronchodilatory effects of DI. The observations made in this study do not allow us to assess the mechanisms through which airway tone exerts its influence, and further studies are required. It should be pointed out that one technical detail may also play a role in the apparent loss of the relationship between airway distensibility and DI-induced bronchodilation after the administration of albuterol. As we previously observed with HRCT, airway caliber increased with albuterol by 23% at FRC but only by 14% at TLC. As a result, the change in size from FRC to TLC (airway distension) is less after relaxation than in the prerelaxed state.
There are at least two components to the increase in airway diameter from FRC to TLC (which we have termed “distensibility”): 1) overcoming airway tone and 2) distending the structure of the airway wall. In the presence of tone, both components lead to a substantial increase in airway diameter from FRC to TLC. With the use of albuterol, the airway diameter at FRC increases, reflecting the removal of airway tone. Thus, in the absence of tone, the only component that is measured as distensibility is the distension of the airway wall from FRC to TLC. The airway wall, obviously, has a limit, which may be influenced by factors such as remodeling and airway wall stiffness. So, we believe that tone plays a major role when distensibility is examined at baseline; however, after relaxation with albuterol, distensibility becomes a reflection of intrinsic airway structure and stiffness. In other words, distensibility at baseline is an entity different from distensibility after removal of tone (13).
Brown et al. (3), using a forced oscillation technique, found no change in airway distensibility in asthmatic individuals after bronchodilator treatment. Our dissimilar results are likely due to methodological differences. Forced oscillation is an indirect method to assess airway caliber. Given the oscillation frequency measured, the exact location of the airways measured is not known, and some fraction of parenchyma is likely included in the measurements. HRCT only measured the conducting airways visualized.
In summary, our results suggest that the major factor in the loss of the bronchodilatory effect of DI in asthma is air trapping. We speculate that reduced airway distensibility with lung inflation, particularly in larger airways, may lead to dynamic hyperinflation, causing air trapping. Airway wall thickness in the conducting airways does not appear to play a role in limiting the bronchodilatory ability of lung inflation.
GRANTS
G. Pyrgos is the recipient of the George Behrakis Hellenic Fellowship in Respiratory Allergy at the Johns Hopkins Asthma and Allergy Center. This work was supported by National Heart, Lung, and Blood Institute Grants RO1 HL-61277, R01 HL-62698, and P01 HL-10342.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Amirav I, Kramer S, Grunstein M, Hoffman E. Assessment of methacholine-induced airway constriction by ultrafast high-resolution computed tomography. J Appl Physiol 75: 2239–2250, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Blackie SP, al-Majed S, Staples CA, Hilliam C, Pare PD. Changes in total lung capacity during acute spontaneous asthma. Am Rev Respir Dis 142: 79–83, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Brown NJ, Salome CM, Berend N, Thorpe CW, King GG. Airway distensibility in adults with asthma and healthy adults, measured by forced oscillation technique. Am J Respir Crit Care Med 176: 129–137, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Brown R, Scichilone N, Mudge B, Diemer F, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 163: 994–1001, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Brown R, Zerhouni E, Mitzner W. Visualization of airway obstruction in vivo during lung vascular engorgement and edema. J Appl Physiol 78: 1070–1078, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Brown RH, Croisille P, Mudge B, Diemer F, Permutt S, Togias A. Airway narrowing in healthy humans inhaling methacholine without deep inspirations demonstrated by HRCT. Am J Respir Crit Care Med 161: 1256–1263, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Brown RH, Georgakopoulos J, Mitzner W. Individual airway responsiveness to aerosol histamine and methacholine in vivo. Am J Respir Crit Care Med 157: 491–497, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Brown RH, Mitzner W. Airway response to deep inspiration: role of inflation pressure. J Appl Physiol 91: 2574–2578, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Brown RH, Mitzner W. Airway response to deep inspiration: role of nitric oxide. Eur Respir J 22: 57–61, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Brown RH, Mitzner W. Duration of deep inspiration and subsequent airway constriction in vivo. J Asthma 40: 119–124, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Brown RH, Mitzner W. Effect of lung inflation and airway muscle tone on airway diameter in vivo. J Appl Physiol 80: 1581–1588, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Brown RH, Mitzner W, Bulut Y, Wagner EM. Effects of lung inflation in vivo on airways with smooth muscle tone or edema. J Appl Physiol 82: 491–499, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Brown RH, Mitzner W, Wagner E, Permutt S, Togias A. Airway distension with lung inflation measured by HRCT. Acad Radiol 10: 1097–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Brown RH, Pearse DB, Pyrgos G, Liu MC, Togias A, Permutt S. The structural basis of airways hyperresponsiveness in asthma. J Appl Physiol 101: 30–39, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Brown RH, Scichilone N, Mudge B, Diemer F, Permutt S, Togias A. High resolution computed tomographic evaluation of airways distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 163: 994–1001, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Fredberg J. Frozen objects: small airways, big breaths, asthma. J Allergy Clin Immunol 106: 615–624, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and the dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 81: 2703–2712, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Gelb AF, Licuanan J, Shinar CM, Zamel N. Unsuspected loss of lung elastic recoil in chronic persistent asthma. Chest 121: 715–721, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Gelb AF, Zamel N. Unsuspected pseudophysiologic emphysema in chronic persistent asthma. Am J Respir Crit Care Med 162: 1778–1782, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med 153: 582–589, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Gunst S, Wu M. Plasticity of airway smooth muscle stiffness and extensibility: role of length-adaptive mechanisms. J Appl Physiol 90: 741–749, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Gunst SJ, Shen X, Ramchandani R, Tepper RS. Bronchoprotective and bronchodilatory effects of deep inspiration in rabbits subjected to bronchial challenge. J Appl Physiol 91: 2511–2516, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. The potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol 89: 711–720, 2000 [DOI] [PubMed] [Google Scholar]
- 26. King GG, Moore BJ, Seow CY, Pare PD. Time course of increased airway narrowing caused by inhibition of deep inspiration during methacholine challenge. Am J Respir Crit Care Med 160: 454–457, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Lambert RK, Codd SL, Alley MR, Pack RJ. Physical determinants of bronchial mucosal folding. J Appl Physiol 77: 1206–1216, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Martin J, Powell E, Shore S, Emrich J, Engel LA. The role of respiratory muscles in the hyperinflation of bronchial asthma. Am Rev Respir Dis 121: 441–447, 1980 [DOI] [PubMed] [Google Scholar]
- 29. Milanese M, Crimi E, Scordamaglia A, Riccio A, Pellegrino R, Canonica GW, Brusasco V. On the functional consequences of bronchial basement membrane thickening. J Appl Physiol 91: 1035–1040, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Pellegrino R, Violante B, Nava S, Rampulla C, Brusasco V, Rodarte JR. Expiratory airflow limitation and hyperinflation during methacholine-induced bronchoconstriction. J Appl Physiol 75: 1720–1727, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Pyrgos G, Kapsali T, Permutt S, Togias A. Absence of deep inspiration-induced bronchoprotection against inhaled allergen. Am J Respir Crit Care Med 167: 1660–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med 162: 910–916, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Scichilone N, Marchese R, Catalano F, Togias A, Vignola AM, Bellia V. The bronchodilatory effect of deep inspiration diminishes with aging. Respir Med 98: 838–843, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Scichilone N, Marchese R, Soresi S, Interrante A, Togias A, Bellia V. Deep inspiration-induced changes in lung volume decrease with severity of asthma. Respir Med 101: 951–956, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Scichilone N, Permutt S, Bellia V, Togias A. Inhaled corticosteroids and the beneficial effect of deep inspiration in asthma. Am J Respir Crit Care Med 172: 693–699, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med 163: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Scichilone N, Pygros G, Kapsali T, Anderlind C, Brown RH, Permutt S, Togias A. Airways hyperresponsiveness and the effects of lung inflation. Int Arch Allergy Immunol 124: 262–266, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Scichilone N, Togias A. The role of lung inflation in airway hyperresponsiveness and in asthma. Curr Allergy Asthma Rep 4: 166–174, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol 24: 55–72, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176: 121–128, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, Jarjour NN, Moore WC, Peters SP, Teague WG, Wenzel SE. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol 104: 394–403, 2008 [DOI] [PubMed] [Google Scholar]